Abstract
Background
Despite a non-obstructive coronary angiogram, many patients may still have an abnormal coronary vasomotor response to provocation and to myocardial demand during stress. The ability of non-invasive stress tests to predict coronary vasomotor dysfunction in patients with non-obstructive coronary artery disease is unknown.
Methods and Results
All patients with non-obstructive coronary artery disease who had invasive coronary vasomotor assessment and a non-invasive stress test (exercise ECG, stress echocardiography or stress nuclear imaging) within 6 months of the cardiac catheterization with provocation at our institution were identified (n=376). Coronary vasomotor dysfunction was defined as a percentage increase in coronary blood flow of ≤50% to intracoronary acetylcholine (endothelium-dependent dysfunction) and/or a coronary flow reserve ratio of ≤2.5 to intracoronary adenosine (endothelium-independent dysfunction). We determined the sensitivity and specificity of various non-invasive stress tests to predict coronary vasomotor dysfunction in these patients.
On invasive testing, 233 (63%) had coronary vasomotor dysfunction of which 187 patients (51%) had endothelium-dependent dysfunction, 109 patients (29%) had endothelium-independent dysfunction and 63 patients (17%) had both. On non-invasive stress testing, 157 (42%) had a positive imaging study and 56 (15%) a positive ECG stress test. The non-invasive stress tests had limited diagnostic accuracy for predicting coronary vasomotor dysfunction (41% sensitivity [95%CI 34–47] and 57% specificity [95%CI 49–66]), endothelium-dependent dysfunction (41% sensitivity [95%CI 34–49] and 58% specificity [95%CI 50–65]) or endothelium-independent dysfunction (46% sensitivity [95%CI 37–56] and 61% specificity [95%CI 54–67]. The exercise ECG test was more specific but less sensitive than the imaging tests.
Conclusion
The current study suggests that a negative non-invasive stress test does not rule out coronary vasomotor dysfunction in symptomatic patients with non-obstructive coronary artery disease. This underscores the need for invasive assessment or novel more sensitive non-invasive imaging for these patients.
Keywords: Coronary disease, Microcirculation, Vasomotor Dysfunction, Endothelium, Stress
Introduction
Approximately 20–30% of patients undergoing invasive coronary angiography for chest pain have normal arteries or minimal atherosclerosis1, 2. Abnormal cardiac pain perception3, 4 has been implicated as the etiology for the chest pain in these patients. However, a substantial number of patients with non-obstructive coronary anatomy may still have abnormalities in coronary vasomotor function in response to stress5–9 and possibly myocardial ischemia10–13.
The diagnosis and treatment of patients with coronary vasomotor dysfunction remain a challenge in contemporary practice. The gold standard for the assessment of coronary vasomotor function is invasive cardiac catheterization with intracoronary infusion of endothelium-dependent and endothelium-independent vasodilators14. However, such techniques have inherent risks and are limited to a few catheterization laboratories with expertise. Standard clinical non-invasive stress tests have been proposed to identify coronary vasomotor dysfunction13, 15 but to date their ability to detect endothelium-dependent dysfunction or endothelium-independent dysfunction in the setting of normal coronary arteries is unknown. This study evaluated the sensitivity and specificity of standard non-invasive stress tests for identifying coronary vasomotor dysfunction as identified by invasive studies in patients without obstructive epicardial coronary artery disease.
Methods
Study Design, Study population and Data Collection (Fig. 1)
Fig. 1. Study Cohort Profile.
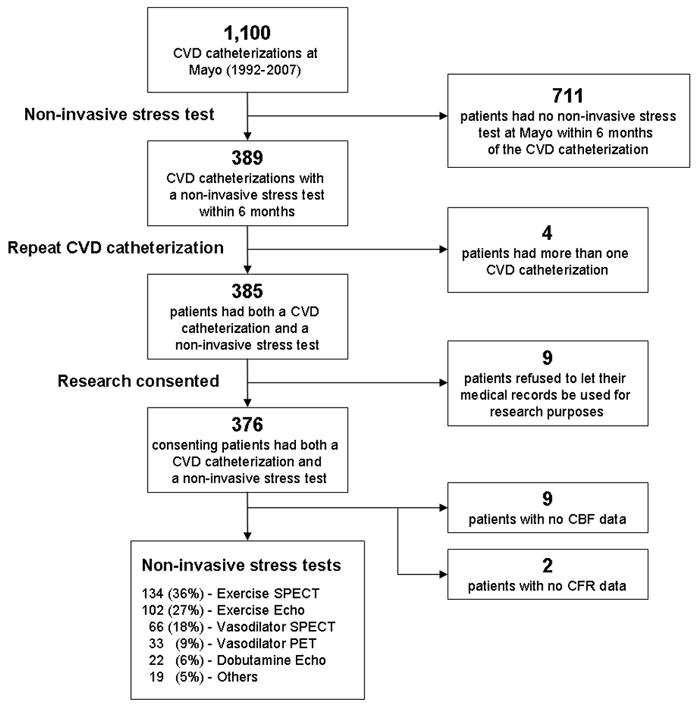
CVD = Coronary Vasomotor Dysfunction, CFR = Coronary Flow Reserve, CBF = Coronary Blood Flow. Exercise SPECT = Exercise Sestamibi (n=114) + Exercise Thallium (n=20); Vasodilator SPECT = Adenosine Sestamibi (n=55) + Adenosine Thallium (n=9) + Dipyridamole Thallium (n=2); Vasodilator PET = Adenosine PET (n=32) + Dipyridamole PET (n=1); Others = Exercise MUGA (n=10) + Dobutamine PET (n=4) + Dobutamine Sestamibi (n=2) + Exercise ECG only (n=2) + Dobutamine Thallium (n=1).
