Abstract
The sympathetic nervous system plays an important role in the regulation of blood pressure. There is increasing evidence for positive and negative interactions between dopamine and adrenergic receptors; the activation of the α-adrenergic receptor induces vasoconstriction, whereas the activation of dopamine receptor induces vasorelaxation. We hypothesize that the D1-like receptor and/or D3 receptor also inhibit α1-adrenergic receptor-mediated proliferation in vascular smooth muscle cells (VSMCs). In this study, VSMC proliferation was determined by measuring [3H]thymidine incorporation, cell number, and uptake of 3-(4,5-dimethylthiazol-2-yl)-diphenyltetrazolium bromide (MTT). Norepinephrine increased VSMC number and MTT uptake, as well as [3H]thymidine incorporation via the α1-adrenergic receptor in aortic VSMCs from Sprague-Dawley rats. The proliferative effects of norepinephrine were attenuated by the activation of D1-like receptors or D3 receptors, although a D1-like receptor agonist, fenoldopam, and a D3 receptor agonist, PD-128907, by themselves, at low concentrations, had no effect on VSMC proliferation. Simultaneous stimulation of both D1-like and D3 receptors had an additive inhibitory effect. The inhibitory effect of D3 receptor was via protein kinase A, whereas the D1-like receptor effect was via protein kinase C-ζ. The interaction between α1-adrenergic and dopamine receptors, especially D1-like and D3 receptors in VSMCs, could be involved in the pathogenesis of hypertension.
Keywords: α1-adrenergic receptor, hypertension
Cardiovascular disease remains the major cause of death in the world, and hypertension accounts for the major cause of these deaths in the United States and Western Europe (46). Hypertension is known to be associated with an increase in sympathetic activity (8, 42). The sympathetic neurotransmitters, norepinephrine and epinephrine, play important roles in the regulation of physiological and pathological processes in the cardiovascular system, via binding to adrenoceptors (21). Vascular smooth muscle cell (VSMC) proliferation is central to the development of vascular diseases, such as restenosis and atherosclerosis. In addition to the fact that prolonged elevation of plasma catecholamines is a risk factor for vascular diseases, recent reports have shown that catecholamines directly induce hypertrophy of the arterial wall by stimulation of α1-adrenoceptors (7, 10). Catecholamines in cell and organ culture induce dose-dependent proliferation of VSMCs, and the trophic effect is dependent on the production of reactive oxygen species (5). Furthermore, the potency of these effects is strongly augmented in hypertensive states (47, 55, 60).
Although dopamine is a precursor of norepinephrine and epinephrine, by itself it also exerts widespread effects both in neuronal and nonneuronal tissues (18, 57). Dopamine receptors, classically, have been divided into D1- and D2-like subtypes based on their interaction with the effector enzyme, adenylyl cyclase. The D1-like receptors, which stimulate adenylyl cyclases, are composed of the receptor subtypes D1 and D5 receptors, whereas the D2-like receptors, which inhibit adenylyl cyclase, are composed of the subtypes D2, D3, and D4 receptors (18, 57). Both D1-like and D3 receptors have been found in conduit and resistance arteries, including the aorta, mesenteric artery, and renal artery (2, 34, 36, 53, 54, 60).
Dopamine, at low concentrations, via D1-like receptors, relaxes blood vessels and decreases blood pressure (18, 57). We have reported that in the rat mesenteric artery, D3 receptor agonist has no effect on vascular contractility. However, when the mesenteric rings are preconstricted with potassium chloride or norepinephrine, stimulation of D1-like receptors or the D3 receptor causes vasorelaxation (60). When compared with vessels preconstricted with norepinephrine, the D1-like receptor- and D3 receptor-mediated vasorelaxation is greater in vessels preconstricted with norepinephrine than in vessels preconstricted with potassium chloride, indicating that norepinephrine could impair the vasodilatory effect of dopamine (60).
Dopamine also has antiproliferative effects, via D1-like receptors in neuronal and VSMCs (41, 53, 54). Dopamine receptors have been reported to inhibit or stimulate cell proliferation that is subtype dependent (3, 27). We hypothesize that in addition to opposing the vasoconstrictor effect of norepinephrine in the artery, activation of D1-like and D3 receptors reduces the proliferative effects mediated by norepinephrine, via α1-adrenergic receptor. To test this hypothesis, we used VSMCs from the aorta of Sprague-Dawley (SD) rats. We found that the activation of D1-like or D3 receptor reduces the proliferative effects mediated by norepinephrine, via the α1-adrenergic receptor; costimulation of D1-like and D3 receptors has an additive antiproliferative effect in VSMCs from SD rats. Moreover, we found that protein kinase A (PKA) is involved in the D3 receptor-mediated antiproliferative effect, whereas protein kinase C (PKC) is involved in the D1-like receptor-mediated antiproliferative effect.
Methods
Aortic smooth muscle cell isolation and culture
The tunica media were dissected from freshly harvested aortic strips from 200-g SD rats and plated in 100-mm petri dishes using standard explant techniques (26). Briefly, the aorta was incised longitudinally, and the endothelial cells were removed by vigorously scraping the surface of the lumen with a scalpel blade to expose the medial layer of the vessel. The vessel was then divided into 1-cm sections and placed lumen-side down in 60-mm petri dishes containing VSMC medium [Dulbecco's modified Eagle's medium (DMEM; GIBCO) supplemented with 20% FBS (HyClone) and 1% penicillin/streptomycin/bFGF/insulin/EGF (all from Sigma, St. Louis, MO)] and cultured at 37°C in a humidified 5% CO2-95% room air atmosphere for 2 wk to allow for VSMC migration from the vessel onto the dish. Explant-derived cells were initially treated with 0.1% trypsin, and 4 mM EDTA for 3 min at 37°C, and thereafter the passage was performed conventionally. Cells from passages 5–15 were used for all growth studies. The cells were characterized as smooth muscle cells by morphology (multilayer sheets, “hills and valleys”) and immunostaining with monoclonal antibody specific for smooth muscle α-actin. Cells were subcultured into 24-well plates in a medium containing 10% serum for 24 h with an initial density of 5 × 104 cells per well. The cells were then placed in the medium without serum for 24 h to render them quiescent. All experiments were approved by the Third Military Medical University Animal Use and Care Committee.
Evaluation of cell number
VSMCs were seeded in six-well plastic culture dishes at a density of 1 × 104/well in DMEM containing 10% FBS and cultured for 24 h. After the induction of quiescence, the medium was replaced with serum-free medium with the indicated drug concentrations (fenoldopam, 10−7 M; PD-128907, 10−7 M; and norepinephrine, 10−6 M) and incubated for another 24 h. The experiment was terminated by aspiration of the medium and washing with phosphate-buffered saline (PBS) followed by the addition of 1 ml of 2% (wt/vol) trypsin to each dish. After the collection of the cells, viable cells, determined by the uptake of 0.4% trypan blue after 5 min of mixing (Invitrogen Life Technologies), were counted using a hemocytometer (trypan blue uptake was observed in <10% of the cells). Counting was performed in triplicate (35, 49).
