Abstract
Background
Survival of ABO-mismatched kidneys with stable renal function despite the persistence of anti-ABO antibodies is called accommodation. The mechanism of accommodation is unclear, but may involve complement regulatory proteins such as CD59. The development of alpha-1,3-Galactosyltransferase knock-out (GalT-KO) swine that produce anti-Gal antibodies provides a large animal model capable of determining the role of complement regulatory proteins in accommodation.
Methods
ELISA and antibody FACS were used to examine the rate of anti-Gal antibody expression as a function of age. MHC-matched kidneys were transplanted from Gal-positive MGH miniature swine to MGH GalT-KO swine with systemic immunosuppression. One recipient underwent adsorbtion of anti-Gal antibodies prior to transplantation. Graft survival, antibody and complement deposition patterns and CD59 expression were determined.
Results
Three animals rejected Gal-positive kidneys via humoral mechanisms. One animal with low titers of anti-Gal Ab displayed spontaneous accommodation and the animal that was treated with Ab adsorbtion also displayed accommodation. Rejected grafts had deposition of IgM, IgG, C3 and C5b-9 with low expression of CD59, while accommodated grafts had low deposition of C5b-9 and high expression of CD59. Re-transplantation of one accommodated graft to a naïve GalT-KO animal confirmed that changes in the graft were responsible for the lack of C5b-9 deposition.
Conclusion
GalT-KO miniature swine produce anti-Gal antibodies and titers increase with age. These anti-Gal antibodies can cause rejection of MHC matched kidneys unless accommodation occurs. CD59 upregulation appears to be involved in the mechanism of accommodation by preventing the formation of the MAC on the accommodated graft.
Keywords: accommodation, CD59, swine, Gal
Introduction
In 1981, due to an error in the initial reporting of a donor blood type, Guy Alexandre and colleagues transplanted a kidney from an A1 blood donor to an O recipient. Despite a progressive rise in the anti-A antibodies after the transplant, the recipient maintained stable renal function (1). This phenomenon of stable organ function despite the presence of antibodies reactive with antigens on the graft is called “accommodation”. The initial observation by Alexandre and colleagues led to the development of protocols designed to induce accommodation across ABO incompatible barriers. Although this procedure has now gained widespread acceptance (2-4), the mechanism of accommodation is not well defined.
In 2006, the American Society of Transplantation sponsored a symposium on B cell immunity in transplantation. At this meeting, accommodation was agreed to have occurred if C4d was detected on the blood vessels, but the function and structure of the graft were relatively normal (5). Several potential mechanisms of accommodation have been proposed and may be divided into changes in the host environment and changes in the graft. Alterations in the host environment that have been proposed include that after initial depletion, the antibodies that return to the circulation may differ in affinity or specificity (6), possibly due to isotype shifting to IgG2 (5), which are thought to bind complement less efficiently. Changes in the graft that could confer protection include alteration of the epitopes recognized by the antibodies or upregulation of protective genes that prevent apoptosis or molecules, such as complement regulatory proteins, that would protect the cells from damage (6).
Models in rodents have been used to examine the contribution of protective genes or antibody production to the mechanism of accommodation. However, an examination of the response of complement regulatory proteins in these models is hindered by the presence of complement receptor 1-related gene/protein y (Crry), that has actions of both CD55 and CD46 and is unique to the mouse and rat (7). Pigs, however, express CD46, CD55 and CD59, but not Crry, which is analogous to humans and would allow direct translation of these findings to clinical allogeneic and xenogeneic transplantation.
Over the course of evolution, humans and Old World monkeys lost the function of alpha-1,3-Galactosyltransferase (GalT) and as a result make antibodies against terminal alpha-1,3-galactose moieties due to exposure to Gal positive bacteria (8). These antibodies were the main obstacle to xenotransplantation of porcine organs until GalT knockout swine were developed in 2004 from our most inbred strain of MHC defined miniature swine (9, 10). Although our lab has previously demonstrated that these animals produce anti-Gal antibodies (11), the level of antibody production relative to age was not well characterized. However, since anti-Gal antibodies share many characteristics with antibodies that react with human blood group antigens, these animals are well-suited to develop a model of accommodation in a large animal model. The existence of MHC defined haplotypes in this herd (12) uniquely allow us to perform Gal mismatched transplants with varying degrees of T cell help depending on the MHC matching.
In this study, we show that GalT-KO animals reject MHC matched Gal-positive kidneys due to the anti-Gal antibody response. We then used this model to determine if accommodation to renal allografts would occur across the Gal barrier and to investigate the mechanism of accommodation in a large animal model.
Materials and Methods
Animals
Transplant donors (SLAdd) were selected from our herd of partially inbred miniature swine. The immunogenetic characteristics of this herd and of the intra-MHC recombinant haplotypes have been described previously (13). Transplant recipients were SLAdd GalT-KO animals aged 8 to 24 months. The generation of these animals has been described in detail previously (9, 10). All animals were cared for according to the guidelines of the Massachusetts General Hospital Institutional Animal Care and Use Committee.
Kidney transplantation
The surgical procedures for primary and retransplantation in miniature swine have been previously described in detail (14). Briefly, kidneys from Gal positive donors were removed and perfused with cold Eurocolins solution. The recipients were unilaterally nephrectomized and the MHC matched, Gal positive kidney was anastomosed to the recipient aorta and vena cava. The ureter on the remaining native kidney was ligated with two silk sutures so that the only functional graft was the transplanted kidney.
Immunosuppression and Rejection monitoring
FK506 was generously provided by Fujisawa Healthcare, Inc. (Deerfield, IL) and administered for 12 days by continuous infusion starting on the day of renal transplant at a dose of 0.15mg/kg/day and subsequently adjusted to maintain blood levels between 30 and 40 ng/ml. Whole blood levels were determined by microparticle enzyme immunoassay (Tacrolimus II, IMX System. Abbot Labs) and the results were expressed in ng/ml. Rejection was monitored by daily serum creatinine levels. Open wedge biopsies were taken at 1 hour, 7, 14 and 28 days or when clinically indicated by a rise in serum creatinine levels.
In vivo depletion of anti-Gal antibodies
Anti-Gal antibodies were depleted in one animal by a process that has been previously reported by out lab (15). Briefly, plasma was separated from cellular components by a COBESpectra pheresis unit (Blood Component Technology, Inc., Lakewood, CO, USA) after anticoagulation with citrate dextrose solution and passed through a Gal affinity column (Alberta Research Council, Edmonton, Alta, Canada) for 4 hours. Adsorption of anti-Gal antibodies was confirmed by elution of the antibodies from the column after pheresis.
Histology
Biopsy specimens were fixed in 1% formaldehyde, embedded in paraffin, and subsequently sectioned. Tissues were then stained using either hematoxylin and eosin (H&E), or periodic acid-Schiff (PAS) stains. Allograft rejection was scored by standard pathologic criteria according to the Cooperative Clinical Trials in Transplantation Criteria (16).
