Abstract
PEPT1 is a high-capacity, low-affinity peptide transporter that mediates the uptake of di- and tripeptides in the intestine and kidney. PEPT1 also has significance in its ability to transport therapeutic agents and because of its potential as a target for anti-inflammatory therapies. To further understand the relevance of specific peptide transporters in intestinal physiology, pharmacology and pathophysiology, we have generated Pept1 null mice by targeted gene disruption. The Pept1 gene was disrupted by insertion of a lacZ reporter gene under the control of the endogenous Pept1 promoter. Phenotypic profiling of wild-type and Pept1 null mice was then performed, along with in vitro intestinal uptake, in situ intestinal perfusion and in vivo pharmacokinetic studies of glycylsarcosine (GlySar). Pept1 null mice lacked expression of PEPT1 protein in the intestine and kidney, tissues in which this peptide transporter is normally expressed. Pept1-deficient mice were found to be viable, fertile, grew to normal size and weight, and were without any obvious abnormalities. Nevertheless, Pept1 deletion dramatically reduced the intestinal uptake and effective permeability of the model dipeptide GlySar (i.e., by at least 80%), and its oral absorption following gastric gavage (i.e., by about 50%). In contrast, the plasma profiles of GlySar were almost superimposable between wild-type and Pept1 null animals after intravenous dosing. These novel findings provide strong evidence that PEPT1 has a major role in the in vivo oral absorption of dipeptides.
Keywords: targeted disruption, knockout mice, Pept1, dipeptides, intestinal absorption
Introduction
In mammals, the proton-coupled oligopeptide transporter (POT) family consists of four members [i.e., PEPT1 (SLC15A1), PEPT2 (SLC15A2), PHT1 (SLC15A4), PHT2 (SLC15A3)] and is responsible for the symport of small peptides/mimetics across biological membranes via an inwardly-directed proton gradient and negative membrane potential.1-4 The POT proteins vary in size from 572-729 amino acids and are predicted to contain 12 transmembrane domains, with the N- and C-termini facing the cytosol. The encoded proteins have a number of potential protein kinase recognition domains (i.e., 0-3 for PKA, 1-11 for PKC) and glycosylation sites (i.e., 2-7); however, their relevance has not been proven experimentally. Whereas PEPT1 and PEPT2 have high homology between species (about 80% in rat, rabbit, human and mouse), the homology between these two transporters for a given species is low (about 50%). Rat PHT1 and PHT2 have an amino acid identity of about 50%, but they show little homology to either PEPT1 or PEPT2 (less than 20%).
PEPT1, a high-capacity low-affinity transporter, has nutritional importance because of its role in the intestinal absorption of small peptides from the diet and because of its role in the reabsorption of peptide-bound amino nitrogen from glomerular filtrate in kidney. PEPT1 also has significance in its ability to transport therapeutic agents (e.g., β-lactam antibiotics, angiotensin-converting enzyme inhibitors, antiviral nucleoside prodrugs) and potentially toxic peptidomimetics (e.g., 5-aminolevulinic acid). Immunolocalization studies demonstrated that PEPT1 was expressed in the apical membrane of enterocytes in the small intestine (i.e., duodenum, jejunum and ileum) with little or no expression in normal colon.5,6 PEPT2, a low-capacity high-affinity transporter, has been reported in glial cells and tissue-resident macrophages of the enteric nervous system.7 However, it is unlikely that PEPT2-mediated absorption is involved in these deep, neuromuscular layers of the gastrointestinal tract. Likewise, transcripts of the peptide-histidine transporters PHT1 and PHT2 have been reported in intestinal tissue segments,8 but their role in peptide/mimetic absorption has not been demonstrated.
More recently, it has been shown that in chronic states of intestinal inflammation, such as in Crohn's disease and ulcerative colitis, PEPT1 expression is induced in colonic epithelia.9 Under such conditions, bacterially-derived chemotactic peptides (e.g., fMet-Leu-Phe and Ac-muramyl-Ala-Glu) can gain access to the intracellular compartment of epithelial cells and/or immune cells within the lamina propria. This unique relationship suggests an involvement of PEPT1 in inflammatory bowel disease and the innate immune response, as well as a novel target for anti-inflammatory therapies.10
While cellular, molecular and physiological studies have made major contributions toward a mechanistic understanding of PEPT1 structure, function and localization, the experimental approaches are often limited by an in vitro design and lack of blood supply, overlapping substrate specificities and contribution of multiple transport systems, some of which are unknown at the time of study. As a result, it is difficult, if not impossible, to define the function of a single specific gene product and its significance in relation to other possible proteins that are present in the tissue or organ of interest.
