Abstract
Purpose
Osteosarcoma is the most frequent primary bone tumor that develops mainly during youth, the median age of diagnosis being 18 years old. Despite improvement in osteosarcoma treatment, survival rate is only 30% after 5 years for patients with pulmonary metastases at diagnosis. This warrants exploration of new therapeutic options. The anti-bone resorption molecule Receptor Activator of NF-κB (RANK) is very promising as it may block the vicious cycle between bone resorption and tumor proliferation that takes place during tumor development in bone site.
Experimental design
The cDNA encoding murine RANK-Fc (mRANK-Fc) was administered by gene transfer using an amphiphilic polymer in a mouse model of osteolytic osteosarcoma. Clinical and bone micro-architecture parameters were assessed by radiography and micro-CT analyses. In vitro experiments were designed to determine the mechanism of action of RANK-Fc on tumor cell proliferation (XTT assays), apoptosis (caspase activation), cell cycle distribution (FACS analysis), or gene expression (RT-PCR).
Results
RANK-Fc was effective in preventing the formation of osteolytic lesions associated with osteosarcoma development, in reducing the tumor incidence, the local tumor growth and the lung metastases dissemination leading to a 3.9-fold augmentation of mice survival 28 days after implantation. On the contrary, mRANK-Fc did not prevent the development of non osseous tumor nodules, suggesting that bone environment is necessary for mRANK-Fc therapeutic efficacy. Furthermore, mRANK-Fc has no dire ct activity on osteosarcoma cells in vitro.
Conclusion
mRANK-Fc exerts an indirect inhibitory effect on osteosarcoma progression through inhibition of bone resorption.
Keywords: Animals; Apoptosis; Cell Line, Tumor; Cell Movement; Cell Proliferation; Disease Models, Animal; Disease Progression; Gene Expression Regulation, Neoplastic; Gene Therapy; Gene Transfer Techniques; Humans; Immunoglobulin Fc Fragments; genetics; Lung; pathology; Mice; Osteolysis; complications; genetics; pathology; therapy; Osteosarcoma; complications; genetics; pathology; therapy; Receptor Activator of Nuclear Factor-kappa B; genetics; therapeutic use; Reproducibility of Results; Solubility; Survival Analysis; Transgenes; Treatment Outcome
Keywords: osteosarcoma, gene transfer, RANK-Fc, bone resorption, RANKL
Introduction
With an estimated incidence of 2 cases per million people per year, osteosarcoma is the most frequent primary bone malignant tumor excluding hematopoietic intraosseous tumors. Osteosarcoma generally affects young patients with a peak incidence at 18 years old. The unifying histologic feature found in all types and subtypes of osteosarcomas is the presence of osteoid tissue produced by the neoplasic cells [1]. As these tumors frequently penetrate and destroy the cortical substance of the bone and extend into the surrounding tissues, a strong osteolytic activity is often associated with osteosarcoma development. In spite of newly devised chemotherapy regimes combined with wide-margin, limb-sparing surgery, osteosarcoma continues to confer a generally poor prognosis in patients with lung metastasis (less than 30%)[2]. As this survival rate has not evolved since two decades, there is an urgent need for new therapeutic strategies, adjuvant to surgery and chemotherapy.
The final effector molecules that ultimately control osteolysis associated with tumor development in bone site are Receptor Activator of NF-κB Ligand (RANKL) and osteoprotegerin (OPG)[3]. RANKL directly stimulates osteoclastic differenciation and osteoclast activation and survival by signalling through its membrane receptor RANK, expressed on the osteoclast precursor surface. Physiologically, RANKL signalling is negatively regulated by the soluble antagonist receptor protein OPG which induces osteoclast apoptosis. Targeting RANKL signalling with OPG inhibits tumor associated osteolysis in several experimental bone tumor models including mouse colon adenocarcinoma, myeloma, breast, lung, prostate cancer and osteosarcoma [4–9]. However, OPG has been shown to bind the TNF receptor TRAIL (TNF Related Apoptosis Inducing Ligand), and to block TRAIL-mediated apoptosis in cancer cells [10–13]. Thus, alternative agents such as soluble RANK-Fc that block RANKL mediated bone resorption but that do not interact with TRAIL, may provide useful therapies to prevent tumor development in bone. RANK-Fc is a chimeric protein formed by fusing the four cysteine-rich pseudorepeats of RANK that are responsible for RANK-L binding with the Fc portion of human immunoglobulin G1 (IgG1). Moreover, RANK-Fc has the potential advantage over OPG of greater specificity for RANKL. Fusion of the four Cystein Rich Domains (CRD) of RANK to the constant region of hIgG1 dictates homodimerization which probably increases its avidity for RANKL. The sRANK differs from OPG because in addition to containing four CRDs, OPG also contains two death domains and a heparin binding domain [14]. Accordingly, RANK-Fc has been recently shown to cause a marked reduction in tumor burden in two SCID-hu-multiple myeloma models [15], and also an inhibition of prostate cancer progression in bone [16]. However, no study was performed on RANK-Fc effects in primary bone tumors.
Previous data showed that it is feasible to provide long-lasting expression of RANK-Fc at bone-protective levels using a retroviral gene therapy approach [17]. However, the toxicity associated with the use of viral vectors is extremely complex involving both the innate and adaptative immune responses. Recently, a new class of synthetic vectors has been reported for in vivo gene transfer in various organs including skeletal and cardiac muscles [18,19] and in lungs [20]. These new synthetic vectors result from the association of plasmid DNA with amphiphilic polymers consisting in blocks of poly(ethylene oxide) and of poly(propylene oxide). Intramuscular injections of these synthetic vectors lead to the synthesis of proteins for local benefit such as dystrophin or for systemic use such as erythropoietin [21].
The aim of this study was to determine the therapeutic relevance of RANK-Fc in a murine osteolytic osteosarcoma model by using a non-viral gene transfer approach.
Material & Methods
Cell lines
- The osteosarcoma cell line POS-1, derived from mouse spontaneous osteosarcoma [22], was cultured in RPMI 1640 medium (Bio Whittaker, Verviers, Belgium) supplemented with 10% fetal bovine serum (FBS, Hyclone, Perbio, France) and 2 mmol/L L-glutamine.
- The osteoclast precursor RAW 264.7 cells from the monocyte macrophage lineage were obtained from the American Tissue and Cell Collection (ATCC) and grown in αMEM (Invitrogen, Cergy-Pontoise, France) supplemented with 10% FBS (Hyclone) and 1% non essential amino acids (Invitrogen).
- The human osteosarcoma MG63 cell line was purchased from the ATCC and used for in vitro assay of TRAIL biological activity and cultured in Dulbecco’s Modified Eagle’s Medium (DMEM, BioWhittaker) supplemented by 10% FBS (Hyclone) and 2 mmol/L of L-glutamine.
In vitro experimentations
Plasmid constructions
The pcDNA3.1-RANK-Fc construction (kindly provided by Dr Choi Y., Philadelphia, USA) contains a DNA sequence encoding the extracellular domain of murine RANK (0.7 kb) fused to the coding sequences of the constant portion of human IgG1 (0.5 kb)[23]. The soluble RANK-Fc cDNA is inserted in the pcDNA3.1 plasmid between XbaI and XhoI under the control of the CMV promoter. For the in vivo and in vitro studies, the empty pcDNA3.1 was used as a control.
Cell transfection
To assess the cellular expression of RANK-Fc, 2 μg of pcDNA3.1 and pcDNA3.1-RANK-Fc were transfected by nucleofection into RAW 264.7 cells using the cell Line nucleofector® Kit V program D-032 (AMAXA biosystems, Köln, Germany) following the manufacturer’s recommendations. The transfection efficacy is controlled by quantification of 2 μg of pmaxGFP™ transfected cells by fluorescence microscopy (AMAXA biosystems).
