Abstract
Background
Reproductive failure is a significant public health concern. Although relatively little is known about factors affecting fertility and early pregnancy loss, a growing body of literature suggests that environmental and lifestyle factors play an important role. There is sufficient evidence to hypothesize that diet, particularly its constituent antioxidants, and oxidative stress (OS) may influence the timing and maintenance of a viable pregnancy. We hypothesize that conditions leading to OS in the female affect time-to-pregnancy and early pregnancy loss.
Methods
We review the epidemiology of female infertility related to antioxidant defenses and oxidation and examine potential sources of OS from the ovarian germ cell through the stages of human pregnancy and pregnancy complications related to infertility. Articles were identified through a search of the PubMed database.
Results
Female OS is a likely mediator of conception and threshold levels for OS exist, dependent on anatomic location and stage of preconception.
Conclusions
Prospective pregnancy studies with dietary assessment and collection of biological samples prior to conception with endpoints of time-to-pregnancy and early pregnancy loss are needed.
Keywords: antioxidants, female infertility, oxidative stress
Introduction
Reproductive failure is a significant public health concern. Infertility, defined as the failure to conceive a recognized pregnancy after 12 months of unprotected intercourse, carries significant personal, societal and financial consequences (Goldman et al., 2000). Human reproduction is inefficient with only one-fourth to one-third of fertilized human embryos likely to survive to produce a term delivery (Witschi, 1968; Baird et al., 1986). The great majority of the failures occur in the early weeks after ovulation, with relatively little loss after clinical detection (Fig. 1). Women who experience a delay before achieving a recognized conception also have elevated rates of early unrecognized pregnancy loss (Hakim et al., 1998) and clinical spontaneous abortion (Gray and Becker, 2000). A review by Gray and Becker (2000) suggests that the delayed conception and the early pregnancy loss may share a common etiology, possibly through events or exposures prior to or during implantation and embryogenesis.
Figure 1.
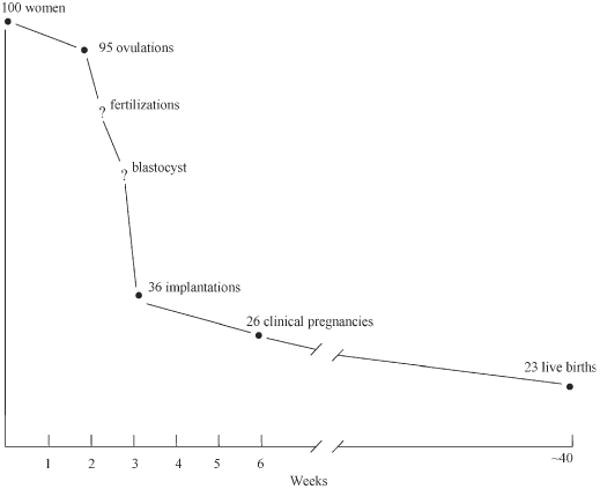
Timeline of reproductive failure. (Baird et al., 1986; reprinted by permission of Oxford University Press).
Although relatively little is known about factors affecting fertility and early pregnancy loss, there is sufficient evidence to hypothesize that dietary antioxidants and oxidative stress (OS) may influence the timing and maintenance of a viable pregnancy (Fig. 2). Evidence from studies of men indicates that diet appears to be crucial in preventing oxidative damage to sperm DNA (Woodall and Ames, 1997). Sperm-related dysfunctions associated with reactive oxygen species (ROS) are decreased sperm number and motility, and inhibition of sperm-oocyte fusion (Sharma and Agarwal, 1996). The female ovary is the source of oocytes and regulating hormones, and OS in the gynecologic environment is likely to be an important mediator of conception. Evaluation of the impact of OS on women's fertility and early pregnancy loss represents a significant gap in our knowledge about reproduction and suggests new research in this area is warranted.
Figure 2.
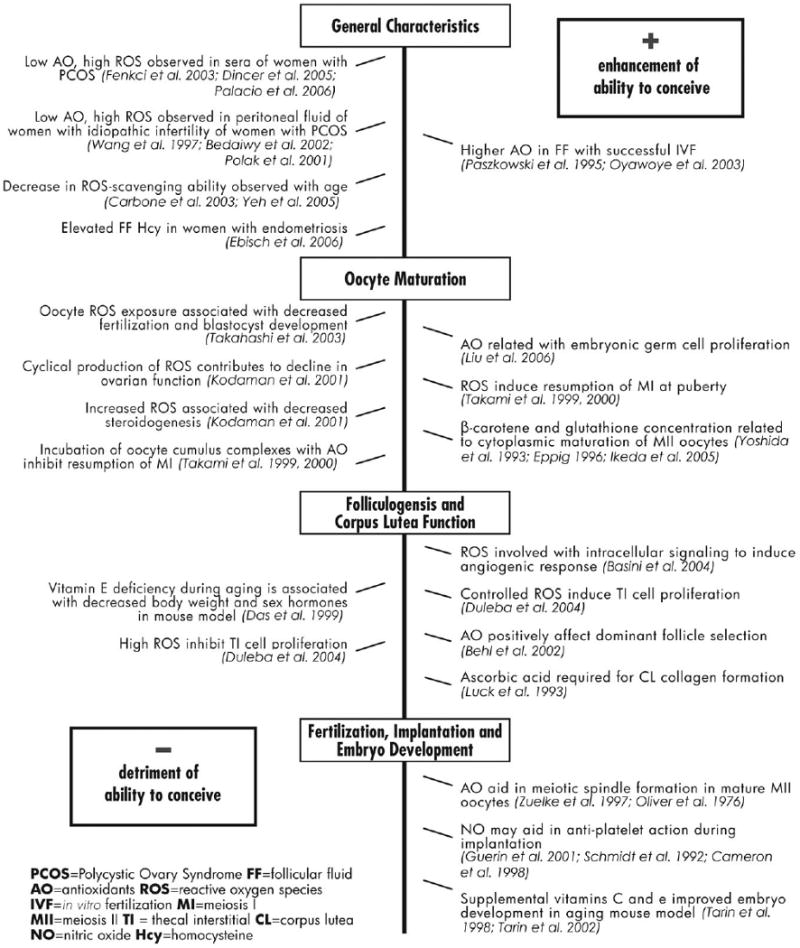
Selected studies on the association of antioxidants, oxidative stress and conception.
Materials and Methods
We searched PubMed database for all articles published in English from January 1966 though October 2006 with evidence relating to OS, antioxidant status and female fertility. Search terms included conception, conception delay, ROS, early pregnancy loss, antioxidant, OS, ovarian aging and infertility. We reviewed the abstract of the identified articles to determine if the article met the scope of our review. The reference lists of articles included in our review were also examined for additional potential articles.
ROS and OS
In living cells, ROS are formed continuously as a consequence of both biochemical reactions, e.g. within the mitochondrial respiratory chain and external factors (Fig. 3). OS induces lipid peroxidation, structurally and functionally alters protein and DNA, promotes apoptosis, and contributes to the risk of chronic diseases like cancer and heart disease via effects on redox status and/or redox-sensitive signaling pathways and gene expression (Ames et al., 1993). Evidence from in vitro, animal model and clinical studies suggests that OS plays a role in the etiology of adverse reproductive events in both women and men (Sharma and Agarwal, 1996; Jozwik et al., 1999; Duru et al., 2000; Shen and Ong, 2000; Vural et al., 2000; Walsh et al., 2000; Acevedo et al., 2001; Sikka, 2001). OS occurs when the generation of ROS and other radical species exceeds scavenging by antioxidants as a result of excessive production of ROS and/or inadequate intakes or increased utilization antioxidants. Antioxidants (such as vitamins C and E) and antioxidant cofactors (such as selenium, zinc and copper) are compounds that are capable of disposing, scavenging or suppressing the formation of ROS. In vitro and animal studies point to several possible avenues through which OS may affect fertility and early pregnancy loss, however, no study has directly addressed the effects of OS on fertility in women, although a recent review has addressed folate, zinc and antioxidants in the pathogenesis of subfertility (Ebisch et al., 2007).
Figure 3.
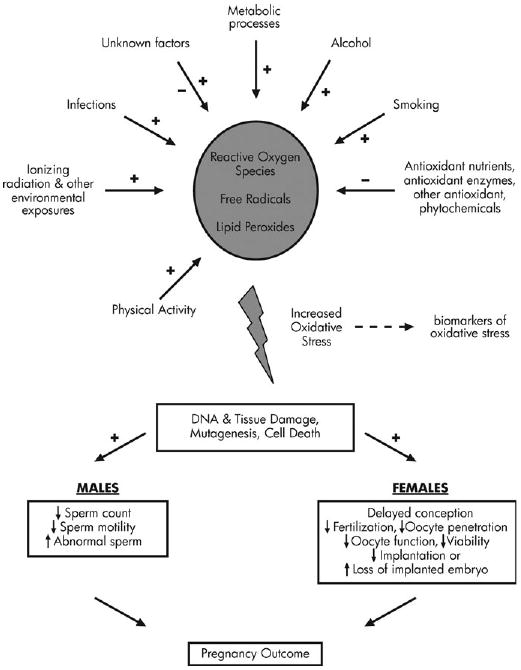
Role of oxidative stress in fertility.
It is interesting to note that some, but not all, antioxidants have increased Dietary Reference Intakes during pregnancy, as issued by the Food and Nutrition Board, Institute of Medicine (2003). For example, the maternal requirement for vitamin C is increased during pregnancy due to hemodilution and active transfer to the fetus. Certain populations, such as cigarette smokers and heavy users of alcohol, may have further increased vitamin C requirements during pregnancy due to increased lipid peroxidation. In contrast, sufficient scientific evidence is not available to support a change in the requirement of vitamin E during pregnancy.
Pregnancy itself may produce OS as a result of increased metabolic activity. Increased plasma thiols in pregnant women (Wisdom et al., 1991) and increased placental lipid peroxides and decreased expression of antioxidants have been reported (Wisdom et al., 1991; Myatt and Cui, 2004). Substantial increases in OS have been hypothesized to lead to acute pregnancy complications (Wang et al., 1997) or spontaneous abortion (Sane et al., 1991; Vural et al., 2000). Successful initiation of pregnancy requires the ovulation of a mature oocyte, production of competent sperm, proximity of sperm and oocyte in the reproductive tract, fertilization of the oocyte, transport of the conceptus into the uterus, and implantation of the embryo into a properly prepared, healthy endometrium. A dysfunction in any one of these complex biological steps can cause infertility (Goldman et al., 2000). In this paper, we hypothesize that conditions leading to OS in the female, including low intake of dietary antioxidants, affect time-to-pregnancy and early pregnancy loss. We review the epidemiology of female infertility related to oxidation and consider potential factors for the production of OS, including diet and body weight status. Cigarette and alcohol use are known to correlate with diet and are also reviewed for completeness of the diet-lifestyle paradigm. Presently, the depth of evidence from animal models provides a strong biological rationale for a growing number of studies involving human subjects. Both animal and human studies are reviewed from the ovarian germ cell through the stages of human pregnancy and pregnancy complications related to infertility.
Epidemiology of dietary and diet-related exposures to oxidation and fertility
Indirect evidence of the importance of OS and its control with antioxidant intake is provided by studies that have shown that preconceptional multivitamin supplementation may enhance fertility, perhaps by increasing menstrual cycle regularity (Czeizel et al., 1994; Dudas et al., 1995) or via prevention of ovulatory disorders (Chavarro et al., 2007). Caffeinated beverages are also associated with decreased fertility (Wilcox et al., 1988; Christianson et al., 1989), possibly mediated by tubal disease or endometriosis, which are characterized by ROS in the hydrosalpingeal fluid (HSF) and peritoneal fluid, respectively (Grodstein et al., 1993; Bedaiwy et al., 2002b; Allaire, 2006).
The effects of body weight and weight change on OS have only recently been investigated. More studies on this topic are needed, since both inadequate and excessive energy intakes have been associated with reduced fertility among women. Research in this area has focused on the effects of energy intake on hormonal patterns and menstrual cycles. Irregular menstrual cycles, ovulatory dysfunction and later age at menarche (Komura et al., 1992) have been associated with both low and high body mass index (BMI, calculated as kg/m2) (Grodstein et al., 1994a; Frisch, 1997), energy intake (Williams, 2003) and high levels of physical activity (Cumming et al., 1994; De Souza and Williams, 2004). Physical activity, in turn, is associated with an increase in ROS (Davies et al., 1982), but appears only to be damaging to tissues when the exercise is exhaustive (Gomez-Cabrera et al., 2003). Kurzer and Calloway (1986) demonstrated that even short-term energy deprivation affected the menstrual cycles and sex hormone levels of normal weight women (n = 6) and had an effect on hypothalamic–pituitary function. Trevisan et al. (2001) suggested that BMI is modestly but positively correlated with OS in women (n = 903, r = 0.09, P < 0.05). These investigators noted that the relationship was strongest among premenopausal women, but did not provide supporting data. Research needs in this area include prospective studies on the influence of other factors related to energy balance and fertility (e.g. body fat distribution, recurrent weight fluctuations) on OS, delayed time-to-pregnancy and early pregnancy loss. The association between OS, delayed onset of menses and irregular menstrual cycles also needs to be explored.