Immunohistochemistry
Immunohistochemical staining for anti-donor immunoglobulin (Ig)M and IgG deposition in renal allografts was examined by fluorescence microscopy using frozen sections stained with saturating concentrations of fluorescent isothiocyante-labeled goat anti-swine IgM or IgG as previously described (14). Complement deposition on the grafts was determined by staining with rabbit anti-human C3b (Biogenesis) and mouse anti-human C5b-9 (DAKO). Staining for CD59 was performed using mouse anti-swine CD59 (MEL) that was generously provided by Dr. B. Paul Morgan of the University of Cardiff, UK (17).
Preparation of PBL
Freshly heparinized whole blood was diluted approximately 1:2 with Hank’s Balanced Salt Solution (HBSS, GIBCO BRL, Grand Island, NY) and the mononuclear cells were obtained by gradient centrifugation using Histopaque 1077 (Sigma). The mononuclear cells were washed once with HBSS, and contaminating red cells were lysed with ammonium chloride potassium (ACK) buffer (Lonza BioWhittaker, Walkersville, MD). Cells were then washed with HBSS and re-suspended in tissue culture medium. All cell suspensions were kept at 4°C until used in cellular assays.
Flow Cytometry
The presence of anti-Gal antibodies in the serum of experimental swine was detected by indirect flow cytometry. Fluorescence-activated cell sorting (FACS) was performed using a Becton Dickinson FACScan microfluorimeter (Sunnyvale, CA) and recombinant SLA PBL to determine the SLA-binding specificity of the antibody. Fluorescein isothiocyanate (FITC) labeled goat anti-swine IgM or IgG polyclonal antibodies were used as secondary reagents (Kirkegaard & Perry Laboratories Inc., Gaithersburg, MD). Staining was performed with 1×106 of donor-type PBL resuspended in 100 μl of HBSS (Life Technologies, Grand Island, NY) containing 0.1% bovine serum albumin (BSA) and 0.05% NaN3. Cells were incubated for 30 minutes at 4°C with 10 μl decomplemented test sera and after two washes a saturating concentration of FITC-labeled goat anti-swine IgM or IgG was added and incubated for 30 minutes at 4°C. After a final wash, cells were analyzed by flow cytometry using propidium iodide gating to exclude dead cells.
ELISA
This procedure has been described in detail (18). Briefly, 96 well plates were coated with 5μg/mL bovine serum albumin (BSA)-Gal (Alberta Research Council, Edmonton, Alberta, Canada) and blocked with 0.45% fish gelatin/ 45% Fish Gelatin in PBS/Tween (Sigma). Porcine serum was diluted to 2% and then serial 5-fold dilutions were made. The plates were then incubated with horseradish peroxidase-conjugated goat anti-swine IgM and IgG antibodies (Accurate Chemical QRL041402 and Accurate Chemical QRL041403) and incubated at 37 degrees for 1 hour. Color development was achieved with the addition of ABTS (Kirkegaard & Perry Lab, Inc.) followed by 13 minute incubation in the dark. 1% SDS was added to stop the reaction, and the plates were read using a Victor2 1420 multilabel counter (Perkins Elmer, USA) at 405 nm.
Cytotoxicity
CDC of recipient sera, in twofold dilutions from 1:2 to 1:256 in M199 with 2% FBS, against Gal positive PBL was measured using the fluorescent viability stain 7-actinoaminomycin D (7-AAD, Sigma). Analysis of cells stained with 7-AAD was collected for 5×10^3 cells per sample using a FACScan on a 256-channel logarithmic scale as previously described (19).
Results
GalTKO animals make anti-Gal Ab and titers increase with age
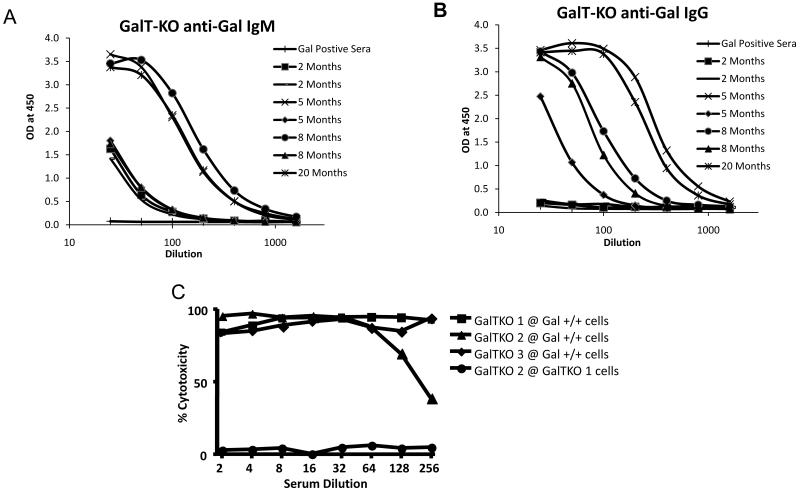
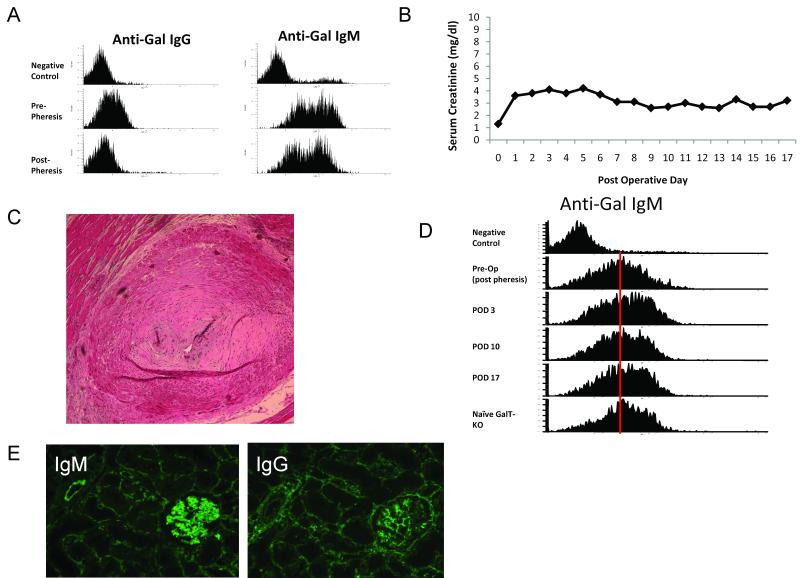
Serum was collected from young animals and breeders from our herd of GalT-KO miniature swine. Anti-Gal ELISA assays demonstrated that young pigs had low-levels of anti-Gal IgM even at two months of age, but did not produce detectable levels of anti-Gal IgG at this age (Fig 1a,b). However, by 5 months of age, the animals began producing a significant amount of anti-Gal IgG as well as increased amounts of anti-Gal IgM.