Osteoclasts differentiation
The biological activity of the transgene was compared between pcDNA3.1-RANK-Fc- and pcDNA3.1-nucleofected RAW 264.7 cells plated in 96-well plates (3000 cells/well) during five days. Media was replaced twice (after 2 and 48 hours) with αMEM 10% FBS, 1% non essential amino acids and increasing concentrations of recombinant human soluble RANKL (50, 75 and 100 ng/ml, kindly provided by Amgen Inc, Thousand Oaks, USA). After five days of culture, multinucleated cells (> 3 nuclei) were counted after a May Grünwald Giemsa staining.
Cell proliferation
Replicate subconfluent cell cultures of POS-1 cells in 96-well plates were treated for 24 to 72 hours with increasing concentrations of murine RANK-Fc (R&D systems, Abingdon, UK) (0, 25, 50 and 100 ng/ml). Cell viability was determined by the sodium 3′[1-(phenylaminocarbonyl)-3,4-tetrazolium]-bis(4-methoxy-6-nitro)benzene sulfonic acid hydrate (XTT) cell proliferation reagent assay kit (Roche Molecular Biomedicals).
Caspase activity
POS-1 cells (2 × 104 per well) cultured in 24-well plates were treated with 100 ng/ml RANK-Fc for 24 to 72 hours, washed and lysed with 50 μl of RIPA buffer for 30 min. in the presence of protease inhibitors. The cells were then scraped off and the protein amount was quantified using the BCA (bicinchominic acid + Copper II sulfate) test (Pierce Chemical Co., Rockford, IL, USA). Caspase-3 activity was assessed on 10 μl of cell lysate with the CaspACE™ assay kit (Promega, Madison, USA) following the manufacturer’s recommendations. Cells treated with 1 μM staurosporin 6 hours before harvesting were used as a positive control for caspase activity.
Cell cycle analysis
Confluent POS-1 cells treated with increasing concentrations of RANK-Fc (0, 50 and 100 ng/ml) for 24, 48 and 72 hours were removed from culture dishes by trypsinization, washed twice in PBS and incubated in PBS containing 0.12% Triton X-100, 0.12 mM EDTA and 1 μg/ml DNase-free ribonuclease A (Sigma Chemical Co, St Quentin Falavier, France). Then each sample was incubated 20 min at 4°C in the dark with 50 μg/ml propidium iodide (Sigma Chemical Co). The stained nuclei were analyzed by flow cytometry (FACScan, BD Biosciences, Franklin Lakes, USA) using the CellQuest software. Cell cycle distribution was based on 2N and 4N DNA content.
TRAIL biological activity
Replicate subconfluent cell cultures of human osteosarcoma MG63 cells in 96-well plates were treated for 72 hours with increasing concentrations of human TRAIL (R&D systems) (0, 50 and 100 ng/ml) in the presence or absence of 100 ng/ml RANK-Fc. Cell viability was determined by the XTT cell proliferation reagent assay kit as described above.
In vivo experimentations
Mouse osteosarcoma models: four-week-old male C3H/He mice (Elevages Janvier, Le Genest St Isle, France) were housed under pathogen-free conditions at the Experimental Therapy Unit (Faculty of Medicine, Nantes, France) in accordance with the institutional guidelines of the French Ethical Committee and under the supervision of authorized investigators. The mice were anaesthetized by inhalation of a combination isoflurane/air (1.5%, 1 L/min) associated with an intramuscular injection of buprenorphine (0.05 mg/kg; Temgesic®, Schering-Plough, Levallois-Perret, France) prior to subcutaneous inoculation of POS-1 cell suspension containing 2 × 106 cells in 50 μl PBS in the hind footpad of the mice. Under these conditions, mice develop a primary tumor at the site of injection in three weeks that can be transplanted to mice of the same strain as a small fragment (2×2×2 mm3) in close contact with the tibia. For this purpose, the periostum of the diaphysis was opened and resected along a length of 5 mm, underlying bone was intact. The osteosarcoma fragment was placed contiguous to the exposed bone surface without periostum, and the cutaneous and muscular wounds were sutured. Tumors appeared at the graft site approximately 8 days later associated with the development of pulmonary metastases in a 3 week-period. The tumor that develops in contact to the tibia induces osteolytic lesions that reproduce the osteolytic form of human osteosarcoma [22]. The tumor volume (V) was calculated from the measurement of two perpendicular diameters using a calliper, according to the following formula: V = 0.5 × L × (S)2, where L and S are respectively the largest and smallest perpendicular tumor diameters. A model of pulmonary metastases was developed to study RANK-Fc effect independent of bone environment, where mice were anaesthetized by inhalation of a mixture of isoflurane/air combined with an intramuscular injection of buprenorphine as described above prior to i.v. injection of 50 μl of POS-1 cell suspension containing 1.5 × 105 cells. In these conditions, pulmonary metastases developed rapidly, leading to the death of the animals in three weeks after POS-1 cell injection.
Formulation preparations: for intramuscular injections, mice were anaesthetized with a combination isoflurane/air. Fifty microliters of block copolymers/DNA formulations were injected into shaved tibial anterior muscles at one site using a microfine syringe (U100, Becton Dickinson, Rungis, France). Lutrol®, a block copolymer consisting of poly(ethyleneoxide)75 -poly(propyleneoxide)30 -poly(ethyleneoxide)75 (PEO75-PPO30-PEO75) was generously provided by BASF (Mount Olive, NJ, USA). Stock solutions were prepared at 6% (w/v) in water and stored at 4°C. Formulations of DNA with block copolymers were prepared by equivolumetric mixing block copolymers in water and DNA solution at the desired concentration (50 μg/muscle), as already reported [21].
Experimental protocols: to determine the effect of RANK-Fc delivered by synthetic vectors on osteosarcoma development, the mice were transplanted with POS-1 osteosarcoma fragments as described above. Groups of 8 mice were assigned respectively as controls (no injection), control vectors (Lutrol®/pcDNA3.1 alone) and RANK-Fc (Lutrol®/pcDNA3.1-RANK-Fc). Preliminary results from the laboratory have already shown that the vector Lutrol® alone does not affect tumor development in the same mouse osteosarcoma model [9]. A preventive treatment was applied, where the Lutrol®/DNA formulations were injected into both tibial anterior muscles once a week, beginning 7 days before osteosarcoma implantation up to 21 days post-implantation. The tumor volume was calculated as described above. Treatment continued until each animal showed signs of morbidity, which included cachexia or respiratory distress, at which point they were sacrificed by cervical dislocation. Lung tumor dissemination was assessed at necropsy.
Radiographs on animals anaesthetized with 50 mg/kg Nesdonal (Merial, Lyon, France) were taken every week and at the time of necropsy with a mammography PLANMED Sophie apparatus (SN RAH 40710, Helsinki, Finland). Analysis of architectural parameters was performed using the high resolution X-ray micro-CT system for small animal imaging SkyScan-1072 (SkyScan, Aartselaar, Belgium). Relative volume (BV/TV) of the tibia [total bone (cortical + trabecular) or trabecular bone] was quantified at necropsy in the osteosarcoma groups that received RANK-Fc compared to that of control mice. The results of one representative experiment out of 3 are shown.
Gene and protein expression analysis
To assess RANK-Fc transgene expression in vitro at the mRNA and protein levels, pcDNA3.1- and pcDNA3.1-RANK-Fc-nucleofected RAW 264.7 cells were cultured in 6-well plates (2 × 106 per well) under standard conditions during 48 hours.
