Abstract
The values of the second dissociation constant pK2 and related thermodynamic quantities of the ampholyte 3-(N-morpholino)-2-hydroxypropanesulfonic acid (MOPSO) have been previously determined at temperatures from (278.15 to 328.15) K. In this study, the pH values of two buffer solutions without NaCl and three buffer solutions with NaCl having ionic strengths (I = 0.16 mol·kg−1) similar to those in blood plasma, have been evaluated at 12 temperatures from (278.15 to 328.15) K using an extended form of the Debye-Hückel equation, since the Bates-Guggenheim convention is valid up to I = 0.1 mol·kg−1. The liquid junction potentials (Ej) between the buffer solutions of MOPSO and saturated KCl solution of the calomel electrode at (298.15 and 310.15) K have been estimated by measurement with a flowing junction cell. These values of Ej have been used to ascertain the operational pH values at (298.15 and 310.15) K. Three buffer solutions of MOPSO are recommended as useful reference solutions for pH measurements in saline media of ionic strength I = 0.16 mol·kg−1.
Introduction
The buffer substances recommended by Good et al.1,2 have proven very useful for the measurement of the pH of blood and the control of pH in the region close to that of physiological solutions. Very recently, we have reported the pH values of 3-[(1, 1-dimethyl-2-hydroxymethyl)amino]-2-hydroxypropanesulfonic acid (AMPSO)3 at temperatures from (278.15 to 328.15) K including 310.15 K. The zwitterionic buffer N-[tris(hydroxymethyl)methyl-3-amino]propanesulfonic acid (TAPS)4 has also been recommended for use as a physiological buffer at (298.15 and 310.15) K. In the present investigation, we are interested in providing reliable pH values for the ampholyte 3-(N-morpholino)-2-hydroxypropanesulfonic acid, (MOPSO), which has the structure as follows:
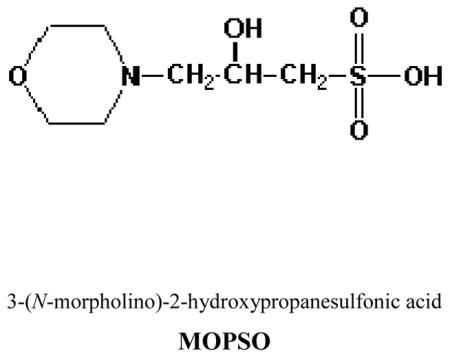
Bates and his associates5 reported pH values of N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid (TES) at (298.15 and 310.15) K. These buffer solutions are recommended as standard buffers for pH measurements.
For the highest reproducibility and accuracy, the glass electrode pH meter assembly at a point close to the pH of blood (7.407) can be obtained within the framework of the National Institute of Standards and Technology (NIST/NBS) by using physiological phosphate pH buffer as a primary standard.6 The pH of this physiological phosphate buffer standard is 7.415 at 298.15 K and 7.395 at 310.15 K, and has been internationally used for standardization at or close to the pH of the clinical sample.
Various attempts to establish a suitable primary reference standard at an isotonic saline solution, I = 0.16 mol·kg−1 and near the pH of blood plasma have been met with difficulty. The commonly accepted physiological phosphate standard solutions are mixtures of KH2PO4 (0.008695 mol·kg−1) and Na2HPO4 (0.03043 mol·kg-1). The problems associated with the use of the physiological phosphate solutions are: (i) phosphates interact unfavorably with biological media, (ii) phosphate precipitates with blood ingredients (Mg2+ and Ca2+), and (iii) the temperature coefficient of blood is (−0.015 pH unit/K) as compared to 1:3.5 phosphate standard (−0.0028 pH unit/K).7 The compound MOPSO is not expected to have any undesirable side effects (no precipitation with Ca2+ and Mg2+), but the possibility of complex formation with cations such as Ca2+ and Mg2+ exists. We have attempted to minimize it with high concentration of sodium chloride-buffer ratio for an isotonic saline solution of I = 0.16 mol·kg−1.
