Abstract
When placed in a novel environment, mice tend to explore for a period of time, and then reduce the level of exploration. This reduction in locomotor or exploratory behavior is known as habituation and can occur within a single session or across sessions, respectively termed intrasession and intersession habituation. Recent research indicates that there is a genetic component to habituation behavior and that some of the genes involved differ between the two types of habituation. The genetic evidence also suggests that intrasession habituation and intersession habituation are measuring somewhat different conceptual entities and with more such evidence may eventually help us understand the different pathways involved. Some of the genetic methods and tools used to unravel the roles of specific genes in both types of habituation are outlined here, with examples from the literature, as well as new data, to illustrate that this seemingly simple behavior is actually very complicated in terms of genetics. Evidence to date suggests that a number of genetic regions play roles in one or both types of habituation, and further research will be necessary to determine the specific genes involved.
Keywords: mouse, genetics, intrasession, intersession, inbred strain, linkage analysis, quantitative trait loci (QTL)
1. Introduction
Habituation, the waning of a response after repeated exposures to the same stimulus, aids organisms in selectively responding to biologically significant stimuli, while ignoring less relevant ones. It is considered one of the simplest forms of learning (Groves and Thompson, 1970; Harris, 1943; Thompson and Spencer, 1966) and is found in all phyla of the animal kingdom (Bailey and Kandel, 2008; Colombo and Mitchell, 2009; Engel and Wu, 2009; Giles and Rankin, 2009; Leussis and Bolivar, 2006). Habituation may appear relatively simple; however, neurobiological, biochemical and genetic studies indicate that the process is very complicated. The level of complexity is also a function of the type of habituation being investigated, and there is much variability throughout the animal kingdom (e.g., the gill-withdrawal reflex in sea slugs, changes in exploratory behavior in a stimulus rich novel environment in rodents). Furthermore, a host of factors, both internal and external to the organism of study, can influence the habituation response, thereby introducing further variability both within and across species. A set of 10 characteristics of habituation were updated and revised at the August 2007 workshop in Vancouver, Canada. These characteristics illustrate the complexity of habituation and the factors influencing it (Rankin, Abrams, Barry, Bhatnagar, Clayton, Colombo, Coppola, Geyer, Glanzman, Marsland, McSweeney, Wilson, Wu, and Thompson, 2009).
One relatively complicated type of habituation is the change in behavior in response to a novel environment over time, a phenomenon that has been studied in rodents for decades. According to cognitive map theory (O'Keefe and Nadel, 1978), whenever an organism explores a novel environment, it constructs an internal representation of that environment in the hippocampus; as the map becomes increasingly complete, exploration is reduced. We say that the organism has habituated to the environment. The level of habituation has been measured in a number of ways; in rodent studies some of the most common measures are distance traveled, number of horizontal beam breaks, number of line crosses and number of vertical beam breaks. The change over time can be measured within a single session (intrasession habituation) or across sessions (intersession). Figure 1 illustrates four different situations, entailing varying degrees of intrasession and intersession habituation. An animal can display no habituation, or intrasession but not intersession habituation, or intersession but not intrasession habituation, or both types of habituation (see Figure 1, Panels A-D). Generally, when repeatedly placed in the same environment, rodents over time will display both intrasession and intersession habituation. There will be a general decrease in activity over time within a single session and across sessions. However, this general pattern can be modulated by various factors, such as genotype (see below for detailed discussion), sex and age.
Fig. 1.
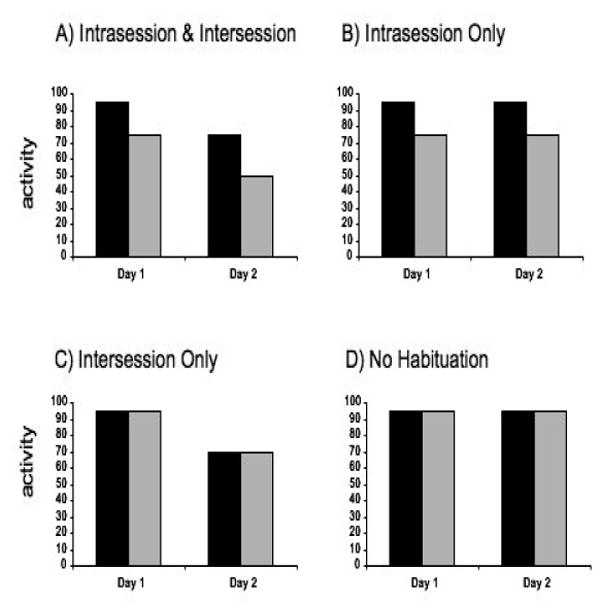
Graphic representations of various combinations of intrasession and intersession habituation. Graphs represent activity over two consecutive days of testing, for a 10-minute period each day. Dark and light bars represent the first five-minute block and last five-minute block of the session, respectively. Four combinations are illustrated: both intersession and intrasession habituation with activity decreasing across days and within each session (Panel A), intrasession but not intersession habituation, activity decreases within a single session but not across days (Panel B), intersession but not intrasession habituation, activity decreases across days but not within a session (Panel C), and neither intrasession or intersession habituation (Panel D).
Several types of evidence indicate that intrasession habituation and intersession habituation measure somewhat different constructs (Peeler, 1990). This may be as simple as short-term and long-term memory, respectively. It has been suggested that intrasession habituation measures adaptivity to the environment, whereas intersession habituation measures long-term memory of previous exposure (Muller, Cristina, Li, Wolfer, Lipp, Rulicke, Brandner, Aguzzi, and Weissmann, 1994). However, this distinction does not preclude a role for memory in intrasession habituation. Simply, more studies have focused on the memory component in intersession habituation. For instance, duration of exposure and retention period between exposures have been found to influence intersession habituation (Fraley and Springer, 1981a; b; Terry, 1979). Furthermore, any number of factors that interfere with formation or retention of a cognitive map (O'Keefe and Nadel, 1978) of the environment can modulate the habituation process.
It is important to remember that a behavior as complicated as habituation will be influenced by many factors. For instance, initial exploratory activity level during an encounter with the novel environment can influence the level of habituation, especially across sessions. An animal that does not explore the novel environment very quickly may not be able to form a complete cognitive map of the area in the allotted time. During a later session, when the animal's performance is not optimal, the deficit may not be the result of memory deficits, but due to incomplete initial exploration of the entire area. This type of situation can result from high levels of anxiety, low levels of locomotor activity, or any number of sensory deficits. Thus, in evaluations of habituation behavior, there must be a consideration of baseline levels of activity, and correct for these differences. A number of methodologies have been developed to take baseline differences in activity levels into consideration, among these are calculation of percentage of baseline, percent change and activity change ratio (Anisman, Kokkinidis, Glazier, and Remington, 1976; Fraley and Springer, 1981b; Nadel, 1968). Although it is less critical to make these sorts of corrections when differences in baseline levels of activity do not exist between strains of interest, as much of our research involves hypoactive and/or high-anxiety strains, and here such a correction becomes very important.
