Abstract
Problem
Women with antiphospholipid antibodies (aPL) are at risk for recurrent miscarriage, preeclampsia and preterm labor. aPL target the placenta directly by binding to Beta2-Glycoprotein I (β2GPI) expressed on the surface of trophoblast cells. The objective of this study was to determine the effects of aPL on trophoblast function and the mechanisms involved.
Method of study
First trimester trophoblast were treated with anti-β2GPI monoclonal antibodies and patient-derived aPL, after which cell survival and function was evaluated.
Results
We report that anti-β2GPI antibodies trigger an inflammatory response in trophoblast, characterized by increased secretion of IL-8, MCP-1, GRO-α and IL-1β, and that this occurs in a TLR-4/MyD88-dependent manner. At high concentrations, these antibodies also induce caspase-mediated cell death. This was attenuated upon disabling of the MyD88 pathway, suggesting that anti-β2GPI-induced inflammatory mediators compromise trophoblast survival by acting in an autocrine/paracrine manner. Enhanced IL-8, GRO-α and IL-1β secretion also occured when trophoblast were incubated with antibodies from patients with antiphospholipid syndrome. Heparin, which acts as a pro-survival factor in human trophoblast, attenuated the anti-β2GPI antibody-mediated cell death, and also the pro-inflammatory response, but only at high concentrations.
Conclusions
These findings demonstrate that aPL triggers a placental inflammatory response via the TLR-4/MyD88 pathway, which in turn compromises trophoblast survival. Thus, the TLR-4/MyD88 pathway may provide a new therapeutic target to improve pregnancy outcome in antiphospholipid syndrome patients.
Keywords: Apoptosis, Autoantibodies, Human, Inflammation, Toll-like receptor
Introduction
The presence of antiphospholipid antibodies (aPL) negatively impacts on a woman's chance of reproductive success. This heterogeneous family of autoantibodies are found in patients with primary antiphospholipid syndrome (APS) which may occur in isolation (primary APS) or in the presence of other autoimmune rheumatic disease (secondary APS); most commonly in patients with systemic lupus erythematosus (SLE) 1. Pregnant women with aPL are at high risk for thrombosis, recurrent pregnancy loss, as well as other pregnancy complications associated with impaired placental function, such as fetal growth restriction and preeclampsia 1–5. While treatment with aspirin and heparin from early pregnancy significantly increases the live birth rate in recurrent miscarriage patients with APS, the incidence of severe late obstetric complications, including preeclampsia, intrauterine growth restriction and prematurity, still remains high 6,7.
Most aPL in APS and SLE are detected by the anti-cardiolipin ELISA or lupus anticoagulant test 1. However, it has become increasingly clear that pathogenic aPL actually recognize epitopes on phospholipid-binding proteins rather than on the phospholipids themselves. The most important of these is the plasma glycoprotein Beta2-Glycoprotein I (β2GPI), which is a major antigen of aPL 2. Trophoblast cells synthesize their own β2GPI and express this protein on the cell surface under normal physiological conditions 8. Furthermore, exogenous β2GPI can bind to the surface of viable trophoblast cells 9. In vivo, β2GPI localizes to the surface of the extravillous trophoblast cells that invade the decidua, and to the syncytiotrophoblast cells that are in direct contact with maternal blood 8,10. Thus, the placenta is a target for anti-β2GPI Abs.
As aPL are one of the most common causes of acquired hypercoagulability in the general population, pregnancy complications in patients with APS are often attributed to thrombotic events at the maternal-fetal interface. This notion is seemingly supported by the success of anticoagulant treatment, and more specifically heparin, in preventing fetal loss in affected patients. However, intravascular or intervillous blood clots are rarely found on histological examination of miscarriage samples from APS patients 11. Instead, the process of placenta formation is compromised, evidenced by reduced endovascular trophoblast invasion, limited spiral artery transformation 11,12, and profound influx of inflammatory immune cells, such as neutrophils, at the maternal-fetal interface 13–15. These observations suggest that aPL may primarily impact on trophoblast function rather than causing a direct insult on the feto-maternal vasculature. Indeed, several in vitro studies have shown that aPL compromise proliferation, invasion and differentiation of term trophoblast and choriocarcinoma cell lines 9,16–22, although the underlying mechanisms are unknown. Furthermore, early pregnancy loss is the commonest pregnancy complication associated with APS, yet the effects of aPL on first trimester trophoblast function and survival have not been studied.
This study demonstrates for the first time that anti-β2GPI Abs elicit a dose-dependent response in human first trimester trophoblast cells. High anti-β2GPI Ab concentrations induce cell death and apoptosis, whereas lower concentrations markedly enhance trophoblast production of IL-8, MCP-1, GRO-α, and IL-1β in a Toll-like receptor 4/myeloid differentiation factor 88 (TLR4/MyD88)-dependent manner. Interestingly, disabling of the TLR4/MyD88 pathway not only attenuated this pro-inflammatory response but also partially protected trophoblast to cell death induced by anti-β2GPI Abs. Furthermore, the effect of anti-β2GPI Abs on trophoblast cytokine and chemokine production was recapitulated with purified Abs from sera of affected patients, indicating that adverse pregnancy outcome in APS may be primarily caused by an excessive placental inflammatory response, leading to trophoblast cell death and tissue injury.
Materials and Methods
Reagents and antibodies
Camptothecin (CPT) and heparin were both purchased from Sigma (St Louis, MO). The mouse antibody for β2GPI was purchased from Abnova (Walnut, CA), and the rabbit anti-β-actin polyclonal antibody was purchased from Sigma. Specific signals for Western blot were detected using a peroxidase-conjugated horse anti-mouse secondary antibody (Vector Laboratories).
Antiphospholipid antibodies
These studies utilized two mouse IgG1 anti-human β2GPI monoclonal Abs (mAb), designated ID2 and IIC5, which were produced by one of us (LWC), under sterile conditions, and were filter-sterilized prior to use. The antibodies, ID2 and IIC5 were cloned from mice immunized with purified human β2GPI. They have been previously characterized 20, and like human aPL, they bind β2GPI, but only when it is immobilized on a suitable negatively charged surface, such the phospholipids, cardiolipin or phosphatidyl serine, or irradiated polystyrene 23. In addition, polyclonal IgG purified from the serum of 18 patients with different clinical manifestations of APS, fulfilling APS criteria 24, under long term follow up at University College London Hospital (UCLH) were studied. All subjects signed consent forms approved by the local ethics committees at UCLH. IgG from the nine subjects was purified by protein G sepharose affinity columns (Amersham Biosciences, Sweden) and then passed through Detoxi-Gel™ Endotoxin removing columns coated with immobilised polymyxin B (Pierce, Perbio, UK) and subsequently determined to be endotoxin free (<0.06 endotoxin units/ml) by the Limulus Amoebocyte Lysate assay (Sigma, UK). The polyclonal APS-IgG was purified from stored (−80) serum samples which were taken close to the time of the APS related clinical event and confirmed to have current anti-cardiolipin/anti-β2GPI activity, as shown in Table I. Anti-β2GPI activity was measured using HCAL (Sapporo Standard, used at 25µg/ml), and anti-cardiolipin activity, was determined using cardiolipin calibrators (APS Diagnostics laboratory, Galveston, TX). The patient samples were then divided into 3 groups: 1) PM+/VT−: patients who have had previous episodes of pregnancy morbidity (PM), but no history of venous thrombosis (VT) (n=6); 2) PM−/VT+: patients who have had VT, but not PM (n=6); and 3) PM+/VT+: patients who have had both VT and PM (n=6). In the VT/PM group, three samples were obtained from close to the PM event, two were close to the VT event, and one sample was obtained from a patient who had recently experienced a pulmonary embolus two weeks post APS related PM. All patient clinical and serological information is shown in Table I.
Table I.
