Abstract
Memory T-cells promote allograft rejection particularly in costimulation blockade (CoB)-based immunosuppressive regimens. Here we show that the CD2-specific fusion protein alefacept (LFA3-Ig) selectively eliminates memory T-cells and when combined with a CoB-based regimen utilizing CTLA4-Ig, prevents renal allograft rejection and alloantibody formation in primates. These results support the development of an immediately translatable regimen for the prevention of allograft rejection without the use of calcineurin inhibitors, steroids, or pan-T-cell depletion.
Calcineurin inhibitors and glucocorticosteroids are commonly used to prevent the rejection of allografted organs; however, both agents promote many side effects (1). Blockade of the CD28/B7 costimulation pathway has been suggested as a means of preventing allograft rejection without these side effects, and indeed, the CD28/B7-specific fusion protein CTLA4-Ig has been shown to induce permanent engraftment of allografts in some rodent models, particularly when combined with the drug sirolimus and/ or donor specific transfusion (DST) (2, 3). Unfortunately, CTLA4-Ig with or without sirolimus is insufficient to prevent rejection in primates (reviewed in 4).
Several mechanisms of CoB resistant rejection have been demonstrated experimentally with many implicating T-cells with a memory phenotype (5). T-cells gain this phenotype through prior cognate antigen exposure, heterologous cross-reactivity between alloantigens and environmental pathogens, or homeostatic expansion, and thereafter either lack CD28 or otherwise have reduced costimulation requirements (6, 7). We have therefore been interested in developing adjuvant therapies that transiently but specifically neutralize memory T (TM)-cells to facilitate the clinical use of CoB in transplantation, preferably adopting maneuvers that avoid TM development through homeostatic activation (e.g. pan T-cell depletion).
One candidate agent is LFA3-Ig (alefacept, Amevive, Astellas, Inc.), a dimeric fusion protein consisting of the CD2-binding portion of the human lymphocyte function-associated antigen-3 (LFA-3) linked to the Fc portion of human IgG1. It is currently approved for the clinical treatment of psoriasis, and its therapeutic effect has been linked to its ability to deplete TM-cells (8–10). We therefore investigated LFA3-Ig as an adjuvant agent for use with the CoB-based regimen of CTLA4-Ig, sirolimus and/or DST.
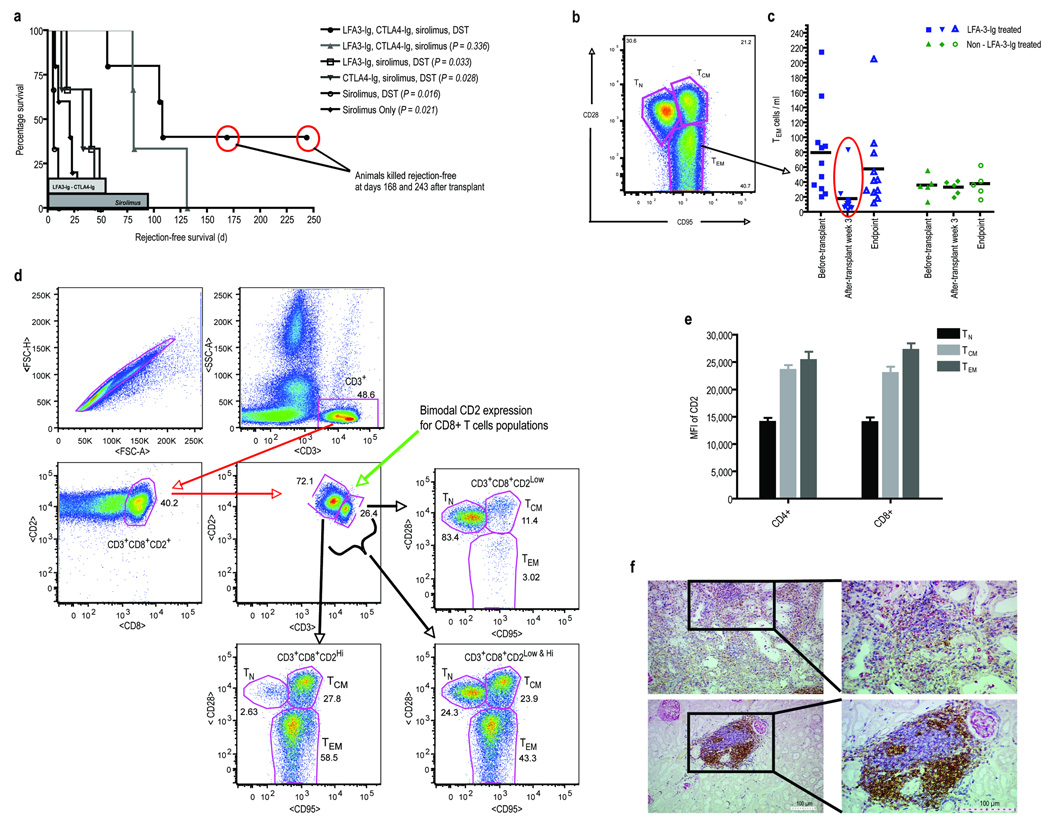
Renal allografted rhesus monkeys were treated with LFA3-Ig and/or CTLA4-Ig weekly for 8 weeks, oral sirolimus daily for 90 days, and pre-transplant whole blood DST (see Supplementary Methods and Supplementary Fig. 1 online). Control animals receiving no treatment, sirolimus alone, sirolimus with DST, sirolimus with DST and CTLA4-Ig, or sirolimus with DST and LFA3-Ig had progressively increased survival; however, no animals remained rejection-free beyond their treatment period (Fig. 1a; Supplementary Table 1 online), and all animals developed alloantibody by the onset of rejection (see Supplementary Fig. 2 online). In comparison, when both LFA3-Ig and CTLA4-Ig were combined with sirolimus with or without DST, significantly prolonged survival was seen compared to all other groups. These animals remained alloantibody-free while receiving both LFA3-Ig and CTLA4-Ig (see Supplementary Fig. 2 online) and 5/8 animals receiving the combination therapy remained rejection-free beyond the period of treatment (> 90 days). Two animals were killed with normally functioning, rejection-free, allografts at days 168 and 243 after developing alloantibody. Thus, LFA3-Ig was clearly additive and potentially synergistic to this CoB-based regimen.