We have used the activity change ratio developed by Nadel (1968) extensively in our research on habituation (Bolivar, Manley, and Messer, 2004; Bolivar, Scott Ganus, and Messer, 2002; Bothe, Bolivar, Vedder, and Geistfeld, 2004; 2005; Cook, Bolivar, McFadyen, and Flaherty, 2002). The activity change ratio score is used to compare activity levels during initial and final periods in the environment. If one is measuring intersession habituation across three days it is calculated as follows: day 3 activity/(day 1 activity + day 3 activity). To examine intrasession habituation in a single 15-minute session, it would be calculated as follows: activity during final 5 minutes/(activity during initial 5 minutes + activity during final 5 minutes). Thus, the ratio score will approach 0.5 if no change in activity has occurred, i.e., no habituation. If the ratio approaches 0, there is evidence of habituation. If the ratio approaches 1.0 activity has actually increased from the initial to final time periods.
The biological underpinnings of habituation to a novel environment are numerous and links have been made to a variety of neurotransmitters, including serotonin, acetylcholine, dopamine and glutamate (for a recent review, see (Leussis and Bolivar, 2006). Furthermore, a plethora of studies, many of which have addressed the roles of specific neurotransmitters, affirm that genetics plays a role in habituation. The existing body of work has been obtained via manipulations of single genes, so as to determine the effects of the manipulations on habituation behavior (for reviews see (Bolivar, Cook, and Flaherty, 2000a; Leussis and Bolivar, 2006). However, habituation is a complicated phenotype, likely under polygenic control. Traditional transgenic and knockout technologies do not generally allow the study of multiple genes at the same time and interactions among these genes, i.e., epistasis. In contrast, the linkage methods outlined below allow researchers to study gene-gene interactions.
Accordingly, other behavioral genetics researchers have taken a different approach to the evaluation of the role of genetics in habituation. The mouse has become the model of choice for many researchers, due to the vast knowledge of its genetics, and also to technological advances in the genetic manipulation of murine embryonic stem cells. However, the foundation upon which all mouse genetics rests, and the principle reason for the ascendancy of the mouse in the field of genetics, is undoubtedly the availability of large numbers of inbred mouse strains. This resource is unparalleled among mammalian models, and allows a level of genetic analysis almost on par with the situation for Drosophila and other invertebrates. The necessary steps in progressing from phenotype – in this case, habituation – to gene will be outlined below. The first step of the procedure entails the comparisons among inbred strains of mice to reveal differences in habituation behavior.
Generally, the first indication for a role played by genetics, in any complex behavior in mice, arises from comparisons among inbred strains. An inbred strain is homozygous at every genetic locus, and the alleles at each locus are identical by descent. Thus, all members of an inbred strain can be considered to be identical twins. A strain is generally classified as inbred after ≥20 consecutive generations of brother X sister matings (Silver, 1995). At this stage, all members of the strain are considered virtually identical, with an inbreeding coefficient of 0.99. Hundreds of inbred strains of mice have been developed (Beck, Lloyd, Hafezparast, Lennon-Pierce, Eppig, Festing, and Fisher, 2000) and they can readily be used to screen genetic variability for any phenotype. Once phenotypic variability has been established, there are a number of routes by which the genetic underpinnings of behavior can be identified. The first step in elucidating the genetics of any behavior is generally to find phenotypic differences among inbred strains.
2. Intrasession habituation
2.1. Inbred strains
In general, most inbred-strain studies of locomotor behavior include some type of measurement of the change in activity over time (i.e., intrasession habituation). A small number of studies entailing in-depth analysis of intrasession habituation have indicated that most mice display intrasession habituation, although inter-strain variability exists (Anisman et al., 1976; Cabib et al., 1990; Kafkafi, Lipkind, Benjamini, Mayo, Elmer, and Golani, 2003; Koyner, Demarest, McCaughran, Cipp, and Hitzemann, 2000; Logue, Owen, Rasmussen, and Wehner, 1997; Miner, 1997; Peeler, 1990). In almost all cases, only two inbred strains are compared at a time; for this reason the studies yield only limited information on the variability of the behavior under study across a variety of genetic backgrounds. Habituation has been most frequently studied in the inbred strains C57BL/6J (B6) and DBA/2J (D2) (Anisman et al., 1976; Cabib et al., 1990; Kafkafi et al., 2003; Koyner et al., 2000; Logue et al., 1997). These two strains have been well studied in behavioral terms, presumably because of their differences in sensitivity to pharmacological agents, physiology, and brain structure are expected to have behavioral correlates. The strains both display intrasession habituation of locomotor activity, for session lengths of 15, 20, 30, or 90 minutes in duration, although the extent of habituation has varied across studies (Anisman et al., 1976; Cabib et al., 1990; Kafkafi et al., 2003; Koyner et al., 2000). Using a short testing session,Logue et al. (1997) reported an increase in activity in D2 mice during the second half of the testing session, in contrast to B6, which displayed a reduction in activity over the five-minute session. It is quite plausible that the habituation behavior measured in a short time period is associated with somewhat different genes than the habitation behavior associated with a longer time period. Further studies are necessary to investigate this issue. In contrast, the 129X1/SvJ strain, displays a more consistent pattern, showing intrasession habituation in both five-minute and thirty-minute session protocols (Miner, 1997; Logue et al., 1997). Thus, session length is likely to have greater effects on some inbred strains of mice than on others. Clearly, selection of the parameters of the habituation protocol is critical.
The capability to draw conclusions about general habituation performance across strains, as reported in the existing literature is hindered by the range of session lengths that have been used by researchers, from 5 to 90 minutes, and from the limited numbers of strains examined in any given study. It is clear that intrasession habituation among a variety of inbred strains is a phenomenon that needs rigorous study. Toward that goal, we recently examined a group of 14 inbred strains, for habituation performance in a darkened activity monitor over a 15-minute session. This investigation is part of a large intrasession and intersession study as the mice were actually exposed to 15 minutes a day over three days. The intersession portion of this study will be described in the intersession habituation section below. A total of 252 adult male mice (18 males each of 129P2/OlaHsd (129P2), 129S1/SvImJ (129S1), 129S2/SvHsd (129S2), 129S6/SvEvTac (129S6), 129T2/SvEmJ (129T2), 129X1/SvJ (129X1), A/J (A), C57BL/6J (B6), BTBR T+ tf/J (BTBR), BALB/cByJ (CBy), C3H/HeJ (C3H), CBA/J (CBA), DBA/2J (D2), FVB/NJ (FVB)) were tested. At the time of testing, mice were 8-12 weeks old (mean age=63.25 days, SEM=0.324). All mice were purchased from Jackson Laboratory (Bar Harbor, ME), Taconic Farms Inc. (Germantown, NY) or Harlan (Indianapolis, IN) and were maintained in our colony at the Wadsworth Center. Mice were housed in clear polycarbonate cages (29 X 18 X 12.5 cm) with stainless steel wire lids and filter tops and were maintained on a 12:12 hour light: dark cycle (lights on at 7:00 a.m.). Mice were tested in AccuScan VersaMax activity monitors (Columbus, OH), which were enclosed in melamine sound-attenuating chambers (65cm X 55cm X 55cm; Med Associates, St. Albans, VT). Mice were tested during the light portion of the light-dark cycle, and all testing procedures had prior approval from the Wadsworth Center IACUC committee.
