Abstract
5-lipoxygenase (5-LOX), an enzyme involved in leukotriene synthesis, is expressed in the brain and has been associated with Alzheimer's disease and depression. Recently, it has been suspected that leukotriene receptor antagonists might be associated with suicide. In this work, we investigated the 5-LOX protein in the brain samples from depressed suicide victims and matching controls. We used Western immunoblotting with an antibody against Ser523-phosphorylated 5-LOX (p5-LOX) to evaluate protein kinase A-mediated 5-LOX phosphorylation, and in addition, an antibody against the total 5-LOX protein. In the total homogenate of the prefrontal cortex samples, 5-LOX content did not differ in the control and suicide groups but p5-LOX was significantly elevated in the suicide samples. The 5-LOX protein content was reduced in the membrane fraction and increased in the cytosol fraction of suicide victims. We propose that further studies of brain 5-LOX are needed to elucidate the functional implications of the protein alterations observed in our present study, and to further explore a putative role of 5-LOX in depression and suicide.
Introduction
5-Lipoxygenase (5-LOX; also called 5-LO) is an enzyme involved in the conversion of arachidonic acid into leukotrienes, lipid molecules that activate leukotriene receptors [1, 2]. 5-LOX is expressed in the central nervous system neurons [3]. Its brain expression increases during aging [4, 5] and in Alzheimer's disease [6, 7]. Brain 5-LOX has been linked to antidepressant activity [8 - 10]. More recently, it has been suspected that leukotriene receptor antagonists might be associated with suicide. Currently, the U.S. Food and Drug Administration (FDA) is investigating a possible association between the use of leukotriene receptor antagonists and mood changes and suicide [11].
The activity of the 5-LOX pathway can be regulated at the level of gene expression and enzyme activity [1]. 5-LOX enzyme activity is increased by ions (e.g., calcium) and inhibited by cholesterol, n-3 polyunsaturated fatty acids, and phosphorylation [1, 12, 13]. Protein kinase A (PKA) phosphorylates 5-LOX on Ser523; this leads to an accumulation of 5-LOX protein in the cytosol [12]. Alterations in phosphorylation pathways have been identified as an important pathobiological mechanism in mood disorders and suicide [14]. We used Western immunobloting with an antibody against Ser523-phosphorylated 5-LOX to evaluate PKA-mediated 5-LOX phosphorylation, and also used an antibody against the total 5-LOX protein to assay 5-LOX content in the cytosol and membrane fractions of samples from controls and suicide victims.
Materials and Methodology
Subjects
The study was performed in samples from the prefrontal cortex (PFC) from the right hemisphere. Biochemical changes in this brain area were previously associated with depression and suicide [15]. Brain tissues were obtained from the Brain Collection Program of the Maryland Psychiatric Research Center, Baltimore, MD [15]. After giving verbal informed consent at least one family member underwent an interview based on the Diagnostic Evaluation After Death (DEAD) and the Structured Clinical Interview for the DSM-IV (SCID). The interviews were conducted by a trained psychiatric social worker. Two psychiatrists independently reviewed the write-up from this interview and the SCID that was completed from it as part of their diagnostic assessment of the case. Their diagnoses were made from the data obtained in this interview, medical records from the case, and records obtained from the Medical Examiner's office. The two diagnoses were compared and discrepancies were resolved by means of a consensus conference. Controls were verified as free from mental illnesses using these consensus diagnostic procedures. This study was approved by the Institutional Review Board of the University of Illinois at Chicago.
Brain Tissue
We analyzed PFC samples (Brodmann's area 9) obtained from depressed suicide subjects (n = 11) and nonpsychiatric control subjects (n = 11), hereafter referred to as normal controls. After removal from the cranium, the brains were cut into six major regions (four cerebral cortical lobes, basal ganglia-diencephalon, and lower brain stem-cerebellum), rapidly frozen on dry ice, and stored at −70°C until dissection. During dissection, the frontal lobes were sliced into 1-1.5 mm thick coronal sections at a temperature between 0-10°C. To keep the samples frozen, the dissections were performed on a metal plate over a container filled with dry ice. The PFC samples were cut out of the coronal sections by a fine microdissecting knife under a stereomicroscope with low magnification. The dorsomedial PFC was taken just dorsal to the frontopolar area including the most polar portion of the superior and partly the middle superior gyrus between the superior and intermediate frontal sulci. In the sections of the dissected cortical area, the gray and white matter was separated, and only the gray matter was used in this study. To ensure that the same area was used for each assay, after dissection, each brain area tissue was cut into very small pieces, mixed, and then used for analysis.