Figure 1.
(a.) Rejection-free survival defined as the interval between the time of transplantation and the first allograft rejection event shown in days by treatment group. The duration of therapy is shown in the shaded bars along the x-axis. P values were determined by Student’s t-test (two-tailed) comparing each individual group versus the treatment group receiving LFA3-Ig, CTLA4-Ig, sirolimus, DST. This group had significantly prolonged rejection-free survival compared to all groups except the group receiving the combined therapy without DST. Similarly, the two groups receiving both LFA3-Ig and CTLA4-Ig, when considered together, had significantly prolonged survival compared to all other groups combined (p=0.002, two tailed Student’s t-test). The groups did not differ by sirolimus level or donor-directed mixed lymphocyte reactivity (See Supplementary Table 1 online). Two animals in the combined therapy group were killed with normal graft function after developing alloantibody. (b.) Polychromatic flow cytometry (PFC) was used to analyze LFA3-Ig’s depletional influence on PBMC T-cell subsets: naïve (TN; CD4+CD28+CD95low/−, β7 integrinint and CD8+CD28+CD95low/−, CD11alow), central memory (TCM; CD28+CD95+ or CD45RA−CD62L+ in both CD4+ and CD8+ cells), and effector memory (TEM; CD4+CD28−CD95+or CD45RAhigh and CD8+CD28−CD95+ or CD11ahigh). Shown is a representative gate defining the three subsets for T-cells previously gated for CD3. (c.) The influence of LFA3-Ig treatment of peripheral TEM-cell count levels is shown for all animals pre-transplant, 3 weeks post-transplant, and at terminal end points. Animals receiving LFA3-Ig are shown to the left in blue, and animals that did not receive LFA3-Ig are shown to the right in green. The mean values for each group are shown as black horizontal bars. TM-cells were significantly reduced after 3 weeks of LFA3-Ig therapy with the greatest depletion seen in the TEM (CD28−CD95+) subset of CD4+ and CD8+ T-cells (p=0.007, two-tailed Student’s t-test comparing pre and post transplant cell counts) without significant alteration in the absolute numbers of naive T-cells. This was not seen in non-LFA3-Ig treated animals. No significant difference in naïve T-cells was observed between LFA3-Ig and non-LFA3-Ig treated animals at each time point. At the time of rejection or sacrifice, TEM counts in LFA3-Ig treated animals had returned to normal. (d.) There is an inverse relationship between the surface expression of CD2 (the target of LFA3-Ig) and CD28 (the relevant receptor blocked with CTLA4-Ig). Shown are results from PFC experiments performed on freshly isolated peripheral blood lymphocytes obtained from untreated animals. T-cells prior to treatment have a bimodal expression of CD2 and CD3, with antigen experienced cells having lower CD3 expression and higher CD2 expression (center panel). Shown is this relationship for CD8+ T-cells based on PFC plots. The CD3hiCD2lo cells are predominently TN (upper right) while the CD3loCD2hi cells are predominently TEM (lower left). TCM cells are less distinctly segregated by these markers. (e.) Summary data from five untreated animals relating the mean fluorescnce intensity (MFI) of CD2 to the surface phenotype for both CD4+ and CD8+ T-cells. (f.) Renal