Abstract
Alginate gel crosslinked by calcium ions (Ca2+) has been widely used in cartilage tissue engineering. However, most studies have been largely performed in vitro in medium with a calcium concentration ([Ca2+]) of 1.8mM, while the calcium level in the synovial fluid of the human knee joints, for example, has been reported to be 4mM or even higher. To simulate the synovial environment, the two studies in this paper were designed to investigate how the alginate scaffold alone, as well as the chondrocytes seeded alginate gel responds to variations in medium [Ca2+]. In Study A, the mechanical properties of 2% alginate hydrogel were tested in 0.15M NaCl and various [Ca2+] (1.0mM, 1.8mM, and 4mM). In Study B, primary bovine chondrocytes was seeded in alginate gel, and biochemical contents and mechanical properties were determined after incubation for 28 days in three [Ca2+] (1.8mM, 4mM, and 8mM). For both studies, it was found that the magnitude of the complex shear modulus (|G*|) at 1Hz doubled and the corresponding phase angle shift angle (δ) increased > 2° as a result of the approximate 4-fold change in [Ca2+]. At high [Ca2+], the chondrocyte glycosaminogylcan (GAG) production inside the chondrocyte-alginate constructs was suppressed significantly. This is likely due to a decrease in the porosity of the chondrocyte-alginate constructs as a result of compaction in structure caused by an increased crosslinking density with [Ca2+]. These may be important considerations in the eventual successful implementation of cartilage tissue-engineered constructs in the clinical setting.
Keywords: Cartilage tissue engineering, Alginate gel, Chondrocytes, Mechanical property, Calcium ion
1 Introduction
Articular cartilage is a dense white connective tissue covering the moving articular ends of diarthrodial joints [1]. With traumatic injuries and progressive degenerative diseases such as osteoarthritis (OA), some articulating joint cartilage loses its essential mechanical functions including load-supporting, wear and friction minimization [2]. Furthermore, since the time of Hippocrates (460BC), it has been known that articular cartilage has limited ability to repair itself, and this observation has been repeated demonstrated over time [3]. Today, autologous chondrocytes implantation (ACI) [4] is the only cell-based therapy for cartilage repair approved by US Food and Drug Administration (FDA). Because of the associated donor-site morbidity and prolonged cell cultural and surgical time, an alternative approach has been proposed with the goal aimed to make reliable and functional substitutes for biological tissues by applying the knowledge and principles of engineering and sciences [5-7]. Alginate gel crosslinked with calcium ions (Ca2+) has also been widely used for tissue engineering studies [e.g. 8, 9, 10] due to its documented biocompatibility, low toxicity, relatively low cost, and spontaneous gelation. Physiologically, calcium concentration ([Ca2+]) plays an important role in the function and organization of articular cartilage. These ions bind to proteoglycans by electrostatic force to maintain structure and function of the tissue [11]. Thus calcium metabolism and the changes in calcium ion concentration are important during the cartilage development and under pathological conditions [12]. In particular, the calcium level in synovial fluid of human knee joints has been reported to be 4mM or higher [13], while (inexplicably) most previous studies have been performed in culture medium with a [Ca2+] of 1.8mM. The increase in [Ca2+] is expected to change the structure and mechanical properties of alginate hydrogels, and therefore affect the overall cellular response of embedded chondrocytes in such tissue engineered cartilage constructs in vivo. Within the in vivo intra-articular situation, these constructs are subjected to very high loading conditions, and as such, the mechanical properties of the hydrogel will have significant influence on the states of stress and strain of the embedded chondrocytes will sustain [1,5].
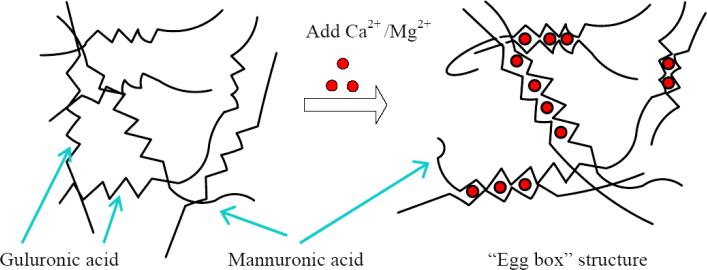
Alginate is a naturally derived linear polysaccharide comprised of (1-4)-linked β-D-mannuronic acid and α-L-guluronic acid. Divalent cations, such as Ca2+, can bind the guluronic residues in adjacent alginate chains to form an egg-box structure [14, 15] and cause the gelation of aqueous alginate solutions (Fig. 1). Monovalent ions such as Na+ can also compete with calcium ions for junction sites of guluronic residues and thereby weakening the alginate gel [16, 17]. The calcium crosslinks can be readily dissolved in the absence of the divalent ions, leading the gel to depolymerize. Alginate hydrogel has been utilized for cartilage repair, ACI and cartilage tissue engineering [9, 10, 18, 19], and has been reported to facilitate maintenance of the chondrocytic phenotype [20]. Within the three-dimensional alginate matrix, the bone-marrow-derived stem cells have been reported to differentiate into chondrocytes in an in vivo rabbit study [21], and embedding dedifferentiated chondrocytes in alginate hydrogel has led to the establishment of the characteristics of chondrocyte phenotype [22].
Figure 1.
Gelation of alginate solution with the addition of calcium or magnesium ions and formation of “Egg box” structure.
Scaffold mechanical properties are important for cartilage tissue engineering studies. Chondrocytes seeded in scaffolds are often stimulated with static or low-frequency dynamic compressive loading (leading to interstitial perfusion which is important for the transport of nutrients and waste products), and oscillatory shear to increase or accelerate tissue regeneration by increasing cell biosynthetic activities [e.g., 23, 24]. The generated mechano-electrochemical signals are highly dependent on the composition of the matrix (scaffold materials, and chondrocyte produced extracellular matrix proteins, i.e., collagen, proteoglycan, etc.) mechanical properties such as compressive modulus, shear modulus, and permeability of the solid, porous-permeable matrix [1]. Therefore, it is important to not only quantify the mechanical properties of these constructs and scaffolds, but to do so under physiological ionic conditions [25]. Most of the previously-reported mechanical properties of the alginate hydrogel have been obtained in various non-physiological conditions at low-osmotic environment [16, 26], therefore, are not directly applicable to tissue engineering for in vivo applications. LeRoux et al [17] was the first to study how the mechanical properties of alginate hydrogel change with the concentration of alginate and storage time of the hydrogel under a physiologically relevant ionic condition (0.15M NaCl and 1.8mM CaCl2). Although it has been observed that alginate hydrogel mechanical properties highly depend on [Ca2+] in the gelation solution [27, 28], no precisely controlled torsional shear and compressional tests have been performed to quantify the effects of this [Ca2+] variation on the true material properties of alginate hydrogel under physiological ionic conditions, which has a [Ca2+] of 4.0mM or higher.1 It is not known how alginate responds in these levels of [Ca2+], and more importantly, how changes in [Ca2+] within the interstitium of an alginate hydrogel could affect chondrocyte biosynthesis and matrix production in engineered cartilage based on the use of such alginate constructs.