In the present study, we describe for the first time the development, validation, and phenotypic analysis of transgenic mice lacking the Pept1 gene. Our findings demonstrate that PEPT1 is responsible for at least 80% of glycylsarcosine (GlySar) uptake during studies using in vitro intestinal rings and in situ single-pass intestinal perfusions. Moreover, the results are corroborated by in vivo studies in which Pept1 deletion substantially reduces the oral absorption and systemic exposure of this dipeptide.
Materials and Methods
Animals
Animal studies were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health. Gender- and weight-matched wild-type (Pept1+/+) and null (Pept1-/-) mice (>99% C57BL/6 genetic background), 8 to 10 weeks of age, were used for all phenotypic analyses unless otherwise noted. The mice were kept in a temperature-controlled environment with a 12-hr light and 12-hr dark cycle, and received a standard diet and water ad libitum (Unit for Laboratory Animal Medicine, University of Michigan, Ann Arbor, MI).
Construction of Targeting Vector and Generation of Pept1 Null Mice
Pept1 null mice were obtained from Deltagen, Inc (San Mateo, CA) and were developed using a conventional transgenic and breeding strategy, as described previously.11 Briefly, a genomic fragment of about 5.6 kb, including protein coding regions of the Pept1 gene (slc15a1), was isolated from a mouse genomic library and subcloned into the Bam HI site of the pBluescript II SK(-) vector. Bases from +77 to +101 were deleted from a segment of the protein coding region and replaced with an IRES-lacZ reporter and neomycin (G418) resistance cassette of about 6.9 kb. This mutation was designed to produce a loss-of-function mutation by deletion of amino acids 16 to 24. The IRES-lacZ-neo cassette was flanked by 2.8 kb of mouse genomic DNA at the 5' arm and by 2.8 kb of genomic DNA at the 3' arm. The targeting vector was then linearized and electroporated into ES cells derived from the 129P2/OlaHsd mouse substrain. The ES cells were selected for G418 resistance, and colonies that had integrated the IRES lacZ-neo DNA were identified by PCR amplification using three neo-specific primers paired with primers located outside the targeting homology arms of vector (i.e., AAGGCTCTTCTTGGCTGACTGTAGC for 5' and TTAAGCATGGAATGGACATGGTCTC for 3', respectively). Male chimeric mice, generated by injection of the targeted ES cells into C57BL/6 blastocysts, were bred with C57BL/6 mice to produce F1 heterozygous (Pept1+/-) mice. F2 homozygous mutant mice were then produced by intercrossing F1 heterozygous males and females. PCR was used to confirm germline transmission and the subsequent production of congenic Pept1 null mice on a C57BL/6 background (≥ 99%).
Taqman Real-Time PCR Analyses
Quantitation of PHT1 and PHT2 transcripts was performed on the small intestine, colon and kidney from wild-type and Pept1 null mice using the 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA). Total RNA was isolated according to the manufacturer's protocol using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA), and then reverse-transcribed. The PHT1 and PHT2 primers and probes were designed with Primer Express 3.0 software (Applied Biosystems, Foster City, CA), and the primers, probes and standard DNA were synthesized by Integrated DNA Technologies (Coralville, IA). The forward and reverse primers and probe for PHT1 were: GGCCATTGGGTGGATGAG, GCAGGTGGCAGCTGTTGA and 5'-/56-FAM/-CTGGCCGCCATCCAGGGAGC-/36-TAMSp/-3', respectively; the forward and reverse primers and probe for PHT2 were: CCTGTGATGGTGACCCTTGTG, GGAGGACATAGGTGGACTGCAT and 5'-/56-FAM/-CTGCTGTCCCTGCCGGAAGTCCT-/36-TAMSp/-3', respectively. The forward and reverse primers and probe for GAPDH were: GAGACAGCCGCATCTTCTTGT, CACACCGACCTTCACCATTTT and 5'-/Joe/-CAGTGCCAGCCTCGTCCCGTAGA-/36-TAMSp/-3', respectively. The thermal profile was 1 cycle at 50°C for 2 min, 1 cycle at 95°C for 10 min, 40 cycles at 95°C for 15 sec and 60°C for 1 min. The absolute amount of PHT1 and PHT2 transcripts was calculated automatically based on the standard curve, and then normalized for GAPDH.