In vivo, blood was drawn intermittently from the retro-orbital vein to monitor serum RANK-Fc level (day 7, 10 or 15 post-injection). At necropsy, the tumor and muscle tissues were lysed in Reporter Lysis Buffer 1X (Promega, Madison, USA) supplemented with protease inhibitor cocktail (Roche Molecular Biomedicals, Manheim, Germany), broyed during 30 sec using Ultraturax®, centrifuged at 10 000 rpm during 5 min at 4°C and were processed for RANK-Fc detection using the ELISA test described below.
RNA extraction and semi-quantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR) analysis
RANK-Fc mRNA expression was determined by RT-PCR after total RNA extraction using Trizol reagent (Invitrogen). First, RNA was reversed-transcribed (RT) using 400 U MMLV-RT from Invitrogen, then two microliters of the RT reaction mixture were subjected to PCR using upstream (5′-GCTGGCTACCACTGGAACTC-3′) and downstream (5′-GTGGGCATGTGTGAGTTTTG-3′) primers (30 pmoles each) of sRANK-Fc and Taq polymerase (1.25 U, Eurobio, Les Ulis, France). The effect of RANK-Fc was assessed on tumor cell phenotype by studying the expression of several bone markers by RT-PCR.
RANK-Fc ELISA
RANK-Fc protein levels were determined by ELISA test: 96-well plates were coated with goat polyclonal anti-mouse RANK extracellular domain (Jackson ImmunoResearch Laboratories, Baltimore, USA) (0.2 μg/well) overnight at room temperature, and then washed and incubated for 1 hour with a blocking solution (1% BSA, 5% sucrose PBS). After washing, wells were incubated at room temperature with dilute serum or media from nucleofected RAW 264.7 cells for 2 hours followed by a 2 hours incubation with a Biotin-SP-conjugate Donkey Anti-Human IgG (1:20000) (R&D systems). Detection was performed with conjugate streptavidin HRP (1:200) (R&D systems) incubation for 30 minutes. Reaction was stopped by 50 μL of 1M H2SO4 then 410nm absorbance was determined thanks to VICTOR2 Multilabel counter (Perkin ELMER, Waltham, USA). The ELISA detection limit was 0.02 ng/ml.
Histology
After sacrifice, tibia were conserved and fixed in 10% neutral buffered formalin at 4°C, and embedded in Glycol Methyl Methacrylate (GMA) for TRAP staining, and 5-μm sections were cut. The sections were stained for TRAP to identify osteoclasts by one hour incubation in a 2 mg/ml napthyl phosphate (Sigma Chemical Co) and 5 mg/ml Fast violet salt (Sigma Chemical Co) solution. The counterstain was performed with toluidine blue after a one hour inactivation of the first solution in sodium fluorure. The number of osteoclasts was evaluated on the cortical bone by manually counting by light microscopy.
Data analyses
For in vivo experimentations, the Tukey/Bonferroni test was used for microscanner and non parametrical test by Kruskal-Wallis analysis for the tumoral progression evaluation. The differences of actuarial survival were determined by the log-rank test on a Kaplan Meier survival curve. Statistical evaluation of the in vitro proliferation data was performed by Student’s t-test. Results are given as mean ± SD and results with p<0.05 were considered significant.
Results
In vitro analysis of transgene expression
mRANK-Fc overexpression was investigated at the mRNA and protein levels by RT-PCR and ELISA analyses performed on RAW 264.7 cells which were transfected with pcDNA3.1 or pcDNA3.1-RANK-Fc using the AMAXA nucleofector system. RT-PCR analysis showed a major expression of RANK-Fc mRNA in pcDNA3.1-RANK-Fc transfected cells whereas no expression was observed in the corresponding pcDNA3.1 transfected cells (Fig. 1a). This result was confirmed at the protein level by ELISA analysis, as supernatant from pcDNA3.1-RANK-Fc transfected cells showed a high level of murine RANK-Fc protein (101.6 ± 15.88 ng/ml) after 48 hours, when compared to pcDNA3.1 transfected cells which showed no protein expression (Fig. 1b).
Figure 1. In vitro and in vivo validation of RANK-Fc transgene overexpression and biological activity.
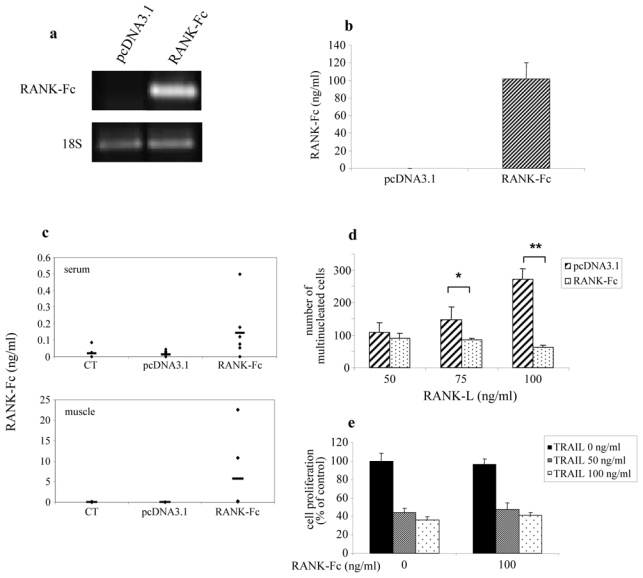
Murine RANK-Fc (mRANK-Fc) transgene expression was assessed 48 hours after transfection in murine RAW 264.7 cells using a AMAXA nucleofector/pcDNA3-mRANK-Fc formulation at the transcript level by RT-PCR (a) and at the protein level by ELISA in the culture medium (b). In vivo, formulations of pcDNA3-mRANK-Fc with Lutrol® block copolymers were assessed. The formulations were prepared by equivolumetric mixing block copolymers in water and DNA solution at the desired concentration (50 μg/muscle). The DNA-Lutrol® formulation was administrated in the tibial anterior muscle of both legs at weekly intervals. Murine RANK-Fc expression was analyzed 7 days post-injection by ELISA test in the muscle and in the serum (c). The biological activity of the mRANK-Fc transgene was assessed by its ability to block the RANKL induced multinucleated cell formation in RAW 264.7 cells (d). RAW 264.7 cells transfected by nucleofection with pcDNA3.1 or pcDNA3.1-mRANK-Fc (RANK-Fc) were cultured in the presence of 50, 75 and 100 ng/ml RANKL during 5 days to assess multinucleated cell formation. *: p=0.048, pcDNA3.1/RANK-Fc vs pcDNA3.1 transfected RAW cells cultured with 75 ng/ml of RANKL. **: p=0.003, pcDNA3.1-mRANK-Fc vs pcDNA3.1 transfected RAW cells cultured with 100 ng/ml of RANKL. Viability assay using XTT test of MG63 cells cultured in the presence of 50 or 100 ng/ml of hTRAIL were realized with recombinant human RANK-Fc (100 ng/ml) which exhibits the same sequence than the RANK-Fc transgene produced in vivo (e).
In vivo validation of transgene expression
After injection of the DNA-Lutrol® complexes into the tibial anterior muscles, an overexpression of the RANK-Fc transgene was measured both at systemic (serum) and local (muscle) levels in mice treated with pcDNA3.1-RANK-Fc. The production varied from 0.053 to 0.499 ng/ml in the serum and from 0.09 to 22.57 ng/ml in the muscle, being significantly higher to the pcDNA3.1 or control groups where no RANK-Fc expression could be detected, either in serum or in muscle (Fig. 1c). No correlation could be established between RANK-Fc concentration (either systemic or local) and its therapeutic efficacy. The immunogenicity of the human Fc part of RANK-Fc was checked previously in a immunocompetent rat model of osteosarcoma, and no antibodies were detected up to 3 weeks (not shown).
