Abstract
Three patients with progressive renal failure and advanced hepatic insufficiency due to cirrhosis of the liver underwent orthotopic liver transplantation. All three patients had immediate improvement in hepatic function and within two weeks after liver replacement regained nearly normal kidney function. However, the renal recovery was delayed in each case, and its course was not uniform. Plasma renin activity was high, and renin substrate was low before transplantation in one case in which these measurements were obtained; both returned to normal soon after liver replacement.
There is no satisfactory treatment for either the liver or the kidney component of the hepatorenal syndrome.1 However, since the renal failure of this disorder is believed to be secondary to hepatic dysfunction, the replacement of the diseased liver by an orthotopic transplantation should coincidentally improve the kidney status. This expectation was realized in the three cirrhotic patients described below, who recovered from the hepatorenal syndrome after orthotopic liver transplantation.
Case Reports
Case 1
A 34-year-old man was transferred to the Colorado General Hospital on September 14, 1972. At the age of 15 he was hospitalized for two months with acute viral hepatitis. At 31 years of age jaundice, ascites and peripheral edema developed. Three months before transplantation, the manifestations of liver disease became refractory to medical therapy, including diuretics. Repeated episodes of hepatic encephalopathy and gastrointestinal hemorrhage now began. After a liver biopsy, the diagnosis of chronic active hepatitis with advanced cirrhosis, hepatitis-B-antigen negative, was made. There was no history of renal disease, and he had a past record, of normal blood urea nitrogen (BUN) and creatinine tests within a month before this admission.
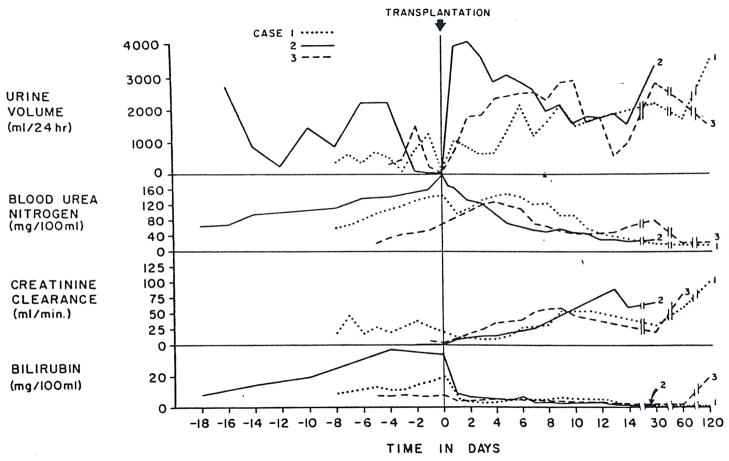
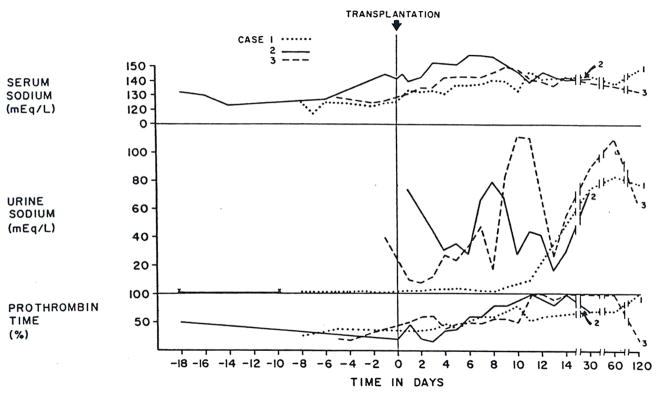
On admission he was mentally alert, with normal vital signs. He had a strong hepatic fetor, several spider angiomas, slight asterixis, marked ascites and splenomegaly. The liver was not palpable. Some of the initial abnormalities in renal and hepatic function and the serum sodium concentrations are summarized in Figures 1 and 2.* The urine had a specific gravity of 1.020, pH 5.0, and was without glycosuria or proteinuria; microscopic examination of the sediment revealed 1 to 5 white cells per high-power field and no red cells.