MTT assay
The number of viable cells in each well was also estimated by the uptake of the tetrazolium salt, 3-(4,5-dimethylthiazol-2-yl)-diphenyltetrazolium bromide (MTT). MTT, selectively taken up by mitochondria, is converted to a dark-blue product by living but not by dead cells. After the induction of quiescence in 96-well plastic culture dishes at the density of 1×103 cells/well, the cells were incubated with the indicated drugs for 24 h. Subsequently, 20 μl of MTT (2.5 g/l) were added to each well, and the incubation continued for an additional 4 h at 37°C. Thereafter, 150 μl DMSO were added to each well, and an absorbance at 490 nm was read on a Microplate reader (model 680, Bio-Rad) (25, 26).
[3H]thymidine incorporation
Cell proliferation was determined by measuring the incorporation of [3H]thymidine (Atomic Energy Research Establishment of China, Beijing City, China) into DNA of cells cultured in 96-well plates. After the induction of quiescence, the cells were stimulated with norepinephrine (Huofen, Shanghai City, China), vehicle (distilled H2O) in the presence or absence of the indicated drugs; PD-128907 (Sigma), a D3 receptor agonist; and fenoldopam (Sigma), a D1-like receptor agonist, for 24 h. All of antagonists or inhibitors were added into the medium 30 min before the corresponding agonists or activators. [3H]thymidine (1 μCi/ml) was then added to the growth medium of each well 6 h before the measurements. At the end of incubation, the medium was removed and the cells were treated with 0.25 ml of 0.05% trypsin-0.53 mM EDTA (GIBCO, Grand Island, NY) for 5 min and diluted to 10 ml with a balanced electrolyte solution (17, 26). The cells were then treated with 10% trichloroacetic acid to precipitate acid-insoluble materials from which the DNA was extracted with 0.1 M NaOH. The DNA was collected on a Whatman GF/B filter and washed twice with 5 ml ice-cold PBS. The filter was then cut and shaken in 3.5 ml scintillation fluid for 24 h before counting in a liquid scintillation counter (Beckman LS6500, Beckman). Data are presented as [3H]thymidine uptake per microgram of protein.
To determine the specificity of the D1-like and D3 receptor agonists, D1-like receptor and D3 receptor antagonists, SCH-23390 (Sigma) and U-99194A (Research Biochemicals International, Natick, MA), respectively, were used to block the effect of fenoldopam or PD-128907. There are no ligands, agonists, or antagonists that can distinguish between D1 and D5 receptors; therefore, the receptors involved in this experiment are D1-like receptors (D1 and D5), which are expressed in the VSMCs of rats. To determine whether the proliferative effect of norepinephrine is exerted at the α1-adrenergic receptor, the α1-adrenergic receptor antagonist prazosin (Sigma) was used.
Determination of the second messenger(s) involved in the antiproliferative effects of D1-like and D3 receptors
To determine the second messenger(s) involved in the effect of D1-like and D3 receptors, cell permeable, myristoylated peptide inhibitors of PKC (peptide 19–31) (20), PKA (14–22 amide) (6) (Calbiochem, Darmstadt, Germany), and myristoylated PKC-ζ pseudosubstrate inhibitor (Calbiochem, Darmstadt, Germany) (37, 51) were used. To determine the specificity of PKC and PKA inhibitors, PKC activator, phorbol 12-myristate 13-acetate (PMA, Sigma), and PKA activator, 8-(4-chlorophenylthio) adenosine-3′,5′-cyclic monophosphorothioate, Sp-isomer sodium salt (Sp-cAMP[S], Sigma) were used.
Statistical analysis
The data are expressed as means ± SE. The comparison within groups was made by repeated-measures ANOVA (or paired t-test when only 2 groups were compared), and the comparison among groups (or t-test when only 2 groups were compared) was made by factorial ANOVA and Duncan's test. A value of P < 0.05 was considered significant.
Results
Norepinephrine, via the α1-adrenergic receptor, increases proliferation of VSMCs from rat aorta of SD rats
Treatment of VSMCs with varying concentrations of norepinephrine (10−9-10−6 M) for 24 h resulted in a concentration-dependent increase in [3H]thymidine incorporation in aortic VSMCs from SD rats (data not shown). The increase in [3H]thymidine incorporation, induced by norepinephrine, was blocked by the α1-adrenergic receptor antagonist, prazosin (Fig. 1), indicating that the norepinephrine-mediated proliferation was via the α1-adrenergic receptor.
Fig. 1.
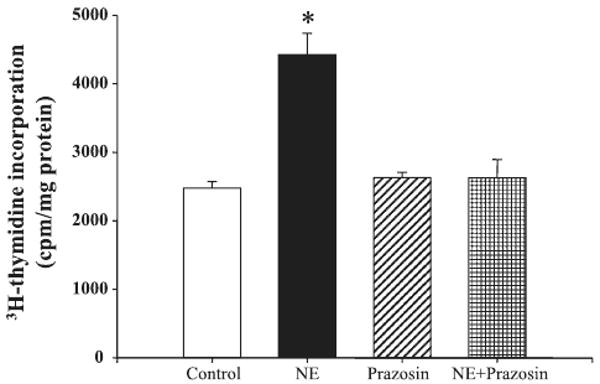
Effect of the α1-adrenergic receptor antagonist prazosin on norepinephrine (NE)-mediated proliferation of aortic vascular smooth muscle cells (VSMCs) from Sprague-Dawley (SD) rats. VSMC proliferation was determined by [3H]thymidine incorporation after incubation with the indicated concentrations of NE (10−6 M) with or without the presence of α1-adrenergic receptor antagonist prazosin (10−6 M). Results are expressed as counts per minute (cpm) per mg protein (n = 5 experiments, *P < 0.05 vs. others; ANOVA, Duncan's test).
The norepinephrine-mediated proliferation of VSMCs from rat aorta of SD rats is attenuated by a D1-like receptor agonist
To investigate whether there is an interaction between α1-adrenergic and D1-like receptors, VSMCs were incubated with norepinephrine and the D1-like receptor agonist, fenoldopam, for 24 h. Fenoldopam, by itself, had no effect on [3H]thymidine incorporation (data not shown), but dose-dependently (10−12-10−5 M) reduced the stimulatory effect of norepinephrine (Fig. 2A). The effect of fenoldopam was via the D1-like receptor, because the inhibitory effect of fenoldopam on norepinephrine-mediated proliferation was blocked by the D1-like receptor antagonist, SCH-23390 (Fig. 2B).
Fig. 2.
