Abstract
We describe a novel class of light-triggerable liposomes prepared from a photo-polymerizable phospholipid DC8,9PC (1,2- bis (tricosa-10,12-diynoyl)-sn-glycero-3-phosphocholine) and DPPC (1,2-Dipalmitoyl-sn-Glycero-3-Phosphocholine). Exposure to UV (254 nm) radiation for 0–45 minutes at 25°C resulted in photo-polymerization of DC8,9PC in these liposomes and the release of an encapsulated fluorescent dye (calcein). Kinetics and extents of calcein release correlated with mol% of DC8,9PC in the liposomes. Photopolymerization and calcein release occurred only from DPPC/DC8,9PC but not from Egg PC/DC8,9PC liposomes. Our data indicate that phase separation and packing of polymerizable lipids in the liposome bilayer are major determinants of photo-activation and triggered contents release.
Keywords: polymerizable lipids, lipid packing, triggered drug release, diacetylene phospholipids, light-sensitive liposomes, lipid modification, phase separation
1. Introduction
Our understanding of domain formation in biological membranes has been furthered by studies with lipid model membranes. Domain formation has been observed in multi-component lipid bilayer mixtures with compositions that give rise to temperature-dependent lateral separation between solid and liquid-ordered phases in the plane of the membrane [1]. It has been widely reported that phase boundary defects will lower the membrane permeability barrier [2]. This feature has been exploited to construct liposomes with temperature-dependent triggered release properties [3]. In this paper we have pursued an alternative approach to create phase boundary defects in lipid model membranes using mixtures of DPPC and a photo-polymerizable phospholipid, DC8,9PC, which bears highly reactive diacetylenic groups that can be polymerized by UV irradiation to form chains of covalently linked lipid molecules in the bilayer [4,5].
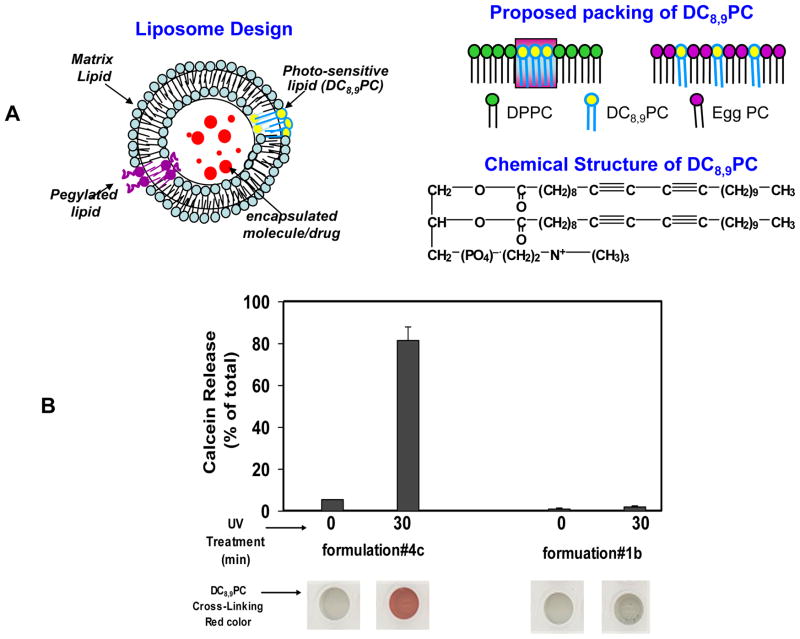
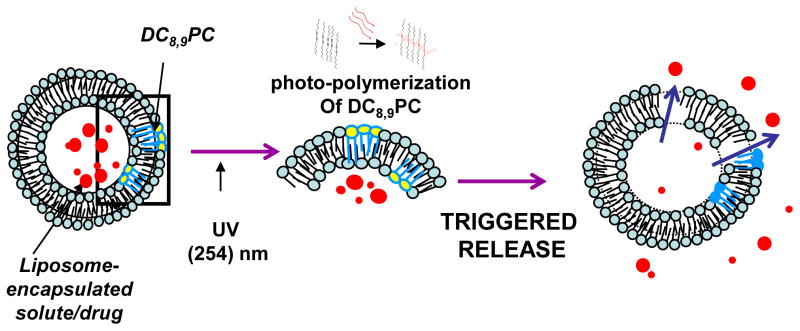
The design principle of the liposomes (Fig. 1, A) described in this report is based on photo cross-linking of DC8,9PC leading to formation of defects in the liposome bilayer upon UV exposure resulting in lateral membrane expansion/destabilization leading to drug release. We hypothesize that DC8,9PC is likely to form aggregates in the bilayer of phospholipids containing saturated acyl chains. This is consistent with our observation that light-triggered calcein release occurs from liposomes containing a mixture of saturated phospholipids and DC8,9PC. The packing properties of DC8,9PC in triggerable formulations described herein are in agreement with the modulation of the melting phase transitions (Tm) in these liposomes as determined by differential scanning calorimetry (DSC). Our formulations are likely to provide a next-generation of radiation-sensitive liposomes for drug delivery applications in the clinic.