After approval by the Mayo Clinic Institutional Review Board, all patients who had invasive coronary vasomotor assessment at the Mayo Clinic, Rochester MN from December 1992 to August 2007 were identified (n=1100). These patients’ medical records were reviewed by one investigator for any type of non-invasive stress test (exercise ECG, stress echocardiography or stress nuclear imaging) performed within 6 months of the invasive test. There were 389 invasive tests with a non-invasive stress test within this time period. Four patients had two invasive tests performed - only the earliest invasive test was included in the analysis. Thirty-two patients had more than one non-invasive stress test - only the non-invasive stress test in closest temporal relation to the invasive catheterization was included in the study. Nine patients refused use of their medical records for research and were excluded. After the exclusions, the final study population consisted of 376 patients.
Invasive Testing
Study Population
The study population included patients with angiographic coronary artery lesions of <40% luminal diameter stenosis. Exclusion criteria for performing the invasive coronary vasomotor assessment at our institution include a history of ischemic heart disease (myocardial infarction, percutaneous coronary revascularization, CABG, unstable angina), heart failure with an ejection fraction <40%, valvular heart disease, stroke or significant hepatic, renal, diabetic or inflammatory disease within 6 months of the invasive study. Pregnant or lactating patients were excluded. Patients who required treatment with positive inotropic agents other than digoxin during the study were excluded. Long-acting nitratesor calcium channel blockers were withheld for 36 to 48 hours before the study to allow assessment of baseline coronary physiology.
Assessment of Coronary Vasomotor Function
Diagnostic coronary angiography and determination of endothelium-dependent and endothelium-independent function were performed as previously described15–17. In brief, a Doppler guide-wire (FloWire, Volcano Corp, Rancho Cordova, CA, USA) within a coronary infusion catheter was positioned in the mid-portion of the left anterior descending coronary artery. Average peak velocity (APV) was obtained from Doppler flow velocity spectra18. Coronary artery diameter (CAD) measurements were made in the segment 5 mm distal to the tip of the Doppler using an online quantitative coronary angiography program (Medis Corporation)19. Analysis of data from our laboratory demonstrates that the interobserver and intraobserver variation is 8±3%.
Intracoronary bolus injections of incremental doses (18 to 42 μg) of adenosine were administered into the guiding catheter until maximal hyperemia was achieved. The endothelium-independent coronary flow reserve (CFR) ratio was calculated by dividing the APV after adenosine injection by the baseline APV20. We performed intracoronary adenosine instead of intravenous adenosine to avoid confounding effects associated with intravenous use such as transient lowering of systemic blood pressure and changes in heart rate, both of which can decrease coronary perfusion pressure and alter coronary flow independent of the functional integrity of the coronary microcirculation. Assessment of the endothelium-dependent vasoreactivity was performed by selective infusion of increasing concentrations of acetylcholine (10−6, 10−5 and 10−4 mol/L) at 1mL/min for 3 minutes with Doppler measurements and coronary angiography after each infusion. Coronary blood flow (CBF) was determined from the equation:
Definition of Coronary Vasomotor Dysfunction by Invasive Testing
Coronary endothelium-dependent dysfunction was defined as an increase in CBF of ≤ 50% in response to maximum dose acetylcholine when compared to baseline. Coronary endothelium-independent dysfunction was defined as a CFR ratio of ≤ 2.5 during infusion of adenosine. Coronary vasomotor dysfunction was defined as endothelium-dependent dysfunction and/or endothelium-independent dysfunction. These parameters have been shown to have prognostic significance in patients without significant obstructive epicardial disease16, 21–23.
Non-Invasive Stress Testing
Patients underwent the following non-invasive stress studies using standard clinical institutional protocols. The choice of the non-invasive test was at the discretion of the attending clinician.
Stress protocols
Exercise stress protocols
235 patients exercised on a treadmill according to the standard Bruce protocol24. Four used the supine bike protocol25 and ten the MUGA exercise protocol26.
Pharmacological stress protocols
Adenosine
Ninety-seven patients received a standard dose of 140 mcg/kg/min for 6 minutes27.
Dipyridamole
Three patients received a dose of 0.56mg/kg to a maximum dose of 60mg over 4 minutes27.
Dobutamine
Twenty-nine patients received dobutamine starting at 5–10mcg/kg/min followed by stepwise increases to 20, 30 and 40mcg/kg/min for each consecutive 3 minute interval. If maximum heart rate was not achieved, 0.5 to 1.0mg of atropine was administered28.
Stress Echocardiogram protocol
Echocardiographic images using the 16-segment model29 were obtained at rest and compared with those obtained immediately (<1minute) after treadmill exercise or during pharmacological stress.
Stress Nuclear protocols
Tc-99m Sestamibi (n=172)
A one-day rest-stress Tc-99m sestamibi protocol was performed30 with a 12 mCi resting Tc-99m sestamibi injection and SPECT acquisition followed by a 48 mCi Tc-99m sestamibi injection at 90 seconds before peak exercise, 3 minutes into adenosine infusion, 4 minutes after dipyridamole infusion or at peak dobutamine infusion, followed by gated SPECT acquisition.
Thallium-201 (n=32)
3mCi thallium-201 was injected 60 seconds before peak exercise, 3 minutes into adenosine infusion, 4 minutes after dipyridamole infusion or at peak dobutamine infusion followed by planar and SPECT acquisitions. At 4 hours, 1mCi thallium-201 was injected followed by image acquisition for redistribution30.
PET (n=37)
All scans were performed on an Advance scanner (General Electric, Waukesha, Wisconsin). Following a 10-minute transmission scan for attenuation correction, N-13 ammonia (n=21; 10–20mCi) or rubidium-82 (n=16; 45–60mCi) was injected at rest and static gated PET images were acquired for 10 minutes. After a 50-minute period of decay, the same tracer dose was injected during stress followed by a 10-minute static stress emission acquisition and a repeat transmission scan for attenuation correction of the stress images31.
MUGA (n=10)
Injection of in-vitro labeled patient’s blood with 30mCi of Tc-99m was performed before starting exercise. Regional wall motion was assessed at rest and after every stage during exercise26.
ECG and Imaging interpretation
ECG
The interpretation of the exercise ECG was performed by an experienced cardiologist. The appearance of horizontal or downsloping ST depression of ≥ 1mm at 0.08s after the J-point was the criterion for a positive stress ECG32. Patients with left bundle branch block, pacemaker, Wolff-Parkinson-White or ≥1mm ST depression at rest or left bundle branch block on stress were considered non-diagnostic.
