Abstract
BACKGROUND:
High prevalence rates of celiac disease (CD) in patients with type-1 diabetes mellitus (T1DM) have been reported in several countries. However, the data regarding this association are scarce in Iran. In this study, we report the prevalence of CD in patients with T1DM in northwest of Iran using tissue transglutaminase antibodies (tTGA) as a screening test.
METHODOLOGY:
One hundred patients with T1DM (58 women and 42 men) aged 21.8 ± 8.86 years (age range: 7–50 years) were compared with 150 healthy people (82 women and 68 men) aged 28.9 ± 9.07 years (age range: 4–50 years). All subjects were serologically screened for the presence of tTGA. Total immunoglobin A (IgA) was obtained to investigate IgA deficiency. Subjects positive for tTGA and deficient for IgA were submitted to upper gastrointestinal endoscopy.
RESULTS:
Eight patients with T1DM (8%) and three of the controls (2%) were positive for tTGA (P = 0.023), while only 3% of the tTGA positive T1DM patients underwent duodenal biopsy and all of them showed partial or total villous atrophy. The mean age of tTGA positive cases was significantly lower than tTGA negative ones (mean difference 7.17; 95% CI: 0.82–13.52). None of the tTGA positive T1DM patients had a history of chronic diarrhea, but one out of eight tTGA positives reported history of dermatitis (P = 0.001). Also, none of the tTGA positive subjects presented IgA deficiency. There was a significant difference in history of chronic diarrhea (P = 0.006) and autoimmune diseases (P = 0.001) between patients with T1DM and controls.
CONCLUSION:
This study showed higher prevalence of CD in patients with T1DM than in general population of northwest Iran and the data lend support to recommend regular screening for CD in all patients with T1DM.
Keywords: Celiac disease, tissue transglutaminase antibodies, type 1 diabetes
Introduction
Type-1 diabetes mellitus (T1DM) is a chronic autoimmune disorder with varying degrees of insulin deficiency resulting from an immune-mediated destruction of pancreatic β-cells, usually presenting in young individuals.[1] T1DM can be associated with other clinical, subclinical, or potential organ-specific autoimmune diseases. Celiac disease (CD) is an autoimmune enteropathy induced by gluten proteins present in wheat, barley, rye; and characterized by small intestinal lesions of variable severity.[2] In its classic form, CD appears with symptoms and signs of intestinal malabsorption. However, the disease may occur in a silent or latent form.[3] Co-existence of T1DM and CD was first suspected in 1954.[4] The same ‘susceptibility genotypes’ are involved in the etiopathogenesis of diabetes mellitus and CD. In both diseases, genetic susceptibility is associated with the HLA-DQ α1*0501, β1*0201 heterodimer, which preferentially presents gluten-derived gliadin peptides on its antigen-presenting groove to stimulate intestinal mucosal T cells.[5] With the existing identical gene location in both diseases, it seems that CD is more frequent in patients with T1DM than in general population. Using different screening procedures for auto antibodies, the reported prevalence of CD in patients with T1DM ranged from 0.6–16.4%.[6] Among different types of serological tests for screening CD, such as anti-gliadin antibodies (AGAs) and antiendomysial IgA antibody (EMA), tissue transglutaminase antibodies (tTGA) has proved to be a very specific indicator to identify subjects with latent CD.[7] It is well known that clinical CD represents only the tip of the iceberg. The subclinical disease is not infrequent in the general population, and serological tests such as tTGA can be used as markers for the identification of these asymptomatic individuals.[8] In several studies, the sensitivity and specificity of this test compared with biopsy-proven disease were 94% and 98%, respectively.[9–11] This is important because the treatment of asymptomatic patients with T1DM having a gluten-free diet seems to have a positive effect on glycemic control and on the growth. Furthermore, it can prevent osteoporosis and the development of autoimmune diseases.[12] The aim of our study was to determine the prevalence of CD in patients with T1DM using tTGA as a screening test.