All tissue from normal controls and suicide subjects was screened for evidence of any neuropathology by experienced neuropathologists. The presence of Alzheimer's disease, infarcts, demyelinating diseases, or atrophy (or a clinical history of these disorders) disqualified subjects from the study. Blood/urine samples from every suicide and control subject were systematically obtained. These biological specimens allow the undertaking of a systematic toxicology screening for alcohol and a comprehensive battery of tests for illicit drug use, as well as screening for antidepressant/psychoactive drugs taken prior to death. This information, along with a medication history (obtained from hospital records) and a compliance history (obtained through interviews with family members), identifies individuals who have taken antidepressants, psychoactive drugs, or substances of abuse prior to suicide. In each case, screening for the presence of the human immunodeficiency virus (HIV) was done in blood samples and all HIV-positive cases were excluded.
5-LOX immunoblotting
The previously prepared and frozen aliquots of total, membrane, and cytosol fractions [15] were used for Western immunoblotting. Briefly, these samples were prepared as follows. To obtain the total, membrane, and cytosol fractions, the brain tissue was homogenized in a homogenizing buffer containing 20 mM Tris-HCl (pH 7.4), 2 mM ethylenediaminetetraacetic acid, 1 mM dithiothreitol, 110 μg/ml aprotinin, 10 μg/ml pepstain, 10 μg/ml leupeptin, and 8.7 μg/ml phenylmethylsulfonyl fluoride. The homogenate was centrifuged at 100 x g for 5 min at 4°C. The pellet was homogenized in homogenizing buffer. A portion of the homogenate was used as total fraction. The homogenate was further centrifuged at 100,000 g for 60 min at 4°C. The resulting supernatant (S1) was saved. The pellet obtained was re-homogenized in the homogenizing buffer and re-centrifuged at 100,000 g for 60 min at 4°C. The resultant supernatant (S2) was combined with S1 and used as the cytosol fraction. The pellet was homogenized in the homogenizing buffer and used as the membrane fraction. For 5-LOX immunoblotting, samples (20-40 μg protein) were electrophoretically separated on a 7.5% acrylamide gel, transferred to a nitrocellulose membrane (Amersham, Piscataway, NJ, USA), and incubated overnight with anti-5-LOX (1:1000; F58420, Transduction Laboratories, Lexington, KY, USA) or anti-Ser523-phosphorylated 5-LOX (1:1000; PA1-4628, Affinity Bioreagents, Golden, CO) antibodies. The blots were washed and incubated with horseradish-peroxidase-linked secondary antibodies (Amersham); anti-mouse IgG (1:1000) and anti-rabbit IgG (1:1000), respectively. They were processed with an enhanced chemiluminescence (ECL) kit (Amersham) and exposed to Hyperfilm ECL (Amersham). To normalize the total 5-LOX signal, the signal for β-actin protein was measured on the same blot (mouse monoclonal anti-β-actin antibody, Sigma, St. Louis, MO, USA; 1:10000). The Ser523-phosphorylated 5-LOX signal was normalized by the total 5-LOX signal. The optical densities of the corresponding bands were measured using the Loats image analysis system (Westminster, MD, USA).
Statistical methods
Data analyses were performed using the SPSS Statistics version 15 (Chicago, IL, USA). All the dependent variables were first subjected to tests of normality. The assumption of normality was tested using the Shapiro-Wilk test. Both normal controls and depressed suicide groups were separately tested for normality. To adjust for multiplicity of testing based on multiple endpoints (i.e., dependent variables), a multiple analysis of covariance (MANCOVA) was applied. Age, gender, and postmortem interval were used as covariates. The assumption of homogeneity of variance was tested using Box's test of equality of covariance matrices. In the presence of a significant MANCOVA, ANCOVAs were performed for each dependent variable. The differences in age, gender, and postmortem interval (PMI), between suicide subjects and normal controls were analyzed using the independent-sample “t” test. In addition, relationships between 5-LOX and PMI and age were also determined using a Pearson product-moment correlation analysis. The effects of gender on various measures were also determined by an independent sample t-test comparing males and females. Similarly, an independent sample t-test was used to compare the depressed subjects who showed the presence of antidepressants at the time of death with depressed subjects who did not.