In vitro validation of transgene biological activity
To confirm that RANK-Fc expressed by non viral gene transfer was biologically active, we investigated its ability to block RANKL induced multinucleated cell formation in the osteoclast precursor RAW 264.7 cells. Inhibition of osteoclast formation was analyzed by multinucleated cell counting after May Grünwald Giemsa staining of pcDNA3.1 and pcDNA3.1-RANK-Fc transfected RAW 264.7 cells cultured in the presence of soluble RANKL. The results presented in Figure 1d show that RAW 264.7 cells transfected with pcDNA3.1-RANK-Fc induced a significant inhibition of multinucleated cell formation in the presence of RANKL as compared to cells transfected with pcDNA3.1 [respectively 84.7±4 and 148.3±39.1 multinucleated cells (p=0.048) in the presence of 75 ng/ml RANKL, and 62.7±7.4 and 272.7±30.3 (p=0.003) for 100 ng/ml RANKL].
To ensure that RANK-Fc does not block TRAIL-mediated apoptosis, the effect of RANK-Fc was assessed on the human osteosarcoma cell line MG63 treated with increasing concentrations of TRAIL. To dispose of great amount of protein for dose and time-dependant analysis in vitro, a recombinant RANK-Fc corresponding to the same sequence as the transgene used in the in vivo study was chosen. No effect of RANK-Fc treatment could be demonstrated on TRAIL induced apoptosis of MG63 cells (Fig. 1e). Similar results were obtained with other TRAIL sensitive cell lines, such as the A673 human Ewing sarcoma cell line.
These results demonstrate that RANK-Fc protein is expressed after non viral gene transfer both in vitro and in vivo, and that it exerts an anti-resorption activity in vivo (against RANKL) and not a pro-tumoral one (unable to block TRAIL induced apoptosis)
Protective effect of mRANK-Fc transgene expression on bone resorption associated to osteosarcoma development
The mouse osteosarcoma POS-1 model used in this study has been previously associated with osteolytic lesions by micro-CT analysis [22]. To demonstrate the inhibitory effect of RANK-Fc on the development of osteolytic lesions, mice transplanted with osteosarcoma were treated with pcDNA3.1-RANK-Fc or pcDNA3.1 alone (control vector) using the amphiphile block copolymer Lutrol® which had previously demonstrated the best transfection efficiency in vivo [20,21]. Because control tumors and pcDNA3.1 (control vector) treated mice exhibit similar bone alterations [9], only pcDNA3.1 treated mice tibiae radiographs and micro-CT analyses were shown. Important osteolytic lesions were observed on the tibia of POS-1 osteosarcoma bearing mice treated with pcDNA3.1 alone as compared to the pcDNA3.1-RANK-Fc group that exhibit minor lesions (Fig 2a). These results were confirmed by the bone microarchitecture analysis, as the quantification of the specific bone volume revealed a reduced trabecular bone loss in RANK-Fc treated mice as compared to the pcDNA3.1 group: 24.01 % versus 44.7 % respectively (Fig 2b,c). The anti-bone resorption activity of the RANK-Fc transgene was thus confirmed in vivo as RANK-Fc treatment induces a decrease of osteolytic lesions in the POS-1 model of osteosarcoma as compared to the control group (CT: without tumor).
Figure 2. mRANK-Fc transgene expression prevents osteosarcoma associated osteolysis.
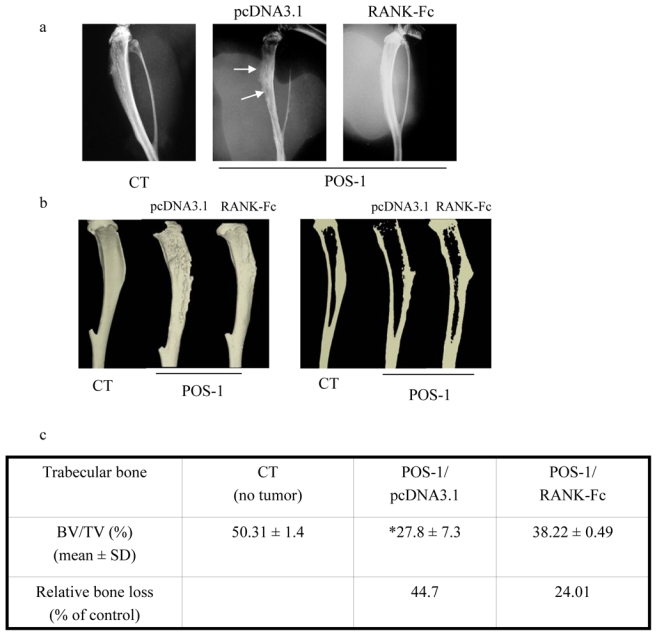
Mice were transplanted with POS-1 osteosarcoma fragments as described in the Material & Methods section. Groups of 6–8 mice were assigned respectively as CT (controls: no tumor), POS-1/pcDNA3.1 (control vectors: Lutrol®/pcDNA3.1 alone in POS-1 osteosarcoma bearing mice) and POS-1/RANK-Fc (Lutrol®/pcDNA3.1-mRANK-Fc in POS-bearing mice). A preventive treatment was applied, where the Lutrol®/DNA formulations were injected into both tibial anterior muscles once a week, beginning at 7 days before osteosarcoma implantation up to 21 days post-implantation (time of sacrifice). At that time, the tumor associated osteolysis was analysed by radiography (a) and the micro-architectural parameters by micro-CT (b and c). The trabecular specific bone volume was quantified in percentage by the following formula: BV/TV (c): BV: bone volume, TV: total volume. Relative bone loss was calculated as percentage of control following the formula: [(BV/TV)CT−(BV/TV)POS or mRANK-Fc]/(BV/TV)CT. *, p < 0.05, POS-1/pcDNA3.1 versus CT.
mRANK-Fc transgene expression limits osteosarcoma progression and increases animal survival
Mice received a preventive treatment with DNA-Lutrol® complexes, the first injections being realized 7 days before osteosarcoma implantation. This strategy was chosen because in vivo transgene production is optimal 7 days after the construct injection, this timepoint corresponding to the day of tumor transplantation. The tumor volume was calculated twice a week following the measure of the two perpendicular diameters. The results show that the tumor volume of each animal was smaller in the RANK-Fc treated group compared to the pcDNA3.1 or the control groups (Fig 3a). As a result, the mean tumor volume 21 days after tumor implantation was significantly lower in the group receiving RANK-Fc compared to the pcDNA3.1 group or the control group (p<0.05), reaching respectively 1652.7±1055 mm3, 4272.7±712.8 mm3 and 4504.1±2162.2 mm3 (Fig 3a). In addition, the incidence of animals bearing progressive tumors (tumor volume > 2000 mm3) is significantly lower at day 21 in the RANK-Fc treated animals (2/6) as compared to controls or pcDNA3.1 treated mice (6/6 for both, Fig. 3a). These results enabled us to calculate the relative tumor progression between day 12 and 21 which was significantly decreased by 62.7% (p<0.05) and 64.3% (p<0.01) in the RANK-Fc treated group as compared respectively to the pcDNA3.1 and untreated group (Fig. 3b). As a consequence, a significant increase (p<0.02) of animal survival was observed with 57.1% of survival at day 28 in the RANK-Fc treated group versus 0% in the pcDNA3.1 group and 14.3% in the untreated group (Fig 3c). Animals died of respiratory distress because of lung metastasis or were sacrificed due to huge tumor development. Animals treated with RANK-Fc showed no pulmonary metastases at day 26.