Methodology
For the current study, 100 patients with T1DM (58 women and 42 men) from diabetes clinic of Medical University of Tabriz, Iran, as study population and 150 nondiabetic healthy people (82 women and 68 men) without having autoimmune and other CD-related diseases as control population were recruited. All patients were interviewed by the doctor about any history of diseases and symptoms compatible with CD and a questionnaire was filled out. After formal consenting, 7 ml of blood was collected from each subject. Samples were centrifuged and the serum was separated, divided into two aliquots and immediately stored at −20°C. Anti-tissue transglutaminase IgA antibodies were determined by enzyme-linked immunosorbent assay (ELISA) with human recombinant tTGA as antigen, using a commercial kit (Eu- tTG IgA, Eurospital, Trieste, Italy). Results were considered positive when the tTGA levels were higher than 7 AU/mL. The tTGA serological test is not appropriate for patients with IgA deficiency and due to the prevalence of 2–3% IgA deficiency in general population,[13] the serum IgA levels should be determined before any serological tests such as tTGA. This helps in eliminating false-negative results. The total serum IgA levels was determined by turbidimetry and IgA deficiency was considered positive when the IgA levels were <70 ng/dl. The tTGA positive and, IgA deficient subjects were clinically evaluated and submitted to upper gastrointestinal endoscopy. Crypt hyperplasia and villous atrophy (VA) were classified as either partial (PVA) or total (TVA), according to Marsh.[14]
The Statistical Package for Social Science (SPSS), version 11.5, was used for the statistical analysis. Simple statistics such as frequency, mean and standard deviation were used. Also, chi-square, t-test and Mann-whitney U test were used for comparison. The results were considered to have a statistical significance when the P values were <0.05.
Results
Serological screening for CD based on tTGA was performed in 100 patients with T1DM (58 women and 42 men) aged 21.8 ± 8.86 years (age range: 7–50 years), and in 150 healthy controls (82 women and 68 men) aged 28.9 ± 9.07 years (age range: 4–50 years).
The results for patients with T1DM and controls are shown in Figure 1 Statistically significant positivity of tTGA was observed in the T1DM patients when compared to the controls (P = 0.023). Eight patients, three men and five women, were positive for tTGA, while three of the 150 control individuals (2%), one man and two women, were positive too.
Figure 1.
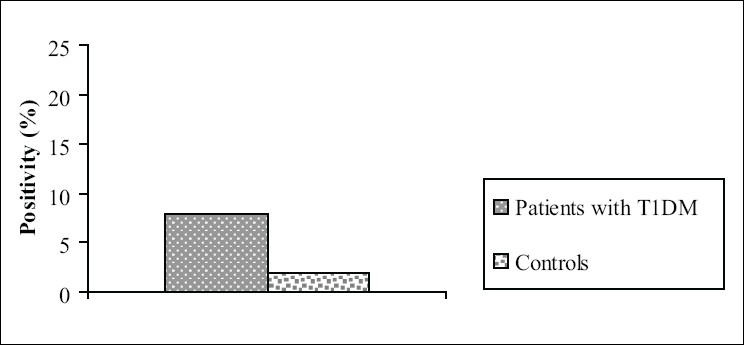
Tissue trnsglutaminase antibodies (tTGA) in patients with type 1 diabetes and controls
Table 1 shows demographic and clinical characteristics of subjects with DM compared with healthy controls. There was no difference based on gender between cases and controls. However, the mean age of controls was significantly higher than the patients with T1DM (P < 0.001). Type 1 patients with DM reported positive history of chronic diarrhea and autoimmune disease significantly more often than controls (P = 0.006 and P = 0.001, respectively). Four percent of T1D patients (n = 4) and 1.3% of controls were IgA deficient (P > 0.05), but none of the tTGA positive individuals in both the groups had a IgA titer of <70 ng/dl.
Table 1.
