Abstract
Background
Depressive symptoms and caregiving stress may contribute to cardiovascular disease (CVD) via chronic platelet activation; however, it remains unclear whether this elevated activation constitutes a trait or state marker. The primary objective was to investigate whether persistent depressive symptoms would relate to elevated platelet activation in response to acute psychological stress over a three-year period.
Methods
Depressive symptoms (Brief Symptom Inventory) were assessed among 99 spousal dementia caregivers (52–88 years). Platelet P-selectin expression was assessed in vivo using flow cytometry at three time-points over the course of an acute stress test: baseline, post-stress, and after 14 minutes of recovery. Two competing structural analytic models of depressive symptoms and platelet hyperactivity with three yearly assessments were compared.
Results
Although depressive symptoms were generally in the subclinical range, their persistent elevation was associated with heightened platelet reactivity and recovery at all three-years while the change in depressive symptoms from the previous year did not predict platelet activity.
Limitations
These results focus on caregivers providing consistent home care, while future studies may extend these results by modeling major caregiving stressors.
Conclusions
Enduring aspects of negative affect, even among those not suffering from clinical depression are related to hemostatic changes, in this case platelet reactivity, which might be one mechanism for previously reported increase in CVD risk among elderly Alzheimer caregivers.
Keywords: P-selectin, cell adhesion molecule, coagulation, cardiovascular disease, spousal dementia caregiving
Introduction
Substantial evidence demonstrates that depression is associated with heightened risk of cardiovascular events (Glassman et al., 2006, Barefoot and Schroll, 1996, Anda et al., 1993, Mausbach et al., 2007a) and all-cause mortality (Penninx et al., 1999, Schulz et al., 2000), independent of sociodemographic, behavioral, and medical factors (Musselman et al., 1998). Heightened platelet activation (i.e., hyperactivation) associated with depressive symptoms may represent a key physiological pathway underlying this link (Nemeroff and Musselman, 2000, von Känel, 2004); however, it remains unclear whether hyperactivation associated with affective distress constitutes a trait or state marker, or may extend to depressive symptoms that do not rise to the severity of clinical depression (Musselman et al., 2000, Piletz et al., 2000). Based on previous findings(Aschbacher et al., 2008), hyperactivation is broadly defined here to include both reactivity (initial magnitude of the increase) and recovery (a sustained increase). A rigid trait-state dichotomy may be oversimplified, given that the current theories of the genetics of depression and cardiovascular disease (CVD) highlight gene-environment interactions, where stressful life events can cause an underlying genetic diathesis to emerge (Lesch, 2004, Bondy, 2007). Nonetheless, negative affect may be composed of both transient fluctuations responding to external events and stable individual differences.
An older review from 1989 on negative affectivity, stress, and health examined the contradictory evidence and concluded, “NA [Negative Affectivity] is largely unrelated to objective indices of cardiac health, including risk factors for heart disease, coronary stenosis and other evidence for cardiac pathology, and heart-related mortality” (Watson and Pennebaker, 1989, p. 240). However, since that time, a substantial body of literature has emerged examining hemostatic and platelet reactivity in vivo. Additionally, more recent literature has examined both transient and persistent models of depressive symptoms prospectively predicting CVD. For example, Wassertheil-Smoller et al.(Wassertheil-Smoller et al., 1996), found each 5-point increase in Center for Epidemiological Studies-Depression (CES-D) scores relative to baseline to be predictive of a 25% increased risk of death and an 18% increased risk for stroke or myocardial infarction. Among dementia caregivers, Mausbach and colleagues(Mausbach et al., 2007a) found that each 5-point increase in CES-D scores was associated with a 20% increase in 18-month risk for all-cause CVD (Mausbach et al., 2007a). In contrast, Ariyo et al.(Ariyo et al., 2000) found that each 5-point increase in the cumulative mean CES-D score was associated with a hazard ratio of 1.15 for development of CHD and 1.29 for all-cause mortality among previously healthy elderly. Importantly, accumulating research has shown that subclinical depressive symptoms have also been associated with increased CVD risk (Tomfohr et al., 2007) and mortality (Wassertheil-Smoller et al., 2004).
Persistent models of depression are supported by substantial literature on the role of serotonin (5HT) in negative affect (Owens and Nemeroff, 1994, Stahl, 2000) and platelet hyperactivity (Whyte et al., 2001). Platelets have previously been utilized as peripheral markers of central 5HT because they store over 99% of the body’s peripheral 5HT (Bianchi et al., 2002), which, upon secretion, augments platelet aggregation and thrombus formation (De Clerck et al., 1988). Moreover, platelets express a genetically identical 5HT transporter region (5HTT) to that expressed in the brain, the region regarded as the initial site of action for many modern antidepressants (Lesch et al., 1993). Research on common genetic factors for depression and CVD implicate various genes involved with the serotonergic pathway (Bondy, 2007), such as the 5HTT polymorphism (Whyte et al., 2001, Williams et al., 2001). Selective serotonin reuptake inhibitors (SSRIs) decrease platelet activity among depressed individuals (Musselman et al., 2000, Pollock et al., 2000, Serebruany et al., 2005), a finding which again points to a common mechanism.