Results
The demographic characteristics of depressed suicide subjects and normal control subjects are provided in Table 1 and Table 2. There were 7 males and 4 females in the control group, and 5 males and 6 females in the suicide group. There were no significant differences in age (t = 1.18, df = 20, P = 0.25) and PMI (t = 0.78, df = 20, P = 0.44). Only the right brain hemisphere samples were used in this study, but the handedness of the subjects has not been recorded; we have no reason to believe that the groups differed in this respect.
Table 1.
Characteristics of suicide victims.
| Group and subject | Age (yrs) | Race | Gender | PMI (hr) | Cause of death | Drug toxicity (at the time of death) |
|---|---|---|---|---|---|---|
| Suicide | ||||||
| 1 | 53 | White | Male | 23 | Jumping | None |
| 2 | 24 | White | Male | 7 | GSW | Ethanol |
| 3 | 36 | White | Female | 10 | GSW | Butalbital, Diphenhydramine, Acetaminophen |
| 4 | 38 | White | Male | 24 | Drug overdose | Ethanol, Diphenhydramine |
| 5 | 46 | White | Female | 16 | Drug overdose | Nortriptyline |
| 6 | 44 | White | Female | 11 | Drug overdose | Nortriptyline |
| 7 | 22 | Black | Female | 16 | Drug overdose | Propranolol |
| 8 | 46 | White | Female | 21 | Drug overdose | Amitriptyline, Desipramine, Ethanol |
| 9 | 43 | White | Male | 12 | Drug overdose | Acetaminophen, Propoxyphen |
| 10 | 27 | White | Male | 24 | GSW | None |
| 11 |
36 |
White |
Female |
18 |
GSW |
None |
| Mean | 38.2 | 17.3 | ||||
| SD | 9.9 | 6.8 |
ASCVD = atherosclerotic cardiovascular disease; GSW = gunshot wound
Table 2.
Characteristics of control subjects.
| Group and subject | Age (yrs) | Race | Gender | PMI (hr) | Cause of death | Drug toxicity (at the time of death) |
|---|---|---|---|---|---|---|
| Controls | ||||||
| 12 | 45 | White | Male | 22 | ASCVD | None |
| 13 | 22 | Black | Male | 19 | GSW | None |
| 14 | 63 | White | Female | 30 | Ovarian cancer | None |
| 15 | 33 | White | Male | 15 | GSW | Acetaminophen |
| 16 | 37 | White | Male | 24 | ASCVD | None |
| 17 | 65 | Black | Female | 23 | ASCVD | None |
| 18 | 38 | Black | Male | 16 | Lung sarcoidosis | None |
| 19 | 40 | White | Female | 7 | ASCVD | None |
| 20 | 42 | White | Female | 23 | Pneumonia | None |
| 21 | 46 | Black | Male | 9 | Multiple injuries | None |
| 22 |
52 |
White |
Male |
30 |
ASCVD |
None |
| Mean | 43.9 | 19.8 | ||||
| SD | 12.5 | 7.5 |
ASCVD = atherosclerotic cardiovascular disease; GSW = gunshot wound
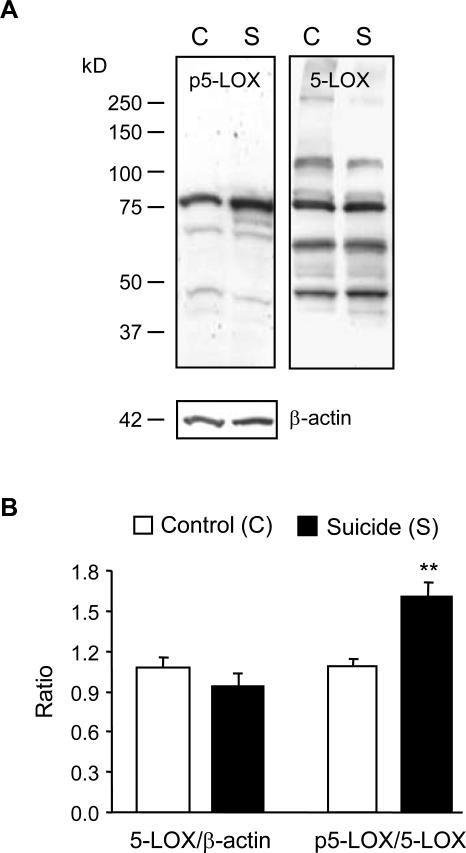
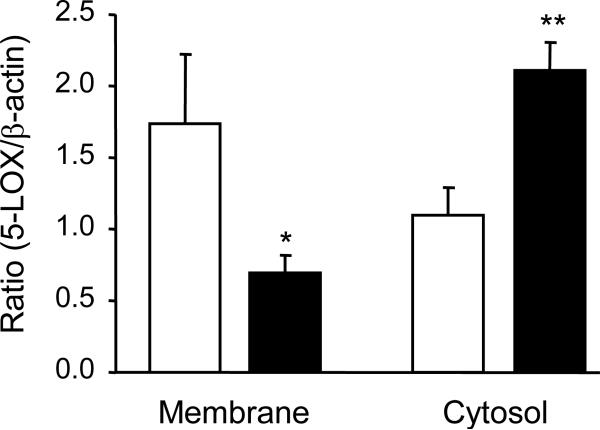