Good and his associates1,2 introduced a series of new hydrogen ion buffers for use in the physiological pH range. The authors took the liberty of citing some published works by various investigators for structurally related zwitterionic buffer compounds with a view to comparing the effects of substituents on pK2 and pH values. Wu and coworkers8 have published the values of pK2 and pH of the zwitterionic buffer N-(2-hydroxyethyl)piperazine-N-2-ethanesulfonic acid (HEPES), and a second zwitterionic buffer, 3-(N-morpholino)-2-hydroxypropanesulfonic acid (MOPSO).9 Roy et al.10 reported results for pK2 and pH for 3-(N-morpholino)propanesulfonic acid (MOPS) and 4-N-(morpholino)butanesulfonic acid (MOBS).11 The pH of these solutions closely match that of the common biological media. In 1973, Bates et al.12 suggested the use of tris(hydroxymethyl)methylglycine (TRICINE) as a secondary buffer standard for the physiological range of pH 7.2 to 8.5. The pH of 0.06 m TRICINE + 0.02 m sodium TRICINEate buffer solution at 310.15 K is 7.407, matching exactly the pH of blood. Goldberg et al.,13 in their excellent review article of the thermodynamic quantities of the biological buffers, indicated that the results for pK2 are available in the literature for MOPSO. To the authors’ knowledge, no reliable pH values of MOPSO for the buffer compositions under study have been reported.
In order to provide accurate and reproducible pH values for physiological pH standards, we have studied the buffer compound, MOPSO, with the following compositions on the scale of molality (m), where m = mol·kg−1, and I is the ionic strength in the unit of mol·kg−1:
MOPSO (0.02 mol·kg−1) + NaMOPSO (0.06 mol·kg−1), I = 0.06 mol·kg−1
MOPSO (0.02 mol·kg−1) + NaMOPSO (0.04 mol·kg−1), I = 0.04 mol·kg−1
MOPSO (0.01 mol·kg−1) + NaMOPSO (0.03 mol·kg−1) + NaCl (0.13 mol·kg−1), I = 0.16 mol·kg−1
MOPSO (0.02 mol·kg−1) + NaMOPSO (0.06 mol·kg−1) + NaCl (0.10 mol·kg−1), I = 0.16 mol·kg−1
MOPSO (0.04 mol·kg−1) + NaMOPSO (0.04 mol·kg−1) + NaCl (0.12 mol·kg−1), I = 0.16 mol·kg−1
The detailed procedure for the preparation of these buffer solutions for MOPSO is described in the following section.
Experimental
MOPSO was purchased from the Sigma Chemical Co. (St. Louis, Missouri). The purification procedure (using further crystallization) and the assay have been reported in a previous paper.5 The assay showed that the MOPSO buffer used was (99.91 to 99.97) % pure. All buffer solutions were prepared by massing the MOPSO, NaCl (ACS reagent grade), a standard solution of NaOH for the preparation of NaMOPSO, and calculated amounts of CO2- free doubly distilled water. Buoyancy corrections were made for all masses used to prepare solutions.
The cell design, the preparation procedure of the hydrogen electrodes using chloroplatinic acid, the silver-silver chloride thermal, electrolytic electrodes,14 hydrogen gas purification, and preparation of the solutions have been described previously.3,10 Details about the control of temperature (within ± 0.005 K)3 using a digital platinum resistance thermometer (Guildline Model 9540), a digital voltmeter (Hewlett-Packard 2000 multimeter), and other experimental procedures, will also be found elsewhere.3
Methods and Results
The values of cell potential for the calculations of pH are given in Tables 1 and 2 for cell (A) containing 2 buffer solutions lacking NaCl, and 3 buffer solutions in which NaCl had been added to make I = 0.16 mol·kg−1, respectively. These values have been corrected to a hydrogen pressure of 101.325 kPa. At 298.15 K, cell potential values are the average of at least 2 readings at the beginning, the middle, and sometimes at the end of the temperature sequence. Duplicate cells usually gave readings on the average within 0.04 mV in the temperature range (278.15 to 328.15) K.
Table 1.