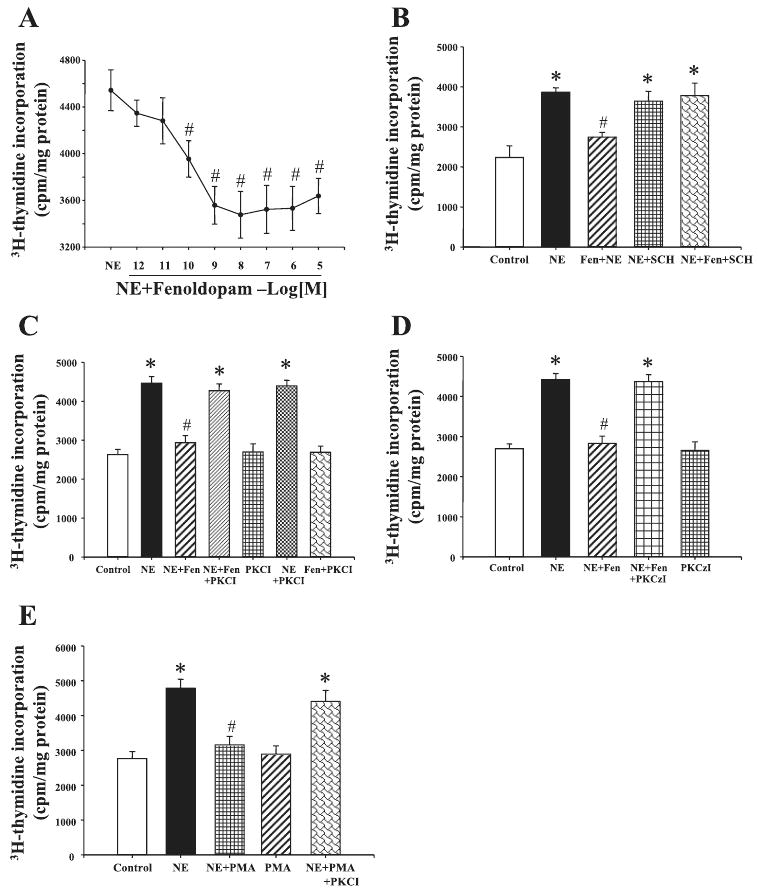
Effect of dopamine D1-like receptor on NE-mediated proliferation of aortic VSMCs from SD rats. A: effect of D1-like receptor agonist fenoldopam (Fen) on NE-mediated proliferation of aortic VSMCs from SD rats. VSMC proliferation was determined by [3H]thymidine incorporation after incubation with the indicated concentrations of NE (10−6 M) with or without the presence of Fen (10−12-10−5 M). B: effect of a D1-like receptor agonist Fen and D1-like receptor antagonist SCH-23390 (SCH) on NE-mediated proliferation of aortic VSMCs from SD rats. The cells were incubated with the indicated reagents: Fen (10−7 M) and SCH (10−7 M) for 24 h. VSMC proliferation was determined by [3H]thymidine incorporation. C: effect of PKC inhibitor, peptide 19–31 (PKCI), on the inhibitory effect of D1-like receptor in aortic VSMCs from SD rats. The cells were incubated with the indicated reagents: NE (10−6 M), Fen (10−7 M), and PKCI (10−6 M) for 24 h. VSMC proliferation was determined by [3H]thymidine incorporation. D: effect of PKC-ζ inhibitor (PKCzI) on the inhibitory effect of D1-like receptor in aortic VSMCs from SD rats. The cells were incubated with the indicated reagents: NE (10−6 M), Fen (10−7 M), and PKCzI (10−5 M) for 24 h. VSMC proliferation was determined by [3H]thymidine incorporation. E: effect of PKC activator, PMA and PKC inhibitor, PKCI on NE-mediated proliferation of aortic VSMCs from SD rats. The cells were incubated with the indicated reagents: NE (10−6 M), PKCI (10−6 M), and PMA (10−7 M) for 24 h. VSMC proliferation was determined by [3H]thymidine incorporation. All results are expressed as cpm/mg protein [n = 10 (A), n = 5 (B), n = 10 (C), n = 6 (D), and n = 6 (E) experiments]. *P < 0.05 vs. control; #P < 0.05 vs. NE (ANOVA, Duncan's test).
The PKC inhibitor peptide 19–31 (10−6 M), by itself, had no effect on VSMC proliferation. The PKC inhibitor also did not affect norepinephrine-mediated VSMC proliferation but blocked the inhibitory effect of fenoldopam on norepinephrine-mediated proliferation (Fig. 2C), indicating that PKC was involved in the inhibitory action of fenoldopam (20). We also used a PKA inhibitor (14–22 amide) and a calcium channel blocker (nicardipine) in this experiment. However, neither 14–22 amide nor nicardipine could block the effect of D1-like receptor on VSMC proliferation (data not shown).
To further investigate which isoform of PKC was involved in the mechanism, myristoylated PKC-ζ pseudosubstrate inhibitor (10−5 M) was used in this experiment. It resulted that myr-RRGARRWRK, by itself, had no effect on VSMC proliferation but blocked the inhibitory effect of fenoldopam on norepinephrine-mediated proliferation (Fig. 2D), indicating that PKC-ζ was involved in the inhibitory action of fenoldopam.
The specificity of PKC inhibitor, peptide 19–31, on norepinephrine-mediated proliferation was also determined. The PKC activator, PMA, by itself, had no effect on VSMC proliferation but reduced the norepinephrine-mediated proliferation in aortic VSMCs from SD rats, which was blocked by the PKC inhibitor peptide 19–31 (Fig. 2E).
The norepinephrine-mediated proliferation of VSMCs from rat aorta of SD rats is reduced by a D3 receptor agonist
To determine whether there is any effect of D3 receptors on cell proliferation, the VSMCs from SD rats were treated with varying concentrations (10−8-10−5 M) of the D3 receptor agonist, PD-128907 (58). We found that at low concentrations (10−8 and 10−7 M), PD-128907 had no effect on cell proliferation but at high concentrations (10−6 and 10−5 M), PD-128907 stimulated VSMC proliferation (data not shown). The stimulatory effect of PD-128907 on VSMC proliferation was blocked by the α1-adrenergic receptor blocker prazosin, but not by D3 receptor antagonist U-99194A, indicating that the stimulatory effect of the high concentrations of PD-128907 is via the α1-adrenergic receptor rather than the D3 receptor (control = 2,399 ± 180; 10−5 M PD-128907 = 3,824 ± 394; 10−5 M PD-128907 + 10−6 M U-99194A = 4,062 ± 349; 10−5 M PD-128907 + 10−6 M prazosin = 2,449 ± 172; 10−6 M U-99194A = 2,391 ± 181; 10−6 M prazosin = 2,424 ± 171 counts·min−1·mg protein−1, n = 5 experiments/group).
To investigate whether there is an interaction between α1-adrenergic and D3 receptors, VSMCs were incubated for 24 h with norepinephrine (10−6 M) and low concentrations of the D3 receptor agonist PD-128907. PD-128907 (10−12-10−7 M), by itself, had no effect but reduced the stimulatory of norepinephrine on [3H]thymidine incorporation in aortic VSMCs from SD rats (Fig. 3A). The effect of PD-128907 was via the D3 receptor, because the inhibitory effect of PD-128907 on norepinephrine-mediated proliferation was blocked by D3 receptor antagonist U-99194A (Fig. 3B).