Figure 3. mRANK-Fc decreases tumor volume, tumor progression and increases mouse survival.
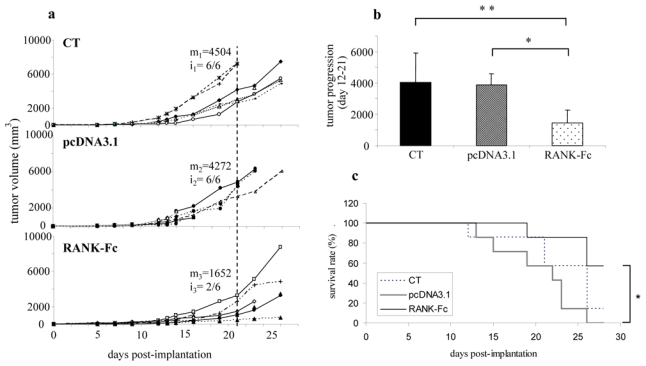
The same protocol was applied as in Figure 2. The tumor volume (V) was calculated from the measurement of two perpendicular diameters, according to the following formula: V = 0.5 × L × (S)2, where L and S are respectively the largest and smallest perpendicular tumor diameters. The tumor volumes of individuals untreated or treated by pcDNA3.1/Lutrol® formulation (named pcDNA3.1) are compared with that of mice treated with pcDNA3.1-mRANK-Fc/Lutrol® (RANK-Fc), 50 μg in both tibial anterior muscles (a). The mean tumor is indicated at day 21 in each group as m1–3. The incidence of animals bearing progressive tumors at day 21 is also indicated as i1–3. The mean tumor progression volume between day 12 and 21 is represented in (b): *: p<0.05, pcDNA3.1-mRANK-Fc treated mice vs pcDNA3.1 mice; **: p<0.01, pcDNA3.1-mRANK-Fc treated mice versus untreated mice (CT). The overall survival rate was compared between control mice and mice that received pcDNA3/Lutrol® formulation (pcDNA3) and pcDNA3.1-mRANK-Fc/Lutrol® (RANK-Fc) over a 28 day-period (c); *: p<0.02.
All these results show that RANK-Fc not only exerts a protective effect on osteosarcoma associated bone lesions but also diminishes the tumor progression in bone, increasing the animal survival in an osteolytic model of osteosarcoma.
Expression of mRANK-Fc inhibits osteosarcoma progression by preventing bone resorption
Complementary to radiographic and micro-CT analyses, TRAP staining was performed on osteosarcoma sections from mice treated or not with RANK-Fc to determine whether this cytokine could inhibit osteosarcoma-induced osteoclast formation. The development of large osteosarcoma tumors in this model is accompanied by increased osteoclastic bone resorption and increased recruitment of osteoclasts to the tumor-bone interface (Fig. 4a, arrows). The presence of TRAP positive osteoclasts was abrogated in the sRANK-Fc group (Fig. 4a,b), confirming at the cellular level the inhibitory effect of RANK-Fc on osteosarcoma associated osteolysis.
Figure 4. mRANK-Fc gene transfer inhibits osteosarcoma progression by preventing bone resorption.
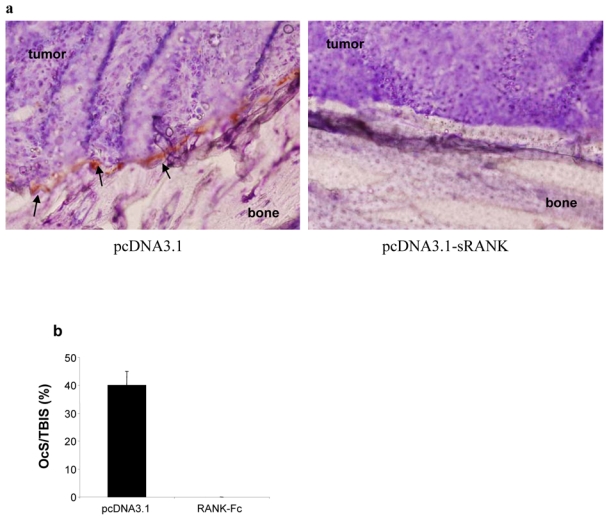
TRAP staining was realized on 6 μm-sections of tibias and tumors from pcDNA3.1 and RANK-Fc treated mice (a). Numerous TRAP positive osteoclasts were observed in POS-1 osteosarcoma bearing mice treated by pcDNA3.1 (arrows). Magnification: x320. Osteoclast numbers at the tumor-bone interface surface (OcS/TBIS; b).
In vitro, RANK-Fc has no effect on tumor cell proliferation, apoptosis, migration and gene expression
To determine whether in vivo RANK-Fc inhibitory effect on tumor progression is direct on tumor cells or not, several in vitro experiments were performed on osteosarcoma cell proliferation, apoptosis, cell migration, gene expression and cell cycle, using the POS-1 mouse osteosarcoma cell line corresponding to the in vivo model.
The viability of POS-1 cells treated with increasing concentrations of RANK-Fc was analyzed from 24 to 72 hours using a XTT based method. No significant effect was noticed even after 72 hours of treatment with 100 ng/ml of RANK-Fc (Fig 5a), nor on POS-1 cell apoptosis as analyzed by caspase activity assay (Fig 5b). Similarly, RANK-Fc treatment had no effect on POS-1 cells migration (data not shown) and on the cell cycle phase distribution (Fig 5c). RT-PCR analysis demonstrates that a 100 ng/ml treatment during 72 hours with RANK-Fc does not induce gene expression modulation of POS-1 cells (data not shown).
Figure 5. murine RANK-Fc exerts no direct effect on osteosarcoma cells in vitro.
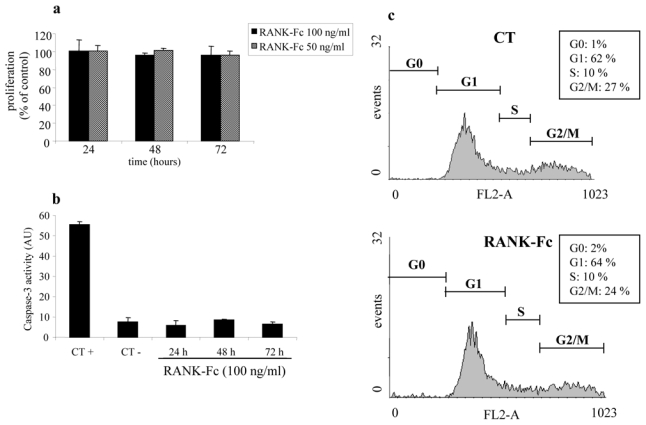
The effects were assessed on POS-1 cell proliferation using an XTT assay as described in the Material & Methods section. POS-1 cells were treated for 24 to 72 hours with increasing concentrations of recombinant murine RANK-Fc (50 and 100 ng/ml), that exhibits the same sequence as the mRANK-Fc transgene used for in vivo experiments (a). To determine the RANK influence on tumor cell apoptosis, the caspase-3 activity was analysed in the cell lysate of POS-1 cells treated with 100 ng/ml mRANK-Fc for 24, 48 and 72 hours (b), CT+: staurosporin (1 μM, 6 hours) was used as positive control; CT−: POS-1 cells alone. Paralleled experiments were performed on cell cycle distribution by FACS analysis in the absence or the presence of 100 ng/ml mRANK-Fc during 24, 48 and 72 hours (c: as no effect could be detected, only the results obtained after 72 hours of incubation are shown).