Smoking and alcohol are both known to decrease fertility in women (Howe et al., 1985; Hakim et al., 1998), likely through an increase in OS. Cigarette smoke contains a number of ROS (Pryor et al., 1983) and ethanol metabolism generates ROS through the electron transport chain. Both cigarette smoking and alcohol consumption may lead to lipid peroxidation, protein oxidation and DNA damage. However, dietary intakes of smokers are different from non-smokers, confounding this relationship (Subar and Harlan, 1993). Among individuals undergoing in vitro fertilization (IVF), smokers (n = 117) were found to have diminished follicular fluid (FF) activity of the antioxidant enzyme glutathione peroxidase (GSHPx) (Paszkowski et al., 1995). The mean GSHPx activity in follicles yielding oocytes that were subsequently fertilized was greater than that of the follicles with non-fertilized oocytes. Overall, smokers have been reported to have higher ROS in FF and have a lower IVF success rate (Paszkowski et al., 2002). Lower concentrations of FF beta-carotene and decreased IVF success have been found in smokers (n = 17) compared with non-smokers (n = 43) (Tiboni et al., 2004). This finding is consistent with a smaller study of 5 smokers and 11 non-smokers (Palan et al., 1995). Tiboni et al. (2004) found no smoking-related differences in FF or plasma concentrations of vitamin E or lycopene, suggesting that follicular loss of beta-carotene occurs in response to OS brought on with smoking. Beta-carotene has previously been reported to be one of the micronutrients whose concentration is most strongly influenced by smoking (Alberg, 2002).
Bolumar et al. (2000) reported associations between tobacco use, BMI and time-to-pregnancy from the European Study of Infertility and Subfecundity. Using information collected at ≥20 weeks gestation on 4035 women, they found that conception was delayed among female smokers with BMI > 30 (Odds Ratio (OR) = 11.5; 95% Confidence Interval (CI) 3.7–36.2) or BMI < 20 (OR = 1.70; 95% CI 1.1–2.8), compared to normal weight smokers (BMI = 20.0–24.9). Analysis of non-smokers in the cohort showed no association between BMI and time-to-pregnancy, however women who did not become pregnant, regardless of smoking status, were not studied.
A study of healthy post-menopausal women (n = 53) who consumed a controlled diet plus each of three treatments (15 or 30 g of alcohol per day or a no-alcohol placebo beverage), during three 8-week periods in random order indicated that consuming 30 g of alcohol per day (equivalent to two drinks per day) was associated with a 4.6% decrease in α-tocopherol, the principal circulating form of vitamin E (P = 0.02) and a 4.9% increase in OS measured by serum isoprostane concentration (P = 0.07) (Hartman et al., 2005). This report indicates that even moderate alcohol consumption can affect OS in certain female populations. It is worth noting that the essentiality of vitamin E for fertility in rodents resulted in its dietary compounds being named ‘tocopherol,’ the Greek word for ‘childbirth’ (tocos) and ‘to bring forth’ (pheros) (Evans, 1963; Gray, 1996).
Female partners of couples (n = 430) planning a first pregnancy who consumed alcohol while they were trying to conceive were found to have reduced odds of conceiving over a period of six menstrual cycles compared to women who voluntarily abstained from alcohol (Jensen et al., 1998). After multivariate adjustment, compared with women who abstained from alcohol, women who consumed 1–5 drinks/week had an OR of becoming pregnant = 0.61 (95% CI 0.40–0.93), whereas women who consumed 6–10 drinks/week had an OR = 0.55 (95% CI 0.36–0.85), and women who reported 11–15 drinks/week had an OR = 0.34 (95% CI 0.22–0.52), (P = 0.03). No relationship with alcohol intake was observed among male partners (Jensen et al., 1998). Self-reported alcohol intake among a cohort of 39 612 pregnant women showed no marked reduction in waiting time to pregnancy with alcohol consumption; however, women who failed to conceive were not available for analysis since women entered the cohort once they were pregnant (Juhl et al., 2001). A positive dose–response relationship between alcohol consumption and ovulatory factor infertility has also been reported in a case–control study in the USA (Grodstein et al., 1994b). Compared with non-drinkers, the OR for ovulatory infertility was 1.3 (95% CI 1.0–1.7) for moderate drinkers (consuming <100 g alcohol or <7 drinks alcohol/week) and 1.6 (95% CI 1.1–2.3) for heavy drinkers (consuming >100 g alcohol/week).
An 18-year prospective examination of Swedish women (n = 7393) revealed an increased risk for proxy measures of infertility among high consumers of alcohol (defined as those responding ‘often’ to the question: ‘Do you drink alcohol’ and responding ‘sometimes’ or ‘often’ to the question: ‘Do you drink at least a half bottle of spirits or a couple bottles of wine per week?’ in a baseline questionnaire) (Eggert et al., 2004). Compared with moderate consumers of alcohol, high consumers were 58% more likely to have undergone an infertility examination and low consumers of alcohol were 36% less likely to have undergone an infertility examination (95% CI 1.07–2.34 and 0.46–0.90, respectively). Both low and high consumers of alcohol had fewer first and second registered births compared with moderate drinkers (RR = 0.87, 95% CI 0.81–0.94 and RR = 0.78, 95% CI 0.69–0.88 for low and high alcohol consumers, respectively).
In addition to alcohol and tobacco use, other exogenous agents such as environmental pollutants acting as endocrine disruptors or in an otherwise antagonistic manner may interfere with ovarian development, folliculogenesis and steroidogenesis (Uzumcu and Zachow, 2007). Although many of these contaminants have pro-oxidant capabilities, oxidation is not the likely mechanism of action causing the endocrine disruption. Pesticides (e.g. dichlorodiphenyltrichloroethane, DDT; methoxychlor, MXC; vinclozolin; and atrazine), detergents and surfactants (e.g. octyphenol, nonylphenol and bisphenol-A) plastics (e.g. phthalates), and industrial compounds (e.g. polychlorinated biphenyl, PCB) have potential estrogenic, anti-estrogenic, and/or anti-androgenic effects thus potentiating the ability to mimic endogenous hormone mediated mechanisms and interfere with normal female fertility (Uzumcu and Zachow, 2007). Moreover, fetal exposure to certain environmental pollutants may alter DNA methylation (Li et al., 1997; Anway et al., 2005), leading to the expression of hormone–responsive genes in adult-life, but also transmission of altered genes to the next generation (Li et al., 1997; Newbold et al., 1998, 2000). In vitro animal work suggests that the exposure to mercury and cadmium decreases the quantity of ATP in the ovary and uterus via a reduction in the ATP-hydrolyzing enzyme and may affect mammalian fertility (Milosevic et al., 2005), however self-reported time-to-pregnancy was not associated with hair mercury concentration among 193 Japanese women (Arakawa et al., 2006).
ROS, antioxidants and reproductive processes in women
Oocytes: ovarian germ cells to secondary oocytes
Liu et al. (2006) investigated the antioxidant effects of daidzen, an isoflavone found principally in soybeans, on germ cell proliferation in ovarian cells extracted from 18-day-old chicken embryos. Exposure to daidzen increased germ cell proliferation (P < 0.05) and helped restore overall antioxidant levels following a challenge by the ROS-producing hypoxanthine/xanthine oxidase (HX/XO) system.
Following hormonal influence at puberty, a number of primary oocytes begin to grow each month. One primary oocyte outgrows the others and resumes meiosis I (MI). Interestingly, resumption of MI is induced by an increase in ROS and inhibited by antioxidants (Takami et al., 1999, 2000; Kodaman and Behrman, 2001), indicating that regulated generation of ROS by the pre-ovulatory follicle is an important promoter of the ovulatory sequence. However, it has been suggested that cyclical ROS production may, over time, contribute to oophoritis associated with autoimmune premature ovarian failure (Behrman et al., 2001) and exacerbated by diminished antioxidant status.
Oocyte maturation occurs with the second meiotic division (MII), which arises in response to an increase in pre-ovulatory luteinizing hormone (LH) (Thibault et al., 1987). The process is suspended in metaphase and does not resume unless fertilization occurs following ovulation of the mature oocyte. In both the human and rat, granulosa and luteal cells respond negatively to ROS and adversely affect MII progression, leading to diminished gonadotrophin and anti-steroidogenic actions, DNA damage, and inhibited protein ATP production (Behrman et al., 2001). Glutathione (GSH), a non-protein sulphydryl tripeptide and key cellular antioxidant, has also been identified as critical for oocyte maturation, particularly in the cytoplasmic maturation required for pre-implantation development and formation of the male sperm pronucleus (Yoshida et al., 1993; Eppig, 1996). In bovine models, beta-carotene has been recognized for its ability to enhance cytoplasmic maturation, further supporting reports in other species (Ikeda et al., 2005). The contrasting relationship of antioxidants, detrimental for the progression of MI, but beneficial for MII, suggests a complex role for antioxidants and ROS in the ovarian environment. Such findings, along with others discussed below suggesting a threshold for ROS beneficence where embryo formation is compromised by ROS concentration during IVF treatment, requires an appreciation of ROS as multifunctional agents in which their effects may vary over the continuum of concentration and developmental stages.
Folliculogenesis
Generation of ROS
Attendant to the increase in steroid hormone production of developing follicles is an increase in the activity of cytochrome P450, which in turn generates ROS such as hydrogen peroxide (H2O2) (Ortega-Camarillo et al., 1999). An investigation of ROS regulation by the preovulatory follicle in response to LH indicated that a gonadotrophin-simulated, protein kinase C-activated, NADPH/NADH oxidase-type superoxide generator in the preovulatory follicle exists and may be a regulating factor in ROS production during ovulation (Kodaman and Behrman, 2001). Behl and Pandey (2002) sought to investigate whether changes in the antioxidant enzyme catalase (which converts H2O2 to H2O and O2) and estradiol (E2) activity of ovarian follicular cells in various stages of development fluctuated with follicle-stimulating hormone (FSH). Concurrent catalase and E2 fluctuation may signal a developmental role of catalase in folliculogenesis. Granulosa cells isolated from dissected goat ovarian follicles indicated that large follicles (>6 mm) exhibited greater catalase activity than granulosa cells from small (<3 mm) or medium (3–6 mm) sized follicles. After a uniform dose of FSH (200 ng/ml), both catalase activity and E2 release were greater in large follicles than in medium or small follicles. Since the dominant follicle will be the follicle with the highest estrogen concentration, the concomitant increases in catalase and E2 in response to FSH suggest a role for catalase in follicular selection and prevention of apoptosis (Behl and Pandey, 2002).
In addition to its primary role as an iron transport protein, transferrin is produced extrahepatically and can prevent the formation of hydroxyl radicals via the Fenton reaction in the ovaries and other tissues by binding ferrous ion (Fe2+). Although the exact role of transferrin in folliculogenesis has not been elucidated, Briggs et al. (1999) reported that transferrin and transferrin receptors are distributed heterogeneously throughout human granulosa cells, with greater expression in mature follicles. Reverse transcription–polymerase chain reaction indicated transferrin mRNA in the ovary but not in the oocyte; suggesting that local production of transferrin by the ovary is likely. FF concentrations of transferrin were found to be similar to serum concentrations.
Hypoxia of the granulosa cells is a normal event during the growth of ovarian follicles (Tropea et al., 2006). Oxygen limitation is known to stimulate follicular angiogenesis, which is important for follicular growth and development. Impairment of angiogenesis within ovarian follicles contributes to follicular atresia (Greenwald and Terranova, 1988). ROS may act as signal transducers (Schroedl et al., 2002) or intracellular messengers (Pearlstein et al., 2002) of the angiogenic response. Basini et al. (2004) investigated whether hypoxia modulates ROS production in granulosa cells isolated from swine follicles. Cells were held in normoxic, hypoxic and anoxic environments followed by measurement of ROS (O2 and H2O2) and scavenging enzymes (superoxide dismutase (SOD), catalase, peroxidase). Hypoxic and anoxic conditions reduced ROS (P < 0.05). SOD and peroxidase activities were increased (P < 0.05) by hypoxic and anoxic conditions, but the difference in activity between the two conditions was not statistically significant. Catalase activity was unaffected by hypoxic or anoxic conditions. Lack of change in catalase may be due to localization of catalase in the peroxisomes (Kinnula et al., 1995), whereas SOD and peroxidase are found in the mitochondria (Fridovich and Freeman, 1986). Mitochondria are the major consumers of cellular oxygen, thereby providing support to the hypothesis that ROS are involved in intracellular signaling between tissue hypoxia and angiogenic response (Basini et al., 2004).