Figure 1.
Renal Function and Bilirubin in Cases 1–3 before and after Orthotopic Liver Transplantation.
Figure 2.
Serum and Urinary Sodium and Prothrombin Time (per Cent of Control) in Cases 1–3 before and after Orthotopic Liver Transplantation.
After admission, and in the eight days before liver transplantation, both hepatic and renal function deteriorated (Fig. 1 and 2). The progressive uremia was associated with oliguria, a high urine specific gravity and low sodium concentration. During these eight days the urinary urea and creatinine concentrations fell from 770 to 340 and 205 to 80 mg per 100 ml. The patient was placed on a diet containing 80 mEq of sodium and 30 g of protein. Except for vitamins and antacids, he did not receive any medication. After seven days Stage III to IV hepatic coma, without signs of gastrointestinal bleeding, developed. Blood ammonia was 238 μg per milliliter. Plasma expansion therapy resulted in an increase in urine volume (Fig. 1) without appreciable change in creatinine clearance. The hepatic coma was unaltered.
Early on the ninth hospital day, a 46-year-old cadaveric donor became available. An orthotopic liver transplantation and splenectomy were carried out; the recipient’s diseased liver had advanced macronodular post-necrotic cirrhosis. By this time the total bilirubin had increased to 19.8 mg per 100 ml; the BUN was 141, and the creatinine 4.8 mg per 100 ml. During the 12-hour operation, 15 U of fresh whole blood were given. The hourly urine volume ranged between 15 and 50 ml throughout the operation, during which the systolic blood pressure was always more than 80 mm Hg; after operation, cardiodynamic indexes were stable. Immunosuppression with cyclophosphamide, prednisolone and horse antilymphocyte globulin was begun during operation. Intravenous ampicillin, methicillin and gentamicin were given prophylactically for one week after operation. The patient was kept on no oral intake for 36 hours after surgery, but on the third day, an unrestricted regular hospital diet was started.
The immediate postoperative course was uneventful. He regained consciousness within a few hours after surgery and was mentally alert. Liver-function tests showed an almost immediate improvement, which continued over the next two weeks (Fig. 1 and 2). However, renal function remained depressed for the first four postoperative days to an even more severe degree than before operation (Fig. 1). There was also a reaccumulation of ascites during this time for which diuretics were not given. Then, a diuresis began on the fifth day after transplantation (Fig. 1) although the urinary sodium concentration remained quite low (Fig. 2). Within 24 hours, the BUN and serum creatinine had begun to fall, and by the 11th day the creatinine was within the normal range. Even after a week and a half, the urinary sodium concentration was only 10 mEq per liter.
Thirteen days after operation, Escherichia coli peritonitis developed, which involved the entire ascites-containing abdominal cavity. Despite therapy with 12 g per day of cephalothin (Keflin) and 240 mg per day of gentamicin, the infection persisted, with clinical deterioration. On the 25th day, the right portion of the previous bilateral subcostal incision was reopened and left open to enable peritoneal lavage. After this drastic step, the peritonitis resolved.
Two weeks after the intravenous administration of gentamicin was begun, renal function deteriorated. The serum creatinine reached a maximum of 4.2 mg per 100 ml, and the creatinine clearance decreased to 11 ml per minute. Urinary findings of a low specific gravity (1.013) and a high urinary sodium concentration (80 mEq per liter) suggested tubular damage consistent with gentamicin toxicity. The renal function gradually improved after the withdrawal of gentamicin. At the end of the second post-transplant month the BUN was 22, and creatinine 0.8 mg per 100 ml, and creatinine clearance was 61 ml per minute. Total bilirubin was 0.4 (direct 0.2) mg per 100 ml (Fig. 1). The patient has not had any documented rejection crises or any episodes of viral hepatitis since transplantation although he has required two secondary operations for bile-duct reconstruction. At present he is doing well in the 12th post-transplant month, with normal kidney function and adequate hepatic function.