Echocardiogram
The echocardiographic images were interpreted by an experienced staff cardiologist using the 16-segment model29. The stress test was considered positive if wall motion abnormalities developed with exercise or pharmacological stress in previously normal territories or worsened in an already abnormal segment. Fixed wall motion abnormalities were considered negative.
Nuclear Scans
All nuclear images were interpreted by a consensus of a nuclear cardiologist and a nuclear medicine specialist, using a 5-point semi-quantitative scale and a 16-segment model27. Images were considered positive if a new perfusion defect of at least one grade developed after stress or a worsening in perfusion of one or more grades was observed after stress compared to the rest images. Fixed defects were considered negative.
Statistical Analysis
The statistical analysis was performed by a statistician (RJL). Sensitivity, specificity, PPV and NPV of the non-invasive stress tests for predicting invasive coronary vasomotor function was performed. Continuous variables are summarized as mean ± standard deviation (unless otherwise noted); discrete variables are presented as frequency (percentage). Exact binomial methods were used to calculate 95% confidence intervals. Logistic regression was used to test whether the association between non-invasive tests and the invasive coronary vasomotor function were different according to the type of non-invasive test done. The model consisted of indicator variables for the different test types with the endpoint being agreement between the non-invasive test and invasive coronary vasomotor function. If the overall likelihood ratio test for the model was non-significant, then all tests were declared to be equivalent. Otherwise, Wald tests were used to determine which pairs of tests were significantly different.
Results
The characteristics of the 376 patients under study are shown in Table 1. Sixty-six percent of patients were female and the average age was 51.3 years (range 17–84). There were no statistical differences between the 2 groups with regards to medication use (aspirin, beta-blockers, lipid lowering drugs, calcium channel blockers, ACE-inhibitors, nitrates). Of the 376 patients, 310 stress tests (82%) were performed within 1 month of the invasive test and the median time between tests was 0.7 weeks. Data on CBF on 9 patients (2%) and CFR on 2 patients (0.5%) was not available and these were excluded from the data analyses (Figure 1). On invasive testing, 233 of 367 patients (63%) had coronary vasomotor dysfunction of which 187 of 367 patients (51%) had endothelium-dependent dysfunction, 109 of 374 patients (29%) had endothelium-independent dysfunction and 63 of 365 patients (17%) had both. On non-invasive stress testing, 157 (42%) had a positive imaging study and 56 (15%) a positive ECG stress test. Forty-two patients (11%) had a non-diagnostic ECG stress test. Two hundred ninety-one patients (77%) had symptoms of chest pain (n=203 (54%)) or shortness of breath (n=185 (49%)) during the non-invasive stress test. The different types of non-invasive tests performed are described in Figure 1. All patients had a stress ECG prior to the imaging test and of these, 249 (66%) were exercise ECG tests.
Table 1.
Patient Characteristics - Vasomotor Function
| Variable | Normal (N=134) | Dysfunctional (N=233) | P-value |
|---|---|---|---|
| Age, yrs | 48.8±11.2 | 52.9±12.0 | 0.001 |
| Men, No. (%) | 48(36%) | 74(32%) | 0.43 |
| Body Mass Index | 28.9±6.4 | 29.2±5.7 | 0.63 |
| Hypertension, No. (%) | 59(44%) | 98(42%) | 0.67 |
| Diabetes, No. (%) | 8(6%) | 28(12%) | 0.06 |
| Smoking status, No. (%) | 0.34 | ||
| • Never smoked | 69(51%) | 127(55%) | |
| • Former smoker | 47(35%) | 84(36%) | |
| • Current smoker | 18(13%) | 20(9%) | |
| Positive Family history, No. (%) | 79(60%) | 148(65%) | 0.38 |
| Hyperlipidemia, No. (%) | 71(53%) | 137(59%) | 0.29 |
| hsCRP, mg/L, Median (Q1, Q3) | 0.4(0.2,1.5) | 0.5(0.3,1.6) | 0.51 |
| Vascular disease*, No. (%) | 6(5%) | 23(10%) | 0.07 |
| Other vasospasm†, No. (%) | 34(26%) | 48(21%) | 0.29 |
| % chg CBF(Ach) | 140.2±79.8 | 11.9±62.5 | <.001 |
| Max CFR | 3.3±0.6 | 2.7±0.7 | <.001 |
| Heart rate at baseline | 70.2±12.3 | 74.1±15.4 | 0.015 |
| Heart rate at hyperemia | 68.9±12.5 | 72.5±14.6 | 0.018 |
| Change in HR(baseline to hyperemia) | −1.4±6.8 | −1.4±8.1 | 0.95 |
| Base MAP | 100.0±14.0 | 100.8±15.1 | 0.62 |
| MAP at hyperemia | 98.2±13.5 | 100.8±14.4 | 0.09 |
| Change in MAP(baseline to hyperemia) | −2.2±8.8 | −0.0±8.9 | 0.031 |
Peripheral Artery Disease, Abdominal aortic aneurysm, Transient Ischemic Attack
Migraine, Raynaud’s phenomenon
Predicting Coronary Vasomotor Function with Non-Invasive Stress Tests
The sensitivity and specificity of all the non-invasive imaging stress tests for predicting coronary vasomotor dysfunction was 41% [95%CI 34–47] and 57% [95%CI 49–66] respectively (Table 2); for endothelium-dependent dysfunction 41% [95%CI 34–49] and 58% [95%CI 50–65] respectively (Table 3) and for endothelium-independent dysfunction 46% [95%CI 37–56] and 61% [95%CI 54–67] respectively (Table 4). By logistic regression, none of the individual non-invasive imaging stress tests was superior for predicting coronary vasomotor dysfunction or endothelium-independent dysfunction. For endothelium-dependent dysfunction, dobutamine echocardiogram was inferior to all other imaging tests except for vasodilator PET. The exercise ECG test was more specific than the imaging tests (80% [95%CI 71–88] for coronary vasomotor dysfunction; 78% [95%CI 69–85] for endothelium-dependent dysfunction and 75% [95%CI 68–81] for endothelium-independent dysfunction) but it was also the least sensitive test (18% [95%CI 12–25] for coronary vasomotor dysfunction; 18% [95%CI 12–27] for endothelium-dependent dysfunction; 16% [95%CI 8–27] for endothelium-independent dysfunction). Combining the results of the stress ECG test with the imaging findings improved specificity but substantially reduced sensitivity (see Tables 2, 3 and 4). On subgroup analysis, there was no significant difference in the sensitivity, specificity, NPV or PPV of the non-invasive tests for predicting invasive coronary vasomotor dysfunction, endothelium-dependent dysfunction or endothelium-independent dysfunction between men and women. One hundred and thirty-seven (63%) of the 219 patients who had a negative non-invasive imaging stress test had one or more abnormalities on invasive catheterization testing. There was no significant difference between the mean percentage change in CBF or mean CFR ratio on invasive testing of all the patients who had positive imaging tests compared to all patients who had negative imaging tests (Fig. 2).