The objective of this study is to investigate how alginate hydrogel structure and mechanical properties change with medium calcium concentration and how chondrocytes embedded in alginate hydrogel respond to the variation in external calcium concentration in culture. To this end, two experiments were conducted: Study A – examine the changes in mechanical properties of alginate hydrogel in concentrations as a function of [Ca2+] (1.0mM, 1.8mM, and 4mM); Study B – determine the chemical contents and mechanical properties of chondrocyte-embedded alginate hydrogel after culturing in media with in various calcium concentrations (1.8mM, 4mM, and 8mM) for 28 days.
2 Methods
2.1 Sample Preparation and Cell Culture
Low-viscosity sodium salt alginic acid (A2158, Sigma, St Louis, MO) was used in these studies. This alginate is derived from Macrocystitis pyrifera, has an M/G ratio of 1.67 and a molecular weight of around 50,000 Dalton. For Macrocystitis pyrifera, the molar fraction of guluronic acid is approximately 0.39 with an average block length of 5.0 [15, 17]. Two percent (2%) alginate solutions were made and sterile filtered (0.22μm porous size).
In Study A, 2% alginate solutions were gelled by diffusion in 50mM CaCl2 solution in a custom-made cylindrical Delrin mold (10mm diameter × 1.6mm thickness). After 1.5 hours, the samples (n=4) were removed from the mold, and put into a solution with 0.15M NaCl and various concentrations of CaCl2: 1mM, 1.8mM to 4mM. Media were exchanged regularly to keep the external ion concentrations outside the samples as prescribed. The samples were immersed in the solutions for 15-20 hours to render them structurally stable and at diffusion equilibrium before performing mechanical tests [17].
In Study B, chondrocytes were obtained from full-thickness articular cartilage of carpometacarpal joints of skeletally immature, 1-4 week old calves from a local abattoir (Rutland, VT) following enzymatic digestion described by Jiang et al [29]. Briefly, articular cartilage was minced into small pieces and was then subjected to serial enzymatic digestion with 0.25% pronase (Calbiochem, San Diego, CA) for 1 hour and 0.05% collagenase (Sigma) for 4 hours at 37°C. The resulting cell suspension was filtered through a sterile membrane with a pore size of ~30 μm and centrifuged for 5 minutes. The cell pellet was then mixed with 2% alginate solution at a density of 30 million cells/mL. The cell-alginate mixture was gelled in a custom mold with 50mM CaCl2 in phosphate buffered saline (PBS) for 30 minutes before the chondrocyte-alginate constructs were removed from the mold. These samples were then incubated at 37°C and 5% CO2 for 28 days in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Cellgro-Mediatech), 1% non-essential amino acids, 100 units/mL penicillin, 100 μg/mL streptomycin, and 20 μg/mL ascorbic acid (Sigma). Concentrated CaCl2 solution (2M) was added to reach calcium levels of 1.8mM, 4mM, and 8mM. During incubation, media were changed every other day.
2.2 Mechanical Testing
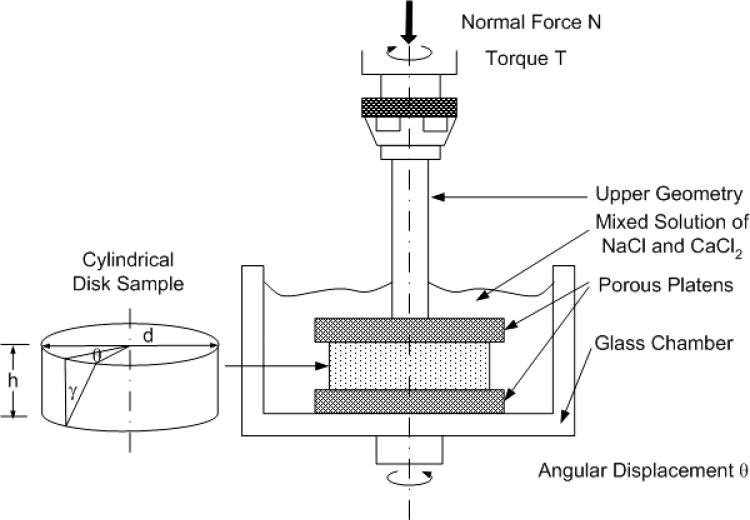
The diameter (d) of each sample from both Study A and Study B was measured with a stereomicroscope (model PP&E 56939; Bausch and Lomb, Rochester, NY). The mechanical tests were performed on a shear-strain controlled rheometer (ARES-LS1, TA instruments, New Castle, DE). The sample was placed between two flat-rough porous platens (rms surface roughness is ±30μm) to prevent it from slipping against the specimen, and immersed, to prevent dehydration, in the same solution for Study A and storage medium at 37°C for Study B [30] (Fig. 2). First, a normal tare load of about 0.025N was administered, and the reference thickness h of the sample was determined from the axial position readings of the rheometer. After 5 minutes of compressive-stress relaxation, the normal (i.e., vertical) force component (F1) was recorded. A 15% (ε) normal strain was then applied, and the normal force (F2) was again determined after another 25 minutes. From this normal force, the equilibrium compressive modulus (Eeq) of this specimen was calculated at 15% compressive strain as follows:
| (1) |
Figure 2.
Schematic illustration of an alginate gel disk in the torsional shear and compression testing device (ARES-LS1, TA instruments).
For Study A, a step shear stress relaxation test was performed after 15% compression, a step shear strain of 0.01 (γ) radian was administered by applying an angular displacement θ = 2γ h / d (see Fig. 2) and followed with a 40-minute shear-stress relaxation. The equilibrium shear moduli (Geq) were then calculated from the equilibrium torque applied (Teq) to the sample with the formula below:
| (2) |
where Ip is the polar moment of inertia of a cylinder and given by Ip = πd4 / 32.