Figure 1.
(A) Design principle of photopolymerization-based radiation-sensitive liposomes. Top Left: Liposomes consist of a matrix phospholipid (cyan), pegylated lipid (purple) and a photopolymerizable (pore forming) phospholipid, DC8,9PC (yellow), loaded with a chemotherapeutic agent in the aqueous milieu (red).
Top Right: Proposed packing of DC8,9PC (yellow) as immiscible patches within the bulk of a saturated matrix lipid (green, DPPC, #4e, Table I), or as miscible patches within the bulk of an unsaturated matrix lipid (pink, Egg PC, #1b, Table I)
Chemical formula of, DC8,9PC is also shown in Figure 1A.
(B) UV (254 nm)-Triggered calcein release from liposomes. Calcein-loaded DPPC (#4e) or Egg PC (#1a) liposomes (Table I) were placed in a 96-well plate (0.1–0.2 ml per well), and treated with UV radiation for 0 or 30 minutes. Calcein release (% of total) is shown in Fig 1B (graph). To confirm DC8,9PC mediated photo-polymerization; the plate was imaged (#4e Fig. 1B, bottom). The results were reproducible from at least 6 independent experiments.
2. Materials and Methods
2.1 Materials
DC8,9PC was synthesized at Naval Research Laboratory using published literature procedure [6]. All other phospholipids were purchased from Avanti Polar lipids, Inc. (Alabaster, AL, USA). Calcein and calcein blue were purchased from Fluka- Sigma-Aldrich (St. Louis, MO, USA). Agarose beads, Bio-Gel A-0.5m were purchased from Bio-Rad Laboratories, Richmond, CA, USA. All other reagents and buffers were of reagent grade.
2.2 Formation of liposomes
Lipids were mixed at desired molar ratios in a glass tube (Table I). A lipid film was formed by removing the solvent under nitrogen and any residual chloroform was removed by placing the films overnight in a vacuum desiccator. Multilamellar vesicles (MLVs) were formed by reconstituting the lipid film with HEPES buffer (10mM HEPES, 140mM NaCl, pH 7.5) by vigorous vortexing. To encapsulate calcein, lipid film was reconstituted in HEPES buffer containing self-quenched concentration of calcein (0.1M at pH=7.2–7.6). Liposomes were formed by sonication at 4°C for 5–10 min (1 min pulses and 1 min rest) using a Probe Sonicator (W-375 Heat Systems-Ultrasonics, New York, USA) [7,8]. The samples were centrifuged at 2000xg for 5–10 minutes to remove any titanium particles and larger aggregates. Calcein-loaded liposomes were separated from un-entrapped calcein using size exclusion gel chromatography column (Bio Gel A0.5m, 1×40 cm). Relative entrapment efficiency of calcein was determined by relief of self-quenching using Triton X-100 at Ex/Em 490/530 nm filter sets (CytoFlour, Series 4000, Perseptive Biosystems, CA, USA). Time and temperature dependent stability of these formulations was assayed by determining calcein leakage. Detailed experimental conditions are given in corresponding figure legends.
TABLE I.
Liposome formulations Used in this Study
| Matrix Lipid | Formulation* | Matrix Lipid (%) | Other Phospholipids |
Calcein Encapsulation Efficiency# | Liposome Stability% | Photo Polymerize effect$ | ||
|---|---|---|---|---|---|---|---|---|
| C22:1 PC | C23:0 PC | DC8,9PC | ||||||
| Egg PC (1) | 1a | 96 | none | none | none | high | good | No |
| 1b | 86 | none | none | 10 (mol %) | high | good | No | |
| DNPC (2) | 2a | 86 | 10 (mol %) | none | none | no entrapment | n.a. | No |
| 2c | 46 | none | none | 50 (mol %) | no entrapment | n.a. | Yes | |
| 2b | 86 | none | 10 (mol %) | none | no entrapment | n.a. | No | |
| DMPC (3) | 3a | 86 | none | none | 10 (mol %) | high | poor | Yes |
| 3b | 66 | none | none | 30 (mol %) | high | poor | Yes | |
| 3c | 46 | none | none | 50 (mol %) | high | poor | Yes | |
| 3d | 96 | none | none | none | no entrapment | n.a. | No | |
| DPPC (4) | 4a | 96 | none | none | none | high | good | No |
| 4b | 91 | none | none | 5 (mol %) | high | good | Yes | |
| 4c | 86 | none | none | 10 (mol %) | high | good | Yes | |
| 4d | 81 | none | none | 15 (mol %) | high | good | Yes | |
| 4e | 76 | none | none | 20 (mol %) | high | good | Yes | |
| 4f | 71 | none | none | 25 (mol %) | high | moderate | Yes | |
| 4g | 66 | none | none | 30 (mol %) | low | Poor | Yes | |
| 4h | 61 | none | none | 40 (mol %) | low | poor | Yes | |
| 4i | 56 | none | none | 50 (mol %) | low | poor | Yes | |
| 4j ^^ | 72 | none | none | 10 (mol %) | no entrapment | n.a. | N.A. | |
| 4k ^^ | 62 | none | none | 20 (mol %) | no entrapment | n.a. | N.A. | |
All preparations contained 4 mol% DSPE-PEG2000 for future in vivo applications.
