Abstract
Acrolein is a toxic, highly reactive α,β-unsaturated aldehyde that is present in high concentrations in cigarette smoke. In the current study, the effect of acrolein on eicosanoid synthesis in stimulated human neutrophils was examined. Eicosanoid synthesis in neutrophils was initiated by priming with granulocyte-macrophage colony-stimulating factor (GM-CSF) and subsequent stimulation with formyl-methionyl-leucyl-phenylalanine (fMLP) and 5-LO products in addition to small amounts of COX products were detected using LC/MS/MS. A dose-dependent decrease in the formation of 5-LO products was observed in GM-CSF/fMLP stimulated neutrophils when acrolein (0-50 μM) was present with almost complete inhibition at ≥25 μM acrolein. The production of COX products was not affected by acrolein in these cells. The effect of acrolein was examined on key parts of the eicosanoid pathway, such as arachidonic acid release, intracellular calcium ion concentration, and adenosine production. In addition, the direct effect of acrolein on 5-LO enzymatic activity was probed using a recombinant enzyme. Some of these factors were affected by acrolein, but did not completely explain the almost complete inhibition of 5-LO product formation in GM-CSF/fMLP treated cells with acrolein. In addition, the effect of acrolein on different stimuli that initiate the 5-LO pathway (platelet-activating factor (PAF)/fMLP, GM-CSF/PAF, opsonized zymosan, and A23187) was examined. Acrolein had no significant effect on the leukotriene production in neutrophils stimulated with PAF/fMLP, GM-CSF/PAF, or OPZ. Additionally, 50% inhibition of the 5-LO pathway was observed in A23187 stimulated neutrophils. Our results suggest that acrolein has a profound effect on the 5-LO pathway in neutrophils, which may have implications in disease states, such as COPD and other pulmonary disease where both activated neutrophils and acrolein are present.
INTRODUCTION
Acrolein is an extremely toxic and reactiveα,β-unsaturated aldehyde that is produced by several different mechanisms relevant to human exposure (1). The sources of acrolein include combustion of fossil fuels (2), cigarette smoke (3), burning of animal and vegetable fats (4), lipid peroxidation (5), neutrophil myeloperoxidase catalyzed amino acid oxidation (6), and drug metabolism (7). It is thought that the main mechanism of acrolein toxicity is by covalent bonding of this highly reactive, electrophilic aldehyde with nucleophilic molecules in the cell, such as DNA (8), proteins (9), aminophospholipids (10) and glutathione (11). Acrolein has been found to have numerous biological effects including the suppression of cytokine (12-14) and redox sensitive transcription factor production (15,16), induction of apoptosis (17-19), and up-regulation (20) or down-regulation (21) of various signaling molecules.
Acrolein has been implicated in various inflammatory disease states, including COPD (22), in which the presence of neutrophils has been noted (23). Neutrophils are activated at the site of inflammation, which leads to production of O2- and eicosanoids. The effect of acrolein on superoxide production in activated neutrophils has been previously examined. In particular, acrolein (10 μM) has been found to inhibit NADPH oxidase activity in fMLP stimulated neutrophils (24,25). However, the effect of acrolein on eicosanoid production in stimulated neutrophils has not been investigated.
Eicosanoid production in activated neutrophils is initiated by the release of arachidonic acid from membrane phospholipids via a Ca2+ dependent cytosolic phospholipase A2α (cPLA2α) (26). For leukotriene synthesis, arachidonic acid (AA) is converted to 5-hydroperoxyeicosatetraenoic acid (5-HpETE) and then to leukotriene A4 (LTA4) by the action of 5-lipoxygenase (5-LO) (27). The presence of calcium, release of AA, and translocation of 5-LO from the cytosol to the nuclear membrane where it interacts with 5-LO activating protein (FLAP) are necessary for activation of 5-LO (28). In neutrophils, LTA4 hydrolase is present and converts LTA4 into biologically active leukotriene B4 (LTB4) (29). This biologically active product is metabolized by a specific cytochrome P-450 enzyme in neutrophils into the ω-oxidation product (20-OH-LTB4) (30).
In this study, the effect of acrolein on the synthesis of eicosanoids, both 5-LO and COX products, by human neutrophils was examined in response to various extracellular stimuli, including GM-CSF/fMLP, PAF/fMLP, GM-CSF/PAF, opsonized zymosan, and A23187. In addition the effect of acrolein on key parts of the eicosanoid pathway, such as arachidonic acid release, intracellular calcium, and adenosine production was examined and the direct effect of acrolein on 5-LO enzymatic activity was probed using a recombinant enzymatic assay. The influence of acrolein on basic neutrophil function, such as NADPH oxidase activation, was also examined. The data reported herein suggest that acrolein does affect the 5-LO pathway initiated by GM-CSF/fMLP in neutrophils.
EXPERIMENTAL
Materials
Hanks' balanced salt solution (1X) without Ca2+ and Mg2+ (HBSS=), Hanks' balanced salt solution (1X) with Ca2+ and Mg2+ (HBSS), and indo-1 AM were obtained from Invitrogen (Carlsbad, CA). Granulocyte-macrophage colony-stimulating factor (GM-CSF), formyl-methionyl-leucyl-phenylalanine (fMLP), acrolein, ATP, A23187, zymosan, and cytochrome c were purchased from Sigma (St. Louis, MO). Human recombinant 5-lipoxygenase (80 units/mg), platelet-activating factor (PAF), arachidonic acid (AA), d4-leukotriene B4 (LTB4) (≥97 atom % D), d8-5-hydroxyeicosatetraeonic acid (5-HETE) (≥98 atom % D), d4-thromboxane B2 (TXB2) (≥98 atom % D), d4-prostaglandin E2 (PGE2) (≥99 atom % D), and d8-AA (≥99 atom % D) were obtained from Cayman Chemical (Ann Arbor, MI). Zileuton was purchased from Abbott Laboratories (Chicago, IL). Salts and HPLC solvents were purchased from Fisher Scientific (Fair Lawn, NJ).
Human neutrophil experiments with GM-CSF/fMLP, GM-CSF/PAF, PAF/fMLP, A23187, and opsonized zymosan
Neutrophils were obtained from the whole blood of volunteers using the Percoll gradient centrifugation technique as previously described (31). The acrolein stock solutions used in these experiments were in HBSS=, which were prepared fresh daily. In some experiments, neutrophils were primed using either GM-CSF or PAF. For GM-CSF priming, the neutrophils were centrifuged and initially suspended in HBSS= at a concentration of 20×106 cells/ml and primed with GM-CSF (1 nM) for 30 min at 37°C. After GM-CSF priming, the cells were diluted to 2×106 cells/ml and CaCl2 (final concentration 2 mM) and MgCl2 (final concentration 500 μM) were added so that the final volume in each tube was 1 ml. Alternatively for PAF priming, the neutrophils were suspended in HBSS at a concentration of 2×106 cells/ml and primed with PAF (100 nM) for 5 min at 37°C with a final volume in each tube of 1 ml. After priming with either GM-CSF or PAF, acrolein (0-50 μM) was added and allowed to incubate at 37°C for 3 min. The GM-CSF primed/acrolein treated neutrophils were stimulated with fMLP (100 nM) or PAF (100 nM) and the samples were allowed to incubate at 37°C for 10 min. Additionally, the PAF primed/acrolein treated cells were stimulated for eicosanoid production by addition of fMLP (100 nM) and incubated at 37°C for 10 min. For the arachidonic acid supplementation of GM-CSF primed neutrophils, the procedure described above was followed and the only difference was that arachidonic acid (0.5 μM final concentration) was added immediately after the addition of fMLP. For the time course of acrolein (25 μM) addition to GM-CSF/fMLP treated cells, acrolein was either added at the same time as GM-CSF or at some point after the 30 min incubation with GM-CSF. For those time course points where the acrolein was added after the 30 min GM-CSF priming event, the acrolein was added either 3 min before fMLP (as described above), at the same time as fMLP addition, or 30 s after fMLP addition.
Additionally, for stimulation of neutrophils with opsonized zymosan, zymosan was opsonized with autologous serum as described previously (32) and added to the neutrophils at a concentration of 200 μg/ml for 30 min at 37°C after a 3 min preincubation with acrolein (25 μM). Finally, neutrophils were stimulated with calcium ionophore A23187 (50 nM) for 10 min at 37°C after a 3 min preincubation with acrolein (25 μM). For both the A23187 and opsonized zymosan experiments the concentration of neutrophils was 2×106 cells/ml in HBSS and the final incubation volume was 1 ml.
All reactions were stopped by the addition of one volume of ice-cold methanol containing d4-LTB4 (2 ng), d8-5-HETE (2 ng), d4-TXB2 (5 ng), and d4-PGE2 (5 ng). The samples were diluted with 4 ml water and extracted using an Oasis HLB solid phase extraction column (30 mg/ml; Waters, Milford, MA). The eicosanoids were eluted with 1 ml of methanol. The eluate was dried down and reconstituted in 40 μl of HPLC solvent A (8.3 mM acetic acid buffered to pH 5.7 with NH4OH) and 20 μl of HPLC solvent B (acetonitrile/methanol, 65/35, v/v) for analysis.