Antioxidants
OS and apoptosis are the consequences of folliculogenesis, follicular atresia and luteal regression. However, the ROS increase can be countered (be it desirable or undesirable) by antioxidant status. Antioxidant properties of E2 were investigated in pig luteal and follicular tissue exposed to in vitro H2O2. High doses of E2 (≥40 pg/ml) protected against apoptosis, but other non-aromatizable steroid hormones (progesterone, testosterone, dihydrotestosterone or cortisol) offered no protection, suggesting that ovarian E2 functions as a ROS scavenger during pregnancy-mediated luteal rescue and folliculogenesis (Murdoch, 1998). Follicular ROS initiate apoptosis whereas follicular GSH, in addition to FSH, protect against apoptosis in cultured preovulatory rat follicles (Tsai-Turton and Luderer, 2006). Oocyte GSH synthesis is believed to be stimulated by low-molecular weight thiol compounds including cysteine, cysteamine and β-mercaptoethanol (de Matos and Furnus, 2000; Luberda, 2005). Supplementation of cysteamine during in vitro maturation (IVM) of sheep oocytes indicated that a single cysteamine supplement of 200 μmol increased morula and blastocyst development (P < 0.05), but no such effect was found with IVM β-mercaptoethanol supplementation. However, both cysteamine and β-mercaptoethanol supplementation were found to decrease intracellular peroxidase content, most likely via increased GSH synthesis (de Matos et al., 2002). Interestingly, increased serum GSH reductase (GSHR) was significantly associated with decreased time-to-pregnancy in 83 female participants in a prospective pregnancy study with preconception enrollment recruited from the New York Angler Cohort. No statistically significant associations were found with GSHPx, SOD, catalase or thiobarbituric acid (Jackson et al., 2005a).
Das and Chowdhury (1999) investigated the effect of a vitamin E deficient diet on uterine estrogen inducing enzymes and gonadal–pituitary axis and ovarian histological changes among pre-pubertal female rats. Animals, which were 30-days old at initiation of the experiment, were assigned to one of four diet regimens for a 70-day experiment period: Group 1 received normal (control) chow diet for the entire period, Group 2 received vitamin E deficient chow for the entire period, Group 3 received normal chow for the first 45 days and deficient chow for the next 25 days and Group 4 received deficient chow for the first 45 days and normal diet for the last 25 days. Interestingly, the mean body weight of animals in Group 4 did not significantly differ from that of control animals at the end of the 70-day experiment. In contrast, animals fed 70-days deficient (Group 2) or 45-days normal chow followed by 25-days vitamin E deficient chow (Group 3) weighed significantly less than control animals at the study's conclusion. Uterine weight was also reduced in Groups 2 and 3 compared with the control group (P < 0.01). Uterine peroxidase and uterine alkaline phosphatase are estrogen-inducible enzymes associated with uterine growth (Manning et al., 1969; Lyttle and DeSombre, 1977). Activity of uterine peroxidase was significantly lower in the three groups that experienced deficiency compared with the control group, indicating that although having the last 25 days as control diet was effective in restoring body weight, it was not effective in restoring peroxidase concentration to control levels. Activity of alkaline phosphatase was reduced in Group 2 (P < 0.01) and Group 3 (P < 0.01), but no difference was found between control and Group 4. These findings suggest that vitamin E deficiency inhibits uterine growth. Among measured hormone levels, plasma LH was significantly lower than the control group in Groups 2 and 4. FSH was not significantly different from the control in any of the groups, but serum estrogen was significantly lower in Groups 2 and 3, but not Group 4, again indicating that 25 days of a vitamin E adequate diet has restorative properties. Histological analysis indicated that the ovaries of animals on the 70-day vitamin E deficient diet showed degenerated follicles, follicles with increased diameter and hypertrophy of the granulosa cells; whereas the control animals exhibited healthy, large follicles. In addition to vitamin E, other antioxidants such as manganese (a cofactor for SOD) are known to influence LH secretion in female rats (Pine et al., 2005; Lee et al., 2007) and ascorbic acid has been shown to stimulate gonadotrophin release in male animals (Karanth et al., 2001), thus suggesting that antioxidants stimulate the release of gonadotrophins from the adenohypophysis.
Basini et al. (2004) reported that ROS under moderate concentrations plays a role in signal transduction processes involved in growth and protection from apoptosis. Duleba et al. (2004) investigated in vitro effects of antioxidants and OS on proliferation of rat thecal-interstitial (T-I) cells. T-I cells develop in the secondary follicle stage and control follicle growth and atresia, regulate ovarian steroidogenesis, and may provide mechanical support for ovarian follicles (Erickson et al., 1985; Spaczynski et al., 1999). ROS were found to induce a biphasic effect with lower (P < 0.01) and higher concentrations inhibiting proliferation (P < 0.01), suggesting that controlled levels of ROS may be needed to maintain DNA synthesis, T-I cell proliferation, and growth of ovarian mesenchyme. However, caution is always warranted in extrapolating the results of in vitro studies to the in vivo milieu.
Ovulation and secondary oocyte quality
Chao et al. (2005) investigated murine oocyte competence, ovarian mitochondrial DNA (mtDNA) mutation and oxidative damage after repeated ovarian stimulation by exogenous gonadotrophin. An increase in degenerative oocytes and ovulated immature oocytes was seen with repeated stimulation, indicating a decrease in oocyte quality. Oxidative damage to the ovaries increased with cycles of stimulation, with a statistically significant increase in lipid peroxides between the first and fifth and first and sixth cycles, and an increase in 8-hydroxydeoxyguanine (8-OH-dG), a biomarker of oxidative DNA damage, between the first and sixth cycles (P < 0.05). In addition, an increase in mtDNA large scale deletions was noted with increased ovarian stimulation. Related work by Tarin et al. (1998a, 2002) suggests that the timing of antioxidant administration may have an effect on the number and quality of ovulated oocytes as assessed by morphological appearance and chromosome distribution in female mice. Mice were given a mixture of vitamins C and E, either after weaning (early administration) or beginning at 32 weeks of age (late administration) and continuing through sacrifice at 40–42, 50–52 or 57–62 weeks after exogenous stimulation (Tarin et al., 2002). To evaluate the overall quality of ovulated oocytes, the number of retrieved oocytes from both antioxidant supplementation groups at all three times points of sacrifice and total percentage of ovaries exhibiting morphological traits indicative of apoptosis were summed and compared with the control. Animals receiving antioxidant supplements showed an increased number of normal MII oocytes compared with the control group (46.7±1.9 versus 41.7±2.1%, P = 0.039), and decreased percentage of apoptotic oocytes (35.9±2.1 versus 43.3±2.5%, P = 0.041, in the antioxidant and control groups, respectively). In general, when supplemental vitamins C and E were given to older mice, the age-associated reduction in ovulation was partially prevented, but the preventive effects of supplementation were greatest when supplementation began after weaning and continued to time of sacrifice.
GSH in mature oocytes is thought to be a highly relevant biochemical marker for the viability of mammalian oocytes (Zuelke et al., 2003; Luberda, 2005). Samples collected during hamster IVM indicate ovulated oocytes suspended in metaphase of MII have approximately twice the concentration of GSH as immature germinal vesicle stage oocytes (Zuelke et al., 2003). GSH was found through the preimplantation stage in bovine oocytes (Furnus et al., 1998; de Matos and Furnus, 2000).
Corpus lutea function
The corpus luteum (CL) has a high concentration of antioxidants, particularly beta-carotene, which gives the CL its bright yellow color (Rodgers et al., 1995). Other carotenoids and vitamins C and E are also present in relatively high concentrations in the CL where they may play an important role in scavenging ROS (Aten et al., 1992, 1994; Matzuk et al., 1998; Behrman et al., 2001). In addition to its antioxidant function, ascorbic acid is a required cofactor in the synthesis of collagen in the luteal extracellular matrix (Luck and Zhao, 1993). ROS are produced during luteal regression (Behrman et al., 2001), in part though cytochrome P450 enzymes which are necessary for the first step of steroidogenesis (Rodgers et al., 1995).
Steroidogenesis
Over-exposure of the ovary to H2O2 causes the LH receptor to uncouple from adenylate cyclase, thereby impairing protein synthesis and cholesterol utilization by mitochondrial P450 side-chain cleavage (P450scc), most likely through impaired production of steroidogenic acute regulatory protein (StAR) (Behrman et al., 2001). StAR is responsible for moving cholesterol to the inner mitochondrial membrane where P450scc converts cholesterol to pregnenolone (Behrman and Aten, 1991; Stocco et al., 1993; Musicki et al., 1994; Behrman et al., 2001). Lecithin–cholesterol acyltransferase (LCAT) plays an important role in reverse cholesterol transport and follicular synthesis of estrogen. Cigliano et al. (2002) investigated the estrogen:progesterone ratio (which decreases near the time of ovulation) and LCAT activity following titration of ascorbate and α-tocopherol in human pre-ovulatory FF. High FF LCAT activity was positively associated with ascorbate and α-tocopherol accumulation, and lower LCAT activity was associated with their consumption, so the mature follicle appears to accumulate these vitamins in the FF to protect LCAT from oxidative damage and promote steroidogenesis.
Fertilization
As noted above, GSH concentrations in mature, metaphase hamster and mouse oocytes are higher than those found in most other tissues, and mature oocytes have a higher concentration than immature or fertilized oocytes (Zuelke et al., 1997). High GSH concentrations may also aid in meiotic spindle formation (Oliver et al., 1976), male pronucleus development and fertilization (Yoshida et al., 1993). Zuelke et al. (1997) exposed MII hamster oocytes to diamide, an oxidant relatively specific to GSH. High-performance liquid chromatography analyses were conducted to measure oxidation of GSH to GSH disulfide (GSSG), recovery of GSH from GSSG after the removal of diamide, and the overall effect of diamide on MII completion and zygote formation following IVF. Diamide oxidized GSH in a time- and concentration-dependent manner. Diamide exposure resulted in disruption of spindle morphology, chromosome clumping and altered oocyte cortex microtubules. Recovery from these aberrations was possible if the diamide exposure was followed by either a 1.5- or 3-h washout period, depending on the combination of exposure concentration, length of exposure and length of washout. Diamide exposure did not affect fertilization or development of the male pronuclei, but oocytes exposed to 50 mm (but not 25 mm) before IVF exhibited abnormal female pronuclei. Thus, exposure to OS before fertilization appears to disrupt the meiotic spindle and increase risk of abnormal zygote formation. The activity of ROS generated during gamete fusion is inhibited, due to increased production of antioxidants, particularly SOD. As a result, it is unlikely that affects gamete fusion when adequate antioxidant defenses are available (Miesel et al., 1993).
Implantation
Nitric oxide (NO), a free radical produced by NO synthases (NOS), functions as an important vasodilator, neurotransmitter, regulator of embryonic development and implantation (Guerin et al., 2001), and may also contribute as an anti-platelet agent during implantation (Schmidt et al., 1992; Cameron and Campbell, 1998). Schmidt et al. (1992) investigated the distribution of NOS in a number of rat organ tissues. NOS type I (NOS-I) and NADPH-diaphorase (NADPH-d) were found to be highly concentrated in endometrial epithelial cells. The function of NO in endometrial epithelial cells is not established, but may include regulation of cyclic GMP, which may mediate the estrogen-stimulated rapid uterine secretory response at the implantation site. Reports on the origin of these enzymes in rat endometrium are inconsistent (Shew et al., 1993), perhaps due to limitations regarding the specificity of antibodies used to identify NOS isoforms (Cameron and Campbell, 1998). No relationship was found between nitrite/nitrate (stable oxidation products of NO) and ovarian response in an analysis of FF of 70 patients undergoing IVF, indicating that although NO may be involved in folliculogenesis and implantation, it is not a useful measure of ovarian response to gonadotrophin stimulation (Manau et al., 2000). SOD in human endometrial stromal cells increases with decidualization and is thought to be an important component of implantation (Sugino et al., 1996, 2000). In addition, both ROS and SOD may act as second messengers to regulate endometrial function (Sugino, 2007). Furthermore, uterine expression of the gene for a-tocopherol transfer protein, a major determinant of serum a-tocopherol status, increases after implantation, suggesting a protective action of α-tocopherol during embryogenesis (Jishage et al., 2001).