Figure 1.
Production of anti-Gal antibodies by GalT-KO swine increases with age. ELISA assays demonstrated that GalT-KO animals produced low-levels of anti-Gal IgM as early as 2 months of age, with the titers increasing at around 5 months of age (A). The level of anti-Gal IgG was not detectable until about 5 months of age and increased rapidly thereafter (B). These antibodies were cytotoxic at high titers to MHC matched Gal positive PBMC, while they displayed no cytotoxic activity to PBMC from other MHC-matched GalT-KO animals (C). Gal positive sera indicates negative control sera taken from a Gal positive miniature swine, which does not contain anti-Gal antibodies.
Porcine anti-Gal Ab are cytotoxic to MHC matched porcine PBL
To confirm that the anti-Gal antibodies were cytotoxic and could be expected to mediate rejection in this model, we performed a cytotoxic assay using sera from aged GalT-KO swine against MHC-matched, but Gal positive PBL (Figure 1c). The anti-Gal antibodies were highly cytotoxic, even at a 1:256 dilution. To confirm that the cytotoxic antibodies were in fact reacting with the Gal antigen, we tested the sera of one GalT-KO animal against the PBL of another GalT-KO in the same assay. The sera of the GalT-KO animal displayed no cytotoxic activity against GalT-KO cells, confirming the specificity of the cytotoxic antibodies.
MHC matched Gal-positive kidney transplantation to GalT-KO miniature swine
The MGH miniature swine model of kidney transplantation has been well-established with highly reproducible results (20, 21). It has been shown that MHC matched kidneys are accepted approximately 66% of the time even if immunosuppression is not given (22). However, we decided to administer FK506 by continuous infusion to achieve a dose that has been shown to uniformly induce tolerance across full MHC mismatched barriers (23) in order to completely eliminate any contribution of T cells to rejection and ensure any renal dysfunction was due to antibody mediated rejection alone.
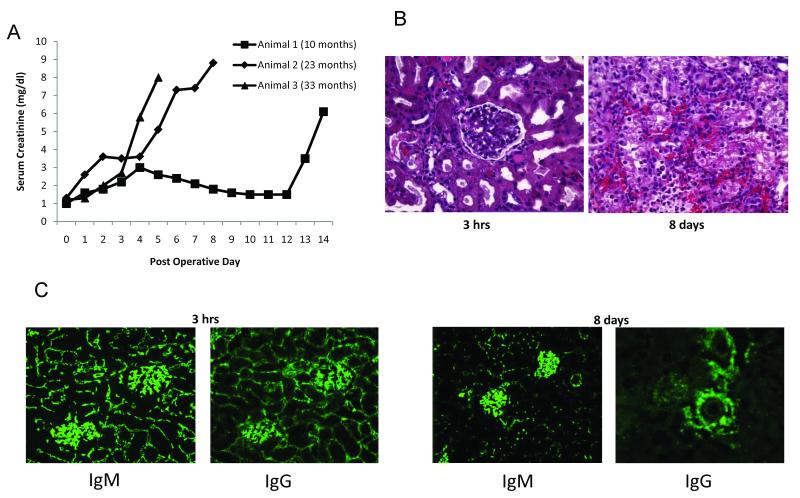
GalT-KO animals rejected Gal positive kidneys by acute humoral mechanisms
We transplanted Gal positive MHC matched kidneys to 3 animals aged 10 months (Animal 1), 23 months (Animal 2) and 33 months (Animal 3), all of which had detectable levels of anti-Gal IgM and IgG. All 3 animals experienced rejection of the Gal positive kidney, with more severe rejection of the ureter than the kidney itself. The grafts from animal 1 and 2 were removed, while in animal 3, we left the graft in place but removed the ligature around the naïve ureter to restore renal function. The rate of rejection was related to the age of the animals, with the older animals rejecting their grafts at an earlier time point (Fig 2a). Histology of the rejected grafts revealed diffuse hemorrhage with a neutrophil infiltrate, consistent with humoral rejection (Figure 2b). Immunohistochemistry showed diffuse deposition of both IgM and IgG at 3 hours after transplantation and the time of rejection (Figure 2c). Diffuse deposition of complement, both C3 and C5b-9 (MAC) was seen at these time points and also on the animal that rejected the graft at day 14 (Figure 2d), indicating the complement cascade was not interrupted.
Figure 2.
Anti-Gal antibodies reject MHC-matched Gal positive kidneys via humoral mechanisms. Following Gal+ kidney transplantation with FK506 administration, creatinine levels rose rapidly in the post-operative period (A). Light microscopy revealed focal hemorrhagic changes and neutrophil infiltration as early as 3 hours post-transplant, with advanced hemorrhagic changes by day 8 (B). Immunohistochemistry showed dense deposition of IgM and IgG antibodies at 3hrs and 8 days post-transplantation (C). The presence of IgM and IgG was associated with deposition of complement C3 and C5b-9 (MAC) in the rejected grafts (D).
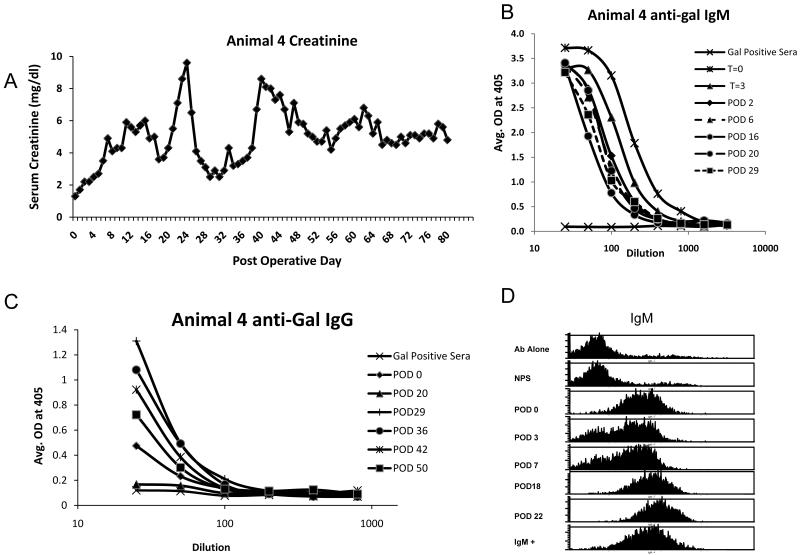
Spontaneous accommodation in a young animal
Animal four, a GalT-KO pig 10 months of age, also received a Gal positive kidney with 12 days of FK506. This animal was notable for a low level of anti-Gal IgG relative to the previous 3 animals. Despite an early period of elevated creatinine, the animal did not reject its graft and maintained a stable creatinine at approximately 5 mg/dl from day 50 to 80, when the graft was removed (Figure 3a). The animal’s creatinine stabilized despite the persistence of cytotoxic anti-Gal IgM in the serum as determined by FACS and ELISA and anti-Gal IgG that decreased in the initial post-operative period, but returned to greater than baseline levels soon thereafter (Fig 3b,c). Antibody FACS also showed the persistence of anti-Gal antibodies in the recipient serum post-operatively (Fig 3d). The high baseline creatinine level was therefore likely due to the initial damage caused by the anti-Gal antibodies before the changes involved in accommodation occurred.