For both Studies A and B, a dynamic shear test was performed over frequencies ranging from 0.01 Hz to 10 Hz on a logarithmic frequency sweep with shear strain amplitude γ0 = 0.01 radian. The complex (dynamic) shear modulus was calculated from G* = Td/(2Ip γ) as a function of frequency (ω), where, γ is a sinusoidal shear strain applied on the sample with the form γ = γ0 sin(ωt), where γ0 is the amplitude and ω is the angular frequency, and T is the torque response. Generally, G* is a complex number for viscoelastic materials such as hydrogels, and can be expressed as, where G* = G'+iG", G' and G" are the storage modulus and loss modulus, respectively. The magnitude of the dynamic shear modulus (|G*|) is given by , and the phase shift angle (δ) between the applied strain and the torque response is calculated from δ = tan−1(G"/G') [30].
2.3 Biochemical Assays and Histology
For Study B, after mechanical tests, each sample was first washed with PBS and then halved with one half frozen for biochemical assays and the other half used for histology. For biochemical assays, the sample was first gently blotted dry and weighed (wet weight), and then the sample was lyophilized and the dry weight was obtained. Water content (%) was calculated as (wet weight – dry weight)/wet weight. Samples were then digested for 16 hours at 60°C with 20 μl/mL papain in 0.1M sodium acetate, 10mM cysteine HCl, and 50mM ethylenediaminetetraacetate (EDTA).
An aliquot of the digest was used to determine the total DNA per sample using the PicoGreen dsDNA assay [31] (Molecular Probes, Eugene, OR) following the protocol provided by the manufacturer. Fluorescence was measured with a microplate reader (Tecan, Maennedorf, Switzerland) with excitation and emission wave-lengths of 485nm and 535nm, respectively.
To quantify the glycosaminogylcan (GAG) content, a modified 1,9-dimethylmethylene blue (DMMB) dye-binding assay [32, 33] with chondroitin-6-sulfate (Sigma) as a standard was used. To account for the anionic nature of the carboxyl groups on the alginate hydrogel, the pH of the DMMB dye was adjusted to be 1.5 with the concentrated formic acid (Sigma) so that only the sulfated GAG-DMB complexes were detectable with the spectrophotometer. The absorbance at both 540nm and 595nm was used to improve signal detection [34].
Sample collagen content was quantified with a simplified hydroxyproline assay [35-37]. Specifically, an aliquot of the digest was hydrolyzed with high concentration sodium hydroxide (2M) at 120°C for 20 minutes. The hydrolyzate was then oxidized by a buffered choloramine-T (Sigma) reagent for 25 minutes before the addition of Ehrlich's reagent (15% p-dimethylaminobenzaldehyde in the mixed solution of isopropanol / percholoric acid (2:1)), and the absorbance was measured at 550 nm (Tecan). The hydroxyproline content was converted to collagen content using a conversion ratio of 1:10 hydroxyproline: collagen [38].
For histology, the sample was fixed with acid formalin (4% formaldehyde in 70% ethanol and 5% acetic acid), dehydrated with a graded series of ethanol, and embedded in paraffin for histological study. The samples were sectioned (7.5 μm) and mounted on microscope slides prior to staining. Sample GAG distribution was stained with Alcian blue staining at pH = 1.5, and collagen distribution was ascertained with Picosirius red staining [39]. Stained sections were imaged using the Axiovert 35 microscope (Zeiss, Oberkochen, Germany).
2.4 Statistical Analysis
Data are presented as means ± standard deviations. In Study A, one-way analysis of variance (ANOVA) was performed on the equilibrium compressive modulus, shear modulus, and the magnitude of dynamic shear modulus and phase shift angle at 1 Hz to assess the effects of varying calcium concentrations on alginate hydrogel. In study B, ANOVA was also performed on chemical contents for the chondrocyte-embedded alginate hydrogel constructs. For both studies, the effects of external ion concentrations and frequency on the magnitude of dynamic shear modulus |G*| and phase shift angle δ were assessed by two-way ANOVA, and the effects of the frequency on |G*| at the lowest frequency (0.01Hz) and the highest frequency (10Hz) were tested with an ANOVA with repeated measures. Student-Newman-Keuls (SNK) tests were performed at a 95% confidence interval to determine specific differences between groups. All statistical analyses were performed using the Statistical Analysis Software (SAS, Cary, NC).
3 Results
3.1 Effects of [Ca2+] on Alginate Hydrogel
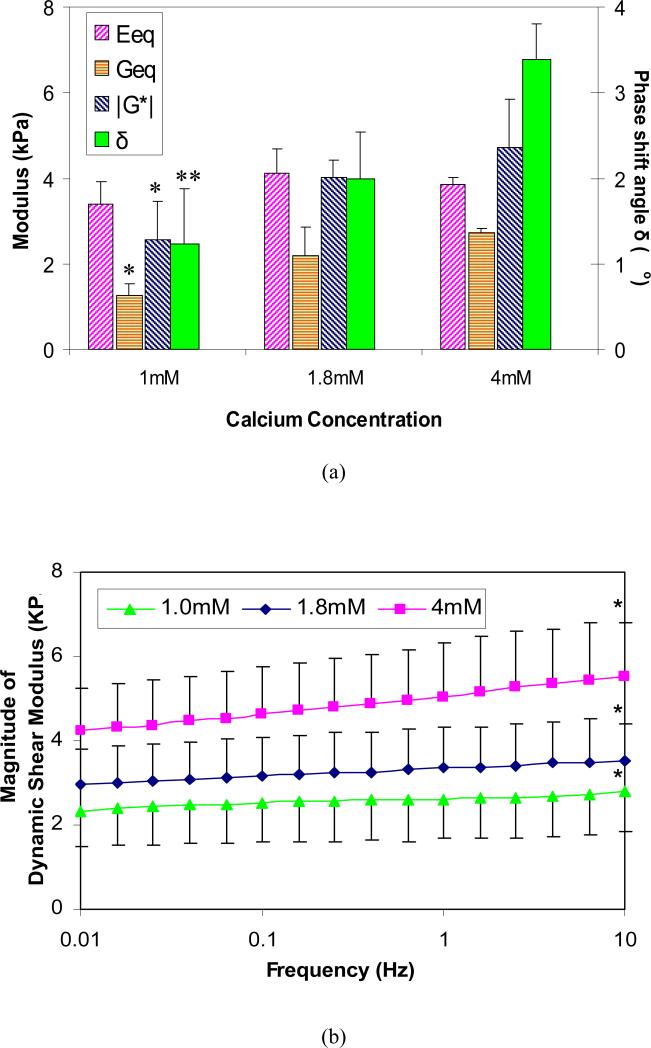
For alginate hydrogel samples, it was found that the equilibrium shear modulus Geq depended significantly on the calcium concentration in the external solutions (p<0.05), while the ion concentration effects on the equilibrium compressive modulus Eeq were not significant (p>0.05; Fig. 3a). The values for Geq more than doubled (1.26 ± 0.28 kPa vs. 2.72 ± 0.11 kPa) when [Ca2+] increased from 1mM to 4mM.
