Abstract
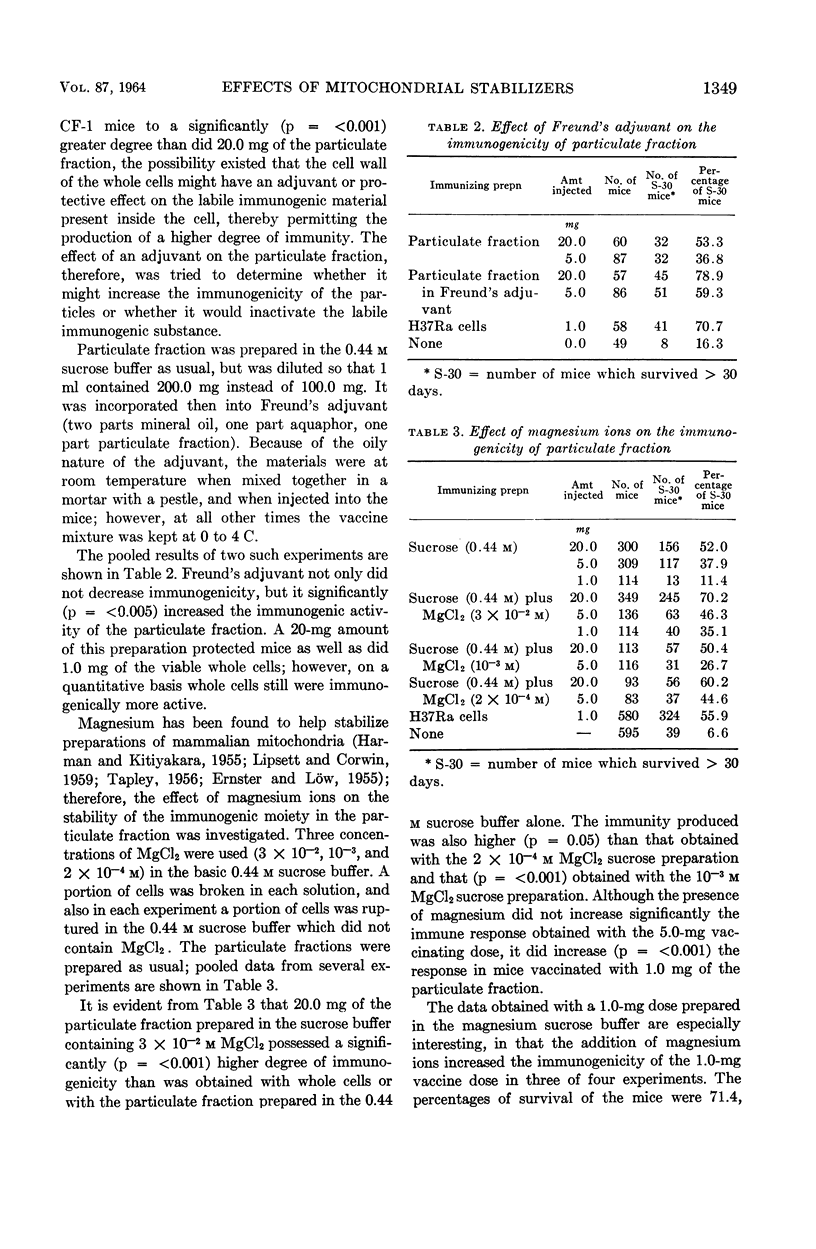
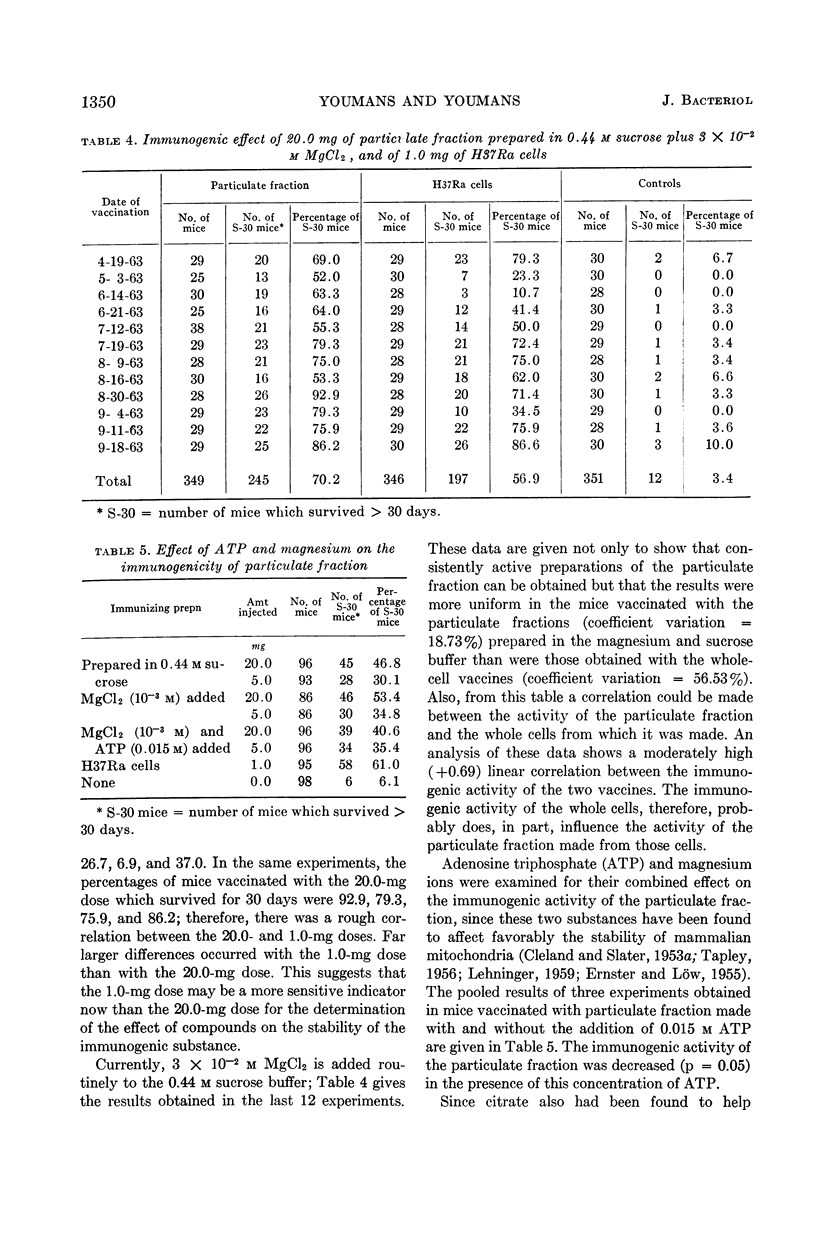
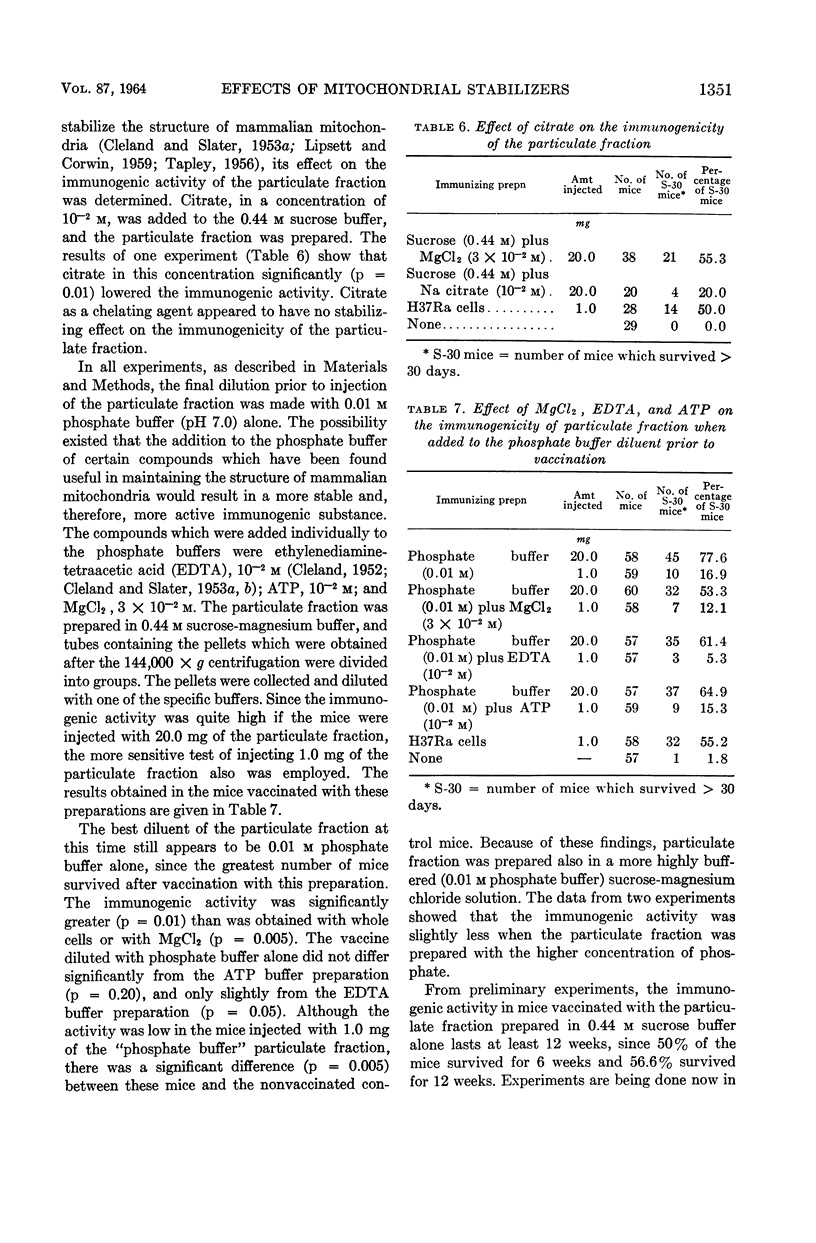
Youmans, Anne S. (Northwestern University Medical School, Chicago, Ill.), and Guy P. Youmans. Effect of mitochondrial stabilizers on the immunogenicity of the particulate fraction isolated from Mycobacterium tuberculosis. J. Bacteriol. 87:1346–1354. 1964.—A number of substances which have been used to stabilize mammalian mitochondrial preparations were tested to determine whether they would similarly affect the immunogenicity of a particulate fraction prepared from ruptured viable attenuated mycobacterial cells. The use of 0.44 m sucrose and the presence of 3 × 10−2m MgCl2 during the preparatory processes markedly increased the immunogenicity of the particulate fraction. The increase was so great that immunogenic preparations were then consistently obtained which, in adequate dosage, were more immunogenic in CF-1 male mice than were viable attenuated mycobacterial cells. On the other hand, adenosine triphosphate (ATP), citrate, and polyvinylpyrrolidone when present during the preparatory processes reduced the immunogenicity. The addition of MgCl2, ethylene-diaminetetraacetate, or ATP to the particulate fraction after it had been prepared did not increase its immunogenicity. When the particles were prepared in the 0.44 m sucrose buffer alone, incorporated in Freund's adjuvant, and injected intraperitoneally, immunogenicity was increased. However, this increase was not significantly greater than that obtained when the particles were prepared in the sucrose buffer containing MgCl2. The immune state engendered in mice by the intraperitoneal injection of the particulate fraction persisted for at least 12 weeks.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A., McNAMARA P. Polynucleotide phosphorylase in isolated bacterial cell membranes. J Biol Chem. 1962 Jan;237:170–175. [PubMed] [Google Scholar]