Summary of clinical data from patients with APSa
| Age | Ethnicity | Other Disease | Live Births |
PM History | VT History | Clinical aCL |
Clinical anti-β2GPI |
Clinical LA |
Lab* aCL (GPLU) |
Lab* anti-β2GPI (% HCAL activity) |
Other autoAbs |
Treatments | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VT1 | 46 | Caucasian | - | 0 | - | 5× TIA | + | NT | − | 50 | >50% | - | Warfarin; Diclofenac |
| VT2 | 39 | Asian | SLE; CAPS; HT | 0 | - | CAPS: Palmar thrombosis | − | + | + | >96 | >50% | ANA ENA DNA | Aspirin; Alendronate; Prednisolone; Losartan; Atorvastatin; Morphine Sulphate; Oramorph |
| VT3 | 25 | Caucasian | SLE; autoimmune hypothyroidism | 1 | - | 1× PE | − | + | − | 85 | >50% | ANA ENA DNA | Warfarin; Aspirin; Hydroxychloroquine; Calcium; Thyroxine |
| VT4 | 60 | Caucasian | - | 0 | - | 1× CVA | + | + | + | >96 | >100% | - | Warfarin; Aspirin |
| VT5 | 72 | Caucasian | SLE | 2 | - | 1× DVT | + | + | + | 90 | >100% | ANA DNA | Warfarin |
| VT6 | 35 | Caucasian | SLE | 0 | - | 1× CVA | + | NT | + | 70 | >100% | ANA DNA | Warfarin; Azathioprine |
| PM1 | 51 | Caucasian | - | 1 | 1× 2nd trimester PL; 1× death in utero due to rhesus incompatibility | - | + | + | + | >96 | >100% | ANA | Aspirin |
| PM2 | 50 | Caucasian | ITP; IDDM | 3 | 1× 2nd trimester PL | - | + | + | + | >96 | >100% | ANA DNA | Aspirin; Rituximab; Insulin |
| PM3 | 60 | Caucasian | HT | 1 | 3× 2nd trimester PL; 1× LB 36/52 weeks (Pre-eclampsia & IUGR) | - | + | NT | + | >90 | >100% | - | Aspirin; Amlodipine; Enalapril |
| PM4 | 64 | Caucasian | - | 0 | 5× 1st trimester PL; 1× 2nd trimester PL | - | − | + | + | 50 | >100% | - | Aspirin; Hydroxychloroquine |
| PM5 | 45 | Oriental | SLE | 0 | 2× 2nd trimester PL; 1× ectopic pregnancy | - | + | NT | + | <30 | - | ANA ENA DNA | Prednisolone; Azathioprine |
| PM6 | 47 | African-Caribbean | SLE | 2 | 1× LB delivered at 5/12 months (low birth weight); 1× ectopic pregnancy | - | − | − | + | 50 | - | ANA ENA DNA | Hydroxychloroquine; Diclofenac |
| VT/PM1 | 51 | Caucasian | HT | 2 | 1× LB at 32/52 weeks | 1× PE | + | NT | + | >90 | >100% | ANA | Warfarin; Aspirin; Nifedipine |
| VT/PM2 | 52 | Caucasian | HT | 0 | 3×1st trimester PL; 1× 2nd trimester PL | 1× DVT | − | NT | + | 90 | 100% | ANA | Warfarin; Telmisartan |
| VT/PM3 | 40 | Caucasian | - | 0 | 1× IUD 23/52 weeks due to placental thrombosis | 1× CVA | + | NT | + | >96 | >100% | - | Aspirin |
| VT/PM4 | 52 | Caucasian | - | 2 | 1× LB at 29/52 weeks (preeclampsia); 1× blighted ovum | 1× PE; 2× CVA | + | + | + | >96 | >100% | - | Warfarin |
| VT/PM5 | 34 | Caucasian | - | 0 | 1× 1st trimester PL; 2× 2nd trimester PL | 1× DVT | + | + | + | 70 | >100% | - | Warfarin |
| VT/PM6 | 51 | Caucasian | - | 3 | 1× 2nd trimester PL (twins) | 3× CVA; 1× DVT; 1× PE | + | NT | + | 90 | >100% | - | Warfarin |
Purified IgG at 500µg/ml was tested for anticardiolipin and anti-β2GPI activity using cardiolipin calibrators and HCAL, respectively. Values are shown in GPLU and as a percentage of HCAL activity. Serum lupus anticoagulant activity was measured using either dRVVT or aPPT. All positive ANA are ≥ 1/80.
Abbreviations: aβ2GPI: anti-β2GPI antibodies; aCL: anticardiolipin antibodies; ANA: antinuclear antibodies; CAPS: catastrophic APS; CNS; central nervous system; CVA: cerebrovascular accident; DNA: anti-DNA antibodies; DVT: deep vein thrombosis; ENA: extractrable nuclear antigens; HT: hypertension; IDDM: insulin-dependent diabetes mellitus; IUD: intrauterine death; IUGR: intra-uterine growth restriction; PE: pulmonary embolism; PL: pregnancy loss PM: Pregnancy Mortality; ITP: idiopathic thrombocytopenic purpura; LA: lupus anticoagulant; LB: live birth; SLE: systemic lupus erythematosus; TIA: transient ischaemic attack; VT: vascular thrombosis.
First trimester trophoblast cell line
The human first trimester extravillous trophoblast cell line, HTR8, was used in these studies. The HTR8 cells were immortalized by SV40 25, and was a kind gift from Dr Charles Graham (Queens University, Kingston, ON, Canada). HTR8 cells were cultured in RPMI 1640 (Gibco) which was supplemented with 10% fetal bovine serum (Hyclone, South Logan, UT), 10mM Hepes, 0.1mM MEM non-essential amino acids, 1mM sodium pyruvate, 100nm penicillin/streptomycin (Gibco). Cells were maintained at 37°C/5% CO2.
Isolation of primary trophoblast cells from first trimester placenta
First trimester placentas (7 – 12 weeks gestation) were obtained from elective terminations of normal pregnancies performed at Bridgeport Hospital. The use of patient samples was approved under both Yale University's HIC and Bridgeport Hospital's IRB. Tissue specimens were washed with cold Hanks Balanced Salt Solution (Gibco) to remove excess blood. Cells were scraped from the membranes, transferred to trypsin-EDTA (Invitrogen, Carlsbad, CA) digestion buffer and incubated at 37°C for 40 minutes with shaking. The mixture was then passed through a nylon strainer and then layered over Lymphocyte Separation Media (ICN Biomedicals, Inc., Aurora, OH) and centrifuged at 2000rpm for 25 minutes. The cellular interface containing the trophoblast cells was collected and resuspended in D-MEM with D-valine (Caisson Labs, North Logan, UT) supplemented with 10% normal human serum (Gemini Bio-Products, Woodland, CA) and cultured at 37°C/5% CO2. Purity of the trophoblast cells was determined by monitoring cytokeratin 7 expression.
Transfection of trophoblast cells with MyD88 and TLR4 dominant negatives
HTR8 cells were transiently transfected with either the pDeNy plasmid containing the human MyD88 dominant negative (MyD88-DN), or the pZERO plasmid containing the human HA-tagged TLR4ΔTIR (Invivogen, San Diego, CA). The TLR4ΔTIR acts as a dominant negative since the TIR domain has been deleted. Thus, TLR4ΔTIR (TLR4-DN) can compete with endogenous TLR4 for ligand binding, but cannot transduce a signal 26. Transfection was performed as previously described 27. Briefly, cells were transfected overnight with 2µg of DNA using Fugene 6 (Roche Diagnostics, Indianapolis, IN) before a treatment experiment was performed. Transfection was monitored by performing Western blot analysis on cell lysates for the HAtag using the mouse anti-HAtag mAb from Invivogen.
Western blot analysis
For analysis of proteins, Western blot analysis was performed. Proteins, diluted to 20µg with gel loading buffer were boiled for 5 minutes and resolved under reducing conditions on 12% SDS-PAGE gels and then transferred onto PVDF paper (PerkinElmer, Boston, MA). Membranes were blocked with 5% fat-free powdered milk (FFPM) in PBS/0.05% Tween-20 (PBS-T). Following washes with PBS-T, membranes were incubated overnight at 4°C with primary antibody in PBS-T/1% FFPM. Following this incubation, membranes were washed as before and then incubated with the appropriate secondary antibody conjugated to peroxidase (Vector Labs) in PBS-T/1% FFPM. Following washes with PBS-T and then with distilled water, the peroxidase-conjugated antibody was detected by enhanced chemiluminescence (PerkinElmer). β-actin was used as internal control, in addition to Ponseau Red, to validate the amount of protein loaded onto the gels. Images were recorded using the Gel Logic 100 (Kodak) and Kodak MI software.