Arachidonic acid metabolite separation by RP-HPLC and analysis by electrospray ionization tandem mass spectrometry
Each sample (25 μl) was injected into a HPLC gradient pump system directly interfaced to the electrospray source of a Sciex API 3000 triple quadrupole mass spectrometer (PE Sciex, Toronto, Canada). A Gemini 5μ C18 (2.0 × 150 mm) column (Phenomenex, Torrance, CA) was used to separate the arachidonic acid metabolites extracted from the supernatant. The initial mobile phase was 45% solvent B at a flow rate of 200 μl/min. A linear gradient was started to 75% solvent B in 13 min and followed by an increase to 98% solvent B in 2 min. This was followed by isocratic elution at 98% solvent B for 7 min. Multiple reaction monitoring (MRM) in the negative ion mode of m/z 369→169 for TXB2, m/z 373→173 for d4-TXB2, m/z 351→271 for PGE2, m/z 355→275 for d4-PGE2, m/z 351→195 for 20-OH-LTB4, m/z 335→195 for LTB4 and the Δ6-trans-LTB4 isomers, m/z 339→197 for d4-LTB4, m/z 319→115 for 5-HETE, and m/z 327→116 for d8-5-HETE was used to detect the eicosanoids eluting from the RP-HPLC column. Turbo ion spray was used with the turbo heater temperature at 350°C and the drying gas delivered at 500 mL/min for eicosanoid analysis. Quantitation was performed using a standard isotope dilution curve as previously described (33).
Adenosine analysis by LC/MS/MS
Adenosine released from neutrophils was also analyzed by LC/MS/MS using the Sciex API 3000 triple quadrupole mass spectrometer (PE Sciex, Toronto, Canada) by a method adapted from Cadieux et al (34). After GM-CSF/acrolein/fMLP treatment, 1×106 neutrophils (0.5 ml) were not lysed with methanol but 15N5-adenosine (50 ng; Spectra Stable Isotopes, Columbia, MD) was added and the cells immediately centrifuged (1000×g, 5 min). Methanol with 0.05% acetic acid (0.3 ml) was added to the supernatant and stored at -20°C until analysis. An aliquot (50 μl) was directly injected onto a reverse phase HPLC column (150 × 2 mm, Gemini 5μ C18 column; Phenomenex), which was eluted at 200 μl/min with an isocratic gradient of methanol/water (40/60, v/v) with 2 mM ammonium acetate for 15 min. Adenosine and 15N5-adenosine were detected in the positive ion mode by the MRM transitions of m/z 268→136 and m/z 273→141, respectively. Turbo ion spray was used with the turbo heater temperature at 275°C and the drying gas delivered at 250 mL/min for adenosine analysis.
Quantitation of Arachidonic Acid by GC/MS
After GM-CSF/acrolein/fMLP treatment, an aliquot of neutrophil lysate (1:1; H2O/MeOH) corresponding to 1×106 cells was acidified with HCl (70 mM final concentration), d8-AA (20 ng) was added, and the acidified neutrophil lysate was extracted with 2 ml of iso-octane. After derivatization with pentafluorobenzyl bromide (35), the quantitation of arachidonic acid released upon GM-CSF/acrolein/fMLP treatment of neutrophils was performed as described previously by negative ion chemical ionization GC/MS on a Finnigan DSQ GC/MS system (Thermo Finnigan, Thousand Oaks, CA) (36).
Calcium Assay
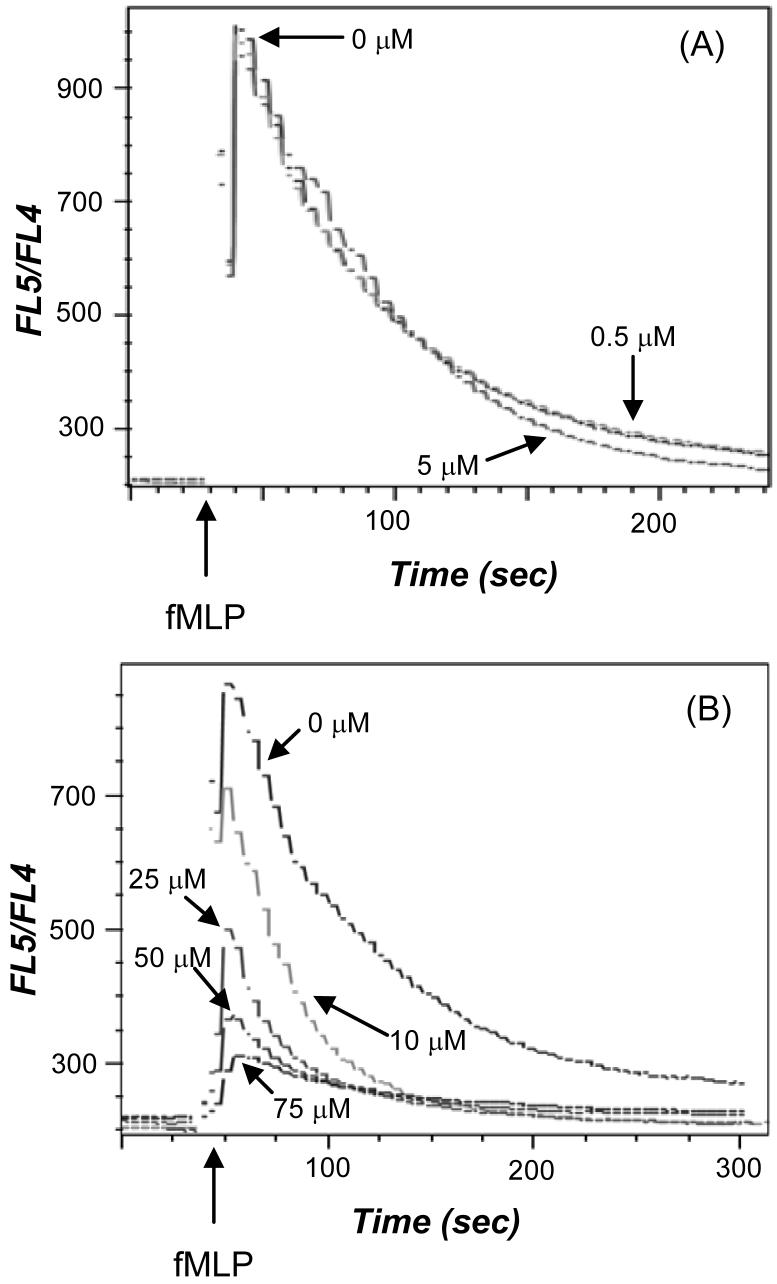
Cells (10×106/ml in HEPES buffer with 0.5% fatty acid-free BSA and 1 mM CaCl2) were loaded with 2 μM indo-1 AM for 30 min at 37°C. Cells were centrifuged and resuspended in HEPES buffer with 0.5% fatty acid-free BSA at 20×106 cells/ml. Priming with GM-CSF (1 nM) was accomplished by incubating at 37°C for 30 min. For the calcium assay, the fatty acid-free BSA was removed from 2×106 cells by centrifugation and resuspension of cells in HEPES buffer. The indo-1 AM labeled, GM-CSF primed neutrophils were resuspended in 1 ml HEPES buffer and acrolein (0-50 μM) was added. The neutrophils and acrolein were incubated at 37°C for 3 min. Intracellular calcium changes were monitored by the change in FL5/FL4 fluorescence (LSR II, Becton Dickinson, Franklin Lakes, NJ) following fMLP (100 nM final concentration) stimulation. All results shown are representative of at least five independent experiments.
Human recombinant 5-lipoxygenase assay
Recombinant 5-lipoxygenase enzyme (0.7 units) was added to 200 μl of 50 mM PBS containing 2 mM ATP and acrolein (0-500 μM) or zileuton (0.5 μM). This mixture was allowed to incubate at 37°C for 3 min (acrolein) or 15 min (zileuton). Arachidonic acid (0.2 μM final concentration) and calcium (2 mM final concentration) were added to each of the samples and allowed to incubate for an additional 10 min at 37°C. The reaction was stopped by the addition of ice cold methanol (200 μl) containing d4-LTB4 (2 ng) and d8-5-HETE (2 ng). These samples were extracted using solid phase extraction and analyzed by LC/MS/MS as described above.
Superoxide assay
For cytochrome c reduction, 2×106 cells in 1ml HEPES buffer were primed with either GM-CSF for 30 min or PAF for 5 min at 37°C and then treated with acrolein for an additional 3 min at 37°C as described above. Following the priming and acrolein preincubation, 100 μl of cytochrome c (14.8 mg/ml) and fMLP (100 nM) were added and allowed to incubate for an additional 10 min. The reaction was stopped by centrifuging the cells at 4°C and the absorbance at 550 nm of the supernatant was determined. The nanomoles of superoxide produced per 2×106 cells per 10 min were calculated from the absorbance by using the extinction coefficient 71.4.
Statistical analysis
The data was expressed as the mean ± standard error of the mean from five independent experiments. Analysis of variance (ANOVA) test followed by Tukey multiple comparisons test using the GraphPad InStat version 3.06 statistical program (GraphPad Software, San Diego, CA).