In vitro fertilization
During IVF, the FF removed from the ovary has no therapeutic use and has become a ‘biological window’ (Wiener-Megnazi et al., 2004) for understanding the environment of the mature oocyte in infertility. A prospective study by Oyawoye et al. (2003) evaluated total antioxidant capacity (TAC) with the Ferric Reducing Antioxidant Power (FRAP) assay, using FF collected from 63 women undergoing oocyte retrieval for IVF after controlled ovarian stimulation. Of the 303 samples, 71.9% (218) contained oocytes, but baseline TAC did not differ with oocyte presence. A total of 77.5% (169/218) of the oocytes were fertilized, of which 79.3% (134/169) survived to the day of transfer. Baseline TAC was significantly higher in FF samples of oocytes that achieved successful fertilization, suggesting that higher TAC may predict increased fertilization potential. However, significantly lower baseline TAC was observed in the FF where the resultant embryo survived to the day of transfer. The observation that higher FF TAC is associated with successful fertilization is consistent with the findings of Paszkowski et al. (1995), who observed higher mean GSHPx activity in follicles yielding oocytes that were successfully fertilized compared to follicles with non-fertilized oocytes (Paszkowski et al., 1995). However, the latter finding of lower baseline TAC among embryos surviving to transfer conflicts with those of Paszkowski and Clarke (1996), Paszkowski et al. (2002) and Yang et al. (1998). Oyawoye et al. suggest that the discrepancy may be due to the effects of ROS being dependent on the stage of embryo development. The percent of TAC loss 72-h post-harvest did not differ significantly between follicles containing oocytes and those that did not, nor did it differ by fertilization status or embryo survival to time of transfer. These results suggest that antioxidant consumption in FF may have little value in predicting successful fertilization and embryo viability up to the time of transfer. However, the variation in outcomes may also reflect differential impact on OS by the various causes of infertility and confounding by indication for IVF.
ROS and TAC were measured by chemiluminescence in the FF of 53 women undergoing IVF by Attaran et al. (2000). Individuals who became pregnant had significantly higher FF ROS levels than those who did not, although TAC did not differ by pregnancy status. However, lack of a reference value for healthy women with unstimulated cycles precludes the comparison of the study population with a healthy fertile population. Nonetheless, this study suggests FF ROS, at physiologic concentrations, may be indicative of a metabolically active system and a potential marker of IVF success.
Wiener-Megnazi et al. (2004) used a thermochemiluminescence (TCL) assay to measure OS in FF samples from 189 women undergoing IVF. After controlling for age, OS was found to be positively correlated with the number of retrieved mature oocytes (P < 0.0001). All pregnancies occurred when the FF TCL amplitude at 50 sec was within the range of 347–569 cps. With 385 and 569 cps as limits for the occurrence of conception, the negative predictive value beyond this range was 96% and the positive predictive value was 32% (P < 0.004). These results suggest a beneficial threshold level for OS. The existence of an acceptable threshold level was also suggested in the evaluation of 208 FF samples from 78 women undergoing controlled ovarian stimulation (Das et al., 2006). Similar to the findings of Pasqualotto et al. (2004) who failed to find an association with lipid peroxidation and TAC, oocyte maturation was not associated with ROS in either Grade II or Grade III oocytes. However, the Das et al. (2006) study found an overall negative correlation between ROS in FF and embryo quality, similar to the association of lower TAC with decreased fertilization potential shown earlier (Oyawoye et al., 2003). The unique finding of the Das et al. investigation was evidence of a favorable effect of ROS on percent embryo formation up to ∼100 cps in both Grade II and Grade III oocytes, after which embryo formation declined. Pasqualotto et al. (2004) reported both lipid peroxidation and TAC to be positively correlated with pregnancy rate, but not fertilization rate. However, the categorical nature of the correlation precludes the ability to detect differences over the continuum of values.
Many thiols are capable of scavenging free radicals. However, homocysteine (Hcy) is a sulfur-containing amino acid produced primarily in vivo during demethylation of methionine during DNA/RNA methylation and appears to possess some pro-oxidant activity (Garry and Vellas, 1996; Tyagi et al., 2005). Plasma Hcy is negatively associated with fruit and vegetable consumption and endurance exercise and positively associated with alcohol intake, caffeine intake and tobacco use (Chrysohoou et al., 2004). Elevated Hcy induces endothelial dysfunction and promotes disease of the vasculature, in part by reducing the availability of NO and activation of protease activated receptors (PARs) to generate ROS (Tyagi et al., 2005). Ebisch et al. (2006) measured concentration of Hcy, GSH, cysteine and cysteinyl-glycine in FF of 156 women undergoing infertility treatment. Hcy was found to be higher in the FF of women with endometriosis compared to women with idiopathic subfertility (P = 0.04). No difference in the FF concentration of Hcy was detected among etiologic subfertility classifications or among the other substances under investigation. In regression analyses, Hcy was negatively associated with embryo quality on culture day three (OR = 0.58, 95% CI 0.35–0.97), suggesting that Hcy is inversely related to fertility outcome.
Impact of ROS on the aging oocyte
Free radical activity of human FF increases with age (Wiener-Megnazi et al., 2004), as does apoptosis of human granulosa and cumulus cells (Sadraie et al., 2000; Moffatt et al., 2002). Takahashi et al. (2003) hypothesized that prolonged exposure of aged oocytes to ROS negatively affects calcium homeostasis and impairs Ca2+ oscillation-dependent signaling, and causing a decline in oocyte developmental ability. At the time of sperm penetration, drastic changes occur in intracellular oocyte free calcium concentration ([Ca2+]i). Occurring first is a single long-lasting increase in [Ca2+]i, followed by short, repetitive transient [Ca2+]i, which last for several hours (Takahashi et al., 2003). These changes in [Ca2+]i stimulate the resumption of meiosis and trigger the release of the cortical granule thereby preventing penetration of the ovum by additional sperm (Kline and Kline, 1992; Takahashi et al., 2003). Treatment of fresh and aged mouse oocytes with 100 μM H2O2 for 10 min confirmed increased frequency of Ca2+ oscillation in aged oocytes compared to untreated fresh oocytes (P < 0.001). In addition, the fresh oocytes had lowered individual Ca2+ transients (P < 0.001). Both aged oocytes and H2O2 treated fresh oocytes exhibited a lower fertilization rate and decreased blastocyst development compared with fresh untreated oocytes.
Follicular fluid aspirates from 12 young women (aged 27–32 years) and 12 older women (aged 39–45 years) undergoing IVF treatment were analysed for the activity and protein expression of catalase, SOD, GSHPx, GSH transferase (GST) and GSHR (Carbone et al., 2003). The specific activity of catalase was ∼60% lower (P < 0.0005) in the older women compared with the younger women. GST was also higher in the younger women (P < 0.05). However, SOD activity was ∼25% higher (P < 0.01) in the older women compared to the younger group. No difference in activity between the two groups was observed for GSHPx or GSHR. The age-related changes caused a reduction in the catalase/SOD ratio and a slight reduction in the GSHPx/SOD ratio, suggesting an overall decrease in ROS scavenging ability with aging. No difference in protein expression of catalase or SOD was observed between the two groups, indicating that the age-associated change is due to a post-translational process. A potential weakness of the study was the failure to describe the reason the women were seeking IVF treatment, because the etiology of the infertility may have influenced FF antioxidant and ROS activity.
Whole ovaries were removed and homogenized from reproductive aging rats (aged 8–9 months) and control animals (26-day old) following CL induction and prostaglandin F2a (PGF2a) administration in the mid-luteal phase to induce luteal regression (Yeh et al., 2005). Analysis of homogenized ovaries at baseline (from animals given a 0.9% NaCl vehicle solution) and 2- and 24-h post-PGF2α administration indicated alterations in antioxidant defense with age. It is hypothesized that diminished antioxidant status may induce apoptosis during luteal regression and lead to decreased progesterone synthesis. The aged ovaries had elevated vitamin E content at 0, 2 and 24 h (P < 0.05), and lower GSHR levels at 2- and 24-h post-PGF2α administration (P < 0.01) compared with control ovaries. No significant differences in GSHPx, catalase or thiobarbituric acid-reacting substances (TBARS), an index of lipid peroxidation, were detected. Thus, a shift toward a higher concentration of vitamin E may occur to help protect the aging ovary during luteolysis and compensate for the decline in the luteal cell ability to quench ROS, as evidenced by lower GSHR.
ROS and fertility-related disease states
Hydrosalpinx
Hydrosalpinx is caused by blockage of the Fallopian tube with serous fluid, typically secondary to Fallopian viral infection, which is known to augment OS. Recent evidence suggests a role for ROS in HSF as a contributor to embryotoxicity. Bedaiwy et al. (2002b) aspirated HSF from 11 infertile women with confirmed hydrosalpinges. ROS and enzymatic TAC (both measured by chemiluminescence), and lipid peroxidation (measured by TBARS) were quantified and 2-cell mouse embryos were incubated with 25, 50 or 75% HSF and observed for blastocyst development. Blastocyst development was inversely correlated with concentration of HSF among embryos cultured in 75% HSF compared to control embryos (P < 0.0001, OR = 0.28, 95% CI 0.17–0.49). Lipid peroxidation was not significantly related to blastocyst development, and TAC was detectable in only 2/11 samples, although TAC was at a level unlikely to affect blastocyst development. Blastocyst development was positively correlated with ROS concentration (P < 0.02), but overall low ROS concentration is suggestive of healthy endosalpinx rather than a concentration potentially deleterious to the developing embryos. Several possible mechanisms for the embryotoxic properties of the HSF exist, including presence of microorganisms, endotoxins, cytokines, OS and lack of nutrients (Strandell and Lindhard, 2002). Alternatively, HSF without ROS may indicate extensive endosalpingeal damage or, as seen in the positive correlation of successful IVF pregnancy with ROS (Attaran et al., 2000), the finding may be indicative of healthy and metabolically active cells.
Endometriosis
Advanced endometriosis may cause tubal occlusion or otherwise interfere with the mechanics of ovulation (Wang et al., 1997), but the reason for infertility in women with mild to moderate endometriosis without mechanical impairment is unclear (Allaire, 2006). It has been hypothesized that endometriosis increases the presence of ROS in the peritoneal and tubal fluid, adversely affecting sperm motility and function (Curtis et al., 1993) and increasing the growth and adhesion of endometrial cells in the peritoneal cavity (Portz et al., 1991; Murphy et al., 1998). Aspirated peritoneal fluid from women with endometriosis in the follicular phase of their menstrual cycle (n = 15), idiopathic infertility (n = 11) or controls undergoing tubal ligation (n = 13) indicated the presence of ROS (measured by chemiluminescence) in the peritoneal fluid of all three groups, but similar concentrations of ROS in the endometriosis and control groups. The idiopathic infertility group was found to exhibit a higher concentration of ROS than the control group. Leukocyte distribution also failed to vary by group. Furthermore, no correlation between ROS and increasing stage of severity of endometriosis was detected in either the unprocessed or processed (cell-free) peritoneal fluid. Patients with endometriosis did exhibit increased peritoneal fluid volume compared to controls (P < 0.01). Sperm motility has been negatively correlated with peritoneal fluid volume (van Furth et al., 1979; Wang et al., 1997), suggesting the possibility that increased fluid volume may be a causative factor in endometriosis-associated infertility. Antioxidant status was not measured, but low concentrations of antioxidant nutrients or low antioxidant enzyme activity could contribute to the level of ROS in each group (Wang et al., 1997). Ho et al. (1997) examined peritoneal fluid total antioxidant status (TAS, a kit assay using 2,2′-azina-di-(3-ethylbenzthiazoline sulfonate)) and products of NO metabolism during the early follicular phase of women with endometriosis (early stage n = 12, late stage n = 12) and fertile controls without endometriosis (n = 10). Similar to the findings of Wang et al. (1997), who found no difference in ROS, Ho and colleagues found no difference among the groups in TAS or NO production. However, compared to the control group, the group with advanced endometriosis exhibited significantly increased peritoneal fluid volume (P = 0.007). Only progesterone peritoneal fluid concentrations were increased in early stage endometriosis compared to controls (P = 0.013) (Ho et al., 1997). In another study, peritoneal fluid was prospectively collected from 130 women undergoing laproscopy for pain, infertility, tubal ligation or sterilization reversal during the proliferative and luteal phases of the menstrual cycle (Bedaiwy et al., 2002a). Concentrations of six cytokines and tumor necrosis factor-α (markers of inflammation positively associated with OS) in serum and peritoneal fluid and ROS in peritoneal fluid (determined by chemiluminescense) were measured and used to compare women categorized by their post-surgery diagnosis. Patients diagnosed with endometriosis exhibited higher median serum interleukin-6 and peritoneal fluid tumor necrosis factor-α. Both of these variables had a high degree of predictive sensitivity and specificity for endometriosis. A number of other studies have also failed to find a difference in ROS or antioxidant concentration in peritoneal fluid of patients with endometriosis and fertile controls or individuals with other causes of infertility (Arumugam and Dip, 1995; Polak et al., 2001; do Amaral et al., 2005). In contrast, Zeller et al. (1987) observed a significant increase in ROS production by resting peritoneal macrophages in peritoneal fluid samples collected during mid-luteal phase of menstruation in women with endometriosis compared with fertile controls. Differences in menstrual cycle phase at time of peritoneal fluid collection could contribute to the divergent findings. OS was measured in 32 women with endometriosis and 52 controls. After controlling for covariates, each 0.5 nmol/ml increase in serum TBARS was weakly but positively associated with the presence of endometriosis, although other measures of antioxidant status and ROS, including serum beta-carotene, vitamin E and 8-F2α-isoprostane were not (Jackson et al., 2005b). However, the control subjects were not free of infertility concerns (30 had idiopathic infertility while the remaining 22 were undergoing tubual ligation), which could influence the results if infertility is associated with increased OS. In addition, blood collection was not standardized within the menstrual cycle, which is known to affect antioxidant concentration (Forman et al., 1998; Lanza et al., 1998).
Polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is characterized by chronic anovulation, oligomenorrhea, obesity, enlarged cystic ovaries, elevated LH, hyperandrogenism, infertility and often, insulin resistance. Women with PCOS have increased difficulty becoming pregnant, and experience higher rates of spontaneous abortion and pregnancy complications. Healthy controls (n = 30) and women with PCOS (n = 31) were examined for differences in OS and cardiovascular disease risk factors (Fenkci et al., 2003). No significant differences were found between the groups for BMI, waist/hip ratio, fasting serum glucose, lipid fractions, serum total testosterone or free testosterone concentrations. The PCOS group had significantly lower serum TAS (P < 0.05) and increased protein carbonyls (P < 0.05), biomarkers of protein oxidation, higher serum fasting insulin, homeostasis model assessment (HOMA, an estimate of insulin resistance), C-reactive protein (a biomarker of inflammation), LH levels, and LH/FSH ratios. In addition, fasting insulin was negatively associated with antioxidant status and positively associated with protein carbonyls.
Dincer et al. (2005) examined DNA damage and H2O2-induced DNA damage in women with PCOS. Results indicted that DNA damage (strand breakage) and H2O2-induced DNA damage were much higher in the PCOS subjects compared to controls (P < 0.01 and < 0.05, respectively). In addition, GSH in whole blood was higher in the healthy controls (P < 0.05). Thus, the susceptibility of DNA to OS may explain the association between PCOS and ovarian cancer, but this finding also offers another possible explanation for PCOS and the association with early pregnancy loss.
Higher levels of anti-endometrial antibodies (P < 0.01), suggesting an autoimmune response, as well as OS indicated by higher protein–malondialdehyde (P < 0.001) were found in sera from ten women with PCOS compared with 21 women with partners experiencing male factor infertility (Palacio et al., 2006). An autoimmune response may inhibit successful implantation among PCOS patients who do achieve fertilization of an ovulated egg. Gonzalez et al. (2006) studied the effects of hyperglycemia following an oral glucose tolerance test on generation of ROS from mononuclear cells (MNC) in patients with PCOS compared to control subjects. Generation of ROS by MNC was increased in patients with PCOS, independent of obesity. Blood glucose at baseline was not reported, but an earlier study from the same cohort indicated that women with PCOS had higher mean fasting serum insulin compared with controls (P < 0.003) (Gonzalez et al., 1999). Thus, OS may contribute to the insulin resistance observed in PCOS. Future investigations in this area should include measurements of OS in relation to time-to-pregnancy among women with PCOS.
Idiopathic infertility
Idiopathic (unexplained) infertility is diagnosed by exclusion and is defined as the inability to conceive after 12 months of timed, unprotected intercourse where tests have been performed on both partners to rule out known causes of infertility, including but not limited to anovulation and sperm defects (Goldman et al., 2000). TAS was found to be lower in peritoneal fluid of women with idiopathic infertility (n = 23) compared to fertile controls (n = 13, P = 0.02) and individuals with tubal infertility (n = 12, P = 0.001) (Polak et al., 2001). This observation offers a possible explanation for the elevated levels of ROS observed in the peritoneal fluid of women with idiopathic infertility reported by Wang et al. (1997) and Polak et al. (2001). Polak et al. (2001) hypothesized that peritoneal fluid diffuses into the Fallopian tubes where it may cause damage to sperm, which are known to be sensitive to OS (Storey, 1997). Peritoneal fluid of women with idiopathic infertility (n = 7) was found to have a higher concentration of ROS compared with fertile controls (n = 27, P = 0.02), but was not different from women with endometriosis (n = 56) (Bedaiwy et al., 2002a). However, these results are based on small numbers and need to be investigated with a larger sample.
Conclusion
The role of OS in female fertility and subfertility is an area deserving of continued research. The available evidence suggests gynecologic OS is an important mediator of conception. However, it appears that threshold levels for the benefit or harm of OS exist, and these thresholds are dependent on anatomic location and stage of preconception. For example, resumption of MI is induced by an increase in ROS and inhibited by a high antioxidant status and low FF ROS are associated with successful IVF procedures, perhaps as an indication of a healthy, metabolically active follicle. In addition, care must be given to acknowledge potential undesirable effects of excessive vitamin supplementation (Tarin et al., 1998b).
Human research investigating OS and dietary antioxidants can be especially challenging. It is not possible to retrospectively collect data on very early pregnancy loss since many women may have been unaware that they were pregnant. Furthermore, in the event a woman was aware that she was pregnant, retrospective dietary data may be biased by the knowledge of a pregnancy loss (Bunin et al., 2001). In vitro studies of humans and other mammals offer promising insight, but the nature of these experiments can introduce additional OS not found in vivo. In addition, most studies of FF composition are conducted in women undergoing IVF where follicle maturation is stimulated with exogenous hormones, creating a milieu which differs from the FF of women not under ovarian stimulation. Prospective pregnancy studies with dietary assessment and collection of biological samples prior to conception that investigate time-to-pregnancy and early pregnancy loss will provide the highest quality scientific evidence.
Acknowledgments
The authors thank Alison Zimon, MD for her assistance and insight during the early development of this manuscript.
Funding: The work described was supported by Grant Number R01HD049762 from the National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health. Jeffrey Blumberg's funding was provided by the United States Department of Agriculture/Agricultural Research Service under Cooperative Agreement No. 58-1950-7-707.
References
- Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Institute of Medicine. 2003 [PubMed] [Google Scholar]
- Acevedo CG, Carrasco G, Burotto M, Rojas S, Bravo I. Ethanol inhibits L-arginine uptake and enhances NO formation in human placenta. Life Sci. 2001;68:2893–2903. doi: 10.1016/s0024-3205(01)01070-0. [DOI] [PubMed] [Google Scholar]
- Alberg A. The influence of cigarette smoking on circulating concentrations of antioxidant micronutrients. Toxicology. 2002;180:121–137. doi: 10.1016/s0300-483x(02)00386-4. [DOI] [PubMed] [Google Scholar]
- Allaire C. Endometriosis and infertility: a review. J Reprod Med. 2006;51:164–168. [PubMed] [Google Scholar]
- Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–1469. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa C, Yoshinaga J, Okamura K, Nakai K, Satoh H. Fish consumption and time to pregnancy in Japanese women. Int J Hyg Environ Health. 2006;209:337–344. doi: 10.1016/j.ijheh.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Arumugam K, Dip YC. Endometriosis and infertility: the role of exogenous lipid peroxides in the peritoneal fluid. Fertil Steril. 1995;63:198–199. doi: 10.1016/s0015-0282(16)57320-8. [DOI] [PubMed] [Google Scholar]
- Aten RF, Duarte KM, Behrman HR. Regulation of ovarian antioxidant vitamins, reduced glutathione, and lipid peroxidation by luteinizing hormone and prostaglandin F2 alpha. Biol Reprod. 1992;46:401–407. doi: 10.1095/biolreprod46.3.401. [DOI] [PubMed] [Google Scholar]
- Aten RF, Kolodecik TR, Behrman HR. Ovarian vitamin E accumulation: evidence for a role of lipoproteins. Endocrinology. 1994;135:533–539. doi: 10.1210/endo.135.2.8033800. [DOI] [PubMed] [Google Scholar]
- Attaran M, Pasqualotto E, Falcone T, Goldberg JM, Miller KF, Agarwal A, Sharma RK. The effect of follicular fluid reactive oxygen species on the outcome of in vitro fertilization. Int J Fertil Womens Med. 2000;45:314–320. [PubMed] [Google Scholar]
- Baird DD, Wilcox AJ, Weinberg CR. Use of time to pregnancy to study environmental exposures. Am J Epidemiol. 1986;124:470–480. doi: 10.1093/oxfordjournals.aje.a114417. [DOI] [PubMed] [Google Scholar]
- Basini G, Grasselli F, Bianco F, Tirelli M, Tamanini C. Effect of reduced oxygen tension on reactive oxygen species production and activity of antioxidant enzymes in swine granulosa cells. Biofactors. 2004;20:61–69. doi: 10.1002/biof.5520200201. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, Falcone T, Sharma RK, Goldberg JM, Attaran M, Nelson DR, Agarwal A. Prediction of endometriosis with serum and peritoneal fluid markers: a prospective controlled trial. Hum Reprod. 2002a;17:426–431. doi: 10.1093/humrep/17.2.426. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, Goldberg JM, Falcone T, Singh M, Nelson D, Azab H, Wang X, Sharma R. Relationship between oxidative stress and embryotoxicity of hydrosalpingeal fluid. Hum Reprod. 2002b;17:601–604. doi: 10.1093/humrep/17.3.601. [DOI] [PubMed] [Google Scholar]
- Behl R, Pandey RS. FSH induced stimulation of catalase activity in goat granulosa cells in vitro. Anim Reprod Sci. 2002;70:215–221. doi: 10.1016/s0378-4320(02)00006-4. [DOI] [PubMed] [Google Scholar]
- Behrman HR, Aten RF. Evidence that hydrogen peroxide blocks hormone-sensitive cholesterol transport into mitochondria of rat luteal cells. Endocrinology. 1991;128:2958–2966. doi: 10.1210/endo-128-6-2958. [DOI] [PubMed] [Google Scholar]
- Behrman HR, Kodaman PH, Preston SL, Gao S. Oxidative stress and the ovary. J Soc Gynecol Investig. 2001;8(Suppl 1 Proceedings):S40–S42. doi: 10.1016/s1071-5576(00)00106-4. [DOI] [PubMed] [Google Scholar]
- Bolumar F, Olsen J, Rebagliato M, Saez-Lloret I, Bisanti L. Body mass index and delayed conception: a European multicenter study on infertility and subfecundity. Am J Epidemiol. 2000;151:1072–1079. doi: 10.1093/oxfordjournals.aje.a010150. [DOI] [PubMed] [Google Scholar]
- Briggs DA, Sharp DJ, Miller D, Gosden RG. Transferrin in the developing ovarian follicle: evidence for de-novo expression by granulosa cells. Mol Hum Reprod. 1999;5:1107–1114. doi: 10.1093/molehr/5.12.1107. [DOI] [PubMed] [Google Scholar]
- Bunin GR, Gyllstrom ME, Brown JE, Kahn EB, Kushi LH. Recall of diet during a past pregnancy. Am J Epidemiol. 2001;154:1136–1142. doi: 10.1093/aje/154.12.1136. [DOI] [PubMed] [Google Scholar]
- Cameron IT, Campbell S. Nitric oxide in the endometrium. Hum Reprod Update. 1998;4:565–569. doi: 10.1093/humupd/4.5.565. [DOI] [PubMed] [Google Scholar]