Figure 3.
Spontaneous accommodation in one animal. After Gal+ kidney transplant, Animal 4 had an initial rise in serum creatinine, but did not reject the graft. Creatinine stabilized in the range of 5-6 mg/dl (A). Animal 4 maintained detectable levels of anti-Gal IgM as determined by ELISA throughout the post-operative period. T=0 indicates sera taken just before kidney revascularization, T=3 indicates sera drawn 3 hours after kidney revascularization. All other sera were drawn at the indicated post-operative day (POD) (B). Although ELISA showed that the level of anti-Gal IgG decreased in the early post-operative period, it rebounded by day 29 and remained detectable for the rest of the post-operative period (C). Antibody FACS also showed that the recipient sera had antibodies to MHC matched, but Gal positive PBMC, in the post-operative period (D).
Pre-transplant depletion of anti-Gal antibodies resulted in stable renal function
Two pheresis procedures were performed on Animal 5, a GalT-KO animal aged 27 months, prior to Gal positive kidney transplantation. The levels of anti-Gal IgM and IgG decreased as determined by FACS and ELISA, but were not completely eliminated (Figure 4a). After the transplant, the level of anti-Gal antibodies remained stable, though slightly below pre-pheresis levels. This animal maintained stable renal function until day 17 (Figure 4b), when the animal developed heart failure and was sacrificed. Histology revealed concentric hyperplasia of the coronary arteries (Fig 4c), consistent with the findings of FK506 toxicity that has been reported in rats (24) and monkeys (25). FACS revealed that this animal also maintained anti-Gal antibodies in its sera throughout the post-transplantation period (Figure 4d) with deposition of IgM and IgG seen at day 17 (Figure 4e). The maintenance of stable renal function despite persistence of anti-Gal antibodies in the serum suggests that accommodation had been achieved.
Figure 4.
Accommodation after pre-transplant immunoadsorbtion. One animal was treated with plasma immunoadsorbtion through a Gal polymer column. After pheresis, anti-Gal IgM and IgG decreased as shown by antibody FACS (A). Following Gal positive kidney transplantation, the animal maintained a stable creatinine level (B). At day 17, the animal developed heart failure and histology showed intimal changes in the coronary artery consistent with FK506 toxicity (H&E, 100×, C). Antibody FACS with post-transplant serum in this animal showed that anti-Gal antibodies persisted throughout the post-operative period (D). Immunohistochemical analysis of the graft at day 17 showed deposition of IgM and IgG antibodies (E).
Lack of membrane attack complex deposition on accommodated grafts
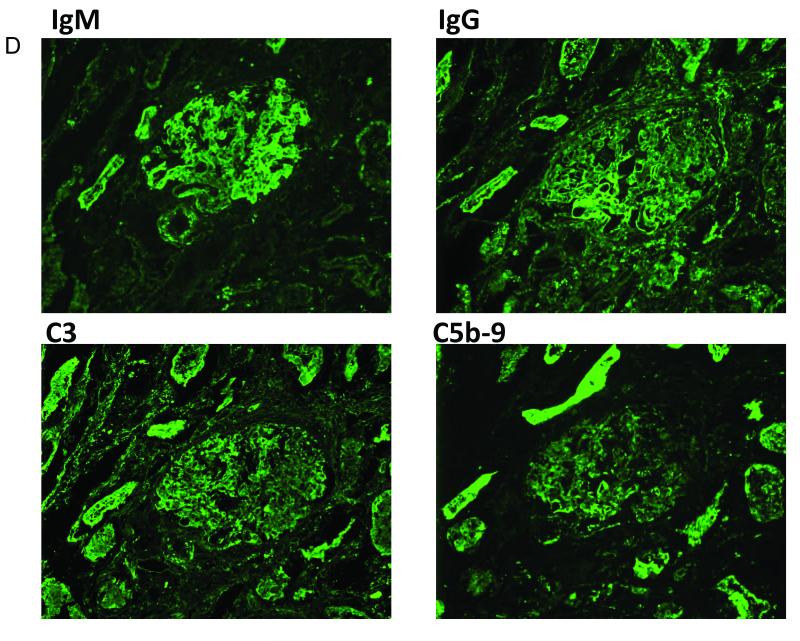
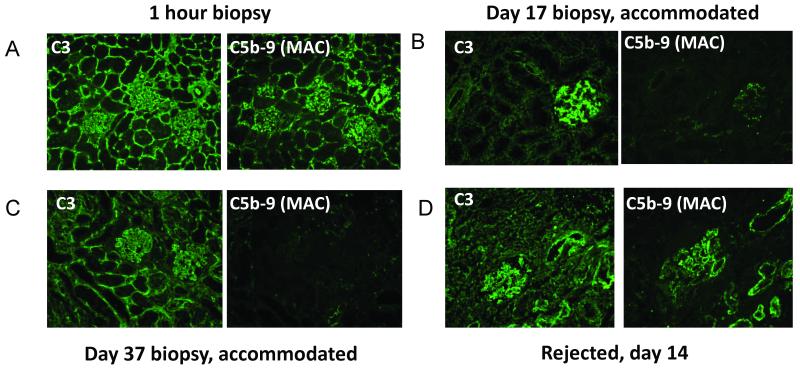
An analysis of the graft that displayed spontaneous accommodation (Animal 4) and the animal that had pre-transplant pheresis (Animal 5) showed that at the 1 hour post-reperfusion biopsies, the deposition of IgM and IgG on the grafts was associated with dense deposits of both C3 and the MAC (Figure 5a), which was also observed in the grafts that rejected. However, by POD 10 for animal 4 and POD 17 for animal 5, although the C3 deposition remained, there was almost no MAC deposition found in the graft (Figure 5b). This complement deposition pattern remained at days 37 and 80 in Animal 4. In the animals that rejected their grafts, both C3 and MAC deposition was seen at the time of rejection (Figure 5c). These data suggest that a disruption of the complement cascade was involved in protecting the graft and the mechanism of accommodation.
Figure 5.
Complement deposition pattern in accommodated grafts. 1 hour biopsies showed deposition of complement C3 and C5b-9 in all grafts (A). Accommodated grafts had persistent depositions of complement C3, but minimal deposition of C5b-9 at day 17 (B) and 37 (C), while rejected kidneys showed continued deposition of both C3 and C5b-9 (D, day 14).