Calcein encapsulation efficiency was compared with Egg PC liposomes (1a/1b).
Formulations that retained more than 75% entrapped calcein upon storage at 4°C up to 10 days were classified as stable and were used for further studies.
Photo polymerizable effect was measured be Appearance of red color after UV treatment
16 mol% DNPC was added to the liposome formulation
2.3 Radiation Treatment
Liposomes placed in a 96-well plate were irradiated with a UV lamp (UVP, SHORT WAVE ASSEMLY 115V, 60Hz 254 nm) at a distance of 1 inch at room temperature for 0–45 minutes. Increase in fluorescence due to release of calcein from liposomes was measured as above. 100% calcein release was obtained after addition of Triton X-100 (Tx-100) (0.02%, final concentration). In some experiments, the samples were imaged using a scanner (HP Precision ScanPro model 3.01, Hewlett Packard) to document appearance of red color upon photo cross-linking of DC8,9PC (Figure 1B, bottom).
2.4 Liposome Characterization
(i) Size
Size and population distribution of liposomes was determined by dynamic light scattering (DLS) measurements using a DynaPro Tytan instrument (Wyatt Technology Corp., Santa Barbara, CA) with a Temperature Controlled Microsampler at a laser wavelength of 830 nm and temperature 23°C [9]. To obtain the structural data, the intensity autocorrelation functions were processed with Dynamics 6.7.7.9 Software (Wyatt Technology Corp. CA, USA). For a typical DLS experiment 30–70 μl of liposome solution were placed into a special quartz microcuvette. Each measurement consisted of ten to sixty acquisitions, 10–20 seconds each. The hydrodynamic radius was calculated from regularization algorithm by the software. The particle hydrodynamic radii Rh were calculated from Stokes-Einstein equation Pη = Kβ/6πη Δτ, (Kb= Boltzmann’s constant, T- temperature, oK, η–viscosity of solvent, Dt –translational diffusion coefficient).
(ii) DSC analysis
Differential scanning calorimetry (DSC) curves of aqueous dispersions of pure lipids and their mixtures were recorded between 298–333/K on DSC 2920 by TA Instruments [10,11–13]. Typically, a 10 μl aliquot of lipid dispersion was placed in a hermatically sealed aluminum pan for recording each curve. Samples were equilibrated at 4°C for 1 hour before starting a heating scan.
(iii) UV-VIS Spectral Analysis
To monitor spectral shifts upon UV treatment, we prepared liposomes in the absence of calcein. Liposomes (0.2 ml each sample) were treated with UV as described above (section 2.2). In our initial analysis, substitution of HEPES buffer with PBS (2.66 mM KCl, 1.47 mM KH2PO4, 138 mM NaCl, and 8.06 mM Na2HPO4-7H2O (pH 7.1) had no effect on spectral measurements, therefore, UV-VIS spectra were routinely run in PBS. The samples were diluted with 9 volumes of PBS and absorption spectra were recorded in a spectrophotometer using quartz curettes (DU-350 Beckman Coulter, Fullerton, CA, USA).
3. Results and Discussion
3.1 Determination of Optimal Lipid Composition for DC8,9PC containing Liposomes
We hypothesize that the packing characteristics of DC8,9PC in the plane of the liposome membrane will guide light-triggered polymerization reaction of this lipid [14,15]. Therefore, our initial studies were focused on the evaluation of phospholipid composition(s) that potentially support light-triggered solute release from liposomes without compromising their stability. Various lipid compositions examined in this study are summarized in Table I. We included a pegylated lipid, DSPE-PEG2000 (18:0 PEG2 PE), in all our formulations for future in vivo applications [16]. Matrix phospholipids 1, Egg PC, 2, DNPC, 3, DMPC, and 4, DPPC were used to prepare liposomes (Table I). Stability and calcein encapsulation efficiency of our formulations was compared relative to Egg PC liposomes (formulation #1a&1b, Table I).