A number of gene-environment studies have noted that vulnerabilities to depression tend to emerge when individuals face significant life stress (Caspi et al., 2003, Eley et al., 2004). Along these lines, a previous study found that the chronic stress of caring for demented spouse moderated the relationship between depressive symptoms and platelet responses to acute stress (Aschbacher et al., 2008). More generally, caregiving strain has been associated with elevated symptoms of depression (Schulz et al., 1995), heightened procoagulant reactivity to acute stress (Aschbacher et al., 2006), increased cardiovascular risk (von Känel et al., 2008), and mortality risk (Schulz and Beach, 1999). Thus, the examination of the depression-platelet link among elderly spousal dementia caregivers adds a crucial environmental-stress component, which is both empirically and theoretically implicated.
Persistent depressive symptoms may yield stronger predictive value for platelet hyperactivity than transient symptoms, particularly among chronically-stressed caregivers. This study hypothesized that persistent depression, represented by aggregating depressive symptoms over 3 years, would be associated with platelet hyperactivity at each year. To better assess model fit, a persistent model of depression was compared to an alternative model of transient depressive symptoms (i.e., the change or increase/decrease from the previous year). These aims were investigated with two competing path analytic models of depressive symptoms and platelet responses to acute stress with 3 yearly assessments among elderly dementia caregivers. In that our community-dwelling caregivers were generally not clinically depressed, this study also hoped to illuminate the effects of subclinical depressive symptomatology.
Methods and Materials
Participants
Ninety-nine individuals continuously providing in-home care to spouses with Alzheimer’s disease participated in a 3-year study of psychobiologic responses to stress with assessments conducted on a roughly yearly basis. The average age of the sample was 73 years (range = 52–88), 68% were female, and 93% were Caucasian. Although participants taking β-blocking or anticoagulant medication at the time of recruitment were excluded, 9 participants began taking β-blockers after enrollment, 2 took anti-aggregation drugs, and 1 took an anti-platelet (clopidogrel). Given that previous cross-sectional research in a similar sample did not find that including these data-points significantly altered the overall pattern of results (Aschbacher et al., 2008), they were not excluded, rather the issue was addressed statistically. Participants were recruited through referrals from the University of California, San Diego, Alzheimer’s Disease Research Center, community support groups, health fairs, and media advertisements. Participants provided written informed consent for this protocol, approved by the UCSD Institutional Review board.
Procedures
All data were collected by research nurses who made annual visits to participants’ homes. Nurses arrived to participants’ homes between 8:00 AM and 10:00 AM and administered a psychosocial interview including assessments for depressive and anxious symptoms, general physical health, and coping strategies. Following the interview, the nurse inserted a venous indwelling 22-gauge catheter into the participant’s forearm in preparation for the acute stress task. Blood samples were collected at 3 time points throughout the protocol: following 20 minutes of rest after catheter insertion (baseline), immediately after the stress task (speech), and 14-minutes after the stress task (recovery). To control for effects of artificial platelet activation evoked by the needle stick, the first 2 ml of blood were discarded (Michelson et al., 2000).
Acute Stress Protocol
Participants were instructed to deliver a brief impromptu speech (3 minutes of preparation and 3 of delivery) in response to one of two randomly assigned stressor vignettes (false accusations of shoplifting (Saab et al., 1992) and a disagreement with a disreputable auto mechanic). Participants repeated the same vignette in year 3 as they received at year 1. An additional study awaiting publication (Aschbacher et al., in press), has found that both tasks elicited comparable platelet reactivity and that no significant habituation occurred over the three yearly assessments. This finding is in keeping with another published study that did not find habituation effects for platelet-leukocyte aggregate responses to acute stress measured at two points, four weeks apart (Hamer et al., 2006).
Platelet Measures and Assay Procedure
Flow cytometry (Harrison, 2000, Michelson and Furman, 1999), a technique frequently used to examine microscopic particles suspended in liquid, was used to assess the percentage of platelets expressing the cell adhesion molecule P-selectin (PSEL) as an index of platelet activation (Blann et al., 2003). As this assessment of PSEL has been thoroughly documented in a previous publication (Aschbacher et al., 2008), it will be abbreviated here. Five μl of whole blood was incubated with 30 μl of fluorochrome-conjugated antibodies, CD61-PerCP and CD62P-PE (Brecton Dickerson Immunocytometry Systems, San Jose, CA), at pre-determined saturating concentrations for 15 minutes in a dark, room temperature setting. Samples were fixed with 1 ml 1% formaldehyde in PBS containing 0.1% NaN3 and analyzed with a Beckman Coulter EPICS Elite flow cytometer and Expo32 Software within 24-hours of staining.