Figure 3.
(a) Effects of calcium concentration on the equilibrium compressive modulus Eeq and equilibrium shear modulus Geq, the magnitude of dynamic shear modulus |G*| and phase shift angle δ of alginate hydrogel at 1Hz (n=4; * significant compared to [Ca2+] = 4mM, ** significantly different compared to [Ca2+] = 1.8mM and 4mM, p<0.05, SNK); (b) Changes in the magnitude of dynamic shear modulus (|G*|) of alginate gel with loading frequency (0.01Hz-10Hz) in 0.15M NaCl with various CaCl2 concentrations (n=4; * Significantly different from 0.01Hz; p<0.05, SNK).
Similar to the equilibrium shear behavior, both the magnitude of the dynamic shear modulus and phase shift angle at 1Hz increased significantly with calcium concentration from 1mM to 4mM (p<0.05) as shown in Fig. 3a. The magnitude of the complex modulus increased from 2.63±0.93 kPa to 5.17±1.25 kPa when [Ca2+] increased from 1mM to 4mM, and the corresponding phase angle shift angle increased from 1.22° ± 0.65 ° to 3.38 ° ± 0.43 °.
The magnitude of dynamic shear modulus (|G*|) increased linearly with the logarithm of frequency (p<0.0001), while phase shift angle δ varied little with frequency. Both |G*| and δ (not shown) increased with [Ca2+] (p<0.0001; Fig. 3b). Shear moduli of alginate hydrogel tested at the highest frequency (10 Hz) and at the lowest frequency (0.01 Hz) was also found to be significantly different for each other (p<0.05).
3.2 Effects of [Ca2+] on Biosynthesis and Related Alginate Hydrogel Mechanical Properties
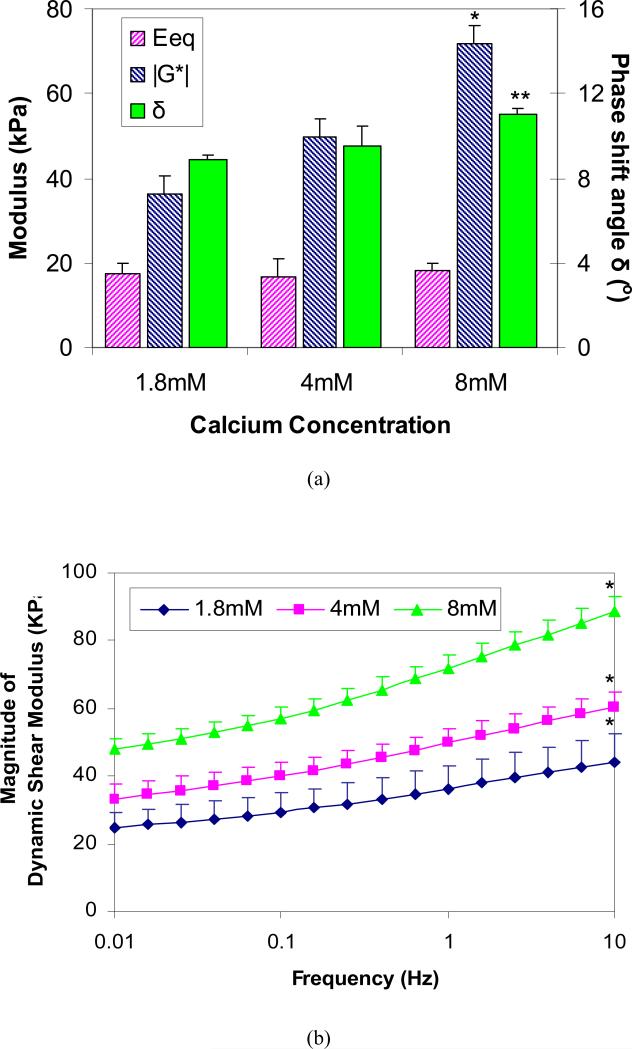
For chondrocyte embedded alginate hydrogel constructs after the 28 days of culture, it was found that the magnitude of the dynamic shear modulus |G*| was strongly dependent on the calcium ion concentration in solution (p<0.05), while the calcium ion effect on the equilibrium compressive modulus Eeq was not significant (Fig. 4a). The value for |G*| at 1Hz doubled (36.4 ± 6.9 kPa vs. 72.0 ± 4.0 kPa) when [Ca2+] increased from 1.8mM to 8mM, while the phase shift angle δ also increased significantly from 8.9 ° ± 0.2° to 11.0 ° ± 0.2°. The dynamic modulus |G*| increased almost linearly with the logarithm of the frequency (Fig. 4b), and significant difference between two extreme frequencies (i.e., 0.01Hz and 10Hz) was detected for all groups (p<0.05).
Figure 4.
(a) Effects of calcium concentration on the equilibrium compressive modulus Eeq and the magnitude of dynamic shear modulus |G*| and phase shift angle δ at 1Hz for chondrocyte-embedded alginate hydrogel constructs after incubation for 28 days (n=4; * Significant differences between all groups, ** Significantly different from groups [Ca2+] = 1.8mM and 4mM; p<0.05, SNK); (b) Effects of calcium concentration on frequency-dependent dynamic shear modulus of chondrocyte- alginate constructs after incubation for 28 days. (* Significantly different from 0.01Hz; p<0.05, SNK).