allograft histological examination of necropsy specimens from a healthy, rejection-free animal with immunohistochemical (IHC) staining for T-cells (blue – CD3+), B-cells (brown – CD79+), and macrophages (pink/red – Ham-56) at low (left images) and higher (right images) magnification. Despite normal renal allograft function and no clinical signs/symptoms of rejection, multiple focal and highly organized cellular infiltrates where observed (lower panels). Interestingly, the cellular infiltrates were predominately aggregates of T- and B-cells, with minimal or no macrophages. In comparison, examination of rejecting animals using identical IHC staining demonstrated diffuse infiltrates without focal organization displaying greater cellular heterogeneity. Importantly, there were greater numbers of macrophages observed per objective field compared to the infiltrates seen in non-rejecting allografts, which has been shown to be of functional significance. Note the Ham-56 macrophage marker is also present on mesangial cells. As a result, glomeruli will also stain positive (pink) for Ham-56 as seen throughout the histological specimens.
We used polychromatic flow cytometry (PFC) to evaluate LFA3-Ig’s influence on naive and TM-cell subsets (11) (Fig. 1b, Supplementary Methods and Supplementary Fig. 3 online). While there was no gross lymphopenia, there was a general trend toward decreased absolute T-cell numbers in LFA3-Ig treated animals, with a greater reduction in CD8+ cells and an increase in the CD4/CD8 ratio that reversed following the withdrawal of LFA3-Ig. This modest decrease in lymphocyte counts could be attributed to a selective depletion in TM-cells without alteration in the numbers of naive T-cells. In particular, the greatest depletion was seen within the T effector (TEM; CD28−CD95+) subset of CD4+ and CD8+ T-cells (Fig. 1c). TEM counts were markedly (p=0.007) reduced after 3 weeks of LFA3-Ig therapy. At the time of rejection or sacrifice, TEM counts in LFA3-Ig treated animals had returned to normal, suggesting that more prolonged treatment would facilitate improved survival. This reduction in TEM-cells was not observed in non-LFA3-Ig treated animals. Interestingly, dual evaluation of peripheral blood T-cells from naïve animals with regard to CD2 and CD3 expression indicated that CD28+ T-cells were overwhelmingly CD2loCD3hi while CD28- T-cells were conversely CD2hiCD3lo, providing an explanation for the TEM-selective effects of LFA3-Ig (Fig. 1d and 1e).
All animals at one month showed modest lymphocytic infiltrates in surveillance biopsies studied using multi-color immunohistochemistry (see Supplementary Methods online). Interestingly, while T-cell infiltrates were present in all animals, animals not receiving LFA3-Ig tended to show a more heterogeneous infiltrate including monocytes in addition to T-cells. All animals reaching endpoint criteria showed allograft rejection on histological analysis following necropsy. However, two animals killed with normally functioning, rejection-free allografts contained focal perivascular aggregates of T-and B-cells with minimal or no monocytes (Fig. 1f and Supplementary Fig. 4 online), without tubulitis or vasculitis. The paucity of monocytes in these infiltrates is consistent with previously reported results of allografts maintaining normal function under CoB-based therapies, in that pure T-cell aggregates behaved in a benign fashion while those with monocytes were associated with functional impairment (12).