Depressive Symptoms
Brief Symptom Inventory (BSI)
The 6-item depression subscale of the BSI (Derogatis and Melisaratos, 1983) was used to index participant depressive symptom severity. The BSI-D has been shown to have excellent reliability and validity among community-dwelling older adults. Specifically, Stuckenberg and colleagues (1990) compared the accuracy of the BSI-D to that of the Hamilton Rating Scale for Depression (HAM-D) for detecting a DSM diagnosis of depression. Results of this study found that the area under the ROC curve was .83 for the BSI-D compared to .85 for the HAM-D, and that these values were not significantly different. This study demonstrated that the BSI-D and the HAM-D are comparable in screening cases of depression in the elderly (Stukenberg et al., 1990). Therefore a brief, 6-item self report scale offers the advantage of constituting an adequate and more parsimonious substitute for a 10-minute clinical interview. The BSI also assesses somatic complaints in a separate subscale, which may be a useful distinction among elderly populations with preexisting medical conditions. Participants rated the frequency of depressive symptoms over the past 6 months on a scale of 0 (not at all) to 4 (extremely), yielding an average symptom score. Mean depression scores are given in Table 1. Based on norms in the elderly (Hale et al., 1984), in any given year 10–14% of the caregivers in the study met the criteria for clinically significant symptomology (t≥53) (Derogatis, 1992) (t-score M±SE: Y1 = 51.81 ± 10.90, Y2 = 51.14 ± 11.96, Y3 = 51.63 ± 12.20).
Table 1.
Correlations Among Raw/Untransformed Independent & Dependent Variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. DEP year 1 | .766** | .605** | −.176 | −.057 | −.090 | .183 | −.039 | .245 | .045 | .212 | .235 | |
| 2. DEP year 2 | .613** | −.012 | −.087 | −.175 | .048 | −.105 | .048 | .127 | −.030 | −.036 | ||
| 3. DEP year 3 | −.308 | .001 | .175 | .023 | .106 | .055 | −.078 | .271 | .172 | |||
| 4. PSEL base year 1 | −.019 | .021 | .340** | .051 | −.049 | .545** | .278† | −.117 | ||||
| 5. PSEL base year 2 | .519** | .060 | .674** | .382* | .031 | .406** | −.065 | |||||
| 6. PSEL base year 3 | .677** | .419* | .576** | .546** | .289 | .289 | ||||||
| 7. PSEL speech year 1 | .301 | .610** | .657** | .398* | .299 | |||||||
| 8. PSEL speech year 2 | .606** | .276 | .708** | .299 | ||||||||
| 9. PSEL speech year 3 | .339 | .497** | .396* | |||||||||
| 10. PSEL recov year 1 | .441** | .117 | ||||||||||
| 11. PSEL recov year 2 | .299 | |||||||||||
| 12. PSEL recov year 3 | ||||||||||||
| Mean (SE) | .61 (.07) | .58 (.09) | .58 (.11) | 2.40 (.37) | 4.68 (1.97) | 2.90 (.55) | 16.95 (2.52) | 13.49 (3.0) | 15.52 (3.93) | 17.95 (2.65) | 23.52 (3.89) | 13.27 (3.79) |
Note. DEP = Brief Symptom Inventory Depression Subscale Score; PSEL = P-Selectin; base = baseline measurement; speech = post-speech measurement; recov = recovery measurement. Means and their standard errors appear in the diagonal (M (SE)).
p ≤.01
p ≤ .05
p ≤ .07
Cardiovascular Health & Medication
The specific covariates chosen were based on previous longitudinal analyses in this sample (Aschbacher et al., in press) that identified the following factors as relevant covariates: previous physician’s diagnosis of myocardial infarction (n=6), use of aspirin (n=36), or use of any anti-depressant (n=31) (i.e., selective serotonin reuptake inhibitors, atypicals, tricyclics, or other). Self-reports of medication use were given and verified by inspection of the medication bottles.
Path Analytic Models
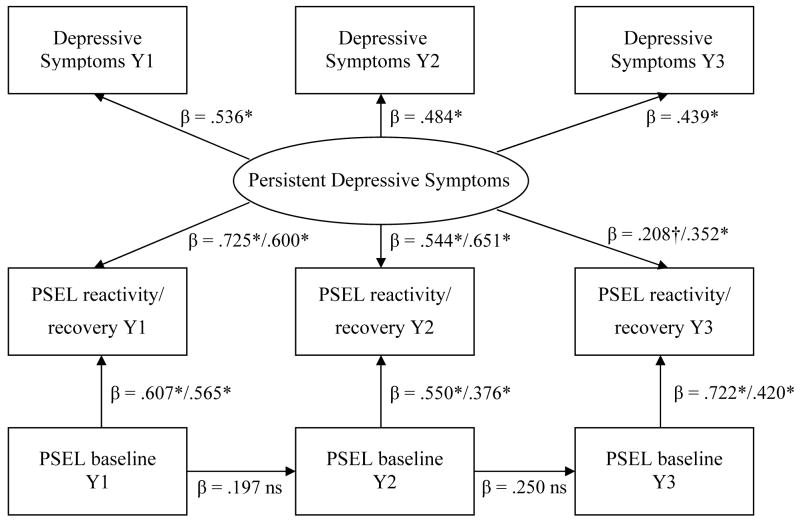
The temporal relationships between affect and platelet activation were evaluated using path analytic models, which provide indices of overall model fit. Two models, one assessing persistent depressive symptoms and the other transient symptoms, were tested using EQS 6.1. The first persistent model (Figure 1) formed a latent variable representing persistent depressive symptoms (DEP) by aggregating the common variance of depressive symptoms among the three time-points (r-values= .605–.766). Paths were specified from DEP to all PSEL reactivity and recovery values (the outcome variables), while controlling for covariates. Specifically, all PSEL reactivity values (Year 1, Year 2, Year 3) were regressed on DEP, while controlling for basal PSEL, aspirin and antidepressant use of the corresponding year and previous myocardial infarction. PSEL recovery was handled identically. Stability paths between baseline PSEL values were included to control for the influence of the previous year. The effects of age and gender on DEP were controlled for (i.e., partialed out) in the path analyses. As age and gender were not significantly associated with PSEL in previous analyses (Aschbacher et al., in press), in the interests of parsimony, they were not included as predictors of PSEL.