Case 2
A 42-year-old man was transferred from another hospital to the Colorado General Hospital on November 22, 1972, in Stage IV hepatic coma with ascites, edema and jaundice. He had been anuric for the preceding 12 hours and had BUN and serum creatinine concentrations of 201 and 6.9 mg per 100 ml, respectively. From the records of his hospitalization elsewhere, it was possible to trace the advancing profile of combined liver and kidney failure back through the preceding 18 days (Fig. 1 and 2).
The hepatic illness was of 10 months’ duration and began with anorexia, fatigue, weight loss and jaundice. Eight months before transplantation, he had the first of many hospitalizations for the treatment of fluid retention, gastrointestinal bleeding and episodic hepatic encephalopathy. There was no history of renal disease, and the serum creatinine a month before transplantation was 1.2 mg per 100 ml. On admission to the other hospital because of melena and hepatic encephalopathy 18 days before transplantation, the urine had a specific gravity of 1.021, a pH of 5.5, sodium concentration of 1 mEq per liter and no glycosuria or proteinuria. Microscopical examination of this urine showed some hyaline and granular casts. Despite treatment with blood transfusions, plasma expansion, 1 g per day of oral or rectal neomycin and 120 mg per day of furosemide for 12 days, his condition rapidly deteriorated. Total plasma volume before the start of plasma-expansion therapy measured 58 ml per kilogram (normal, 35 to 45) with the 125I-radioactive iodinated serum albumin method. The treatment caused a slight increase in urine output. Beginning five days before transfer, the furosemide dose was increased to 160 mg every six hours in conjunction with further plasma infusions. Despite such severe bleeding that a Sengstaken-Blakemore tube was required, as well as blood transfusion, there were no documented examples of blood pressure below 90 mm Hg. Two days before transfer and transplantation he had become essentially anuric. By this time, the BUN had increased from 65 to 201, the serum creatinine from 3.0 to 6.9, and the total bilirubin from 7.9 to 34 mg per 100 ml.
Orthotopic liver transplantation was carried out on the day of admission. The diseased host liver weighed 2130 g and was found to have both cirrhosis and massive necrosis of inapparent cause. During operation, 8 U of blood were given. Except for 15 minutes during the anhepatic phase when the blood pressure fell to 60 mm Hg, systolic pressure was always more than 80 mm Hg. Intraoperative urine output was 35 ml, but with two extra transfusions in the recovery room, the blood pressure rose to over 100 mm Hg and a diuresis began, with 4140 ml excreted during the first day. This urine contained 75 mEq per liter of sodium, 40 mEq per liter of potassium, 350 mg per 100 ml of urea, and 18 mg per 100 ml of creatinine. Within an hour after completion of the operation the patient began to respond to stimuli; after a day he was awake, and in three days he was able to eat. Except for hypernatremia, which was corrected with nonelectrolyte intravenous infusions, his course was satisfactory, and his clinical condition improved for the next week. On the seventh postoperative day, the plasma volume was 45 ml per kilogram.
On the 10th postoperative day, despite continuing improvement in both liver and kidney function, the patient became confused and slowly lapsed into coma. A tracheotomy was performed on the 21st postoperative day. Pneumonitis followed, with mixed tracheal cultures of klebsiella, aspergillus, Candida albicans, Esch. coli and enterococci. He died of respiratory insufficiency 42 days after transplantation. Post-mortem examination revealed widespread bronchopneumonia, with no abnormalities in either the graft liver or the host kidneys.
Case 3
A 44-year-old man with Laennec’s cirrhosis was transferred from another hospital to the Denver Veterans Administration Hospital on December 28, 1972, and received an orthotopic liver homograft on the following day. He had drunk large amounts of alcohol for 25 years and for one year had had progressive jaundice, as-cites and peripheral edema. He had had episodic hepatic encephalopathy but no gastrointestinal hemorrhage. During frequent hospitalizations in the year before transplantation he was sporadically treated with furosemide and spironolactone. There was no history of renal disease, and urinalysis five days before arrival in Denver showed a pH of 5.5, specific gravity of 1.014 and no glycosuria or proteinuria. When admitted to the hospital on December 28, he had cellulitis of the left leg and a partially necrotic scrotum, from which Esch. coli and pseudomonas were cultured. He was jaundiced, had anasarca and massive ascites, but was oriented. The blood pressure was 96/60 mm Hg, with a pulse of 88 per minute. The liver was not palpable, and there was splenomegaly.