Immunohistochemistry
The binding of the anti-β2GPI mAbs, ID2 and IIC5, to the trophoblast was determined by immunocytochemistry. In short, trophoblast cells, previously adhered to glass slides, were fixed with 4% paraformaldehyde. They were then blocked with 10% horse serum in PBS for 1 hour at room temperature. Following three washes with PBS, samples were incubated overnight at 4°C with either ID2 or IIC5 (40µg/ml). Mouse IgG1 served as a negative control. After three washes with PBS, specific staining was detected by incubating with a peroxidase-conjugated horse anti-mouse antibody (1:500 dilution) for 1 hour followed by a five minute incubation with DAB substrate (Vector Laboratories). Cells were then counterstained with haematoxylin (Sigma) before dehydration with ethanol and Histosolve (Shandon Inc., Pittsburg, PA). Slides were then mounted with Permount (Fisher Scientific, Pittsburg, PA) and visualized by light microscopy.
Cell viability assay
Trophoblast cell viability was determined using the CellTiter 96™ viability assay (Promega, Madison, WI). Trophoblast cells were plated in wells of a 96 well plate at 1 × 104 cells per well in growth media and cultured until 70% confluent after which the media was then replaced with the reduced serum medium, Opti-MEM (Gibco) and cultured for another 4 hours prior to treatment. Following treatment, the CellTiter 96™ substrate was added to all wells and following a 1–4 hour incubation at 37°C, optical densities were read at 490nm. All samples were assayed in triplicate and cell viability was presented as a percentage relative to the untreated control.
Caspase activity assay
The effect of the anti-β2GPI Abs on trophoblast caspase activity was determined using the Caspase-Glo™ assay (Promega, Madison, WI). Briefly, 10µg of whole cell lysates were incubated at room temperature in the dark for 1 hour with either the caspase 3 substrate, the caspase-8 substrate or the caspase-9 substrate. Following incubation, luminescence was measured using a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA). The amount of luminescence detected as relative light units was proportional to caspase activity.
Cytokine studies
Trophoblast cells were treated with or without the anti-β2GPI Abs. Following a culture of 72 hours, the cell-free supernatants, also termed conditioned media (CM), were collected by centrifugation at 400g for 10 minutes and stored at −80°C until analysis was performed. The concentrations of IL-8 were evaluated by ELISA (Assay Designs, Ann Arbor, MI). Subsequently, multiplex analysis was performed on the supernatants using the BioPLex assay (BioRad) for the following cytokines/chemokines: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p70), IL-13, IL-17, G-CSF, GM-CSF, GRO-α, IFNγ, MCP-1, MIP-1α, RANTES and TNFα. Detection and analysis were performed using the Luminex 100 IS system (Upstate Biotechnology, Charlottesville, VA).
Statistical analysis
Experiments were performed at least three times in triplicate. Data are expressed as mean ± standard deviation (S.D.) of either representative or pooled experiments. Statistical significance (p< 0.05) was determined using either the one-way ANOVA with the Bonferroni correction for multiple comparisons, or the paired student's t-test.
Results
Anti-β2GPI antibodies induce trophoblast cell apoptosis
Since anti-β2GPI Abs have been shown to impair choriocarcinoma cell proliferation 20, the first objective of this study was to determine whether the two anti-β2GPI Abs, ID2 and IIC5, also impact on first trimester trophoblast cell viability, using HTR8 cells as a model cell system. To validate this model, we first evaluated the expression of β2GPI by the HTR8 trophoblast cells, and assessed whether these cells would bind the two anti-β2GPI Abs. As shown in Figure 1A, Western blot analysis confirmed the expression of β2GPI in HTR8 cells. Moreover, both ID2 and IIC5 were able to bind to the trophoblast cells, as evidenced by positive immunoreactivity (Figure 1B). Thus, the effect of these antibodies on HTR8 cell viability was evaluated next. As shown in Figure 2A, neither antibody had an adverse effect on trophoblast viability at 5, 10, or 20µg/ml. If anything, proliferation was modestly enhanced at the lower two doses. However, at a concentration of 40µg/ml, both antibodies significantly reduced cell viability after 48 hours of incubation, indicative of cell death (Figure 2A). While ID2 and IIC5 (40µg/ml) significantly reduced trophoblast viability, the mouse IgG1 (IgG) mAb isotype control at the same concentration had no effect (Figure 2A and B). Camptothecin, which is cytotoxic to trophoblast cells, was used a positive control. It is noteworthy that ID2 was modestly more potent at reducing trophoblast cell viability than IIC5. Since heparin has been shown to protect human trophoblast against a variety of cytotoxic stimuli by enhancing heparin-binding growth factor activation of the phosphoinositide 3-kinase/AKT survival pathway 28, we examined if simultaneous treatment with heparin would also antagonize cell death induced by these anti-β2GPI Abs in first trimester trophoblast. As expected, the loss in cell viability induced by ID2 and IIC5 was significantly attenuated when cells were treated heparin at 100ng/ml (Figure 2C). Higher heparin concentrations had no additional inhibitory effect (data not shown). In order to determine whether the decrease in trophoblast cell viability in response to the anti-β2GPI Abs was the result of apoptosis, the activity of various caspases was measured. As shown in Figure 2D, following treatment of the trophoblast cells with ID2 and IIC5 (40µg/ml) for 48 hours, there was a significant increase in the activities of caspase-8, caspase-9 and caspase-3. Interestingly, ID2 induced significantly more capase-3 activity than IIC5 whereas the reverse was true for activation of caspase-8 and caspase-9 (p<0.05).
Figure 1. Expression of β2GPI by first trimester trophoblast cells.
A) Lysates of the first trimester trophoblast cells (HTR8) and, as a positive control, placental tissue, were analyzed for β2GPI expression by Western blot. The trophoblast cells were positive β2GPI.
B) First trimester trophoblast cells (HTR8) were assessed for ID2 and IIC5 binding by immunocytochemistry. Cell were incubated with ID2 or IIC5 (40µg/ml) followed by a peroxidase-conjugated horse anti-mouse antibody (magnification ×60). Note the strong positive immunoreactivity of the trophoblast cells for ID2 and IIC5, localized to both the cytoplasm and plasma membrane. Control cells (Neg; magnification ×40) showed no staining.
Figure 2. Effects of anti-β2GPI Abs on trophoblast cell viability and apoptosis.
(A) First trimester trophoblast cells (HTR8) were incubated with ID2, IIC5 or the mouse IgG1 isotype control (IgG) at 0, 5, 10, 20 and 40µg/ml for 48 hours. Cell viability was then determined using the CellTiter 96 assay. Line graph shows the percentage cell viability relative to the untreated control (0 µg/ml). Treatment with ID2 or IIC5 at the high dose of 40µg/ml significantly reduced trophoblast cell viability when compared to the untreated control (*p<0.05, **p<0.001). This figure is representative of at least three independent experiments performed in triplicate. Significance was determined using ANOVA.
(B) First trimester trophoblast cells (HTR8) were incubated with no treatment (NT); ID2 (40µg/ml); IIC5 (40µg/ml); mouse IgG (40µg/ml), as a negative isotype control (IgG); or CPT, (4µM) as a positive control for cell death, for 48 hours. Cell viability was then determined using the CellTiter 96 assay. Barchart shows the percentage cell viability relative to the NT control *p <0.05, **p <0.001. Significance was determined using ANOVA. Data are pooled from five individual experiments.
(C) First trimester trophoblast cells (HTR8) were incubated with NT, ID2 (40µg/ml); IIC5 (40µg/ml) or mIgG (IgG; 40µg/ml) in the presence and absence of heparin (100ng/ml) for 48 hours. Cell viability was then determined using the CellTiter 96 assay. Barchart shows the percentage cell viability relative to the NT control unless otherwise indicated and significance was determined using ANOVA. The presence of heparin significantly reduced the amount of cell death induced by ID2 and IIC5 (*p <0.01, **p <0.001), as determined using a paired t-test. Data are pooled from three individual experiments.
(D) Trophoblast cells (HTR8) were incubated with either no treatment (NT), ID2 (40µg/ml) or IIC5 (40µg/ml). After 48 hours, caspase-3; caspase-8; and caspase-9 activation was determined. Barchart shows caspase activity in relative light units (RLU) relative to the NT control (*p<0.001). Significance was determined using ANOVA. Data are pooled from three individual experiments.