RESULTS
Eicosanoid production in acrolein treated neutrophils with GM-CSF/fMLP stimulation
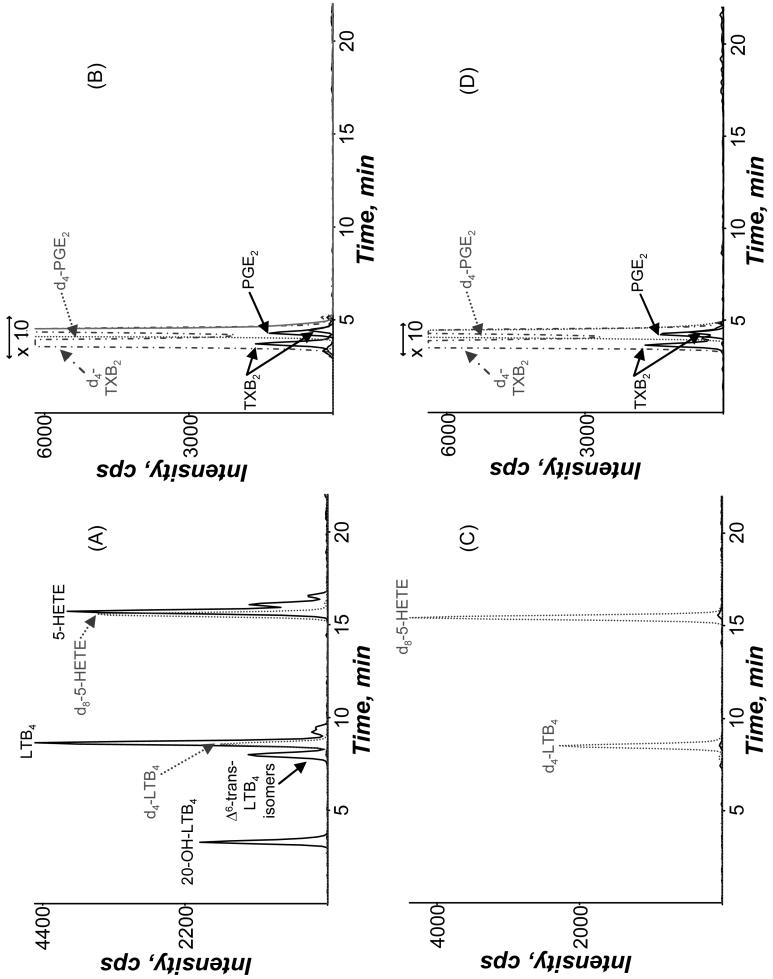
Stimulation of human neutrophils with GM-CSF (1 nM) as a priming agent followed by treatment with fMLP (100 nM) resulted in an activation of the 5-LO cascade with formation of LTB4, 20-OH-LTB4, Δ6-trans-LTB4 isomers, and 5-HETE (Figure 1A). In addition to 5-LO products, cyclooxygenase (COX) products, TXB2 and PGE2, were formed upon the stimulation of neutrophils with GM-CSF/fMLP although in substantially lower abundance (Figure 1B). Eicosanoid production was not detectable when the neutrophils were treated with only GM-CSF or fMLP, but only observed when both GM-CSF and fMLP were used for stimulation (data not shown). Treatment of the GM-CSF primed cells with 25 μM acrolein for 3 min before fMLP addition resulted in a complete disappearance of all 5-LO products (Figure 1C), without much change in the COX products (Figure 1D). Due to the dramatic decrease in 5-LO products, the effect of acrolein on eicosanoid production in GM-CSF/fMLP treated neutrophils was investigated.
Figure 1.
Mass spectrometric analysis of eicosanoids from extracted supernatants of human neutrophils primed with GM-CSF (1 nM, 30 min) and then stimulated with fMLP (100 nM, 10 min) at 37°C in (A and B) the absence and (C and D) the presence of 25 μM acrolein. The GM-CSF primed cells were treated with acrolein for 3 min before fMLP was added. Elution of the eicosanoids was detected in the negative ion mode using MRM transitions specific to each metabolite as described in the Methods. The Δ6-trans-LTB4 isomers peak includes both Δ6-trans-LTB4 and Δ6-trans-12-epi-LTB4.
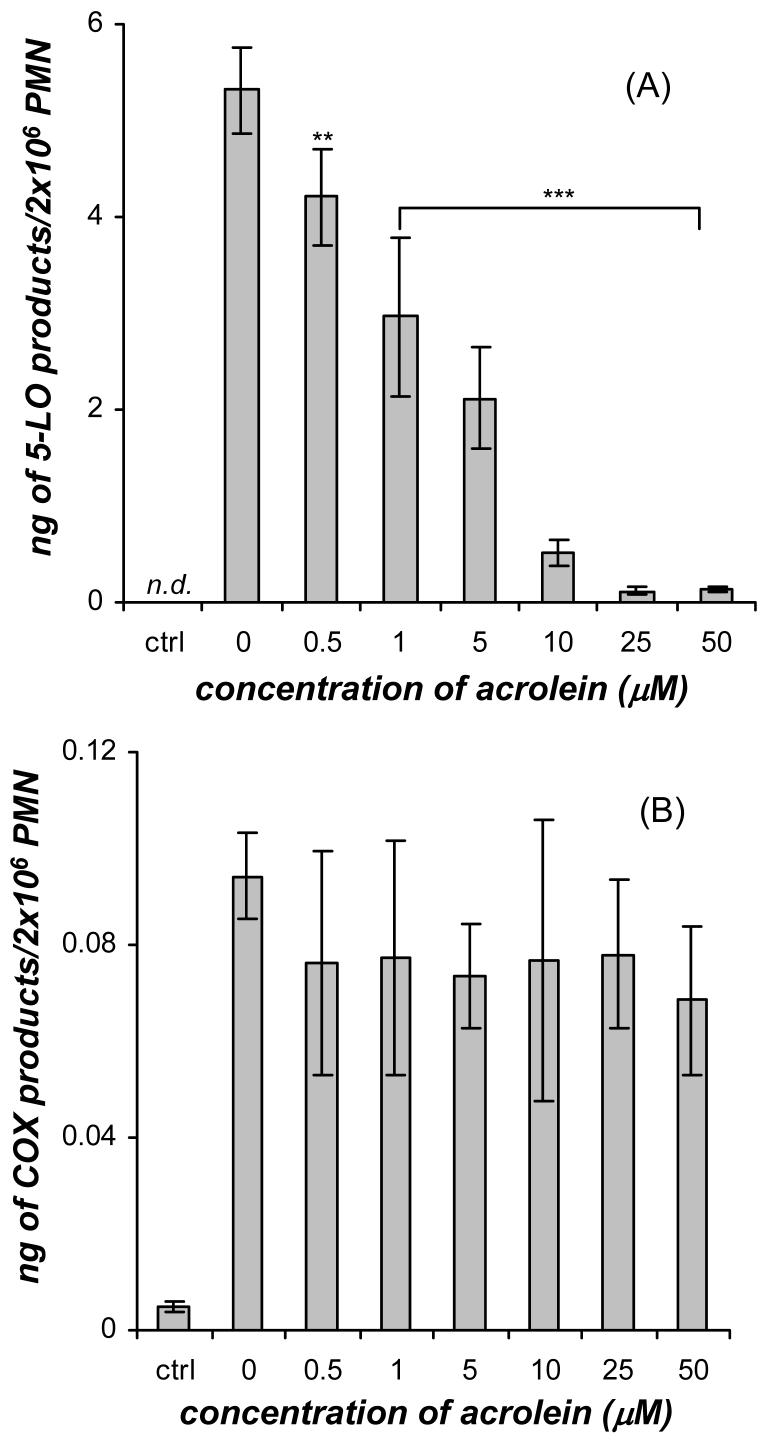
Acrolein treatment (0-50 μM) significantly decreased the amount of 5-LO products in GM-CSF/fMLP stimulated neutrophils in a dose-dependent manner (Figure 2A). The GM-CSF/fMLP treated neutrophils produced 5.32±0.45 ng 5-LO products/2×106 cells. A significant decrease in the amount of 5-LO products to 4.21±0.49 ng/2×106 cells was observed at the lowest dose of acrolein (0.5 μM) tested. In addition, a near total disappearance of 5-LO products to 0.12±0.03 and 0.14±0.03 ng/2×106 cells was observed at 25 μM and 50 μM acrolein, respectively, in the dose response. Conversely, the dose response of GM-CSF/fMLP treated neutrophils with acrolein did not show a significant change in COX metabolite production (Figure 2B) over the concentration range of 0-50 μM acrolein. In previous studies, eicosanoids have been found to be released from HUVECs after 16 h exposure to acrolein (10 μM) (37) and from bovine airway epithelial cells after 20 min treatment with acrolein (100 μM) (38). However, the addition of acrolein alone (without GM-CSF and fMLP) to neutrophils did not result in the production of detectable amounts of 5-LO or COX products (data not shown). From the dose response data (Figure 2A and 2B) it was clear that the addition of acrolein to GM-CSF/fMLP treated neutrophils resulted in significant alteration of the complex set of events that ultimately leads to leukotriene production, but did not affect the COX cascade.
Figure 2.
Dose response of acrolein (0-50 μM) on the synthesis of (A) 5-LO products (20-OHLTB4, Δ6-trans-LTB4 isomers, LTB4, and 5-HETE) and (B) COX products (PGE2 and TXB2) formed in human neutrophils (PMN) primed with GM-CSF (1 nM, 30 min) and stimulated with fMLP (100 nM, 10 min) at 37°C. The GM-CSF primed cells were treated with acrolein for 3 min before fMLP was added. The control neutrophils were not treated with GM-CSF or fMLP. The 5-LO metabolites were analyzed by mass spectrometry and absolute quantities were calculated using relative abundances of the specific MRM relative to the deuterium labeled internal standard. Values (ng/2×106 cells) are expressed as mean ± S.E. (n = 5; ** p<0.01 and *** p<0.001).