Immunoblot Analyses
Brush border membrane vesicles were prepared from the intestine and kidneys from wild-type and Pept1 null mice, as described previously.12,13 The membrane proteins were resolved on 7.5% SDS-PAGE, transferred to a PVDF membrane, and blotted with specific polyclonal rat PEPT1 antisera14,15 (1:500 dilution) or mouse PEPT2 antisera (raised against the COOH-terminal region, KQIPHIQGNMINLETKNTRL, amino acids 721-740; Lampire Biological Laboratories, Pipersville, PA) (1:5000 dilution). The filters were washed three times with TBS-T and then incubated with goat anti-rabbit IgG conjugated to horseradish peroxidase (Bio-Rad, Hercules, CA) (1:3000 dilution). For β-actin, the membrane was blotted with a mouse monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) (1:1000 dilution) followed by the secondary antibody, goat anti-mouse IgG conjugated to horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA) (1:3000 dilution). The membranes were then washed five times in TBS-T, and the bound antibody was detected with Immobilon Western Chemiluminescent Substrate (Millipore, Billerica, MA).
In Vitro Intestinal Uptake Studies
Following an overnight fast (>12 hr), gender-matched Pept1+/+ and Pept1-/- mice were anesthetized with sodium pentobarbital (40-60 mg/kg ip). Everted ring experiments were then performed according to methods described previously.16 In brief, the abdomen was opened, the jejunum isolated (i.e., ~ 2 cm distal to the ligament of Treitz), and two 2-cm segments transferred to an ice-cold incubation medium. Following a rapid wash, the jejunal segments were everted and fixed over glass rods (3 mm outer diameter) with surgical thread. Everted segments were then equilibrated for 5 min with incubation medium, which was gassed continuously with 5%CO2-95%O2 in a 37°C shaking water bath. Following the equilibration period, each jejunal segment was placed in 1 ml of incubation medium containing 4 μM [14C]GlySar and 4 μM [3H]mannitol (an extracellular marker). After incubating for 20 sec at 37°C, each segment was rapidly washed with ice-cold incubation medium, blotted on filter paper, weighed, and solubilized in 0.33 ml of 1 M hyamine hydroxide. The contents were then transferred to a vial containing scintillation fluid for measurement of radioactivity by a dual-channel liquid scintillation counter. The incubation medium contained 129 mM NaCl, 5.1 mM KCl, 1.4 mM CaCl2, 1.3 mM NaH2PO4, and 1.3 mM Na2HPO4 (pH 6.0).
In Situ Single-Pass Intestinal Perfusion Studies
Following an overnight fast (>12 hr), gender-matched Pept1+/+ and Pept1-/- mice were anesthetized with sodium pentobarbital (40-60 mg/kg ip). Jejunal perfusion experiments were then performed according to methods described previously for normal and mdr1a/1b (-/-) mice.17 In brief, surgery was performed on animals after placing them on a heating pad to maintain body temperature. The abdomen was opened and a midline longitudinal incision was made to expose the small intestine. An 8-cm segment of proximal jejunum was isolated (i.e., ~ 2 cm distal to the ligament of Treitz) followed by incisions at both the proximal and distal ends. A glass cannula (2.0 mm outer diameter) was inserted at each end of the jejunal segment and secured in place with silk sutures. Following cannulation, the isolated intestinal segment was rinsed with isotonic saline solution, and covered with saline-wetted gauze and parafilm to prevent dehydration. After the surgical procedure, the animals was transferred to a temperature-controlled chamber (31°C) to maintain body temperature during the actual perfusion experiment. The inlet cannula was connected to a 10-ml syringe placed on a perfusion pump.
