Abstract
OBJECTIVE
To develop and validate a model of cutaneous allodynia triggered by dural inflammation for pain associated with headaches. To explore neural mechanisms underlying cephalic and extracephalic allodynia.
METHODS
Inflammatory mediators (IM) were applied to the dura of unanesthetized rats via previously implanted cannulas and sensory thresholds of the face and hindpaws were characterized.
RESULTS
IM elicited robust facial and hindpaw allodynia which peaked within 3 hr. These effects were reminiscent of cutaneous allodynia seen in patients with migraine or other primary headache conditions, and were reversed by agents used clinically in treatment of migraine, including sumatriptan, naproxen, and a CGRP-antagonist. Consistent with clinical observations the allodynia was unaffected by an NK-1 antagonist. Having established facial and hindpaw allodynia as a useful animal surrogate of headache-associated allodynia, we next showed that blocking pain-facilitating processes in the rostral ventromedial medulla (RVM) interfered with its expression. Bupivacaine, destruction of putative pain-facilitating neurons or block of cholecystokinin receptors prevented or significantly attenuated IM-induced allodynia. Electrophysiological studies confirmed activation of pain-facilitating RVM ON cells and transient suppression of RVM OFF cells following IM.
INTERPRETATION
Facial and hindpaw allodynia associated with dural stimulation is a useful surrogate of pain associated with primary headache including migraine and may be exploited mechanistically for development of novel therapeutic strategies for headache pain. The data also demonstrate the requirement for activation of descending facilitation from the RVM for the expression of cranial and extracranial cutaneous allodynia and are consistent with a brainstem generator of allodynia associated with headache disorders.
Keywords: headache; migraine; cutaneous allodynia; sumatriptan; naproxen; central sensitization, RVM
INTRODUCTION
Mechanisms underlying migraine headache remain poorly understood. While multiple mechanistic theories of have been advanced1 none has proven consistent with all of the available data. Recent limited imaging data from human migraineurs suggests the possibility that the brainstem could play a critical role2 in headache and associated pain
Some patients demonstrate cutaneous allodynia affecting the periorbital region which spreads to extracephalic regions over the course of their migraine or primary headache episode. Extracranial symptoms include increased sensitivity of the skin to normally non-noxious stimuli associated with ordinary daily activities1,3. The development of allodynia within the referred pain area occurs after a significant delay in the migraine attack1,3, and is believed to reflect the sensitization of second-order neurons in the trigeminal system and higher structures. Testing for allodynia has been suggested as a strategy to optimize therapy, since its absence typically indicates a more robust response to abortive treatments, presumably reflecting the correlation between headache pain and expression of central sensitization1,3.
Here, we explored the time-related development of cutaneous allodynia following IM application to the dura as a model for intracranial pain in awake behaving animals. Dural IM has previously been used in acute electrophysiological studies in anesthetized preparations4 and in models of pain-induced loss of appetite5 and recurrent headache6. The present studies employed a chronically implanted cannula placed superficial to the dura. This minimized potential mechanical trauma to the dura, and allowed recovery from surgery with subsequent measurement of changes in tactile thresholds following dural stimulation. A single application of dural IM elicited time-related allodynia of the facial region that was suppressed by medications effective for acute migraine headache, but not by treatments shown to be ineffective clinically. Critically, expression of allodynia following IM was generalized to other regions of the body including the hindpaws.
Having established dural IM-induced cutaneous allodynia as a useful model of headache-related pain, we next examined the possible contribution of the rostral ventromedial medulla (RVM) to this response. The RVM has been shown to facilitate as well as inhibit pain7,8. Both cranial and extracranial hypersensitivity required the activation of the RVM, and electrophysiological studies showed that dural IM activated RVM pain-facilitating ON cells. These data provide direct evidence for a medullary brainstem mediator of cutaneous allodynia associated with headache. Additionally, tactile allodynia resulting from dural IM may be useful for exploration of mechanisms for novel therapies of headache-related pain.
MATERIALS AND METHODS
Animals
Male, Sprague-Dawley rats (250–300 g; Harlan) were maintained on a 12-hr light/dark cycle with food and water ad libitum. All procedures were performed according to the policies and recommendations of the IASP, the NIH guidelines for laboratory animals, and by the IACUC recommendations of the University of Arizona and Oregon Health and Science University.
Surgical Preparation
Dura cannulation
Anesthesia was induced with ketamine/xylazine (80 mg/kg and 12 mg/kg i.p., respectively). Rats were placed in a stereotactic headholder and a 2 cm incision was made to expose the skull. A 1 mm hole (1 mm left of midline, 1 mm anterior to bregma) was made with a hand drill (DH-0 Pin Vise, Plastics One Inc., Roanoke, VA) to carefully expose the dura. A guide cannula (22 GA, #C313G, Plastics One Inc.), designed to extend 0.5 mm from the pedestal to avoid irritation of the dural tissue, was inserted into the hole and sealed into place with glue. Two additional 1 mm holes were made caudal to the cannula to receive stainless steel screws (#MPX-080-3F-1M, Small Parts Inc., Miami Lakes, FL) and dental acrylic was used to fix the cannula to the screws. A dummy cannula (#C313DC, Plastics One Inc.) was inserted to ensure patency of the guide cannula, the skin was sutured closed around the dried acrylic and Amikacin C (5 mg/kg, i.m.) was administered. Rats were housed separately and allowed 6–8 days recovery. Cannula placement and integrity of the dura was confirmed with microinjection of 10 µL of India ink, which spread 3–5 mm on the dorsal aspect of the dura and did not penetrate to the brain.
RVM cannulation
Some rats also received an additional bilateral guide cannula (26GA, #C235G-1.2mm, Plastics One Inc.) directed to the RVM. The cannula was placed at: −11.0 mm from bregma, −7.5 mm from the dura and 0.6 mm on either side of the midline. Injections were made by expelling 0.5 µL through an injection cannula protruding 1 mm beyond the tip of the guide. Cannula placement was confirmed with India ink and microscopic examination of Nissl-stained medullary sections. Acute single injections into the RVM were performed by inserting a 30 gauge needle attached to a Hamilton syringe and expelling 0.5 µL at the same coordinates.
Behavioral Testing
Facial and hindpaw sensory thresholds to non-noxious tactile stimuli in rats
Rats were acclimated to suspended plexiglass chambers (30 cm L × 15 cm W × 20 cm H) with a wire mesh bottom (1cm2). A behavioral response to calibrated von Frey filaments applied to the midline of the forehead, at the level of the eyes, was indicated by a sharp withdrawal of the head. Paw withdrawal thresholds were determined by applying von Frey filaments to the plantar aspect of the hindpaw and a response was indicated by a withdrawal of the paw. The withdrawal thresholds were determined by the Dixon up-down method. Maximum filament strengths were 8 g and 15 g for the face and hindpaw, respectively.
Dural Inflammation
Inflammation of the dura was produced by injecting 10 µL of a cocktail of inflammatory mediators through an injection cannula (28GA, #C313I, Plastics One Inc.) cut to fit the guide cannula. Composition of synthetic interstitial fluid (SIF) and inflammatory mediators (IM) was modified from previous reports4,5. The SIF consisted of 10 mM Hepes, 5 mM KCl, 1 mM MgCl2, 5 mM CaCl2, and 135 mM NaCl, pH 7.3. The IM solution was formulated with 2 mM histamine, serotonin, bradykinin and 0.2 mM PGE2 in 10 mM Hepes buffer, pH 5.0, representing twice that used by Burstein and colleagues4,5. Pilot studies were performed with multiples of these concentrations, and the formulation providing a robust and consistent, yet submaximal, response was employed.