Figure 1.
Persistent Depressive Symptoms are Significantly Associated with P-Selectin Reactivity & Recovery Following Acute Psychological Stress
* p ≤ .01, † p ≤ .07, ns = not significant at alpha of .05, using robust standard errors. Y1 = Year 1, Y2 = Year 2, Y3 = Year 3, PSEL = Platelet P-Selectin Expression. Persistent Depressive Symptoms controlled for age and gender. Reactivity & Recovery values controlled for aspirin, antidepressants, and previous myocardial infarction.
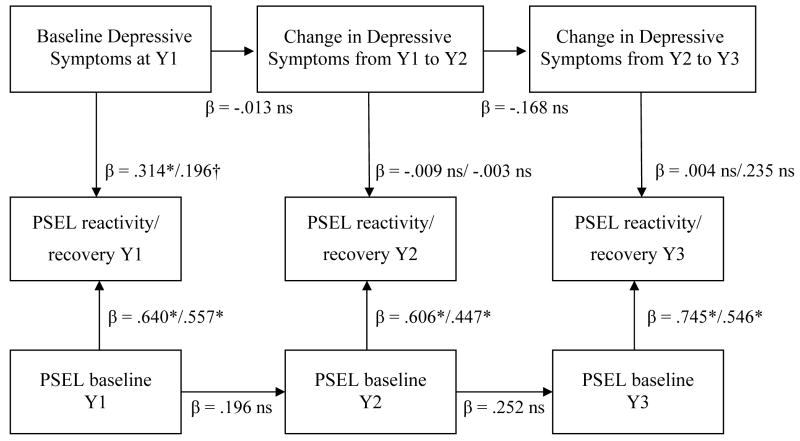
The second model (Figure 2) was identical, except for the specification of depressive symptoms. Year 1 (Y1) depressive symptoms were entered predicting PSEL reactivity/recovery at Y1, having controlled for the effects of gender and age as in the previous model. Transient depressive symptoms were represented by forming two change scores, i.e., Y2 – Y1 & Y3 – Y2. Values above 0 indicate increases in depressive symptoms relative to the previous year and vice versa. Paths were specified with the Y1 to Y2 symptom increase predicting PSEL reactivity/recovery at Y2, and the Y2 to Y3 symptom increase predicting PSEL reactivity/recovery at Y3.
Figure 2.
Change in Depressive Symptoms is not Significantly Associated with P-Selectin Reactivity & Recovery Following Acute Psychological Stress
* p ≤ .01, † p ≤ .06, ns = not significant at alpha of .05.
All coefficients are standardized and significance values are based on robust standard errors. Y1 = Year 1, Y2 = Year 2, Y3 = Year 3, PSEL = Platelet P-Selectin Expression. Baseline Depressive Symptoms controlled for age and gender. Reactivity & Recovery values controlled for aspirin, antidepressants, and previous myocardial infarction.
Prior to analysis, age was standardized and all PSEL variables were log-transformed to approximate normality (all p’s>.05, Kolmogorov-Smirnov’s Test). Depressive symptoms were positively skewed, but could not be normalized by square-root or logarithmic transformations. Therefore, robust standard error procedures were employed, which correct for multivariate non-normality. To accommodate unavailable data, intent-to-treat analyses with missing values imputation and robust standard errors were utilized, which does not inflate correlation values as does carrying forward the xmean.
Results
Analyses of Sample Attrition
A total of twenty-five individuals elected to drop out of the study, 15 between year 1 and year 2, and 10 between year 2 and year 3, citing reasons such as having insufficient time or interest in continued participation. Statistical comparisons revealed that participants who dropped did not significantly differ from those who remained active on any variable in the analyses, i.e., age, gender, previous myocardial infarction, taking aspirin or antidepressants, depressive symptoms or platelet outcomes.
Model Fit & Comparison
The persistent model of depression provided stronger and more consistent associations with platelet activation than the transient model. Akaike’s Information Criteria (AIC(Akaike, 1974) compares goodness of fit, identifying the model that best explains the data using the fewest number of parameters. As lower values indicate the preferred model, the persistent model performed better than the transient model (Table 2). The Yuan-Bentler corrected Comparative Fit Index (CFI), an index of descriptive model fit where higher values indicate superior fit (Bentler and Yuan, 1999, Hu and Bentler, 1999), was also preferable for the persistent model (Table 2). The Yuan-Bentler corrected Chi-Square for the persistent and transient models were both significant: persistent → χ2 [177] = 580.27, p < .001; transient → χ2 [178] = 701.73, p < .001. Table 1 depicts the correlations, means and standard errors for all untransformed independent and dependent variables. The proportion of variance in the platelet outcomes accounted for provides additional effect size comparisons (Table 2), illustrating the persistent model better explains platelet responses to acute stress.