Increased expression of CD59 on accommodated grafts
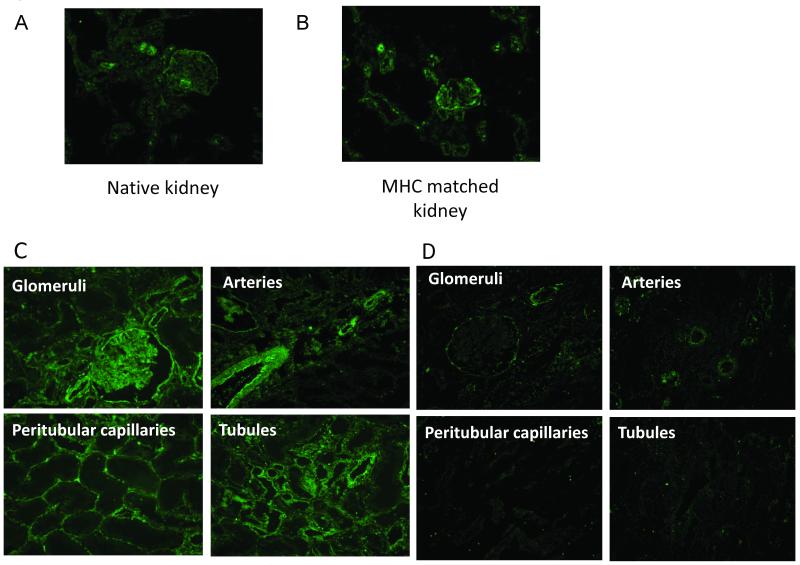
Since CD59 is known to prevent the formation of the MAC, but should not affect the deposition of C3 or C4, we examined if increased expression of this complement regulatory protein was associated with decreased deposition of MAC on accommodated grafts. Since this antigen is constitutively expressed, we first examined the expression on native kidneys and on kidneys that were transplanted across MHC matched barriers to Gal positive recipients and therefore did not have antibody deposition. We found no difference in the expression of CD59 between the native kidneys and the MHC matched Gal positive to Gal positive transplanted kidneys (Figure 6a,b). When we examined biopsy specimens from the accommodated grafts, we found that at 1 hour after transplantation, the expression of CD59 was similar to native kidneys. However, by day 10, the expression of CD59 was significantly elevated on the glomeruli, tubules, peritubular capillaries and arteries and remained elevated throughout the post-transplantation period (Figure 6c). To confirm that these changes were specific to accommodated grafts, we examined the expression of CD59 on the rejected kidneys. These grafts did not have an upregulation of CD59, even in the kidney that rejected at day 14 (Figure 6d), demonstrating that the upregulation of CD59 was specific to accommodated kidneys.
Figure 6.
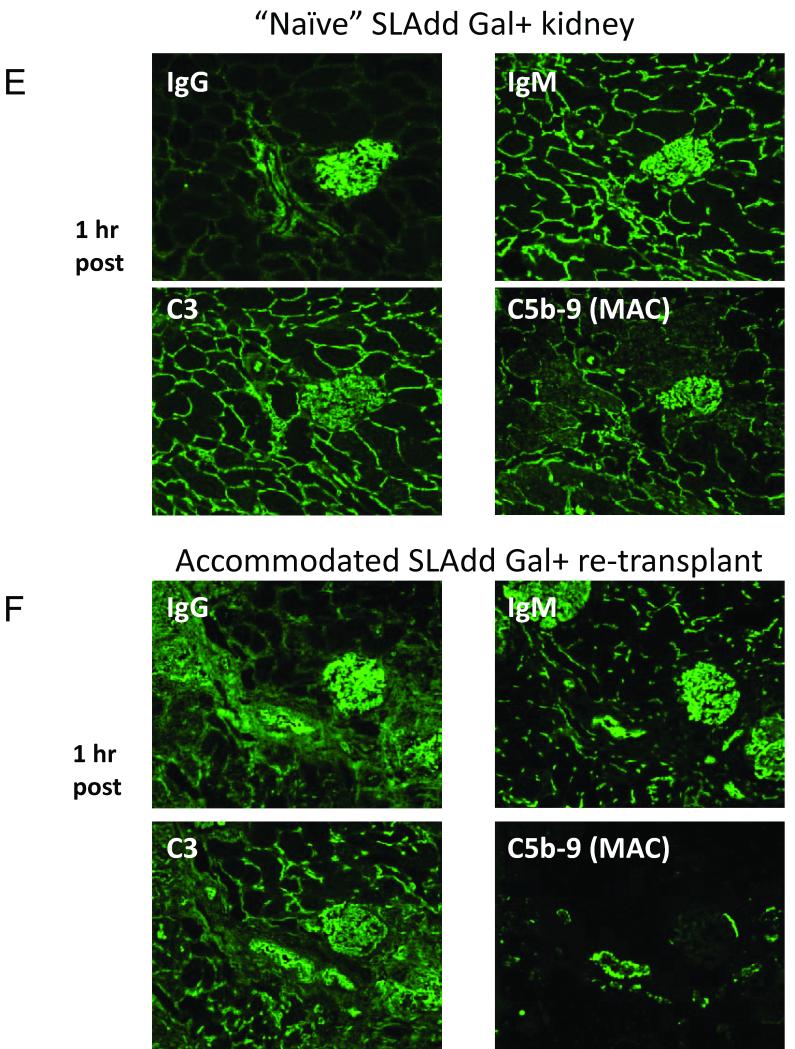
Analysis of CD59 expression on transplanted kidneys. To control for normal CD59 expression, we stained native kidneys and a kidney transplanted across an MHC matched barrier into a Gal positive animal (i.e. no anti-Gal antibodies involved). CD59 expression in the native kidney (A) was the same as in the MHC matched kidney transplanted to a Gal positive recipient (B). Ten days after transplantation to a GalT-KO animal, accommodated kidneys showed increased expression of CD59 (C). Gal positive kidneys that rejected did not show increased expression of CD59 even at day 14 (D). In order to confirm that CD59 expression was responsible for decreased C5b-9 deposition, we removed the spontaneously accommodated graft from the original recipient and re-transplanted it to a naïve GalT-KO animal along with a fresh Gal positive graft. The fresh graft showed deposition of IgM, IgG, C3 and C5b-9 at 1 hour (E). At the same time point, the accommodated graft showed deposition of IgM, IgG and C3 at similar levels to the fresh graft, but C5b-9 deposition was markedly decreased relative to the fresh graft (F).
CD59 may be responsible for decreased deposition of MAC
To confirm that the upregulation of CD59 was responsible for the decreased deposition of MAC on accommodated kidneys, and not due to an alteration in the level or affinity of complement in the animals, we removed the accommodated kidney from Animal 4 at day 80 and re-transplanted the graft, along with a naïve MHC matched Gal positive kidney, to animal 6. The biopsies of the kidneys at 1 hour after re-perfusion showed deposition of IgM, IgG and C3 on both grafts (Figure 6e,f). However, while the naïve kidney had diffuse deposition of MAC, the accommodated kidney had very low deposition of MAC, indicating that the lack of deposition in Animal 4 was due to a change in the graft, and not due to an alteration in the host. Thus, the protection from complement-mediated cell lysis in the accommodated graft appears to be due to upregulation of CD59 by the kidney that is specific to accommodated grafts.