These data demonstrate that the inhibitory effects observed on tumor progression in vivo for the RANK-Fc treated group are not due to a direct effect on POS-1 tumor cells.
RANK-Fc treatment does not affect tumor nodule development in lungs
As RANK-Fc exerts no direct effect on tumor cells, we hypothesize that the inhibitory effect observed in osteosarcoma development is the indirect consequence of bone resorption inhibition, thus depending on bone microenvironment. To demonstrate this hypothesis, another set of experiments was performed in mice developing tumor lesions in lungs. This model was induced after POS-1 cell injection in the retro-orbital vein. The results presented in Figure 6 show no significant differences between the different groups in the overall mouse survival rate, indicating that RANK-Fc exerts no inhibitory effect on tumor development outside the bone micro-environment.
Figure 6. mRANK-Fc exerts no therapeutic effect in a model of tumor lesions that develop in lungs.
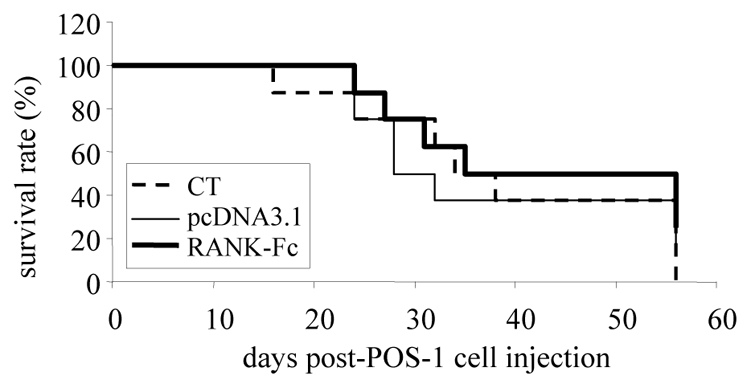
A model of pulmonary tumor lesions was induced in C3H/HeN mice by the intraveinous injection of 200 000 POS-1 cells in the retro-orbital vein. The Lutrol®/DNA formulations were injected into both tibial anterior muscles once a week, beginning at 7 days before POS-1 cells injection up to 21 days post-implantation. The mice were separated into 3 groups (n = 8) treated as follows: control mice received vehicle (PBS) alone (CT), pcDNA3.1/Lutrol® (pcDNA3.1) or pcDNA3.1-mRANK-Fc/Lutrol® (RANK-Fc). The overall survival rate was compared the three groups over a 56 day-period.
Discussion
The mouse model of osteosarcoma used in this study is characterized by the presence of osteolytic lesions [24]. The release of latent growth factors (such as insulin growth factor, TGF-β, BMP, PDGF and VEGF) stored in the bone matrix during osteolysis stimulates the tumor growth in a vicious cycle that leads to tumor cell proliferation and tumor progression in bone sites [25,26]. Bone resorption inhibitors thus appear as one of the most promising tools to manage osteolytic lesions associated to primary or secondary bone tumors. The OPG/RANKL/RANK pathway offers multiple molecular checkpoints for therapeutic targeting in osteolytic tumors. Inhibition of this axis has demonstrated therapeutic efficacy in restricting tumor-mediated osteolysis in vitro as well as in animal models of both bone metastasis [16,27–29] and osteosarcoma [9]. Indeed, previous results obtained in our laboratory in the same mouse osteosarcoma model with OPG gene transfer demonstrated the relevance of using such anti-resorptive factors as promising therapeutic approaches for primitive osteolytic tumors. However, the ability of OPG to block the TRAIL apoptosis pathway in cancer cells was noticed and there were concerns that this could lead to a burst in tumor growth [30]. Denosumab is a fully human mAb directed against RANKL and is currently under investigation in phase III clinical trials [31,32]. Denosumab was generated by immunizing the XenoMouse [33] with full-length human RANKL protein, producing a fully human IgG1 mAb which recognizes an epitope with a single amino acid difference between the mouse and the human sequences. Because human IgG1 can induce complement-dependent cytotoxicity or antibody-dependent cell cytotoxicity to target cells [34], it was converted to a noncytotoxic IgG2 mAb, known as Denosumab which has an extremely high affinity (Kd approximately 10−12 M) for human RANKL. The fact that Denosumab does not recognize rodent RANKL has complicated preclinical development, with only one relevant animal study conducted in cynomolgus monkeys [35]. Therefore, as these anti-RANKL antibodies cannot be used in our mouse model of osteosarcoma, RANK-Fc, another member of the TNF receptor family was used in the present study. Indeed, RANK-Fc, the fusion of the extracellular domain of RANK (amino acids 22-209) with the constant region of human immunoglobulin G1 (IgG1) has the potential advantage over OPG of greater specificity for RANKL [15]. Delivery of RANK-Fc as a recombinant protein has shown promising results as a potential therapy through experiments in animal models, in that RANK-Fc limits hypercalcemia and osteolysis induced by myeloma or prostate cancer and reduces bone tumor establishment in these models [15,16,29,36]. Moreover, a recent study reported that RANK-Fc inhibition of RANKL has an anti-osteoclast activity at doses that have no detectable immunoregulatory activity [37]. Even if long-lasting expression of RANK-Fc could be provided at bone-protective levels using a retrovirus-mediated gene transfer approach by the use of genetically modified mesenchymal stem cells [17], the toxicity associated with the use of such viral vectors is extremely complex.
The results from the present study validate the non viral gene transfer method and the therapeutic interest of RANK-Fc in osteosarcoma. This methodology has previously proved its efficacy to deliver and express therapeutic gene in the same mouse model of osteosarcoma [9]. In the present study, we demonstrate that when injected every week, the DNA-Lutrol® complexes can induce RANK-Fc over-expression both at local (muscle) and systemic levels in sufficient quantities to induce anti-bone resorption activity. The overproduction reached its maximum 7 days after injection and lasted for 15 days at the systemic level. The intra-muscular injection of the RANK-Fc/Lutrol® complexes induces a local RANK-Fc transgene production that blocks bone resorption, as confirmed by radiography, histology, and quantified by micro-CT analysis in an osteolytic model of osteosarcoma. Moreover, the same inhibitory effect on bone osteolytic lesions has been obtained by adenoviral delivery of RANK-Fc in a rat osteocondensant osteosarcoma (data already published; [3,27]).
Given its role in osteoclastogenesis, it is likely that inhibition of osteosarcoma progression by RANK-Fc reflects, at least in part, its ability to inhibit bone resorption. Indeed, RANK-Fc has no direct activity on osteosarcoma cells, as demonstrated by in vitro studies on proliferation, migration, apoptosis, cell cycle or phenotype analysis. Furthermore, the observation that RANK-Fc did not diminish development of non osseous tumors (pulmonary tumor lesions induced by the same osteosarcoma POS-1 cells injected i.v.) suggests that the ability of RANK-Fc to inhibit osteosarcoma development was not caused by a direct effect on tumor but rather specific to factors in the bone microenvironment. Given that RANKL expression has been shown previously in the POS-1 tumor by immunohistochemical analyses [9], the present data suggest that inhibition of RANKL activity diminishes the osteosarcoma progression. Moreover, other experiments performed in our laboratory have shown that osteosarcoma cells express RANK, and that RANKL is able to induce modulation of gene expression in these cells [24,38,39]. Targeting RANKL is therefore a promising approach in bone tumor therapy. It has been demonstrated that RANKL is implicated in the pathogenesis of bone metastasis at several levels: increased bone resorption as a result of excess RANKL results in the release of growth factors that facilitate tumor cell division and survival, and a recent study suggested a role for RANKL as a chemoattractant for certain cancer cells metastatic to bone [40]. Furthermore, RANKL can stimulate both angiogenesis [41] and endothelial cell survival [42], suggesting a role for RANKL in supporting vascularization of bone metastases. This role could also be extended to osteosarcoma, which is a naturally highly vascularized tumor. As RANKL participates to the regulation of Treg function, it could also interfere in tumor response at this level [43,44].