Cell Potential of Cell A (in Volts): Pt(s); H2 (g), 101.325 kPa | MOPSO (m1), NaMOPSO (m2), NaCl (m3) | AgCl(s), Ag(s)
| mol·kg−1 | T/K | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m1 | m2 | m3 | 278.15 | 283.15 | 288.15 | 293.15 | 298.15 | 303.15 | 308.15 | 310.15 | 313.15 | 318.15 | 323.15 | 328.15 |
| 0.02 | 0.06 | 0.005 | 0.78522 | 0.78765 | 0.79014 | 0.79235 | 0.79454 | 0.79663 | 0.79830 | 0.79933 | 0.80035 | 0.80232 | 0.80380 | 0.80535 |
| 0.02 | 0.06 | 0.010 | 0.76992 | 0.77207 | 0.77410 | 0.77617 | 0.77823 | 0.78003 | 0.78121 | 0.78229 | 0.78327 | 0.78461 | 0.78576 | 0.78689 |
| 0.02 | 0.06 | 0.015 | 0.76144 | 0.76351 | 0.76535 | 0.76706 | 0.76951 | 0.77114 | 0.77179 | 0.77318 | 0.77395 | 0.77514 | 0.77614 | 0.77704 |
| 0.02 | 0.06 | 0.020 | 0.75575 | 0.75787 | 0.75966 | 0.76130 | 0.76347 | 0.76500 | 0.76522 | 0.76684 | 0.76750 | 0.76854 | 0.76941 | 0.77017 |
| 0.02 | 0.04 | 0.005 | 0.77350 | 0.77583 | 0.77798 | 0.78017 | 0.78180 | 0.78395 | 0.78552 | 0.78626 | 0.78709 | 0.78859 | 0.78954 | 0.79070 |
| 0.02 | 0.04 | 0.010 | 0.75766 | 0.75980 | 0.76168 | 0.76337 | 0.76456 | 0.76681 | 0.76821 | 0.76883 | 0.76953 | 0.77118 | 0.77149 | 0.77203 |
| 0.02 | 0.04 | 0.015 | 0.74873 | 0.75053 | 0.75232 | 0.75389 | 0.75495 | 0.75704 | 0.75830 | 0.75885 | 0.75946 | 0.76139 | 0.76116 | 0.76182 |
| 0.02 | 0.04 | 0.020 | 0.74283 | 0.74447 | 0.74609 | 0.74752 | 0.74821 | 0.75063 | 0.75175 | 0.75220 | 0.75274 | 0.75489 | 0.75422 | 0.75478 |
Table 2.
Cell Voltage of Cell A (in Volts): Pt(s); H2 (g), 101.325 kPa | MOPSO (m1), NaMOPSO (m2), NaCl (m3) | AgCl(s), Ag(s)
| mol·kg−1 | T/K | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m1 | m2 | m3 | 278.15 | 283.15 | 288.15 | 293.15 | 298.15 | 303.15 | 308.15 | 310.15 | 313.15 | 318.15 | 323.15 | 328.15 |
| 0.01 | 0.03 | 0.13 | 0.71369 | 0.71524 | 0.71636 | 0.71726 | 0.71798 | 0.71858 | 0.71905 | 0.71918 | 0.71932 | 0.71944 | 0.71945 | 0.71934 |
| 0.02 | 0.06 | 0.10 | 0.71951 | 0.72100 | 0.72226 | 0.72333 | 0.72429 | 0.72504 | 0.72568 | 0.72592 | 0.72616 | 0.72651 | 0.72666 | 0.72680 |
| 0.04 | 0.04 | 0.12 | 0.74072 | 0.74249 | 0.74407 | 0.74558 | 0.74704 | 0.74812 | 0.74920 | 0.74955 | 0.75007 | 0.75078 | 0.75132 | 0.75171 |
The pH of MOPSO Buffer
The conventional standard pH values have been evaluated by the method of Bates et al. 3, 9–10, 15–17 for 5 standard buffer solutions, stated in the introduction section, (a to e). For accurate calculations of the second dissociation constants, pK2; and pH values of the 5 buffer solutions, the following cell (A) is used for the collection of cell potential data
| (A) |
where m1, m2, and m3 indicate molalities of the respective species, and 1 atm = 101.325 kPa in SI units. The cell (A) is known as the Harned-type cell.