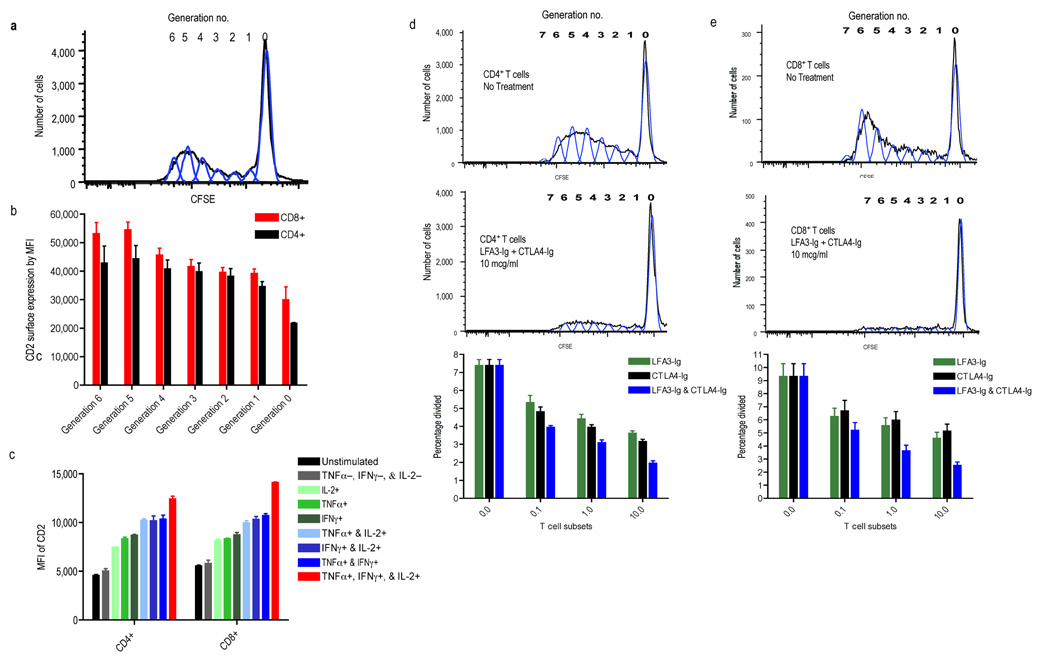
To investigate LFA3-Ig’s effect on alloreactive TM-cells, we performed mixed lymphocyte cultures (MLCs) specifically evaluating the relationship between the alloresponsiveness of CD4+ and CD8+ T-cells and their surface expression of CD2. Responder lymphocytes were labeled with CFSE to enable the identification of responding cells by generation of cell division (Fig. 2a; and Supplementary Methods), and each division was individually interrogated for maturation phenotype and CD2 expression. Responding cells progressively increased CD2 expression leaving highly alloresponsive cells markedly decorated with the LFA3-Ig target compared to non-responding cells (Fig. 2b). Similarly, cells that differentiated into cytokine producing cells (IFN-γ, TNF-α, and/or IL-2) in response to alloantigen stimulation progressively gained CD2 expression commensurate with their acquisition of cytokine secretion capabilities with the highest CD2 expression seen in those cells with poly-functionality (Fig. 2c), cells previously shown to be more robust and terminally differentiated effectors (13). In keeping with this target pattern, CTLA4-Ig and LFA3-Ig each mediated a dose-dependent inhibition of proliferation for both CD4+ and CD8+ T-cells when added to MLCs, and the combination induced an additive reduction in percent cells divided across 3 logs of concentration tested (Fig. 2d and 2e).
Figure 2.