Demographic and clinical characteristics of patients with type 1 diabetes compared with healthy controls
| Characteristics | Patients* | Healthy controls** | P value |
|---|---|---|---|
| Sex (female/male) | 58/42 | 82/68 | 0.603 |
| Age (mean ± SD) | 21.85 ± 8.86 | 28.91 ± 9.07 | 0.001 |
| FBS mg/dl (mean ± SD) | 184/86 ± 75/03 | 77.46 ± 10.78 | < 0.001 |
| IgA deficiency (Pos/Neg) | 4/96 | 2/148 | 0.177 |
| History of chronic diarrhea (Y/N) | 5/95 | 0/150 | 0.006 |
| History of anemia (Y/N) | 10/90 | 10/140 | 0.341 |
| History of autoimmune disease (Y/N) | 7/93 | 0/150 | 0.001 |
n = 100
n = 150, FBS: fasting blood sugar; SD: standard deviation; Pos: positive; Neg: negative; Y: yes; N: no
Table 2 represents characteristics of tTGA positives compared with tTGA negatives in patients with T1DM. The mean age of tTGA positive cases was significantly lower than tTGA negative ones (mean difference 7.17; 95% CI: 0.82–13.52 years). Furthermore, the mean age of diabetes diagnosis was lower in tTGA positive than tTGA negative subjects, but was not statistically significant (P > 0.1). Positive history of dermatitis was reported in only one of eight tTGA positive cases (P = 0.001). Of the tTGA and IgA deficient patients with T1DM only three (all of them were tTGA positive) underwent duodenal mucosa biopsy. The biopsy showed PVA or TVA in all three patients.
Table 2.
Characteristics of tTGA positives compared with tTGA negatives in T1DM patients
| Characteristics | tTGA positive T1DM patients* | tTGA negative T1DM patients** | P value |
|---|---|---|---|
| Sex (female/male) | 5/3 | 53/39 | 0.788 |
| Age (mean ± SD) | 15.25 ± 5.54 | 22.42 ± 8.88 | 0.027 |
| FBS mg/dl (mean ± SD) | 172.40 ± 36.81 | 185.79 ± 77.21 | 0.703 |
| IgA deficiency (Pos/Neg) | 0/8 | 4/88 | 0.547 |
| History of chronic diarrhea (Y/N) | 0/8 | 5/87 | 0.499 |
| History of anemia (Y/N) | 0/8 | 10/82 | 0.326 |
| History of ketoacidosis (Y/N) | 1/7 | 29/63 | 0.260 |
| History of dermatitis (Y/N) | 1/7 | 0/92 | 0.001 |
| History of autoimmune disease (Y/N) | 1/7 | 6/86 | 0.525 |
n = 8
n = 96; FBS: fasting blood sugar; SD: standard deviation; Pos: positive; Neg: negative; Y: yes; N: no
Discussion
The prevalence of CD in patients with T1DM who underwent tTGA testing was 8%. There was a significant difference in frequency of the tTGA positivity between cases and controls. Results of studies in Western, African and Middle-East countries showed high variation of CD prevalence in patients. In European and American countries, the prevalence ranged from 1–8% by serology.[15] A recent study conducted in UK, of total 113 children and adolescents with T1DM, 6.2% were tested antibody positive.[16] In addition, 12.3% of Danish children with T1DM were positive for CD.[17] These values were remarkably higher among Africans. The prevalence of CD in Libya and Algeria was 21.3% and 16.3% respectively.[18,19] In the Middle-East countries, positive serology tests for CD was detected in 20.9% of Saudi children with T1DM.[20] Apparently, the prevalence of the disease in the present study (8%) is similar to those reported in European countries using serological tests. However, this prevalence is relatively higher than those previously reported in Iran among patients with T1DM. In a study by Shahbazkhani et al,[21] EMA was positive in 2.4% of the patients. Two other studies in Iran compared CD prevalence between the cases (with T1DM) and controls (without T1DM). In one of them, 3.8% of 80 patients with T1DM had positive serology test for CD[22] and in other study, 3.3% of patients with T1DM were tTGA positive,[23] but unlike in our study, these data were not significantly different between cases and controls. The higher CD prevalence in the present study might be explained by differences in study conditions: (1) present study was performed in northwest of Iran; therefore, genetic and environmental factors might account for some of the regional differences, (2) we used tTGA which is proven to be a very specific indicator for CD in contrast with other studies wherein either AGA or EMA was used. Consequently, the prevalence of CD determined by tTGA would be higher than those determined by either AGA or EMA as a screening test. The test for IgA antibodies against tTGA is proven to be highly accurate, the ELISA that has less potential for interpretational error and thus, represents an improvement over the EMA that relies on indirect immunofluorescence, thus, carrying an inherent intraobserver subjectivity in interpretation of the test.[11] It seems that the lower prevalence of CD in patients with T1DM found in previous studies in Iran might be underestimates of the true prevalence in the population. Also, the observed prevalence of CD in our study might have been underestimated. Had all tTGA positive patients with T1DM and IgA deficient ones underwent duodenal biopsy, the true prevalence of CD might have been >8%. Although not all tTGA positives in our study underwent an intestinal biopsy, the confirmed CD prevalence in patients with T1DM was still high (3%) compared with the general population (0.86%).[24]
Since the control group was representative of general population, where CD may develop at any age, both during childhood or adolescence and is relatively common in the adult and elderly patients,[3,25–27] the significant higher mean age of controls than cases does not alter the significant difference of CD prevalence between them.