Anti-β2GPI antibodies induce a trophoblast inflammatory response
Although not overtly cytotoxic, we speculated that lower concentrations of anti-β2GPI Abs may, still adversely affect trophoblast function; for instance by altering cellular cytokine or chemokine production. To test this hypothesis, we first monitored the effects of ID2 and IIC5 on IL-8 production, an inflammatory mediator constitutively expressed by primary first trimester trophoblast and HTR8 cells 29. As shown in Figure 3A, exposure of HTR8 cells to low concentrations of the anti-β2GPI Abs (1 – 20µg/ml) significantly increased IL-8 secretion in a dose-dependent manner. After 72 hours of treatment, trophoblast IL-8 production in response to ID2 or IIC5 (20µg/ml) was increased by 12.2-fold and 6.6-fold, respectively, whereas the mIgG isotype control had no effect (Figure 3B). Since heparin is also known to have anti-inflammatory properties 30–32, we examined if heparin also protects first trimester trophoblast against the inflammatory cytokine response induced by anti-β2GPI antibodies. While, the presence of heparin at 100ng/ml had no effect on IL-8 production upon treatment with ID2 or IIC5 (data not shown), at the higher dose of 10µg/ml, heparin significantly reduced the anti-β2GPI Ab-induced IL-8 response (Figure 3C).
Figure 3. Effects of anti-β2GPI Abs on trophoblast IL-8 secretion.
Trophoblast cells (HTR8) were treated with ID2 and IIC5 (0 – 20µg/ml) or the mouse IgG isotype control (mIgG) (20µg/ml) for 72 hours after which cell-free supernatants were collected and analyzed for IL-8 by ELISA. (A) Line graph shows that both ID2 and IIC5 treatment upregulated trophoblast production of IL-8 in a dose dependent manner (*p<0.05, **p<0.001). (B) Barchart shows that while ID2 and IIC5 at 20µg/ml significantly increased trophoblast IL-8 secretion relative the no treatment (NT) control (*p <0.05, **p<0.001), the mIgG had no effect. Significance was determined using ANOVA. (C) Trophoblast cells (HTR8) were incubated with NT, ID2 (20µg/ml) or IIC5 (20µg/ml) in the presence and absence of heparin (10µg/ml) for 72 hours after which cell-free supernatants were collected and analyzed for IL-8 by ELISA. Barchart shows that while ID2 and IIC5 significantly increased trophoblast IL-8 secretion relative the no treatment (NT) control (*p <0.05; **p<0.001), as determined using ANOVA, the presence of heparin significantly inhibited this response as determine by paired t-test (*p <0.05; **p<0.001). Data are representative of at least three independent experiments.
We next extended our search for other cytokines and chemokines upregulated or induced by the anti-β2GPI Abs by performing multiplex analysis on the trophoblast culture supernatants. This screen allowed us to analyze the following cytokines/chemokines: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12 (p70), IL-13, IL-17, G-CSF, GM-CSF, GRO-α, IFNγ, MCP-1, MIP-1α, RANTES and TNFα. This screen revealed that, in addition to IL-8, ID2 and IIC5 (20µg/ml) also significantly increased the secretion of MCP-1, GRO-α, and IL-1β, by the HTR8 cells (Figure 4A). The anti-β2GPI Abs had no significant effect on the remaining cytokines and chemokines tested. Furthermore, there was a significant increase in the secretion of these same cytokines and chemokines when primary trophoblast cultures were treated with the same concentration of ID2 or IIC5 (Figure 4B).
Figure 4. Effects of anti-β2GPI Abs on trophoblast cytokine and chemokine production.
(A) HTR8 cells and (B) primary trophoblast cells were treated with either no treatment (NT), ID2 (20µg/ml), IIC5 (20µg/ml) or mouse IgG1 (mIgG; 20µg/ml) for 48 hours after which cell-free supernatants were collected and analyzed by multiplex analysis. Barcharts show changes in the secretion of i) IL-8; ii) MCP-1; iii) GROα; and iv) IL-1β relative to the NT control (*p<0.05; **p<0.001). Data are representative of at least three independent experiments. Significance was determined using ANOVA.
Anti-β2GPI antibodies upregulate trophoblast cytokine and chemokine production via the TLR-4/MyD88 pathway
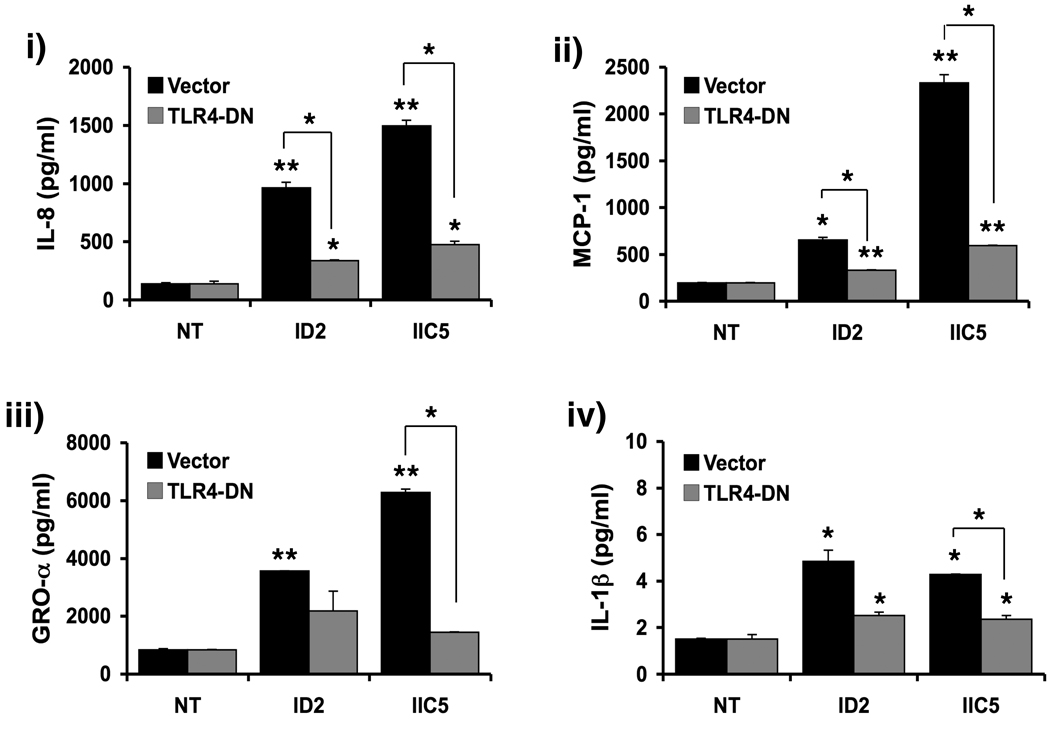
The next objective was to elucidate the mechanisms by which anti-β2GPI Abs induce a pro-inflammatory response in human trophoblast. A recent study in monocytes reported that anti-β2GPI Abs may recruit the innate immune receptor, TLR4, upon interaction with the β2GPI 33. Since first trimester trophoblast cells express functional TLR4 34, we questioned whether this signaling pathway is involved in the trophoblast response to the anti-β2GPI Abs. Thus, HTR8 cells were transfected with an expression vector that encodes for a dominant-negative TLR4 mutant (TLR4-DN) before treatment with ID2 and IIC5 (20µg/ml). The TLR4-DN lacks the TIR (Toll-IL-1R) domain and therefore competes with the endogenous receptor for ligand binding but cannot signal. As shown in Figure 5A, expression of TLR4-DN in trophoblast cells significantly inhibited the ability of the anti-β2GPI Abs to upregulate IL-8, MCP-1, GRO-α and IL-1β secretion.
Figure 5. Effects of a TLR4-DN on the modulation of trophoblast cytokine/chemokine production by anti-β2GPI Abs.
Trophoblast cells (HTR8) were transiently transfected to express the TLR4-DN. Following transfection with either the TLR4-DN or the vector control, trophoblast cells were treated with either no treatment (NT), ID2 (20µg/ml), or IIC5 (20µg/ml) for 48 hours after which cell-free supernatants were collected and analyzed by multiplex analysis. Barcharts show changes in the secretion of i) IL-8; ii) MCP-1; iii) GROα; and iv) IL-1β. *p<0.05; **p<0.001 relative to the NT control was determined using ANOVA. Significant differences between the TLR4-DN and vector control were determined by paired t-test (*p<0.05; **p<0.001). Data are a representative of at least three independent experiments.