Arachidonic acid release and supplementation of GM-CSF/fMLP treated neutrophils
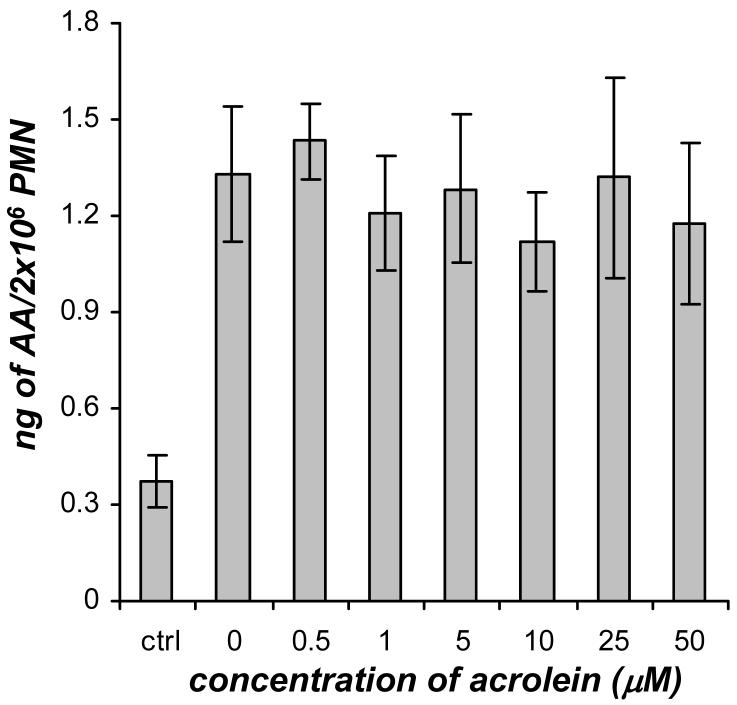
The amount of AA released in GM-CSF/fMLP treated neutrophils in the presence or absence of acrolein was directly quantitated. The dose response of GM-CSF/fMLP treated neutrophils with acrolein did not show a significant change in AA release (Figure 3) over the concentration range of 0-50 μM acrolein. The AA levels in the all of the GM-CSF/fMLP treated cells (with and without acrolein) were higher than the control neutrophils (Figure 3).
Figure 3.
The effect of acrolein (0-50 μM) on AA release in human neutrophils (PMN) primed with GM-CSF (1 nM, 30 min) and stimulated with fMLP (100 nM). The control neutrophils were not treated with GM-CSF or fMLP. The AA present in these samples was analyzed using GC/MS and quantitation was achieved by stable isotope dilution of added internal standards. Values (ng/2×106 cells) are expressed as mean ± S.E. (n = 3).
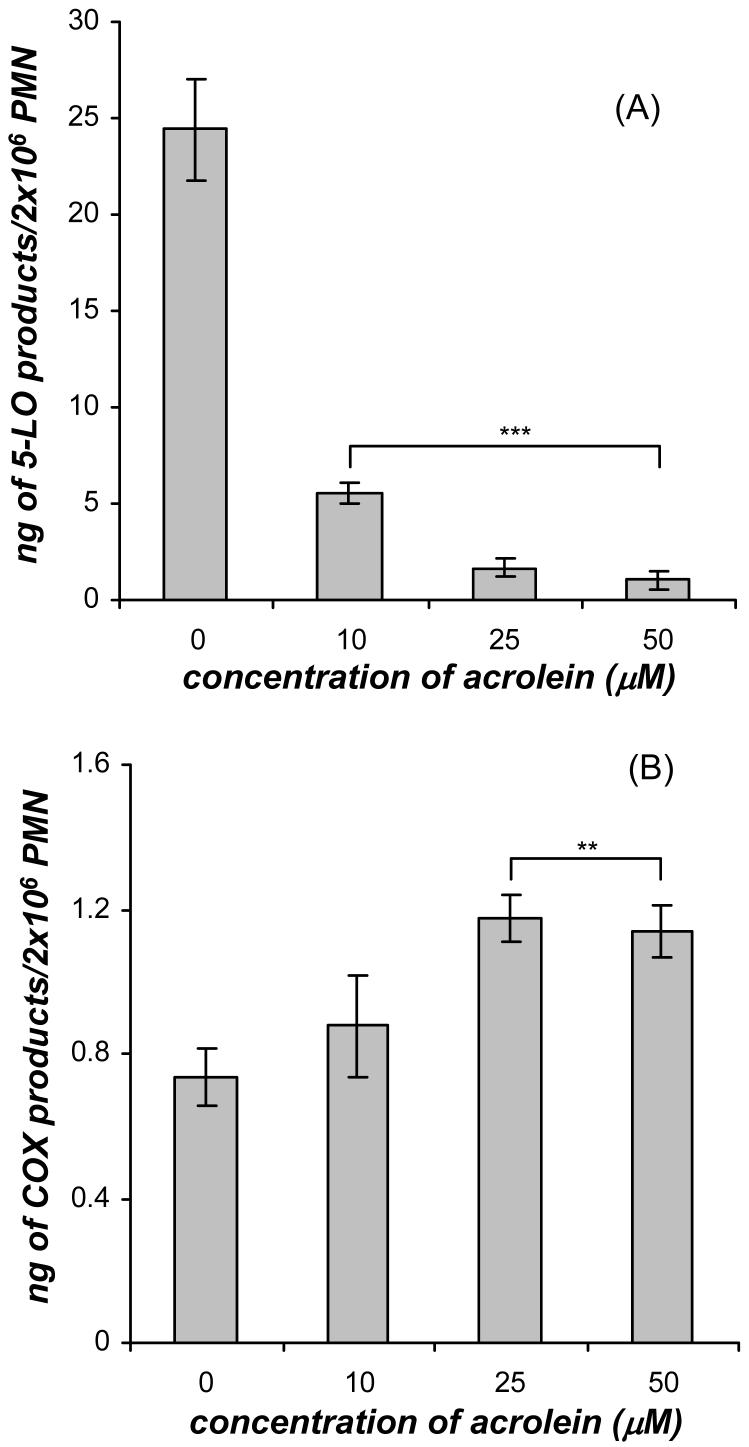
In separate experiments, exogenous AA (0.5 μM) was added to the GM-CSF/acrolein treated neutrophils immediately after the addition of fMLP. AA supplementation of GM-CSF/fMLP treated neutrophils caused the amount of 5-LO products to increase by 5-fold and the amount of COX products to increase by 10-fold compared to GM-CSF/fMLP treated neutrophils without AA supplementation. The supplementation of AA after fMLP addition to GM-CSF primed neutrophils did not alter the inhibition of leukotriene biosynthesis by acrolein and a significant decrease in the amount of 5-LO products in the presence of 10, 25, and 50 μM acrolein was observed compared to the cells not treated with acrolein (Figure 4A). This dose response was very similar to the dose response observed in GM-CSF/acrolein/fMLP treated neutrophils without AA supplementation (Figure 2A). AA supplementation after fMLP addition to GM-CSF primed neutrophils caused a significant increase in the amount of COX products in the presence of 25 and 50 μM acrolein compared to the cells not treated with acrolein (Figure 4B). This response was different from the dose response observed for GM-CSF/fMLP treated cells in the presence of acrolein with no AA supplementation where there was no significant change in the COX products (Figure 2B). The AA supplementation data along with the AA release data for both 5-LO and COX products suggested that the release of arachidonate was not altered by acrolein and the level of free arachidonate could not alter the effect of acrolein.
Figure 4.
Dose response of acrolein (0-50 μM) on the synthesis of (A) 5-LO products (20-OHLTB4, Δ6-trans-LTB4 isomers, LTB4, and 5-HETE) and (B) COX products (PGE2 and TXB2) formed in human neutrophils (PMN) primed with GM-CSF (1 nM, 30 min) and stimulated with fMLP (100 nM) and supplemented with AA (0.5 μM) at 37°C. The GM-CSF primed cells were treated with acrolein for 3 min before fMLP and AA were added. The 5-LO metabolites were analyzed by mass spectrometry and absolute quantities were calculated using relative abundances of the specific MRM relative to the deuterium labeled internal standard. Values (ng/2×106 cells) are expressed as mean ± S.E. (n = 5; ** p<0.01 and *** p<0.001).
Effect of acrolein on intracellular Ca2+
Since calcium plays a crucial role in the activation of enzymes in the leukotriene synthesis pathway, the effect of acrolein on intracellular calcium levels in neutrophils was studied in cells loaded with indo-1 AM and primed with GM-CSF. When fMLP was added in the presence of 0.5 and 5 μM acrolein, their Ca2+ responses were similar to the magnitude of the initial calcium response in the absence of acrolein (Figure 5A). In contrast, when fMLP was added in the presence of 10, 25, 50, and 75 μM acrolein, the initial Ca2+ spike was reduced in a dose-dependent manner compared to the cells without acrolein (Figure 5B). In addition, acrolein reduced the amount of time that it took for the calcium to get back to pre-fMLP levels. The effect of acrolein without fMLP on intracellular calcium was examined and it was determined that acrolein did not elicit a calcium response by itself in indo-1 AM labeled neutrophils. Significant inhibition of 5-LO product formation was observed at 0.5 and 5 μM acrolein (Figure 2A), but there was no effect of acrolein at these concentrations on the release of intracellular calcium stimulated by GM-CSF/fMLP treated neutrophils. Since, the intracellular calcium response was affected by acrolein only at higher concentrations (10, 25, and 50 μM), it was concluded that modulation of calcium signaling came into play only when acrolein is present at 10 μM or greater.
Figure 5.
The effect of acrolein on intracellular calcium was examined in neutrophils loaded with indo-1 AM and primed with GM-CSF (1 nM). The loaded, primed neutrophils were treated with 0, 0.5, and 5 μM acrolein (A) and 0, 10, 25, 50, and 75 μM acrolein (B) for 3 min and then stimulated with fMLP (100 nM). The arrow indicates when fMLP was added. The calcium flux was determined by monitoring the change in FL5/FL4 fluorescence. All calcium responses are representative of five experiments.
Adenosine measurements
The effect of acrolein on extracellular adenosine levels was examined because 5-LO product biosynthesis is known to be regulated by the adenosine receptor and stimulation of cAMP (39). The adenosine released from neutrophils treated with GM-CSF/fMLP was 5.60±0.48 ng/2×106 cells. Additionally, the amount of adenosine released in unstimulated neutrophils was 4.98±0.37 ng/2×106 cells. The levels of adenosine released in GM-CSF/fMLP and unstimulated neutrophils were measured in the presence of 0.5, 1, 5, 10, 25, and 50 μM and there was no change in the adenosine released compared to the GM-CSF/fMLP treated neutrophils reported above (data not shown).