Experimental Protocols
Baseline behavioral responses to probing of the face and hindpaws were obtained from all rats prior to drug administration. Rats then received either SIF or IM, and behavioral responses were determined at 1 hr intervals for 6 hrs. Drug administration was performed either 10 min prior to dural inflammation or at time points after inflammation by either systemic injection or RVM microinjection. Sumatriptan succinate (GlaxoSmithKline), naproxen (Sigma), L-732,138 (NK-1 antagonist, Tocris), α-CGRP(8–37) (CGRP-antagonist, Bachem), YMO22 (CCK2-antagonist, Tocris), CCK-8(s) (American Peptide Inc.), bupivacaine HCl (Sigma), dermorphin-saporin conjugate and saporin alone (Advanced Targeting Systems) were used. Doses used were kept within published literature.
Immunolabeling
Following behavioral evaluation, some rats were used for determination of FOS expression. Animals were anesthetized with ketamine/xylazine, perfused with phosphate-buffered saline (PBS; pH 7.4) and fixed by perfusion with 500 ml of 4% paraformaldehyde in PBS using standard immunohistochemical methods. Sections 20 µm thick were cut through the caudal medulla at the level of trigeminal nucleus caudalis (TNC) and immunolabeled for FOS with standard methods. Images were acquired with a digital camera and analyzed with MetaMorph imaging software (Molecular Devices, Downington, PA). A total of 5 sections were obtained from each rat, and 4 rats were used for each treatment group.
Electrophysiological Studies
Male Sprague-Dawley rats (Taconic, Germantown, NY, 250–300g) were anesthetized with pentobarbital (60 mg/kg, i.p.), and prepared for RVM extracellular single unit recording as previously described8. A cannula was implanted over the left frontal bone for infusion of IM as described above. Once surgery was complete, the animals were maintained in a lightly anesthetized state using a continuous infusion of methohexital (15–30 mg/kg per hr, i.v.). RVM neurons were classified as previously described8. “OFF cells” showed an abrupt pause in ongoing activity beginning just prior to the occurrence of paw withdrawal (PW). “ON cells” were identified by a sudden burst of activity beginning just prior to PW. “Neutral cells” were identified by no change in activity associated with PW. Following three baseline PW trials (5 min intervals), IM or vehicle was applied to the dura. Cell activity and PW latency were then monitored for an additional 105 min. Only one protocol was performed in each animal. At the conclusion of the experiments, recording sites were marked with an electrolytic lesion and patency of the dural cannula verified by dye injection. Recording sites were distributed in the RVM as in previous reports8.
Data Analysis
Withdrawal thresholds to probing the face and hindpaws were determined at 1 hr intervals after administration of IM or SIF. Data were converted to area under the time-effect (AUC) curve and normalized as a percent of the vehicle-treated control group to allow for multiple comparisons. Comparisons among several treatment groups were performed by 2-factor ANOVA for repeated measures, and comparisons within a treatment group were determined by ANOVA. The post-hoc Student-Neuman-Keuls test was applied to find significant differences among means. Cell counts among treatment groups were evaluated with ANOVA followed by Student-Neuman-Keuls test. Electrophysiological data were analyzed using Friedman’s analysis of variance by ranks followed by Wilcoxon’s signed ranks test for post-hoc analysis. Statistical analyses were performed with FlashCalc, a visual basic-based program developed in-house by M.H.O..
RESULTS
Dural IM Elicits Cutaneous Allodynia
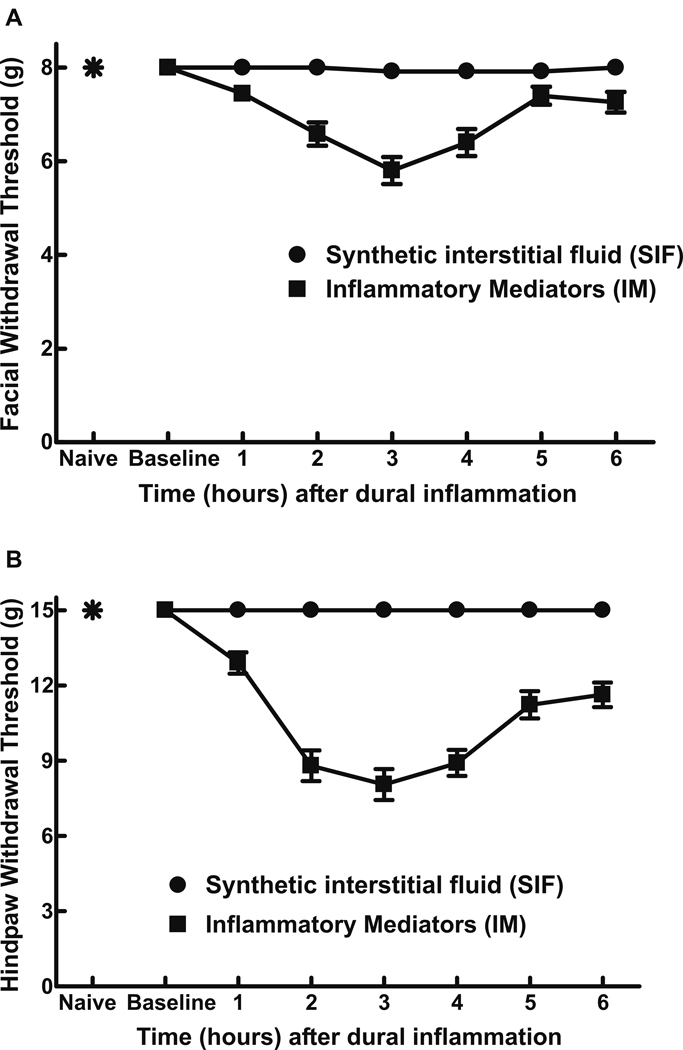
Dural IM produced significant (P < 0.0001) time-dependent and reversible reductions in withdrawal thresholds to tactile stimuli applied to the face or the hindpaws (Fig 1A,B). Maximal effects occurred 3 hrs after IM, and facial and hindpaw responses approached baseline values by 5 and 6 hrs after IM, respectively (Fig 1). Cannula implantation alone or dural SIF did not significantly change facial or hindpaw thresholds from their respective baseline values (Fig 1, P > 0.05). Pilot experiments with varying IM concentration of up to a four-fold multiple of that previously reported4,5 were performed. A concentration of 2 mM histamine, serotonin, bradykinin and 0.2 mM PGE2 elicited robust and reproducible behavioral effects and was used for all further experiments.
Fig 1.