Table 2.
Predicting Coronary Vasomotor Dysfunction
| Test | N | % (+) | Sensitivity (95% CI) | Specificity (95% CI) | NPV (95% CI) | PPV (95% CI) |
|---|---|---|---|---|---|---|
| Exercise Echocardiogram | 99 | 40.4 | 38(26–51) | 55(38–71) | 36(24–49) | 58(41–73) |
| Dobutamine Echocardiogram | 21 | 33.3 | 29(8–58) | 57(18–90) | 29(8–58) | 57(18–90) |
| Exercise SPECT | 131 | 38.2 | 40(29–51) | 65(49–78) | 38(28–50) | 66(51–79) |
| Vasodilator SPECT | 64 | 50.0 | 51(35–68) | 52(31–72) | 41(24–59) | 63(44–79) |
| Vasodilator PET | 33 | 36.4 | 35(16–57) | 60(26–88) | 29(11–52) | 67(35–90) |
| All imaging | 365 | 41.4 | 41(34–47) | 57(49–66) | 36(30–43) | 62(54–70) |
| Exercise ECG | 242 | 16.1 | 18(12–25) | 80(71–88) | 41(33–48) | 69(52–83) |
| All imaging + ECG | 365 | 6.3 | 6(3–10) | 90(83–94) | 37(32–43) | 61(39–80) |
Table 3.
Predicting Endothelium-dependent Dysfunction
| Test | N | % (+) | Sensitivity (95% CI) | Specificity (95% CI) | NPV (95% CI) | PPV (95% CI) |
|---|---|---|---|---|---|---|
| Exercise Echocardiogram | 100 | 40.0 | 42(28–57) | 62(47–75) | 52(38–65) | 53(36–68) |
| Dobutamine Echocardiogram | 21 | 38.1 | 18(2–52) | 40(12–74) | 31(9–61) | 25(3–65) |
| Exercise SPECT | 131 | 38.2 | 37(26–50) | 61(48–73) | 48(37–60) | 50(36–64) |
| Vasodilator SPECT | 63 | 50.8 | 61(42–78) | 59(41–76) | 61(42–78) | 59(41–76) |
| Vasodilator PET | 33 | 36.4 | 20(4–48) | 50(26–74) | 43(22–66) | 25(5–57) |
| All imaging | 365 | 41.6 | 41(34–49) | 58(50–65) | 49(42–56) | 50(42–58) |
| Exercise ECG | 233 | 15.5 | 18(12–27) | 78(69–85) | 51(43–59) | 61(43–77) |
| All imaging + ECG | 365 | 6.3 | 8(4–12) | 90(85–94) | 50(45–56) | 61(39–80) |
Table 4.
Predicting Endothelium-independent Dysfunction
| Test | N | % (+) | Sensitivity (95% CI) | Specificity (95% CI) | NPV (95% CI) | PPV (95% CI) |
|---|---|---|---|---|---|---|
| Exercise Echocardiogram | 101 | 40.6 | 42(25–61) | 60(48–72) | 70(57–81) | 32(18–48) |
| Dobutamine Echocardiogram | 21 | 33.3 | 33(4–78) | 67(38–88) | 71(42–92) | 29(4–71) |
| Exercise SPECT | 134 | 38.8 | 51(34–68) | 66(56–75) | 78(68–86) | 37(24–51) |
| Vasodilator SPECT | 66 | 48.5 | 41(18–67) | 49(34–64) | 71(53–85) | 22(9–40) |
| Vasodilator PET | 33 | 36.4 | 50(23–77) | 74(49–91) | 67(43–85) | 58(28–85) |
| All imaging | 372 | 41.4 | 46(37–56) | 61(54–67) | 73(67–79) | 32(25–40) |
| Exercise ECG | 237 | 15.2 | 16(8–27) | 75(68–81) | 71(64–77) | 31(16–48) |
| All imaging + ECG | 372 | 6.2 | 6(3–13) | 89(85–93) | 71(66–76) | 30(13–53) |
Fig. 2. Mean Percentage Change in CBF and Mean CFR Ratio in all Patients with Positive Imaging compared to all Patients with Negative Imaging Stress Tests.
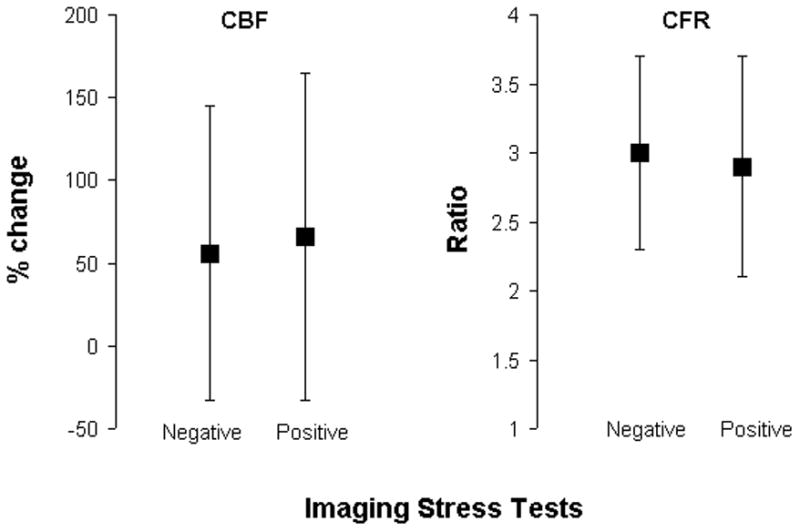
No significant difference (p=0.30 for mean percentage change CBF and p=0.45 for mean CFR ratio) by T-test.