Human recombinant 5-LO activity assay
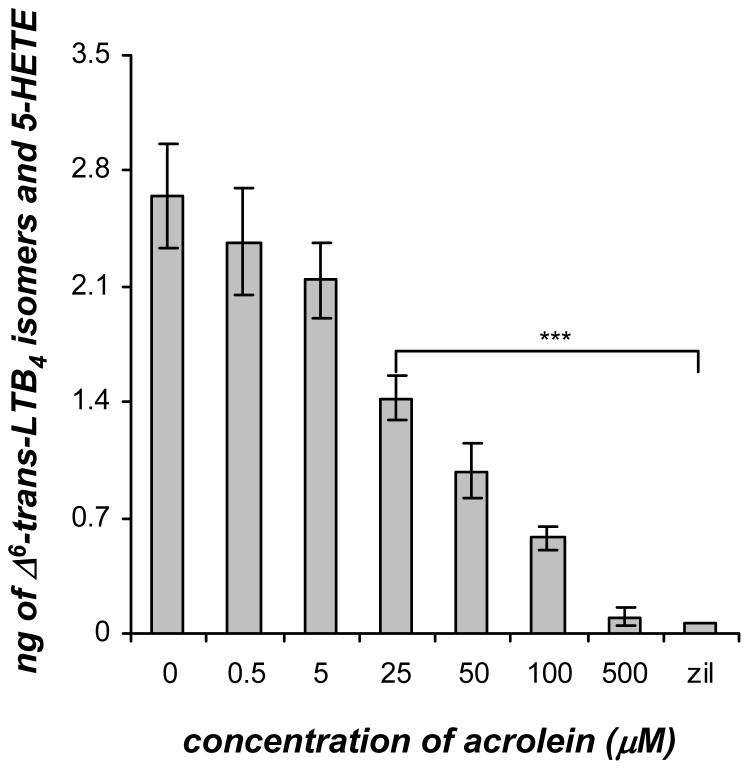
Human recombinant 5-LO was assayed in the presence of acrolein (0-500 μM) to determine whether acrolein could directly inhibit 5-LO activity. Acrolein treatment of recombinant 5-LO significantly decreased the amount of Δ6-trans-LTB4 isomers and 5-HETE in a dose-dependent manner (Figure 6). In the in vitro 5-LO assay without the addition of acrolein, 2.65±0.32 ng of Δ6-trans-LTB4 isomers and 5-HETE were produced. The synthesis of Δ6-trans-LTB4 isomers and 5-HETE was not significantly affected at the doses of 0.5 and 5 μM acrolein. However, the amount of Δ6-trans-LTB4 isomers and 5-HETE were decreased by about half to 1.43±0.13 ng at a dose of 25 μM acrolein. Near complete inhibition of 5-LO was achieved at a dose of 500 μM acrolein. In addition, zileuton was used a positive control for complete inhibition of 5-LO activity. This in vitro recombinant 5-LO assay revealed that acrolein did directly affect 5-LO activity but the dose response of the inhibition of 5-LO product formation by acrolein was shifted compared to that observed in neutrophils (Figure 2A).
Figure 6.
Dose response of acrolein (0-500 μM) on the synthesis of Δ6-trans-LTB4 isomers and 5-HETE formed in a human recombinant 5-LO enzymatic assay. Human recombinant 5-LO (0.7 units) was incubated with ATP (2 mM) and acrolein (0-500 μM) for 3 min or zileuton (0.5 μM) for 15 min. After the preincubation, AA (0.2 μM) and Ca2+ (2 mM) were added and allowed to react for 10 min. The 5-LO metabolites were analyzed by mass spectrometry and absolute quantities were calculated using relative abundances of the specific MRM relative to the deuterium labeled internal standard. Values are expressed as mean ± S.E. (n = 3; *** p<0.001).
While some factors such as direct inhibition of 5-LO activity and reduction of the initial intracellular calcium spike are affected by acrolein, the doses of this agent required to inhibit either process were higher doses than those observed to cause a significant inhibition of 5-LO product formation in GM-CSF/fMLP treated neutrophils (Figure 2A). For this reason it was thought that other factors might contribute to the almost complete inhibition of 5-LO product formation in GM-CSF/fMLP neutrophils treated with acrolein (≥25 μM), such as the signaling pathways of GM-CSF and fMLP.
Time course of acrolein addition into GM-CSF/fMLP treated cells
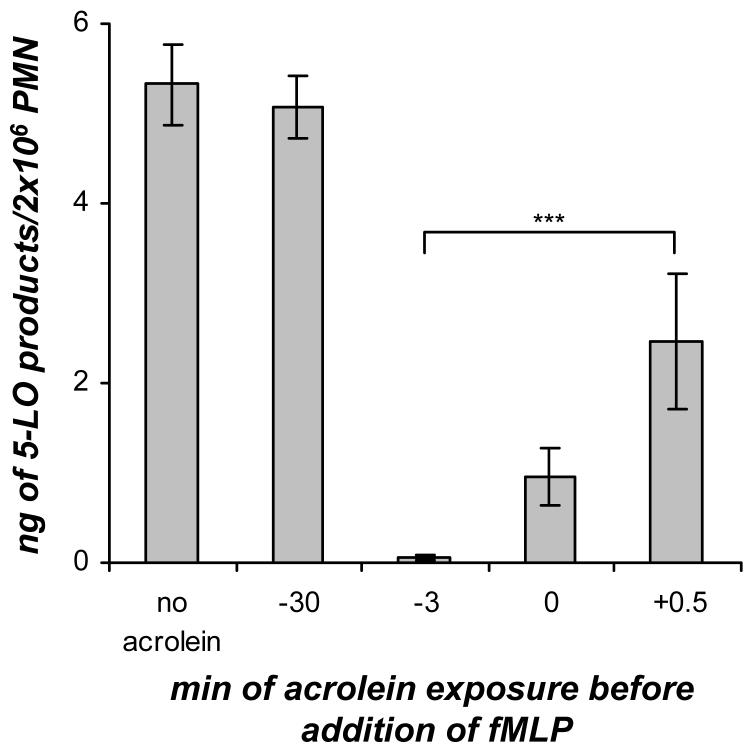
In order to determine whether acrolein affects the priming or the stimulation events when added to GM-CSF/fMLP treated neutrophils, the time-dependent effect of acrolein (25 μM) on 5-LO product formation in GM-CSF/fMLP treated neutrophils was examined (Figure 7). For this time course the acrolein was either added to the cells at the same time as GM-CSF or at some point after the 30 min incubation with GM-CSF. The time course revealed that the addition of acrolein at the same time as GM-CSF (-30 min) did not have any effect on 5-LO product formation compared to the GM-CSF/fMLP treated neutrophils with no acrolein added. From previous studies, it was found that acrolein (≤30 μM) rapidly disappears as soon as 3 min after being added to cells (10). Therefore, no acrolein remained when fMLP was added after the 30 min of incubation of human neutrophils with GM-CSF and acrolein. When acrolein was added to GM-CSF primed cells 3 min before fMLP addition (-3 min), at the same time as fMLP (0 min), or 0.5 min after fMLP (+0.5 min), a significant inhibition of 5-LO products was observed compared to GM-CSF/fMLP treated cells without acrolein added. This time course (Figure 7) suggested that the addition of acrolein (25 μM) had an effect on the fMLP signaling pathway in GM-CSF/fMLP treated human neutrophils and that it did not interrupt the GM-CSF priming.
Figure 7.
Time course of acrolein (25 μM) addition to GM-CSF/fMLP treated human neutrophils (PMN). The acrolein was either added at the same time as GM-CSF (-30 min) or at some point after the 30 min incubation with GM-CSF. For those time course points where the acrolein was added after the 30 min GM-CSF priming event, the acrolein was added either 3 min before fMLP (-3 min), at the same time as fMLP addition (0 min), or 30 s after fMLP addition (+0.5 min). Values are expressed as mean + S.E. (n = 5; *** p<0.001).
Eicosanoid production and acrolein in neutrophils with PAF/fMLP, GM-CSF/PAF, opsonized zymosan, and A23187
PAF/fMLP, GM-CSF/PAF, OPZ, and A23187 were each used as a stimulus of the 5-LO pathway in neutrophils in order to determine whether the effect of acrolein on 5-LO product formation neutrophils is stimulus specific (Table 1). For these studies the acrolein was added after the priming was completed and 3 min before the stimulus was added. As mentioned above, GM-CSF/fMLP treated neutrophils produced 5-LO products which were substantially decreased when acrolein (25 μM) was present. When the PAF/fMLP stimulus was used with neutrophils, very small amounts of 5-LO products were detected compared to the GM-CSF/fMLP stimulus and these products were not diminished significantly in the presence of acrolein (25 μM) (Table 1). Using the GM-CSF/PAF stimulus with neutrophils, an increase in 5-LO products were observed compared to the GM-CSF/fMLP stimulus but these products were not diminished by the addition of acrolein (25 μM). Additionally, phagocytosis of OPZ was used as a stimulus of neutrophils for eicosanoid production and the 5-LO products were only slightly diminished by exposure of the neutrophils to acrolein (25 μM). Additional experiments revealed that the phagocytic ability of neutrophils was not altered by acrolein (data not shown). When A23187 was used as a stimulus of eicosanoid production, a significant increase in 5-LO products were observed compared to the GM-CSF/fMLP stimulus and these 5-LO products did decrease by about half when acrolein (25 μM) was added 3 min prior to A23187. These results suggested that the effect of acrolein on eicosanoid synthesis was dependent on the exact method of stimulation and possibly the signal transduction pathway engaged by the GM-CSF/fMLP stimulated neutrophils was uniquely affected by acrolein.