At the other hospital during the five preceding days, liver function was very poor, and there was rapidly developing renal failure (Fig. 1 and 2). Before transfer to Denver, plasma-volume expansion with albumin and glucose-saline solutions had been tried along with intravenous furosemide. Although the urine volume increased to 1515 ml for one day, the progression of the kidney failure was not halted (Fig. 1).
The orthotopic liver transplantation on December 29, 1972, required 12 hours and 12 U of blood. The diseased host liver weighed 1150 g, grossly had micronodular cirrhosis, and was confirmed histopathologically to have Laennec’s cirrhosis. Immunosuppression was similar to that in Case 1.
The new liver provided slowly improving life-supporting function (Fig. 1 and 2). During the 12-hour operation, the urine output was only 50 ml in spite of the fact that the systolic blood pressure was never lower than 80 mm Hg, and there was no urine at all during the last half of the procedure. The patient remained anuric for another 12 hours after operation, but then urine flow started and gradually increased (Fig. 1). On the 11th day, when the creatinine clearance was 49 ml per minute, amphotericin B was started because of the appearance of C. albicans in the peritoneal fluid coming from a drain site. In the next 10 days, kidney function secondarily deteriorated. Low urine specific gravity and proteinuria suggested tubular damage due to amphotericin toxicity. The drug was stopped, with recovery of nearly normal renal function.
Plasma renin activity and renin substrate were measured before and after transplantation (Table 1). High preoperative renin and low renin-substrate values returned to and remained normal afterwards.
Table 1.
Plasma Renin Activity and Renin Substrate in Case 3 before and after Liver Transplantation.
| Day | Plasma Renin Activity*ng/ml/hr | Renin Substrate †ng/ml |
|---|---|---|
| − 1 | 11.89 | 110.68 |
| ‡ | ||
| + 1 | 9.76 | 546.40 |
| + 2 | 10.59 | 808.16 |
| + 4 | 1.46 | 739.69 |
| + 6 | 1.58 | 677.82 |
| + 9 | 0.67 | 683.23 |
| + 11 | 5.69 | 792.15 |
Normal renin activity in the supine position is 0.2–3.6 ng angiotensin 1/ml/hr on a diet containing 113 mEq of sodium & 2.1–13.8 ng/ml/hr on one containing 10 mEq.
Normal renin substrate level is >800 ng angiotensin 1/ml.
Day of transplantation.
Biliary-duct reconstruction in this patient was with choledochocholedochostomy. After operation a bile fistula developed, requiring attempts at late repair as well as drastic reductions in immunosuppression in the third postoperative month to minimize the risk of infection. When indolent rejection supervened, retransplantation of another orthotopic liver was carried out on April 19, 1973. The second homograft did not function properly, and the patient died on the 124th day, 10 days after the retransplantation. Renal function had not deteriorated before the provision of the second homograft, but after the retransplantation, progressively deteriorated in the last five days of life. The last BUN and creatinine were 105 and 3.4 mg per 100 ml, respectively. Post-mortem examination revealed normal kidneys except for bile staining and mild ischemic tubular damage.