Fig. 3.
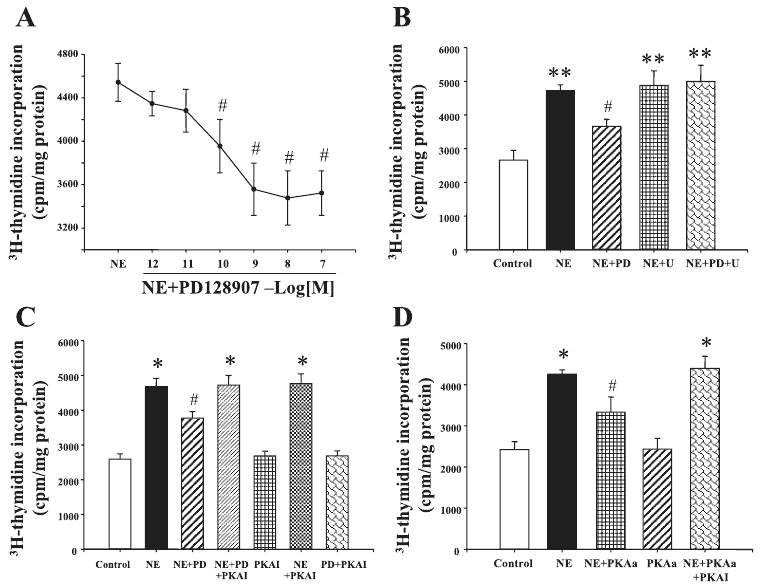
Effect of D3 receptor on NE-mediated proliferation of aortic VSMCs from SD rats. A: effect of D3 receptor agonist PD-128907 (PD) on NE-mediated proliferation of aortic VSMCs from SD rats. VSMC proliferation was determined by [3H]thymidine incorporation after incubation with the indicated concentrations of NE (10−6 M) with or without the presence of PD (10−12-10−7 M). B: effect of a D3 receptor agonist PD and D3 receptor antagonist U-99194A (U) on NE-mediated proliferation of aortic VSMCs from SD rats. The cells were incubated with the indicated reagents: PD (10−7 M) and U (10−7 M) for 24 h. VSMC proliferation was determined by [3H]thymidine incorporation. C: effect of PKA inhibitor, protein kinase A inhibitor 14–22 (PKAI), on the inhibitory effect of D3 receptor in aortic VSMCs from SD rats. The cells were incubated with the indicated reagents: NE (10−6 M), PD (10−7 M), and PKAI (10−6 M) for 24 h. VSMC proliferation was determined by [3H]thymidine incorporation. D: effect of PKA activator 8-(4-chlorophenylthio)adenosine-3′,5′-cyclic monophosphorothioate, Sp-isomer sodium salt (Sp-cAMP[S]; PKAa) and PKAI on NE-mediated proliferation of aortic VSMCs from SD rats. The cells were incubated with the indicated reagents: NE (10−6 M), PKAI (10−6 M), and Sp-cAMP[S] (10−7 M) for 24 h. VSMC proliferation was determined by [3H]thymidine incorporation. All results are expressed as cpm/mg protein [n = 9 (A), n = 5 (B), n = 8 (C), and n = 4 (D) experiments]. *P < 0.05 vs. control; **P < 0.01 vs. control; #P < 0.05 vs. NE (ANOVA, Duncan's test).
The PKA inhibitor 14–22 (10−6 M), by itself, had no effect on VSMC proliferation. The PKA inhibitor also did not affect norepinephrine-mediated VSMC proliferation but blocked the inhibitory effect of PD-128907, indicating that PKA was engaged in the inhibitory action of PD-128907 (Fig. 3C). We also used a PKC inhibitor (19–31) and a calcium channel blocker (nicardipine) in this experiment. However, neither 19–31 nor nicardipine could block the effect of D3 receptor on VSMC proliferation (data not shown).
The specificity of PKA inhibitor 14–22 on norepinephrine-mediated proliferation was also determined in this experiment. PKA activator, Sp-cAMP[S], by itself had no effect on VSMC proliferation but reduced the norepinephrine-mediated proliferation in aortic VSMCs from SD rats, which was blocked by the PKA inhibitor 14–22 (Fig. 3D).
D1-like and D3 receptors have additive inhibitory effect on norepinephrine-mediated VSMC proliferation
In additional studies, we found that costimulation with D1-like and D3 receptor agonists, fenoldopam (10−7 M) and PD-128907 (10−7 M), reduced norepinephrine-mediated proliferation to a greater degree than those observed with fenoldopam or PD-128907, indicating an additive effect of D1-like and D3 receptors (Fig. 4A).
Fig. 4.
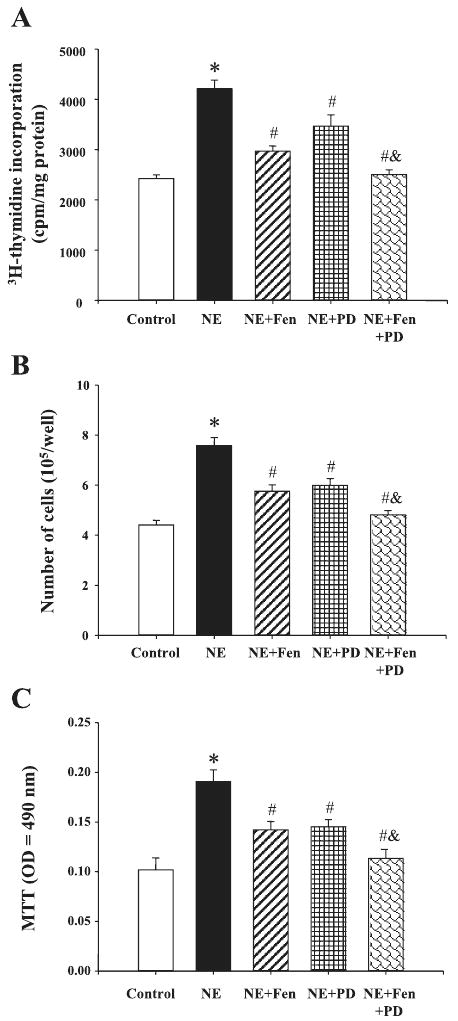
A: Effect of D1-like and D3 receptors on NE-mediated proliferation of aortic VSMCs from SD rats. A: VSMC proliferation was determined by [3H]thymidine incorporation after incubation with the indicated concentrations of NE (10−6 M) with or without the presence of PD (10−7 M) and Fen (10−7 M). Results are expressed as cpm/mg protein (n = 8 experiments). B: VSMC proliferation was determined by cell number after incubation with the indicated concentrations of NE (10−6 M) with or without the presence of PD (10−7 M) and Fen (10−7 M). Results are expressed as cell number per well (n = 4 experiments). C: VSMC proliferation was determined by the 3-(4,5-dimethyl-thiazol-2-yl)-diphenyltetrazolium bromide (MTT) method after incubation with the indicated concentrations of NE (10−6 M) with or without the presence of PD (10−7 M) and Fen (10−7 M). Results are expressed as MTT optical density (OD) = 490 nm (n = 14 experiments). *P < 0.01 vs. control; #P < 0.05 vs. NE, &P < 0.05 vs. NE + Fen or NE + PD (ANOVA, Duncan's test).