Withdrawal thresholds to tactile stimuli applied to the face (A) and the hindpaw (B) were measured in rats prior to any surgical manipulations (naïve) and immediately prior to dural application of inflammatory mediators (IM) or synthetic interstitial fluid (SIF) (baseline). There were no significant differences (P > 0.05) between the responses of naïve rats and those receiving SIF, indicating that surgery alone did not produce a sensitization of the face or hindpaws to tactile stimuli. Withdrawal responses to tactile stimuli applied to the face and hindpaws developed slowly over time and reached a maximal decrease in threshold 3 hr after administration. Withdrawal responses to stimuli applied to the face and hindpaws approached baseline values 5 hr and 6 hr, respectively, after IM administration. For both facial and hindpaw responses, 2-factor ANOVA indicated that response thresholds of IM-treated rats were significantly (P < 0.0001) less than those of SIF-treated rats.
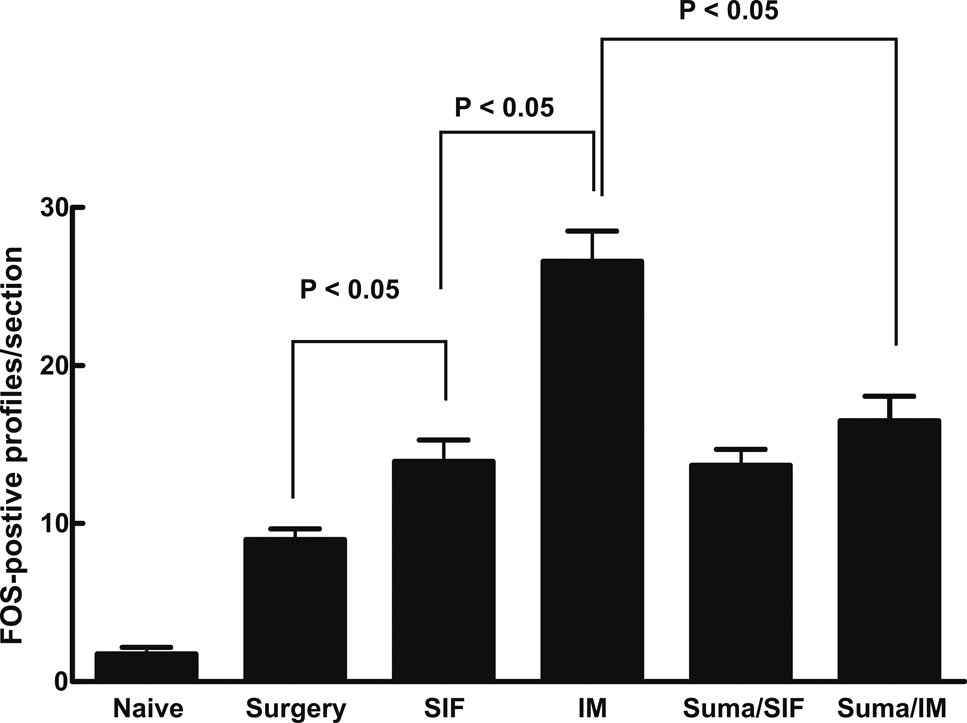
FOS Expression in TNC
The dural cannulation surgery alone elicited a significant increase in FOS expression, expressed as 9.0 ± 0.7 positive profiles per section, relative to naïve animals, which showed only 1.7 ± 0.4 FOS positive neuronal profiles per section of TNC (Fig 2). This result suggests surgery produces an inflammatory response with the likelihood of some associated sensitization in the TNC. The dural administration of SIF produced a moderate, but significant (P < 0.05) increase in FOS expression relative to the surgery only group that was further doubled by dural IM (Fig 2). Sumatriptan abolished IM-induced increased FOS expression (Fig 2).
Fig 2.
Medullary sections (20 µm thick) within 1 mm caudal to the Vi/Vc border were harvested from naïve rats, rats with dural cannulation only and rats receiving either SIF or IM in the absence or presence of systemic sumatriptan. Sections were prepared for DAB staining to visualize FOS expression, and the numbers of FOS-positive profiles within the TNC were counted. Significant (P < 0.05) differences among mean counts for each group were determined by ANOVA followed by Student-Neuman-Keuls post-hoc test. Each group represents a total of 20 sections obtained from 4 rats. The results indicate that cannulation surgery alone produced a significant elevation in trigeminal FOS expression compared to the naïve rats, and SIF administration further enhanced FOS expression. Dural administration of IM produced a significantly greater increase in FOS expression when compared to SIF-treated rats, and this expression was reduced to the same level as the SIF-treated group by administration of sumatriptan.
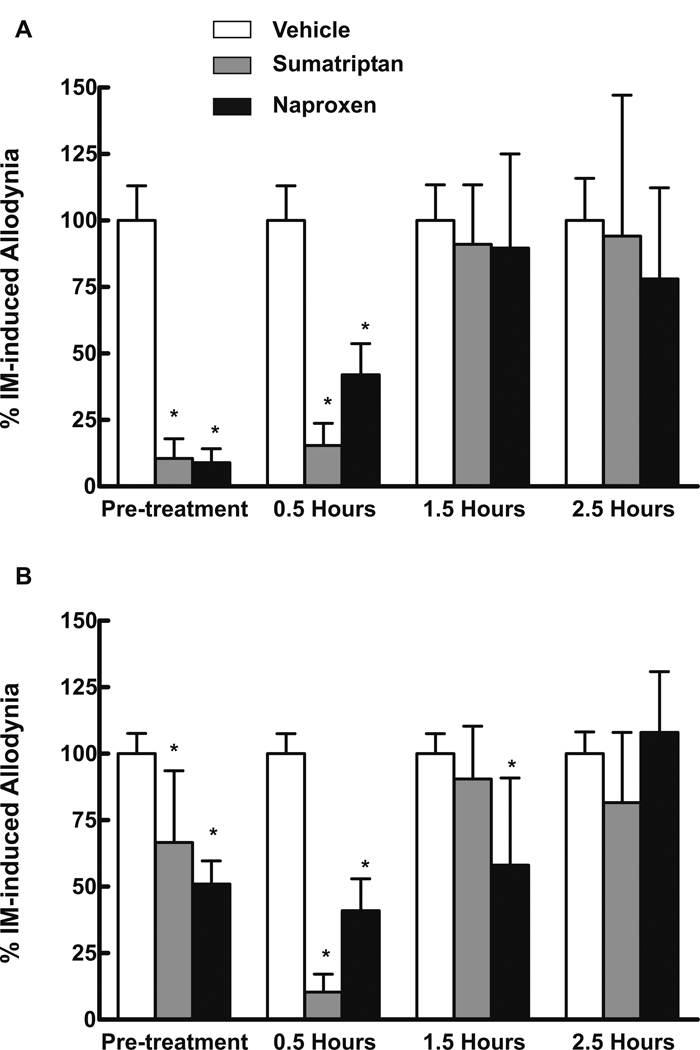
Systemic Drug Treatment of IM-induced Cutaneous Allodynia
Sumatriptan (0.6 mg/kg) or naproxen (100 mg/kg) administered s.c., either 10 min prior to or 30 min after dural IM, prevented and reversed facial and hindpaw cutaneous allodynia (Fig 3A,B respectively). Thresholds were significantly (P < 0.001) reduced in the presence of either sumatriptan or naproxen. Sumatriptan administered 1.5 or 2.5 hr after IM, and naproxen at 2.5 hr after IM, did not significantly change behavioral responses (Fig 3). Neither sumatriptan nor naproxen altered responses in SIF-treated animals.