Discussion
Hecker and Sherlock2 and later Papper et al.3 called attention to renal failure in patients with hepatic cirrhosis. The consequent hepatorenal syndrome usually heralds the final illness of patients with end-stage liver disease. However, the functional nature and inherent reversibility of the renal disorder has been suggested or proved by the virtual absence of abnormalities on light microscopy in the post-mortem kidneys,3,4 by the disappearance after death of vasospastic changes demonstrated during life with angiography,5 by occasional spontaneous recoveries from the complication,6,7 and by the successful transplantation of cadaveric kidneys donated to noncirrhotic recipients by victims of the hepatorenal syndrome.8,9
Other evidence has been strong that major changes in the volume of renal blood flow or regional redistributions of renal blood flow away from the cortex have a crucial role in the pathogenesis of the hepatorenal syndrome.5,10,11 Baldus et al.12 found inappropriate increases of renal vascular resistance, and several authors have speculated that the numerous extrarenal arteriovenous shunts characteristic of these patients contribute to relative deprivation of blood flow to the high-resistance kidney vascular bed in spite of an increased total cardiac output.13–15 It has also been suggested that effective plasma volume may be partially sequestered in the splanchnic bed because of portal hypertension.14 If this were true, portacaval shunt would relieve the hepatorenal syndrome as Schroeder et al.16 have described, but such amelioration is uncommon. However, because of the possibility of a low effective blood volume, even though the plasma14,17–19 and whole-blood20 volumes are usually elevated in patients with cirrhosis, plasma expansion has been proposed by Tristani and Cohn14 as a means of increasing renal blood flow. This therapy was tried before operation in all three of our patients, but with little effect.
The ultimate explanation for hemodynamic changes in the kidney during hepatic failure has been the subject of much speculation. Two such hypotheses have been that vasoactive substances in the blood are no longer inactivated by the failing liver20 or alternatively that such substances are actually released by the damaged hepatic tissue.21 Recently, a potentially unifying explanation for the renal hemodynamic alterations in the hepatorenal syndrome has been advanced. In cirrhotic patients, it has been shown that plasma renin concentrations are high and that plasma concentrations of renin substrate, which is normally produced by the liver, are low.20, 22, 23 From these observations vasoconstriction and flow redistribution within the kidney might be expected. Berkowitz et al.24 showed in isolated dog-kidney perfusion experiments that renal cortical flow decreased with the depletion of renin substrate in the perfusate and that addition of renin substrate to the depleted perfusate increased the cortical flow. They concluded that the failure of intrarenal angiotensin production secondary to low circulating renin substrate levels could underlie the reduced renal cortical perfusion in the hepatorenal syndrome owing to the consequent inability to constrict efferent arterioles selectively. In Case 3 of our series high renin and low renin substrate values were corrected to normal soon after liver replacement. In this patient, as well as the other two, recovery of renal function required a number of days, however.
Since successful orthotopic liver transplantation should correct all the foregoing factors suspected of causing functional renal changes, experience with our cases cannot be said to clarify the mechanism of recovery. In fact, there is not even proof that all the patients had exactly the same kind of functional renal failure. The diagnosis of hepatorenal syndrome is based partly on ruling out renal impairment of demonstrable origin, including pre-existing chronic disease or such acute events as hypotension, septicemia or drug nephrotoxicity. As summarized by Papper,25 the remaining patients usually have progressive azotemia, oliguria, a concentrated urine, a low urinary sodium concentration and mild proteinuria. Case 1 met all the criteria for this diagnosis; Cases 2 and 3 met most of the criteria but at some previous time while in another hospital. However, in Case 2 septicemia, gastrointestinal hemorrhage or the prior attempt at diuretic therapy26 could have contributed to the final picture, and diuretics may also have been involved in Case 3.
After liver replacement, all three patients had renal recovery that evolved over many days, suggesting that reversal of the conditions causing the hepatorenal syndrome, at least in these three cases, required more time than has been realized. There were wide variations in the recovery course in terms of diuresis, urinary sodium excretion and concentration of urine. In Case 1 urine volume was relatively small, with a high specific gravity and a persistently low urinary sodium excretion, whereas in Case 2, the recovery started with a brisk diuresis of over 4000 ml per day and a high urinary sodium concentration. The recovery of Case 3 was intermediate in terms of these urinary findings. The reasons are not readily apparent for this difference in recovery pattern, which could not be correlated with liver function before or after transplantation.