Previously it has been shown that nanoscale structures derived from DC8,9PC and DNPC (a C9-chain length lipid) significantly enhance kinetics of the photo-crosslinking of DC8,9PC [14]. Lipid mixtures prepared from DNPC/DC8,9PC (formulation #2c, 1:1 mol ratio), exhibited rapid photo-crosslinking upon exposure to UV radiation as indicated by spectral shifts in the UV-VIS spectrum [14] (data not shown) [17,18]. However, none of the DNPC (matrix lipid 2) formulations entrapped any calcein. Replacement of DC8,9PC with either C23:0 PC (formulation #2b) or C22:1 PC (formulation #2b) in DNPC liposomes did not result in any calcein encapsulation either, presumably due to their inability to form stable liposomes. We also examined a C14-chain length lipid, DMPC as the matrix lipid. These liposomes (formulation #3a–3c), entrapped calcein at high efficiency, that was released upon exposure to UV light at 25°C (Methods section, and data not shown). However, we also observed massive leakage of entrapped calcein upon storage at 4°C within 6–12 hours without any radiation treatment, rendering these formulations unsuitable for further testing.
Our next set of formulations included DPPC, a C16-chain length lipid (matrix lipid, formulation #4). Liposomes prepared from DPPC and DC8,9PC entrapped calcein similar to that for Egg PC (formulation #1a) or Egg PC/DC8,9PC (formulation #1b) liposomes. However, inclusion of >20 mol% DC8,9PC in DPPC liposomes resulted in spontaneous release of calcein within 24 hours at 4°C. Therefore, we used DPPC liposomes that included 10 or 20 mol% DC8,9PC for further studies.
3.2 UV-triggered Photopolymerization and Calcein Release from Liposomes
UV-triggered photo-cross linking of DC8,9PC molecular assemblies [19],[20] has been reported earlier based on spectral shifts in the UV-visible spectrum [18,21]. We observed photo-polymerization of DC8,9PC in our liposome formulations by visualization of red color following UV treatment for 30 min at 25°C (imaging with a scanner - HP Precision ScanPro model 3.01, Hewlett Packard). Fig. 1B (bottom section) shows representative wells of UV-treated and untreated DPPC/DC8,9PC and Egg PC/DC8,9PC mixtures. Appearance of red color confirmed photo-polymerization of DC8,9PC only in the liposome formulation #4e. Previously photo-polymerization using a similar formulation had been demonstrated in monolayers [22]. In contrast, Egg PC/DC8,9PC liposomes under identical conditions did not show any evidence of photopolymerization above background. Based on these data, we cannot rule out the possibility photo-crosslinking of a very limited number of DC8,9PC molecules in Egg PC/DC8,9PC liposomes. The rate of DC8,9PC photopolymerization in liposomes was dependent on the choice of matrix lipid and presumably the self-assembly and packing properties of DC8,9PC were critical (Fig 1A,) for the observed effect. Photo-triggering of DPPC/DC8,9PC liposomes resulted in massive calcein leakage, whereas the Egg PC/DC8,9PC liposomes remained stable under the identical conditions (Fig. 1B). These results are consistent with the notion that the packing of DC8,9PC in the liposomes profoundly affects photo-polymerization and that the polymerization of DC8,9PC in the plane of the membrane results in release of liposome contents. Since our leakage experiments were done at temperature (25°C), below the Tm of both lipids (DPPC, 314 K, and DC8,9PC 316 K), it is expected that the lipids in these liposomes will be in the gel crystalline phase. Therefore, we propose that in the liposomes formulations sensitive to UV-triggered photo-crosslinking and calcein leakage, DC8,9PC remains immiscible with DPPC (Fig. 1A), consistent with monolayer studies of lipids with similar compositions [22,23]. Natural leakage of calcein (no radiation) from these liposomes was negligible when incubated at various temperatures (data not shown). Therefore, these formulations may serve as promising carriers for radiation-triggered release of drugs and pharmaceuticals in future.
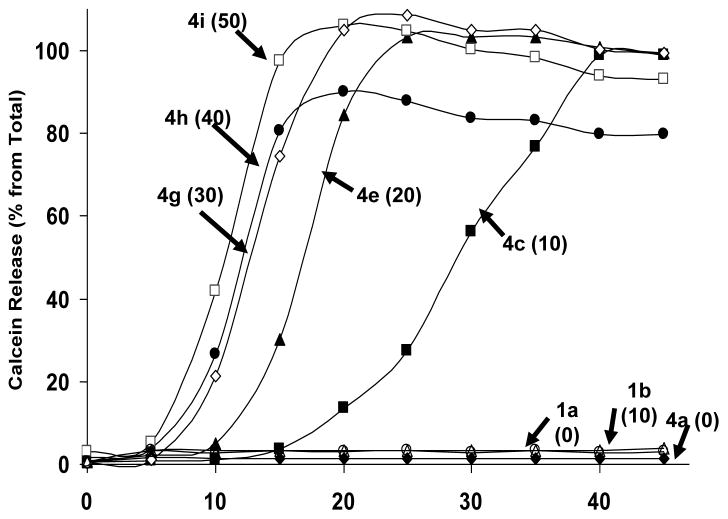
Next, we examined the kinetics of initial trigger and release rates of entrapped solutes from DPPC/DC8,9PC liposomes. The results shown in Fig. 2 observed an increase in rates of calcein release with increasing mol% of DC8,9PC following UV-treatment. Liposomes containing 10 or 20 mol% DC8,9PC (formulation #4c and #4e respectively), showed an initial delay of 10 and 15 minutes on the onset of calcein release, but resulted in maximum extents upon longer exposures (45 minutes). Kinetics of calcein release from liposomes containing >20 mol% DC8,9PC (formulation #4g, 4h and 4i) was very rapid and 100% calcein release reached a plateau within 15 minutes (Fig. 2). However, significant spontaneous leakage from liposomes that contained higher concentrations of DC8, 9PC (≥20 mol%) was observed making them unsuitable for further examinations (Table 1). In contrast, formulations 4c (10 mol% DC8,9PC) and 4e (20 mol% DC8,9PC) retained majority of encapsulated calcein in the absence of any radiation treatment upon storage, and were therefore used for further studies. Egg PC liposomes (formulation #1a or #1b) or DPPC liposomes without any DC8,9PC (formulation #4a) did not release any significant amount of entrapped calcein upon UV treatment under identical conditions (Fig. 2).