The flowing junction cell (B), was used for the evaluation of the liquid junction potential at the contact between the buffer solution and the heavier saturated KCl solution shown with a double vertical line.
| (B) |
where the abbreviations (s), (l), and (g) denote solid, liquid, and gaseous state, respectively. In routine laboratory measurements, the hydrogen electrode is commonly replaced by a glass electrode. For the cell (B), the values of the standard electrode potential, , of the saturated calomel electrode were taken as: −0.2415 V, and −0.2335 V at (298.15 and 310.15) K, respectively. These values are periodically rechecked with experiments.
For cell (C), the phosphate salts were NIST standard reference materials with the composition [KH2PO4 (0.008695 mol·kg−1) + Na2HPO4 (0.03043 mol·kg−1)] and its solutions are recommended for pH measurements in physiological solutions.
| (C) |
It should be emphasized that the difference in values between the liquid junction potential when one solution (the pH standard) is replaced by another (the unknown) is important. The values of the liquid junction potential, Ej, for the physiological phosphate solutions and other buffer solutions of MOPSO from cell (B) were obtained8,10 using the flowing junction cell. The equation for the calculation of Ej10 is
| (1) |
where , k = 0.059156, and pH = 7.415 (physiological phosphate buffer solution) at 298.15 K; , k = 0.061538, and pH = 7.395 at 310.15 K. The operational definition of pH, designated as pH(x), is
| (2) |
where “x” refers to the unknown buffer MOPSO + NaMOPSO; “s” is the reference solution (NBS/NIST physiological phosphate buffer) of known pH, and δEj = Ej(s) − Ej(x). If δEj = 0, then eq 3 takes the form
| (3) |
It is important to mention that eq 3 is more common, as δEj (the difference) is all that is needed, not Ej in mV.
In order to calculate pH(s) values for all 5 buffer solutions, calculations for the acidity function p(aHγCl) values were made in the temperature range (278.15 to 328.15) K, from the cell potential (E) listed in Tables 1 and 2, the molality of the chloride ion, and E°, the standard potential of the silver-silver chloride electrode.3 The Nernst equation12,14,17 for cell (A) is given by:
| (4) |
where k is the Nernst slope.
From the plot of the acidity function, p(aHγCl), for each buffer solution against the molality of the chloride ion employing linear regression analysis, the intercept, p(aHγCl)°, at mCl = 0 was obtained. These values of p(aHγCl)° for 2 chloride-free buffer solutions listed above are given in Table 3. The uncertainty (mean deviation) introduced in this type of graphical extrapolation appeared to be slightly greater than 0.001 from the lines drawn. For 3 buffer solutions in the presence of NaCl (c to e), the values of p(aHγCl) are entered in Table 4 from (278.15 to 328.15) K.
Table 3.
p(aHγCl)o of (MOPSO + NaMOPSO) Buffer Solutions from (278.15 to 328.15) K Obtained by Extrapolation for Chloride-Free Solutions a
| T(K) | 0.02 m MOPSO + 0.04 m NaMOPSO |
0.02 m MOPSO + 0.06 m NaMOPSO |
|---|---|---|
| I = 0.04 m | I = 0.06 m | |
| 278.15 | 7.455 | 7.662 |
| 283.15 | 7.374 | 7.575 |
| 288.15 | 7.293 | 7.496 |
| 293.15 | 7.219 | 7.420 |
| 298.15 | 7.142 | 7.344 |
| 303.15 | 7.073 | 7.275 |
| 308.15 | 7.002 | 7.208 |
| 310.15 | 6.978 | 7.181 |
| 313.15 | 6.937 | 7.142 |
| 318.15 | 6.868 | 7.084 |
| 323.15 | 6.807 | 7.022 |
| 328.15 | 6.743 | 6.964 |
m = 1 mol·kg−1
Table 4.