Hyponatremia, usually seen in the hepatorenal syndrome, was present in all three cases before transplantation, and in all was reversed by massive blood transfusions during surgery. However, in Cases 2 and 3 hypernatremia, which could not be explained by parenteral fluid replacement, developed after transplantation. The hypernatremia observed during recovery from acute renal failure or after renal transplantation27 has been attributed to osmotic diuresis, which causes a urinary water loss in excess of sodium excretion. In our cases, massive urinary excretion of retained urea could, in fact, have resulted in such an osmotic diuresis, thereby correcting the hyponatremia or even leading to hypernatremia.
Acknowledgments
Supported by research grants from the Veterans Administration, by grants (AI-AM-08898 and AM-07772) from the National Institutes of Health, and by grants (RR-00051 and RR-00069) from the General Clinical Research Centers Program of the Division of Research Resources, National Institutes of Health.
Footnotes
For more detailed information order NAPS Document 02246 from National Auxiliary Publications Service, c/o Microfiche Publications. 305 E. 46th St.. New York. N.Y. 10017; remitting $1.50 for each microfiche-copy reproduction or $5 for each photocopy. Checks or money orders should be made payable to Microfiche Publications.
References
- 1.Lieberman FL. Functional renal failure in cirrhosis. Gastroenterology. 1970;58:108–110. [PubMed] [Google Scholar]
- 2.Hecker R, Sherlock S. Electrolyte and circulatory changes in terminal liver failure. Lancet. 1956;2:1121–1125. doi: 10.1016/s0140-6736(56)90149-0. [DOI] [PubMed] [Google Scholar]
- 3.Papper S, Belsky JL, Bleifer KH. Renal failure in Laennec’s cirrhosis of the liver. I. Description of clinical and laboratory features. Ann Intern Med. 1959;51:759–773. doi: 10.7326/0003-4819-51-4-759. [DOI] [PubMed] [Google Scholar]
- 4.Shear L, Kleinerman J, Gabuzda GJ. Renal failure in patients with cirrhosis of the liver. I. Clinical and pathologic characteristics. Am J Med. 1965;39:184–198. doi: 10.1016/0002-9343(65)90041-0. [DOI] [PubMed] [Google Scholar]
- 5.Epstein M, Berk DP, Hollenberg NK, et al. Renal failure in the patient with cirrhosis. Am J Med. 1970;49:175–185. doi: 10.1016/s0002-9343(70)80073-0. [DOI] [PubMed] [Google Scholar]
- 6.Lieberman FL, Ito S, Reynolds TB. Effective plasma volume in cirrhosis with ascites: evidence that a decreased value does not account for renal sodium retention, a spontaneous reduction in glomerular filtration rate (GFR), and a fall in GFR during drug-induced diuresis. J Clin Invest. 1969;48:975–981. doi: 10.1172/JCI106078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein H, Boyle JD. Spontaneous recovery from the hepatorenal syndrome: report of four cases. N Engl J Med. 1965;272:895–898. doi: 10.1056/NEJM196504292721706. [DOI] [PubMed] [Google Scholar]
- 8.Koppel MH, Coburn JW, Mims MM, et al. Transplantation of cadaveric kidneys from patients with hepatorenal syndrome: evidence for the functional nature of renal failure in advanced liver disease. N Engl J Med. 1969;280:1367–1371. doi: 10.1056/NEJM196906192802501. [DOI] [PubMed] [Google Scholar]
- 9.McDonald FD, Brennan LA, Turcotte JG. Severe hypertension and elevated plasma renin activity following transplantation of “hepatorenal donor” kidneys into anephric recipients. Am J Med. 1973;54:39–43. doi: 10.1016/0002-9343(73)90081-8. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder ET, Shear L, Sancetta SM, et al. Renal failure in patients with cirrhosis of the liver, III. Evaluation of intrarenal blood flow by para- aminohippurate extraction and response to angiotensin. Am J Med. 1967;43:887–896. doi: 10.1016/0002-9343(67)90247-1. [DOI] [PubMed] [Google Scholar]