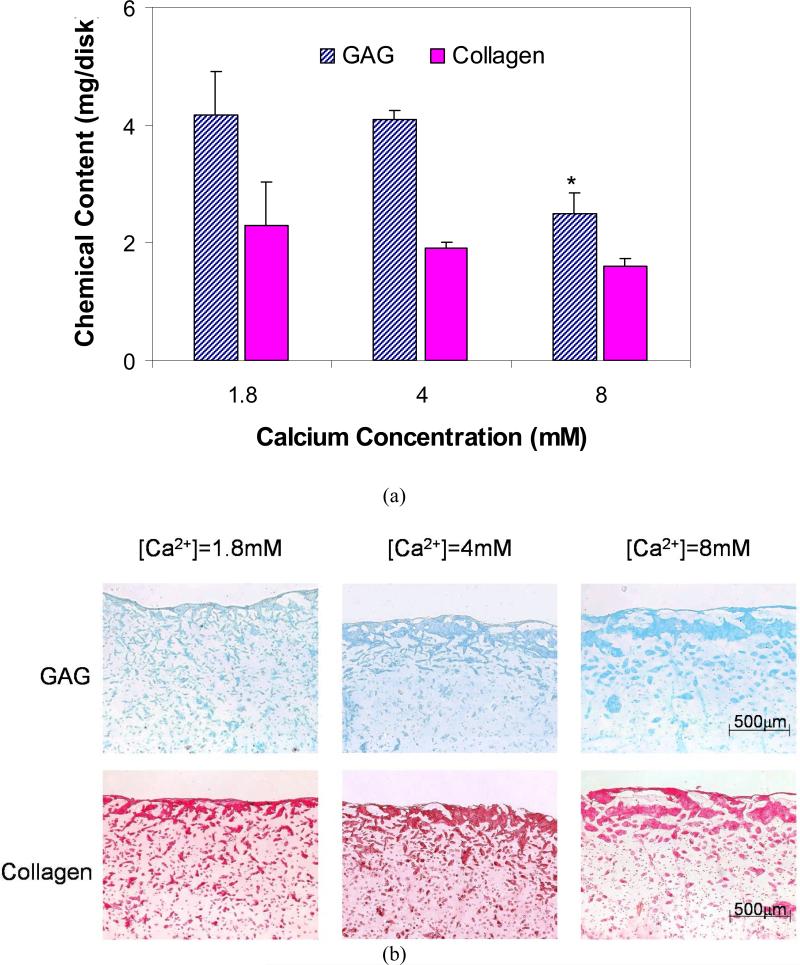
The diameter, wet weight, and total GAG amount of samples were found to decrease significantly with increasing [Ca2+] (Table 1, Fig. 5a). The total DNA and collagen amount decreased slightly but not significantly. These variations of GAG and collagen with [Ca2+] were supported by the appearance of the histological staining sections (Fig. 5b). Both the GAG distribution stained with Alcian blue (Fig. 5b top) and the collagen distribution stained with Picosirius Red (Fig. 5b bottom) showed that there was apparently more deposition at the exteriors of the constructs than the interiors and that the deposition in the interiors decreases with increasing [Ca2+]. Two important differences are noted between these chondrocyte-embedded alginate hydrogels tissue engineering constructs and native articular cartilage: 1) the water content of these tissue engineering constructs are very high, i.e., > 93%, whereas in normal articular cartilage it is ~ 78% [44]; and 2) the collagen does not appear to be organized in a layered-anisotropic manner as in native articular cartilage [1].
Table 1.
Chemical contents (total weights) and dimension of chondrocyte-alginate constructs (n=4) after incubation for 28 days.
| [Ca2+] (mM) | |||
|---|---|---|---|
| 1.8 | 4.0 | 8.0 | |
| Diameter (mm) | 10.79±0.38 | 10.86±0.51 | 9.53±0.12* |
| Wet Weight (mg) | 302±10 | 286±2 | 236±3* |
| Water (mg) | 287±9 | 269±2 | 221±2* |
| Water Content (%) | 95.1±0.4 | 94.4±0.4 | 93.7±0.4 |
| DNA (μg) | 25.85±5.54 | 29.35±7.23 | 22.21±9.71 |
| Collagen (mg) | 2.29±0.73 | 1.89±0.10 | 1.61±0.11 |
| GAG (mg) | 4.17±0.73 | 4.09±0.15 | 2.49±0.35* |
Significantly different from [Ca2+]=1.8mM and [Ca2+]=4.0mM; p<0.05, SNK
Figure 5.
Effects of calcium concentration (a) on glycosaminoglycan (GAG) and collagen contents inside chondrocyte-embedded alginate hydrogel constructs after incubation for 28 days. (n=4; * Significant differences relative to [Ca2+] = 1.8mM and 4.0mM; p<0.05, SNK) and (b) on the distribution of GAG and collagen inside constructs (Top: Alcian Blue staining; Bottom: Picosirius Red staining).
4 Discussion
The objective of this study was to investigate the effects of the physiologic synovial level [Ca2+] on alginate hydrogel and alginate hydrogel seeded with chondrocytes. Specifically, compression and shear properties of the samples were determined as a function of [Ca2+]. In addition, the effects of the [Ca2+] increase on matrix synthesis and distribution by chondrocytes in alginate hydrogel were investigated. Overall, it was found that changes in Ca2+ concentration significantly altered the shear properties of alginate hydrogel and chondrocyte-embedded alginate hydrogel constructs under both the equilibrium and dynamic tests, while surprisingly no significant changes in the compressive properties were detected. This had occurred even with the modification of the samples swelling pressure (Donnan osmotic pressure). The phase shift angle of dynamic shear tests, a measure of the matrix viscoelasticity, was found to increase significantly with calcium concentration. The GAG content for chondrocyte-embedded alginate hydrogel constructs decreased significantly with calcium concentration, while their shear properties increased. The variation of scaffold shear properties with calcium concentration could significantly alter the magnitude of mechano-electrochemical signals in situ (such as stresses, strains, fluid flow and electrical potential, i.e., with a decrease of the charged GAG molecules) and therefore the chondrocyte biosynthetic response, and also affect the continued integrity of the constructs and the stress-strains fields surrounding of host cartilage tissue under joint loading [1].