In the present study, the age of diabetes mellitus diagnosis was lower in tTGA positive subjects compared to tTGA negative ones in patients with T1DM, but the difference was not statistically significant. However, the tTGA positive cases had a significant lower age than tTGA negative cases. These observations are in agreement with some studies which revealed that the risk of CD and T1DM is higher in younger age groups than in older ones.[17,28,29]
Dermatitis is reported to occur in CD patients especially between 15 and 40 years.[7] In our research, one out of eight (12.5%) tTGA positive patients with T1DM had a positive history of dermatitis (P = 0.001). The autoimmune disease history did not differ between the tTGA positives and tTGA negatives in with T1DM patients and the significant difference in having history of autoimmune diseases between cases and controls might be described by the fact that the risk of autoimmune disease increases in patients with T1DM.[30]
Diarrhea is another common symptom among CD patients, but none of tTGA positive patients in our study reported chronic diarrhea. As mentioned before, tTGA screens patients with latent CD, in whom there is absence of diarrhea.[31] Furthermore, Iranian diet contains wheat as a major component, therefore, exposure to a high level of wheat proteins induces some degree of immune tolerance, leading to milder symptoms. This observation supports recommendation of CD screening in patients with T1DM. It should be noted that the significant difference of chronic diarrhea history between the patients with T1DM and the controls was not the result of age difference between the two groups because there was no association between age and reporting history of chronic diarrhea in our research. Probably this finding is due to higher prevalence of chronic diarrhea in patients with T1DM. In a study performed by Lysy et al, nondiabetic and diabetic diarrheas have high prevalence in T1D patients and the most common cause of nondiabetic diarrhea is drug therapy.[32]
Longitudinal prospective studies compared with cross-sectional ones can better show the true prevalence of CD in patients with T1DM. Moreover, the benefits of a gluten-free diet (GFD) in these patients are not well established in Iran. So, it is necessary to conduct a short-term and long-term clinical randomized control trials to investigate the effect of GFD. Obviously, since most of the patients with CD are asymptomatic, many studies recommend serologic testing for diagnosis of T1D and every two years after that.[30,33,34] Thus, the younger individuals with T1DM could benefit from GFD to improve their quality of life.