| T(K) | 0.01 m MOPSO + 0.03 m NaMOPSO + 0.13 m NaCl |
0.02 m MOPSO + 0.06 m NaMOPSO + 0.10 m NaCl |
0.04 m MOPSO + 0.12 m NaMOPSO + 0.04 m NaCl |
|---|---|---|---|
| I = 0.16 ma | I = 0.16 ma | I = 0.16 ma | |
| 278.15 | 7.803 | 7.794 | 7.781 |
| 283.15 | 7.725 | 7.713 | 7.698 |
| 288.15 | 7.645 | 7.634 | 7.617 |
| 293.15 | 7.566 | 7.557 | 7.541 |
| 298.15 | 7.491 | 7.483 | 7.470 |
| 303.15 | 7.417 | 7.411 | 7.397 |
| 308.15 | 7.346 | 7.341 | 7.327 |
| 310.15 | 7.318 | 7.314 | 7.300 |
| 313.15 | 7.277 | 7.273 | 7.260 |
| 318.15 | 7.210 | 7.208 | 7.194 |
| 323.15 | 7.144 | 7.143 | 7.130 |
| 328.15 | 7.080 | 7.081 | 7.066 |
m = 1 mol·kg−1
Conventional pH(s) values determined from the cell potential of cells without liquid junction for the solution without the presence of the chloride ion were determined by the equation
| (5) |
where the single-ion activity coefficient, , cannot be measured experimentally. The estimation of for the calculation of pH(s) by eq 6 has been outlined before.10 The pH values obtained from the liquid junction cell are indicated by pH, whereas the “conventional” pH calculated from eq 6 is designated as pH(s). The “pH convention,” commonly known as Bates-Guggenheim convention,18 is expressed by the following equation
| (6) |
The International Union of Pure and Applied Chemistry19 has recommended this convention. It has been assumed that eq 6 is valid up to I = 0.1 mol·kg−1. For I > 0.1 mol·kg−1, there is no widely accepted (agreed-upon) convention. Perhaps a linear dependent CI term from eq 7 along with a variation of the ion-size parameter as a function of temperature would provide a more logical choice when I > 0.1 mol·kg−1.
Thus a “pH convention”8,10 based on an extended Debye-Hückel equation8 has been assumed to be more logical when I > 0.1 mol·kg−1 up to I = 1.0 mol·kg−1 in the calculation of for all of the buffer-chloride solutions. The following equation is preferred:
| (7) |
where I is the ionic strength of the buffer solution, A and B are the Debye-Hückel constants, C is an adjustable parameter, Ba° was assumed to be 1.38 kg½·mol−½ for all the experimental temperatures, corresponding to an (ion-size parameter) a° of 4.2 Å.8, 10 The empirical equation given below for the calculation of the parameter C8,10 was obtained from a curve-fitting method:
| (8) |
where C298.15 = 0.0328 kg·mol−1 at 298.15 K and T is the absolute temperature.
The values of pH(s), listed in Table 5 for two buffer solutions of MOPSO without NaCl were computed from eqs 4 – 8 and are represented by the following equations:
| (9) |
| (10) |
where (278.15 ≤ T ≤ 328.15) K. The standard deviations of regression for the pH(s) of the chloride-free buffer solutions, obtained from the fits with eqs 12 – 13, are 0.0019, and 0.0015, respectively.
Table 5.
| T(K) | 0.02 m MOPSO + 0.06 m NaMOPSO |
0.02 m MOPSO + 0.04 m NaMOPSO |
|---|---|---|
| I = 0.06 ma | I = 0.04 ma | |
| 278.15 | 7.572 | 7.378 |
| 283.15 | 7.484 | 7.297 |
| 288.15 | 7.405 | 7.215 |
| 293.15 | 7.329 | 7.141 |
| 298.15 | 7.252 | 7.065 |
| 303.15 | 7.182 | 7.994 |
| 308.15 | 7.115 | 7.922 |
| 310.15 | 7.088 | 7.898 |
| 313.15 | 7.049 | 7.857 |
| 318.15 | 6.990 | 6.787 |
| 323.15 | 6.927 | 6.725 |
| 328.15 | 6.869 | 6.661 |
m = 1 mol·kg−1
For 3 buffer solutions containing NaCl at an isotonic saline media total ionic strength of I = 0.16 mol·kg−1, the values of pH(s) calculated using eq 4 – 8 and from the acidity function data listed in Table 4 are entered in Table 6. These values of pH(s) are expressed by the following equations:
| (11) |
| (12) |
| (13) |
where T is the temperature in K. The standard deviations for regression of the “observed” results from eqs 11 – 13 are 0.0009, 0.0004, and 0.0014, respectively.