In the total homogenate, 5-LOX immunoreactivity (corrected by β-actin) did not differ in the control and suicide groups but Ser523-phosphorylated 5-LOX immunoreactivity (corrected by 5-LOX) was significantly elevated in the suicide samples (Fig. 1). On the other hand, 5-LOX immunoreactivity was significantly reduced in the membrane fraction and increased in the cytosol fraction of suicide victims (Fig. 2).
Figure 1.
5-LOX phosphorylation in the total homogenate from post-mortem PFC of control subjects and suicide victims. Upper panel (A) shows typical examples of Ser523-phosphorylated 5-LOX (p5-LOX), total 5-LOX (both at the expected size of ~78 kDa), and β-actin (~ 42 kDa) immunoreactive bands.In the total homogenate, 5-LOX content did not differ between two groups but the p5-LOX content was elevated in the suicide samples (B). Results are the mean ± S.E.M. (n = 11; **p<0.01 vs. corresponding controls). Open bars, control (C); closed bars, suicide (S).
Figure 2.
5-LOX protein content in the membrane and cytosol fractions from postmortem PFC of control subjects and suicide victims. 5-LOX content was significantly reduced in the membrane fraction and increased in the cytosol fraction of the suicide PFC. Results are the mean ± S.E.M. (n = 11; *p<0.05, **p<0.01 vs. corresponding controls). Open bars, control; closed bars, suicide.
The effects of potential confounding variables, namely, age, gender, PMI, antidepressant treatment, presence of alcohol, and method of suicide, were evaluated with respect to the levels of cytosolic 5-LOX immunoreactivity and p5-LOX immunoreactivity, i.e., in the samples where we found changes in suicide subjects. We found no significant effects of age on cytosol 5-LOX (r = 0.38, P = 0.07), membrane 5-LOX (r = 0.38, P = 0.08), and p5-LOX (r = 0.22, P = 0.32). The PMI also did not have any effect on protein levels of cytosolic 5-LOX (r = 0.21, P = 0.33), membrane 5-LOX (r = 0.25, P = 0.25), and p5-LOX (r = 0.06, P = 0.76).
There were 7 males and 4 females in the control group. Comparison studies showed no significant differences in protein levels of cytosolic 5-LOX (t = 0.22, df = 9, P = 0.83), membrane 5-LOX (t = 0.34, df = 9, P = 0.74) and p5-LOX (t = 0.47, df = 9, P = 0.65) between males and females.
To examine whether the observed changes in cytosol 5-LOX and p5-LOX in the depressed suicide group were related to the presence of antidepressant(s), we compared the suicide subjects who tested positive for antidepressants during the screen at the time of death and those who did not. We did not find significant differences in protein levels of cytosolic 5-LOX (t = 1.36, df = 9, P = 0.20), membrane 5-LOX (t = 1.11, df = 9, P = 0.29), and p5-LOX (t = 1.38, df = , P = 0.19) between those who showed a presence of antidepressants during the screen at the time of death and those who did not.