- Carbone MC, Tatone C, Delle Monache S, Marci R, Caserta D, Colonna R, Amicarelli F. Antioxidant enzymatic defences in human follicular fluid: characterization and age-dependent changes. Mol Hum Reprod. 2003;9:639–643. doi: 10.1093/molehr/gag090. [DOI] [PubMed] [Google Scholar]
- Chao HT, Lee SY, Lee HM, Liao TL, Wei YH, Kao SH. Repeated ovarian stimulations induce oxidative damage and mitochondrial DNA mutations in mouse ovaries. Ann N Y Acad Sci. 2005;1042:148–156. doi: 10.1196/annals.1338.016. [DOI] [PubMed] [Google Scholar]
- Chavarro JE, Rich-Edwards JW, Rosner BA, Willett WC. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet Gynecol. 2007;110:1050–1058. doi: 10.1097/01.AOG.0000287293.25465.e1. [DOI] [PubMed] [Google Scholar]
- Christianson RE, Oechsli FW, van den Berg BJ. Caffeinated beverages and decreased fertility. Lancet. 1989;1:378. doi: 10.1016/s0140-6736(89)91745-5. [DOI] [PubMed] [Google Scholar]
- Chrysohoou C, Panagiotakos DB, Pitsavos C, Zeimbekis A, Zampelas A, Papademetriou L, Masoura C, Stefanadis C. The associations between smoking, physical activity, dietary habits and plasma homocysteine levels in cardiovascular disease-free people: the ‘ATTICA’ study. Vasc Med. 2004;9:117–123. doi: 10.1191/1358863x04vm542oa. [DOI] [PubMed] [Google Scholar]
- Cigliano L, Balestrieri M, Spagnuolo MS, Dale B, Abrescia P. Lecithin-cholesterol acyltransferase activity during maturation of human preovulatory follicles with different concentrations of ascorbate, alpha-tocopherol and nitrotyrosine. Reprod Fertil Dev. 2002;14:15–21. doi: 10.1071/rd01044. [DOI] [PubMed] [Google Scholar]
- Cumming DC, Wheeler GD, Harber VJ. Physical activity, nutrition, and reproduction. Ann N Y Acad Sci. 1994;709:55–76. doi: 10.1111/j.1749-6632.1994.tb30388.x. [DOI] [PubMed] [Google Scholar]
- Curtis P, Lindsay P, Jackson AE, Shaw RW. Adverse effects on sperm movement characteristics in women with minimal and mild endometriosis. Br J Obstet Gynaecol. 1993;100:165–169. doi: 10.1111/j.1471-0528.1993.tb15215.x. [DOI] [PubMed] [Google Scholar]
- Czeizel AE, Metneki J, Dudas I. Higher rate of multiple births after periconceptional vitamin supplementation. N Engl J Med. 1994;330:1687–1688. doi: 10.1056/NEJM199406093302314. [DOI] [PubMed] [Google Scholar]
- Das P, Chowdhury M. Vitamin E-deficiency induced changes in ovary and uterus. Mol Cell Biochem. 1999;198:151–156. doi: 10.1023/a:1006954032164. [DOI] [PubMed] [Google Scholar]
- Das S, Chattopadhyay R, Ghosh S, Ghosh S, Goswami SK, Chakravarty BN, Chaudhury K. Reactive oxygen species level in follicular fluid–embryo quality marker in IVF? Hum Reprod. 2006;21:2403–2407. doi: 10.1093/humrep/del156. [DOI] [PubMed] [Google Scholar]
- Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- de Matos DG, Furnus CC. The importance of having high glutathione (GSH) level after bovine in vitro maturation on embryo development effect of beta-mercaptoethanol, cysteine and cystine. Theriogenology. 2000;53:761–771. doi: 10.1016/S0093-691X(99)00278-2. [DOI] [PubMed] [Google Scholar]
- de Matos DG, Gasparrini B, Pasqualini SR, Thompson JG. Effect of glutathione synthesis stimulation during in vitro maturation of ovine oocytes on embryo development and intracellular peroxide content. Theriogenology. 2002;57:1443–1451. doi: 10.1016/s0093-691x(02)00643-x. [DOI] [PubMed] [Google Scholar]
- De Souza MJ, Williams NI. Physiological aspects and clinical sequelae of energy deficiency and hypoestrogenism in exercising women. Hum Reprod Update. 2004;10:433–448. doi: 10.1093/humupd/dmh033. [DOI] [PubMed] [Google Scholar]
- Dincer Y, Akcay T, Erdem T, Ilker Saygili E, Gundogdu S. DNA damage, DNA susceptibility to oxidation and glutathione level in women with polycystic ovary syndrome. Scand J Clin Lab Invest. 2005;65:721–728. doi: 10.1080/00365510500375263. [DOI] [PubMed] [Google Scholar]
- do Amaral VF, Bydlowski SP, Peranovich TC, Navarro PA, Subbiah MT, Ferriani RA. Lipid peroxidation in the peritoneal fluid of infertile women with peritoneal endometriosis. Eur J Obstet Gynecol Reprod Biol. 2005;119:72–75. doi: 10.1016/j.ejogrb.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Dudas I, Rockenbauer M, Czeizel AE. The effect of preconceptional multivitamin supplementation on the menstrual cycle. Arch Gynecol Obstet. 1995;256:115–123. doi: 10.1007/BF01314639. [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Foyouzi N, Karaca M, Pehlivan T, Kwintkiewicz J, Behrman HR. Proliferation of ovarian theca-interstitial cells is modulated by antioxidants and oxidative stress. Hum Reprod. 2004;19:1519–1524. doi: 10.1093/humrep/deh299. [DOI] [PubMed] [Google Scholar]
- Duru NK, Morshedi M, Schuffner A, Oehninger S. Semen treatment with progesterone and/or acetyl-L-carnitine does not improve sperm motility or membrane damage after cryopreservation-thawing. Fertil Steril. 2000;74:715–720. doi: 10.1016/s0015-0282(00)01494-1. [DOI] [PubMed] [Google Scholar]
- Ebisch IM, Peters WH, Thomas CM, Wetzels AM, Peer PG, Steegers-Theunissen RP. Homocysteine, glutathione and related thiols affect fertility parameters in the (sub)fertile couple. Hum Reprod. 2006;21:1725–1733. doi: 10.1093/humrep/del081. [DOI] [PubMed] [Google Scholar]
- Ebisch IM, Thomas CM, Peters WH, Braat DD, Steegers-Theunissen RP. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod Update. 2007;13:163–174. doi: 10.1093/humupd/dml054. [DOI] [PubMed] [Google Scholar]
- Eggert J, Theobald H, Engfeldt P. Effects of alcohol consumption on female fertility during an 18-year period. Fertil Steril. 2004;81:379–383. doi: 10.1016/j.fertnstert.2003.06.018. [DOI] [PubMed] [Google Scholar]
- Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev. 1996;8:485–489. doi: 10.1071/rd9960485. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Magoffin DA, Dyer CA, Hofeditz C. The ovarian androgen producing cells: a review of structure/function relationships. Endocr Rev. 1985;6:371–399. doi: 10.1210/edrv-6-3-371. [DOI] [PubMed] [Google Scholar]
- Evans H. The pioneer history of vitamin E. Vitam Horm. 1963;20:379–387. [Google Scholar]
- Fenkci V, Fenkci S, Yilmazer M, Serteser M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil Steril. 2003;80:123–127. doi: 10.1016/s0015-0282(03)00571-5. [DOI] [PubMed] [Google Scholar]
- Forman MR, Johnson EJ, Lanza E, Graubard BI, Beecher GR, Muesing R. Effect of menstrual cycle phase on the concentration of individual carotenoids in lipoproteins of premenopausal women: a controlled dietary study. Am J Clin Nutr. 1998;67:81–87. doi: 10.1093/ajcn/67.1.81. [DOI] [PubMed] [Google Scholar]
- Fridovich I, Freeman B. Antioxidant defenses in the lung. Annu Rev Physiol. 1986;48:693–702. doi: 10.1146/annurev.ph.48.030186.003401. [DOI] [PubMed] [Google Scholar]
- Frisch RE. Critical fatness hypothesis. Am J Physiol. 1997;273:E231–E232. doi: 10.1152/ajpendo.1997.273.1.E231. [DOI] [PubMed] [Google Scholar]
- Furnus CC, de Matos DG, Moses DF. Cumulus expansion during in vitro maturation of bovine oocytes: relationship with intracellular glutathione level and its role on subsequent embryo development. Mol Reprod Dev. 1998;51:76–83. doi: 10.1002/(SICI)1098-2795(199809)51:1<76::AID-MRD9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Garry PJ, Vellas BJ. Present Knowledge in Nutrition. Washington, DC: ILSI Press; 1996. Aging and Nutrition; pp. 414–419. [Google Scholar]
- Goldman MB, Missmer SA, Barberi RL. Infertility. In: Goldman MB, Hatch MC, editors. Women and Helath. San Diego: Academic Press; 2000. pp. 196–214. [Google Scholar]
- Gomez-Cabrera MC, Pallardo FV, Sastre J, Vina J, Garcia-del-Moral L. Allopurinol and markers of muscle damage among participants in the Tour de France. JAMA. 2003;289:2503–2504. doi: 10.1001/jama.289.19.2503-b. [DOI] [PubMed] [Google Scholar]
- Gonzalez F, Thusu K, Abdel-Rahman E, Prabhala A, Tomani M, Dandona P. Elevated serum levels of tumor necrosis factor alpha in normal-weight women with polycystic ovary syndrome. Metabolism. 1999;48:437–441. doi: 10.1016/s0026-0495(99)90100-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez F, Rote NS, Minium J, Kirwan JP. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:336–340. doi: 10.1210/jc.2005-1696. [DOI] [PubMed] [Google Scholar]
- Gray MA. Vitamin E: hype or hope. Orthop Nurs. 1996;15:55–57. [PubMed] [Google Scholar]
- Gray RH, Becker S. Selected topics in the epidemiology of reproductive outcomes. Epidemiol Rev. 2000;22:71–75. doi: 10.1093/oxfordjournals.epirev.a018027. [DOI] [PubMed] [Google Scholar]
- Greenwald GS, Terranova PF. The Physiology of Reproduction. New York: Raven Press; 1988. Follicular selection and its control; pp. 387–445. [Google Scholar]
- Grodstein F, Goldman MB, Ryan L, Cramer DW. Relation of female infertility to consumption of caffeinated beverages. Am J Epidemiol. 1993;137:1353–1360. doi: 10.1093/oxfordjournals.aje.a116644. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994a;5:247–250. doi: 10.1097/00001648-199403000-00016. [DOI] [PubMed] [Google Scholar]
- Grodstein F, Goldman MB, Cramer DW. Infertility in women and moderate alcohol use. Am J Public Health. 1994b;84:1429–1432. doi: 10.2105/ajph.84.9.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7:175–189. doi: 10.1093/humupd/7.2.175. [DOI] [PubMed] [Google Scholar]
- Hakim RB, Gray RH, Zacur H. Alcohol and caffeine consumption and decreased fertility. Fertil Steril. 1998;70:632–637. doi: 10.1016/s0015-0282(98)00257-x. [DOI] [PubMed] [Google Scholar]
- Hartman TJ, Baer DJ, Graham LB, Stone WL, Gunter EW, Parker CE, Albert PS, Dorgan JF, Clevidence BA, Campbell WS, et al. Moderate alcohol consumption and levels of antioxidant vitamins and isoprostanes in postmenopausal women. Eur J Clin Nutr. 2005;59:161–168. doi: 10.1038/sj.ejcn.1602051. [DOI] [PubMed] [Google Scholar]
- Ho HN, Wu MY, Chen SU, Chao KH, Chen CD, Yang YS. Total antioxidant status and nitric oxide do not increase in peritoneal fluids from women with endometriosis. Hum Reprod. 1997;12:2810–2815. doi: 10.1093/humrep/12.12.2810. [DOI] [PubMed] [Google Scholar]
- Howe G, Westhoff C, Vessey M, Yeates D. Effects of age, cigarette smoking, and other factors on fertility: findings in a large prospective study. Br Med J (Clin Res Ed) 1985;290:1697–1700. doi: 10.1136/bmj.290.6483.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Kitagawa M, Imai H, Yamada M. The roles of vitamin A for cytoplasmic maturation of bovine oocytes. J Reprod Dev. 2005;51:23–35. doi: 10.1262/jrd.51.23. [DOI] [PubMed] [Google Scholar]
- Jackson LW, Schisterman EF, Browne RW, Armstrong D. Oxidative stress and female fecundity. Society of Pediatric and Perinatal Reproductive Epidemiologic Research; 2005a. Abstract #106. [Google Scholar]