TLR4 is known to signal via the adapter protein MyD88, but can also transduce signals in a MyD88-independent manner 35. Therefore, in order to determine if the TLR4-mediated trophoblast responses to the anti-β2GPI Abs were occurring through MyD88, HTR8 cells were transiently transfected to express a dominant-negative MyD88 mutant (MyD88-DN). As shown in Figure 6, the ability of ID2, as well as IIC5, to upregulate IL-8, MCP-1, GRO-α and IL-1β secretion was significantly attenuated in trophoblast cells expressing MyD88-DN when compared to the vector control cells. Together, the results establish that anti-β2GPI Abs induce a pro-inflammatory response in human trophoblast cells by activating the TLR-4/MyD88 pathway.
Figure 6. Effects of a MyD88-DN on the modulation of trophoblast cytokine/chemokine production by anti-β2GPI Abs.
Trophoblast cells (HTR8) were transiently transfected to express the MyD88-DN. Following transfection with either the MyD88-DN or the vector control, trophoblast cells were treated with either no treatment (NT), ID2 (20µg/ml), or IIC5 (20µg/ml) for 48 hours after which cell-free supernatants were collected and analyzed by multiplex analysis. Barcharts show changes in the secretion of i) IL-8; ii) MCP-1; iii) GROα; and iv) IL-1β. *p<0.05 and **p<0.001 relative to the NT was determined using ANOVA. Significant differences between the MyD88-DN and vector control were determined by paired t-test (*p<0.05; **p<0.001). Data are a representative of at least three independent experiments.
Anti-β2GPI antibody-induced inflammation triggers trophoblast cell death
Inflammatory mediators, such as IL-1β, TNFα and IFNγ, are known to induce trophoblast cell death 36,37. As anti-β2GPI Abs markedly upregulated IL-1β production in first trimester trophoblast cells, we hypothesized that this inflammatory response may feed back onto the trophoblast cells in an autocrine/paracine manner to induce this cell death. To test this conjecture, conditioned media (CM) collected from untreated trophoblast cultures (CM/NT) or cells incubated for 72 hours with 20µg/ml ID2 (CM/ID2) or IIC5 (CM/IIC5) were diluted to 50% in serum-free medium and then added to fresh trophoblast cultures. As shown in Figure 7A, while the supernatant from the untreated cells (CM/NT) did induce some trophoblast cell death, this response was significantly less than that induced by the conditioned media from cells treated with ID2 or IIC5 (CM/ID2; CM/IIC5). In addition, trophoblast cells exposed to CM/ID2 and CM/IIC5 had significantly higher levels of active caspase-3 than cells exposed to either CM/NT or medium alone (NT), confirming that apoptosis was being induced by the trophoblast CM (Figure 7B). In order to confirm that the apoptotic response was mediated indirectly via the antibody-induced inflammatory factors, trophoblast cells were transiently transfected to express the MyD88-DN after which cells were treated with ID2 or IIC5 at 40 µg/ml for 48 hours. The presence of the MyD88-DN significantly reduced that amount of anti-β2GPI Ab-induced trophoblast cell death (Figure 7C).
Figure 7. Effects of anti-β2GPI Abs induced inflammation on trophoblast cell death.
First trimester trophoblast cells (HTR8) were incubated with no treatment (NT) or the CM from trophoblast cells treated with NT (CM/NT), ID2 at 20µg/ml (CM/ID2) or IIC5 at 20µg/ml (CM/IIC5). After 96 hours, cell viability was determined using the CellTiter 96 assay and after 72 hours caspase-3 activity was determined using the caspase-Glo assay. Barcharts shows (A) the differences in trophoblast cell death; and (B) the differences in trophoblast caspase-3 activity, relative to the NT, as determined using ANOVA (*p <0.05, **p <0.001). Statistical differences between the treatment groups was determined by a paired t-test (*p <0.05, **p <0.001). Data are pooled from three independent experiments.
(C) Trophoblast cells (HTR8) were transiently transfected to express the MyD88-DN. Following transfection, trophoblast cells were treated with either no treatment (NT), ID2 (40µg/ml), or IIC5 (40µg/ml) for 48 hours, after which cell viability was determined using the CellTiter 96 assay. Barcharts show the differences in trophoblast cell death relative to the NT control. *p<0.05 and **p<0.001 relative to the NT control was determined using ANOVA. Statistical differences between the MyD88-DN and vector control were determined by a paired t-test (*p <0.05, **p <0.001). Data are pooled from three independent experiments.
Patient-derived aPL enhance trophoblast cytokine/chemokine production
Having found that the two mouse anti-β2GPI mAbs were able to upregulate first trimester trophoblast cytokine and chemokine production, we next sought to validate the clinical relevance of these results by determining the effects of polyclonal IgG derived from patients with APS on trophoblast cell function. For this experiment, polyclonal APS-IgG were purified from the serum of APS patients with a history of pregnancy morbidity, venous thrombosis, or both (PM+/VT−; PM−/VT+; or PM+/VT+, respectively). HTR8 cells were incubated with the APS-IgG at 12.5µg/ml for 72 hours, after which the supernatants were collected, pooled on the basis of patient group, and then evaluated by multiplex analysis. As with ID2 and IIC5, the APS-IgG from all three clinical groups significantly upregulated trophoblast secretion of IL-8, GRO-α, and IL-1β, although not MCP-1 (Figure 8). Interestingly, the pro-inflammatory trophoblast response tended to be more pronounced upon incubation of polyclonal APS-IgG purified from patients with a history of pregnancy morbidity. Specifically, polyclonal APS-IgG from the PM+/VT+ group induced significantly more trophoblast IL-8 and GRO-α secretion than the IgG from the PM−/VT+ group. Similarly, polyclonal APS-IgG from the PM+/VT− group induced significantly more trophoblast IL-8 production than the IgG from the PM−/VT+ group (Figure 8).
Figure 8. Effects of patient derived aPL on trophoblast cytokine/chemokine production.
Trophoblast cells were incubated with either no treatment (NT) or polyclonal IgG aPL (12.5µg/ml) from patients with PM+/VT− (n=6); PM−/VT+ (n=6) or PM+/VT+ (n=6). After 72 hours, the supernatants were collected, pooled on the basis of patient group, and than evaluated by multiplex analysis. Barcharts show the effects of aPL on trophoblast secretion of i) IL-8; ii) MCP-1; iii) GROα; and iv) IL-1β after 72 hours. *p<0.05 relative to the NT control, as determined using ANOVA.
Discussion
Women with aPL are at high risk of early pregnancy loss and late pregnancy complications, such as preeclampsia and prematurity, as well as vascular thrombosis. However, the pathogenesis of these pregnancy-associated complications in patients with APS remains largely undefined. Both clinical and experimental observations suggest that pregnancy failure in these patients may involve an inflammatory response at the maternal-fetal interface, as well as disruption of normal trophoblast survival and function. Yet, to date, the mechanisms involved have not been studied in any depth, and little is known about the effect of aPL on human first trimester trophoblast cells. In this study we demonstrate, for the first time, that anti-β2GPI Abs can modulate first trimester trophoblast cytokine and chemokine production, which in turn compromises cell viability.
Animal models have supported a role for aPL in the pathogenesis of pregnancy failure and pregnancy complications. Several groups have shown that either immunization of animals with β2GPI or the passive transfer of aPL to mice promotes fetal resorption, fetal death, reduced litter sizes and IUGR 38–41. Moreover, one study showed that while the passive transfer of human anti-β2GPI I Abs to ®2GPI+/+ mice triggered fetal loss, β2GPI−/− mice were resistant to this antibody-induced effect, highlighting the importance of β2GPI as a major antigen in APS 42. Exposure of pregnant mice to human aPL results in complement activation and elevated levels of TNFα at the maternal-fetal interface, which are key players in subsequent pregnancy failure 13,43. In order to better understand how aPL might impact human placental function, and thus pregnancy outcome, a number of in vitro studies have evaluated the effects of aPL on trophoblast cell function. These studies have shown, using either anti-β2GPI Abs or aPL Ab containing IgG, that these autoantibodies can inhibit trophoblast expression and secretion of human chorionic gonadotrophin; reduce trophoblast proliferation; decrease trophoblast fusion and differentiation; alter their expression of cell adhesion molecules; and limit trophoblast chemotaxis and invasiveness 9,16–22. While important, these studies have been limited by their use of term trophoblast cells or malignant choriocarcinoma cell lines and the lack of mechanistic analysis of the aPL Ab response.