Table 2.
Comparison of the Persistent & Transient Models: Goodness of Fit of Overall Model & Multiple R2 for Platelet Outcomes.
| Goodness of Fit Statistics | Persistent Model | Transient Model |
|---|---|---|
| AIC | 226.27 | 345.73 |
| CFI | 0.973 | 0.830 |
| Variance in Platelet Outcomes Accounted for by the Model, R2 | ||
| P-Selectin Variable | Persistent Model | Transient Model |
| Y1 Reactivity | 90% | 53% |
| Y2 Reactivity | 64% | 41% |
| Y3 Reactivity | 62% | 59% |
| Y1 Recovery | 70% | 39% |
| Y2 Recovery | 57% | 22% |
| Y3 Recovery | 33% | 40% |
Note: AIC = Akaike’s Information Criterion; lower values indicate better model fit (Akaike, 1974). CFI = Yuan-Bentler Corrected Comparative Fit Index; values above 0.95 are generally considered to exhibit good fit (Bentler and Yuan, 1999, Hu and Bentler, 1999). Y1 = Year 1, Y2= Year 2, Y3 = Year 3.
The Persistent Model of Depressive Symptoms
In the persistent model, DEP consistently predicted PSEL reactivity and recovery (all p’s ≤ .01, except reactivity at Y3: p = .066), while controlling for the influences of aspirin, antidepressants, and myocardial infarction on platelet activation. Standardized coefficients are provided in Figure 1. The stability paths for baseline PSEL values were not significant. The loadings of depressive symptoms on DEP were moderate to strong (β’s = .441–.533). Older age and male gender were associated with lower levels of DEP (β = −.679; β = −.238, respectively, p’s < .01). Myocardial infarction was not significantly associated with platelet outcomes; however, 5 of these 6 individuals were taking either antidepressants or aspirin. Aspirin use was not a significant independent predictor of platelet outcomes. Antidepressant use was associated with lower PSEL reactivity at Y2 (β = −.195, p = .01), and non-significant in relation to the remaining outcomes. In order to confirm that the presence of several participants who began taking exclusionary medications (primarily beta-blockers) after the study began had not significantly altered the pattern of results, the final model was rerun, adding an additional covariate at each year (1=exclusionary meds used, 0=not used). As expected based on previous reports (Aschbacher et al., 2008), taking exclusionary medications was not significantly related any PSEL outcome, and the overall pattern of significance remained the same for all variables.
The Transient Model of Depressive Symptoms
In the transient model, consistent with previous cross-sectional analyses(Aschbacher et al., 2008), higher initial depressive symptoms at Y1 were associated with increased PSEL reactivity at Y1 (β = .314, p < .01) and marginally increased PSEL recovery at Y1 (β = .196, p = .055). However, increases/decreases in depressive symptoms from one year to the next were not significantly associated with platelet outcomes in Y2 or Y3.
Discussion
These findings demonstrate that persistent depressive symptoms (i.e., the enduring symptoms over three yearly assessments), were significantly related to increased platelet P-selectin expression in response to acute psychological stress among older dementia caregivers. This association held for both immediate reactivity and 14-minute recovery over three assessments taken at yearly intervals, controlling for aspirin, antidepressant use, and previous history of myocardial infarction. In contrast, transient depressive symptoms (i.e., change from a previous year), were not significantly associated with platelet activation. Given that P-selectin facilitates inflammation of the vascular endothelium (Chen and Geng, 2006) and thrombus formation (Merten and Thiagarajan, 2000), these results suggest the possibility that platelet hyperactivity mediated by depressive symptoms may be associated with accelerated atherosclerotic progression and increased risk of acute coronary events (Blann et al., 2003, Libby et al., 2002). Moreover, platelet P-selectin assessed by flow cytometry has been associated with atherosclerotic wall changes and the occurrence of carotid plaque among human participants (Koyama et al., 2003).
These results extend upon previous research identifying cross-sectional associations between depressive symptoms and P-selectin among caregivers (Aschbacher et al., 2008), by demonstrating longitudinal associations. That study found no associations between negative affect and platelet responses in the non-caregiving group. These combined findings highlight the role of life stress in trait-environment interactions (Caspi et al., 2003). For example, certain types of genotypes are associated with certain types of 5HT regulation (Lesch, 2004), which may provide a common mechanism associated with mood and platelet hyperactivation. Although speculative, as this study did not directly assess 5HT functioning, the roles of 5HT in relation to stress, negative affect, platelet hyperactivity, antidepressant use, and CVD (Caspi et al., 2003, Otte et al., 2007, Williams et al., 2001, Piletz et al., 2000), are consistent with the possibility that stress may invite genetic diatheses in serotonergic functioning to manifest. Future research should attempt to integrate research in serotonin transporter genotypes with assessments of negative affect, chronic stress, and procoagulant reactivity.
The two models presented here were selected based on theoretical and empirical evidence; however, future research could consider additional models, if appropriate support for such models is available. Also of potential value would be to explore whether altered platelet activation is associated with major stressors related to caregiving, such as the death or placement of a spouse. Previous research has found that caregiving transitions can exert additional effects on mood and coagulation (Mausbach et al., 2007b) therefore, future research should investigate the best way to model these transitions.