Figure 2. Effect of various concentrations of DC8,9PC on kinetics and extents of UV-induced calcein release from liposomes.
Liposomes containing varying concentrations of DC8,9PC (#4a–4h, Table I), were treated with UV for 0–45 minutes and calcein release was measured. The mole% of DC8,9PC in the formulations are given in parentheses. The data are reproducible from at least three independent experiments.
3.3 Melting Transitions of pure and mixed DPPC/DC8,9PC liposomes
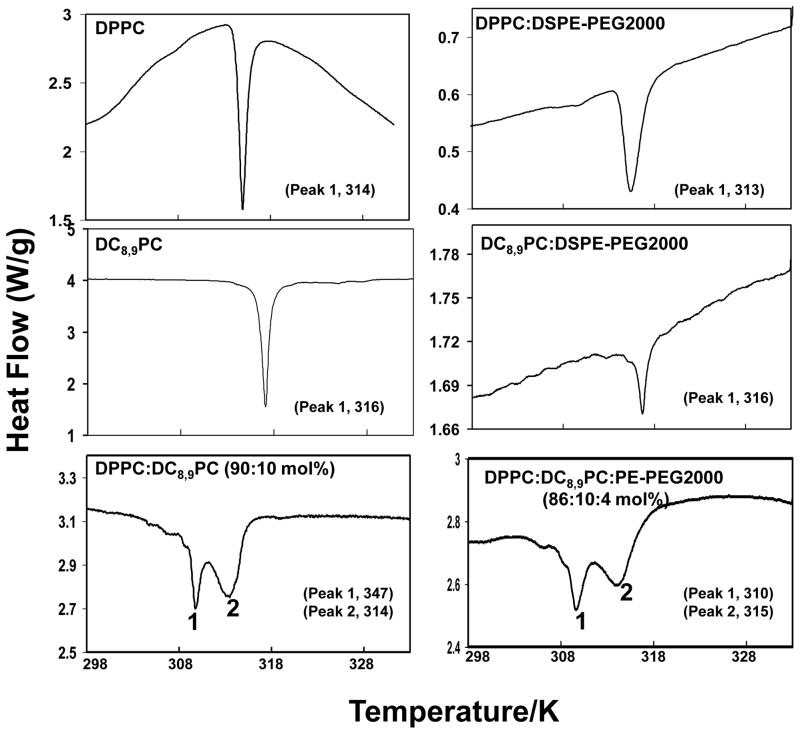
Results presented above (Fig. 1 and 2) indicate that preferential partitioning of DC8,9PC into DPPC (but not Egg PC) liposomes is essential for UV-triggered release of entrapped calcein. This observation led us to propose that DC8,9PC self-assembles in DPPC liposomes (Fig. 1A). Therefore, we examined the phase transition temperatures of these liposomes. A single broad peak in a DSC run of a lipid mixture would indicate well-mixed components in the lipid bilayer, whereas separate melting peaks indicate phase separation of the acetylenic lipid into separate domains within the lipid bilayer.
Calorimetric results are summarized in the Fig. 3. Tm of aqueous dispersions from pure lipids, DC8,9PC and DPPC, showed single sharp melting transitions (Tm) a 316 and 314 K respectively (Fig. 3, top panels) consistent with literature reports [24]. Incorporation of DSPE-PEG2000 (4 mol%) in DPPC or DC8,9PC aqueous dispersion had only moderate effect on Tm of resulting lipid mixtures (313 K,, DPPC:DSPE-PEG2000, and 316 K, DC8,9PC: DSPE-PEG2000). Next, we examined phase transition in DC8,9PC/DPPC mixtures (Fig. 3, bottom panel, left). DPPC:DC8,9PC (90:10 mol ratio) lipid dispersion showed two transition peaks at 310 K (peak 1) and 314 K (peak 2) indicating phase separation within the bilayer (Figure 3, lower panel, right). Addition of 4 mol % DSPE-PEG2000 had no significant effect on phase transition of this lipid mixture (peak 1, 310 K, and peak 2, 315 K). DSC analysis of DPPC:DC8,9PC mixtures (80:20 mol%) gave similar results (data not shown). Taken together, these results support our proposal that DC8,9PC segregates in DPPC liposomes.