Discussion
A relative increase of cytosolic 5-LOX and the increased 5-LOX Ser523 phosphorylation in suicide PFC samples suggests that PKA-mediated phosphorylation of 5-LOX is increased in these subjects compared with controls. Namely, PKA is responsible for Ser523 phosphorylation of the 5-LOX protein, a process that leads to a cytosolic accumulation of 5-LOX [12]. Previously, it was found that PKA-mediated phosphorylation of some proteins is increased in the PFC of suicide victims [16]. However, previous studies in our brain samples revealed a significant reduction of PKA activity in the PFC of suicide subjects, which was associated with a selective reduction in the expression of the PKA regulatory RIIβ and catalytic Cβ subunits [15]. Hence, it appears that certain PKA substrates are selectively hyperphosphorylated in the PFC of suicide subjects in spite of a general reduction of the PFC PKA activity. Furthermore, 5-LOX phosphorylation can be modified by other kinase that were not analyzed in our brain samples; for example, p38-dependent MAPKAP kinase [17].
Although 5-LOX is considered less active in the cytosol, little is known about 5-LOX regulation in brain cells. Furthermore, there is evidence that 5-LOX can be active in the cytosol through an association with a coactosin-like protein [18]. Thus, further studies are needed to ascertain whether our findings indicate an increased or decreased 5-LOX activity in the suicide PFC.
On one hand, compounds that inhibit 5-LOX, such as n-3 polyunsaturated fatty acids [13], have been reported to be preventive for depression/suicide [19], and animal studies indicate that 5-LOX inhibitors produce antidepressant-like effects [8, 10]. On the other, anecdotal clinical observations suggest that leukotriene receptor antagonists might have a pro-suicide activity. If these suspicions are confirmed, further research into the physiological relevance of neuronal leukotriene receptors would become warranted. Currently, the knowledge about central nervous system leukotriene receptors is limited [20].
Conclusion
In conclusion, for the first time we demonstrated changes in the leukotriene-producing 5-LOX system in the brain samples of depressed suicide victims compared to normal controls. Because it has been suspected that leukotriene receptor antagonists might be associated with suicide, we propose that further studies of brain 5-LOX may contribute to a better understanding of the pathobiology of depression and suicide.
Acknowledgements
We acknowledge the support from the Department of Psychiatry, UIC and by the NIH grants MH068777 (Y.D.), MH48153 (G.N.P.), MH60744 and MH66123 (RCR), MH074139 (R.M.), and AG015347 (H.M.).
References
- 1.Rådmark O, Werz O, Steinhilber D, Samuelsson B. 5-Lipoxygenase: regulation of expression and enzyme activity. Trends Biochem Sci. 2007;32:332–41. doi: 10.1016/j.tibs.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Soberman RJ, Christmas P. The organization and consequences of eicosanoid signaling. J Clin Invest. 2003;111:1107–13. doi: 10.1172/JCI18338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lammers CH, Schweitzer P, Facchinetti P, Arrang JM, Madamba SG, Siggins GR, Piomelli D. Arachidonate 5-lipoxygenase and its activating protein: prominent hippocampal expression and role in somatostatin signaling. J Neurochem. 1996;66:147–52. doi: 10.1046/j.1471-4159.1996.66010147.x. [DOI] [PubMed] [Google Scholar]
- 4.Chinnici CM, Yao Y, Praticò D. The 5-lipoxygenase enzymatic pathway in the mouse brain: young versus old. Neurobiol Aging. 2007;28:1457–62. doi: 10.1016/j.neurobiolaging.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Uz T, Pesold C, Longone P, Manev H. Aging-associated up-regulation of neuronal 5-lipoxygenase expression: putative role in neuronal vulnerability. FASEB J. 1998;12:439–49. doi: 10.1096/fasebj.12.6.439. [DOI] [PubMed] [Google Scholar]
- 6.Firuzi O, Zhuo J, Chinnici CM, Wisniewski T, Praticò D. 5-Lipoxygenase gene disruption reduces amyloid-β pathology in a mouse model of Alzheimer's disease. FASEB J. 2008;22:1169–78. doi: 10.1096/fj.07-9131.com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikonomovic MD, Abrahamson EE, Uz T, Manev H, DeKosky ST. Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with Alzheimer's disease. J Histochem Cytochem. 2008 doi: 10.1369/jhc.2008.951855. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dzitoyeva S, Imbesi M, Uz T, Dimitrijevic N, Manev H, Manev R. Caffeic acid attenuates the decrease of cortical BDNF transcript IV mRNA induced by swim stress in wild-type but not in 5-lipoxygenase-deficient mice. J Neural Transm. 2008;115:823–7. doi: 10.1007/s00702-008-0034-7. [DOI] [PubMed] [Google Scholar]
- 9.Manev H, Manev R. 5-lipoxygenase as a possible biological link between depressive symptoms and atherosclerosis. Arch Gen Psychiatry. 2007;64:1333. doi: 10.1001/archpsyc.64.11.1333. [DOI] [PubMed] [Google Scholar]
- 10.Uz T, Dimitrijevic N, Imbesi M, Manev H, Manev R. Effects of MK-886, a 5-lipoxygenase activating protein (FLAP) inhibitor, and 5-lipoxygenase deficiency on the forced swimming behavior of mice. Neurosci Lett. 2008;436:269–72. doi: 10.1016/j.neulet.2008.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration [website on the Internet] Early Communication About an Ongoing Safety Review of Montelukast (Singulair) Available from: http://www.fda.gov/cder/drug/early_comm/montelukast.htm.