We found that antidepressants were inconsistently related to platelet hyperactivation; however, antidepressant use was heterogeneously defined to include drugs with differing dosages and mechanisms of action (e.g., tricyclics, SSRIs, and atypicals). Therefore, these findings are consistent with evidence that not all antidepressants affect P-selectin reactivity (Musselman et al., 2000, Piletz et al., 2000). The fact that aspirin was not significantly related to increases in P-selectin responses using flow cytometric assay has been supported by other studies (Li et al., 2003, Li et al., 1999). Li et al. (2003) argue that, unlike other platelet assay methodologies, flow cytometry does not result in artifactual thromboxane synthesis, and therefore, the use of platelet activation assessment by flow cytometry may be associated with lesser effects of aspirin on platelet activation.
In sum, the current results suggest that persistent depressive symptoms, even when they are subclinical in severity, assessed three times at yearly intervals, are strongly associated with heightened platelet activation in response to acute stress among a group of older adults who are experiencing the chronic stress of caring for a spouse with Alzheimer’s disease. Exaggerated platelet responses to acute stress may constitute an important mechanism to account for prospective associations between negative affect and cardiovascular events or mortality. Finally, these findings suggest that the enduring aspects of negative affect may provide stronger predictive value for platelet-mediated processes as they contribute to overall cardiovascular disease risk.
References
- Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–23. [Google Scholar]
- Anda R, Williamson D, Jones D, Macera C, Eaker E, Glassman A, Marks J. Depressed affect, hopelessness, and the risk of ischemic heart disease in a cohort of U.S. adults. Epidemiology. 1993;4:285–94. doi: 10.1097/00001648-199307000-00003. [DOI] [PubMed] [Google Scholar]
- Ariyo AA, Haan M, Tangen CM, Rutledge JC, Cushman M, Dobs A, Furberg CD. Depressive symptoms and risks of coronary heart disease and mortality in elderly Americans. Cardiovascular Health Study Collaborative Research Group. Circulation. 2000;102:1773–1779. doi: 10.1161/01.cir.102.15.1773. [DOI] [PubMed] [Google Scholar]
- Aschbacher K, Von Känel R, Dimsdale JE, Patterson TL, Mills PJ, Mausbach BT, Ancoli-Israel S, Grant I. Increasing dementia of the care receiver predicts procoagulant response in Alzheimer caregivers. American Journal of Geriatric Psychiatry. 2006;14:694–703. doi: 10.1097/01.JGP.0000227969.36850.eb. [DOI] [PubMed] [Google Scholar]
- Aschbacher K, Von Känel R, Mills PJ, Hong S, Patterson TL, Roepke SK, Mausbach BT, Ziegler MG, Dimsdale JE, Ancoli-Israel S, Grant I. Effects of depressive and anxious symptoms on norepinephrine and platelet p-selectin responses to acute psychological stress among elderly caregivers. Brain, Behavior & Immunity. 2008;22:493–502. doi: 10.1016/j.bbi.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschbacher K, Von Känel R, Mills PJ, Roepke SK, Hong S, Dimsdale JE, Mausbach BT, Patterson TL, Ziegler MG, Ancoli-Israel S, Grant I. Longitudinal platelet reactivity to acute psychological stress among older men and women. Stress. doi: 10.1080/10253890802574993. in press. under review. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Schroll M. Symptoms of depression, acute myocardial infarction, and total mortality in a community sample. Circulation. 1996;93:1976–1980. doi: 10.1161/01.cir.93.11.1976. [DOI] [PubMed] [Google Scholar]
- Bentler PM, Yuan KH. Structural equation modeling with small samples: test statistics. Multivariate Behavioral Research. 1999;34:181–197. doi: 10.1207/S15327906Mb340203. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Moser C, Lazzarini C, Vecchiato E, Crespi F. Forced swimming test and fluoxetine treatment: in vivo evidence that peripheral 5-HT in rat platelet-rich plasma mirrors cerebral extracellular 5-HT levels, whilst 5-HT in isolated platelets mirrors neuronal 5-HT changes. Experimental Brain Research. 2002;143:191–197. doi: 10.1007/s00221-001-0979-3. [DOI] [PubMed] [Google Scholar]
- Blann AD, Nadar SK, Lip GY. The adhesion molecule P-selectin and cardiovascular disease. European Heart Journal. 2003;24:2166–2179. doi: 10.1016/j.ehj.2003.08.021. [DOI] [PubMed] [Google Scholar]
- Bondy B. Common genetic factors for depression and cardiovascular disease. Dialogues in Clinical Neuroscience. 2007;9:19–28. doi: 10.31887/DCNS.2007.9.1/bbondy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, Mcclay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chen M, Geng J. P-selectin mediates adhesion of leukocytes, platelets, and cancer cells in inflammation, thrombosis and cancer growth and metastasis. Archivum Immunologiae et Therapiae Experimentalis. 2006:54. doi: 10.1007/s00005-006-0010-6. [DOI] [PubMed] [Google Scholar]