To inhibit RANKL activity, three candidates are potentially promising: the decoy receptor OPG, the soluble receptor RANK-Fc constructed as the fusion of RANK extracellular domain with the constant region of the human IgG1, and antibodies directed against human RANKL. The advantage of using anti-RANKL antibodies is that they do not inhibit the TRAIL apoptosis pathway. In a recent randomized, double-blind, double dummy, active-controlled multicenter phase I clinical study, Body et al [45] demonstrated the efficacy of denosumab, A single subcutaneous dose of denosumab given to patients with multiple myeloma or bone metastasis from breast cancer yielded a dose dependent and sustained reduction in bone resorption. However, as this therapeutic agent cannot be used in immuno-competent models of bone tumors in rodents, RANK-Fc which is more specific of RANKL binding than OPG seems therefore a better therapeutic tool for these pathologies.
In conclusion, these data demonstrate that RANK-Fc delivered by non viral gene transfer is a promising therapeutic approach for osteolytic bone tumors such as osteosarcoma by targeting RANKL in the bone microenvironment.
Acknowledgments
Financial support: This study was financially supported by the Institut National du Cancer (INCa), the « Fondation pour l’Avenir de la Recherche Médicale Appliquée » (study n°ET 6-418, Paris, France), the Ligue Contre le Cancer (Comité des Pays de la Loire) and the INSERM - Région Pays de la Loire (grant for JR). FL was financially supported by the Association de Recherche sur le Cancer, GP by the INCa.
The authors wish to thank Christelle Bailly, Marie-Noëlle Hervé and Cyril Le Corre from the Experimental Therapy Unit and Paul Pilet (INSERM U791) from the microscopy platforms of the IFR26 (Nantes, France) for their technical assistance. We thank G. Odri for critical review of this manuscript.
Abbreviations list
- OPG
osteoprotegerin
- TNF
Tumor Necrosis Factor
- RANK
Receptor Activator of NF-kappaB
- RANKL
RANK Ligand
- TRAIL
TNF Related Apoptosis Inducing Ligand
Footnotes
The authors declare no conflict of interest
References
- 1.Klein MJ, Siegal GP. Osteosarcoma. Anatomic and histologic variants. Am J Clin Pathol. 2006;125:555–81. doi: 10.1309/UC6K-QHLD-9LV2-KENN. [DOI] [PubMed] [Google Scholar]
- 2.Campanacci M, Bacci G, Bertoni F, Picci P, Minutillo A, Franceschi C. The treatment of osteosarcoma of the extremities: twenty year’s experience at Istituto Rizzoli. Cancer. 1981;48:1569–81. doi: 10.1002/1097-0142(19811001)48:7<1569::aid-cncr2820480717>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 3.Baud’huin M, Lamoureux F, Duplomb L, Rédini F, Heymann D. RANKL, RANK, osteoprotegerin: key partners of osteoimmunology and vascular diseases. Cell Mol Life Sci. 2007;64:2334–50. doi: 10.1007/s00018-007-7104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morony S, Capparelli C, Sarosi I, Lacey DL, Dunstan CR, Kostenuik PJ. Osteoprotegerin inhibits osteolysis and decreases skeletal tumor burden in syngeneic and nude mouse models of experimental bone metastasis. Cancer Res. 2001;61:4432–6. [PubMed] [Google Scholar]
- 5.Croucher PI, Shipman CM, Lippitt J, et al. Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood. 2001;98:3534–40. doi: 10.1182/blood.v98.13.3534. [DOI] [PubMed] [Google Scholar]
- 6.Yonou H, Kanomata N, Goya M, et al. Osteoprotegerin/osteoclastogenesis inhibitory factor decreases human prostate cancer burden in human adult bone implanted into nonobese diabetic/severe combined immunodeficient mice. Cancer Res. 2003;63:2096–102. [PubMed] [Google Scholar]
- 7.Capparelli C, Kostenuik PJ, Morony S, et al. Osteoprotegerin prevents and reverses hypercalcemia in a murine model of humoral hypercalcemia of malignancy. Cancer Res. 2000;60:783–7. [PubMed] [Google Scholar]
- 8.Zhang J, Dai J, Qi Y, et al. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest. 2001;107:1235–44. doi: 10.1172/JCI11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamoureux F, Richard P, Wittrant Y, et al. Therapeutic relevance of osteoprotegerin gene therapy in osteosarcoma: prevention of bone resorption, inhibition of tumor progression, increase of animal survival. Cancer Res. 2007;67:7308–18. doi: 10.1158/0008-5472.CAN-06-4130. [DOI] [PubMed] [Google Scholar]
- 10.Holen I, Shipman CM. Role of osteoprotegerin (OPG) in cancer. Clin Sci (Lond) 2006;110:279–91. doi: 10.1042/CS20050175. [DOI] [PubMed] [Google Scholar]
- 11.Holen I, Croucher PI, Hamdy FC, Eaton CL. Osteoprotegerin (OPG) is a survival factor for human prostate cancer cells. Cancer Res. 2002;62:1619–23. [PubMed] [Google Scholar]
- 12.Shipman CM, Croucher PI. Osteoprotegerin is a soluble decoy receptor for tumor necrosis factor-related apoptosis-inducing ligand/Apo2 ligand and can function as a paracrine survival factor for human myeloma cells. Cancer Res. 2003;63:912–6. [PubMed] [Google Scholar]
- 13.Kapoor P, Suva LJ, Welch DR, Donahue HJ. Osteoprotegerin and the bone homing and colonization potential of breast cancer. J Cell Biochem. 2008:1030–41. doi: 10.1002/jcb.21382. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi K, Kinosaki M, Goto M, et al. Characterization of Structural Domains of Human Osteoclastogenesis Inhibitory Factor. J Biol Chem. 1998;273:5117–23. doi: 10.1074/jbc.273.9.5117. [DOI] [PubMed] [Google Scholar]
- 15.Sordillo EM, Pearse RN. RANK-Fc: a therapeutic antagonist for RANK-L in myeloma. Cancer. 2003;97(3 Suppl):802–12. doi: 10.1002/cncr.11134. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Dai J, Yao Z, Lu Y, Dougall W, Keller ET. Soluble Receptor Activator of Nuclear Factor kB diminishes prostate cancer progression in bone. Cancer Res. 2003;63:7883–90. [PubMed] [Google Scholar]
- 17.Kim D, Cho SW, Her SJ, et al. Retrovirus-mediated gene transfer of receptor activator of nuclear factor-kappaB-Fc prevents bone loss in ovariectomized mice. Stem Cells. 2006;24:1798–805. doi: 10.1634/stemcells.2005-0480. [DOI] [PubMed] [Google Scholar]
- 18.Pitard B, Pollard H, Agbulut O, et al. A nonionic amphiphile agent promotes gene delivery in vivo to skeletal and cardiac muscles. Hum Gene Ther. 2002;13:1767–75. doi: 10.1089/104303402760293592. [DOI] [PubMed] [Google Scholar]