To substantiate the above-mentioned phenomena determined by [3H]thymidine incorporation, we investigated VSMC proliferation further by counting the number of viable cells, determined by counting 0.4% trypan blue-positive cells and by the MTT method. In agreement with the [3H]thymidine incorporation method, norepinephrine increased the number of VSMCs that was reduced in the presence of D1-like and D3 receptor agonists, fenoldopam (10−7 M) and PD-128907 (10−7 M), respectively. Moreover, costimulation with fenoldopam and PD-128907 reduced the norepinephrine-mediated increase in cell number to a greater extent than those observed with fenoldopam or PD-128907, indicating an additive effect of D1-like and D3 receptors (Figs. 4, B and C).
Discussion
There are several novel observations in our study. We show that the proliferative effect of norepinephrine, via an α1-adrenergic receptor, in VSMCs from the aorta of SD rats is reduced by the activation of D1-like or D3 receptors, although the D1-like and D3 receptors, by themselves, have no effect on VSMC proliferation. The D1-like and D3 receptors have an additive inhibitory effect on norepinephrine-mediated proliferation of VSMCs. The inhibitory effect of D3 receptor is via PKA, whereas D1-like receptor effect is via PKC.
The sympathetic neurotransmitters, norepinephrine and epinephrine, via α-adrenergic receptors, induce vasoconstriction and proliferation of VSMCs, whereas the opposite effects (vasodilation and inhibition of the proliferation of VSMCs) occur via β-adrenergic receptors (16, 60). Dopamine at low concentrations, via dopamine receptors, dilates the resistance and capacitance arteries (18, 23, 57); at high concentrations, dopamine stimulates other G protein-coupled receptors, including α,β-adrenergic receptors (18, 57) and serotonin receptors (14, 50).
There are increasing pieces of evidence for interactions among catecholamines and their receptors in neural and non-neural tissues. The D2-like receptors, D2, D3, and D4, are located pre- and postsynaptically/junctionally (2, 30, 45, 60). Prejunctional D2 dopamine receptors in postganglionic sympathetic nerves innervating various organs, including human gastric and uterine arteries, can inhibit the release of catecholamine (28). In contrast, the blockade of the presynaptic α2-adrenergic receptor enhances brain cortical dopamine output (15). An activation of human peripheral blood mononuclear cells triggers endogenous production of catecholamines, an effect that is blocked by an activation of D1-like receptor. The counterregulatory actions of the α-adrenergic and dopamine receptors extend to the effects on vascular proliferation.
Our previous study showed that D1 and D3 receptors exist in the artery. Stimulation of D1-like or D3 receptor can increase both receptor protein expression in rat aorta A10 cells; the activation of D1-like or D3 receptor relaxes rat mesenteric arteries preconstricted by potassium chloride, and the simultaneous stimulation of D1-like and D3 receptors has an additive relaxant effect (60). The present results show that the D1-like or D3 receptor has an inhibitory effect on norepinephrine-induced VSMC proliferation, and costimulation of D1-like and D3 receptors has an additive inhibitory effect, which further indicates the interaction between D1-like and D3 receptors in the artery.
α1-Adrenergic receptors, like dopamine receptors, are members of the class A of G protein-coupled receptors and mediate many of the physiological effects of catecholamines. The α1-adrenergic receptor plays a particularly important role in the regulation of blood pressure via the activation of smooth muscle replication and contraction. Consistent with our findings in this study, the activation of α1-receptors stimulates vascular smooth muscle growth (19). Human VSMCs express at least three subtypes of α1-receptors, namely, α1A-, α1B-, and α1D-receptors. It is generally accepted that the activation of all three subtypes of α1-receptors increases the hydrolysis of phosphatidylinositol 4,5-bisphosphate to form inositol 1,4,5-trisphosphate and diacylglycerol via Gq, leading to the activation of PKC and an increase in intracellular Ca2+ (29). Substantial evidence indicates that the activation of PKC or Ca2+ channel is involved in the α1-adrenergic receptor induction of mitogenesis in VSMCs (22). In this study, we show that the activation of D1-like or D3 receptors has an inhibitory effect on norepinephrine-induced proliferation in VSMCs. However, the mechanism(s) of the antiproliferative action of D1-like or D3 receptor is not clear. Our studies show that the inhibitory effect of D3 receptor is blocked by treatment with PKA inhibitor, whereas the D1-like receptor inhibitory effect is blocked by the inhibition of PKC activity, indicating that PKA and PKC are involved in these actions. High concentrations of D1-like or D3 receptor agonists can also compete with norepinephrine for adrenergic receptor occupancy to some degree. However, our studies used low concentrations of D1-like or D3 receptor agonists that can be blocked by their respective antagonists but not by an α1-adrenergic receptor antagonist. However, it is interesting to find that PKA and PKC are involved in the D1-like and D3 receptor-mediated antiproliferative effects.
PKC isoforms are classified into four groups, the conventional PKCs (α, βI, βII, and γ), the novel PKCs (δ, ε, η, and θ), the atypical PKCs (ζ and λ/ι), and PKD or PKC-μ. In this study, we could not determine which one is involved in the antiproliferative effects by D1-like receptor. The activities of novel and atypical PKCs are not affected by Ca2+, whereas conventional PKCs can bind Ca2+ (39, 44). In renal proximal tubule cells, the activation of D1-like receptor can stimulate (θ and ζ) or inhibit (δ) new PKCs or translocate specific PKC isoforms from cytosol to membrane (α, β, and ε) and membrane to cytosol (δ) (9, 32, 52, 56). There are reports that PKC-β1, PKC-δ, PKC-ε, PKC-ζ, and PKC-η have proliferative VSMC effects (1, 56). In our studies, a Ca2+ channel blocker has no effect on the α1-adrenoceptor-mediated proliferation effect; therefore, it is possible that atypical and novel PKCs, and not conventional PKCs, are involved in this action. In this experiment, we found that the inhibition of PKC-ζ blocks the inhibitory effect of fenoldopam on norepinephrine-mediated proliferation, indicating that PKC-ζ is involved in the inhibitory action of fenoldopam.