Table 6.
| T(K) | 0.01 m MOPSO + 0.03 m NaMOPSO + 0.13 m NaCl |
0.02 m MOPSO + 0.06 m NaMOPSO + 0.10 m NaCl |
0.04 m MOPSO + 0.12 m NaMOPSO + 0.04 m NaCl |
|---|---|---|---|
| I = 0.16 ma | I = 0.16 ma | I = 0.16 ma | |
| 278.15 | 7.677 | 7.669 | 7.655 |
| 283.15 | 7.599 | 7.588 | 7.572 |
| 288.15 | 7.519 | 7.508 | 7.492 |
| 293.15 | 7.441 | 7.432 | 7.416 |
| 298.15 | 7.364 | 7.357 | 7.343 |
| 303.15 | 7.290 | 7.284 | 7.270 |
| 308.15 | 7.219 | 7.213 | 7.200 |
| 310.15 | 7.190 | 7.186 | 7.172 |
| 313.15 | 7.148 | 7.144 | 7.131 |
| 318.15 | 7.080 | 7.078 | 7.065 |
| 323.15 | 7.014 | 7.013 | 7.999 |
| 328.15 | 7.949 | 6.950 | 7.934 |
m = 1 mol·kg−1
The operational pH values at (298.15 and 310.15) K were evaluated from cells with liquid junctions cells (B and C) by means of the flowing junction cell.8,10 The cell potential values of the cells (B and C) at (298.15 and 310.15) K are given in Table 7. The values of Ej listed in Table 8 were obtained by using eq 1. The widely used equation for the calculation of is based on the Bates - Guggenheim convention,3, 5, 7–8 and is valid up to I = 0.1 mol·kg−1.6,17–19 The combined standard uncertainty for the pH(s) values was accounted for by combining the various known sources of error: (i) assumption for the calculation of the using eq 7 (± 0.004 pH unit), (ii) extrapolation of the p(aHγCl)° plot for chloride-free solutions (less than ± 0.002 pH unit), and (iii) error in the experimental measurement from the multimeter (± 0.02 mV). Thus the overall estimated uncertainty is ± 0.006 and ± 0.012 pH unit for buffers without the presence of NaCl and with the ionic strength I = 0.16 mol·kg−1, respectively. Errors in the values of Ej are irrelevant to the values of pH(s) determined from cell (A) without liquid junction; however, the δEj of eq 2 does affect the operational pH values listed in Table 9 at (298.15 and 310.15) K. These are recommended as useful secondary pH standards for calibrating electrodes for pH measuring assembly in the range of physiological interest. The consistency of the three sets of experiments listed in Table 9 leads credence in the pH values of MOPSO buffer solutions.
Table 7.
Cell Voltage of Cell B for MOPSO buffer
| mol·kg−1 | E/V | |||
|---|---|---|---|---|
| m1 | m2 | m3 | 298.15 K | 310.15 K |
| 0.02 | 0.06 | 0.10 | 0.67722 | 0.69514 |
| 0.04 | 0.04 | 0.12 | 0.67633 | 0.67556 |
| 0.01 | 0.03 | 0.13 | 0.67745 | 0.67672 |
| Cell Voltage of Cell Ca | ||
|---|---|---|
| Cell C | E, V | |
| 0.008695 m KH2PO4 | 298.15 K | 310.15 K |
| + 0.03043 m Na2HPO4 | 0.68275 | 0.69147 |
Corrected to a hydrogen pressure of 101.325 kPa, for physiological phosphate buffer solutions (primary reference standard buffer) at (298.15 and 310.15) K.
Table 8.