Discussion
The use of ABO incompatible transplants has become widespread since the serendipitous result of Alexandre and colleagues in 1981 (1). Here, we report the development of a large animal model of accommodation that additionally allows for selective transplantation MHC matched or mismatched kidneys. While the anti-Gal antibody titers in the older recipients in this series were high enough to reject MHC matched kidneys despite the lack of T cell help, one young animal with low IgG titers of anti-Gal antibodies accepted its Gal positive kidney long-term. In a protocol that is similar to current ABOi protocols in humans, an older animal that underwent two pre-transplant adsorbtions of anti-Gal antibodies accepted a kidney with stable renal function for 17 days when it died due to complications related to FK506. Both grafts that maintained function had a pattern of complement deposition, notably the lack of MAC deposits, which was unique to accommodated grafts and was not seen on the grafts that rejected or on Gal positive grafts that were transplanted to Gal positive animals. When we analyzed the expression of CD59 on the accommodated grafts, we found it was significantly upregulated, while there was no increased expression of CD59 on the rejected grafts. This upregulation prevented MAC deposition in one accommodated graft that was re-transplanted to a naïve GalT-KO animal to determine if alterations in complement or antibodies in the first recipient were contributing to the pattern of complement deposition after the primary transplant. A fresh graft transplanted to this same animal had IgM, IgG, C3 and MAC deposition at 1 hour. The accommodated graft, while having strong deposition of IgM, IgG and C3, indicating no downregulation of Gal expression, had minimal MAC deposition demonstrating a disruption of the complement cascade due to alterations in the graft and not due to host factors.
A recent report describing accommodation to anti-Gal antibodies in a rodent model of heart grafts reported the upregulation of three complement regulatory proteins: CD55, CD59 and Crry (26). Although we were not able to examine the expression of CD46 and CD55 in the grafts because of the lack of an appropriate antibody for swine, the pattern of complement deposition suggests that they did not play a significant role in the protection of the grafts from complement damage. High levels of CD55 would be expected to result in decreased deposition of C3 due to its ability to decrease the formation and increase the degradation of C3 convertase (27). Likewise, since CD46 is a cofactor for the cleavage of C3b and C4b, it is also unlikely that its expression is substantially involved in producing the complement deposition pattern that was observed in our study (28). The differences in complement regulatory protein expression may be due likewise to species differences between rodents, which have a several different complement regulatory genes, and our large animal model (27). Furthermore, since the pig has been identified as the most appropriate donor species for xenotransplantation, the finding that porcine grafts upregulate CD59 in accommodation has particular implications for that field.
CD59 is an 18-kD cell surface protein that inhibits the formation of the MAC, and has been shown to inhibit complement-mediated lysis of glomerular epithelial cells both in vitro and in vivo (29). Although it is constitutively expressed on the endothelium, studies have shown that ligation of α-Gal by B. simplicifolia lectin I isolectin B4 upregulated the expression of CD59 on porcine endothelial cells and induced resistance to complement mediated lysis (30). Others have examined the ability of porcine CD59 to inhibit human complement mediated cell lysis to determine the potential for this protein to mediate accommodation in xenotransplantation. One study examined the expression of porcine CD59 and CD46, (membrane co-factor protein, which degrades C3b and C4b (31)) on pig aortic endothelial cells (PAEC) during culture (32) and found that CD59 was down-regulated after 5 passages, while CD46 expression doubled. The PAEC became more susceptible to lysis by human complement during culture as CD59 expression decreased. Further highlighting its importance to inhibiting complement-mediated lysis, blocking antibodies to CD59, but not CD46, added to cells in early passages increased the susceptibility of PAEC to human complement-mediated lysis. Additionally, mouse heart grafts that expressed high levels of either human or pig CD59 were found to be protected against human complement mediated injury with improved function (33). These data suggest that elevated CD59 expression could protect both allografts and xenografts from MAC induced cell lysis and mediate accommodation. In vitro studies have been performed to test this hypothesis using human sera containing anti-pig antibodies and human complement (30). These studies demonstrated that following a short period of B. Simplificolia lectin I stimulation, protection against complement-mediated lysis seemed to be dependent on increased expression of CD59 and this protection was reversed after removal of CD59 from the cell surface following cleavage via phosphatidylinositol-specific phospholipase. However, following prolonged stimulation with B. Simplificolia lectin I, sensitivity to complement lysis was not restored following CD59 removal with phosphatidylinositol-specific phospholipase, suggesting an alternate and perhaps complementary pathway of protection against complement that was still associated with upregulation of CD59. Other groups have reported that in humans, accommodation is associated with upregulation and downregulation of multiple genes (34), suggesting that accommodation may involve several pathways.
In this study, we observed strong deposition of MAC at 1 hour in all grafts. In kidneys that went on to demonstrate accommodation, however, the deposition of MAC decreased at later time points. This deposition of MAC at 1 hour is likely due to the low-level of expression of CD59 that exists at the time the graft is implanted and reperfused. As shown in Figure 6a, although CD59 is constitutively expressed in the porcine kidney, it is not expressed at high levels. Thus, the deposition of MAC was not greatly inhibited in the 1 hour specimens. Other groups have studied the kinetics of CD59 upregulation following αGal ligation by B. Simplificolia lectin I and found increased expression on the cell surface at 1 hour, followed by increased mRNA levels at approximately 4 hours (30). Other groups have shown that cells that survive complement mediated injury remove cell membrane bound MAC via ectocytosis (35) (36) (37). In accommodated grafts, deposition of MAC markedly decreased by POD 10 concomitant with a rise in the expression of CD59. This was likely due to both loss of previously deposited MAC via ectocytosis and prevention of new deposition of MAC via upregulation of CD59.
The development of the GalT-KO animal represents a significant step forward for xenotransplantation. However, the importance of preformed xenoreactive antibodies to non-Gal antigens remains to be determined. Previous studies have suggested that if the response of anti-Gal antibodies was eliminated, that grafts expressing high levels of complement regulatory proteins survived longer than wild-type pig organs (38) (39) (40) (41) (42). Since the major target or targets of non-Gal antibodies has not been determined, immunoadsorbtion of the xenoreactive antibodies seems impractical at this time. Thus, the development of a GalT-KO pig that has over expression of CD59 could result xenograft accommodation to non-Gal antibodies. This, combined with a strategy for T cell tolerance, may make clinical xenotransplantation a reality.