Figure 3. Differential scanning calorimetry (DSC) of DPPC and DC8,9PC dispersions.
Cooling cycles of curves of aqueous dispersions of pure lipids and lipid mixtures (1–2 mg) were recorded (Methods section). The phase transition peaks of individual lipids are indicated in the respective graphs. Left panels show without the inclusion of DSPE-PEG2000. Right panels show the effect of inclusion of 4 mol% DSPE-PEG2000 on Tm of DPPC, DC8,9PC or the lipid mixtures.
It may be noted that our leakage experiments were performed at 25°C (below the Tm of both lipids), in the gel-crystalline state of these lipids without any phase boundaries. Therefore we propose that DC8,9PC co-exists with DPPC in our liposomes as the immiscible phases (Fig. 1A), despite being in the gel crystalline state [22,23]. In contrast, such immiscibility of DC8,9PC in Egg PC is not a predominant mode of packing. Detailed studies are needed to evaluate exact nature of packing properties of DC8,9PC in these liposomes.
3.4 Effects of Lipid Composition on the Size Distribution of DC8,9PC Liposomes
The hydrodynamic diameter of various liposome preparations (Table I) was determined by dynamic light scattering (DLS). The size distribution of various liposomes preparations was as follows: 75.0±8.6 nm (formulation #4a), 73.4±17 nm (formulation #4c), 63.6±10.3 nm (formulation #4e), 73.8±9.5 nm (formulation #4g), 73.4±11.5 nm (formulation #4h), and 62.4±6.3 nm (formulation #1b). The DLS method measures diffusive properties of particles, which, in part, depend on their size. When the sample is illuminated with laser light, the particle’s Brownian motion causes light scattering fluctuations. For larger particles these fluctuations will be longer, than for small ones. [9].
3.5 Effects of UV Treatment on Size distribution and Integrity of Liposomes
The effect of radiation treatments on hydrodynamic size distribution was determined as described above. UV treatment of liposomes (that released entrapped calcein from DPPC:DC8,9PC (formulation# 4c, Table I)), did not result in any significant modification of liposome size distribution (untreated liposomes, 73.4±17 nm; UV-treated liposomes, 76.8±19.8 nm). We made similar observations when liposomes, without any entrapped calcein were treated with radiation under identical conditions (data not shown). In addition, we did not observe any significant changes in the shape of liposomes following UV treatment by negative staining electron microscopy (data not shown). Therefore, radiation treatment of liposomes does not result in disruption of liposome architecture suggesting that leakage of calcein occurred due to pore formation in liposomes upon UV treatment.
3.6 Photo-polymerization occurs only in DPPC/DC8,9PC Liposomes: A phase-separation effect
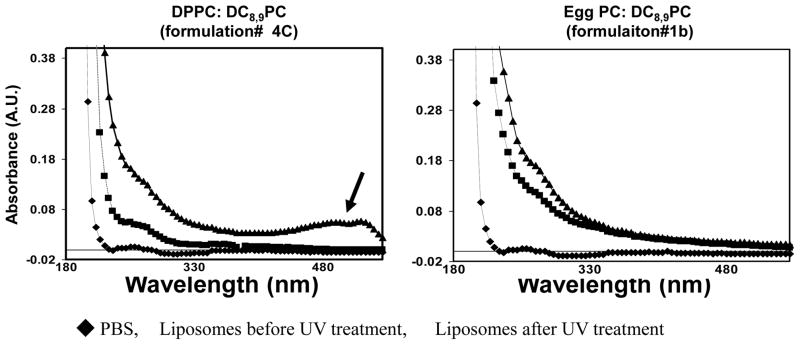
It is well-documented that DC8,9PC undergoes photo-crosslinking upon treatments with 254 nm radiation [18,25], resulting in spectral shifts in the UV-VIS spectrum [18]. To understand mechanisms of calcein leakage, we monitored spectral shifts in UV-treated liposomes (These experiments were conducted without calcein entrapment). The results are shown in Fig. 4. It is clear that a change in absorption spectrum (indicated by arrow) was observed only in UV-treated DPPC/DC8,9PC liposomes (triangles, left panel, Fig. 4), but not in egg PC/DC8,9PC liposomes (triangles, right panel, Fig. 4). Either liposome preparations did not show any significant absorbance in visible spectrum in the absence of UV radiation (squares). Buffer only (PBS control) is also shown (diamonds). This data taken together with DSC results, strongly indicate that DC8,9PC-triggered solute release from liposomes occurs when this lipid is organized as segregated patches in the lipid bilayer.
Figure 4. Spectral Properties of UV-treated liposomes.