- BRODIE A. F., GRAY C. T. Bacterial particles in oxidative phosphorylation. Science. 1957 Mar 22;125(3247):534–537. doi: 10.1126/science.125.3247.534. [DOI] [PubMed] [Google Scholar]
- CHAPMAN G. B., HANKS J. H., WALLACE J. H. An electron microscope study of the disposition and fine structure of Mycobacterium lepraemurium in mouse spleen. J Bacteriol. 1959 Feb;77(2):205–211. doi: 10.1128/jb.77.2.205-211.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND K. W. Permeability of isolated rat heart sarcosomes. Nature. 1952 Sep 20;170(4325):497–499. doi: 10.1038/170497a0. [DOI] [PubMed] [Google Scholar]
- CLELAND K. W., SLATER E. C. Respiratory granules of heart muscle. Biochem J. 1953 Mar;53(4):547–556. doi: 10.1042/bj0530547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARTER R. W., MILLMAN I. Localization of mycobacterial enzymes. Proc Soc Exp Biol Med. 1957 Jul;95(3):440–446. doi: 10.3181/00379727-95-23245. [DOI] [PubMed] [Google Scholar]
- DOUNCE A. L., WITTER R. F., MONTY K. J., PATE S., COTTONE M. A. A method for isolating intact mitochondria and nuclei from the same homogenate, and the influence of mitochondrial destruction on the properties of cell nuclei. J Biophys Biochem Cytol. 1955 Mar;1(2):139–153. doi: 10.1083/jcb.1.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERNSTER L., LOW H. Reconstruction of oxidative phosphorylation in aged mitochondrial systems. Exp Cell Res. 1955;(Suppl 3):133–153. [PubMed] [Google Scholar]
- FREDERIC J. Action of various substances on the mitochondria of living cells cultivated in vitro. Ann N Y Acad Sci. 1954 Nov 17;58(7):1246–1263. doi: 10.1111/j.1749-6632.1954.tb45906.x. [DOI] [PubMed] [Google Scholar]