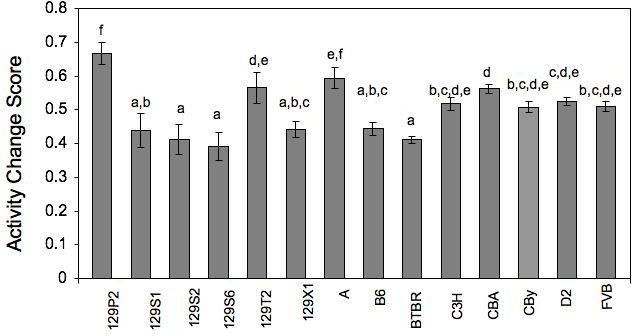
From our data, it is clear that most of these 14 inbred strains of mice displayed intrasession habituation during the 15-minute session, although there were differences among the strains (see Fig. 2). Inbred strain differences in performance across the three time periods (min 1-5, min 6-10 and min 11-15) were analyzed by a mixed ANOVA. We found a main effect of strain (F(13,238)=48.15,p<0.0001), a main effect of time tested (F(2,476)=904.028,p<0.0001) and an interaction between strain and time tested (F(26,476)=24.401,p<0.0001). Habituation scores were calculated using the Nadel (1968) equation, and data were analyzed by one-way ANOVA. We found a significant effect of strain (F(13,238)=44.611, p<0.0001; Fig.3) and used Fishers posthoc tests (after having first obtained a corresponding significant F-value) to further examine genotype differences. The 129 substrains all tended to perform similarly (with the exception of 129X1, a strain known to have become contaminated at some point during development), as reflects their common genetic heritage. However, even within those 129 substrains, one can see genetic variability. The three strains displaying the lowest degree of habituation were FVB, D2 and CBy. Our data confirm the findings of Logue et al. (1997) for many of strains, although with our longer session length, most inbred strains displayed better habituation than reported by Logue and colleagues with their shorter session. Both studies found that D2 and FVB display lower levels of intrasession habituation than other strains, independent of session length, making these two strains of particular interest for future genetic studies.
Fig. 2.
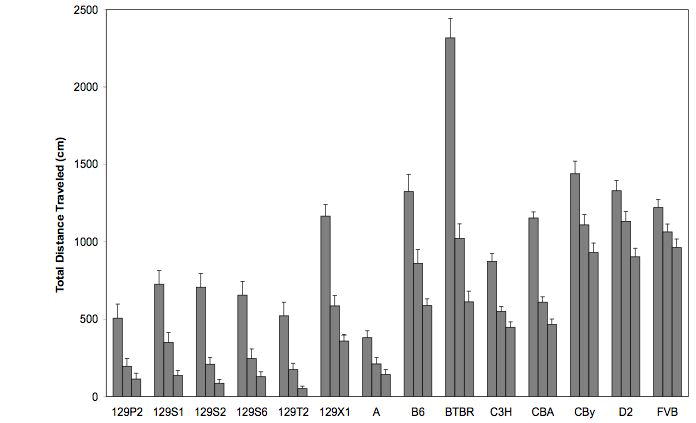
Total distance traveled in the activity monitor over the 15-minute period for the 14 inbred strains of mice tested. Three bars over each inbred strain represent total distance traveled during the first, second and third five-minute bins, respectively. Error bars indicate standard error. 129P2/OlaHsd (129P2), 129S1/SvImJ (129S1), 129S2/SvHsd (129S2), 129S6/SvEvTac (129S6), 129T2/SvEmJ (129T2), 129X1/SvJ (129X1), A/J (A), C57BL/6J (B6), BTBR T+ tf/J (BTBR), BALB/cByJ (CBy), C3H/HeJ (C3H), CBA/J (CBA), DBA/2J (D2), FVB/NJ (FVB).
Fig. 3.
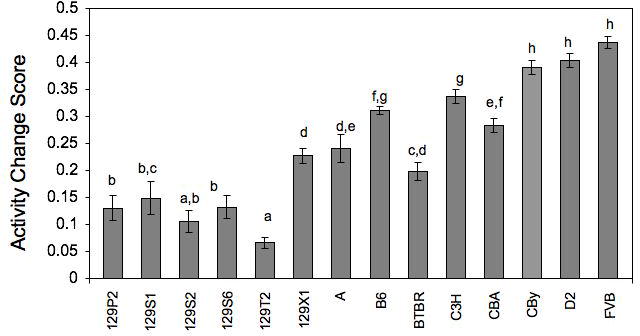
Activity change scores from the first five-minute block to the last five-minute block of the session, for the 14 inbred strains of mice tested. Those not having a common letter above their bars are significantly different (p≤0.05) from one other. Error bars indicate standard error. 129P2/OlaHsd (129P2), 129S1/SvImJ (129S1), 129S2/SvHsd (129S2), 129S6/SvEvTac (129S6), 129T2/SvEmJ (129T2), 129X1/SvJ (129X1), A/J (A), C57BL/6J (B6), BTBR T+ tf/J (BTBR), BALB/cByJ (CBy), C3H/HeJ (C3H), CBA/J (CBA), DBA/2J (D2), FVB/NJ (FVB).
2.2 Linkage Analysis
Inbred strain differences, although informative, really only provide evidence of genetic variability for a phenotype. Unfortunately, environmental factors cannot at this stage be eliminated. In an inbred-strain analysis, evidence cannot be obtained for the involvement of specific gene(s), or even chromosome(s) in the behavior under study. Traditionally, linkage analysis is the next step in the process. For intrasession habituation linkage analysis has been conducted in two studies (Koyner et al., 2000; Radcliffe, Jones, and Erwin, 1998).
In the Radcliffe et al. (1998) study, the mapping was performed with long-sleep and short-sleep recombinant inbred (RI) lines of mice. An RI strain is developed from an initial outcross between two parental inbred strains (the first offspring are deemed a first filial, or F1 generation), followed by at least 20 successive brother-sister matings. Thus, because the different RI lines are derived from the same initial cross, the general assumption is that, for two lines that differ in terms of behavior, the genetic regions that differ between the two strains will be responsible for the behavioral difference, and not the common genetic regions. If enough RI lines are available for study, this set of strains is a very powerful method for detecting genetic loci of interest. A total of 26 RI lines were tested in the Radcliff et al. study. Mice were exposed to an automated testing apparatus for 30 minutes, with the session broken into five-minute blocks for the purpose of analysis. Intrasession habituation, or adaptation as it was termed in that study, was quantified as the difference in activity level between the first five-minute and last five-minute block in a session. The authors found genetic loci, or quantitative trait loci (QTLs), of interest, on Chromosomes 3, 7, 11,12, 13, and 19.
In another RI line study Koyner et al. (2000) used lines originally generated from B6 and D2 strains, which, as noted above, differ with respect to intrasession habituation. With a 20-minute session, Koyner and colleagues compared activity levels during the first five-minute block and the last five-minute block in the session. They did not find the same loci as has been found by Radcliffe et al. (1998), but instead reported loci on Chromosomes 2 and 6. Unfortunately, when they tried to replicate their findings with a F2 hybrid analysis, they were unable to do so. The F2 analysis is a common means by which to confirm findings from RI line studies. The procedure to develop the F2 hybrid entails two steps. First the two founder strains are intercrossed, to produce the F1 progeny, as in the case of the RI line. Next, the F1 mice are interbred, to generate F2 progeny. Each of these F2 mice is genetically unique, as genes are segregating, but all genes come only from the two founder strains. Here, then, a behavioral phenotype can be correlated with one or more chromosomal regions. Although Koyner et al (2000) were not able to confirm their own RI line findings they did find loci on Chromosomes 9 and 12 that could be involved in intrasession habituation. Across three different linkage analyses there was no common chromosomal region of interest. This is interesting, although not surprising, as different parental strains were used to generate the populations in these two studies. Given that Chromosome 12 was implicated in both the Radcliffe et al. (1998) study and the F2 hybrid analysis of Koyner et al. (2000), this region may warrant further genetic analysis. However, Chromosome 12 is unlikely to be the only region involved in the behavior, and future linkage studies with different strains are warranted.