Our present study found that the D3 receptor mediated the inhibition of VSMC proliferation is via PKA. Whereas the D3 receptor is usually linked to adenylyl cyclase inhibition, the D3 receptor can also be linked to its stimulation (33). The D1-like receptor-mediated inhibitory effect on VSMC proliferation is blocked by the inhibition of PKC activity, indicating that PKC is involved in this action. This is in contrast to the study of Yasunari et al. (53), who found that the antiproliferative effect of D1-like receptors is mediated, in part, by the inhibition of PKC activity. The reason for this difference between these two studies is not known; however, there are differences between our study and those reported by Yasunari et al. (53). First, we used VSMCs from the aorta of SD rats, whereas Yasunari et al. used VSMCs from the renal artery of Wistar rats. The differences may be vessel specific (conduit and resistance vessels or differences among resistance vessels, e.g., mesenteric vs. renal VSMCs). It should be noted, however, that it would be unlikely for D1 receptors to inhibit PKC activity because the D1 receptor, by itself, or via an interaction with the D2 receptor (24, 40) has been consistently reported to stimulate and not inhibit phospholipase C, the enzyme proximal to PKC activation, initially reported by Felder et al. in renal tubules (4, 11, 12) and subsequently confirmed in other tissues, including neural tissue (31, 48) and VSMCs (43). D1-like receptors have also been reported to stimulate (e.g., PKC-ε) (10, 14, 33) or inhibit (e.g., PKC-δ) specific PKC isoforms (52, 59) that are cell specific. Second, the proliferative factors are different: we used norepinephrine, whereas Yasunari et al. used PDGF.
In summary, we have demonstrated that the proliferative effect of norepinephrine, via the α1-receptor, on VSMCs from rat aorta is reduced by the activation of D1-like or D3 receptors. A simultaneous stimulation of D1-like and D3 receptors has an additive inhibitory effect on norepinephrine-mediated proliferation of VSMCs. The inhibitory effect of D3 receptor is via PKA, whereas the D1-like receptor effect is via PKC-ζ.
Perspectives
VSMC proliferation plays an important role in the pathogenesis of hypertension. Norepinephrine, via the α1-receptor, increases VSMC proliferation, whereas dopamine, via the D1-like and D3 receptors, reduces norepinephrine-mediated proliferative effects. The development of drugs, which selectively activate dopamine receptors, might be helpful to prevent vessel wall hypertrophy and hyperplasia, reduce peripheral arterial resistance, and normalize blood pressure.
Acknowledgments
Grants: These studies were supported in part by National Institutes of Health Grants HL-23081, DK-39308, HL-68686, HL-62211, and HL-074940, National Natural Science Foundation of China Grants 30470728 and 30672199, and the National Basic Research Program of China (973 Program, 2008CB517308).
References
- 1.Allen TR, Krueger KD, Hunter WJ, 3rd, Agrawal DK. Evidence that insulin-like growth factor-1 requires protein kinase C-epsilon, PI3-kinase and mitogen-activated protein kinase pathways to protect human vascular smooth muscle cells from apoptosis. Immunol Cell Biol. 2005;83:651–667. doi: 10.1111/j.1440-1711.2005.01387.x. [DOI] [PubMed] [Google Scholar]
- 2.Amenta F, Ricci A, Tayebati SK, Zaccheo D. The peripheral dopaminergic system: morphological analysis, functional and clinical applications. Ital J Anat Embryol. 2002;107:145–167. [PubMed] [Google Scholar]
- 3.An JJ, Cho SR, Jeong DW, Park KW, Ahn YS, Baik JH. Antiproliferative effects and cell death mediated by two isoforms of dopamine D2 receptors in pituitary tumor cells. Mol Cell Endocrinol. 2003;206:49–62. doi: 10.1016/s0303-7207(03)00236-3. [DOI] [PubMed] [Google Scholar]
- 4.Banday AA, Lokhandwala MF. Oxidative stress reduces renal dopamine D1 receptor-Gq/11alpha G protein-phospholipase C signaling involving G protein-coupled receptor kinase 2. Am J Physiol Renal Physiol. 2007;293:F306–F315. doi: 10.1152/ajprenal.00108.2007. [DOI] [PubMed] [Google Scholar]
- 5.Bleeke T, Zhang H, Madamanchi N, Patterson C, Faber JE. Cate-cholamine-induced vascular wall growth is dependent on generation of reactive oxygen species. Circ Res. 2004;94:37–45. doi: 10.1161/01.RES.0000109412.80157.7D. [DOI] [PubMed] [Google Scholar]
- 6.Bobalova J, Mutafova-Yambolieva VN. Activation of the adenylyl cyclase/protein kinase A pathway facilitates neural release of β-nicotin-amide adenine dinucleotide in canine mesenteric artery. Eur J Pharmacol. 2006;536:128–132. doi: 10.1016/j.ejphar.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 7.Brodde OE, Michel MC. Adrenergic receptors and their signal transduction mechanisms in hypertension. J Hypertens Suppl. 1992;10:S133–S145. [PubMed] [Google Scholar]
- 8.DiBona GF. Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypertens. 2002;11:197–200. doi: 10.1097/00041552-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Efendiev R, Bertorello AM, Pedemonte CH. PKC-beta and PKC-zeta mediate opposing effects on proximal tubule Na+,K+-ATPase activity. FEBS Lett. 1999;456:45–48. doi: 10.1016/s0014-5793(99)00925-4. [DOI] [PubMed] [Google Scholar]
- 10.Erami C, Zhang H, Tanoue A, Tsujimoto G, Thomas SA, Faber JE. Adrenergic catecholamine trophic activity contributes to flow-mediated arterial remodeling. Am J Physiol Heart Circ Physiol. 2005;289:H744–H753. doi: 10.1152/ajpheart.00129.2005. [DOI] [PubMed] [Google Scholar]
- 11.Felder CC, Blecher M, Jose PA. Dopamine-1-mediated stimulation of phospholipase C activity in rat renal cortical membranes. J Biol Chem. 1989;264:8739–8745. [PubMed] [Google Scholar]
- 12.Felder CC, Jose PA, Axelrod J. The dopamine-1 agonist, SKF 82526, stimulates phospholipase-C activity independent of adenylate cyclase. J Pharmacol Exp Ther. 1989;248:171–175. [PubMed] [Google Scholar]