Values of the liquid junction potentials for MOPSO at (298.15 and 310.15) K
|
Eja/mV |
||
|---|---|---|
| System | 298.15 K | 310.15 K |
| Physiological phosphate (0.008695 m KH2PO4 + 0.03043 m NaCl) | 2.6 | 2.9 |
| 0.02 m MOPSO + 0.06 m NaMOPSO + 0.10 m NaCl | 0.5 | 0.7 |
| 0.04 m MOPSO + 0.04 m NaMOPSO + 0.12 m NaCl | 0.5 | 0.8 |
| 0.01 m MOPSO + 0.03 m NaMOPSO + 0.13 m NaCl | 0.4 | 0.8 |
- k pH from eq 1, E is the cell voltage from Table 5, k = Nernst slope with values 0.059156 at 298.15 K, and 0.061538 at 310.15 K; the pH of the primary reference standard phosphate buffer is 7.415 and 7.395 at (298.15 and 310.15) K; = electrode potential of the saturated calomel electrode = −0.2415 V and −0.2335 V at (298.15 and 310.15) K, respectively.
Table 9.
Values of pH at (298.15 to 310.15) K for MOPSO Buffer Solutions
| Cell B |
T = 298.15 K |
T = 310.15 K |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| mol·kg−1 |
pH |
||||||||
| m1 | m2 | m3 | I | WithoutaEj corr | WithbEj corr | Calcc | WithoutaEj corr | WithbEj corr | Calcc |
| 0.02 | 0.06 | 0.10 | 0.16 | 7.322 | 7.357 | 7.357 | 7.149 | 7.185 | 7.186 |
| 0.04 | 0.04 | 0.12 | 0.16 | 7.307 | 7.342 | 7.343 | 7.137 | 7.171 | 7.172 |
| 0.01 | 0.03 | 0.13 | 0.16 | 7.326 | 7.363 | 7.364 | 7.156 | 7.190 | 7.190 |
pH = 7.415 + [(E − 0.68275)/0.059156] at 298.15 K, and pH = 7.395 + [(E − 0.69144)/0.061538] at 310.15 K; the cell potential values (Table 7) are 0.68275 V and 0.69144 V at (298.15 to 310.15) K for the physiological phosphate buffer standard solution.
Acknowledgments
The authors are grateful to Dr. R. G. Bates for useful discussions for the project funded by the National Institutes of Health (AREA), under grant R15 GM 066866-01.
References
- 1.Good NE, Winget GD, Winter W, Connolly TN, Izawa S, Singh RMM. Hydrogen Ion Buffers for Biological Research. Biochemistry. 1966;5:467 – 477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson WJ, Braunschweiger KI, Braunschweiger WR, Smith JR, McCormick JJ, Wasmann CC, Jarvis NP, Bell DH, Good NE. Hydrogen Ion Buffers for Biological Research. Anal Biochem. 1980;104:300 – 310. doi: 10.1016/0003-2697(80)90079-2. [DOI] [PubMed] [Google Scholar]
- 3.Roy RN, Roy LN, Denton CE, LeNoue SR, Ashkenazi S, Fuge MS, Dunseth CD, Durden JL, Roy CN, Bwashi A, Wollen JT, DeArmon SJ. Buffer Standards for the Physiological pH of 3-[(1,1-Dimethyl-2-hydroxymethyl)amino]-2-hydroxypropanesulfonic Acid from (278.15 K to 328.15) K. J Chem Eng Data. 2008 doi: 10.1021/je800983y. Accepted for publication, special issue “Robin H. Stokes Festschrift”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy LN, Roy RN, Denton CE, LeNoue SR, Himes CA, Richards SJ, Simon AN, Roy CN, Somal VS. Buffer Standards for the Physiological pH of the Zwitterionic Compound, TAPS from 278.15 to 328.15 K. J Solution Chem. 2006;35:551 – 566. [Google Scholar]
- 5.Bates RG, Vega CA, White DR., Jr Standards for pH Measurements in Isotonic Saline Media of Ionic Strength I = 0.16. Anal Chem. 1978;50:1295 – 1300. [Google Scholar]