3. Intersession habituation
3.1. Inbred strains
The investigation of the role of genetics in intersession habituation has followed a path similar to that used to study intrasession habitation. However, intersession habituation has been extensively investigated in a large number of laboratories, with, consequently, large variations in protocols and testing conditions (Ammassari-Teule and Passino, 1997; Ammassari-Teule, Tozzi, Rossi-Arnaud, Save, and Thinus-Blanc, 1995; Anisman et al., 1976; Bolivar et al., 2000b; Bothe et al., 2004; 2005; Contet, Rawlins, and Deacon, 2001; Cook et al., 2002; Crabbe, Rigter, and Kerbusch, 1982; Isles, Humby, Walters, and Wilkinson, 2004; Podhorna and Brown, 2002; Roullet, Mele, and Ammassari-Teule, 1997; Thinus-Blanc, Save, Rossi-Arnaud, Tozzi, and Ammassari-Teule, 1996). Once again, many studies have examined only two inbred strains at a time, very often B6 and D2. Despite the initial disparity seen between these two strains, the actual magnitude of the differences appears to be dependent upon procedural variables such as session length, intersession interval, time of testing in light/dark cycle, and presence/absence of objects in the novel environment (Ammassari-Teule and Passino, 1997; Ammassari-Teule et al., 1995; Anisman et al., 1976; Podhorna and Brown, 2002; Roullet et al., 1997; Thinus-Blanc et al., 1996). In contrast to the other studies entailing relatively short sessions (15 minutes or less), Isles et al (2004) reported for two hour sessions that CBA/Ca, 1299S2/SvHsd, and B6 displayed habituation intersession but BALB/c did not. These authors' finding of B6 habituation contrasts with findings in other studies listed above; the discrepancy could be the result of session length or novel environment complexity.
Almost a decade ago, we began our investigations of intersession habituation in mice, using a very simple short (5-minute) session, once per day repeated across three days. In our first survey, comparing eight common inbred strains, we found that strains such as B6, 129S1, and C3H showed declines in total distance traveled in the activity monitor over the three days of testing (Bolivar, Caldarone, Reilly, and Flaherty, 2000b) (Fig. 4). In contrast, strains such as A, CBA, D2, and FVB showed no such decline. These data provide strong evidence of genetic variability across strains. Recently, we reexamined those data, calculated activity change scores (Nadel, 1968), and analyzed them by one-way ANOVA. We found a significant effect of strain (F(7,88)=7.798, p<0.0001). This reanalysis, controlling for differences in baseline activity levels, confirms that inbred strain genetic variability in intersession habituation exists, if the data have been corrected for baseline (Fig. 5). It is interesting to note that some strains (e.g., A) actually increase activity across days (see Fig. 4). When taking the activity change score as a sole determination of habituation, one may not fully appreciate the complexity of the situation. In the case of the A strain, although activity level is increasing from day 1 to day 3, habituating to the environment may still be occurring. This strain is known for its high anxiety response (Crawley, Belknap, Collins, Crabbe, Frankel, Henderson, Hitzemann, Maxson, Miner, Silva, Wehner, Wynshaw-Boris, and Paylor, 1997; Trullas and Skolnick, 1993), and activity levels may increase as mice become less anxious, i.e., display habituation of anxiety in the environment. Thus, although change in activity is the more traditional measure of habituation, it is important to consider other underlying factors that can influence behavioral performance.
Fig. 4.
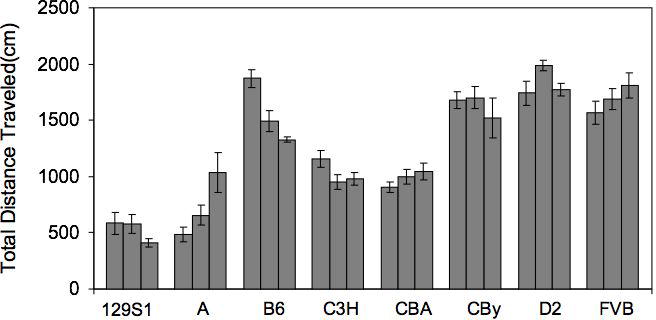
Total distance traveled in the activity monitor over the three days of testing. Three bars over each inbred strain represent total distance traveled on Days 1, 2 and 3, respectively. Error bars indicate standard error. A=A/J, B6=C57BL/6J, CBy=BALB/cByJ, C3H=C3H/HeJ, CBA=CBA/J, D2=DBA/2J, FVB=FVB/NJ, 129S1=129S1/SvImJ. Data reanalyzed from Bolivar et al (2000b).
Fig. 5.
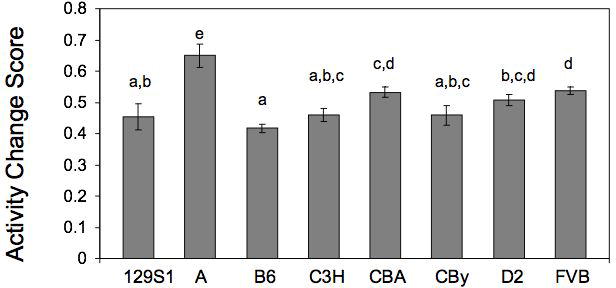
Activity change scores from Day 1 to Day 3 for the eight inbred strains of mice tested. Those without a letter in common, above their bars are significantly different (p≤0.05) from one other. Error bars indicate standard error. A=A/J, B6=C57BL/6J, CBy=BALB/cByJ, C3H=C3H/HeJ, CBA=CBA/J, D2=DBA/2J, FVB=FVB/NJ, 129S1=129S1/SvImJ. Data reanalyzed from Bolivar et al (2000b).
To further examine the performance of strains that differ in habituation performance (e.g., B6, D2), we lengthened the testing duration to four days, and altered the floor of the activity monitor (i.e., changed the actual physical characteristics of the floor) on the fourth day of testing (Bolivar et al., 2000b). We found that B6 mice changed their pattern of habituation in response to the altered floor, whereas D2 mice did not (Fig. 6).
Fig. 6.