Fig 3.
Rats received dural administration of IM or SIF and also received either sumatriptan (0.6 mg/kg, s.c.), naproxen (100 mg/kg, s.c.), or saline. Animals were injected systemically either 10 min prior to the dural administrations or 0.5, 1.5 or 2.5 hrs after dural administration. Pretreatment with sumatriptan or with naproxen prevented the development of tactile allodynia of the face (A) or hindpaws (B), indicated by significant (P < 0.05) reductions in normalized allodynic responses. Administration of sumatriptan or naproxen 0.5 hr after dural inflammation abolished facial and hindpaw indications of tactile allodynia. In contrast, the administration of sumatriptan 1.5 and 2.5 hrs after IM or naproxen 2.5 hrs after IM did not significantly alter the withdrawal thresholds to light tactile stimuli applied to the face (A) or hindpaws (B). Asterisks indicate significant (P < 0.05) differences from the corresponding vehicle-treated group within each experiment. Animals treated with SIF and then treated with either saline, sumatriptan or naproxen did not demonstrate any evidence of tactile allodynia of the face or hindpaws (data not shown).
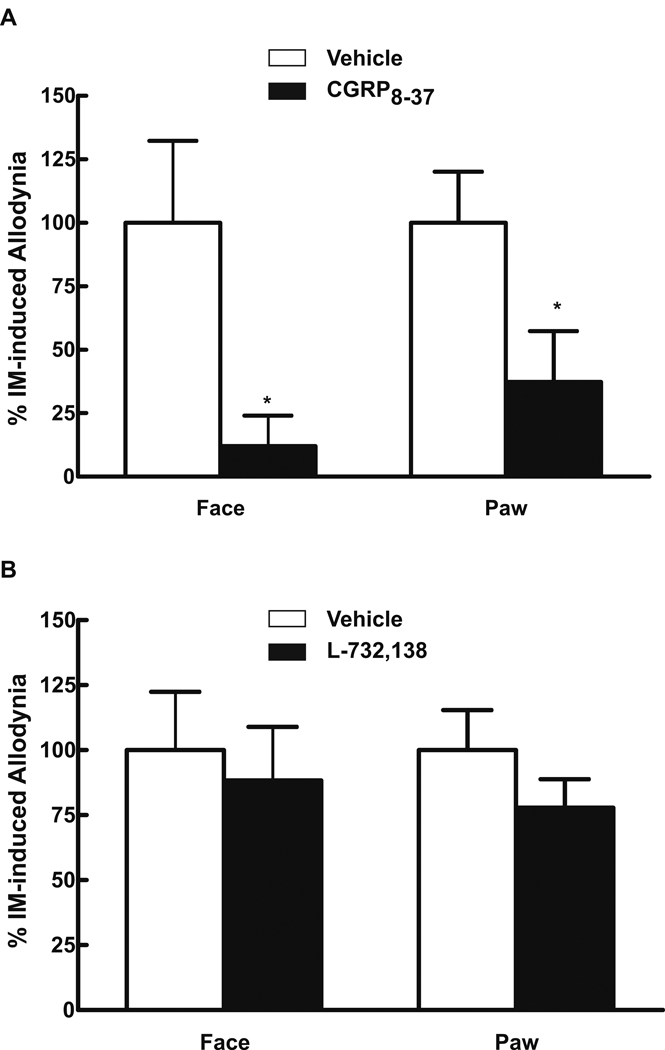
The i.v. administration of 0.45 mg/kg of the CGRP receptor antagonist, α-CGRP(8–37), 30 min after dural IM significantly reduced both facial (P = 0.0032) and hindpaw (P = 0.0002) allodynia (Fig 4A). Systemic α-CGRP(8–37) did not alter sensory thresholds in rats without dural IM. In contrast, L-732,138 (NK-1 antagonist, 10 mg/kg, s.c.) given 10 minutes prior to dural IM did not prevent facial or hindpaw allodynia (Fig 4B). The dose employed abolished nocifensive behaviors elicited by hindpaw injection of capsaicin indicating blockade of NK-1 receptors.
Fig 4.
Rats received dural administration of IM or SIF and α-CGRP(8–37) (0.45 mg/kg, i.v.) 30 min afterwards (A). Systemic administration of α-CGRP(8–37) abolished behavioral signs of tactile allodynia of the face and hindpaws, indicated by significant (P < 0.05, shown as *) reductions in normalized allodynic responses. In a second set of studies, the NK-1 antagonist L-732,138 (10 mg/kg, s.c.) was administered 10 min before dural administration of IM (B). Pretreatment with L-732,138 failed to prevent development of behavioral signs of tactile allodynia of the face or hindpaws (B). In order to test that the dose employed was sufficient to block NK-1 agonist activity, male rats received an intraplantar injection of 100 µl containing 10 µg of capsaicin and challenged with either vehicle (70% DMSO in saline) or L-732,138. Time spent licking or guarding the hindpaw was determined over a 10 min period. Capsaicin treatment after vehicle injection produced a mean cumulative response time of 133 ± 33.3 sec. Rats injected with L-732,138 showed a significantly (P < 0.05) reduced mean cumulative response time of 46 ± 14.7 sec, suggesting that the dose of L-732,138 was sufficient to block NK-1 mediated nociceptive activity (data not shown).
RVM Studies
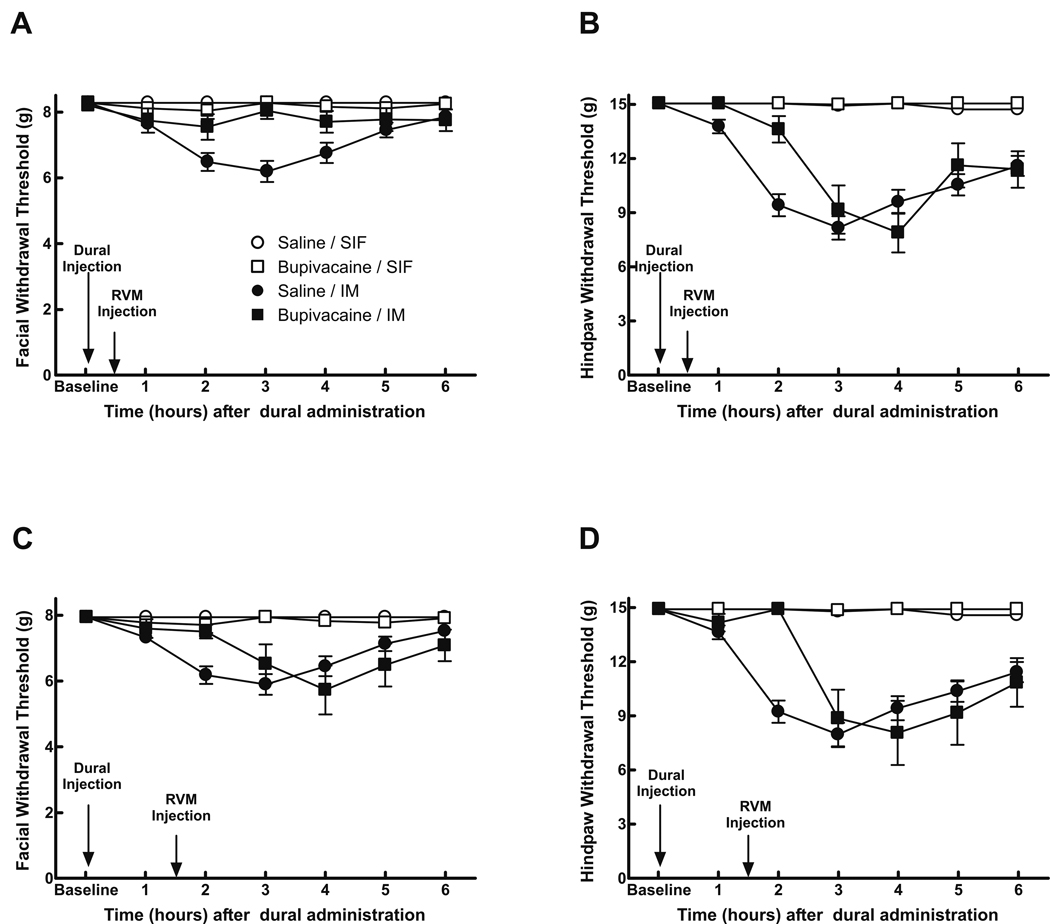
The bilateral microinjection of 0.5 µl of 0.5% w/v bupivacaine into the RVM 30 min after dural IM reversibly prevented, or significantly reduced, facial and hindpaw allodynia (Fig 5A,B). Administration of bupivacaine 1.5 hr after IM significantly (P < 0.05) and reversibly attenuated facial and hindpaw allodynia (Fig. 5C,D). RVM bupivacaine did not alter behavioral responses of rats without IM.