- 12.Luo M, Jones SM, Flamand N, Aronoff DM, Peters-Golden M, Brock TG. Phosphorylation by protein kinase A inhibits nuclear import of 5-lipoxygenase. J Biol Chem. 2005;280:40609–16. doi: 10.1074/jbc.M507045200. (2005) [DOI] [PubMed] [Google Scholar]
- 13.Taccone-Gallucci M, Manca-di-Villahermosa S, Battistini L, Stuffler RG, Tedesco M, Maccarrone M. N-3 PUFAs reduce oxidative stress in ESRD patients on maintenance HD by inhibiting 5-lipoxygenase activity. Kidney Int. 2006;69:1450–4. doi: 10.1038/sj.ki.5000291. [DOI] [PubMed] [Google Scholar]
- 14.Pandey GN, Dwivedi Y. Focus on protein kinase A and protein kinase C, critical components of signal transduction system, in mood disorders and suicide. International Journal of Neuropsychopharmacology. 2005;8:1–4. doi: 10.1017/S1461145704004936. [DOI] [PubMed] [Google Scholar]
- 15.Dwivedi Y, Rizavi HS, Shukla PK, Lyons J, Faludi G, Palkovits M, Sarosi A, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Protein kinase A in postmortem brain of depressed suicide victims: altered expression of specific regulatory and catalytic subunits. Biol Psychiatry. 2004;55:234–43. doi: 10.1016/j.biopsych.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Odagaki Y, García-Sevilla JA, Huguelet P, La Harpe R, Koyama T, Guimón J. Cyclic AMP-mediated signaling components are upregulated in the prefrontal cortex of depressed suicide victims. Brain Res. 2001;898:224–31. doi: 10.1016/s0006-8993(01)02188-6. [DOI] [PubMed] [Google Scholar]
- 17.Werz O, Klemm J, Samuelsson B, Rådmark O. 5-lipoxygenase is phosphorylated by p38 kinase-dependent MAPKAP kinases. Proc Natl Acad Sci USA. 2000;97:5261–6. doi: 10.1073/pnas.050588997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rakonjac M, Fischer L, Provost P, Werz O, Steinhilber D, Samuelsson B, Rådmark Coactosin-like protein supports 5-lipoxygenase enzyme activity and up-regulates leukotriene A4 production. Proc Natl Acad Sci USA. 2006;103:13150–15. doi: 10.1073/pnas.0605150103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallahan B, Hibbeln JR, Davis JM, Garland MR. Omega-3 fatty acid supplementation in patients with recurrent self-harm. Single-centre double-blind randomised controlled trial. Br J Psychiatry. 2007;190:118–122. doi: 10.1192/bjp.bp.106.022707. [DOI] [PubMed] [Google Scholar]
- 20.Ding Q, Wei EQ, Zhang YJ, Zhang WP, Chen Z. Cysteinyl leukotriene receptor 1 is involved in N-methyl-D-aspartate-mediated neuronal injury in mice. Acta Pharmacol Sin. 2006;27:1526–36. doi: 10.1111/j.1745-7254.2006.00438.x. [DOI] [PubMed] [Google Scholar]