- HARMAN J. W., KITIYAKARA A. Studies on mitochondria. VI. The relationship between the structure, osmotic reactivity and ATPase activity of mitochondria from pigeon skeletal muscle. Exp Cell Res. 1955 Jun;8(3):411–435. doi: 10.1016/0014-4827(55)90119-1. [DOI] [PubMed] [Google Scholar]
- HUGHES D. E. The bacterial cytoplasmic membrane. J Gen Microbiol. 1962 Sep;29:39–46. doi: 10.1099/00221287-29-1-39. [DOI] [PubMed] [Google Scholar]
- KALTENBACH J. C., HARMAN J. W. Studies on mitochondria. VII. The relationship between the control of structure and the dinitrophenol stimulation of adenosinetriphosphatase in liver mitochondria. Exp Cell Res. 1955 Jun;8(3):435–452. doi: 10.1016/0014-4827(55)90120-8. [DOI] [PubMed] [Google Scholar]
- KANAI K., YOUMANS G. P. Immunogenicity of intracellular particles and cell walls from Mycobacterium tuberculosis. J Bacteriol. 1960 Nov;80:607–614. doi: 10.1128/jb.80.5.607-614.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANAI K., YOUMANS G. P., YOUMANS A. S. Allergenicity of intracellular particles, cell walls, and cytoplasmic fluid from Mycobacterium tuberculosis. J Bacteriol. 1960 Nov;80:615–621. doi: 10.1128/jb.80.5.615-621.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIELLEY W. W., KIELLEY R. K. A specific adenosinetriphosphatase of liver mitochondria. J Biol Chem. 1953 Jan;200(1):213–222. [PubMed] [Google Scholar]
- LEHNINGER A. L., REMMERT L. F. An endogenous uncoupling and swelling agent in liver mitochondria and its enzymic formation. J Biol Chem. 1959 Sep;234:2459–2464. [PubMed] [Google Scholar]
- LEHNINGER A. L. Reversal of various types of mitochondrial swelling by adenosine triphosphate. J Biol Chem. 1959 Sep;234:2465–2471. [PubMed] [Google Scholar]