Liposomes (prepared without calcein) were treated with UV (254 nm) for 30 min, and absorption spectra were recorded (Methods section). Traingles, UV-treated liposomes, Squares, untreated liposomes, Diamonds, PBS alone.
4. Conclusion
An important requirement for effective drug delivery is the precise spatial and temporal release of therapeutic agents at the target site. Our strategically designed liposomes offer promising vehicles for future development of clinically viable second generation drug delivery systems. The design is based on lateral segregation (and/or self-assembly) of DC8,9PC (yellow) in the lipid bilayer, containing DPPC as a matrix lipid (blue), and calcein as entrapped solute (red) (Fig. 5). We have demonstrated that photo-triggering by UV light occurs via direct photopolymerization mechanism. The triggerable liposomes described here bear the potential for further adaptation to electromagnetic radiations for in vivo applications.
Figure 5. A Schematic representation of a Proposed Mechanism of light- Triggered Release of entrapped Solutes from Liposomes.
Liposome-encapsulated calcein containing DPPC and DC8,9PC can be released by UV-triggered photopolymerization of DC8,9PC.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research and NCI Alliance for Nanotechnology (Piotr Grodzinski). We would like to thank Dr. Julie M. Belanger for critical reading of the manuscript.
Abbreviations
- DNPC (09:0 PC)
1,2-Dinonanoyl-sn-Glycero-3-Phosphocholine
- DMPC (14:0 PC)
1,2-Dimyristoyl-sn-Glycero-3-Phosphocholine
- DPPC (16:0 PC)
1,2-Dipalmitoyl-sn-Glycero-3-Phosphocholine
- Egg PC
L-α-Phosphatidylcholine (Egg, Chicken)
- C23:0 PC
1,2-Ditricosanoyl-sn-Glycero-3-Phosphocholine
- C22:1 PC (cis)
1,2-Dierucoyl-sn-Glycero-3-Phosphocholine
- DC8,9PC
(1,2 bis (tricosa-10, 12-diynoyl)-sn-glycero-3-phosphocholine)
- DSPE-PEG2000 (18:0 PEG2 PE),
1,2-Distearoyl-sn-Glycero-3 Phosphoethanolamine-N-[Methoxy(Polyethylene glycol)-2000] (Ammonium Salt)
- HEPES buffer
10 mM HEPES, 140 mM NaCl (pH 7.5)
- PBS
2.66 mM KCl, 1.47 mM KH2PO4, 138 mM NaCl, 8.06 mM Na2HPO4-7H2O (pH 7.1)
References
- 1.Shimshic EJ, Mcconnell HM. Lateral Phase Separations in Binary-Mixtures of Cholesterol and Phospholipids. Biochemical and Biophysical Research Communications. 1973;53(2):446–451. doi: 10.1016/0006-291x(73)90682-7. [DOI] [PubMed] [Google Scholar]
- 2.Shimshic EJ, Mcconnell HM. Lateral Phase Separation in Phospholipid Membranes. Biochemistry. 1973;12(12):2351–2360. doi: 10.1021/bi00736a026. [DOI] [PubMed] [Google Scholar]
- 3.Yatvin MB, Weinstein JN, Dennis WH, Blumenthal R. Design of Liposomes for Enhanced Local Release of Drugs by Hyperthermia. Science. 1978;202(4374):1290–1293. doi: 10.1126/science.364652. [DOI] [PubMed] [Google Scholar]
- 4.Regen SL, Singh A, Oehme G, Singh M. Polymerized phosphatidyl choline vesicles. Stabilized and controllable time-release carriers. Biochem Biophys Res Commun. 1981;101(1):131–136. doi: 10.1016/s0006-291x(81)80020-4. [DOI] [PubMed] [Google Scholar]
- 5.Rhodes DG, Blechner SL, Yager P, Schoen PE. Structure of polymerizable lipid bilayers. I--1,2-bis(10,12-tricosadiynoyl)-sn-glycero-3-phosphocholine, a tubule-forming phosphatidylcholine. Chem Phys Lipids. 1988;49(1–2):39–47. doi: 10.1016/0009-3084(88)90062-x. [DOI] [PubMed] [Google Scholar]
- 6.Singh A. An efficient synthesis of phosphatidylcholines. J Lipid Res. 1990;31(8):1522–1525. [PubMed] [Google Scholar]
- 7.Fu FN, Singh BR. Calcein permeability of liposomes mediated by type A botulinum neurotoxin and its light and heavy chains. J Protein Chem. 1999;18(6):701–707. doi: 10.1023/a:1020614525534. [DOI] [PubMed] [Google Scholar]
- 8.Puri A, Kramer-Marek G, Campbell-Massa R, et al. HER2-specific affibody-conjugated thermosensitive liposomes (Affisomes) for improved delivery of anticancer agents. J Liposome Res. 2008;18(4):293–307. doi: 10.1080/08982100802457377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dejaeger N, Demeyere H, Finsy R, et al. Particle Sizing by Photon-Correlation Spectroscopy.1. Monodisperse Lattices - Influence of Scattering Angle and Concentration of Dispersed Material. Particle & Particle Systems Characterization. 1991;8(3):179–186. [Google Scholar]