Discussion
Our study shows that in a selected population with chest pain and angiographically normal or non-significant obstructive coronary artery disease, non-invasive stress tests used in contemporary practice have limited diagnostic accuracy for detecting coronary vasomotor dysfunction. These results highlight the continuing challenges in the diagnosis of patients with chest pain in the absence of obstructive epicardial coronary arteries.
Within the coronary circulation, vasomotor dysfunction results in lack of appropriate coronary vasodilatation in response to stress due to mental33 or physical34 exercise or to pharmacological stimuli (such as acetylcholine7 or adenosine9). This may occur at the level of the epicardial vessels or the microcirculation leading to impairment of coronary blood flow during stress and a subsequent imbalance between oxygen demand and supply (myocardial ischemia5–13). The underlying mechanism for coronary vasomotor dysfunction may be endothelium-dependent or endothelium-independent. Endothelium-dependent dysfunction occurs with loss of balance between endothelium-derived relaxing factors (e.g. nitric oxide) and endothelium-derived constrictors (e.g. endothelin). In a functional endothelium, acetylcholine causes vasodilatation at both the epicardium and microcirculation by stimulating the synthesis of nitric oxide via muscarinic receptors. In a dysfunctional endothelium, acetylcholine causes paradoxical vasoconstriction7. Endothelium-independent function depends on myocyte tone and adenosine causes microvascular dilatation by increasing intracellular cyclic AMP9. Coronary vasomotor dysfunction has been implicated in the pathogenesis and clinical course of atherosclerosis35, 36 and is associated with a ten-fold increased risk of cardiovascular events16, 21–23.
To our knowledge, this is the first study to address the sensitivity and specificity of the various clinically available non-invasive stress tests in identifying invasively determined coronary vasomotor dysfunction, specifically endothelium-dependent dysfunction and endothelium-independent dysfunction. Prior studies examining the ability of non-invasive stress tests to identify patients with coronary vasomotor dysfunction often did not perform direct coronary physiological assessment invasively as in our study and have reported conflicting results. Palinkas et al37 correlated stress ECG and echocardiography to assess for endothelial dysfunction measured by flow mediated dilatation of the brachial artery during reactive hyperemia by ultrasound. They found stress induced ST-segment depression but not stress echocardiography to be a predictor of endothelial dysfunction. Youn et al38 demonstrated a sensitivity of 58% and specificity of 95% for stress induced ST-segment depression on ECG to predict a CFR <2.1 detected by Doppler echocardiography. Similar findings were reported in another study39. The current study extends these previous observations and demonstrates that the stress ECG is the most specific (≥75%) non-invasive test for detecting coronary vasomotor dysfunction. Similar to the prior study, sensitivity of the exercise ECG in the current study was also low (<20%). These findings are in contrast to the study by Camici et al40 who demonstrated high sensitivity (86%) of the exercise ECG in identifying patients with blunted CFR but a rather low specificity (45%). This latter study, however, used quantitative myocardial blood flow by PET rather than invasive physiological measurements as the gold standard. The ECG stress test is speculatively the most specific test for coronary vasomotor dysfunction since the latter may cause heterogeneous non-transmural ischemia resulting in small changes in depolarization which in turn produce ST-segment depression. Its lack of sensitivity may be explained by the hypothesis that the minor changes in depolarization may not produce a sufficient ST-segment depression to reach criteria for positivity (≥1mm at 0.08s from the J-point).
Coronary vasomotor dysfunction is not associated with stress-induced myocardial contractile dysfunction on echocardiography37, 41. The latter requires not only flow reduction of >50%, but also reduced flow in at least 20% of trans-mural wall thickness and in 5% of the total cardiac mass42. In patients with abnormal coronary vasomotion, absence of wall motion abnormalities by stress echocardiography may be due to impaired myocardial perfusion limited to only the subendocardium, with preserved transmural perfusion, as demonstrated on myocardial perfusion MRI43.
Studies with myocardial perfusion have shown inconsistent correlation with coronary vasomotor dysfunction. Some studies have reported an association between coronary vasomotor dysfunction and perfusion defects on SPECT perfusion imaging13, 15, 44. However, myocardial blood flow is abnormally heterogeneous in patients with cardiac syndrome X, compatible with the presence of dynamic alterations of the microcirculation45. These alterations are sparse and may not be detected when myocardial perfusion is assessed using conventional methods that do not detect small myocardial regions. Alternatively, decreased myocardial perfusion throughout the entire myocardium may not be detected on SPECT perfusion scans, similar to patients with 3-vessel disease, due to the relative nature of the technique.
In the current study, all non-invasive stress tests performed less favorably for the diagnosis of invasive coronary vasomotor dysfunction than for the diagnosis of angiographic epicardial obstructive coronary artery disease. Our experience with non-invasive stress tests for identifying significant epicardial obstructive coronary artery disease has been previously published (Table 5)28–32. Despite their limitations and only moderately high diagnostic accuracy for some techniques, these non-invasive tests remain useful tools for the diagnosis, management, and risk stratification of patients with known or suspected epicardial coronary artery disease46. On the other hand, the significantly lower diagnostic accuracy of these non-invasive tests for the detection of coronary vasomotor dysfunction suggests more limited usefulness of these non-invasive tests in the subset of patients referred for possible coronary vasomotor dysfunction. This underscores the importance of using invasive assessment or novel more sensitive non-invasive imaging modalities for symptomatic patients with non-obstructive coronary artery disease.
Table 5.