Hydrogels have often been modeled as biphasic materials consisting of a mixture of two incompressible phases: solid phase and water phase [40, 41]. Hydrogels exhibit viscoelastic behavior when the water or fluid inside the porous-permeable hydrogel is forced to gradually flow out under compression. Previously, the linear isotropic biphasic model [42] has been used to describe the mechanical behavior of agarose and study nutrient transport through the pores of an agarose matrix [40, 41]. For this study on the effects of [Ca2+] on alginate hydrogel, however, the situation is much more complex because the hydrogel matrix is negatively charged due to the carboxyl groups present on guluronic and mannuronic residues at the physiological pH range and mechano-electrochemical effects may not be ignored [1]. Similar to articular cartilage, the alginate hydrogel can be, and has been successfully, treated as a triphasic material which includes an additional phase, mobile ions flowing through a charged, porous-permeable matrix. Due to the existence of fixed negative charges attached to the solid matrix of alginate hydrogel, a Donnan osmotic pressure will be generated within the hydrogel, and complex mechano-electrochemical events will occur inside the hydrogel upon mechanical or osmotic loading [1, 13]. In this context, the torsional shear test has special advantages in its ability to determine the shear properties of the specimen since it is an equivoluminal test and thus interstitial fluid flow is minimized. No fluid flow relative to the solid matrix occurs when the applied shear strain is kept small during the test. Therefore, unlike the compressive response, the viscoelastic behavior under torsional shear tests comes solely from the solid matrix of the hydrogel. In other words, the pure shear test is particularly advantageous for quantifying the viscoelastic behavior of the solid phase of alginate hydrogel.
For the alginate hydrogel, the measured equilibrium compressive modulus (~ 4 kPa) and shear modulus (~ 2 kPa) at blood plasma ionic condition (0.15M NaCl and 1.8mM CaCl2) agree well with previous experimental results obtained by LeRoux et al [17]. In this study, the extent to which the mechanical properties change under physiological ionic conditions has been quantified as well, and the results indicate that variations in calcium concentration may significantly affect the shear properties of alginate hydrogel. Increased calcium concentration will promote the formation of additional crosslinks inside the alginate hydrogel and stiffen the alginate samples. Intuitively, the phase shift angle would accordingly decrease. However, the measured phase shift angle was shown to significantly increase with [Ca2+] (δ : 1.2° ±0.6° vs. 3.4° ± 0.4°, at [Ca2+] = 1.0mM vs. 4.0mM). This might be due to the increase in solid volume fraction as a result of the sample shrinkage (swollen diameter: 9.67 ± 0.06mm vs. 9.19 ± 0.07mm, at [Ca2+] = 1.0mM vs. 4.0mM). It led to higher friction between polymer chains inside the alginate hydrogel which dominated the effect of increased Ca2+ cross-linking density, resulting in the increase of the phase shift angle [17].
For chondrocyte-embedded alginate hydrogel constructs, mechanical properties increase with time as a result of the longitudinal cellular production of extracelluar matrix collagen and GAG. The compressive properties of these composite constructs in the medium with a [Ca2+] of 1.8mM at Day 28 are comparable to those reported in several previous studies [19, 40]. Awad et al [43] measured shear properties of alginate tissue engineered constructs using human adipose-derived stem cells and focused on the potential of these cells for chondrogenic differentiation. However, no shear properties of alginate constructs seeded with primary chondrocytes have been reported.
From Day 1 to Day 28, the equilibrium compressive modulus increased about 5 folds from 4 kPa to 18 kPa, while the magnitude of dynamic shear modulus at 1Hz increased about 12 folds from 3 kPa to 36 kPa. This increase in both compressive and shear properties with time is directly associated with the deposition of the GAG and collagen inside the constructs and the resulting decrease in the water content or matrix porosity. The dependence of mechanical properties on the hydrogel porosity has been reported for both soft tissue such as articular cartilage [1, 44] and hard tissue such as bone [45]. Furthermore, the phase shift angle at 1 Hz increased from 2° to 9° from Day 1 to Day 28, which indicates the internal energy dissipation increase may be the result of the increase in cell matrix deposition with time. Previously, it was reported that the phase shift angle of alginate hydrogel remains essentially unchanged when the alginate concentration varied as a result of the counteracting effects from the increase of calcium crosslinks and the solid content [17]. The calculated increase in phase shift angle suggests that in the chondrocyte-embedded alginate hydrogels, the deposited GAG and collagen have yet to form effective crosslinks as strong as the linkages between guluronic residues of the alginate chains [30].
When compared to the native articular cartilage, the equilibrium mechanical properties of these cell-seeded constructs are relatively low even after 28 days of incubation. Specifically, the compressive modulus of these chondrocyte-laden alginate constructs is about 25 times lower than that of native cartilage, while the dynamic shear modulus is about 30 times lower [1]. This is largely due to the relatively lower chemical contents in comparison with native cartilage, with the GAG content of the constructs being 3 times lower, and the collagen content approximately 10 times lower. This may be due to their very high water content (>93%) when compared to normal native articular cartilage (~78%). It is known that for osteoarthritic cartilage, the % hydration increases as the histochemical measures increase [1, 13, 44]; when the hydration of such tissues reaches > 90%, the tissue no longer can provide load support. For the native cartilage, the phase shift angle at 1Hz is about 10° [30], while it is around 9° for chondrocyte-embedded alginate hydrogel constructs after incubation for 28 days. For native articular cartilage, the shear property is highly dependent on the collagen content [30], while for chondrocyte-alginate constructs it is dependent on the density of Ca2+ crosslinks, rather than the collagen content, as indicated by the increase of dynamic shear modulus with increased [Ca2+] despite the decrease of the collagen and GAG contents. This difference is likely due to the small amount of collagen fibrils produced by chondrocytes in vitro, and its restricted deposition to a small region surrounding the cells (Fig. 5b bottom). Consequently, collagen fibers have not been well assembled nor well organized within the alginate hydrogel to contribute to the increase in shear modulus. The lack of a collagen ultrastructural architecture defeats the tension-compression nonlinearity that is needed for effective interstitial fluid pressurization essential for hydrodynamic load support [1, 25, 42].
In this study, no significant effects of [Ca2+] on the equilibrium compressive modulus have been detected for the alginate hydrogel and chondrocyte-embedded alginate hydrogel constructs, but the shear modulus did increase significantly with [Ca2+]. The exact cause is not known and needs future investigation. It could be due to the bimodular response of polymer chains and collagen fibrils, as they exhibit much higher modulus in tension while lower modulus under compression. The polymer chains and collagen fibrils aligned in the tensile principal stress direction significantly contribute to the shear modulus of the solid matrix [1], and therefore shear tests are more sensitive to the crosslinking density changes caused by variations of [Ca2+]. It could also be due to matrix inhomogeneities inside the samples. As indicated by our histological staining (Fig. 5b), the high calcium level group usually contains a thicker exterior or surface region with high GAG and collagen contents. The dense extracellular matrix there may significantly increase the shear modulus of the whole constructs while contributing little to the compressive modulus.