- 10.Stanish I, Singh A. Highly stable vesicles composed of a new chain-terminus acetylenic photopolymeric phospholipid. Chem Phys Lipids. 2001;112(2):99–108. doi: 10.1016/s0009-3084(01)00173-6. [DOI] [PubMed] [Google Scholar]
- 11.Markowitz MA, Singh A. Microstructure formation properties of 1,2-bis(15-thia-pentacosa-10,12-diynoyl)-sn-3-phosphocholine: An acyl chain modified diacetylenic phospholipid. Chemistry and Physics of Lipids. 1996;84(1):65–74. [Google Scholar]
- 12.Pappalardo M, Milardi D, Grasso D, La Rosa C. Phase behaviour of polymer-grafted DPPC membranes for drug delivery systems design. Journal of Thermal Analysis and Calorimetry. 2005;80(2):413–418. [Google Scholar]
- 13.Pentak D, Kowski WWS, Sulkowska A. Calorimetric and EPR studies of the thermotropic phase behavior of phospholipid membranes. Journal of Thermal Analysis and Calorimetry. 2008;93(2):471–477. [Google Scholar]
- 14.Rhodes DG, Singh A. Structure of polymerizable lipid bilayers IV. Mixtures of long chain diacetylenic and short chain saturated phosphatidylcholines and analogous asymmetric isomers. Chem Phys Lipids. 1991;59(3):215–224. doi: 10.1016/0009-3084(91)90021-3. [DOI] [PubMed] [Google Scholar]
- 15.Rhodes DG, Hui SW, Xu YH, Byun HS, Singh M, Bittman R. Structure of polymerizable lipid bilayers VII: lateral organization of diacetylenic phosphatidylcholines with short proximal acyl chains. Biochim Biophys Acta. 1994;1215(3):237–244. doi: 10.1016/0005-2760(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 16.Gabizon A, Martin F. Polyethylene glycol-coated (pegylated) liposomal doxorubicin. Rationale for use in solid tumours. Drugs. 1997;54 (Suppl 4):15–21. doi: 10.2165/00003495-199700544-00005. [DOI] [PubMed] [Google Scholar]
- 17.Hayward JA, Levine DM, Neufeld L, Simon SR, Johnston DS, Chapman D. Polymerized liposomes as stable oxygen-carriers. FEBS Lett. 1985;187(2):261–266. doi: 10.1016/0014-5793(85)81255-2. [DOI] [PubMed] [Google Scholar]
- 18.Johnston DS, McLean LR, Whittam MA, Clark AD, Chapman D. Spectra and physical properties of liposomes and monolayers of polymerizable phospholipids containing diacetylene groups in one or both acyl chains. Biochemistry. 1983;22(13):3194–3202. doi: 10.1021/bi00282a024. [DOI] [PubMed] [Google Scholar]
- 19.Singh A, Wong EM, Schnur JM. Toward the rational control of nanoscale structures using chiral self-assembly: Diacetylenic phosphocholines. Langmuir. 2003;19(5):1888–1898. [Google Scholar]
- 20.Markowitz MA, Singh A, Chang EL. Formation and properties of a network gel formed from mixtures of diacetylenic and short-chain phosphocholine lipids. Biochem Biophys Res Commun. 1994;203(1):296–305. doi: 10.1006/bbrc.1994.2181. [DOI] [PubMed] [Google Scholar]
- 21.Johnston DS, Sanghera S, Pons M, Chapman D. Phospholipid polymers--synthesis and spectral characteristics. Biochim Biophys Acta. 1980;602(1):57–69. doi: 10.1016/0005-2736(80)90289-8. [DOI] [PubMed] [Google Scholar]
- 22.Hupfer B, Ringsdorf H, Schupp H. Polyreactions in Oriented Systems.21. Polymeric Phospholipid Monolayers. Macromolecular Chemistry and Physics-Makromolekulare Chemie. 1981;182(1):247–253. [Google Scholar]
- 23.Hupfer B, Ringsdorf H, Schupp H. Liposomes from polymerizable phospholipids. Chem Phys Lipids. 1983;33(4):355–374. doi: 10.1016/0009-3084(83)90028-2. [DOI] [PubMed] [Google Scholar]
- 24.Burke TG, Rudolph AS, Price RR, et al. Differential scanning calorimetric study of the thermotropic phase behavior of a polymerizable, tubule-forming lipid. Chem Phys Lipids. 1988;48(3–4):215–230. doi: 10.1016/0009-3084(88)90092-8. [DOI] [PubMed] [Google Scholar]
- 25.Leaver J, Alonso A, Durrani AA, Chapman D. The Physical-Properties and Photo-Polymerization of Diacetylene-Containing Phospholipid Liposomes. Biochimica et Biophysica Acta. 1983;732(1):210–218. [Google Scholar]