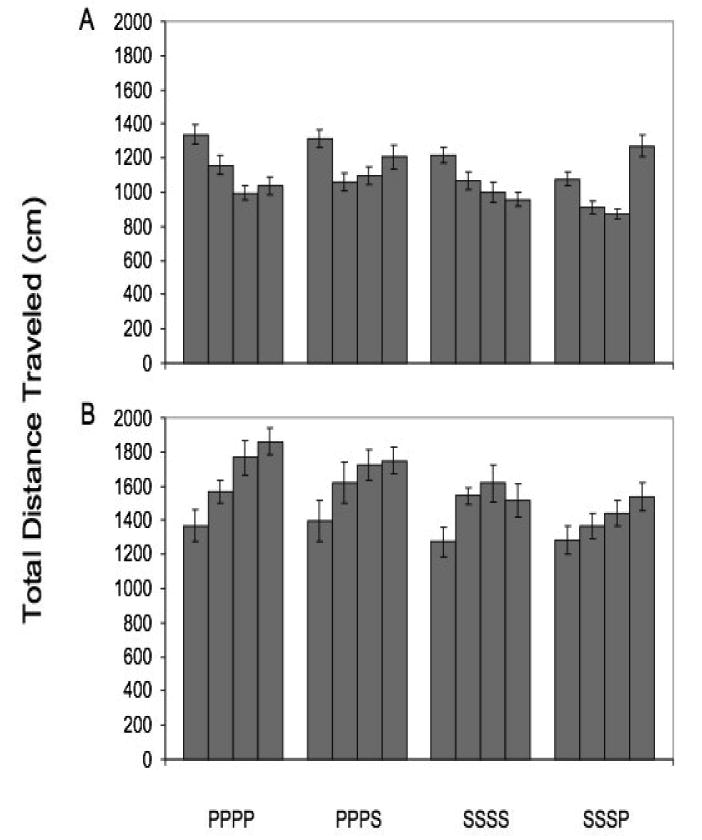
Total distance traveled in the activity monitor over the four days of testing for C57BL/6J (Panel A) and DBA/2J (Panel B). The four bars over each inbred strain represent total distance traveled on days 1, 2, 3, and 4 respectively. PPPP=plain flooring for four days, PPPS=plain flooring for three days followed by screened flooring on day 4, SSSS=screened flooring for four days, and SSSP=screened flooring for three days followed by plain flooring for day 4. Error bars indicate standard error. Data reanalyzed from Bolivar et al (2000b).
Since the Bolivar et al. (2000b) we, and our collaborators, have used the five-minute per day for three days habituation protocol to evaluate the performance of a variety of other inbred strains (Bothe et al., 2004; 2005; Cook et al., 2002). Because 129 substrains are commonly used in the development of knockout mice, we evaluated a group of them for intersession habituation performance (Cook et al., 2002). Due to genetic contamination, there is substantial genetic variability among the 129 family of substrains (Simpson, Linder, Sargent, Davisson, Mobraaten, and Sharp, 1997; Threadgill, Yee, Matin, Nadeau, and Magnuson, 1997). There are three basic lineages: Parental (P; white-bellied, pink-eyed, light chinchilla), Steel (S; white-bellied pink-eyed, light chinchilla or albino), and Ter (T; white-bellied chinchilla), plus genetically contaminated (X; white-bellied pink-eyed, light chinchilla or albino) (http://www.jax.org); we evaluated members of each lineage. We used our three day, five minutes per day procedure to evaluate 129S1, 129S2/SvHsd (129S2), 129S6/SvEvTac (129S6), 129X1, 129P1/ReJ (129P1), 129P3/J (129P3) and 129T2/SvEmsJ (129T2) substrains. Of note is that members of the Parental lineage (129P1 and 129P3) did not display habituation, nor did members of the Ter lineage (129T2), whereas the 129X1 and some of the Steel lineage (129S6, 129S1 but not 129S2) substrains did decrease activity over the three days. Thus, genetic differences among these lineages appear to be involved in intersession habituation. Differences in performance within a lineage are not unexpected either, given that the Steel lineage representatives that we selected for this study included mice from three different suppliers. Because mice from the different suppliers have been separated for a number of years, both genetic and environmental factors could be influencing performance and introducing differences. Once again, genes that differ across substrains within a given lineage could be involved in intersession habituation.
In consideration of the study that found inbred strain performance in behavioral assays could be influenced by inter-laboratory differences (Crabbe, Wahlsten, and Dudek, 1999), we, in collaboration with Dr. Gerald Bothe of Taconic Farms, Inc., re-evaluated the performance of some of the strains that we had tested previously, as well as investigating additional strains from the Taconic facility (Bothe et al., 2004; 2005). However, we also took advantage of this opportunity to examine the effects of adding an additional day of testing and the evaluation of a number of activity parameters (vertical activity, center distance traveled and velocity) in addition to the total distance traveled measure. We found that despite protocol and laboratory variations, B6 mice still displayed habituation. Moreover, the C57BL/6Tac strain behaves similarly to B6, to which it is closely related. Thus, at least for the B6 strain, habituation behavior is robust across moderate protocol manipulations. Comparing data from the 129 substrains in these studies to the findings of Cook et al. (2002), we can see that in many cases strains behaved similarly across studies (i.e., 129T2, 129P3), although in other cases (i.e., 129X1), there were differences. The discrepancies may be a function of the length of the protocol or other laboratory conditions. Furthermore, multiparametric assessment revealed that parameters other than total distance (e.g., vertical activity habituation in FVB/NTac) can be indicative of habituation. Taken together these studies indicate that, for many strains, the habituation protocol that we have devised gives robust results across conditions, although some strains (i.e., B6) show more consistency than others (129S1).
We recently modified our intersession habituation protocol, to determine the effects of increased session length on inbred strain performance. We examined the total distance traveled across three days in the same 14 strains described previously in the intrasession habituation section (see Fig 2 and Fig 3 data and related description in text). Each mouse was given one 15-minute session per day, across three days. In general, mice did not display equivalent levels of intersession habituation and intrasession habituation (compare Figs. 2 and 3 to Figs 7 and 8). This lack of parallelism again indicates that the two types of habituation are not measuring the same construct. Intersession habituation seems to be more challenging for mice, no doubt due to the retention interval (in our case, 24 hours).
Fig. 7.
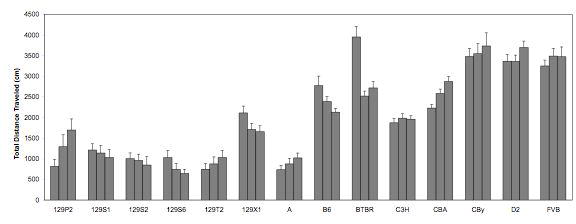
Total distance traveled in the activity monitor over the three days of testing, for the 14 inbred strains tested. Three bars over each inbred strain represent total distance traveled on days 1, 2 and 3, respectively. Error bars indicate standard error. 129P2/OlaHsd (129P2), 129S1/SvImJ (129S1), 129S2/SvHsd (129S2), 129S6/SvEvTac (129S6), 129T2/SvEmJ (129T2), 129X1/SvJ (129X1), A/J (A), C57BL/6J (B6), BTBR T+ tf/J (BTBR), BALB/cByJ (CBy), C3H/HeJ (C3H), CBA/J (CBA), DBA/2J (D2), FVB/NJ (FVB).
Fig. 8.
Activity change scores from Day 1 to Day 3 for the 14 inbred strains of mice tested. Those without a letter in common above their bars, are significantly different (p≤0.05) from one other. Error bars indicate standard error. 129P2/OlaHsd (129P2), 129S1/SvImJ (129S1), 129S2/SvHsd (129S2), 129S6/SvEvTac (129S6), 129T2/SvEmJ (129T2), 129X1/SvJ (129X1), A/J (A), C57BL/6J (B6), BTBR T+ tf/J (BTBR), BALB/cByJ (CBy), C3H/HeJ (C3H), CBA/J (CBA), DBA/2J (D2), FVB/NJ (FVB).