Generally, a calcium concentration larger than 10 mM is considered to be toxic to cells, but the direct effects of calcium level below 10 mM on primary chondrocytes have not been well-studied. Collagen production was reported to be the lowest at [Ca2+] = 1 to 2mM in a chondrocyte-monolayer study, and an increase of [Ca2+] from 2mM stimulated collagen production [12]. Other studies show that a lower calcium level can stimulate the Type X collagen synthesis and suppress the Type I collagen synthesis while leaving the Type II collagen unchanged [46, 47]. No significant effect of calcium concentration on GAG production has been reported. Based on these studies, no significant decrease of ECM (i.e., collagen and GAG) production has ever been reported when [Ca2+] increases from 1.8mM to 8.0mM. In contrast, we noticed in this study that both GAG and collagen in the histological staining of Study B (Fig. 5b) are present in lower amounts at 8mM than 1.8mM in the construct interior, whereas their distribution are comparable for all groups at the rim of the samples. These observations suggest that the variation in [Ca2+] has negligible direct effects on chondrocyte synthetic response and that the difference in matrix production is probably caused by nutrient transport suppression at higher [Ca2+] due to increased hydrogel cross-linking density. Bioreactors coupled with perfusion and dynamic loading devices can be used to test this hypothesis, and possibly to improve the nutrient transport through these tissue engineered constructs.
5 Conclusion
This study has provided new insight into the changes of the basic structure and viscoelastic properties of the alginate hydrogels subject to varying calcium concentration under physiologically relevant ionic conditions as well as its influence on the response of primary bovine chondrocytes seeded in this alginate scaffold. These findings have enhanced our understanding of how the outcome of alginate-based cartilage repair therapies are affected by the elevated calcium level as would be in the knee joints in both autologous chondrocytes implantation and cartilage tissue engineering. It was found that moderate increases in calcium concentration can significantly elevate the shear modulus of alginate hydrogel and chondrocyte-alginate constructs. The compaction in structure due to loss of total volume as a result of increased [Ca2+] decreases the porosity of the chondrocyte-alginate constructs, which impedes nutrient transport, resulting in lower ECM production and cell proliferation. These are important considerations in the implementation of tissue-engineered constructs for cartilage repair.
Acknowledgements
This study is supported by Whitaker Foundation Special Development Award (Mow), Stanley Dicker and Shelly Ping Liu endowments (Mow), NIH grants R01 AR048287 and R21 AR052417 (Guo), and R21 AR052402-01A1 (Lu). The authors thank Drs. Gordana Vunjak-Novakovic and Jeremy J. Mao for their helpful suggestions and discussion of results.
Footnotes
References
- 1.Mow VC, Gu WY, Chen FH. Structure and function of articular cartilage and meniscus. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechano-Biology. Lippincott Williams and Wilkins; Philadelphia: 2005. pp. 181–258. [Google Scholar]
- 2.Mankin HJ, Mow VC, Buckwalter JA, Iannotti JP, Ratcliffe A. Articular cartilage structure, composition, and function. In: Buckwalter JA, Einhorn TA, Simon SR, editors. Orthopaedic Basic Science: Biology and Biomechanics of the Musculoskeletal System. American Academy of Orthopaedic Surgeons Publishers; Rosemont, IL, USA: 2000. pp. 443–470. [Google Scholar]
- 3.Hunziker EB. Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthritis Cartilage. 1999;7:15–28. doi: 10.1053/joca.1998.0159. [DOI] [PubMed] [Google Scholar]
- 4.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 5.Guilak F, Mow VC. The biomechanical environment of the chondrocyte: A biphasic finite element model of cell-matrix interactions in articular cartilage. J Biomechanics. 2000;33:1663–73. [PubMed] [Google Scholar]
- 6.Butler DL, Goldstein SA, Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122:570–5. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 7.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 8.Masuda K, Sah RL, Hejna MJ, Thonar EJ. A novel two-step method for the formation of tissue-engineered cartilage by mature bovine chondrocytes: the alginate-recovered-chondrocyte (ARC) method. J Orthop Res. 2003;21:139–48. doi: 10.1016/S0736-0266(02)00109-2. [DOI] [PubMed] [Google Scholar]
- 9.Paige KT, Cima LG, Yaremchuk MJ, Schloo BL, Vacanti JP, Vacanti CA. De novo cartilage generation using calcium alginate-chondrocyte constructs. Plast Reconstr Surg. 1996;97:168–78. doi: 10.1097/00006534-199601000-00027. discussion 179-80. [DOI] [PubMed] [Google Scholar]
- 10.Wong M, Siegrist M, Gaschen V, Park Y, Graber W, Studer D. Collagen fibrillogenesis by chondrocytes in alginate. Tissue Eng. 2002;8:979–87. doi: 10.1089/107632702320934074. [DOI] [PubMed] [Google Scholar]
- 11.Campo RD. Effects of cations on cartilage structure: swelling of growth plate and degradation of proteoglycans induced by chelators of divalent cations. Calcif Tissue Int. 1988;43:108–21. doi: 10.1007/BF02555156. [DOI] [PubMed] [Google Scholar]
- 12.Koyano Y, Hejna M, Flechtenmacher J, Schmid TM, Thonar EJ, Mollenhauer J. Collagen and proteoglycan production by bovine fetal and adult chondrocytes under low levels of calcium and zinc ions. Connect Tissue Res. 1996;34:213–25. doi: 10.3109/03008209609000700. [DOI] [PubMed] [Google Scholar]
- 13.Maroudas A. Physicochemical properties of articular cartilage. In: Freeman MAR, editor. Adult Articular Cartilage. Pitman Medical; Kent, UK: 1979. pp. 215–290. [Google Scholar]
- 14.Grant GT, Morris ER, Rees DA, Smith PJC, Thom D. Biological Interactions between polysaccharides and divalent cations - egg-box model. Febs Letters. 1973;32:195–198. [Google Scholar]
- 15.Smidsrod O, Skjak-Braek G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990;8:71–8. doi: 10.1016/0167-7799(90)90139-o. [DOI] [PubMed] [Google Scholar]
- 16.Segeren AJM, Boskamp JV, Vandentempel M. Rheological and swelling properties of alginate gels. Faraday Discussions. 1975;1974:255–262. [Google Scholar]