- 13.Gomes P, Soares-da-Silva P. Dopamine acutely decreases type 3 Na+/H+ exchanger activity in renal OK cells through the activation of protein kinases A and C signalling cascades. Eur J Pharmacol. 2004;488:51–59. doi: 10.1016/j.ejphar.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Gretler DD, Jones KC, Murphy MB. 5-Hydroxytryptamine receptor activity of the dopamine receptor agonist fenoldopam in canine tracheal smooth muscle. J Pharmacol Exp Ther. 1992;260:491–498. [PubMed] [Google Scholar]
- 15.Hertel P, Fagerquist MV, Svensson TH. Enhanced cortical dopamine output and antipsychotic-like effects of raclopride by alpha2 adrenoceptor blockade. Science. 1999;286:105–107. doi: 10.1126/science.286.5437.105. [DOI] [PubMed] [Google Scholar]
- 16.Honda H, Iwata T, Matsuda H, Moroe H, Kumasaka K, Kondo M. Comparison of muscarinic receptor- and beta-adrenoceptor-mediated vasorelaxation between euthyroid and acute hyperthyroid rats. Comp Biochem Physiol C Toxicol Pharmacol. 2005;141:241–247. doi: 10.1016/j.cca.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Hu ZW, Shi XY, Lin RZ, Chen J, Hoffman BB. α1-Adrenergic receptor stimulation of mitogenesis in human vascular smooth muscle cells: role of tyrosine protein kinases and calcium in activation of mitogen-activated protein kinase. J Pharmacol Exp Ther. 1999;290:28–37. [PubMed] [Google Scholar]
- 18.Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood) 2003;228:134–142. doi: 10.1177/153537020322800202. [DOI] [PubMed] [Google Scholar]
- 19.Jackson CL, Schwartz SM. Pharmacology of smooth muscle cell replication. Hypertension. 1992;20:713–736. doi: 10.1161/01.hyp.20.6.713. [DOI] [PubMed] [Google Scholar]
- 20.Johnson EA, Oldfield S, Braksator E, Gonzalez-Cuello A, Couch D, Hall KJ, Mundell SJ, Bailey CP, Kelly E, Henderson G. Agonist-selective mechanisms of mu-opioid receptor desensitization in human embryonic kidney 293 cells. Mol Pharmacol. 2006;70:676–685. doi: 10.1124/mol.106.022376. [DOI] [PubMed] [Google Scholar]
- 21.Kaaja RJ, Poyhonen-Alho MK. Insulin resistance and sympathetic overactivity in women. J Hypertens. 2006;24:131–141. doi: 10.1097/01.hjh.0000194121.19851.e5. [DOI] [PubMed] [Google Scholar]
- 22.Kariya K, Karns LR, Simpson PC. An enhancer core element mediates stimulation of the rat beta-myosin heavy chain promoter by an alpha 1-adrenergic agonist and activated beta-protein kinase C in hypertrophy of cardiac myocytes. J Biol Chem. 1994;269:3775–3782. [PubMed] [Google Scholar]
- 23.Kelly MJ. Evidence for the presence of vascular dopamine receptors in the rabbit. Methods Find Exp Clin Pharmacol. 1982;4:365–370. [PubMed] [Google Scholar]
- 24.Lee SP, So CH, Rashid AJ, Varghese G, Cheng R, Lanca AJ, O'Dowd BF, George SR. Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J Biol Chem. 2004;279:35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- 25.Lin H, Lee JL, Hou HH, Chung CP, Hsu SP, Juan SH. Molecular mechanisms of the antiproliferative effect of beraprost, a prostacyclin agonist, in murine vascular smooth muscle cells. J Cell Physiol. 2008;214:434–441. doi: 10.1002/jcp.21214. [DOI] [PubMed] [Google Scholar]
- 26.Lu SY, Zhu MZ, Wang DS, Chen SY, Zhang WD, Dong H, Yu J, Guo HT. Inhibition of the proliferation of smooth muscle cells from human coronary bypass vessels by vasonatrin peptide. Physiol Res. 2004;53:387–393. [PubMed] [Google Scholar]
- 27.Luo Y, Kokkonen GC, Wang X, Neve KA, Roth GS. D2 dopamine receptors stimulate mitogenesis through pertussis toxin-sensitive G proteins and Ras-involved ERK and SAP/JNK pathways in rat C6-D2L glioma cells. J Neurochem. 1998;71:980–989. doi: 10.1046/j.1471-4159.1998.71030980.x. [DOI] [PubMed] [Google Scholar]
- 28.Morgadinho MT, Fontes Ribeiro CA, Macedo TR. Presynaptic dopamine receptors involved in the inhibition of noradrenaline and dopamine release in the human gastric and uterine arteries. Fundam Clin Pharmacol. 1999;13:662–670. doi: 10.1111/j.1472-8206.1999.tb00378.x. [DOI] [PubMed] [Google Scholar]
- 29.Muller S, Lohse MJ. The role of G-protein beta gamma subunits in signal transduction. Biochem Soc Trans. 1995;23:141–148. doi: 10.1042/bst0230141. [DOI] [PubMed] [Google Scholar]
- 30.Narkar VA, Hussain T, Pedemonte C, Lokhandwala MF. Dopamine D2 receptor activation causes mitogenesis via p44/42 mitogen-activated protein kinase in opossum kidney cells. J Am Soc Nephrol. 2001;12:1844–1852. doi: 10.1681/ASN.V1291844. [DOI] [PubMed] [Google Scholar]
- 31.Noriyama Y, Ogawa Y, Yoshino H, Yamashita M, Kishimoto T. Dopamine profoundly suppresses excitatory transmission in neonatal rat hippocampus via phosphatidylinositol-linked D1-like receptor. Neuro-science. 2006;138:475–485. doi: 10.1016/j.neuroscience.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 32.Nowicki S, Kruse MS, Brismar H, Aperia A. Dopamine-induced translocation of protein kinase C isoforms visualized in renal epithelial cells. Am J Physiol Cell Physiol. 2000;279:C1812–C1818. doi: 10.1152/ajpcell.2000.279.6.C1812. [DOI] [PubMed] [Google Scholar]
- 33.Obadiah J, Avidor-Reiss T, Fishburn CS, Carmon S, Bayewitch M, Vogel Z, Fuchs S, Levavi-Sivan B. Adenylyl cyclase interaction with the D2 dopamine receptor family; differential coupling to Gi, Gz, and Gs. Cell Mol Neurobiol. 1999;19:653–664. doi: 10.1023/A:1006988603199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connell DP, Vaughan CJ, Aherne AM, Botkin SJ, Wang ZQ, Felder RA, Carey RM. Expression of the dopamine D3 receptor protein in the rat kidney. Hypertension. 1998;32:886–895. doi: 10.1161/01.hyp.32.5.886. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto H, Fujioka Y, Takahashi A, Takahashi T, Taniguchi T, Ishikawa Y, Yokoyama M. Trichostatin A, an inhibitor of histone deacetylase, inhibits smooth muscle cell proliferation via induction of p21(WAF1) J Atheroscler Thromb. 2006;13:183–191. doi: 10.5551/jat.13.183. [DOI] [PubMed] [Google Scholar]
- 36.Ozono R, O'Connell DP, Wang ZQ, Moore AF, Sanada H, Felder RA, Carey RM. Localization of the dopamine D1 receptor protein in the human heart and kidney. Hypertension. 1997;30:725–729. doi: 10.1161/01.hyp.30.3.725. [DOI] [PubMed] [Google Scholar]