Table 1.
5-LO product synthesis in human neutrophils
| Treatment | 5-LO products* (ng/2×106 cells) | 5-LO products* (ng/2×106 cells) with 25μM acrolein |
|---|---|---|
| GM-CSF/fMLP | 5.32 ± 0.45 | 0.12 ± 0.03 |
| PAF/fMLP | 0.66 ± 0.04 | 0.55 ± 0.14 |
| GM-CSF/PAF | 7.79 ± 0.73 | 7.24 ± 0.51 |
| OPZ | 4.19 ± 0.41 | 3.63 ± 0.37 |
| A23187 |
48.13 ± 6.03 |
21.66 ± 3.42 |
Values (ng/2×106 cells) are expressed as mean ± S.E. (n = 5).
5-LO products include 20-OH-LTB4, Δ6-trans-LTB4 isomers, LTB4, and 5-HETE.
Effect of acrolein on superoxide generation
NADPH oxidase activation was examined in order to determine if this basic neutrophil function is affected by acrolein. The superoxide release from neutrophils was monitored using cytochrome c reduction and acrolein (1, 5, 10, and 25 μM) was found to inhibit GM-CSF/fMLP and PAF/fMLP stimulated neutrophils (Table 2). The superoxide released in GM-CSF/fMLP and PAF/fMLP treated neutrophils without acrolein was quite similar. The superoxide release using both of these stimuli was affected by the addition of 1 and 5 μM acrolein in a dose dependent manner. At doses of 10 μM or higher of acrolein, the neutrophil respiratory burst was completely inhibited to control levels (0.319±0.023 pmol/106 cells/10 min of O2) in neutrophils treated with GM-CSF/fMLP and PAF/fMLP. In neutrophils treated with acrolein (1-25 μM) alone, superoxide levels were similar to control neutrophils. These results agree well with previously published results on superoxide production in unprimed, fMLP treated neutrophils with acrolein (24).
Table 2.
Superoxide release from human neutrophils
| Acrolein concentration | O2- production in GM-CSF/fMLP PMN (pmol/106 cells/10 min) | O2- production in PAF/fMLP PMN (pmol/106 cells/10 min) |
|---|---|---|
| 0 μM | 6.94 ± 1.52 | 6.19 ± 1.63 |
| 1 μM | 4.41 ± 1.24 | 3.99 ± 1.32 |
| 5 μM | 2.36 ± 1.15 | 2.19 ± 0.85 |
| 10 μM | 0.24 ± 0.05 | 0.31 ± 0.07 |
| 25 μM |
0.27 ± 0.03 |
0.26 ± 0.04 |
Values (ng/2×106 cells) are expressed as mean ± S.E. (n = 3).
DISCUSSION
Acrolein is known to be a highly reactive and toxic α,β-unsaturated aldehyde produced by various mechanisms including burning of tobacco which results in high concentrations of this compound in cigarette smoke. Estimations of the concentration of acrolein that can accumulate in the epithelial lining fluid in the bronchial airway of smokers can be as high as 50-80 μM (14). While these estimates are based upon model systems, it is clear that acrolein can be present in very high concentrations exposed to cells as well as fluids in the lung. Acrolein is also known to be highly reactive for proteins (6) and lipids (10), resulting in the covalent binding of acrolein either through Schiff base or Michael addition reactions to target molecules. It has been suggested that such adducts may play an important role in the toxicity of acrolein.
This work focused on the unexpected finding that acrolein at concentrations relevant to that found in the human lung airway fluid of a cigarette smoker can cause profound inhibition of the ability of the GM-CSF/fMLP stimulated polymorphonuclear leukocyte to produce leukotriene B4. While the expression of cyclooxygenase in neutrophils is somewhat low (40), the very sensitive and accurate means to assess the level of both cyclooxygenase and lipoxygenase products used in this study revealed that cyclooxygenase products were not diminished by acrolein despite the profound inhibition of leukotriene products at doses of acrolein between 10-50 μM exposed to the isolated GM-CSF/fMLP treated human neutrophil. This potent inhibition of 5-LO product formation was unique to GM-CSF/fMLP neutrophils treated with acrolein. Other biologically relevant stimuli that activate the 5-LO pathway in neutrophils (PAF/fMLP, GM-CSF/PAF, and OPZ) were tested and acrolein did not effect the 5-LO product formation. It can be concluded that the inhibition on 5-LO product formation in GM-CSF/fMLP neutrophils treated with acrolein could not be simply attributed to covalent modification of the priming agent (GM-CSF) or the stimulant (fMLP). Inhibition of 5-LO product formation was not observed with the PAF/fMLP and GM-CSF/PAF treated neutrophils exposed to acrolein (25 μM) and the activity of either GM-CSF or fMLP was not altered when acrolein was added directly to these compounds prior to neutrophil treatment. Additionally, neutrophils that were pre-incubated with acrolein and then treated with the robust stimulus, A23187, showed an inhibition of 5-LO product formation by about half compared to the A23187 treated cells. While acrolein is known to be cytotoxic (17-19), concentrations employed in our study (25 μM) have been reported not to be cytotoxic in HL-60 cells after 30 min exposure and in human neutrophils for up to 4 h at 50 μM (41).
The biologically relevant stimulus used to activate neutrophils in this study that was susceptible to acrolein treatment was GM-CSF priming followed by fMLP receptor activation. Cigarette smoke is known to greatly enhance the expression of GM-CSF (42) and this cytokine has a multitude of biological effects including the priming of the neutrophil for arachidonic acid metabolism by 5-LO (38). While the exact mechanism of GM-CSF priming of neutrophils is not entirely clear, it is also known that GM-CSF primes neutrophils to increase their production of superoxide anion (43). Cigarette smoke is known to cause neutrophils to accumulate in the bronchial airways in the chronic smoker as well elevate fMLP receptor numbers expressed by neutrophils (44,45). Even at fairly low concentrations of acrolein (1-5 μM) our studies found a significant reduction in the ability of isolated neutrophils to generate LTB4 by GM-CSF/fMLP stimulation.
The mechanism by which the 5-LO pathway generates LTB4 is known to be quite complex and has been the subject of many studies and reviews (30,46). Briefly, cytosolic 5-LO as well as phospholipase A2 (Group IV) are translocated to the nuclear membrane where arachidonic acid is released, presented to 5-LO, and converted into LTA4. This LTA4 is either released from the neutrophil or metabolized by LTA4 hydrolase into the biologically active LTB4. LTB4 is known to be a potent chemotactic factor for the human neutrophil through action of the BLT1 receptor (47). The inhibition of this pathway by acrolein could have profound effects on the ability to recruit neutrophils, but more importantly for them to release leukotrienes following activation. Both LTB4 as well as LTC4, which can result from transcellular biosynthesis and cell-cell cooperation (48), are known to have profound effects in the lung, and in particular cysteinyl leukotrienes have been suggested to be important mediators in asthma (49).
The mechanism by which 5-LO is inhibited by acrolein is also complicated by the 5-LO activation process itself. Stimulation of neutrophils for 5-LO activation results in elevation of intracellular free calcium levels, which were found to be decreased by acrolein in GM-CSF/fMLP cells. However, this decrease in intracellular calcium levels was only observed at acrolein concentrations of 10 μM or greater, which did not coincide well with the inhibition of 5-LO product formation observed in GM-CSF/fMLP treated neutrophils where significant inhibition was observed at 1 μM acrolein. While it is thought that the decrease in intracellular concentration of calcium might play some role in the inhibition of 5-LO product formation, most likely additional factors work in tandem to contribute to the almost complete inhibition of 5-LO product formation in GM-CSF/fMLP neutrophils when acrolein is present.
In these studies it was determined that recombinant 5-LO was also inhibited by acrolein, which was consistent with acrolein covalent binding to the 5-LO protein. Previous studies of apolipoprotein A-I and thioredoxin-1 have demonstrated that covalent modification of certain amino acid residues of these proteins causes inhibition of enzymatic activity (50,51). While the direct covalent binding of acrolein to 5-LO is under current investigation, it is clear that the exposure of 5-LO to acrolein reduces the ability of 5-LO to oxygenate arachidonic acid. However, the doses of acrolein required to alter 5-LO recombinant activity were higher than those needed to observe a significant inhibition of 5-LO product formation. While the direct covalent binding of acrolein to 5-LO might occur to some extent in neutrophils treated with acrolein, it is not thought that this alone is the main mechanism of action of acrolein in the inhibition of 5-LO activity. Furthermore, if covalent binding to 5-LO was a major process, leukotriene production would be inhibited independent of the type of stimulation used for leukotriene production in neutrophils.
A necessary component for 5-LO activation in cells is 5-LO activating protein (FLAP). FLAP interacts with 5-LO on the nuclear membrane in cells and is necessary for leukotriene synthesis. It can not be ruled out that acrolein does directly affect FLAP, but it is not thought that the inhibition of 5-LO activity in neutrophils is due to a direct effect on FLAP because leukotriene production would be inhibited independent of the stimulus used. Therefore, the effect of acrolein on FLAP was not examined.