Fig 5.
Rats received dural administration of IM or SIF and also received bupivacaine (0.5% w/v) microinjected into the RVM either 0.5 or 1.5 hr afterwards. Bupivacaine given 0.5 hr after IM blocked the appearance of behavioral signs of facial allodynia (A) and attenuated the development of hindpaw allodynia in a time-dependent, reversible manner (P = 0.0007) (B). When bupivacaine was given 1.5 hr following dural administration of IM, it also significantly (P < 0.05) and reversibly attenuated behavioral signs of allodynia in both the facial region (C) and the hindpaws (D). Behavioral responses were not altered by bupivacaine in SIF rats or saline in SIF rats (data not shown).
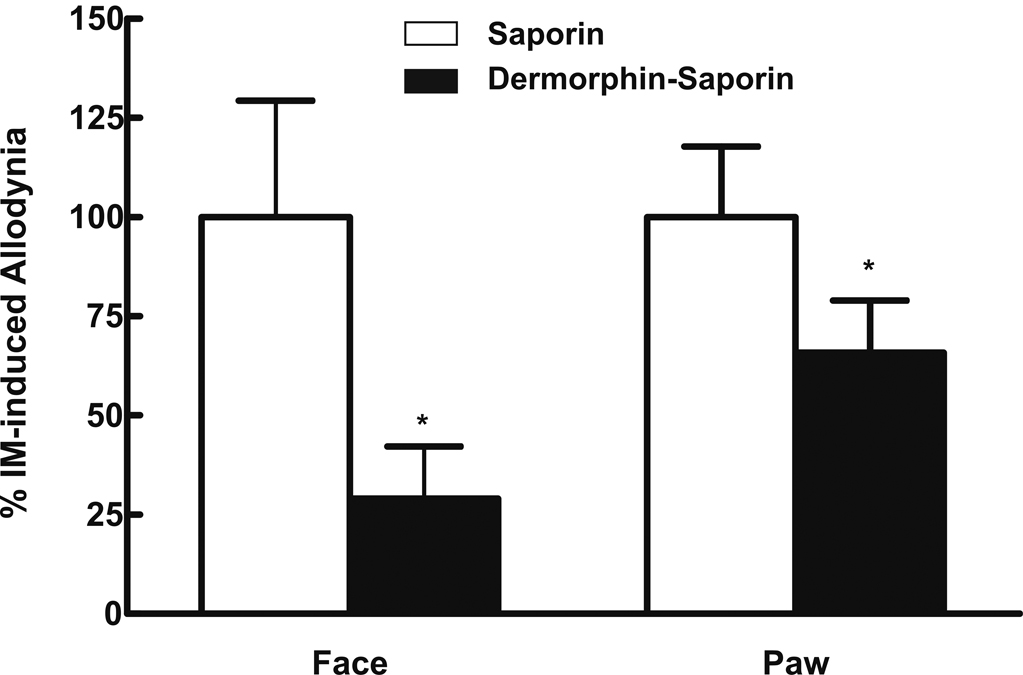
Selective lesion of RVM µ-opioid receptor expressing (i.e., putative pain facilitation) cells was produced by microinjection of dermorphin-saporin conjugate as previously described9. Twenty-eight days following RVM dermorphin-saporin or saporin alone rats were tested with dural IM. While dural IM elicited allodynia in saporin pretreated rats, dermorphin-saporin significantly abolished or diminished IM-induced facial and hindpaw allodynia (Fig 6) with no effect in dural SIF rats.
Fig 6.
Rats were pretreated with dermorphin-saporin (derm-sap) (0.1 µg/µl) or saporin (sap) (0.1 µg/µl) 28 days before behavioral testing. Animals then received either SIF or IM, and withdrawal thresholds to stimuli applied to the face were determined at hourly intervals for 6 hrs. Rats pretreated with saporin and challenged with IM developed facial and hindpaw tactile allodynia (P < 0.05), as indicated by normalized maximal allodynic response. Pretreatment with derm-sap significantly * (P < 0.05) attenuated both facial and hindpaw IM-induced tactile allodynia. Behavioral responses were not altered by derm-sap in SIF rats or sap in SIF rats (data not shown).
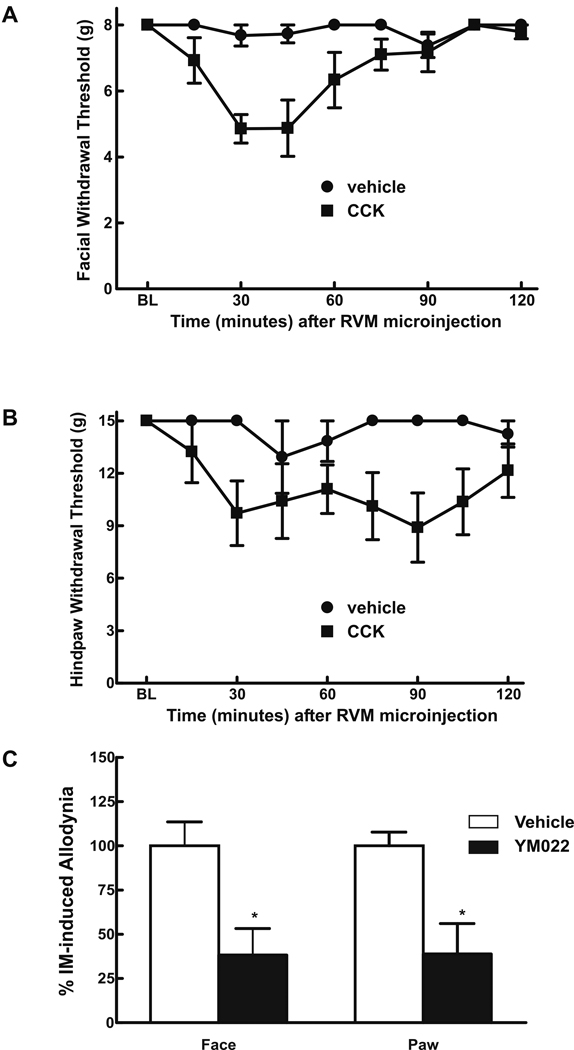
Microinjection of CCK-8 into the RVM of untreated rats produced time-dependent hindpaw10 and facial allodynia (Fig 7A,B). Conversely, YM022 (CCK2-antagonist) microinjected into the RVM abolished IM-induced facial and hindpaw allodynia without affecting thresholds in SIF rats (Fig 7C).