- LIPSETT M. N., CORWIN L. M. Studies on stability of rat liver mitochondria. I. Role of oxidative phosphorylation in swelling. J Biol Chem. 1959 Sep;234:2448–2452. [PubMed] [Google Scholar]
- MARR A. G. Enzyme localization in bacteria. Annu Rev Microbiol. 1960;14:241–260. doi: 10.1146/annurev.mi.14.100160.001325. [DOI] [PubMed] [Google Scholar]
- MILLMAN I., DARTER R. W. Intracellular localization of enzymes in Mycobacterium tuberculosis var. hominis. Proc Soc Exp Biol Med. 1956 Feb;91(2):271–275. doi: 10.3181/00379727-91-22235. [DOI] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. The glycerol-phospho-protein complex envelope of Micrococcus pyogenes. J Gen Microbiol. 1951 Nov;5(5 Suppl):981–992. doi: 10.1099/00221287-5-5-981. [DOI] [PubMed] [Google Scholar]
- MUDD S. The internal organization of the mycobacterial cell. Am Rev Respir Dis. 1962 Feb;85:272–274. doi: 10.1164/arrd.1962.85.2.272. [DOI] [PubMed] [Google Scholar]
- Mudd S. CELLULAR ORGANIZATION IN RELATION TO FUNCTION. Bacteriol Rev. 1956 Dec;20(4):268–271. doi: 10.1128/br.20.4.268-271.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVIKOFF A. B. Preservation of the fine structure of isolated liver cell particulates with polyvinylpyrrollidone-sucrose. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):65–66. doi: 10.1083/jcb.2.4.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATO T. ELECTRON MICROSCOPIC STUDIES ON THE INTRACYTOPLASMIC MEMBRANOUS ORGANELLES OF MYCOBACTERIA. Sci Rep Res Inst Tohoku Univ Med. 1963 Mar;11:143–165. [PubMed] [Google Scholar]