- 17.LeRoux MA, Guilak F, Setton LA. Compressive and shear properties of alginate gel: effects of sodium ions and alginate concentration. J Biomed Mater Res. 1999;47:46–53. doi: 10.1002/(sici)1097-4636(199910)47:1<46::aid-jbm6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Guo JF, Jourdian GW, MacCallum DK. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect Tissue Res. 1989;19:277–97. doi: 10.3109/03008208909043901. [DOI] [PubMed] [Google Scholar]
- 19.Wong M, Siegrist M, Wang X, Hunziker E. Development of mechanically stable alginate/chondrocyte constructs: effects of guluronic acid content and matrix synthesis. J Orthop Res. 2001;19:493–9. doi: 10.1016/S0736-0266(00)90023-8. [DOI] [PubMed] [Google Scholar]
- 20.Hauselmann HJ, Fernandes RJ, Mok SS, Schmid TM, Block JA, Aydelotte MB, Kuettner KE, Thonar EJ. Phenotypic stability of bovine articular chondrocytes after long-term culture in alginate beads. J Cell Sci. 1994;107(Pt 1):17–27. doi: 10.1242/jcs.107.1.17. [DOI] [PubMed] [Google Scholar]
- 21.Diduch DR, Jordan LC, Mierisch CM, Balian G. Marrow stromal cells embedded in alginate for repair of osteochondral defects. Arthroscopy. 2000;16:571–7. doi: 10.1053/jars.2000.4827. [DOI] [PubMed] [Google Scholar]
- 22.Bonaventure J, Kadhom N, Cohen-Solal L, Ng KH, Bourguignon J, Lasselin C, Freisinger P. Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp Cell Res. 1994;212:97–104. doi: 10.1006/excr.1994.1123. [DOI] [PubMed] [Google Scholar]
- 23.Guilak F, Hung CT. Physical regulation of cartilage metablism. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechano-Biology. Lippincott Williams and Wilkins; Philadelphia: 2005. pp. 259–300. [Google Scholar]
- 24.Vunjak-Novakovic G, Goldstein SA. Biomechanical principles of cartilage and bone tissue engineering. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechano-Biology. Lippincott Williams and Wilkins; Philadelphia: 2005. pp. 343–408. [Google Scholar]
- 25.Mow VC, Wang CC, Hung CT. The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage. Osteoarthritis Cartilage. 1999;7:41–58. doi: 10.1053/joca.1998.0161. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell JR, Blanshard JMV. Rheological properties of alginate gels. Rheol Acta. 1974;13:180–184. [Google Scholar]
- 27.Thu B, Bruheim P, Espevik T, Smidsrod O, Soon-Shiong P, Skjak-Braek G. Alginate polycation microcapsules. I. Interaction between alginate and polycation. Biomaterials. 1996;17:1031–40. doi: 10.1016/0142-9612(96)84680-1. [DOI] [PubMed] [Google Scholar]
- 28.Velings NM, Mestdagh MM. Physicochemical properties of alginate gel beads. Polymer Gels and Networks. 1995;3:311–330. [Google Scholar]
- 29.Jiang J, Nicoll SB, Lu HH. Co-culture of osteoblasts and chondrocytes modulates cellular differentiation in vitro. Biochemical and biophysical research communications. 2005;338:762–70. doi: 10.1016/j.bbrc.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 30.Zhu W, Mow VC, Koob TJ, Eyre DR. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J Orthop Res. 1993;11:771–81. doi: 10.1002/jor.1100110602. [DOI] [PubMed] [Google Scholar]
- 31.McGowan KB, Kurtis MS, Lottman LM, Watson D, Sah RL. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen and Hoechst 33258. Osteoarthritis Cartilage. 2002;10:580–7. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- 32.Enobakhare BO, Bader DL, Lee DA. Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1,9-dimethylmethylene blue. Anal Biochem. 1996;243:189–91. doi: 10.1006/abio.1996.0502. [DOI] [PubMed] [Google Scholar]
- 33.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–8. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 34.Seibel MJ, Macaulay W, Jelsma R, Saed-Nejad F, Ratcliffe A. Antigenic properties of keratan sulfate: influence of antigen structure, monoclonal antibodies, and antibody valency. Arch Biochem Biophys. 1992;296:410–8. doi: 10.1016/0003-9861(92)90591-j. [DOI] [PubMed] [Google Scholar]
- 35.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–9. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 36.Huszar G, Maiocco J, Naftolin F. Monitoring of collagen and collagen fragments in chromatography of protein mixtures. Anal Biochem. 1980;105:424–9. doi: 10.1016/0003-2697(80)90481-9. [DOI] [PubMed] [Google Scholar]
- 37.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–73. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 38.Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130–8. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 39.Bancroft JD, Gamble M. Theory and Practice of Histological Techniques. Churchill Livingstone; New York: 2002. [Google Scholar]
- 40.Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, Hung CT, Ateshian GA. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252–60. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 41.Goldsmith AA, Clift SE. Investigation into the biphasic properties of a hydrogel for use in a cushion form replacement joint. J Biomech Eng. 1998;120:362–9. doi: 10.1115/1.2798003. [DOI] [PubMed] [Google Scholar]
- 42.Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression: theory and experiments. J Biomech Eng. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 43.Awad HA, Wickham MQ, Leddy HA, Gimble JM, Guilak F. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials. 2004;25:3211–22. doi: 10.1016/j.biomaterials.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 44.Armstrong CG, Mow VC. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J Bone Joint Surg Am. 1982;64:88–94. [PubMed] [Google Scholar]
- 45.Guo XE. Mechanical properties of cortical bone and cancelllous bone tissue. In: Cowin SC, editor. Bone Mechanics Handbook. CRC press; Boca Raton, FL: 2001. pp. 10–1. [Google Scholar]
- 46.Bonen DK, Schmid TM. Elevated extracellular calcium concentrations induce type X collagen synthesis in chondrocyte cultures. J Cell Biol. 1991;115:1171–8. doi: 10.1083/jcb.115.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gigout A, Jolicoeur M, Buschmann MD. Low calcium levels in serum-free media maintain chondrocyte phenotype in monolayer culture and reduce chondrocyte aggregation in suspension culture. Osteoarthritis Cartilage. 2005;13:1012–24. doi: 10.1016/j.joca.2005.06.003. [DOI] [PubMed] [Google Scholar]