Fig 7.
Rats received microinjection of CCK-8(s) (0.06µg/µl) or dH20 into the RVM. Tactile allodynia of the face (A) and hindpaw (B), indicated by significant decreases in response thresholds, were measured at 15 min intervals for 2 hr following CCK-8(s) or dH20 injection. CCK-8(s) injection produced significant tactile allodynia of both the face (A) and hindpaws (B) that peaked at the 30–45 min time-point and returned towards baseline by 2 hr. Behavioral responses were not altered by dH20 injection. In addition, male rats received microinjection of CCK2-antagonist, YMO22 (0.5 ng/µl) or saline into the RVM 30 min after dural inflammation (C). YMO22 significantly attenuated facial (P = 0.028) and hindpaw (P = 0.0001) allodynia, as indicated by asterisks, when compared to the vehicle-treated group. Behavioral responses were not altered by YMO22 in rats treated with SIF (data not shown).
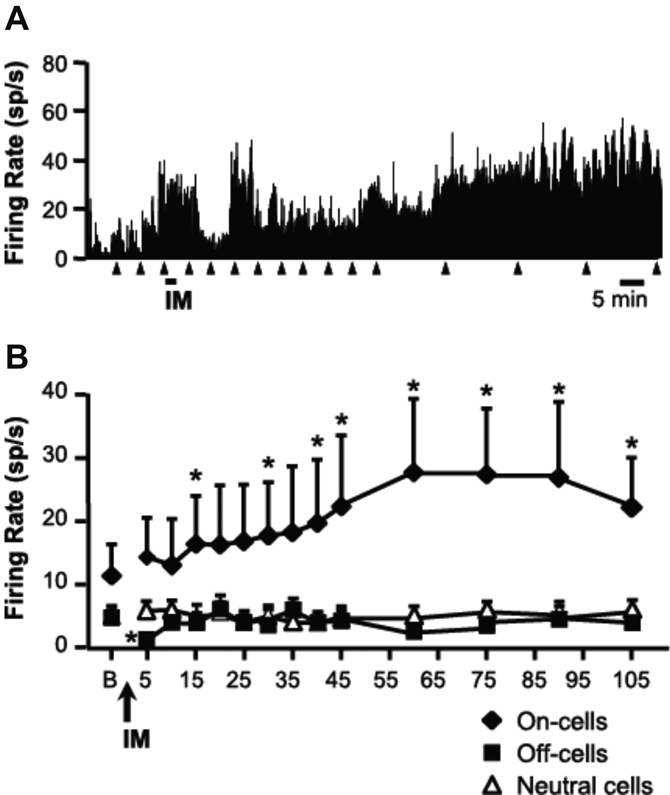
RVM Electrophysiology
Responses of physiologically identified ON cells, OFF cells and neutral cells were recorded following dural IM or SIF (Fig 8). ON cells were potently activated during IM application, and this was followed by a progressive increase in ongoing firing rate which was maintained for 105 min (Fig 8B). Reflex-related firing rate was increased in parallel with ongoing activity. By contrast, OFF cells displayed a transient inhibition during and immediately after the IM infusion, but this was not maintained and activity recovered to baseline within 10 min (Fig 8B). Infusion of SIF had no effect on the discharges of ON or OFF cells (data not shown). Firing of neutral cells was unaffected by IM (Fig 8B).
Fig 8.
A. Effect of dural IM on the discharge of an identified RVM ON cell. The cell responded vigorously during the infusion of IM, but then showed a progressive increase in overall firing to approximately three times baseline at 60 min post infusion. IM was injected at time indicated by underscore, triangles indicate paw withdrawal (PW) trials. Data were collected in 1 s bins. B. Ongoing firing of RVM ON and OFF cells and neutral cells following IM infusion. Mean (+ SEM) overall ongoing discharge of RVM neurons in baseline (“B”) and following infusion of IM. OFF cells (n = 9) displayed a transient inhibition with IM application, whereas ON cells (n = 8) showed a gradual increase in firing that peaked between 1 and 2 hrs post-IM. NEUTRAL cell firing (n = 6) was unchanged following IM. Vehicle infusion did not alter ON or OFF cell firing (data not shown). *P < 0.05 compared to baseline, Friedman’s analysis of variance by ranks followed by Wilcoxon’s signed ranks test for post-hoc analysis.
DISCUSSION
Cutaneous allodynia triggered by dural inflammation as a model for headache-related pain
We have demonstrated that chemical inflammation of the dura elicits cutaneous allodynia that extends beyond the trigeminal dermatome. These responses occur over several hours following IM, consistent with the time-course of cutaneous allodynia seen in migraine patients11,12 and complementing previously reported acute electrophysiological responses4,11.
The pharmacology of IM-induced allodynia showed important parallels with the clinical pharmacology of pain associated with migraine headache. Pretreatment and early post-treatment with sumatriptan and naproxen, current first-line therapies for migraine headache, were effective against IM-induced allodynia. Similarly, IM-induced allodynia was blocked by a CGRP receptor antagonist, consistent with clinical trials13,14. By contrast, allodynia was not altered by a dose of NK-1 antagonist demonstrated to be efficacious in multiple animal models of hyperalgesia15. This observation is consistent with the clinical failure of NK-1 antagonists in treatment of migraine headache16,17. These data thus establish dural IM-induced facial and hindpaw allodynia as a useful animal surrogate of migraine headache-associated pain, as well as for allodynia associated with primary headaches including cluster headache, SUNCT, and other paraoxysmal types of headaches18,19.
IM-induced cutaneous allodynia and central sensitization
Enhanced behavioral responses to light touch and increased FOS expression are indicators of central sensitization in pain models20. Here, IM-induced allodynia developed over several hours, and was found not only on the face, which like the dura is innervated by the trigeminal system21, but also extrasegmentally, on the hindpaws. These observations suggest that the allodynia was not a direct effect of the chemical stimulus, consistent with electrophysiological data following dural inflammation. In the latter experiments, application of lidocaine to the dura attenuated responses of sensitized trigeminal neurons to stimulation of the dura but not of the skin, indicating that sensitization of central trigeminal pathways was not dependent on ongoing activity from the dura4. Dural IM also produced a significant increase in FOS expression in TNC, consistent with previous work5.