- SHINOHARA C., FUKUSHI K., SUZUKI J. Mitochondria-like structures in ultrathin sections of Mycobacterium avium. J Bacteriol. 1957 Sep;74(3):413–415. doi: 10.1128/jb.74.3.413-415.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLATER E. C., CLELAND K. W. Stabilization of oxidative phosphorylation in heart-muscle sarcosomes. Nature. 1952 Jul 19;170(4316):118–119. doi: 10.1038/170118b0. [DOI] [PubMed] [Google Scholar]
- TAPLEY D. F. The effect of thyroxine and other substances on the swelling of isolated rat liver mitochondria. J Biol Chem. 1956 Sep;222(1):325–339. [PubMed] [Google Scholar]
- TODA T., KOIKE M., HIRAKI N., TAKEYA K. The intracellular structures of a mycobacterium. J Bacteriol. 1957 Mar;73(3):442–443. doi: 10.1128/jb.73.3.442-443.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. FURTHER STUDIES ON A LABILE IMMUNOGENIC PARTICULATE SUBSTANCE ISOLATED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1964 Feb;87:278–285. doi: 10.1128/jb.87.2.278-285.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P., MILLMAN I. Immunogenicity of particles isolated from Mycobacterium tuberculosis. Proc Soc Exp Biol Med. 1957 Dec;96(3):762–768. doi: 10.3181/00379727-96-23602. [DOI] [PubMed] [Google Scholar]
- YOUMANS G. P., MILLMAN I., YOUMANS A. S. The immunizing activity against tuberculous infection in mice of enzymatically active particles isolated from extracts of Mycobacterium tuberculosis. J Bacteriol. 1955 Nov;70(5):557–562. doi: 10.1128/jb.70.5.557-562.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUMANS G. P., YOUMANS A. S., KANAI K. The difference in response of four strains of mice to immunization against tuberculous infection. Am Rev Respir Dis. 1959 Nov;80:753–756. doi: 10.1164/arrd.1959.80.5.753. [DOI] [PubMed] [Google Scholar]
- YOUMANS G. P., YOUMANS A. S. The measurement of the response of immunized mice to infection with Mycobacterium tuberculosis va. hominis. J Immunol. 1957 May;78(5):318–329. [PubMed] [Google Scholar]