- De Clerck F, Xhonneux B, De Chaffoy De Courcelles D. Functional expression of the amplification reaction between serotonin and epinephrine on platelets. Journal of Cardiovascular Pharmacology. 1988;11:S1–S5. [PubMed] [Google Scholar]
- Derogatis LR. The Brief Symptom Inventory: Administration, Scoring and Procedures Manual-II. New York: Clinical Psychometric Research, Inc; 1992. [Google Scholar]
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychological medicine. 1983;13:595–605. [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, Mcguffin P, Plomin R, Craig IW. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Molecular Psychiatry. 2004;9:908–915. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Bigger JT, Gaffney M, Shapiro PA, Swenson R. Onset of major depression associated with acute coronary syndromes. Archives of General Psychiatry. 2006;63:283–288. doi: 10.1001/archpsyc.63.3.283. [DOI] [PubMed] [Google Scholar]
- Hale WD, Cochran CD, Hedgepeth BE. Norms for the elderly on the Brief Symptom Inventory. Journal of Consulting & Clinical Psychology. 1984;52:321–322. doi: 10.1037//0022-006x.52.2.321. [DOI] [PubMed] [Google Scholar]
- Hamer M, Gibson EL, Vuononvirta R, Williams E, Steptoe A. Inflammatory and hemostatic responses to repeated mental stress: Individual stability and habituation over time. Brain, Behavior & Immunity. 2006;20:456–459. doi: 10.1016/j.bbi.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Harrison P. Progress in the assessment of platelet function. British Journal of Haematology. 2000;111:733–744. [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Koyama H, Maeno T, Fukumoto S, Shoji T, Yamane T, Yokoyama H, Emoto M, Shoji T, Tahara H, Inaba M, Hino M, Shioi A, Miki T, Nishizawa Y. Platelet P-selectin expression is associated with atherosclerotic wall thickness in carotid artery in humans. Circulation. 2003;108:524–9. doi: 10.1161/01.CIR.0000081765.88440.51. [DOI] [PubMed] [Google Scholar]
- Lesch KP. Gene-environment interaction and the genetics of depression. Review, Journal of Psychiatry & Neuroscience. 2004:3. [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Wolozin BL, Murphy DL, Riederer P. Primary structure of the human platelet serotonin uptake site-identity with the brain serotonin transporter. Journal of Neurochemistry. 1993;60:2319–2322. doi: 10.1111/j.1471-4159.1993.tb03522.x. [DOI] [PubMed] [Google Scholar]
- Li N, Hu H, Hjemdahl P. Aspirin treatment does not attenuate platelet or leukocyte activation as monitored by whole blood flow cytometry. Thrombosis Research. 2003;111:165–170. doi: 10.1016/j.thromres.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Li N, Wallen H, Hjemdahl P. Evidence for prothrombotic effects of exercise and limited protection by aspirin. Circulation. 1999;100:1374–1379. doi: 10.1161/01.cir.100.13.1374. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Mausbach B, Patterson T, Rabinowitz Y, Grant I, Schulz R. Depression and distress predict time to cardiovascular disease in dementia caregivers. Health Psychology. 2007a;26:539–544. doi: 10.1037/0278-6133.26.5.539. [DOI] [PubMed] [Google Scholar]
- Mausbach BT, Aschbacher K, Patterson TL, Von Känel R, Dimsdale JE, Mills PJ, Ancoli-Israel S, Grant I. Effects of placement and bereavement 17 on psychological well-being and cardiovascular risk in Alzheimer’s caregivers: a longitudinal analysis. Journal of Psychosomatic Research. 2007b;62:439–445. doi: 10.1016/j.jpsychores.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Merten M, Thiagarajan P. P-selectin expression on platelets determines size and stability of platelet aggregates. Circulation. 2000;102:1931–1936. doi: 10.1161/01.cir.102.16.1931. [DOI] [PubMed] [Google Scholar]
- Michelson A, Furman M. Laboratory markers of platelet activation and their clinical significance. Current Opinion in Hematology. 1999;6:342–348. doi: 10.1097/00062752-199909000-00012. [DOI] [PubMed] [Google Scholar]
- Michelson AD, Barnard MR, Krueger LA, Frelinger AL, Furman MI. Evaluation of platelet function by flow cytometry. Methods. 2000;21:259–270. doi: 10.1006/meth.2000.1006. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: Epidemiology, biology, and treatment. Archives of General Psychiatry. 1998;55:580–92. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Marzec UM, Manatunga A, Penna S, Reemsnyder A, Knight BT, Baron A, Hanson SR, Nemeroff CB. Platelet reactivity in depressed patients treated with paroxetine: Preliminary findings. Arch Gen Psychiatry. 2000;57:875–882. doi: 10.1001/archpsyc.57.9.875. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Musselman DL. Are platelets the link between depression and ischemic heart disease? American Heart Journal. 2000;140:S57–62. doi: 10.1067/mhj.2000.109978. [DOI] [PubMed] [Google Scholar]