Predicting Obstructive Coronary Artery Disease
| Test | Sensitivity | Specificity |
|---|---|---|
| Exercise Echocardiogram | 88% | 72% |
| Dobutamine Echocardiogram | 97% | 65% |
| Exercise/Vasodilator SPECT | 98%* | 13%† |
| Vasodilator PET | 96%‡ | 34%§ |
| Exercise ECG | 53–69% | 69–74% |
After correction for referral bias: 67%,
75%,
82%,
73%
Limitations to our study include its retrospective nature but this should not alter the results of the invasive or non-invasive tests. A major limitation is referral bias - the study population was referred by physicians due to more ‘worrying’ chest pain than in the general population since they all underwent invasive coronary angiography, many (58%) despite their negative non-invasive stress test. The period of time between the invasive and non-invasive tests of up to 6 months may have resulted in changes in the coronary circulation. However 82% of our patients had less than a 1-month period of time between the two tests. Coronary flow reserve interrogates both the microcirculation as well as the epicardial vessels and thus not measuring epicardial resistance or fractional flow reserve may have misclassified some patients with silent epicardial atherosclerosis as having vasomotor dysfunction. Several additional non-invasive tests have been used for the measurement of coronary vasomotor function including Doppler echocardiography47, phase contrast MRI48 and electron beam computed tomography49. These have been shown to correlate with invasive coronary vasomotor measurements but have not gained widespread application for stress imaging in the evaluation of chest pain. While results of static PET acquisition and semi-quantitative visual analysis were reported in this study, PET dynamic acquisition and quantification of coronary flow reserve has been shown to have good correlation with invasive coronary physiology measurements50 but is more time-consuming, lacks standardization and is not clinically available at most centers. The absence of follow-up does not allow us to compare the prognostic validity of the invasive or non-invasive stress tests as predictors of future cardiovascular events in this study population.
Conclusions
The current study suggests that the majority of widely used non-invasive stress tests have limited diagnostic accuracy for identifying coronary vasomotor dysfunction in patients with non-obstructive coronary artery disease, with the exercise ECG test being more specific but less sensitive than imaging tests. The presence of a negative non-invasive stress test does not rule out coronary vasomotor dysfunction in symptomatic patients with non-obstructive coronary artery disease. This underscores the need for invasive assessment or novel more sensitive non-invasive imaging for these patients.
Acknowledgments
The authors would like to thanks Jonella M. Tilford, Teresa L. Jarland for their valuable help in collecting the data.
Funding Sources: The study was supported by the NIH Grant R01 HL63911 and the Mayo Foundation. Dr. Lerman is an Established Investigator of the American Heart Association.
Abbreviations list
- APV
average peak velocity
- CAD
coronary artery diameter
- CBF
coronary blood flow
- CFR
coronary flow reserve
- ECG
electrocardiogram
- MUGA
multiple gated acquisition scan
- NPV
negative predictive value
- PET
positron emission tomography
- PPV
positive predictive value
- SPECT
single photon emission computed tomography
Footnotes
Disclosures: No conflicts of interest.
References
- 1.Kemp HG, Kronmal RA, Vlietstra RE, Frye RL. Seven year survival of patients with normal or near normal coronary arteriograms: a CASS registry study. J Am Coll Cardiol. 1986;7:479–483. doi: 10.1016/s0735-1097(86)80456-9. [DOI] [PubMed] [Google Scholar]
- 2.Proudfit WL, Shirey EK, Sones FM., Jr Selective cine coronary arteriography. Correlation with clinical findings in 1,000 patients. Circulation. 1966;33:901–910. doi: 10.1161/01.cir.33.6.901. [DOI] [PubMed] [Google Scholar]
- 3.Cannon RO, 3rd, Quyyumi AA, Schenke WH, Fananapazir L, Tucker EE, Gaughan AM, Gracely RH, Cattau EL, Jr, Epstein SE. Abnormal cardiac sensitivity in patients with chest pain and normal coronary arteries. J Am Coll Cardiol. 1990;16:1359–1366. doi: 10.1016/0735-1097(90)90377-2. [DOI] [PubMed] [Google Scholar]
- 4.Chauhan A, Mullins PA, Thuraisingham SI, Taylor G, Petch MC, Schofield PM. Abnormal cardiac pain perception in syndrome X. J Am Coll Cardiol. 1994;24:329–335. doi: 10.1016/0735-1097(94)90284-4. [DOI] [PubMed] [Google Scholar]
- 5.Hasdai D, Holmes DR, Jr, Higano ST, Burnett JC, Jr, Lerman A. Prevalence of coronary blood flow reserve abnormalities among patients with nonobstructive coronary artery disease and chest pain. Mayo Clin Proc. 1998;73:1133–1140. doi: 10.4065/73.12.1133. [DOI] [PubMed] [Google Scholar]
- 6.Reddy KG, Nair RN, Sheehan HM, Hodgson JM. Evidence that selective endothelial dysfunction may occur in the absence of angiographic or ultrasound atherosclerosis in patients with risk factors for atherosclerosis. J Am Coll Cardiol. 1994;23:833–843. doi: 10.1016/0735-1097(94)90627-0. [DOI] [PubMed] [Google Scholar]
- 7.Egashira K, Inou T, Hirooka Y, Yamada A, Urabe Y, Takeshita A. Evidence of impaired endothelium-dependent coronary vasodilatation in patients with angina pectoris and normal coronary angiograms. N Engl J Med. 1993;328:1659–1664. doi: 10.1056/NEJM199306103282302. [DOI] [PubMed] [Google Scholar]