- Jackson LW, Schisterman EF, Dey-Rao R, Browne R, Armstrong D. Oxidative stress and endometriosis. Hum Reprod. 2005b;20:2014–2020. doi: 10.1093/humrep/dei001. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Hjollund NH, Henriksen TB, Scheike T, Kolstad H, Giwercman A, Ernst E, Bonde JP, Skakkebaek NE, Olsen J. Does moderate alcohol consumption affect fertility? Follow up study among couples planning first pregnancy. BMJ. 1998;317:505–510. doi: 10.1136/bmj.317.7157.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jishage K, Arita M, Igarashi K, Iwata T, Watanabe M, Ogawa M, Ueda O, Kamada N, Inoue K, Arai H, et al. Alpha-tocopherol transfer protein is important for the normal development of placental labyrinthine trophoblasts in mice. J Biol Chem. 2001;276:1669–1672. doi: 10.1074/jbc.C000676200. [DOI] [PubMed] [Google Scholar]
- Jozwik M, Wolczynski S, Jozwik M, Szamatowicz M. Oxidative stress markers in preovulatory follicular fluid in humans. Mol Hum Reprod. 1999;5:409–413. doi: 10.1093/molehr/5.5.409. [DOI] [PubMed] [Google Scholar]
- Juhl M, Nyboe Andersen AM, Gronbaek M, Olsen J. Moderate alcohol consumption and waiting time to pregnancy. Hum Reprod. 2001;16:2705–2709. doi: 10.1093/humrep/16.12.2705. [DOI] [PubMed] [Google Scholar]
- Karanth S, Yu WH, Walczewska A, Mastronardi CA, McCann SM. Ascorbic acid stimulates gonadotropin release by autocrine action by means of NO. Proc Natl Acad Sci USA. 2001;98:11783–11788. doi: 10.1073/pnas.191369398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnula VL, Crapo JD, Raivio KO. Generation and disposal of reactive oxygen metabolites in the lung. Lab Invest. 1995;73:3–19. [PubMed] [Google Scholar]
- Kline D, Kline JT. Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J Biol Chem. 1992;267:17624–17630. [PubMed] [Google Scholar]
- Kodaman PH, Behrman HR. Endocrine-regulated and protein kinase C-dependent generation of superoxide by rat preovulatory follicles. Endocrinology. 2001;142:687–693. doi: 10.1210/endo.142.2.7961. [DOI] [PubMed] [Google Scholar]
- Komura H, Miyake A, Chen CF, Tanizawa O, Yoshikawa H. Relationship of age at menarche and subsequent fertility. Eur J Obstet Gynecol Reprod Biol. 1992;44:201–203. doi: 10.1016/0028-2243(92)90099-k. [DOI] [PubMed] [Google Scholar]
- Kurzer MS, Calloway DH. Effects of energy deprivation on sex hormone patterns in healthy menstruating women. Am J Physiol. 1986;251:E483–E488. doi: 10.1152/ajpendo.1986.251.4.E483. [DOI] [PubMed] [Google Scholar]
- Lanza E, Forman MR, Johnson EJ, Muesing RA, Graubard BI, Beecher GR. Alpha-tocopherol concentrations in plasma but not in lipoproteins fluctuate during the menstrual cycle in healthy premenopausal women. J Nutr. 1998;128:1150–1155. doi: 10.1093/jn/128.7.1150. [DOI] [PubMed] [Google Scholar]
- Lee B, Hiney JK, Pine MD, Srivastava VK, Dees WL. Manganese stimulates luteinizing hormone releasing hormone secretion in prepubertal female rats: hypothalamic site and mechanism of action. J Physiol. 2007;578:765–772. doi: 10.1113/jphysiol.2006.123083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Washburn KA, Moore R, Uno T, Teng C, Newbold RR, McLachlan JA, Negishi M. Developmental exposure to diethylstilbestrol elicits demethylation of estrogen-responsive lactoferrin gene in mouse uterus. Cancer Res. 1997;57:4356–4359. [PubMed] [Google Scholar]
- Liu H, Zhang C, Zeng W. Estrogenic and antioxidant effects of a phytoestrogen daidzein on ovarian germ cells in embryonic chickens. Domest Anim Endocrinol. 2006;31:258–268. doi: 10.1016/j.domaniend.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Luberda Z. The role of glutathione in mammalian gametes. Reprod Biol. 2005;5:5–17. [PubMed] [Google Scholar]
- Luck MR, Zhao Y. Identification and measurement of collagen in the bovine corpus luteum and its relationship with ascorbic acid and tissue development. J Reprod Fertil. 1993;99:647–652. doi: 10.1530/jrf.0.0990647. [DOI] [PubMed] [Google Scholar]
- Lyttle CR, DeSombre ER. Uterine peroxidase as a marker for estrogen action. Proc Natl Acad Sci USA. 1977;74:3162–3166. doi: 10.1073/pnas.74.8.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manau D, Balasch J, Jimenez W, Fabregues F, Civico S, Casamitjana R, Creus M, Vanrell JA. Follicular fluid concentrations of adrenomedullin, vascular endothelial growth factor and nitric oxide in IVF cycles: relationship to ovarian response. Hum Reprod. 2000;15:1295–1299. doi: 10.1093/humrep/15.6.1295. [DOI] [PubMed] [Google Scholar]
- Manning JP, Steinetz BG, Giannina T. Decidual alkaline phosphatase activity in the pregnant and pseudopregnant rat. Ann N Y Acad Sci. 1969;166:482–509. doi: 10.1111/j.1749-6632.1969.tb46416.x. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology. 1998;139:4008–4011. doi: 10.1210/endo.139.9.6289. [DOI] [PubMed] [Google Scholar]
- Miesel R, Drzejczak PJ, Kurpisz M. Oxidative stress during the interaction of gametes. Biol Reprod. 1993;49:918–923. doi: 10.1095/biolreprod49.5.918. [DOI] [PubMed] [Google Scholar]
- Milosevic M, Petrovic S, Demajo M, Horvat A. Effects of metal ions on plasma membrane Mg2+-ATPase in rat uterus and ovaries. Ann N Y Acad Sci. 2005;1048:445–448. doi: 10.1196/annals.1342.061. [DOI] [PubMed] [Google Scholar]
- Moffatt O, Drury S, Tomlinson M, Afnan M, Sakkas D. The apoptotic profile of human cumulus cells changes with patient age and after exposure to sperm but not in relation to oocyte maturity. Fertil Steril. 2002;77:1006–1011. doi: 10.1016/s0015-0282(02)02951-5. [DOI] [PubMed] [Google Scholar]
- Murdoch WJ. Inhibition by oestradiol of oxidative stress-induced apoptosis in pig ovarian tissues. J Reprod Fertil. 1998;114:127–130. doi: 10.1530/jrf.0.1140127. [DOI] [PubMed] [Google Scholar]
- Murphy AA, Palinski W, Rankin S, Morales AJ, Parthasarathy S. Evidence for oxidatively modified lipid-protein complexes in endometrium and endometriosis. Fertil Steril. 1998;69:1092–1094. doi: 10.1016/s0015-0282(98)00087-9. [DOI] [PubMed] [Google Scholar]
- Musicki B, Aten RF, Behrman HR. Inhibition of protein synthesis and hormone-sensitive steroidogenesis in response to hydrogen peroxide in rat luteal cells. Endocrinology. 1994;134:588–595. doi: 10.1210/endo.134.2.7507829. [DOI] [PubMed] [Google Scholar]
- Myatt L, Cui X. Oxidative stress in the placenta. Histochem Cell Biol. 2004;122:369–382. doi: 10.1007/s00418-004-0677-x. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Hanson RB, Jefferson WN, Bullock BC, Haseman J, McLachlan JA. Increased tumors but uncompromised fertility in the female descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis. 1998;19:1655–1663. doi: 10.1093/carcin/19.9.1655. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Hanson RB, Jefferson WN, Bullock BC, Haseman J, McLachlan JA. Proliferative lesions and reproductive tract tumors in male descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis. 2000;21:1355–1363. [PubMed] [Google Scholar]
- Oliver JM, Albertini DF, Berlin RD. Effects of glutathione-oxidizing agents on microtubule assembly and microtubule-dependent surface properties of human neutrophils. J Cell Biol. 1976;71:921–932. doi: 10.1083/jcb.71.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Camarillo C, Guzman-Grenfell AM, Hicks JJ. Oxidation of gonadotrophin (PMSG) by oxygen free radicals alters its structure and hormonal activity. Mol Reprod Dev. 1999;52:264–268. doi: 10.1002/(SICI)1098-2795(199903)52:3<264::AID-MRD3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Oyawoye O, Abdel Gadir A, Garner A, Constantinovici N, Perrett C, Hardiman P. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: relationship to outcome. Hum Reprod. 2003;18:2270–2274. doi: 10.1093/humrep/deg450. [DOI] [PubMed] [Google Scholar]
- Palacio JR, Iborra A, Ulcova-Gallova Z, Badia R, Martinez P. The presence of antibodies to oxidative modified proteins in serum from polycystic ovary syndrome patients. Clin Exp Immunol. 2006;144:217–222. doi: 10.1111/j.1365-2249.2006.03061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palan PR, Cohen BL, Barad DH, Romney SL. Effects of smoking on the levels of antioxidant beta carotene, alpha tocopherol and retinol in human ovarian follicular fluid. Gynecol Obstet Invest. 1995;39:43–46. doi: 10.1159/000292374. [DOI] [PubMed] [Google Scholar]
- Pasqualotto EB, Agarwal A, Sharma RK, Izzo VM, Pinotti JA, Joshi NJ, Rose I. Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril. 2004;81:973–976. doi: 10.1016/j.fertnstert.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Paszkowski T, Clarke RN. Antioxidative capacity of preimplantation embryo culture medium declines following the incubation of poor quality embryos. Hum Reprod. 1996;11:2493–2495. doi: 10.1093/oxfordjournals.humrep.a019146. [DOI] [PubMed] [Google Scholar]
- Paszkowski T, Traub AI, Robinson SY, McMaster D. Selenium dependent glutathione peroxidase activity in human follicular fluid. Clin Chim Acta. 1995;236:173–180. doi: 10.1016/0009-8981(95)98130-9. [DOI] [PubMed] [Google Scholar]
- Paszkowski T, Clarke RN, Hornstein MD. Smoking induces oxidative stress inside the Graafian follicle. Hum Reprod. 2002;17:921–925. doi: 10.1093/humrep/17.4.921. [DOI] [PubMed] [Google Scholar]
- Pearlstein DP, Ali MH, Mungai PT, Hynes KL, Gewertz BL, Schumacker PT. Role of mitochondrial oxidant generation in endothelial cell responses to hypoxia. Arterioscler Thromb Vasc Biol. 2002;22:566–573. doi: 10.1161/01.atv.0000012262.76205.6a. [DOI] [PubMed] [Google Scholar]
- Pine M, Lee B, Dearth R, Hiney JK, Dees WL. Manganese acts centrally to stimulate luteinizing hormone secretion: a potential influence on female pubertal development. Toxicol Sci. 2005;85:880–885. doi: 10.1093/toxsci/kfi134. [DOI] [PubMed] [Google Scholar]
- Polak G, Koziol-Montewka M, Gogacz M, Blaszkowska I, Kotarski J. Total antioxidant status of peritoneal fluid in infertile women. Eur J Obstet Gynecol Reprod Biol. 2001;94:261–263. doi: 10.1016/s0301-2115(00)00352-3. [DOI] [PubMed] [Google Scholar]
- Portz DM, Elkins TE, White R, Warren J, Adadevoh S, Randolph J. Oxygen free radicals and pelvic adhesion formation: I. Blocking oxygen free radical toxicity to prevent adhesion formation in an endometriosis model. Int J Fertil. 1991;36:39–42. [PubMed] [Google Scholar]
- Pryor WA, Prier DG, Church DF. Electron-spin resonance study of mainstream and sidestream cigarette smoke: nature of the free radicals in gas-phase smoke and in cigarette tar. Environ Health Perspect. 1983;47:345–355. doi: 10.1289/ehp.8347345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Lavranos TC, Rodgers HF, Young FM, Vella CA. The physiology of the ovary: maturation of ovarian granulosa cells and a novel role for antioxidants in the corpus luteum. J Steroid Biochem Mol Biol. 1995;53:241–246. doi: 10.1016/0960-0760(95)00054-4. [DOI] [PubMed] [Google Scholar]