IM-induced central sensitization is supported by the effectiveness of sumatriptan in suppressing allodynia only when given as a pretreatment or early post-treatment. These data parallel some12,22 but not all23,24 clinical studies showing that triptan administration blocked cutaneous allodynia when administered upon migraine onset, but are relatively less effective when administered 2 hrs later. A similar time course was seen in electrophysiological studies25, suggesting that triptans block transmission from primary afferents to second-order neurons by actions on presynaptic 5HT1B/1D receptors in this pathway. If so, the triptans would not be expected to reverse sensitization of second-order neurons in the trigeminal system once it had been established25,26. Early intervention with triptans has generally been more successful than late intervention, regardless of presence of allodynia23,24.
The present studies extend the idea of sensitization beyond the trigeminal sensory system to include pain-facilitating systems arising from the RVM8. Thus, inactivation of the RVM with a local anesthetic resulted in attenuation of allodynia during the time-course of action of bupivacaine, at least when given within 2 hrs of IM. Dural IM also elicited a slowly developing but prolonged activation of RVM ON cells, a cell class known to facilitate nociceptive processes at the level of the dorsal horn. Destruction of µ-opioid receptor-expressing neurons in the RVM, which are likely to include ON cells, prevented allodynia in this model. Descending pain-facilitatory projections may be driven, in part, by RVM CCK10,27. Previous studies have shown that RVM microinjection of CCK to uninjured rats can elicit allodynia without the need for afferent input10. Here, RVM CCK, which activates ON cells27, mimicked the effects of dural IM administration in evoking widespread allodynia. Conversely, an RVM CCK-receptor antagonist, which blocks activation of ON cells27 similarly prevented IM-induced allodynia. Notably, RVM OFF cells, which suppress nociceptive transmission, showed only a transient suppression of firing during, and immediately after, dural IM application. . This further supports the idea that once established, processes of central sensitization do not depend on ongoing dural input. As in injury-induced pain states allodynia, in this surrogate for migraine headache-associated pain, thus depends in large part on the activation of a descending facilitatory system arising from the RVM8,9,27.
Implications for mechanisms of headache-related pain
Despite the high prevalence of migraine and other types of primary headache in the general population, our understanding of the underlying mechanisms remains incomplete. For migraine, theories of pain include dilation of extracranial vessels, and neurogenic inflammation, in which activation of trigeminal meningeal afferents through unknown triggers evokes plasma extravasation and vasodilation via interactions within the neurovascular unit28,29. Additionally, theories of cortical spreading depression, parasympathetic vasodilation, and activation of meningeal mast cells have all been put forward. Whereas these theories of migraine pain address important aspects associated with migraine headaches, a unified mechanistic concept has not yet emerged 30–32.
Another proposal is that migraine represents a dysfunction of brainstem mechanisms of pain modulation, or more generally, sensory gating33. This idea is attractive because a central dysfunction could potentially explain the multiple triggers for migraine attacks and the range of symptoms associated with migraine (nausea, photophobia, and phonophobia)33,34. Functional imaging studies performed with migraineurs showed activation of pontine brainstem structures that became active with the onset of a migraine headache, and which remained active when the pain was resolved with triptans, suggesting the possible existence of a “brainstem generator” or a “migraine center”2,33–35. Although not universally accepted, the concept of a brainstem generator of migraine is intriguing in that the proposed sites (i.e., periaqueductal grey (PAG), reticular formation/RVM and locus coeruleus) are prominent components of pain modulatory pathways36. The RVM receives inputs from the PAG and exerts bi-directional control over nociception under different physiological and pathophysiological conditions36. A facilitating influence from the RVM has been implicated in models of hyperalgesia and persistent pain including sickness, acute opiate withdrawal, opioid-induced hyperalgesia, inflammation, and neuropathic pain7,8,9,36,37. Based on our observations, we propose that the pronociceptive role of the RVM in sensitized pain states is critical for cutaneous allodynia associated with headache pain. This suggestion is consistent with the observations that electrical stimulation of the PAG or microinjection of naratriptan into the PAG inhibits responses of trigeminal neurons to dural stimulation38,39.
Conclusions
Animal models of chronic pain are critical in order to aid our understanding of fundamental mechanisms and to further development of novel and effective therapies. To this end, the present study explored allodynia resulting from application of IM to the dura as a possible surrogate for migraine-associated pain. Dural inflammation gave rise to a slowly developing allodynia expressed not only in the trigeminal dermatome, but at the hindpaws. Therapies clinically effective in migraine headache (early sumatriptan, CGRP-antagonist and COX-inhibitor) were efficacious in this model, whereas those proven to be less or ineffective in clinical situations (i.e.; late sumatriptan, NK-1 antagonist) were unsuccessful here. Facial and hindpaw allodynia associated with dural inflammation is thus a useful surrogate of migraine-associated pain. The slow development and extensive distribution of the tactile allodynia are consistent with development of central sensitization following dural inflammation that is dependent on a brainstem generator of cutaneous allodynia associated with headache-related pain.
ACKNOWLEDGEMENT
Supported by Grants from the National Institutes on Health (DA023513, FP; NS052364, MMH) and from GSK.
Contributor Information
R. M. Edelmayer, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ.
T.W. Vanderah, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ.
L. Majuta, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ.
E.-T. Zhang, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ.
B. Fioravanti, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ.
M. De Felice, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ.
J. G. Chichorro, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ.
M. H. Ossipov, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ.
T. King, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ.
J. Lai, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ.
S. H. Kori, Clinical Development, Neurosciences MDC, GlaxoSmithKline, Research Triangle Park, NC.
A.C. Nelsen, Clinical Development, Neurosciences MDC, GlaxoSmithKline, Research Triangle Park, NC
K.E. Cannon, Department of Neurosurgery, Oregon Health and Science University, Portland OR.
M.M. Heinricher, Department of Neurosurgery, Oregon Health and Science University, Portland OR.