- 6.Bower VE, Paabo M, Bates RG. A Standard for the Measurement of the pH of Blood and Other Physiological Media. J Res Natl Bur Stand. 1961;65A:267 – 270. doi: 10.6028/jres.065A.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durst RA, Staples BR. Tris/Tris HC1: Standard Buffer for Use in the Physiological pH Range. Clin Chem. 1972;18:206 – 208. [PubMed] [Google Scholar]
- 8.Feng D, Koch WF, Wu YC. Second Dissociation Constant and pH of N-(2-Hydroxyethyl)piperazine-N′-2-ethanesulfonic Acid from 0 to 50 K. Anal Chem. 1989;61:1400 – 1405. [Google Scholar]
- 9.Wu YC, Berezansky PA, Feng D, Koch WF. Second Dissociation Constant of 3-(N-Morpholino)-2-hydroxypropanesulfonic Acid and pH of Its Buffer Solutions. Anal Chem. 1993;65:1084 – 1087. [Google Scholar]
- 10.Roy RN, Mrad DR, Lord PA, Carlsten JA, Good WS, Allsup P, Roy LN, Kuhler KM, Koch WF, Wu YC. Thermodynamics of the Second Dissociation Constant and Standards for pH of 3-(N-Morpholino)propanesulfonic Acid (MOPS) from 278.15 to 328.15 K. J Solution Chem. 1998;27:73 – 87. [Google Scholar]
- 11.Roy RN, Roy LN, Grant JG, Cummins MP, Tabor BJ, Richards SJ, Himes CA, Lively BR, Blackwell PL, Simon AN. Second Dissociation Constants of 4-[N-Morpholino]butanesulfonic Acid and N-[2-Hydroxyethyl]piperazine-N′-4-butanesulfonic Acid from 278.15 to 328.15 K. J Solution Chem. 2002;31:861 – 872. [Google Scholar]
- 12.Bates RG, Roy RN, Robinson RA. Buffer Standards of Tris(hydroxymethyl)methylglycine (“Tricine”) for the Physiological Range pH 7.2 to 8.5. Anal Chem. 1973;45:1663 – 1666. doi: 10.1021/ac60331a022. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg RN, Kishore N, Lennen RM. Thermodynamic quantities for the Ionization Reactions of Buffers. J Phys Chem Ref Data. 2002;31:231 – 370. [Google Scholar]
- 14.Bates RG. Determination of pH. 2. Vol. 4. Wiley; New York: 1973. p. 10. [Google Scholar]
- 15.Roy RN, Roy LN, Fuge MS, Roy CN, Himes CA, Bryant PA, Robinson KT, Kaufmann DA, II, Grove CH, Ghosh T, Bwashi A. Buffer Standards for the Physiological pH of the Zwitterionic Compound, ACES from 5 to 55°C. J Solution Chem. 2008 doi: 10.1007/s10953-009-9380-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy LN, Roy RN, Denton CE, LeNoue SR, Roy CN, Ashkenazi S, Williams TR, Church DR, Fuge MS, Sreepada KN. Second Dissociation Constant of Bis[(2-hydroxyethyl)amino]acetic Acid (BICINE) and pH of Its Buffer Solutions from 5 to 55°C. J Solution Chem. 2006;35:605 – 624. [Google Scholar]
- 17.Roy RN, Roy LN, Ashkenazi S, Wollen JT, Dunseth CD, Fuge MS, Roy CN, Hughes HM, Morris BT, Cline KL. Buffer Standards for pH Measurement of N-(2-Hydroxyethyl)piperazine-N′-2-ethanesulfonic Acid (HEPES) for I=0.16 mol·kg−1 from 5 to 55°C. J Solution Chem. 2008 doi: 10.1007/s10953-009-9378-3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bates RG, Guggenheim EA. Report on the Standardization of pH and Related Terminology. Pure Applied Chem. 1960;1:163 – 168. [Google Scholar]
- 19.Buck RP, Rondinini S, Covington AK, Baucke FGK, Brett CMA, Camões MF, Milton MJT, Mussini T, Naumann R, Pratt KW, Spitzer P, Wilson GS. Measurement of pH. Definition, Standards, and Procedures. Pure Applied Chem. 2002;74:2169 – 2200. [Google Scholar]