- 8.Quyyumi AA, Cannon RO, 3rd, Panza JA, Diodati JG, Epstein SE. Endothelial dysfunction in patients with chest pain and normal coronary arteries. Circulation. 1992;86:1864–1871. doi: 10.1161/01.cir.86.6.1864. [DOI] [PubMed] [Google Scholar]
- 9.Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J. 2001;141:735–741. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 10.Opherk D, Zebe H, Weihe E, Mall G, Durr C, Gravert B, Mehmel HC, Schwarz F, Kubler W. Reduced coronary dilatory capacity and ultrastructural changes of the myocardium in patients with angina pectoris but normal coronary arteriograms. Circulation. 1981;63:817–825. doi: 10.1161/01.cir.63.4.817. [DOI] [PubMed] [Google Scholar]
- 11.Cannon RO, 3rd, Schenke WH, Leon MB, Rosing DR, Urqhart J, Epstein SE. Limited coronary flow reserve after dipyridamole in patients with ergonovine-induced coronary vasoconstriction. Circulation. 1987;75:163–174. doi: 10.1161/01.cir.75.1.163. [DOI] [PubMed] [Google Scholar]
- 12.Buchthal SD, den Hollander JA, Merz CN, Rogers WJ, Pepine CJ, Reichek N, Sharaf BL, Reis S, Kelsey SF, Pohost GM. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med. 2000;342:829–835. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- 13.Inobe Y, Kugiyama K, Morita E, Kawano H, Okumura K, Tomiguchi S, Tsuji A, Kojima A, Takahashi M, Yasue H. Role of adenosine in pathogenesis of syndrome X: assessment with coronary hemodynamic measurements and thallium-201 myocardial single-photon emission computed tomography. J Am Coll Cardiol. 1996;28:890–896. doi: 10.1016/s0735-1097(96)00271-9. [DOI] [PubMed] [Google Scholar]
- 14.Kern MJ, Lerman A, Bech JW, De Bruyne B, Eeckhout E, Fearon WF, Higano ST, Lim MJ, Meuwissen M, Piek JJ, Pijls NH, Siebes M, Spaan JA. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation. 2006;114:1321–1341. doi: 10.1161/CIRCULATIONAHA.106.177276. [DOI] [PubMed] [Google Scholar]
- 15.Hasdai D, Gibbons RJ, Holmes DR, Jr, Higano ST, Lerman A. Coronary endothelial dysfunction in humans is associated with myocardial perfusion defects. Circulation. 1997;96:3390–3395. doi: 10.1161/01.cir.96.10.3390. [DOI] [PubMed] [Google Scholar]
- 16.Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 17.Lavi S, McConnell JP, Rihal CS, Prasad A, Mathew V, Lerman LO, Lerman A. Local production of lipoprotein-associated phospholipase A2 and lysophosphatidylcholine in the coronary circulation: association with early coronary atherosclerosis and endothelial dysfunction in humans. Circulation. 2007;115:2715–2721. doi: 10.1161/CIRCULATIONAHA.106.671420. [DOI] [PubMed] [Google Scholar]
- 18.Doucette JW, Corl PD, Payne HM, Flynn AE, Goto M, Nassi M, Segal J. Validation of a Doppler guide wire for intravascular measurement of coronary artery flow velocity. Circulation. 1992;85:1899–1911. doi: 10.1161/01.cir.85.5.1899. [DOI] [PubMed] [Google Scholar]
- 19.Reiber JH, Serruys PW, Kooijman CJ, Wijns W, Slager CJ, Gerbrands JJ, Schuurbiers JC, den Boer A, Hugenholtz PG. Assessment of short-, medium-, and long-term variations in arterial dimensions from computer-assisted quantitation of coronary cineangiograms. Circulation. 1985;71:280–288. doi: 10.1161/01.cir.71.2.280. [DOI] [PubMed] [Google Scholar]
- 20.Kern MJ. Curriculum in interventional cardiology: coronary pressure and flow measurements in the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2001;54:378–400. doi: 10.1002/ccd.1303. [DOI] [PubMed] [Google Scholar]
- 21.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 22.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. Journal of the American College of Cardiology. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 23.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 24.Bruce RA. Exercise testing of patients with coronary heart disease. Principles and normal standards for evaluation. Annals of clinical research. 1971;3:323–332. [PubMed] [Google Scholar]
- 25.Modesto KM, Rainbird A, Klarich KW, Mahoney DW, Chandrasekaran K, Pellikka PA. Comparison of supine bicycle exercise and treadmill exercise Doppler echocardiography in evaluation of patients with coronary artery disease. Am J Cardiol. 2003;91:1245–1248. doi: 10.1016/s0002-9149(03)00275-3. [DOI] [PubMed] [Google Scholar]
- 26.Gibbons RJ. Rest and exercise radionuclide angiography for diagnosis in chronic ischemic heart disease. Circulation. 1991;84 (Suppl):I93–99. [PubMed] [Google Scholar]
- 27.Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS. Stress protocols and tracers. J Nucl Cardiol. 2006;13:e80–90. doi: 10.1016/j.nuclcard.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 28.Pellikka PA, Roger VL, Oh JK, Miller FA, Seward JB, Tajik AJ. Stress echocardiography. Part II. Dobutamine stress echocardiography: techniques, implementation, clinical applications, and correlations. Mayo Clin Proc. 1995;70:16–27. doi: 10.1016/S0025-6196(11)64660-0. [DOI] [PubMed] [Google Scholar]
- 29.Roger VL, Pellikka PA, Oh JK, Miller FA, Seward JB, Tajik AJ. Stress echocardiography. Part I. Exercise echocardiography: techniques, implementation, clinical applications, and correlations. Mayo Clinic proceedings. 1995;70:5–15. doi: 10.1016/S0025-6196(11)64659-4. [DOI] [PubMed] [Google Scholar]
- 30.Miller TD, Hodge DO, Christian TF, Milavetz JJ, Bailey KR, Gibbons RJ. Effects of adjustment for referral bias on the sensitivity and specificity of single photon emission computed tomography for the diagnosis of coronary artery disease. Am J Med. 2002;112:290–297. doi: 10.1016/s0002-9343(01)01111-1. [DOI] [PubMed] [Google Scholar]