In this study, we have characterized the effects of anti-β2GPI Abs on trophoblast function and survival and identified the molecular pathway involved. Initially, we tested the effects of two anti-β2GPI mAbs, ID2 and IIC5, on trophoblast survival and apoptosis. At high concentrations, the anti-β2GPI Abs caused trophoblast cell death through the induction of caspase-mediated apoptosis. In support of our findings, Orney et al. have shown that first trimester placental villi explants cultured in the serum of patients with SLE/APS and recurrent pregnancy loss exhibit increased levels of apoptosis 44, an observation confirmed by Bose et al. using first trimester placental explants cultures 45. Notably, ID2 activated significantly more caspase-3 but less caspase-8 and caspase-9, when compared to IIC5. Since caspase-8 and caspase-9 are upstream of caspase-3, these findings suggest that kinetics of the apoptotic response may differ between these antibodies, with ID2 activating the caspase pathway more rapidly than IIC5. This is further supported by the observation that ID2 has slightly more detrimental effects on trophoblast cell viability than IIC5. This suggests that these two anti-β2GPI Abs may recognize different, or perhaps overlapping, epitopes 22. Indeed small differences in antibody fine specificity can result in diverse biological effects 46,47.
Since lower concentrations of the anti-β2GPI Abs had no effect on trophoblast cell death, and even seemed to promote proliferation, we next investigated their effects on trophoblast function. It is known that trophoblast cells constitutively produce chemokines that are important for crosstalk with local immune cells 48, and that in the presence of bacterial and viral components trophoblast generate an inflammatory cytokine/chemokine response 26,29,48. Therefore, we explored if anti-β2GPI Abs affect the cytokine/chemokine expression profile of placental cells. Indeed, lower concentrations of the anti-β2GPI Abs significantly increased IL-8, MCP-1, IL-1β and GROα secretion by first trimester trophoblast cells. This response is not a result of LPS contamination of the antibody preparation, as very high doses of LPS (50 –100 µg/ml) are required to induce an inflammatory response in the trophoblast 48. Moreover, the cytokine/chemokine profile triggered by LPS is very different from that induced by anti-β2GPI Abs 29.
When effects of polyclonal IgG, derived from patients with different clinical manifestations of APS, were tested on trophoblast cells, we found the response was similar to that observed with the monoclonal anti-β2GPI Abs. Polyclonal APS-IgG from patients with and without a history of pregnancy mortality significantly increased trophoblast cell secretion of IL-8, GRO-α, and IL-1β. Furthermore, this inflammatory response was more pronounced upon incubation with polyclonal APS-IgG purified from patients with a history of pregnancy morbidity. In particular, the strong increase in IL-8 secretion may account for the increased recruitment of inflammatory immune cells, such as neutrophils, at the maternal-fetal interface 48. In agreement, in both clinical samples from women with APS and experimental models of APS-complicated pregnancies, the maternal-fetal interface is characterized by a neutrophil infiltrate 13,15,43,49. Having found anti-β2GPI Abs perturb the cytokine/chemokine profile of placental cells, we next investigated the underlying signaling mechanisms. In a recent study using monocytes, Sorice et al. demonstrated that β2GPI recruits the innate immune receptor TLR4 in the presence of anti-β2GPI Abs 33. This finding suggests molecular mimicry between β2GPI and bacterial components that can be potentiated by aPL 50. We and others have shown that trophoblast cells express functional TLR4 and its signaling adapter protein MyD88 34,51. Using dominant negative mutants for TLR4 and MyD88, we demonstrate that the inflammatory trophoblast response triggered by anti-β2GPI Abs is dependent upon activation of the TLR4/MyD88 pathway. This finding is in keeping with studies in endothelial cells, in which anti-β2GPI Abs activate NFκB via MyD88 52. In addition, in a mouse model of aPL Ab-induced thrombosis, TLR4 was shown to play a role in thrombus formation and endothelial damage 53.
Our next objective was to establish if the anti-β2GPI Ab-dependent cytokine/chemokine response, characterized by induction of IL-8, MCP-1, GRO-α, and IL-1β, plays a role in subsequent trophoblast cell death. The pro-inflammatory cytokine, IL-1β, has recently been shown to induce trophoblast apoptosis 36, raising the possibility that the anti-β2GPI Abs-induced inflammatory response induces trophoblast cell death in an autocrine/paracrine fashion. To test this hypothesis, trophoblast cells were exposed to the conditioned media from cells treated with or without the anti-β2GPI Abs. The conditioned media from anti-β2GPI Ab-treated trophoblast cells, which contains higher levels of IL-1β, significantly induced more trophoblast cell death and apoptosis than the conditioned media from unstimulated trophoblast cells. These results are indicative of an indirect effect of a soluble factor, rather than direct effect of any residual antibodies in the conditioned media. Although anti-β2GPI Ab were likely still present in the trophoblast conditioned media, they were now diluted to a concentration, at best 10 µg/ml, insufficient to elicit a cell death response. Moreover, the anti-β2GPI Ab-induced trophoblast cell death was inhibited in the presence of the MyD88-DN, the adapter protein that is used by both TLR4 and IL-1R 54. Therefore, TLR4/MyD88-mediated of induction IL-1β likely contributes to enhanced trophoblast cell death in APS. Indeed, IL-1β has been shown to induce beta cell apoptosis via MyD88 55.
Since women with APS are at high risk for pregnancy loss and late gestational complications, heparin is administered with the intention to block the pro-thrombotic events associated with aPL. However, heparin is also known to have anti-inflammatory properties 30–32 and reportedly binds directly to β2GPI, thereby antagonizing β2GPI-phospholipid interactions 56. This study further confirms that heparin blocks the anti-β2GPI Ab-induced trophoblast cell death, in agreement with others 45. Indeed, we have recently reported that heparin inhibits first trimester trophoblast apoptosis triggered by the cytokines TNFα and IFNγ, as well as the TLR-2 agonist, peptidoglycan, by activating pro-survival pathways 28. We now also show that heparin inhibits the effects of these antibodies on the trophoblast cytokine/chemokine response, albeit only at a concentration much higher than needed for cytoprotection. Since Girardi et al., found in vivo that heparin did not alter the binding of aPL to the trophoblast 31, our findings suggest an anti-inflammatory role for heparin. Indeed, a recent study suggests that heparin can bind TLR4 and block dendritic cell activation 57. Thus, while the anti-inflammatory properties of heparin may contribute to improved pregnancy outcome in pregnant women with APS, our observations also raise the possibility that its efficacy could be enhanced by simultaneously and specifically disabling the TLR4/MyD88 pathway.
In summary, we have found that anti-β2GPI Abs modulate human first trimester trophoblast cytokine/chemokine production, a response mediated by the TLR4/MyD88 pathway. Our findings suggest that early pregnancy loss and late obstetric complications in APS may arise from anti-β2GPI Abs acting on first trimester trophoblast cells, thereby triggering placental inflammation and cell death.
Acknowledgments
The authors would like to thank Dr Carol Presnick for her help with tissue procurement, Mrs Paulomi Aldo for help with the multiplex assay, Ms Deidre Jones for technical support. This study was supported in part by grants from the Lupus Foundation of America (VMA) and the NICHD, NIH (RO1HD049446 to VMA).