F. Porreca, Department of Pharmacology, College of Medicine, University of Arizona, Tucson, AZ.
REFERENCES
- 1.Dodick D, Silberstein S. Central sensitization theory of migraine: clinical implications. Headache. 2006;46 Suppl 4:182–191. doi: 10.1111/j.1526-4610.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 2.Diener HC, May A. New aspects of migraine pathophysiology: lessons learned from positron emission tomography. Curr Opin Neurol. 1996;9:199–201. doi: 10.1097/00019052-199606000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Burstein R, Yarnitsky D, Goor-Aryeh I, et al. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–624. [PubMed] [Google Scholar]
- 4.Burstein R, Yamamura H, Malick A, et al. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol. 1998;79:964–982. doi: 10.1152/jn.1998.79.2.964. [DOI] [PubMed] [Google Scholar]
- 5.Malick A, Jakubowski M, Elmquist JK, et al. A neurohistochemical blueprint for pain-induced loss of appetite. Proc Natl Acad Sci U S A. 2001;98:9930–9935. doi: 10.1073/pnas.171616898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache. 2007;47:1026–1036. doi: 10.1111/j.1526-4610.2007.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ossipov MH, Porreca F. Mechanisms of pain facilitation systems: implications for medication overuse headache. In: Jensen TS, Olesen J, editors. From Basic Pain Mechanisms to Headache. Vol. 14. Oxford: Oxford University Press; 2006. [Google Scholar]
- 8.Heinricher MM, Ingram SL. The brainstem and nociceptive modulation. In: Bushnell MC, Basbaum AI, editors. The Senses, A Comprehensive Reference, Vol. 5, Pain. San Diego: Academic Press; 2008. pp. 593–626. [Google Scholar]
- 9.Porreca F, Burgess SE, Gardell LR, et al. Inhibition of neuropathic pain by selective ablation of brainstem medullary cells expressing the mu-opioid receptor. J Neurosci. 2001;21:5281–5288. doi: 10.1523/JNEUROSCI.21-14-05281.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie JY, Herman DS, Stiller CO, et al. Cholecystokinin in the rostral ventromedial medulla mediates opioid-induced hyperalgesia and antinociceptive tolerance. J Neurosci. 2005;25:409–416. doi: 10.1523/JNEUROSCI.4054-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jakubowski M, Levy D, Goor-Aryeh I, et al. Terminating migraine with allodynia and ongoing central sensitization using parenteral administration of COX1/COX2 inhibitors. Headache. 2005;45:850–861. doi: 10.1111/j.1526-4610.2005.05153.x. [DOI] [PubMed] [Google Scholar]
- 12.Landy SH, McGinnis JE, McDonald SA. Clarification of developing and established clinical allodynia and pain-free outcomes. Headache. 2007;47:247–252. doi: 10.1111/j.1526-4610.2006.00689.x. [DOI] [PubMed] [Google Scholar]
- 13.Doods H, Arndt K, Rudolf K, et al. CGRP antagonists: unravelling the role of CGRP in migraine. Trends Pharmacol Sci. 2007;28:580–587. doi: 10.1016/j.tips.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Ho TW, Mannix LK, Fan X, et al. Randomized controlled trial of an oral CGRP antagonist, MK-0974, in acute treatment of migraine. Neurology. 2007 doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- 15.King T, Ossipov MH, Vanderah TW, et al. Is paradoxical pain induced by sustained opioid exposure an underlying mechanism of opioid antinociceptive tolerance? Neurosignals. 2005;14:194–205. doi: 10.1159/000087658. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein DJ, Wang O, Saper JR, et al. Ineffectiveness of neurokinin-1 antagonist in acute migraine: a crossover study. Cephalalgia. 1997;17:785–790. doi: 10.1046/j.1468-2982.1997.1707785.x. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein DJ, Offen WW, Klein EG, et al. Lanepitant, an NK-1 antagonist, in migraine prevention. Cephalalgia. 2001;21:102–106. doi: 10.1046/j.1468-2982.2001.00161.x. [DOI] [PubMed] [Google Scholar]
- 18.Sjaastad O, Bakketeig LS. The rare, unilateral headaches. Vaga study of headache epidemiology. J Headache Pain. 2007;8:19–27. doi: 10.1007/s10194-006-0292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pareja JA, Sjaastad O. SUNCT syndrome. A clinical review. Headache. 1997;37:195–202. doi: 10.1046/j.1526-4610.1997.3704195.x. [DOI] [PubMed] [Google Scholar]
- 20.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 21.Bereiter DA, Hargreaves KM, Hu JW. Trigeminal mechanisms of nociception: Peripheral and Brainstem organization. In: Bushnell MC, Basbaum AI, editors. The Senses, A Comprehensive Reference, Vol. 5, Pain. San Diego: Academic Press; 2008. pp. 435–460. [Google Scholar]
- 22.Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol. 2004;55:19–26. doi: 10.1002/ana.10786. [DOI] [PubMed] [Google Scholar]
- 23.Cady R, Martin V, Mauskop A, et al. Symptoms of cutaneous sensitivity pre-treatment and post-treatment: results from the rizatriptan TAME studies. Cephalalgia. 2007;27:1055–1060. doi: 10.1111/j.1468-2982.2007.01391.x. [DOI] [PubMed] [Google Scholar]
- 24.Goadsby PJ, Zanchin G, Geraud G, et al. Early vs. non-early intervention in acute migraine-'Act when Mild (AwM)'. A double-blind, placebo-controlled trial of almotriptan. Cephalalgia. 2008;28:383–391. doi: 10.1111/j.1468-2982.2008.01546.x. [DOI] [PubMed] [Google Scholar]
- 25.Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004;55:27–36. doi: 10.1002/ana.10785. [DOI] [PubMed] [Google Scholar]
- 26.Levy D, Jakubowski M, Burstein R. Disruption of communication between peripheral and central trigeminovascular neurons mediates the antimigraine action of 5HT 1B/1D receptor agonists. Proc Natl Acad Sci U S A. 2004;101:4274–4279. doi: 10.1073/pnas.0306147101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinricher MM, Neubert MJ. Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J Neurophysiol. 2004 doi: 10.1152/jn.00411.2004. [DOI] [PubMed] [Google Scholar]
- 28.Moskowitz MA. Neurogenic versus vascular mechanisms of sumatriptan and ergot alkaloids in migraine. Trends Pharmacol Sci. 1992;13:307–311. doi: 10.1016/0165-6147(92)90097-p. [DOI] [PubMed] [Google Scholar]
- 29.Williamson DJ, Hargreaves RJ. Neurogenic inflammation in the context of migraine. Microsc Res Tech. 2001;53:167–178. doi: 10.1002/jemt.1081. [DOI] [PubMed] [Google Scholar]
- 30.Levy D, Burstein R, Strassman AM. Mast cell involvement in the pathophysiology of migraine headache: A hypothesis. Headache. 2006;46 Suppl 1:13–18. doi: 10.1111/j.1526-4610.2006.00485.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang XC, Strassman AM, Burstein R, et al. Sensitization and activation of intracranial meningeal nociceptors by mast cell mediators. J Pharmacol Exp Ther. 2007;322:806–812. doi: 10.1124/jpet.107.123745. [DOI] [PubMed] [Google Scholar]
- 32.Bolay H, Reuter U, Dunn AK, et al. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002;8:136–142. doi: 10.1038/nm0202-136. [DOI] [PubMed] [Google Scholar]
- 33.Goadsby PJ. Recent advances in understanding migraine mechanisms, molecules and therapeutics. Trends Mol Med. 2006 doi: 10.1016/j.molmed.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 34.May A, Goadsby PJ. The trigeminovascular system in humans: pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. J Cereb Blood Flow Metab. 1999;19:115–127. doi: 10.1097/00004647-199902000-00001. [DOI] [PubMed] [Google Scholar]
- 35.May A, Matharu M. New insights into migraine: application of functional and structural imaging. Curr Opin Neurol. 2007;20:306–309. doi: 10.1097/WCO.0b013e328136c20b. [DOI] [PubMed] [Google Scholar]
- 36.Fields HL, Basbaum AI. Central nervous system mechanisms of pain modulation. In: Wall PD, Melzack R, editors. Textbook of Pain. 4 ed. Edinburgh: Churchill Livingstone; 1999. pp. 309–329. [Google Scholar]
- 37.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 38.Bartsch T, Knight YE, Goadsby PJ. Activation of 5-HT(1B/1D) receptor in the periaqueductal gray inhibits nociception. Ann Neurol. 2004;56:371–381. doi: 10.1002/ana.20193. [DOI] [PubMed] [Google Scholar]
- 39.Knight YE, Goadsby PJ. The periaqueductal grey matter modulates trigeminovascular input: a role in migraine? Neuroscience. 2001;106:793–800. doi: 10.1016/s0306-4522(01)00303-7. [DOI] [PubMed] [Google Scholar]