In summary, this study demonstrates that porcine kidney grafts demonstrate accommodation to anti-Gal antibodies. These accommodated grafts have increased expression of CD59 accompanied by decreased expression of MAC. This suggests that GalT-KO grafts that overexpress either porcine or human CD59 may show accommodation to xenoreactive non-Gal antibodies.
ACKNOWLEDGMENTS
The authors would like to thank Fujisawa Healthcare, Inc (Deerfield, IL) for generously providing FK506 and Dr. B. Paul Morgan of the University of Cardiff, UK for generously providing the anti-swine CD59 antibody. We would also like to thank Drs. David Sachs and Isabel Hanekamp for their helpful advice and for review of this manuscript. This work was supported by the NIH Program Project 5PO1-A145897 (Project 1) and the Japanese Society for the Promotion of Science, Grant-in-Aid for Scientific Research (A-19209043) and Adam Griesemer received support from the American Society of Transplantation/Juvenile Diabetes Research Foundation Fellowship.
Abbreviations
- Ab
antibody
- ACK
ammonium chloride potassium lysing
- BSA
bovine serum albumin ()
- ELISA
Enzyme-Linked ImmunoSorbent Assay
- FACS
fluorescence activated cell sorting
- FITC
Fluorescein isothiocyanate
- GalT-KO
Galactosyltransferase knock-out
- H&E
hematoxylin and eosin
- HBSS
Hank’s Balanced Salt Solution
- HRP
horseradish peroxidase; Ig, immunoglobulin
- MHC
major histocompatibility complex
- PBL
peripheral blood lymphocytes
- PBS
phosphate-buffered saline
- POD
post-operative day
- SLA
swine leukocyte antigen
References
- 1.Alexandre GP. From ABO-incompatible human kidney transplantation to xenotransplantation. Xenotransplantation. 2004;11(3):233. doi: 10.1111/j.1399-3089.2004.00105.x. [DOI] [PubMed] [Google Scholar]
- 2.Warren DS, Zachary AA, Sonnenday CJ, et al. Successful renal transplantation across simultaneous ABO incompatible and positive crossmatch barriers. Am J Transplant. 2004;4(4):561. doi: 10.1111/j.1600-6143.2004.00364.x. [DOI] [PubMed] [Google Scholar]
- 3.Gloor JM, Lager DJ, Moore SB, et al. ABO-incompatible kidney transplantation using both A2 and non-A2 living donors. Transplantation. 2003;75(7):971. doi: 10.1097/01.TP.0000058226.39732.32. [DOI] [PubMed] [Google Scholar]
- 4.Tanabe K, Takahashi K, Sonda K, et al. Long-term results of ABO-incompatible living kidney transplantation: a single-center experience. Transplantation. 1998;65(2):224. doi: 10.1097/00007890-199801270-00014. [DOI] [PubMed] [Google Scholar]
- 5.Kirk AD, Baldwin WM, Cascalho MI, Chong AS, Sykes M, West LJ. American society of transplantation symposium on B cells in transplantation: harnessing humoral immunity from rodent models to clinical practice. Am J Transplant. 2007;7(6):1464. doi: 10.1111/j.1600-6143.2007.01815.x. [DOI] [PubMed] [Google Scholar]
- 6.Platt JL, Vercellotti GM, Dalmasso AP, et al. Transplantation of discordant xenografts: A review of progress. Immunol.Today. 1990;11:450. doi: 10.1016/0167-5699(90)90174-8. [DOI] [PubMed] [Google Scholar]
- 7.Song WC. Complement regulatory proteins and autoimmunity. Autoimmunity. 2006;39(5):403. doi: 10.1080/08916930600739647. [DOI] [PubMed] [Google Scholar]
- 8.Yamada K, Griesemer AD, Okumi M. Pigs as xenogeneic donors. Transplantation Reviews. 2005;19(3):164. [Google Scholar]
- 9.Lai L, Kolber-Simonds D, Park KW, et al. Production of alpha-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002;295(5557):1089. doi: 10.1126/science.1068228. [DOI] [PubMed] [Google Scholar]
- 10.Kolber-Simonds D, Siangxue L, Watt SR, Denaro M, et al. Production of · 1,3-galactosyltransferase null pigs via nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. PNAS. 2004 doi: 10.1073/pnas.0307819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dor FJ, Tseng YL, Cheng J, et al. alpha1,3-Galactosyltransferase gene-knockout miniature swine produce natural cytotoxic anti-Gal antibodies. Transplantation. 2004;78(1):15. doi: 10.1097/01.tp.0000130487.68051.eb. [DOI] [PubMed] [Google Scholar]
- 12.Sachs DH, Swindle MM, Moody DC, Phillips LD. Swine as Models in Biomedical Research. Vol. 1. Iowa State University Press; Ames, Iowa: 1992. MHC Homozygous Miniature Swine; p. 3. [Google Scholar]
- 13.Sachs DH, Leight G, Cone J, Schwartz S, Stuart L, Rosenberg S. Transplantation in miniature swine. I. Fixation of the major histocompatibility complex. Transplantation. 1976;22:559. doi: 10.1097/00007890-197612000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Yamada K, Gianello PR, Ierino FL, et al. Role of the thymus in transplantation tolerance in miniature swine. I. Requirement of the thymus for rapid and stable induction of tolerance to class I-mismatched renal allografts. J Exp Med. 1997;186(4):497. doi: 10.1084/jem.186.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watts A, Foley A, Awwad M, et al. Plasma perfusion by apheresis through a Gal immunoaffinity column successfully depletes anti-Gal antibody: experience with 320 aphereses in baboons. Xenotransplantation. 2000;7(3):181. doi: 10.1034/j.1399-3089.2000.00068.x. [DOI] [PubMed] [Google Scholar]
- 16.Colvin RB. The renal allograft biopsy. Kidney International. 1996;50:1069. doi: 10.1038/ki.1996.410. [DOI] [PubMed] [Google Scholar]
- 17.Hanna SM, Williams GT, Van Den Berg CW, Morgan BP. Characterization in vitro and in vivo of the pig analogue of human CD59 using new monoclonal antibodies. Immunology. 1998;95(3):450. doi: 10.1046/j.1365-2567.1998.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Y, Lorf T, Sablinski T, et al. Removal of anti-porcine natural antibodies from human and nonhuman primate plasma in vitro and in vivo by a Galalpha1-3Galbeta1-4betaGlc-X immunoaffinity column. Transplantation. 1998;65(2):172. doi: 10.1097/00007890-199801270-00005. [DOI] [PubMed] [Google Scholar]
- 19.Wong BS, Yamada K, Okumi M, et al. Allosensitization does not increase the risk of xenoreactivity to alpha1,3-galactosyltransferase gene-knockout miniature swine in patients on transplantation waiting lists. Transplantation. 2006;82(3):314. doi: 10.1097/01.tp.0000228907.12073.0b. [DOI] [PubMed] [Google Scholar]