Abbreviations
- aPL
antiphospholipid antibody
- APS
antiphospholipid syndrome
- β2GPI
Beta2-Glycoprotein I
- CPT
camptothecin
- NT
no treatment
- PM
pregnancy morbidity
- SLE
systemic lupus erythematosus
- TLR
Toll-like receptor
- VT
venous thrombosis
References
- 1.D'Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. 2007;369:587–596. doi: 10.1016/S0140-6736(07)60279-7. [DOI] [PubMed] [Google Scholar]
- 2.Valesini G, Alessandri C. New facet of antiphospholipid antibodies. Ann N Y Acad Sci. 2005;1051:487–497. doi: 10.1196/annals.1361.089. [DOI] [PubMed] [Google Scholar]
- 3.Rai RS, Regan L, Clifford K, Pickering W, Dave M, Mackie I, McNally T, Cohen H. Antiphospholipid antibodies and beta 2-glycoprotein-I in 500 women with recurrent miscarriage: results of a comprehensive screening approach. Hum Reprod. 1995;10:2001–2005. doi: 10.1093/oxfordjournals.humrep.a136224. [DOI] [PubMed] [Google Scholar]
- 4.Regan L, Rai R. Epidemiology and the medical causes of miscarriage. Baillieres Best Pract Res Clin Obstet Gynaecol. 2000;14:839–854. doi: 10.1053/beog.2000.0123. [DOI] [PubMed] [Google Scholar]
- 5.Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, Jacobsen S, Lakos G, Tincani A, Kontopoulou-Griva I, Galeazzi M, Meroni PL, Derksen RH, de Groot PG, Gromnica-Ihle E, Baleva M, Mosca M, Bombardieri S, Houssiau F, Gris JC, Quere I, Hachulla E, Vasconcelos C, Roch B, Fernandez-Nebro A, Boffa MC, Hughes GR, Ingelmo M. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019–1027. doi: 10.1002/art.10187. [DOI] [PubMed] [Google Scholar]
- 6.Backos M, Rai R, Baxter N, Chilcott IT, Cohen H, Regan L. Pregnancy complications in women with recurrent miscarriage associated with antiphospholipid antibodies treated with low dose aspirin and heparin. Br J Obstet Gynaecol. 1999;106:102–107. doi: 10.1111/j.1471-0528.1999.tb08208.x. [DOI] [PubMed] [Google Scholar]
- 7.Branch DW, Khamashta MA. Antiphospholipid syndrome: obstetric diagnosis, management, and controversies. Obstet Gynecol. 2003;101:1333–1344. doi: 10.1016/s0029-7844(03)00363-6. [DOI] [PubMed] [Google Scholar]
- 8.Chamley LW, Allen JL, Johnson PM. Synthesis of beta2 glycoprotein 1 by the human placenta. Placenta. 1997;18:403–410. doi: 10.1016/s0143-4004(97)80040-9. [DOI] [PubMed] [Google Scholar]
- 9.Di Simone N, Raschi E, Testoni C, Castellani R, D'Asta M, Shi T, Krilis SA, Caruso A, Meroni PL. Pathogenic role of anti-beta 2-glycoprotein I antibodies in antiphospholipid associated fetal loss: characterisation of beta 2-glycoprotein I binding to trophoblast cells and functional effects of anti-beta 2-glycoprotein I antibodies in vitro. Ann Rheum Dis. 2005;64:462–467. doi: 10.1136/ard.2004.021444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Rosa L, Meroni PL, Tincani A, Balestrieri G, Faden D, Lojacono A, Morassi L, Brocchi E, Del Papa N, Gharavi A, et al. Beta 2 glycoprotein I and placental anticoagulant protein I in placentae from patients with antiphospholipid syndrome. J Rheumatol. 1994;21:1684–1693. [PubMed] [Google Scholar]
- 11.Sebire NJ, Fox H, Backos M, Rai R, Paterson C, Regan L. Defective endovascular trophoblast invasion in primary antiphospholipid antibody syndrome-associated early pregnancy failure. Hum Reprod. 2002;17:1067–1071. doi: 10.1093/humrep/17.4.1067. [DOI] [PubMed] [Google Scholar]
- 12.Bose P, Kadyrov M, Goldin R, Hahn S, Backos M, Regan L, Huppertz B. Aberrations of early trophoblast differentiation predispose to pregnancy failure: lessons from the anti-phospholipid syndrome. Placenta. 2006;27:869–875. doi: 10.1016/j.placenta.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, Hollmann TJ, Casali P, Caroll MC, Wetsel RA, Lambris JD, Holers VM, Salmon JE. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112:1644–1654. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redecha P, Tilley R, Tencati M, Salmon JE, Kirchhofer D, Mackman N, Girardi G. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 2007;110:2423–2431. doi: 10.1182/blood-2007-01-070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Horn JT, Craven C, Ward K, Branch DW, Silver RM. Histologic features of placentas and abortion specimens from women with antiphospholipid and antiphospholipid-like syndromes. Placenta. 2004;25:642–648. doi: 10.1016/j.placenta.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Chamley LW, Duncalf AM, Mitchell MD, Johnson PM. Action of anticardiolipin and antibodies to beta2-glycoprotein-I on trophoblast proliferation as a mechanism for fetal death. Lancet. 1998;352:1037–1038. doi: 10.1016/s0140-6736(05)60080-3. [DOI] [PubMed] [Google Scholar]
- 17.Di Simone N, Caliandro D, Castellani R, Ferrazzani S, De Carolis S, Caruso A. Low-molecular weight heparin restores in-vitro trophoblast invasiveness and differentiation in presence of immunoglobulin G fractions obtained from patients with antiphospholipid syndrome. Hum Reprod. 1999;14:489–495. doi: 10.1093/humrep/14.2.489. [DOI] [PubMed] [Google Scholar]
- 18.Di Simone N, Meroni PL, de Papa N, Raschi E, Caliandro D, De Carolis CS, Khamashta MA, Atsumi T, Hughes GR, Balestrieri G, Tincani A, Casali P, Caruso A. Antiphospholipid antibodies affect trophoblast gonadotropin secretion and invasiveness by binding directly and through adhered beta2-glycoprotein I. Arthritis Rheum. 2000;43:140–150. doi: 10.1002/1529-0131(200001)43:1<140::AID-ANR18>3.0.CO;2-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Simone N, Caliandro D, Castellani R, Ferrazzani S, Caruso A. Interleukin-3 and human trophoblast: in vitro explanations for the effect of interleukin in patients with antiphospholipid antibody syndrome. Fertil Steril. 2000;73:1194–1200. doi: 10.1016/s0015-0282(00)00533-1. [DOI] [PubMed] [Google Scholar]
- 20.Chamley LW, Konarkowska B, Duncalf AM, Mitchell MD, Johnson PM. Is interleukin-3 important in antiphospholipid antibody-mediated pregnancy failure? Fertil Steril. 2001;76:700–706. doi: 10.1016/s0015-0282(01)01984-7. [DOI] [PubMed] [Google Scholar]
- 21.Di Simone N, Castellani R, Caliandro D, Caruso A. Antiphospholid antibodies regulate the expression of trophoblast cell adhesion molecules. Fertil Steril. 2002;77:805–811. doi: 10.1016/s0015-0282(01)03258-7. [DOI] [PubMed] [Google Scholar]
- 22.Quenby S, Mountfield S, Cartwright JE, Whitley GS, Chamley L, Vince G. Antiphospholipid antibodies prevent extravillous trophoblast differentiation. Fertil Steril. 2005;83:691–698. doi: 10.1016/j.fertnstert.2004.07.978. [DOI] [PubMed] [Google Scholar]
- 23.Chamley LW, Duncalf AM, Konarkowska B, Mitchell MD, Johnson PM. Conformationally altered beta 2-glycoprotein I is the antigen for anti-cardiolipin autoantibodies. Clin Exp Immunol. 1999;115:571–576. doi: 10.1046/j.1365-2249.1999.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, PG DEG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 25.Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- 26.Abrahams VM, Aldo PB, Murphy SP, Visintin I, Koga K, Wilson G, Romero R, Sharma S, Mor G. TLR6 Modulates First Trimester Trophoblast Responses to Peptidoglycan. J Immunol. 2008;180:6035–6043. doi: 10.4049/jimmunol.180.9.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Guller S, Huang Y. Method to enhance transfection efficiency of cell lines and placental fibroblasts. Placenta. 2007;28:779–782. doi: 10.1016/j.placenta.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Hills FA, Abrahams VM, Gonzalez-Timon B, Francis J, Cloke B, Hinkson L, Rai R, Mor G, Regan L, Sullivan M, Lam EW, Brosens JJ. Heparin prevents programmed cell death in human trophoblast. Mol Hum Reprod. 2006;12:237–243. doi: 10.1093/molehr/gal026. [DOI] [PubMed] [Google Scholar]