- 11.Berk DP, Epstein M, Hollenberg NK, et al. A continuum of renal circulatory changes associated with the hepatorenal syndrome. Gastroenterology. 1969;56:420. [Google Scholar]
- 12.Baldus WP, Summerskill WHJ, Hunt JC, et al. Renal circulation in cirrhosis: observations based on catheterization of the renal vein. J Clin Invest. 1964;43:1090–1097. doi: 10.1172/JCI104993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancestremere RG, Davidson PL, Earley LE, et al. Renal failure in Laennec’s cirrhosis. II. Simultaneous determination of cardiac output and renal hemodynamics. J Clin Invest. 1962;41:1922–1927. doi: 10.1172/JCI104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tristani FE, Cohn JN. Systemic and renal hemodynamics in oliguric hepatic failure: effect of volume expansion. J Clin Invest. 1967;46:1894–1906. doi: 10.1172/JCI105679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shear L, Hall PW, III, Gabuzda GJ. Renal failure in patients with cirrhosis of the liver. II. Factors influencing maximal urinary flow rate. Am J Med. 1965;39:199–209. doi: 10.1016/0002-9343(65)90042-2. [DOI] [PubMed] [Google Scholar]
- 16.Schroeder ET, Numann PJ, Chamberlain BE. Functional renal failure in cirrhosis: recovery after portacaval shunt. Ann Intern Med. 1970;72:923–928. doi: 10.7326/0003-4819-72-6-923. [DOI] [PubMed] [Google Scholar]
- 17.Lieberman FL, Reynolds TB. Plasma volume in cirrhosis of the liver: its relation to portal hypertension, ascites, and renal failure. J Clin Invest. 1967;46:1297–1308. doi: 10.1172/JCI105622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds TB, Lieberman FL, Redeker AG. Functional renal failure with cirrhosis: the effect of plasma expansion therapy. Medicine (Baltimore) 1967;46:191–196. doi: 10.1097/00005792-196703000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Papper S, Vaamonde CA. Renal failure in cirrhosis – role of plasma volume. Ann Intern Med. 1968;68:958–959. doi: 10.7326/0003-4819-68-4-958. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder ET, Eich RH, Smulyan H, et al. Plasma renin level in hepatic cirrhosis: relation to functional renal failure. Am J Med. 1970;49:186–191. doi: 10.1016/s0002-9343(70)80074-2. [DOI] [PubMed] [Google Scholar]
- 21.Vargish T, Levin R, Cotton J, et al. Changes in renal function and intrarenal blood flow distribution due to a humoral factor produced by hepatic ischemia. Ann Surg. doi: 10.1097/00000658-197310000-00012. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berkowitz HD, Miller LD, Itskovitz HD. Liver disease, renal function, and the renin-angiotensin system. Surg Forum. 1968;19:391–392. [PubMed] [Google Scholar]
- 23.Ayers CR. Plasma renin activity and renin-substrate concentration in patients with liver disease. Circ Res. 1967;20:594–598. doi: 10.1161/01.res.20.6.594. [DOI] [PubMed] [Google Scholar]
- 24.Berkowitz HD, Galvin C, Miller LD. Significance of altered renin substrate in the hepatorenal syndrome. Surg Forum. 1972;23:342–343. [PubMed] [Google Scholar]
- 25.Vaamonde Papper S. The kidney in liver disease. In: Strauss MB, Welt LG, editors. Diseases of the Kidney. Boston: Little, Brown and Company; 1971. pp. 1139–1154. [Google Scholar]
- 26.Lieberman FL, Reynolds TB. Renal failure with cirrhosis: observations on the role of diuretics. Ann Intern Med. 1966;64:1221–1228. doi: 10.7326/0003-4819-64-6-1221. [DOI] [PubMed] [Google Scholar]
- 27.Popovtzer MM, Pinggera WF, Holmes JH, et al. Hypernatremia: complications of renal homotransplantation. Arch Intern Med. 1971;127:1129–1132. doi: 10.1001/archinte.127.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]