- 19.Pitard B, Bello-Roufai M, Lambert O, et al. Negatively charged self-assembling DNA/poloxamine nanospheres for in vivo gene transfer. Nucleic Acids Res. 2004;32:e159. doi: 10.1093/nar/gnh153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desigaux L, Gourden C, Bello-Roufai M, et al. Nonionic amphiphilic block copolymers promote gene transfer to the lung. Hum Gene Ther. 2005;16:821–9. doi: 10.1089/hum.2005.16.821. [DOI] [PubMed] [Google Scholar]
- 21.Richard P, Bossard F, Desigaux L, Lanctin C, Bello-Roufai M, Pitard B. Amphiphilic block copolymers promote gene delivery in vivo to pathological skeletal muscles. Hum Gene Ther. 2005;16:1318–24. doi: 10.1089/hum.2005.16.1318. [DOI] [PubMed] [Google Scholar]
- 22.Kamijo A, Koshino T, Uesugi M, Nitto H, Saito T. Inhibition of lung metastasis of osteosarcoma cell line POS-1 transplanted into mice by thigh ligation. Cancer Lett. 2002;188:213–9. doi: 10.1016/s0304-3835(02)00433-0. [DOI] [PubMed] [Google Scholar]
- 23.Guilloneau C, Louvet C, Renaudin K, et al. The role of TNF-related activation-induced cytokine-receptor activating NF-kappa B interaction in acute allograft rejection and CD40L-independent chronic allograft rejection. J Immunol. 2004;172:1619–29. doi: 10.4049/jimmunol.172.3.1619. [DOI] [PubMed] [Google Scholar]
- 24.Wittrant Y, Lamoureux F, Mori K, et al. RANKL directly induces bone morphogenetic protein-2 expression in RANK-expressing POS-1 osteosarcoma cells. Int J Oncol. 2006;28:261–9. [PubMed] [Google Scholar]
- 25.Guise TA, Kozlow WM, Heras-Herzig A, Padalecki SS, Yin JJ, Chirgwin JM. Molecular mechanisms of breast cancer metastases to bone. Clin Breast Cancer. 2005;5(Suppl 2):S46–53. doi: 10.3816/cbc.2005.s.004. [DOI] [PubMed] [Google Scholar]
- 26.Yin JJ, Pollock CB, Kelly K. Mechanisms of cancer metastasis to the bone. Cell Res. 2005;15:57–62. doi: 10.1038/sj.cr.7290266. [DOI] [PubMed] [Google Scholar]
- 27.Wittrant Y, Theoleyre S, Chipoy C, et al. RANKL/RANK/OPG: new therapeutic targets in bone tumours and associated osteolysis. Biochim Biophys Acta. 2004;170:49–57. doi: 10.1016/j.bbcan.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Whang PG, Schwarz EM, Gamradt SC, Dougall WC, Lieberman JR. The effects of RANK blockade and osteoclast depletion in a model of pure osteoblastic prostate cancer metastasis in bone. J Orthop Res. 2005;23:1475–83. doi: 10.1016/j.orthres.2005.05.004.1100230634. [DOI] [PubMed] [Google Scholar]
- 29.Feeley BT, Liu NO, Conduah AH, et al. Mixed metastatic lung cancer lesions in bone are inhibited by noggin overexpression and Rank:Fc administration. J Bone Miner Res. 2006;21:1571–80. doi: 10.1359/jbmr.060706. [DOI] [PubMed] [Google Scholar]
- 30.Neville-Webbe HL, Cross NA, Eaton CL, et al. Osteoprotegerin (OPG) produced by bone marrow stromal cells protects breast cancer cells from TRAIL-induced apoptosis. Breast Cancer Res Treat. 2004;86:269–79. doi: 10.1023/b:brea.0000036900.48763.b3. [DOI] [PubMed] [Google Scholar]
- 31.Weiner LM. Fully human therapeutic monoclonal antibodies. J Immunother. 2006;29:1–9. doi: 10.1097/01.cji.0000192105.24583.83. [DOI] [PubMed] [Google Scholar]
- 32.Lonberg N. Human antibodies from transgenic animals. Nat Biotechnol. 2005;23:1117–25. doi: 10.1038/nbt1135. [DOI] [PubMed] [Google Scholar]
- 33.Green LL. Antibody engineering via genetic engineering of the mouse: XenoMouse strains are a vehicle for the facile generation of therapeutic human monoclonal antibodies. J Immunol Methods. 1999;231:11–23. doi: 10.1016/s0022-1759(99)00137-4. [DOI] [PubMed] [Google Scholar]
- 34.Scallon BJ, Moore MA, Trinh H, Knight DM, Ghrayeb J. Chimeric anti-TNF-alpha monoclonal antibody cA2 binds recombinant transmembrane TNF-alpha and activates immune effector functions. Cytokine. 1995;7:251–9. doi: 10.1006/cyto.1995.0029. [DOI] [PubMed] [Google Scholar]
- 35.Kostenuik PJ. Osteoprotegerin and RANKL regulate bone resorption, density, geometry and strength. Curr Opin Pharmacol. 2005;5:618–25. doi: 10.1016/j.coph.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Oyajobi BO, Anderson DM, Traianedes K, Williams PJ, Yoneda T, Mundy GR. Therapeutic efficacy of a soluble receptor activator of nuclear factor kappaB-IgG Fc fusion protein in suppressing bone resorption and hypercalcemia in a model of humoral hypercalcemia of malignancy. Cancer Res. 2006;61:2572–8. [PubMed] [Google Scholar]
- 37.Miller RE, Branstetter D, Armstrong A, et al. Receptor activator of NF-kappa B ligand inhibition suppresses bone resorption and hypercalcemia but does not affect host immune responses to influenza infection. J Immunol. 2007;179:266–74. doi: 10.4049/jimmunol.179.1.266. [DOI] [PubMed] [Google Scholar]
- 38.Mori K, Le Goff B, Berreur M, et al. Human osteosarcoma cells express functional receptor activator of nuclear factor-kappa B. J Pathol. 2007;211:555–62. doi: 10.1002/path.2140. [DOI] [PubMed] [Google Scholar]
- 39.Mori K, Berreur M, Blanchard F, et al. Receptor activator of nuclear factor-kappaB ligand (RANKL) directly modulates the gene expression profile of RANK-positive Saos-2 human osteosarcoma cells. Oncol Rep. 2007;18:1365–71. [PubMed] [Google Scholar]
- 40.Jones DH, Nakashima T, Sanchez OH, et al. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440:692–6. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- 41.Min JK, Kim YM, Kim YM, et al. Vascular endothelial growth factor up-regulates expression of receptor activator of NF-kappa B (RANK) in endothelial cells. Concomitant increase of angiogenic responses to RANK ligand. J Biol Chem. 2003;278:39548–57. doi: 10.1074/jbc.M300539200. [DOI] [PubMed] [Google Scholar]
- 42.Kim YM, Kim YM, Lee YM, et al. TNF-related activation-induced cytokine (TRANCE) induces angiogenesis through the activation of Src and phospholipase C (PLC) in human endothelial cells. J Biol Chem. 2002;277:6799–805. doi: 10.1074/jbc.M109434200. [DOI] [PubMed] [Google Scholar]
- 43.Antony PA, Restifo NP. Do CD4+CD25+ immunoregulatory T cells hinder tumor immunotherapy ? J Immunother. 1997;25:202–6. doi: 10.1097/00002371-200205000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Curotto de Lafaille MA, Lafaille JJ. CD4(+) regulatory T cells in autoimmunity and allergy. Curr Opin Immunol. 2002;14:771–8. doi: 10.1016/s0952-7915(02)00408-9. [DOI] [PubMed] [Google Scholar]
- 45.Body JJ. Breast cancer: bisphosphonate therapy for metastatic bone disease. Clin Cancer Res. 2006;12:6258s–6263s. doi: 10.1158/1078-0432.CCR-06-0840. [DOI] [PubMed] [Google Scholar]