- 20.Utsugi R, Barth RN, Lee RS, et al. Induction of transplantation tolerance with a short course of tacrolimus (FK506): I. Rapid and stable tolerance to two-haplotype fully mhc-mismatched kidney allografts in miniature swine. Transplantation. 2001;71(10):1368. doi: 10.1097/00007890-200105270-00003. [DOI] [PubMed] [Google Scholar]
- 21.Gianello PR, Fishbein JM, Sachs DH. Tolerance to primarily vascularized allografts in miniature swine. Immunological Reviews. 1993;133:19. doi: 10.1111/j.1600-065x.1993.tb01508.x. [DOI] [PubMed] [Google Scholar]
- 22.Gianello P, Fishbein JM, Sachs DH. Tolerance to primarily vascularized allografts in miniature swine. Immunol.Rev. 1993;133:19. doi: 10.1111/j.1600-065x.1993.tb01508.x. [DOI] [PubMed] [Google Scholar]
- 23.Utsugi R, Barth RN, Kitamura H, Ambroz J, Sachs DH, Yamada K. Tolerance across a two-haplotype, fully MHC-mismatched barrier induced in miniature swine renal allografts treated with a 12-day course of tacrolimus. Transplant Proc. 2001;33(12):101. doi: 10.1016/s0041-1345(00)01925-4. [DOI] [PubMed] [Google Scholar]
- 24.Yamada K, Sugisaki Y, Suzuki S, Akimoto M, Yamanaka N. New morphological changes induced by FK506 in a short period in the rat kidney and the effect of superoxide dismutase and OKY-046 on them: the relationship of FK506 nephrotoxicity to lipid peroxidation and change in production of thromboxane A 2 in the kidney. Transpl.Int. 1992;5:564. doi: 10.1007/978-3-642-77423-2_166. [DOI] [PubMed] [Google Scholar]
- 25.Wijnen RM, Ericzon BG, Tiebosch AT, Buurman WA, Groth CG, Kootstra G. Toxicology of FK506 in the cynomolgus monkey: a clinical, biochemical, and histopathological study. Transpl Int. 1992;5(Suppl 1):S454. doi: 10.1007/978-3-642-77423-2_132. [DOI] [PubMed] [Google Scholar]
- 26.Ding JW, Zhou T, Ma L, et al. Expression of complement regulatory proteins in accommodated xenografts induced by anti-alpha-Gal IgG1 in a rat-to-mouse model. Am J Transplant. 2008;8(1):32. doi: 10.1111/j.1600-6143.2007.02016.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim DD, Song WC. Membrane complement regulatory proteins. Clin Immunol. 2006;118(23):127. doi: 10.1016/j.clim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Liszewski MK, Post TW, Atkinson JP. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 29.Rooney IA, Davies A, Griffiths D, et al. The complement-inhibiting protein, protectin (CD59 antigen), is present and functionally active on glomerular epithelial cells. Clin Exp Immunol. 1991;83(2):251. doi: 10.1111/j.1365-2249.1991.tb05623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grubbs BC, Benson BA, Dalmasso AP. Characteristics of CD59 up-regulation induced in porcine endothelial cells by alphaGal ligation and its association with protection from complement. Xenotransplantation. 2003;10(5):387. doi: 10.1034/j.1399-3089.2003.02088.x. [DOI] [PubMed] [Google Scholar]
- 31.Nangaku M. Complement regulatory proteins in glomerular diseases. Kidney Int. 1998;54(5):1419. doi: 10.1046/j.1523-1755.1998.00130.x. [DOI] [PubMed] [Google Scholar]
- 32.van den Berg CW, Rix C, Hanna SM, Perez de la Lastra JM, Morgan BP. Role and regulation of pig CD59 and membrane cofactor protein/CD46 expressed on pig aortic endothelial cells. Transplantation. 2000;70(4):667. doi: 10.1097/00007890-200008270-00022. [DOI] [PubMed] [Google Scholar]
- 33.Fisicaro N, Aminian A, Hinchliffe SJ, et al. The pig analogue of CD59 protects transgenic mouse hearts from injury by human complement. Transplantation. 2000;70(6):963. doi: 10.1097/00007890-200009270-00014. [DOI] [PubMed] [Google Scholar]
- 34.Park WD, Grande JP, Ninova D, et al. Accommodation in ABO-incompatible kidney allografts, a novel mechanism of self-protection against antibody-mediated injury. Am J Transplant. 2003;3(8):952. doi: 10.1034/j.1600-6143.2003.00179.x. [DOI] [PubMed] [Google Scholar]
- 35.Scolding NJ, Morgan BP, Houston WA, Linington C, Campbell AK, Compston DA. Vesicular removal by oligodendrocytes of membrane attack complexes formed by activated complement. Nature. 1989;339(6226):620. doi: 10.1038/339620a0. [DOI] [PubMed] [Google Scholar]
- 36.Pilzer D, Gasser O, Moskovich O, Schifferli JA, Fishelson Z. Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin Immunopathol. 2005;27(3):375. doi: 10.1007/s00281-005-0004-1. [DOI] [PubMed] [Google Scholar]
- 37.Morgan BP, Dankert JR, Esser AF. Recovery of human neutrophils from complement attack: removal of the membrane attack complex by endocytosis and exocytosis. J Immunol. 1987;138(1):246. [PubMed] [Google Scholar]
- 38.Lin SS, Hanaway MJ, Gonzalez-Stawinski GV, et al. The role of anti-Galalpha1-3Gal antibodies in acute vascular rejection and accommodation of xenografts. Transplantation. 2000;70(12):1667. doi: 10.1097/00007890-200012270-00002. [DOI] [PubMed] [Google Scholar]
- 39.Cowan PJ, Somerville CA, Shinkel TA, et al. High-level endothelial expression of human CD59 prolongs heart function in an ex vivo model of xenograft rejection. Transplantation. 1998;65(6):826. doi: 10.1097/00007890-199803270-00010. [DOI] [PubMed] [Google Scholar]
- 40.Fodor WL, Williams BL, Matis LA, et al. Expression of a functional human complement inhibitor in a transgenic pig as a model for the prevention of xenogeneic hyperacute organ rejection. Proc.Natl.Acad.Sci.U.S.A. 1994;91:11153. doi: 10.1073/pnas.91.23.11153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kroshus TJ, Bolman RM, III, Dalmasso AP, et al. Expression of human CD59 in transgenic pig organs enhances organ survival in an ex vivo xenogeneic perfusion model. Transplantation. 1996;61(10):1513. doi: 10.1097/00007890-199605270-00018. [DOI] [PubMed] [Google Scholar]
- 42.Byrne GW, McCurry KR, Martin MJ, McClellan SM, Platt JL, Logan JS. Transgenic pigs expressing human CD59 and decay-accelerating factor produce an intrinsic barrier to complement-mediated damage. Transplantation. 1997;63(1):149. doi: 10.1097/00007890-199701150-00027. [DOI] [PubMed] [Google Scholar]