Inbred strain differences in performance across the three days of testing were analyzed by a mixed ANOVA. We found a main effect of strain (F(13,238)=53.992,p<0.0001), and an interaction between strain and time tested (F(26,476)=7.063,p<0.0001), but no main effect of day tested. For some inbred strains, we found a reduction in total distance traveled, across the three days (e.g., B6, BTBR, 129X1), but for other strains, there was no difference (e.g., FVB, C3H) (Fig. 7). When activity change scores were calculated using the Nadel (1968) equation and analyzed by one-way ANOVA, a significant inbred strain differences in intersession habituation emerged (F(13,238)=7.144, p<0.0001; Fig. 8). For the longer session length (15 min), many of the same patterns emerged as had been seen for the 5-min session (See Fig. 5). For instance, B6 displayed habituation in both situations, whereas CBA, D2 and FVB strains did not habituate in either situation. In fact, most of the inbred strains that were tested in both session lengths did not show sensitivity to the time parameter variation. Thus, we can hypothesize that after five minutes of exploring a stimulus-free novel environment, such as the activity monitor used in our studies, mice from the inbred strains that we have tested have gathered as much information for their cognitive maps of the area that they are likely to gather in the whole session. Addition of an additional 10 minutes to each day's session does not significantly alter intersession habituation performance.
3.2 Linkage Analysis
To determine the specific locations of genes involved in intersession habituation we evaluated BXD RI lines, which are derived from the crossing of B6 and D2 inbred strains (Bolivar and Flaherty, 2003). When comparing these RI lines to the two parental strains, we found, not surprisingly, that some strains behaved more like B6 and others more like D2 (Fig. 9). Also, the performance of some strains fell outside the range bounded by the performances of the two parental strains. Our linkage analysis indicated a highly significant association exists between a region on Chromosome 15 and intersession habituation behavior. This single QTL or region, accounting for 80% of the genetic variance, is located between 32cM and 46cM. We subsequently confirmed this QTL with a small F2 linkage analysis (Bolivar and Flaherty, 2003). This genetic region is completely different from the regions previously identified for intrasession habituation (Koyner et al., 2000; Radcliffe et al., 1998). A different QTL is expected, if the two types of habituation are indeed measuring somewhat different conceptual entities and under the control of different neural pathways. It is critical to note that the finding of the single QTL does not indicate that a behavior as complex as intersession habituation is governed by a single gene or group of genes in just one chromosomal region. Rather, it suggests that the region plays a significant role in the behavioral phenotype. It is important to consider the habituation QTL in the context of the other QTLs reported for this chromosome in other studies. Chromosome 15 has been linked to locomotor activity and anxiety in a number of studies (Gershenfeld, Neumann, Mathis, Crawley, Li, and Paul, 1997; Gershenfeld and Paul, 1997; Henderson, Turri, DeFries, and Flint, 2004; Kas, de Mooij-van Malsen, Olivier, Spruijt, and van Ree, 2008; Steinberger, Reynolds, Ferris, Lincoln, Datta, Stanley, Paterson, Dawson, and Flint, 2003; Turri, Datta, DeFries, Henderson, and Flint, 2001a; Turri, Henderson, DeFries, and Flint, 2001b), although this linkage has been suggested to arise more in the context of avoidance behavior than that of ambulation (Turri et al., 2001a). Current studies in our laboratory, with a variety of genetic tools, including lines of mice selected on the basis that they differ only within subsections of the area identified in the QTL (congenics), microarray analyses, and full genome scans, are underway to determine the specific genes on Chromosome 15 that are involved in intersession habituation.
Fig. 9.
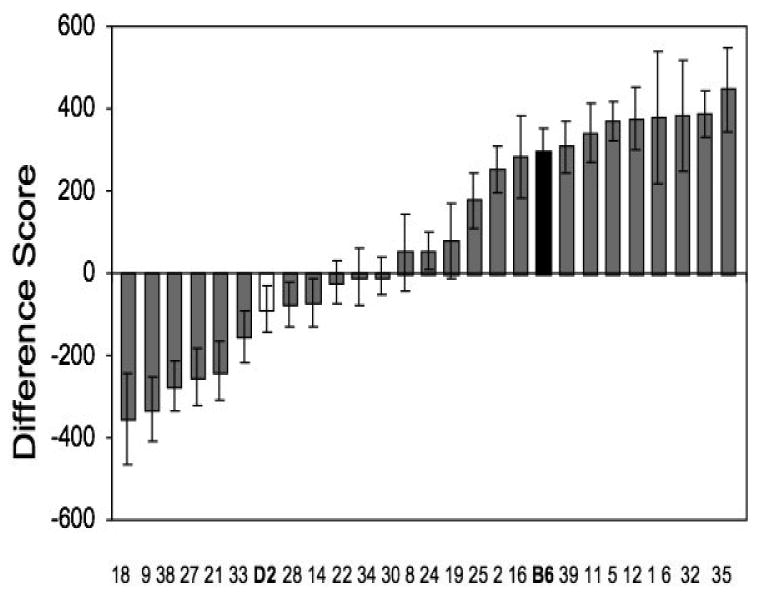
Change in activity from Day 1 to Day 3, for the 27 strains tested. Error bars indicate standard error. Difference score is calculated by subtraction of the total distance traveled on Day 3 from the total distance traveled on Day 1. Twenty-five recombinant inbred lines are each represented by gray shaded bar with individual line numbers (e.g., BXD18=18), C57BL/6J=B6 (black bar), DBA/2J=D2 (white bar). Data reanalyzed from Bolivar and Flaherty (2003).
4. Summary
Based on the findings from inbred strain studies to date, it is clear that genetic variability exists in both intrasession and intersession habituation, although the latter behavior shows greater variability across strains. B6 is generally considered to be one of the strains that displays high levels of both intrasession and intersession habituation, although not in situations when the novel environment contains a number of stimuli to be explored. Thus, B6 is a good strain to use for continued genetic analyses. In contrast, D2 and FVB do not perform as well as B6 in most habituation paradigms. Thus, linkage studies often involve mice derived from interbreeding of B6 and D2: the magnitude of the differences in performance that are seen between these strains is likely to facilitate the finding of relevant chromosomal regions and then the actual genes involved.
A number of QTL or regions on Chromosomes 2,3,6,7,9,11,12,13, and19 have been linked to intrasession habituation (Koyner et al., 2000; Radcliffe et al., 1998), although only the QTL on Chromosome 12 was identified in both of these studies. The region will clearly receive further investigation. The remaining regions may prove to be important for habituation, but they will need to be confirmed through future genetic analyses. Although recombinant inbred strains are a very useful tool for linkage analysis, false-positive results are often obtained with them, unless large numbers of lines are assessed (Belknap, Mitchell, O'Toole, Helms, and Crabbe, 1996). Small RI line studies must be followed up with confirmation using F2 or other populations, the latter approach is one of the most effective ways in which to eliminate false positives.