- Sadraie SH, Saito H, Kaneko T, Saito T, Hiroi M. Effects of aging on ovarian fecundity in terms of the incidence of apoptotic granulosa cells. J Assist Reprod Genet. 2000;17:168–173. doi: 10.1023/A:1009422323306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sane AS, Chokshi SA, Mishra VV, Barad DP, Shah VC, Nagpal S. Serum lipoperoxides in induced and spontaneous abortions. Gynecol Obstet Invest. 1991;31:172–175. doi: 10.1159/000293145. [DOI] [PubMed] [Google Scholar]
- Schmidt HH, Gagne GD, Nakane M, Pollock JS, Miller MF, Murad F. Mapping of neural nitric oxide synthase in the rat suggests frequent co-localization with NADPH diaphorase but not with soluble guanylyl cyclase, and novel paraneural functions for nitrinergic signal transduction. J Histochem Cytochem. 1992;40:1439–1456. doi: 10.1177/40.10.1382087. [DOI] [PubMed] [Google Scholar]
- Schroedl C, McClintock DS, Budinger GR, Chandel NS. Hypoxic but not anoxic stabilization of HIF-1alpha requires mitochondrial reactive oxygen species. Am J Physiol Lung Cell Mol Physiol. 2002;283:L922–L931. doi: 10.1152/ajplung.00014.2002. [DOI] [PubMed] [Google Scholar]
- Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996;48:835–850. doi: 10.1016/s0090-4295(96)00313-5. [DOI] [PubMed] [Google Scholar]
- Shen H, Ong C. Detection of oxidative DNA damage in human sperm and its association with sperm function and male infertility. Free Radic Biol Med. 2000;28:529–536. doi: 10.1016/s0891-5849(99)00234-8. [DOI] [PubMed] [Google Scholar]
- Shew RL, Papka RE, McNeill DL, Yee JA. NADPH-diaphorase-positive nerves and the role of nitric oxide in CGRP relaxation of uterine contraction. Peptides. 1993;14:637–641. doi: 10.1016/0196-9781(93)90157-c. [DOI] [PubMed] [Google Scholar]
- Sikka SC. Relative impact of oxidative stress on male reproductive function. Curr Med Chem. 2001;8:851–862. doi: 10.2174/0929867013373039. [DOI] [PubMed] [Google Scholar]
- Spaczynski RZ, Arici A, Duleba AJ. Tumor necrosis factor-alpha stimulates proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 1999;61:993–998. doi: 10.1095/biolreprod61.4.993. [DOI] [PubMed] [Google Scholar]
- Stocco DM, Wells J, Clark BJ. The effects of hydrogen peroxide on steroidogenesis in mouse Leydig tumor cells. Endocrinology. 1993;133:2827–2832. doi: 10.1210/endo.133.6.8243310. [DOI] [PubMed] [Google Scholar]
- Storey BT. Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Mol Hum Reprod. 1997;3:203–213. doi: 10.1093/molehr/3.3.203. [DOI] [PubMed] [Google Scholar]
- Strandell A, Lindhard A. Why does hydrosalpinx reduce fertility? The importance of hydrosalpinx fluid. Hum Reprod. 2002;17:1141–1145. doi: 10.1093/humrep/17.5.1141. [DOI] [PubMed] [Google Scholar]
- Subar AF, Harlan LC. Nutrient and food group intake by tobacco use status: the 1987 National Health Interview Survey. Ann N Y Acad Sci. 1993;686:310–321. doi: 10.1111/j.1749-6632.1993.tb39193.x. discussion 321-2. [DOI] [PubMed] [Google Scholar]
- Sugino N. The role of oxygen radical-mediated signaling pathways in endometrial function. Placenta. 2007;28(Suppl A):S133–S136. doi: 10.1016/j.placenta.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Sugino N, Shimamura K, Takiguchi S, Tamura H, Ono M, Nakata M, Nakamura Y, Ogino K, Uda T, Kato H. Changes in activity of superoxide dismutase in the human endometrium throughout the menstrual cycle and in early pregnancy. Hum Reprod. 1996;11:1073–1078. doi: 10.1093/oxfordjournals.humrep.a019299. [DOI] [PubMed] [Google Scholar]
- Sugino N, Kashida S, Takiguchi S, Nakamura Y, Kato H. Induction of superoxide dismutase by decidualization in human endometrial stromal cells. Mol Hum Reprod. 2000;6:178–184. doi: 10.1093/molehr/6.2.178. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Takahashi E, Igarashi H, Tezuka N, Kurachi H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol Reprod Dev. 2003;66:143–152. doi: 10.1002/mrd.10341. [DOI] [PubMed] [Google Scholar]
- Takami M, Preston SL, Toyloy VA, Behrman HR. Antioxidants reversibly inhibit the spontaneous resumption of meiosis. Am J Physiol. 1999;276:E684–E688. doi: 10.1152/ajpendo.1999.276.4.E684. [DOI] [PubMed] [Google Scholar]
- Takami M, Preston SL, Behrman HR. Eicosatetraynoic and eicosatriynoic acids, lipoxygenase inhibitors, block meiosis via antioxidant action. Am J Physiol Cell Physiol. 2000;278:C646–C650. doi: 10.1152/ajpcell.2000.278.4.C646. [DOI] [PubMed] [Google Scholar]
- Tarin J, Ten J, Vendrell FJ, de Oliveira MN, Cano A. Effects of maternal ageing and dietary antioxidant supplementation on ovulation, fertilisation and embryo development in vitro in the mouse. Reprod Nutr Dev. 1998a;38:499–508. doi: 10.1051/rnd:19980502. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Brines J, Cano A. Antioxidants may protect against infertility. Hum Reprod. 1998b;13:1415–1416. doi: 10.1093/humrep/13.6.1415. [DOI] [PubMed] [Google Scholar]
- Tarin JJ, Perez-Albala S, Cano A. Oral antioxidants counteract the negative effects of female aging on oocyte quantity and quality in the mouse. Mol Reprod Dev. 2002;61:385–397. doi: 10.1002/mrd.10041. [DOI] [PubMed] [Google Scholar]
- Thibault C, Szollosi D, Gerard M. Mammalian oocyte maturation. Reprod Nutr Dev. 1987;27:865–896. doi: 10.1051/rnd:19870701. [DOI] [PubMed] [Google Scholar]
- Tiboni GM, Bucciarelli T, Giampietro F, Sulpizio M, Di Ilio C. Influence of cigarette smoking on vitamin E, vitamin A, beta-carotene and lycopene concentrations in human pre-ovulatory follicular fluid. Int J Immunopathol Pharmacol. 2004;17:389–393. doi: 10.1177/039463200401700319. [DOI] [PubMed] [Google Scholar]
- Trevisan M, Browne R, Ram M, Muti P, Freudenheim J, Carosella AM, Armstrong D. Correlates of markers of oxidative status in the general population. Am J Epidemiol. 2001;154:348–356. doi: 10.1093/aje/154.4.348. [DOI] [PubMed] [Google Scholar]
- Tropea A, Miceli F, Minici F, Tiberi F, Orlando M, Gangale MF, Romani F, Catino S, Mancuso S, Navarra P, et al. Regulation of vascular endothelial growth factor synthesis and release by human luteal cells in vitro. J Clin Endocrinol Metab. 2006;91:2303–2309. doi: 10.1210/jc.2005-2457. [DOI] [PubMed] [Google Scholar]
- Tsai-Turton M, Luderer U. Opposing effects of glutathione depletion and follicle-stimulating hormone on reactive oxygen species and apoptosis in cultured preovulatory rat follicles. Endocrinology. 2006;147:1224–1236. doi: 10.1210/en.2005-1281. [DOI] [PubMed] [Google Scholar]
- Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC. Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol. 2005;289:H2649–H2656. doi: 10.1152/ajpheart.00548.2005. [DOI] [PubMed] [Google Scholar]
- Uzumcu M, Zachow R. Developmental exposure to environmental endocrine disruptors: consequences within the ovary and on female reproductive function. Reprod Toxicol. 2007;23:337–352. doi: 10.1016/j.reprotox.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R, Raeburn JA, van Zwet TL. Characteristics of human mononuclear phagocytes. Blood. 1979;54:485–500. [PubMed] [Google Scholar]
- Vural P, Akgul C, Yildirim A, Canbaz M. Antioxidant defence in recurrent abortion. Clin Chim Acta. 2000;295:169–177. doi: 10.1016/s0009-8981(99)00255-7. [DOI] [PubMed] [Google Scholar]
- Walsh SW, Vaughan JE, Wang Y, Roberts LJ., 2nd Placental isoprostane is significantly increased in preeclampsia. FASEB J. 2000;14:1289–1296. doi: 10.1096/fj.14.10.1289. [DOI] [PubMed] [Google Scholar]
- Wang Y, Sharma RK, Falcone T, Goldberg J, Agarwal A. Importance of reactive oxygen species in the peritoneal fluid of women with endometriosis or idiopathic infertility. Fertil Steril. 1997;68:826–830. doi: 10.1016/s0015-0282(97)00343-9. [DOI] [PubMed] [Google Scholar]
- Wiener-Megnazi Z, Vardi L, Lissak A, Shnizer S, Reznick AZ, Ishai D, Lahav-Baratz S, Shiloh H, Koifman M, Dirnfeld M. Oxidative stress indices in follicular fluid as measured by the thermochemiluminescence assay correlate with outcome parameters in in vitro fertilization. Fertil Steril. 2004;82(Suppl 3):1171–1176. doi: 10.1016/j.fertnstert.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Wilcox A, Weinberg C, Baird D. Caffeinated beverages and decreased fertility. Lancet. 1988;2:1453–1456. doi: 10.1016/s0140-6736(88)90933-6. [DOI] [PubMed] [Google Scholar]
- Williams NI. Lessons from experimental disruptions of the menstrual cycle in humans and monkeys. Med Sci Sports Exerc. 2003;35:1564–1572. doi: 10.1249/01.MSS.0000084528.13358.67. [DOI] [PubMed] [Google Scholar]
- Wisdom SJ, Wilson R, McKillop JH, Walker JJ. Antioxidant systems in normal pregnancy and in pregnancy-induced hypertension. Am J Obstet Gynecol. 1991;165:1701–1704. doi: 10.1016/0002-9378(91)90018-m. [DOI] [PubMed] [Google Scholar]
- Witschi E. Natural control of fertility. Fertil Steril. 1968;19:1–14. doi: 10.1016/s0015-0282(16)36540-2. [DOI] [PubMed] [Google Scholar]
- Woodall AA, Ames B. Preventive Nutrition: The Comprehensive Guide for Health Professionals. Totowa, NJ: Humana Press Inc.; 1997. Nutritional prevention of DNA damage to sperm and consequent risk reduction in birth defects and cancer in offspring; pp. 373–385. [Google Scholar]
- Yang HW, Hwang KJ, Kwon HC, Kim HS, Choi KW, Oh KS. Detection of reactive oxygen species (ROS) and apoptosis in human fragmented embryos. Hum Reprod. 1998;13:998–1002. doi: 10.1093/humrep/13.4.998. [DOI] [PubMed] [Google Scholar]
- Yeh J, Bowman MJ, Browne RW, Chen N. Reproductive aging results in a reconfigured ovarian antioxidant defense profile in rats. Fertil Steril. 2005;84(Suppl 2):1109–1113. doi: 10.1016/j.fertnstert.2005.02.054. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Ishigaki K, Nagai T, Chikyu M, Pursel VG. Glutathione concentration during maturation and after fertilization in pig oocytes: relevance to the ability of oocytes to form male pronucleus. Biol Reprod. 1993;49:89–94. doi: 10.1095/biolreprod49.1.89. [DOI] [PubMed] [Google Scholar]
- Zeller JM, Henig I, Radwanska E, Dmowski WP. Enhancement of human monocyte and peritoneal macrophage chemiluminescence activities in women with endometriosis. Am J Reprod Immunol Microbiol. 1987;13:78–82. doi: 10.1111/j.1600-0897.1987.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Zuelke KA, Jones DP, Perreault SD. Glutathione oxidation is associated with altered microtubule function and disrupted fertilization in mature hamster oocytes. Biol Reprod. 1997;57:1413–1419. doi: 10.1095/biolreprod57.6.1413. [DOI] [PubMed] [Google Scholar]
- Zuelke KA, Jeffay SC, Zucker RM, Perreault SD. Glutathione (GSH) concentrations vary with the cell cycle in maturing hamster oocytes, zygotes, and pre-implantation stage embryos. Mol Reprod Dev. 2003;64:106–112. doi: 10.1002/mrd.10214. [DOI] [PubMed] [Google Scholar]