Shown are studies investigating the relationship between CD2 expression and alloresponsiveness in mixed lymphocyte culture (MLC) as measured by proliferation and cytokine production. (a.) CFSE-labeled T-cells responding in MLC progressively lose fluorescence intensity by a factor of two permitting alloresponsive cells to be distinguished from non-dividing cells and each generation of division (generation #) to be separately evaluated by PFC. (b.) PFC evaluation of each CFSE generation for accessory molecule expression (CD4 or CD8) and CD2, the target of LFA3-Ig demonstrates that each progressive cell division is associated with a concomitant increase in surface CD2 expression, suggesting that the most alloresponsive cells are the most susceptible to LFA3-Ig treatment. (c.) Intracellular cytokine staining relating CD2 expression and cytokine secretion (IFN-γ, TNF-α, and/or IL-2) produced following allostimulation shows that T-cells with the most developed cytokine production capabilities express the highest density of surface CD2, with poly-functional cells, those expressing IFN-γ, TNF-α, and IL-2 (red bars), having the greatest CD2 density. (d.) Proliferation of both CD4+ and (e.) CD8+ T-cells in CFSE MLC is inhibited with CTLA4-Ig and LFA3-Ig, and the combination of both agents is more effective than either agent alone across a 3-log concentration range. Results of MLCs are shown for untreated cells (top), and cells treated with both agents at 10ug/ml (middle) demonstrating that treatment markedly attenuated proliferations. The bottom panels display summary data expressed as percent cells divided during MLC across 4 different concentrations of CTLA4-Ig and/or LFA3-Ig, (0.0, 0.1, 1.0, and 10 ug/ml). A dose dependent decrease in proliferation is seen with the combination therapy having the lowest proliferative capacity at all concentrations tested.
These results demonstrate that a combination of LFA3-Ig, CTLA4-Ig, and sirolimus markedly prolongs renal allograft survival and delays the onset of alloantibody formation in primates and that the effect lasts beyond the period of therapy in most animals. All components of the regimen appear to be involved in the regimen’s efficacy, as individual agents were ineffective in preventing rejection or antibody formation. The mechanism of LFA3-Ig’s salutary effect appears to be related to increasing CD2 density on alloresponsive cells, particularly CD28− TEM-cells, cells that are least susceptible to CoB and most alloresponsive. These data are consistent with a model in which the transient elimination of CoB-resistant TM-cells fosters an environment that more closely resembles that seen in naïve rodent models. They support the development of a clinically applicable regimen for the prevention of allograft rejection in humans without the use of calcineurin inhibitors, steroids, or gross T-cell depletion.
Supplementary Material
Acknowledgments
This study was funded in part by the Division of Intramural Research, National Institute of Diabetes, Digestive and Kidney Disease, National Institutes of Health (Z01 DK062007-06). The authors have no conflicting financial interests. Salary support for TAW was provided by the Howard Hughes Medical Institute through the NIH Research Scholars Program. ADK is supported by the National Institutes of Health (1U01AI079223-01A1), the Georgia Research Alliance, and the McKelvey Foundation. The authors gratefully acknowledge the expert assistance of John Bacher, and the staff of the NIH Veterinary Pathology Department.
References
- 1.Meier-Kriesche HU, et al. Am J Transplant. 2006;6:1111–1131. doi: 10.1111/j.1600-6143.2006.01270.x. [DOI] [PubMed] [Google Scholar]
- 2.Lin H, et al. J Exp Med. 1993;178:1801–1806. doi: 10.1084/jem.178.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, Zheng XX, Li XC, Zand MS, Strom TB. Transplantation. 1998;66:1387–1388. doi: 10.1097/00007890-199811270-00021. [DOI] [PubMed] [Google Scholar]
- 4.Kirk AD, et al. Imm Rev. 2003;196:176–196. doi: 10.1046/j.1600-065x.2003.00081.x. [DOI] [PubMed] [Google Scholar]
- 5.Valujskikh A, Li XC. J Am Soc Nephrol. 2007;18:2252–2261. doi: 10.1681/ASN.2007020151. [DOI] [PubMed] [Google Scholar]
- 6.Adams AB, et al. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu Z, et al. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ortonne JP, Lebwohl M, Em Griffiths C Alefacept Clinical Study Group. Eur J Derm. 2003;13:117–123. [PubMed] [Google Scholar]
- 9.Ellis CN, Krueger GG Alefacept Clinical Study Group. N Engl J Med. 2001;345:248–255. doi: 10.1056/NEJM200107263450403. [DOI] [PubMed] [Google Scholar]
- 10.Chamian F, et al. Proc Natl Acad Sci USA. 2005;102:2075–2080. doi: 10.1073/pnas.0409569102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitcher CJ, et al. J Immunol. 2002;162:29–24. [Google Scholar]
- 12.Preston EH, et al. Am J Transplant. 2005;5:1032–1041. doi: 10.1111/j.1600-6143.2005.00796.x. [DOI] [PubMed] [Google Scholar]
- 13.Harari A, et al. Immunol Rev. 2006;211:236–254. doi: 10.1111/j.0105-2896.2006.00395.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.