There has only been one linkage study of intersession habituation (Bolivar and Flaherty, 2003), and the QTL on Chromosome 15 obtained in that study does not match any of the QTLs that have been associated with intrasession habituation (Koyner et al., 2000; Radcliffe et al., 1998). Negative evidence to some extent supports the hypothesis that the two types of habituation are measuring somewhat different constructs. Perhaps, the Chromosome 15 QTL is associated with anxiety-like behavior; anxiety QTLs have been identified on this chromosome (Gershenfeld et al., 1997; Gershenfeld and Paul, 1997; Henderson et al., 2004; Kas et al., 2008; Steinberger et al., 2003; Turri et al., 2001a; Turri et al., 2001b). Overlapping QTLs, i.e., some of the same genes, would not be surprising; internal factors such as anxiety can play a role in the development of the cognitive map: an animal cannot form a full map of the area if it is too anxious to explore it. Additional studies are necessary to determine whether this region contains anxiety related gene(s). Whatever the actual role of the chromosomal region in intersession habituation, there is clearly some involvement. Ongoing studies in our laboratory, examining select lines of mice, are directed toward determining the specific gene(s) in that region of Chromosome 15 that play a role in intersession habituation.
Once the genes from the loci listed above have been identified, we can begin to elucidate the pathways involved in habituation behavior. However, elucidating all the genes involved in habituation will not be an easy task. Linkage analyses generally identify genes that are strongly associated with a behavior, whereas many genes with smaller roles may not be easily uncovered. Therefore, it will be important to combine linkage analysis with gene expression analyses, so that pathways can be identified. Confirmation of the role of specific genes will necessitate the development of double, triple or even more complicated transgenic and/or knockout mice. To determine the role of development in these processes, conditional knockout mice may also be necessary. These and other sophisticated genetic tools, currently being refined, will aid in the process of identifying the genetics underlying this complex behavior.
It is important to also note here that open field habituation (both intrasession and intersession) may not involve the same processes as response habituation outlined in many papers in this special issue. Based on the habituation characteristics updated and revised during the August 2007 workshop and outlined in this issue (Rankin et al., 2009), there is much about open field habituation that remains unstudied. Experiments evaluating spontaneous recovery, dishabituation, training schedule effects, stimulus generalization, and habituation of dishabituation in mice are needed to evaluate overlap between open field habituation and response habituation. One can use the tools of mouse genetics to study these, as well as other, issues. Taking the strains that display higher and lower levels of open field habituation, one can then test them for response habituation. Subsequently, one can see if there is genetic overlap among different types of habituation or different characteristics of the phenomenon. Should there be genetic overlap, the common genes would be the subjects of intense study, as they might tell us something about the very biological foundation of the basic habituation response.
Acknowledgments
The new research reported here was supported by NIH grants MH067850 and MH068013 to VB. I thank Dr Catharine Rankin, for her invitation to participate in this special issue on habituation, and Kevin Manley, for proofreading.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ammassari-Teule M, Passino E. The dorsal hippocampus is selectively involved in the processing of spatial information even in mice with a genetic hippocampal dysfunction. Psychobiology. 1997;25:118–125. [Google Scholar]
- Ammassari-Teule M, Tozzi A, Rossi-Arnaud C, Save E, Thinus-Blanc C. Reactions to spatial and nonspatial change in two inbred strains of mice: Further evidence supporting the hippocampal dysfunction hypothesis in the DBA/2 strain. Psychobiology. 1995;23:284–289. [Google Scholar]
- Anisman H, Kokkinidis L, Glazier S, Remington G. Differentiation of response biases elicited by scopolamine and d-amphetamine: effects on habituation. Behav Biol. 1976;18:401–417. doi: 10.1016/s0091-6773(76)92407-x. [DOI] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER. Synaptic remodeling, synaptic growth and the storage of long-term memory in Aplysia. Prog Brain Res. 2008;169:179–198. doi: 10.1016/S0079-6123(07)00010-6. [DOI] [PubMed] [Google Scholar]
- Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MF, Fisher EM. Genealogies of mouse inbred strains. Nat Genet. 2000;24:23–25. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Mitchell SR, O'Toole LA, Helms ML, Crabbe JC. Type I and type II error rates for quantitative trait loci (QTL) mapping studies using recombinant inbred mouse strains. Behav Genet. 1996;26:149–160. doi: 10.1007/BF02359892. [DOI] [PubMed] [Google Scholar]
- Bolivar V, Cook M, Flaherty L. List of transgenic and knockout mice: behavioral profiles. Mamm Genome. 2000a;11:260–274. doi: 10.1007/s003350010051. [DOI] [PubMed] [Google Scholar]
- Bolivar V, Flaherty L. A region on chromosome 15 controls intersession habituation in mice. J Neurosci. 2003;23:9435–9438. doi: 10.1523/JNEUROSCI.23-28-09435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar VJ, Caldarone BJ, Reilly AA, Flaherty L. Habituation of activity in an open field: A survey of inbred strains and F1 hybrids. Behav Genet. 2000b;30:285–293. doi: 10.1023/a:1026545316455. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Manley K, Messer A. Exploratory activity and fear conditioning abnormalities develop early in R6/2 Huntington's disease transgenic mice. Behav Neurosci. 2003;117:1233–1242. doi: 10.1037/0735-7044.117.6.1233. [DOI] [PubMed] [Google Scholar]
- Bolivar VJ, Ganus JS, Messer A. The development of behavioral abnormalities in the motor neuron degeneration (mnd) mouse. Brain Res. 2002;937:74–82. doi: 10.1016/s0006-8993(02)02470-8. [DOI] [PubMed] [Google Scholar]
- Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG. Genetic and behavioral differences among five inbred mouse strains commonly used in the production of transgenic and knockout mice. Genes Brain Behav. 2004;3:149–157. doi: 10.1111/j.1601-183x.2004.00064.x. [DOI] [PubMed] [Google Scholar]
- Bothe GW, Bolivar VJ, Vedder MJ, Geistfeld JG. Behavioral differences among fourteen inbred mouse strains commonly used as disease models. Comp Med. 2005;55:326–334. [PubMed] [Google Scholar]
- Cabib S, Algeri S, Perego C, Puglisi-Allegra S. Behavioral and biochemical changes monitored in two inbred strains of mice during exploration of an unfamiliar environment. Physiol Behav. 1990;47:749–753. doi: 10.1016/0031-9384(90)90089-m. [DOI] [PubMed] [Google Scholar]
- Colombo J, Mitchell DW. Infant visual habituation. Neurobiol Learn Mem. 2009 doi: 10.1016/j.nlm.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Rawlins JN, Deacon RM. A comparison of 129S2/SvHsd and C57BL/6JOlaHsd mice on a test battery assessing sensorimotor, affective and cognitive behaviours: implications for the study of genetically modified mice. Behav Brain Res. 2001;124:33–46. doi: 10.1016/s0166-4328(01)00231-5. [DOI] [PubMed] [Google Scholar]
- Cook MN, Bolivar VJ, McFadyen MP, Flaherty L. Behavioral differences among 129 substrains: implications for knockout and transgenic mice. Behav Neurosci. 2002;116:600–611. [PubMed] [Google Scholar]