Conclusion
This study showed the higher prevalence of CD in patients with T1DM than the general population in northwest of Iran and the data lend support to recommend regular screening for CD in all patients with T1DM.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Atkinson MA, Eisenbarth GS. Type 1 diabetes: New perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–9. doi: 10.1016/S0140-6736(01)05415-0. [DOI] [PubMed] [Google Scholar]
- 2.Ciclitira PJ, Moodie SJ. Transition of care between paediatric and adult gastroenterology: Coeliac disease. Best Pract Res Clin Gastroenterol. 2003;17:181–95. doi: 10.1016/s1521-6918(02)00147-6. [DOI] [PubMed] [Google Scholar]
- 3.Rewers M. Epidemiology of celiac disease: What are the prevalence, incidence and progression of celiac disease? Gastroenterology. 2005;128:S47–51. doi: 10.1053/j.gastro.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Payne WW. Coeliac disease and diabetes mellitus in the same patients. Great Ormond St J. 1954;8:118–22. [PubMed] [Google Scholar]
- 5.Farrel R, Kelly CP. Celiac sprue. N Engl J Med. 2002;346:180–8. doi: 10.1056/NEJMra010852. [DOI] [PubMed] [Google Scholar]
- 6.Lazzarotto F, Basso D, Plebani M, Moscon A, Zanchetta R, Betterle C. Celiac disease and type 1 diabetes. Diabetes Care. 2003;26:248–9. doi: 10.2337/diacare.26.1.248. [DOI] [PubMed] [Google Scholar]
- 7.Rossi T. Celiac disease. Adolesc Med Clinics. 2004;15:91–103. doi: 10.1016/j.admecli.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 8.Catassi C, Ratsch IM, Fabiani E, Rossini M, Bordicchia F, Candela F, et al. Celiac disease in the year 2000: Exploring the iceberg. Lancet. 1994;343:200–3. doi: 10.1016/s0140-6736(94)90989-x. [DOI] [PubMed] [Google Scholar]
- 9.Dieterikh W, Storch WB, Schuppan D. Serum antibodies in celiac disease. Clin Lab. 2000;46:361–4. [PubMed] [Google Scholar]
- 10.Sulkanen S, Halttunen T, Laurila K, Kolho KL, Korponay-Szabo IR, Sarnesto A, et al. Tissue transglutaminase autoantibody enzyme-linked immunosorbent assay in detecting celiac disease. Gastroenterology. 1998;115:1322–8. doi: 10.1016/s0016-5085(98)70008-3. [DOI] [PubMed] [Google Scholar]
- 11.Rossi TM, Tjota A. Serologic indicators of celiac disease. J Pediatr Gastroenterol Nutr. 1998;26:205–10. doi: 10.1097/00005176-199802000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Mohn A, Cerruto M, Iafusco D, Prisco F, Tumini S, Stppoloni O, et al. Celiac disease in children and adolescents with type 1 diabetes: Importance of hypoglycemia. J Pediatr Gastroenterol Nutr. 2001;32:37–40. doi: 10.1097/00005176-200101000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Cataldo F, Marino V, Ventura A, Bottaro G, Corazza GR. Prevalence and clinical features of selective immunoglobulin A deficiency in celiac disease: An Italian multi-centre study. Gut. 1998;42:362–5. doi: 10.1136/gut.42.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marsh MN. Mucosal pathology in gluten sensitivity. In: Marsh MN, editor. Celiac disease. Oxford: Blackwell Scientific Publication; 1992. pp. 136–91. [Google Scholar]
- 15.Holmes GK. Celiac disease and type 1 diabetes mellitus-the case for screening. Diabetes Med. 2001;18:169–77. doi: 10.1046/j.1464-5491.2001.00498.x. [DOI] [PubMed] [Google Scholar]
- 16.Goh C, Banerjee K. Prevalence of celiac disease in children and adolescents with type1 diabetes mellitus in a clinic based population. Postgrad Med J. 2007;83:132–6. doi: 10.1136/pgmj.2006.049189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen D, Brock-Jacobsen B, Lund E, Bjorn C, Hansen LP, Nielsen C, et al. Clinical benefit of a gluten-free diet in type 1 diabetic children with screening detected celiac disease. Diabetes Care. 2006;29:2452–6. doi: 10.2337/dc06-0990. [DOI] [PubMed] [Google Scholar]
- 18.Ashabani A, Abushafa U, Abusrewill S, Abdelazez M, Tuckova L, Tlaskalova-Hogenova H. The prevalence of celiac disease in Libyan children with type 1 diabetes mellitus. Diabetes Metab Res Rev. 2003;19:69–75. doi: 10.1002/dmrr.333. [DOI] [PubMed] [Google Scholar]