In addition to decreasing intracellular calcium and covalent modification of 5-LO, it also appeared that signaling events may be altered by acrolein. This is evident in the PAF/fMLP, OPZ, and GM-CSF/PAF results where the formation of 5-LO products was not altered by the addition of acrolein (25 μM) 3 min before stimulation, even though almost complete inhibition of 5-LO product formation was observed in GM-CSF/fMLP treated cells with acrolein (25 μM) added 3 min before fMLP stimulation. Additionally, the superoxide generation in GM-CSF/fMLP and PAF/fMLP treated neutrophils in the presence of acrolein decreased with both of these stimuli with a similar dose response, even though acrolein did not inactivate 5-LO during PAF/fMLP stimulation. Finally, a time course of acrolein addition to GM-CSF/fMLP treated neutrophils indicated that the GM-CSF priming itself was not affected by acrolein but the fMLP stimulation event was affected by acrolein. This time course data in conjunction with the PAF/fMLP and GM-CSF/PAF data and superoxide data suggested that the GM-CSF/fMLP 5-LO signaling pathway was uniquely affected by acrolein and that the signaling through the fMLP receptor after GM-CSF priming of the neutrophil was profoundly effected by acrolein. These complex series of events that occur during priming and stimulation make it difficult to ascertain a single mechanism by which acrolein causes inactivation of 5-LO, but this is not unexpected when considering the complexity of events which are required for LTB4 to be synthesized and the potential chemical reactivity of acrolein. Therefore, it is thought that all of these factors mentioned above might contribute to some extent to the inhibition of 5-LO product formation observed in neutrophils with certain stimuli in the presence of acrolein.
There has been previous evidence to suggest that cigarette smoke either directly or indirectly can effect the production of LTB4 in humans and animal models. The activation of alveolar macrophages isolated from bronchoalveolar lavage fluid from human subjects revealed a significant reduction in the ability of cells from smokers to convert exogenously added arachidonic acid into LTB4 compared to non-smokers (52). Furthermore, the BAL fluid isolated from animals which was exposed to chronic cigarette smoke had significantly less LTB4 when compared to LTB4 concentrations present in rats not exposed to cigarette smoke (53). In some cases, up to 10-fold reduction in the presence of LTB4 detected in the BAL fluid was observed. Furthermore, it is interesting to point out that a large percentage of human asthmatics continue to smoke in spite of the irritant effect of cigarette smoke on airways (54). While anecdotal evidence suggests that for these individuals cigarette smoking improves their asthmatic condition, it is possible that the cigarette smoke is reducing leukotriene generation in these subjects. However, it is important to point out that there has been no direct evidence for this in vivo effect of acrolein specifically on the 5-LO lipid mediator pathway.
In summary, acrolein is a highly reactive and toxic α/β-unsaturated aldehyde which is known to have pleiotropic effects both as a reactive oxygen species that can covalently bind to target molecules, but also a toxicant present in high concentrations of cigarette smoke that can inhibit the 5-LO pathway when human neutrophils are stimulated with GM-CSF and fMLP. These results might explain previous studies on the effect of cigarette smoke on inhibiting LTB4 production in both human and animal airways.
ACKNOWLEDGEMENTS
We thank Charis Uhlson for GC/MS analysis of free fatty acids. This work was supported by a grant from the National Institutes of Health (HL034303).
Abbreviations
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- fMLP
formyl-methionyl-leucyl-phenylalanine
- FLAP
5-lipoxygenase activating protein
- 5-LO
5-lipoxygenase
- COX
cyclooxygenase
- OPZ
opsonized zymosan
- PAF
platelet-activating factor
- HBSS
Hanks' balanced salt solution (1X) with Ca2+ and Mg22+
- HBSS=
Hanks' balanced salt solution (1X) without Ca2+ and Mg2+
- PMN
polymorphonuclear neutrophil
REFERENCES
- 1.Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol. Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- 2.Aldini G, Dalle-Donne I, Facino RM, Milzani A, Carini M. Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls. Med. Res. Rev. 2007;27:817–868. doi: 10.1002/med.20073. [DOI] [PubMed] [Google Scholar]
- 3.Fujioka K, Shibamoto T. Determination of toxic carbonyl compounds in cigarette smoke. Environ. Toxicol. 2006;21:47–54. doi: 10.1002/tox.20153. [DOI] [PubMed] [Google Scholar]
- 4.Umano K, Shibamoto T. Analysis of acrolein from heated cooking oils and beef fat. J. Agric. Food Chem. 1987;35:909–912. [Google Scholar]
- 5.Uchida K, Kanematsu M, Morimitsu Y, Osawa T, Noguchi N, Niki E. Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J. Biol. Chem. 1998;273:16058–16066. doi: 10.1074/jbc.273.26.16058. [DOI] [PubMed] [Google Scholar]
- 6.Anderson MM, Hazen S, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxyl-amino acids into glycoaldehyde, 2-hydroxypropanal, and acrolein. J. Clin. Invest. 1997;99:424–432. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel JM. Metabolism and pulmonary toxicity of cyclophosphamide. Pharmacol. Ther. 1990;47:137–146. doi: 10.1016/0163-7258(90)90049-8. [DOI] [PubMed] [Google Scholar]
- 8.Chung FL, Young R, Hecht SS. Formation of cyclic 1,N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res. 1984;44:990–995. [PubMed] [Google Scholar]
- 9.Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, Suzuki D, Miyata T, Noguchi N, Niki E, Osawa T. Protein-bound acrolein: potential markers for oxidative stress. Proc. Natl. Acad. Sci. 1998;95:4882–4887. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zemski Berry KA, Murphy RC. Characterization of acroleinglycerophosphoethanolamine lipid adducts using electrospray mass spectrometry. Chem. Res. Toxicol. 2007;20:1342–1351. doi: 10.1021/tx700102n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van der Toorn M, Smit-de Vries MP, Slebos DJ, de Bruin HG, Abello N, van Oosterhout AJM, Bischoff R, Kauffman HF. Cigarette smoke irreversibly modifies glutathione in airway epithelial cells. Am. J. Physiol. Lung Cell Mol Physiol. 2007;293:L1156–L1162. doi: 10.1152/ajplung.00081.2007. [DOI] [PubMed] [Google Scholar]
- 12.Ouyang Y, Virasch N, Hao P, Aubrey MT, Mukerjee N, Bierer BE, Freed BM. Suppression of human IL-1β, IL-2, IFN-γ, and TNF-α production by cigarette smoke extracts. J. Allergy Clin. Immunol. 2000;106:280–287. doi: 10.1067/mai.2000.107751. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Hamilton RF, Taylor DE, Holian A. Acrolein-induced cell death in human alveolar macrophages. Toxicol. Appl. Pharmacol. 1997;145:331–339. doi: 10.1006/taap.1997.8189. [DOI] [PubMed] [Google Scholar]
- 14.Lambert C, McCue J, Portas M, Ouyang Y, Li J, Rosano TG, Lazis A, Freed BM. Acrolein in cigarette smoke inhibits T-cell responses. J. Allergy Clin. Immunol. 2005;116:916–922. doi: 10.1016/j.jaci.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 15.Biswal S, Acquaah-Mensah G, Datta K, Wu X, Kehrer JP. Inhibition of cell proliferation and AP-1 activity by acrolein in human A549 lung adenocarcinoma cells due to thiol imbalance and covalent modifications. Chem. Res. Toxicol. 2002;15:180–186. doi: 10.1021/tx015552p. [DOI] [PubMed] [Google Scholar]
- 16.Li L, Hamilton RF, Holian A. Effect of acrolein on human alveolar macrophage NF-κB activity. Am. J. Physiol. Lung Cell Mol Physiol. 1999;277:L550–L557. doi: 10.1152/ajplung.1999.277.3.L550. [DOI] [PubMed] [Google Scholar]
- 17.Nardini M, Finkelstein EI, Reddy S, Valacchi G, Traber M, Cross CE, van der Vliet A. Acrolein-induced cytotoxicity in cultured human bronchial epithelial cells. Modulation by alpha-tocopherol and ascorbic acid. Toxicology. 2002;170:173–185. doi: 10.1016/s0300-483x(01)00540-6. [DOI] [PubMed] [Google Scholar]
- 18.Takeuchi K, Kato M, Suzuki H, Akhand AA, Wu J, Hossain K, Miyata T, Matsumoto Y, Nimura Y, Nakashima I. Acrolein induces activation of the epidermal growth factor receptor of human keratinocytes for cell death. J. Cell. Biochem. 2001;81:679–688. doi: 10.1002/jcb.1105. [DOI] [PubMed] [Google Scholar]
- 19.Tanel T, Averill-Bates DA. Activation of the death receptor pathway of apoptosis by the aldehyde acrolein. Free Radic. Biol. Med. 2007;42:798–810. doi: 10.1016/j.freeradbiomed.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Ranganna K, Yousefipour Z, Nasif R, Yatsu FM, Milton SG, Hayes BE. Acrolein activates mitogen-activated protein kinase signal transduction pathways in rat vascular smooth muscle cells. Mol. Cell. Biochem. 2002;240:83–98. doi: 10.1023/a:1020659808981. [DOI] [PubMed] [Google Scholar]