- Crabbe JC, Rigter H, Kerbusch S. Analysis of behavioural responses to an ACTH analog in CXB/By recombinant inbred mice. Behav Brain Res. 1982;4:289–314. doi: 10.1016/0166-4328(82)90006-7. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, Wehner JM, Wynshaw-Boris A, Paylor R. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- Engel JE, Wu CF. Neurogenetic approaches to habituation and dishabituation in Drosophila. Neurobiol Learn Mem. 2009 doi: 10.1016/j.nlm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley SM, Springer AD. Duration of exposure to a novel environment affects retention in aging mice. Behav Neural Biol. 1981a;33:293–302. [Google Scholar]
- Fraley SM, Springer AD. Memory of simple learning in young, middle-aged, and aged C57/BL6 mice. Behav Neural Biol. 1981b;31:1–7. doi: 10.1016/s0163-1047(81)90986-9. [DOI] [PubMed] [Google Scholar]
- Gershenfeld HK, Neumann PE, Mathis C, Crawley JN, Li X, Paul SM. Mapping quantitative trait loci for open-field behavior in mice. Behav Genet. 1997;27:201–210. doi: 10.1023/a:1025653812535. [DOI] [PubMed] [Google Scholar]
- Gershenfeld HK, Paul SM. Mapping quantitative trait loci for fear-like behaviors in mice. Genomics. 1997;46:1–8. doi: 10.1006/geno.1997.5002. [DOI] [PubMed] [Google Scholar]
- Giles AC, Rankin CH. Behavioral and genetic characterization of habituation using Caenorhabditis elegans. Neurobiol Learn Mem. 2009 doi: 10.1016/j.nlm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: a dual-process theory. Psychol Rev. 1970;77:419–450. doi: 10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Harris JD. Habituatory response decrement in the intact organism. Psychol Bull. 1943;40:385–422. [Google Scholar]
- Henderson ND, Turri MG, DeFries JC, Flint J. QTL analysis of multiple behavioral measures of anxiety in mice. Behav Genet. 2004;34:267–293. doi: 10.1023/B:BEGE.0000017872.25069.44. [DOI] [PubMed] [Google Scholar]
- Isles AR, Humby T, Walters E, Wilkinson LS. Common genetic effects on variation in impulsivity and activity in mice. J Neurosci. 2004;24:6733–6740. doi: 10.1523/JNEUROSCI.1650-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafkafi N, Lipkind D, Benjamini Y, Mayo CL, Elmer GI, Golani I. SEE locomotor behavior test discriminates C57BL/6J and DBA/2J mouse inbred strains across laboratories and protocol conditions. Behav Neurosci. 2003;117:464–477. doi: 10.1037/0735-7044.117.3.464. [DOI] [PubMed] [Google Scholar]
- Kas M, de Mooij-van Malsen A, Olivier B, Spruijt B, van Ree J. Differential genetic regulation of motor activity and anxiety-related behaviors in mice using an automated home cage task. Behav Neurosci. 2008;122:769–776. doi: 10.1037/0735-7044.122.4.769. [DOI] [PubMed] [Google Scholar]
- Koyner J, Demarest K, McCaughran J, Jr, Cipp L, Hitzemann R. Identification and time dependence of quantitative trait loci for basal locomotor activity in the BXD recombinant inbred series and a B6D2 F2 intercross. Behav Genet. 2000;30:159–170. doi: 10.1023/a:1001963906258. [DOI] [PubMed] [Google Scholar]
- Leussis MP, Bolivar VJ. Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci Biobehav Rev. 2006;30:1045–1064. doi: 10.1016/j.neubiorev.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Logue SF, Owen EH, Rasmussen DL, Wehner JM. Assessment of locomotor activity, acoustic and tactile startle, and prepulse inhibition of startle in inbred mouse strains and F1 hybrids: implications of genetic background for single gene and quantitative trait loci analyses. Neuroscience. 1997;80:1075–1086. doi: 10.1016/s0306-4522(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Miner LL. Cocaine reward and locomotor activity in C57BL/6J and 129/SvJ inbred mice and their F1 cross. Pharmacol Biochem Behav. 1997;58:25–30. doi: 10.1016/s0091-3057(96)00465-0. [DOI] [PubMed] [Google Scholar]
- Muller U, Cristina N, Li ZW, Wolfer DP, Lipp HP, Rulicke T, Brandner S, Aguzzi A, Weissmann C. Behavioral and anatomical deficits in mice homozygous for a modified beta-amyloid precursor protein gene. Cell. 1994;79:755–765. doi: 10.1016/0092-8674(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Nadel L. Dorsal and ventral hippocampal lesions and behavior. Physiol Behav. 1968;3:891–900. [Google Scholar]
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon Press; 1978. [Google Scholar]
- Peeler DF. Two measures of activity in genetically defined mice as a function of strain, time of day, and previous experience. Psychobiology. 1990;18:327–338. [Google Scholar]
- Podhorna J, Brown RE. Strain differences in activity and emotionality do not account for differences in learning and memory performance between C57BL/6 and DBA/2 mice. Genes Brain Behav. 2002;1:96–110. doi: 10.1034/j.1601-183x.2002.10205.x. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Jones BC, Erwin VG. Mapping of provisional quantitative trait loci influencing temporal variation in locomotor activity in the LS × SS recombinant inbred strains. Behav Genet. 1998;28:39–47. doi: 10.1023/a:1021456731470. [DOI] [PubMed] [Google Scholar]
- Rankin CH, Abrams T, Barry RJ, Bhatnagar S, Clayton DF, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, McSweeney FK, Wilson DA, Wu CF, Thompson RF. Habituation revisited: An updated and revised description of the behavioral characteristics of habituation. Neurobiol Learn Mem. 2009 doi: 10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roullet P, Mele A, Ammassari-Teule M. Ibotenic lesions of the nucleus accumbens promote reactivity to spatial novelty in nonreactive DBA mice: implications for neural mechanisms subserving spatial information encoding. Behav Neurosci. 1997;111:976–984. doi: 10.1037//0735-7044.111.5.976. [DOI] [PubMed] [Google Scholar]
- Silver LM. Mouse Genetics: Concepts and Applications. New York: Oxford University Press; 1995. [Google Scholar]
- Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- Steinberger D, Reynolds DS, Ferris P, Lincoln R, Datta S, Stanley J, Paterson A, Dawson GR, Flint J. Genetic mapping of variation in spatial learning in the mouse. J Neurosci. 2003;23:2426–2433. doi: 10.1523/JNEUROSCI.23-06-02426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry WS. Habituation and dishabituation of rats' exploration of a novel environment. Anim Learn Behav. 1979;7:525–536. [Google Scholar]
- Thinus-Blanc C, Save E, Rossi-Arnaud C, Tozzi A, Ammassari-Teule M. The differences shown by C57BL/6 and DBA/2 inbred mice in detecting spatial novelty are subserved by a different hippocampal and parietal cortex interplay. Behav Brain Res. 1996;80:33–40. doi: 10.1016/0166-4328(96)00016-2. [DOI] [PubMed] [Google Scholar]
- Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- Threadgill DW, Yee D, Matin A, Nadeau JH, Magnuson T. Genealogy of the 129 inbred strains: 129/SvJ is a contaminated inbred strain. Mamm Genome. 1997;8:390–393. doi: 10.1007/s003359900453. [DOI] [PubMed] [Google Scholar]
- Trullas R, Skolnick P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology. 1993;111:323–331. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- Turri MG, Datta SR, DeFries J, Henderson ND, Flint J. QTL analysis identifies multiple behavioral dimensions in ethological tests of anxiety in laboratory mice. Curr Biol. 2001a;11:725–734. doi: 10.1016/s0960-9822(01)00206-8. [DOI] [PubMed] [Google Scholar]
- Turri MG, Henderson ND, DeFries JC, Flint J. Quantitative trait locus mapping in laboratory mice derived from a replicated selection experiment for open-field activity. Genetics. 2001b;158:1217–1226. doi: 10.1093/genetics/158.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]