- 19.Boudraa G, Hachelaf W, Benbouabdellah M, Belkadi M, Benmansour FL, Touhami M. Prevalence of celiac disease in children and their first-degree relatives in West Algeria: Screening with serological markers. Acta Paediatr. 1996;412:58–60. doi: 10.1111/j.1651-2227.1996.tb14254.x. [DOI] [PubMed] [Google Scholar]
- 20.Saadah OI, Al Agha AE, Albokhari SM, Almoghales JA. Prevalence of celiac disease in Saudi children with type 1 diabetes mellitus. J Pediatr Gastroenol Nutr. 2004;39:S211. [Google Scholar]
- 21.Shahbazkhani B, Faezi T, Akbari MR, Mohamadnejad M, Sotoudeh M, Rajab A, et al. Celiac disease in Iranian type 1 diabetic patients. Dig Liver Dis. 2004;36:191–4. doi: 10.1016/j.dld.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Ebrahimi-Daryani N, Fakharian A, Nakhjavani M, Mirmomen S, Bahrami H, Haghpanah B. Frequency of antigliadin and anti endomysial antibodies in type1 diabetic adults. Iranian J Endocrinol Metab. 2003;4:225–8. [Google Scholar]
- 23.Sheikholeslami H, Boostani K, Hashemipour S, Hajmanoochehri F, Ziaee A. Prevalence of celiac disease in type1 diabetic patients comparing with nondiabetic healthy individuals. J Iran Diabetes Lipid. 2005;4:49–55. [Google Scholar]
- 24.Shahbazkhani B, Malekzadeh R, Sotoudeh M, Moghadam KF, Farhadi M, Ansari R, et al. High prevalence of celiac disease in apparently healthy Iranian blood donors. Eur J Gastroenterol Hepatol. 2003;15:475–8. doi: 10.1097/01.meg.0000059118.41030.96. [DOI] [PubMed] [Google Scholar]
- 25.Rodrigo Sáez L. Celiac disease in the adult. Rev Esp Enferm Dig. 2006;98:397–407. doi: 10.4321/s1130-01082006000600001. [DOI] [PubMed] [Google Scholar]
- 26.Casellas F, López Vivancos J, Malagelada JR. Current epidemiology and accessibility to diet compliance in adult celiac disease. Rev Esp Enferm Dig. 2006;98:408–19. doi: 10.4321/s1130-01082006000600002. [DOI] [PubMed] [Google Scholar]
- 27.Sachdev A, Srinivasan V, Maheswary S, Mohan H, Ashish B, Singh LS. Adult onset celiac disease in North India. Trop Gastroenterol. 2002;23:117–9. [PubMed] [Google Scholar]
- 28.Cerutti F, Bruno G, Chiarelli F, Lorini R, Meschi F, Sacchetti C, et al. Younger age at onset and sex predict celiac disease in children and adolescents with type 1 diabetes: An Italian multicenter study. Diabetes Care. 2004;27:1294–8. doi: 10.2337/diacare.27.6.1294. [DOI] [PubMed] [Google Scholar]
- 29.Kaspers S, Kordonouri O, Schober E, Grabert M, Hauffa BP, Holl RW. Anthropometry, metabolic control and thyroid autoimmunity in type 1 diabetes with celiac disease: A multicenter survey. J Pediatr. 2004;145:790–5. doi: 10.1016/j.jpeds.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 30.Barker JM. Clinical Review: Type 1 diabetes-associated autoimmunity: Natural history, genetic association, and screening. J Clin Endocrinol Metab. 2006;91:1210–7. doi: 10.1210/jc.2005-1679. [DOI] [PubMed] [Google Scholar]
- 31.Green PH. The many faces of celiac disease: Clinical presentation of celiac disease in the adult population. Gastroenterology. 2005;128:S74–8. doi: 10.1053/j.gastro.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 32.Lysy J, Israeli E, Goldin E. The prevalence of chronic diarrhea among diabetic patients. Am J Gastroenterol. 1999;94:2165–70. doi: 10.1111/j.1572-0241.1999.01289.x. [DOI] [PubMed] [Google Scholar]
- 33.Hill ID, Bhatnagar S, Cameron DJ, De Rosa S, Maki M, Russell GJ, et al. Celiac disease: working group report of the first world congress of pediatric gastroenterology, hepathology and nutrition. J Pediatr Gastroenterol Nutr. 2002;35:S78–88. doi: 10.1097/00005176-200208002-00004. [DOI] [PubMed] [Google Scholar]
- 34.Barker CC, Mittan C, Jevon G, Mock T. Can tissue transglutaminase antibody titers replace small-bowel biopsy to diagnosis celiac disease in select pediatric populations? Pediatrics. 2005;115:1341–6. doi: 10.1542/peds.2004-1392. [DOI] [PubMed] [Google Scholar]