- Otte C, Mccaffery J, Ali S, Whooley MA. Association of a serotonin transporter polymorphism (5-HTTLPR) with depression, perceived stress, and norepinephrine in patients with coronary disease: the heart and soul study. American Journal of Psychiatry. 2007;164:1379–1384. doi: 10.1176/appi.ajp.2007.06101617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Role of serotonin in the pathophysiology of depression: Focus on the serotonin transporter. Clinical Chemistry. 1994;40:288–295. [PubMed] [Google Scholar]
- Penninx BW, Geerlins SW, Deeg DJ, Van Eijk JT, Van Tilburg W, Beekman AT. Minor and major depression and the risk of death in older persons. Archives of General Psychiatry. 1999;56:889–895. doi: 10.1001/archpsyc.56.10.889. [DOI] [PubMed] [Google Scholar]
- Piletz JE, Zhu H, Madakasira S, Pazzaglia P, Devane CL, Goldman N, Halaris A. Elevated p-selectin on platelets in depression: Response to bupropion. Journal of Psychiatric Research. 2000;34:397–404. doi: 10.1016/s0022-3956(00)00034-0. [DOI] [PubMed] [Google Scholar]
- Pollock BG, Laghrissi-Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. Journal of Clinical Psychopharmacology. 2000;20:137–140. doi: 10.1097/00004714-200004000-00004. [DOI] [PubMed] [Google Scholar]
- Saab PG, Llabre MM, Hurwitz BE, Frame CA, Reineke LJ, Fins AI, Mccalla J, Cieply LK, Schneiderman N. Myocardial and peripheral vascular responses to behavioral challenges and their stability in black and white Americans. Psychophysiology. 1992;29:384–97. doi: 10.1111/j.1469-8986.1992.tb01712.x. [DOI] [PubMed] [Google Scholar]
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282:2215–9. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: The Cardiovascular Health Study. Archives of Internal Medicine. 2000;160:1761–8. doi: 10.1001/archinte.160.12.1761. [DOI] [PubMed] [Google Scholar]
- Schulz R, O’brien AT, Bookwala J, Fleissner K. Psychiatric and physical morbidity effects of dementia caregiving: prevalence, correlates and causes. Gerontologist. 1995;35:771–91. doi: 10.1093/geront/35.6.771. [DOI] [PubMed] [Google Scholar]
- Serebruany VL, Suckow RF, Cooper TB, O’connor CM, Malinin AI, Krishnan KR, Van Zyl LT, Lekht V, Glassman AH. Relationship between release of platelet/endothelial biomarkers and plasma levels of sertraline and N-desmethylsertraline in acute coronary syndrome patients receiving SSRI treatment for depression. American Journal of Psychiatry. 2005;162:1165–1170. doi: 10.1176/appi.ajp.162.6.1165. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Essential Pharmocology: Neuroscientific Basis and Practical Applications. Cambridge, UK: Cambridge University Press; 2000. [Google Scholar]
- Stukenberg KW, Dura JR, Kiecolt-Glaser JK. Depression Screening Scale Validation in an Elderly, Community-Dwelling Population. Psychological Assessment. 1990;2:134–138. [Google Scholar]
- Tomfohr LM, Martin TM, Miller GE. Symptoms of depression and impaired endothilial function in healthy adolescent women. Journal of Behavioral Medicine. 2007 doi: 10.1007/s10865-007-9141-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Von Känel R. Platelet hyperactivity in clinical depression and the beneficial effect of antidepressant drug treatment: How strong is the evidence? Acta Psychiatrica Scandinavica. 2004;110:163–177. doi: 10.1111/j.1600-0447.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- Von Känel R, Mausbach B, Patterson A, Dimsdale J, Aschbacher K, Mills MJ, Ziegler M, Ancoli-Israel S, Grant I. Increased Framingham coronary heart disease risk score in dementia caregivers relative to non-caregiving controls. Gerontology. 2008;54:131–7. doi: 10.1159/000113649. [DOI] [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Applegate WB, Berge K, Chang CJ, Davis BR, Grimm RJ, Kostis J, Pressel S, Schron E. Change in depression as a precursor of cardiovascular events. SHEP Cooperative Research Group (Systoloc Hypertension in the elderly) Archives of Internal Medicine. 1996;156:553–61. [PubMed] [Google Scholar]
- Wassertheil-Smoller S, Shumaker S, Ockene J, Talavera GA, Greenland P, Cochrane B, Robbins J, Aragaki A, Dunbar-Jacob J. Depression and cardiovascular sequelae in postmenopausal women. The Women’s Health Initiative (WHI) Archives of internal medicine. 2004;164:289–298. doi: 10.1001/archinte.164.3.289. [DOI] [PubMed] [Google Scholar]
- Watson D, Pennebaker JW. Health complaints, stress and distress: exploring the central role of negative affectivity. Psychological Review. 1989;96:234–254. doi: 10.1037/0033-295x.96.2.234. [DOI] [PubMed] [Google Scholar]
- Whyte EM, Pollock BG, Wagner WR, Mulsant BH, Ferrell RE, Mazumdar S, Reynolds CF., 3rd Influence of serotonin-transporter-linked promoter region polymorphism on platelet activation in geriatric depression. American Journal of Psychiatry. 2001;158:2074–2076. doi: 10.1176/appi.ajp.158.12.2074. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Carefoot JC, Grichnik K, Helms MJ, Kuhm C, Lewis JG. Central nervous system serotonin function and cardiovascular responses to stress. Psychosomatic Medicine. 2001;63:300–305. doi: 10.1097/00006842-200103000-00016. [DOI] [PubMed] [Google Scholar]