- 21.Seiner DR, LaButti JN, Gates KS. Kinetics and mechanism of protein tyrosine phosphatase 1B inactivation by acrolein. Chem. Res. Toxicol. 2007;20:1315–1320. doi: 10.1021/tx700213s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borchers MT, Carty MP, Leikauf GD. Regulation of human airway mucins by acrolein and inflammatory mediators. Am. J. Physiol. Lung Cell Mol Physiol. 1999;276:L549–L555. doi: 10.1152/ajplung.1999.276.4.L549. [DOI] [PubMed] [Google Scholar]
- 23.Barnes PJ. Chronic obstructive pulmonary disease. N. Eng. J. Med. 2000;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen H, Finkelstein E, Reznick A, Cross C, van der Vliet A. Cigarette smoke impairs neutrophil respiratory burst activation by aldehyde-induced thiol modifications. Toxicology. 2001;160:207–217. doi: 10.1016/s0300-483x(00)00450-9. [DOI] [PubMed] [Google Scholar]
- 25.Witz G, Lawrie NJ, Amoruso MA, Goldstein BD. Inhibition by reactive aldehydes of superoxide anion radical production from stimulated polymorphonuclear leukocytes and pulmonary alveolar macrophages. Effects on cellular sulfhydryl groups and NADPH oxidase activity. Biochem. Pharmacol. 1987;36:721–726. doi: 10.1016/0006-2952(87)90725-8. [DOI] [PubMed] [Google Scholar]
- 26.Leslie CC. Regulation of arachidonic acid availability for eicosanoid production. Biochem. Cell Biol. 2004;82:1–17. doi: 10.1139/o03-080. [DOI] [PubMed] [Google Scholar]
- 27.Rouzer CA, Matsumoto T, Samuelsson B. Single protein from human leukocytes possesses 5-lipoxygenase and leukotriene A4 synthase activities. Proc. Natl. Acad. Sci. 1986;83:857–861. doi: 10.1073/pnas.83.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy RC, Gijón MA. Biosynthesis and metabolism of leukotrienes. Biochem. J. 2007;405:379–395. doi: 10.1042/BJ20070289. [DOI] [PubMed] [Google Scholar]
- 29.Haeggstrom JZ. Leukotriene A4 hydrolase/aminopeptidase, the gatekeeper of chemotactic leukotriene B4 biosynthesis. J. Biol. Chem. 2004;279:50639–50642. doi: 10.1074/jbc.R400027200. [DOI] [PubMed] [Google Scholar]
- 30.Kikuta Y, Kusunose E, Kusunose M. Prostaglandin and leukotriene omegahydroxylases. Prostaglandins Other Lipid Mediat. 2002;68:345–362. doi: 10.1016/s0090-6980(02)00039-4. [DOI] [PubMed] [Google Scholar]
- 31.Haslett C, Guthrie LA, Kopaniak M, Johnston RB, Jr., Henson PM. Modulation of multiple neutrophil functions by preparative methods or trace concentrations of bacterial lipopolysaccharide. Am. J. Pathol. 1985;119:101–110. [PMC free article] [PubMed] [Google Scholar]
- 32.Lynch JM, Henson PM. The intracellular retention of newly synthesized platelet-activating factor. J. Immunol. 1986;137:2653–2661. [PubMed] [Google Scholar]
- 33.Hall LM, Murphy RC. Electrospray mass spectrometric analysis of 5-hydroperoxy and 5-hydroxyeicosatetraenoic acids generated by lipid peroxidation of red blood cell ghost phospholipids. J. Am. Soc. Mass Spectrom. 1998;9:527–532. doi: 10.1016/S1044-0305(98)00013-0. [DOI] [PubMed] [Google Scholar]
- 34.Cadieux J-S, Leclerc P, St-Onge M, Dussault AA, Laflamme C, Picard S, Ledent C, Borgeat P, Pouliot M. Potentiation of neutrophil cyclooxygenase-2 by adenosine: an early anti-inflammatory signal. J. Cell. Sci. 2005;118:1437–1447. doi: 10.1242/jcs.01737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fiedler J, Wheelan P, Henson PM, Murphy RC. Exogenous leukotriene B4 (LTB4) inhibits human neutrophil generation of LTB4 from endogenous arachidonic acid during opsonized zymosan phagocytosis. J. Pharmacol. Exp. Ther. 1998;287:150–156. [PubMed] [Google Scholar]
- 36.Zarini S, Gijón MA, Folco G, Murphy RC. Effect of arachidonic acid reacylation on leukotriene biosynthesis in human neutrophils stimulated with granulocyte macrophage colony-stimulating factor and formyl-methionyl-leucyl-phenylalanine. J. Biol. Chem. 2006;281:10134–10142. doi: 10.1074/jbc.M510783200. [DOI] [PubMed] [Google Scholar]
- 37.Park YS, Kim J, Misonou Y, Takamiya R, Takahashi M, Freeman MR, Taniguchi N. Acrolein induces cyclooxygenase-2 and prostaglandin production in human umbilical vein endothelial cells: roles of p38 MAP kinase. Arterioscler. Throm. Vasc. Biol. 2007;27:1319–1325. doi: 10.1161/ATVBAHA.106.132837. [DOI] [PubMed] [Google Scholar]
- 38.Doupnik CA, Leikauf GD. Acrolein stimulates eicosanoid release from bovine airway epithelial cells. Am. J. Physiol. Lung Cell Mol Physiol. 1990;259:L222–L229. doi: 10.1152/ajplung.1990.259.4.L222. [DOI] [PubMed] [Google Scholar]
- 39.Flamand N, Boudreault S, Picard S, Austin M, Surette ME, Plante H, Krump E, Vallée M-J, Gilbert C, Naccache P, Laviolette M, Borgeat P. Adenosine, a potent natural suppressor of arachidonic acid release and leukotriene biosynthesis in human neutrophils. Am. J. Respir. Crit. Care Med. 2000;161:S88–S94. doi: 10.1164/ajrccm.161.supplement_1.ltta-18. [DOI] [PubMed] [Google Scholar]
- 40.Maloney CG, Kutchera WA, Albertine KH, McIntyre TM, Prescott SM, Zimmerman GA. Inflammatory agonists induce cyclooxygenase type 2 expression by human neutrophils. J. Immunol. 1998;160:1402–1410. [PubMed] [Google Scholar]
- 41.Finkelstein EI, Nardini M, van der Vliet A. Inhibition of neutrophil apoptosis by acrolein: a mechanism of tobacco-related lung disease? Am. J. Physiol. Lung Cell Mol. Physiol. 2001;281:L732–L739. doi: 10.1152/ajplung.2001.281.3.L732. [DOI] [PubMed] [Google Scholar]
- 42.Vlahos R, Bozinovski S, Hamilton JA, Anderson GP. Therapeutic potential of treating chronic obstructive pulmonary disease (COPD) by neutralising granulocyte macrophage-colony stimulating factor (GM-CSF) Pharmacol. Ther. 2006;112:106–115. doi: 10.1016/j.pharmthera.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Dang PM, Dewas C, Gaudry M, Fay M, Pedruzzi E, Gougerot-Pocidalo MA, El Benna J. Priming of human neutrophil respiratory burst by granulocyte/macrophage colony-stimulating factor (GM-CSF) involves partial phosphorylation of p47(phox) J. Biol. Chem. 1999;274:20704–20708. doi: 10.1074/jbc.274.29.20704. [DOI] [PubMed] [Google Scholar]
- 44.Matheson M, Rynell A-C, McClean M, Berend N. Cigarette smoking increases neutrophil formyl methionyl leucyl phenylalanine receptor numbers. Chest. 2003;123:1642–1646. doi: 10.1378/chest.123.5.1642. [DOI] [PubMed] [Google Scholar]
- 45.Hunninghake GW, Crystal RG. Cigarette smoking and lung destruction. Accumulation of neutrophils in the lungs of cigarette smokers. Am. Rev. Respir. Dis. 1983;128:833–838. doi: 10.1164/arrd.1983.128.5.833. [DOI] [PubMed] [Google Scholar]
- 46.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 47.Haribabu B, Verghese MW, Steeber DA, Sellars DD, Bock CB, Snyderman R. Targeted disruption of the leukotriene B4 receptor in mice reveals its role in inflammation and platelet-activating factor-induced anaphylaxis. J. Exp. Med. 2000;192:433–438. doi: 10.1084/jem.192.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Folco G, Murphy RC. Eicosanoid transcellular biosynthesis: From cell-cell interactions to in vivo tissue responses. Pharmacol. Rev. 2006;58:375–388. doi: 10.1124/pr.58.3.8. [DOI] [PubMed] [Google Scholar]
- 49.Capra V, Thompson MD, Sala A, Cole DE, Folco G, Rovati GE. Cysteinyl-leukotrienes and their receptors in asthma and other inflammatory diseases: critical update and emerging trends. Med. Res. Rev. 2007;27:469–527. doi: 10.1002/med.20071. [DOI] [PubMed] [Google Scholar]
- 50.Shao B, Fu X, McDonald TO, Green PS, Uchida K, O'Brien KD, Oram JF, Heinecke JW. Acrolein impairs ATP binding cassette transporter A1-dependent cholesterol export from cells through site-specific modification of apolipoprotein A-1. J. Biol. Chem. 2005;280:36386–36396. doi: 10.1074/jbc.M508169200. [DOI] [PubMed] [Google Scholar]
- 51.Go YM, Halvey PJ, Hansen JM, Reed M, Phol J, Jones DP. Reactive aldehyde modification of thioredoxin-1 activates early steps of inflammation and cell adhesion. Am. J. Pathol. 2007;171:1670–1681. doi: 10.2353/ajpath.2007.070218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laviolette M, Coulombe R, Picard S, Braquet P, Borgeat P. Decreased leukotriene B4 synthesis in smokers' alveolar macrophages in vitro. J. Clin. Invest. 1986;77:54–60. doi: 10.1172/JCI112301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mobley A, Tanizawa H, Iwanaga T, Tai CL, Tai HH. Selective inhibition of 5-lipoxygenase pathway in rat pulmonary alveolar macrophages by cigarette smoking. Biochim. Biophys. Acta. 1987;918:115–119. doi: 10.1016/0005-2760(87)90185-8. [DOI] [PubMed] [Google Scholar]
- 54.Silverman RA, Boudreaux ED, Woodruff PG, Clark S, Camargo CA., Jr. Cigarette smoking among asthmatic adults presenting to 64 emergency departments. Chest. 2003;23:1472–1479. doi: 10.1378/chest.123.5.1472. [DOI] [PubMed] [Google Scholar]