- 29.Costello MJ, Joyce SK, Abrahams VM. NOD protein expression and function in first trimester trophoblast cells. Am J Reprod Immunol. 2007;57:67–80. doi: 10.1111/j.1600-0897.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- 30.Fritchley SJ, Kirby JA, Ali S. The antagonism of interferon-gamma (IFN-gamma) by heparin: examination of the blockade of class II MHC antigen and heat shock protein-70 expression. Clin Exp Immunol. 2000;120:247–252. doi: 10.1046/j.1365-2249.2000.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med. 2004;10:1222–1226. doi: 10.1038/nm1121. [DOI] [PubMed] [Google Scholar]
- 32.Johann S, Zoller C, Haas S, Blumel G, Lipp M, Forster R. Sulfated polysaccharide anticoagulants suppress natural killer cell activity in vitro. Thromb Haemost. 1995;74:998–1002. [PubMed] [Google Scholar]
- 33.Sorice M, Longo A, Capozzi A, Garofalo T, Misasi R, Alessandri C, Conti F, Buttari B, Rigano R, Ortona E, Valesini G. Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheum. 2007;56:2687–2697. doi: 10.1002/art.22802. [DOI] [PubMed] [Google Scholar]
- 34.Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R, Mor G. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol. 2004;173:4286–4296. doi: 10.4049/jimmunol.173.7.4286. [DOI] [PubMed] [Google Scholar]
- 35.Kawai T, Akira S. TLR signaling. Cell Death Differ. 2006;13:816–825. doi: 10.1038/sj.cdd.4401850. [DOI] [PubMed] [Google Scholar]
- 36.Nilkaeo A, Bhuvanath S. Interleukin-1 modulation of human placental trophoblast proliferation. Mediators Inflamm. 2006;2006:79359. doi: 10.1155/MI/2006/79359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aschkenazi S, Straszewski S, Verwer KM, Foellmer H, Rutherford T, Mor G. Differential regulation and function of the Fas/Fas ligand system in human trophoblast cells. Biol Reprod. 2002;66:1853–1861. doi: 10.1095/biolreprod66.6.1853. [DOI] [PubMed] [Google Scholar]
- 38.Garcia CO, Kanbour-Shakir A, Tang H, Molina JF, Espinoza LR, Gharavi AE. Induction of experimental antiphospholipid antibody syndrome in PL/J mice following immunization with beta 2 GPI. Am J Reprod Immunol. 1997;37:118–124. doi: 10.1111/j.1600-0897.1997.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 39.Blank M, Cohen J, Toder V, Shoenfeld Y. Induction of anti-phospholipid syndrome in naive mice with mouse lupus monoclonal and human polyclonal anti-cardiolipin antibodies. Proc Natl Acad Sci U S A. 1991;88:3069–3073. doi: 10.1073/pnas.88.8.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bakimer R, Fishman P, Blank M, Sredni B, Djaldetti M, Shoenfeld Y. Induction of primary antiphospholipid syndrome in mice by immunization with a human monoclonal anticardiolipin antibody (H-3) J Clin Invest. 1992;89:1558–1563. doi: 10.1172/JCI115749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blank M, Tincani A, Shoenfeld Y. Induction of experimental antiphospholipid syndrome in naive mice with purified IgG antiphosphatidylserine antibodies. J Rheumatol. 1994;21:100–104. [PubMed] [Google Scholar]
- 42.Robertson SA, Roberts CT, van Beijering E, Pensa K, Sheng Y, Shi T, Krilis SA. Effect of beta2-glycoprotein I null mutation on reproductive outcome and antiphospholipid antibody-mediated pregnancy pathology in mice. Mol Hum Reprod. 2004;10:409–416. doi: 10.1093/molehr/gah058. [DOI] [PubMed] [Google Scholar]
- 43.Berman J, Girardi G, Salmon JE. TNF-alpha is a critical effector and a target for therapy in antiphospholipid antibody-induced pregnancy loss. J Immunol. 2005;174:485–490. doi: 10.4049/jimmunol.174.1.485. [DOI] [PubMed] [Google Scholar]
- 44.Ornoy A, Yacobi S, Matalon ST, Blank M, Blumenfeld Z, Miller RK, Shoenfeld Y. The effects of antiphospholipid antibodies obtained from women with SLE/APS and associated pregnancy loss on rat embryos and placental explants in culture. Lupus. 2003;12:573–578. doi: 10.1191/0961203303lu405oa. [DOI] [PubMed] [Google Scholar]
- 45.Bose P, Black S, Kadyrov M, Bartz C, Shlebak A, Regan L, Huppertz B. Adverse effects of lupus anticoagulant positive blood sera on placental viability can be prevented by heparin in vitro. Am J Obstet Gynecol. 2004;191:2125–2131. doi: 10.1016/j.ajog.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 46.Chukwuocha RU, Zhu M, Cho CS, Visvanathan S, Hwang KK, Rahman A, Chen PP. Molecular and genetic characterizations of five pathogenic and two non-pathogenic monoclonal antiphospholipid antibodies. Mol Immunol. 2002;39:299–311. doi: 10.1016/s0161-5890(02)00115-3. [DOI] [PubMed] [Google Scholar]
- 47.Katz JB, Limpanasithikul W, Diamond B. Mutational analysis of an autoantibody: differential binding and pathogenicity. J Exp Med. 1994;180:925–932. doi: 10.1084/jem.180.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abrahams VM, Visintin I, Aldo PB, Guller S, Romero R, Mor G. A role for TLRs in the regulation of immune cell migration by first trimester trophoblast cells. J Immunol. 2005;175:8096–8104. doi: 10.4049/jimmunol.175.12.8096. [DOI] [PubMed] [Google Scholar]
- 49.Magid MS, Kaplan C, Sammaritano LR, Peterson M, Druzin ML, Lockshin MD. Placental pathology in systemic lupus erythematosus: a prospective study. Am J Obstet Gynecol. 1998;179:226–234. doi: 10.1016/s0002-9378(98)70277-7. [DOI] [PubMed] [Google Scholar]
- 50.Blank M, Shoenfeld Y. Beta-2-glycoprotein-I, infections, antiphospholipid syndrome and therapeutic considerations. Clin Immunol. 2004;112:190–199. doi: 10.1016/j.clim.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 51.Holmlund U, Cebers G, Dahlfors AR, Sandstedt B, Bremme K, Ekstrom ES, Scheynius A. Expression and regulation of the pattern recognition receptors Tolllike receptor-2 and Toll-like receptor-4 in the human placenta. Immunology. 2002;107:145–151. doi: 10.1046/j.1365-2567.2002.01491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raschi E, Testoni C, Bosisio D, Borghi MO, Koike T, Mantovani A, Meroni PL. Role of the MyD88 transduction signaling pathway in endothelial activation by antiphospholipid antibodies. Blood. 2003;101:3495–3500. doi: 10.1182/blood-2002-08-2349. [DOI] [PubMed] [Google Scholar]
- 53.Pierangeli SS, Vega-Ostertag ME, Raschi E, Liu X, Romay-Penabad Z, De Micheli V, Galli M, Moia M, Tincani A, Borghi MO, Nguyen-Oghalai T, Meroni PL. Toll like Receptor 4 is involved in antiphospholipid- mediated thrombosis: In vivo studies. Ann Rheum Dis. 2007 doi: 10.1136/ard.2006.065037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeuchi O, Akira S. MyD88 as a bottle neck in Toll/IL-1 signaling. Curr Top Microbiol Immunol. 2002;270:155–167. doi: 10.1007/978-3-642-59430-4_10. [DOI] [PubMed] [Google Scholar]
- 55.Dupraz P, Cottet S, Hamburger F, Dolci W, Felley-Bosco E, Thorens B. Dominant negative MyD88 proteins inhibit interleukin-1beta /interferon-gamma -mediated induction of nuclear factor kappa B-dependent nitrite production and apoptosis in beta cells. J Biol Chem. 2000;275:37672–37678. doi: 10.1074/jbc.M005150200. [DOI] [PubMed] [Google Scholar]
- 56.Guerin J, Sheng Y, Reddel S, Iverson GM, Chapman MG, Krilis SA. Heparin inhibits the binding of beta 2-glycoprotein I to phospholipids and promotes the plasmin-mediated inactivation of this blood protein. Elucidation of the consequences of the two biological events in patients with the anti-phospholipid syndrome. J Biol Chem. 2002;277:2644–2649. doi: 10.1074/jbc.M110176200. [DOI] [PubMed] [Google Scholar]
- 57.Yan M, Peng J, Jabbar IA, Liu X, Filgueira L, Frazer IH, Thomas R. Activation of dendritic cells by human papillomavirus-like particles through TLR4 and NF-kappaB-mediated signalling, moderated by TGF-beta. Immunol Cell Biol. 2005;83:83–91. doi: 10.1111/j.1440-1711.2004.01291.x. [DOI] [PubMed] [Google Scholar]