- 31.Syed SS, Miller TD, Gibbons RJ, Askew JW, Chareonthaitawee P. Effect of referral bias on the diagnostic accuracy of N-13 ammonia and rubidium-82 myocardial perfusion imaging with positron emission tomography in the detection of coronary artery disease. Circulation. 2008;118:S608. [Google Scholar]
- 32.Miller TD, Roger VL, Milavetz JJ, Hopfenspirger MR, Milavetz DL, Hodge DO, Gibbons RJ. Assessment of the exercise electrocardiogram in women versus men using tomographic myocardial perfusion imaging as the reference standard. Am J Cardiol. 2001;87:868–873. doi: 10.1016/s0002-9149(00)01528-9. [DOI] [PubMed] [Google Scholar]
- 33.Yeung AC, Vekshtein VI, Krantz DS, Vita JA, Ryan TJ, Jr, Ganz P, Selwyn AP. The effect of atherosclerosis on the vasomotor response of coronary arteries to mental stress. The New England journal of medicine. 1991;325:1551–1556. doi: 10.1056/NEJM199111283252205. [DOI] [PubMed] [Google Scholar]
- 34.Gordon JB, Ganz P, Nabel EG, Fish RD, Zebede J, Mudge GH, Alexander RW, Selwyn AP. Atherosclerosis influences the vasomotor response of epicardial coronary arteries to exercise. The Journal of clinical investigation. 1989;83:1946–1952. doi: 10.1172/JCI114103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (1) N Engl J Med. 1992;326:242–250. doi: 10.1056/NEJM199201233260406. [DOI] [PubMed] [Google Scholar]
- 36.Ross R. Atherosclerosis--an inflammatory disease. The New England journal of medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 37.Palinkas A, Toth E, Amyot R, Rigo F, Venneri L, Picano E. The value of ECG and echocardiography during stress testing for identifying systemic endothelial dysfunction and epicardial artery stenosis. Eur Heart J. 2002;23:1587–1595. doi: 10.1053/euhj.2002.3170. [DOI] [PubMed] [Google Scholar]
- 38.Youn HJ, Park CS, Cho EJ, Jung HO, Jeon HK, Lee JM, Oh YS, Chung WS, Kim JH, Choi KB, Hong SJ. Pattern of exercise-induced ST change is related to coronary flow reserve in patients with chest pain and normal coronary angiogram. Int J Cardiol. 2005;101:299–304. doi: 10.1016/j.ijcard.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 39.Mitsugi M, Kaneko H, Yamaki T, Kunii H, Maruyama Y. Correlation between exercise electrocardiography test and coronary flow reserve in patients without organic coronary artery stenosis. J Cardiol. 2007;49:109–114. [PubMed] [Google Scholar]
- 40.Camici PG, Gistri R, Lorenzoni R, Sorace O, Michelassi C, Bongiorni MG, Salvadori PA, L’Abbate A. Coronary reserve and exercise ECG in patients with chest pain and normal coronary angiograms. Circulation. 1992;86:179–186. doi: 10.1161/01.cir.86.1.179. [DOI] [PubMed] [Google Scholar]
- 41.Panza JA, Laurienzo JM, Curiel RV, Unger EF, Quyyumi AA, Dilsizian V, Cannon RO., 3rd Investigation of the mechanism of chest pain in patients with angiographically normal coronary arteries using transesophageal dobutamine stress echocardiography. J Am Coll Cardiol. 1997;29:293–301. doi: 10.1016/s0735-1097(96)00481-0. [DOI] [PubMed] [Google Scholar]
- 42.Picano E. Stress echocardiography. From pathophysiological toy to diagnostic tool. Circulation. 1992;85:1604–1612. doi: 10.1161/01.cir.85.4.1604. [DOI] [PubMed] [Google Scholar]
- 43.Panting JR, Gatehouse PD, Yang GZ, Grothues F, Firmin DN, Collins P, Pennell DJ. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002;346:1948–1953. doi: 10.1056/NEJMoa012369. [DOI] [PubMed] [Google Scholar]
- 44.Zeiher AM, Krause T, Schachinger V, Minners J, Moser E. Impaired endothelium-dependent vasodilation of coronary resistance vessels is associated with exercise-induced myocardial ischemia. Circulation. 1995;91:2345–2352. doi: 10.1161/01.cir.91.9.2345. [DOI] [PubMed] [Google Scholar]
- 45.Galassi AR, Crea F, Araujo LI, Lammertsma AA, Pupita G, Yamamoto Y, Rechavia E, Jones T, Kaski JC, Maseri A. Comparison of regional myocardial blood flow in syndrome X and one-vessel coronary artery disease. The American journal of cardiology. 1993;72:134–139. doi: 10.1016/0002-9149(93)90148-6. [DOI] [PubMed] [Google Scholar]
- 46.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, Ferguson TB, Jr, Fihn SD, Fraker TD, Jr, Gardin JM, O’Rourke RA, Pasternak RC, Williams SV. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina) J Am Coll Cardiol. 2003;41:159–168. doi: 10.1016/s0735-1097(02)02848-6. [DOI] [PubMed] [Google Scholar]
- 47.Hozumi T, Yoshida K, Akasaka T, Asami Y, Ogata Y, Takagi T, Kaji S, Kawamoto T, Ueda Y, Morioka S. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography: comparison with invasive technique. J Am Coll Cardiol. 1998;32:1251–1259. doi: 10.1016/s0735-1097(98)00389-1. [DOI] [PubMed] [Google Scholar]
- 48.Lanza GA, Buffon A, Sestito A, Natale L, Sgueglia GA, Galiuto L, Infusino F, Mariani L, Centola A, Crea F. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol. 2008;51:466–472. doi: 10.1016/j.jacc.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 49.Mohlenkamp S, Lerman LO, Lerman A, Behrenbeck TR, Katusic ZS, Sheedy PF, 2nd, Ritman EL. Minimally invasive evaluation of coronary microvascular function by electron beam computed tomography. Circulation. 2000;102:2411–2416. doi: 10.1161/01.cir.102.19.2411. [DOI] [PubMed] [Google Scholar]
- 50.Frouin F, Merlet P, Bouchareb Y, Frouin V, Dubois-Rande JL, De Cesare A, Herment A, Syrota A, Todd-Pokropek A. Validation of myocardial perfusion reserve measurements using regularized factor images of H(2)(15)O dynamic PET scans. J Nucl Med. 2001;42:1737–1746. [PubMed] [Google Scholar]