- 37.Park JI, Kim SG, Chun JS, Seo YM, Jeon MJ, Ohba M, Kim HJ, Chun SY. Activation of protein kinase Czeta mediates luteinizing hormone- or forskolin-induced NGFI-B expression in preovulatory granulosa cells of rat ovary. Mol Cell Endocrinol. 2007;270:79–86. doi: 10.1016/j.mce.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 38.Parmentier JH, Smelcer P, Pavicevic Z, Basic E, Idrizovic A, Estes A, Malik KU. PKC-zeta mediates norepinephrine-induced phospholipase D activation and cell proliferation in VSMC. Hypertension. 2003;41:794–800. doi: 10.1161/01.HYP.0000047873.76255.0B. [DOI] [PubMed] [Google Scholar]
- 39.Parmentier JH, Zhang C, Estes A, Schaefer S, Malik KU. Essential role of PKC-zeta in normal and angiotensin II-accelerated neointimal growth after vascular injury. Am J Physiol Heart Circ Physiol. 2006;291:H1602–H1613. doi: 10.1152/ajpheart.01363.2005. [DOI] [PubMed] [Google Scholar]
- 40.Pollack A. Coactivation of D1 and D2 dopamine receptors: in marriage, a case of his, hers, and theirs. Sci STKE 2004. 2004:pe50. doi: 10.1126/stke.2552004pe50. [DOI] [PubMed] [Google Scholar]
- 41.Popolo M, McCarthy DM, Bhide PG. Influence of dopamine on precursor cell proliferation and differentiation in the embryonic mouse telencephalon. Dev Neurosci. 2004;26:229–244. doi: 10.1159/000082140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raizada MK, Der Sarkissian S. Potential of gene therapy strategy for the treatment of hypertension. Hypertension. 2006;47:6–9. doi: 10.1161/01.HYP.0000196685.91424.01. [DOI] [PubMed] [Google Scholar]
- 43.Rashed SM, Songu-Mize E. Regulation of Na+,K+-ATPase activity by dopamine in cultured rat aortic smooth muscle cells. Eur J Pharmacol. 1996;305:223–230. doi: 10.1016/0014-2999(96)00179-3. [DOI] [PubMed] [Google Scholar]
- 44.Salamanca DA, Khalil RA. Protein kinase C isoforms as specific targets for modulation of vascular smooth muscle function in hypertension. Biochem Pharmacol. 2005;70:1537–1547. doi: 10.1016/j.bcp.2005.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun D, Wilborn TW, Schafer JA. Dopamine D4 receptor isoform mRNA and protein are expressed in the rat cortical collecting duct. Am J Physiol Renal Physiol. 1998;275:F742–F745. doi: 10.1152/ajprenal.1998.275.5.F742. [DOI] [PubMed] [Google Scholar]
- 46.The World Health Organization. The World Health Report 2002: Reducing Risks, Promoting Healthy Life Geneva. Switzerland: World Health Organization; 2002. [Google Scholar]
- 47.Villalobos-Molina R, Ibarra M. Increased expression and function of vascular alpha1D-adrenoceptors may mediate the prohypertensive effects of angiotensin II. Mol Interv. 2005;5:340–342. doi: 10.1124/mi.5.6.6. [DOI] [PubMed] [Google Scholar]
- 48.Wang HY, Undie AS, Friedman E. Evidence for the coupling of Gq protein to D1-like dopamine sites in rat striatum: possible role in dopamine-mediated inositol phosphate formation. Mol Pharmacol. 1995;48:988–994. [PubMed] [Google Scholar]
- 49.Wang JY, Chuang HN, Chiu JH, Fu SL, Tsai TH, Tsou AP, Hu CP, Chi CW, Yeh SF, Lui WY, Wu CW, Chou CK. Effects of Scutellaria baicalensis Georgi on macrophage-hepatocyte interaction through cytokines related to growth control of murine hepatocytes. Exp Biol Med (Maywood) 2006;231:444–455. doi: 10.1177/153537020623100410. [DOI] [PubMed] [Google Scholar]
- 50.Woodward RM, Panicker MM, Miledi R. Actions of dopamine and dopaminergic drugs on cloned serotonin receptors expressed in Xenopus oocytes. Proc Natl Acad Sci USA. 1992;89:4708–4712. doi: 10.1073/pnas.89.10.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xin M, Gao F, May WS, Flagg T, Deng X. Protein kinase Czeta abrogates the proapoptotic function of Bax through phosphorylation. J Biol Chem. 2007;282:21268–21277. doi: 10.1074/jbc.M701613200. [DOI] [PubMed] [Google Scholar]
- 52.Yao LP, Li XX, Yu PY, Xu J, Asico LD, Jose PA. Dopamine D1 receptor and protein kinase C isoforms in spontaneously hypertensive rats. Hypertension. 1998;32:1049–1053. doi: 10.1161/01.hyp.32.6.1049. [DOI] [PubMed] [Google Scholar]
- 53.Yasunari K, Kohno M, Hasuma T, Horio T, Kano H, Yokokawa K, Minami M, Yoshikawa J. Dopamine as a novel antimigration and antiproliferative factor of vascular smooth muscle cells through dopamine D1-like receptors. Arterioscler Thromb Vasc Biol. 1997;17:3164–3173. doi: 10.1161/01.atv.17.11.3164. [DOI] [PubMed] [Google Scholar]
- 54.Yasunari K, Kohno M, Kano H, Hanehira T, Minami M, Yoshikawa J. Anti-atherosclerotic action of vascular D1 receptors. Clin Exp Pharmacol Physiol Suppl. 1999;26:S36–S40. [PubMed] [Google Scholar]
- 55.Yasunari K, Matsui T, Maeda K, Nakamura M, Watanabe T, Kiriike N. Anxiety-induced plasma norepinephrine augmentation increases reactive oxygen species formation by monocytes in essential hypertension. Am J Hypertens. 2006;19:573–578. doi: 10.1016/j.amjhyper.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 56.Yu PY, Asico LD, Eisner GM, Jose PA. Differential regulation of renal phospholipase C isoforms by catecholamines. J Clin Invest. 1995;95:304–308. doi: 10.1172/JCI117656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng C, Felder RA, Jose PA. A new approach for treatment of hypertension: modifying D1 dopamine receptor function. Cardiovasc Hematol Agents Med Chem. 2006;4:369–377. doi: 10.2174/187152506778520727. [DOI] [PubMed] [Google Scholar]
- 58.Zeng C, Liu Y, Wang Z, He D, Huang L, Yu P, Zheng S, Jones JE, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Activation of D3 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Circ Res. 2006;99:494–500. doi: 10.1161/01.RES.0000240500.96746.ec. [DOI] [PubMed] [Google Scholar]
- 59.Zeng C, Sanada H, Watanabe H, Eisner GM, Felder RA, Jose PA. Functional genomics of the dopaminergic system in hypertension. Physiol Genomics. 2004;19:233–246. doi: 10.1152/physiolgenomics.00127.2004. [DOI] [PubMed] [Google Scholar]
- 60.Zeng C, Wang D, Yang Z, Wang Z, Asico LD, Wilcox CS, Eisner GM, Welch WJ, Felder RA, Jose PA. D1 dopamine receptor augmentation of D3 receptor action in rat aortic or mesenteric vascular smooth muscles. Hypertension. 2004;43:673–679. doi: 10.1161/01.HYP.0000118958.27649.6f. [DOI] [PubMed] [Google Scholar]