The perfusate solution contained 135 mM NaCl, 5 mM KCl, and 10 mM MES (pH 6.5) plus 10 μM [3H]GlySar and 0.01% [14C]PEG 4000 (which served as a nonabsorble marker to correct for water flux). The solution was perfused through the intestinal segment at a rate of 0.1 ml/min, and the exiting perfusate collected every 10 min for 90 min. A 100-μl aliquot of each sample was added to a vial containing scintillation fluid for measurement of radioactivity by a dual-channel liquid scintillation counter. At the conclusion of the experiment, the intestinal segment was measured for its length.
In Vivo Pharmacokinetic Studies
Following an overnight fast (>12 hr), gender-matched Pept1+/+ and Pept1-/- mice were anesthetized briefly with isoflurance prior to administration of [14C]GlySar (10 nmol/g body weight) by tail vein injection (intravenous) or gastric lavage (oral). After intravenous dosing, serial blood samples were collected at 0.25, 2, 5, 15, 30, 60, 120, 240, 360 and 480 min; after oral dosing, serial blood samples were collected at 15, 30, 45, 60, 90, 120, 180, 240, 360 and 480 min. Blood samples (15-20 μl) were obtained via tail transections and the plasma harvested. Animals were returned to their cages in between blood sampling where they had free access to water; food was provided in their cages four hours after dosing. Radioactivity in plasma was measured by a dual-channel liquid scintillation counter.
Data Analysis
Jejunal transport was determined from the steady-state loss of drug from the perfusate as it flowed through the intestine, which was achieved approximately 30-40 min after the start of perfusion. The effective permeability (Peff) of GlySar was calculated according to a complete mixing model:18,19 Peff = - Q/(2πRL)•ln(Cout/Cin), where Q is the perfusate flow rate, R the intestinal radius, L the length of intestine, Cout the outlet drug concentration (corrected for water flux), and Cin the inlet drug concentration.
The pharmacokinetics of GlySar were determined by noncompartmental analysis20 after oral and intravenous administrations using WinNonlin (version 5.0; Pharsight, Mountain View, CA).
Data are reported as mean ± SE, unless otherwise noted. Statistical differences between the wild-type and Pept1 null mice were determined using a two sample t-test; p ≤ 0.05 was considered significant.
Results
Targeted Disruption of the Pept1 Gene
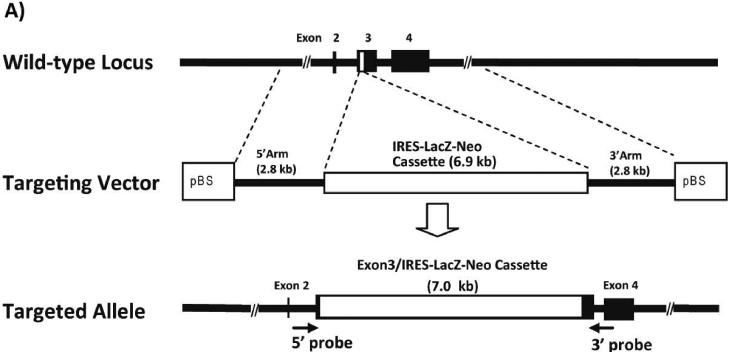
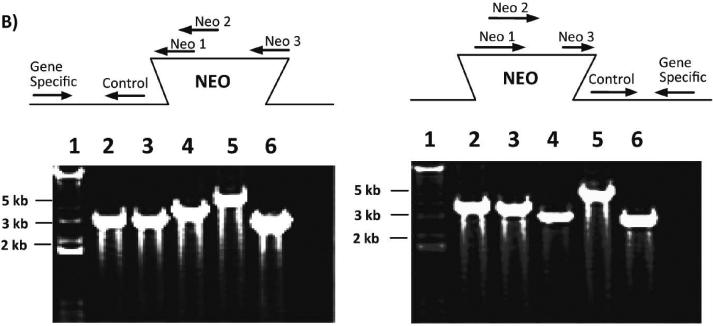
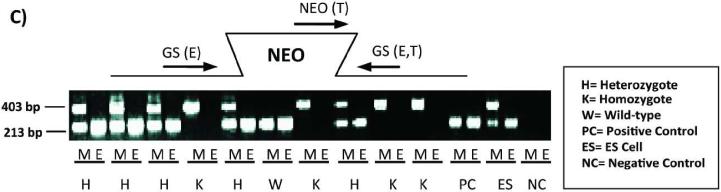
The mouse Pept1 gene was disrupted by replacing part of the coding region of exon 3 with the IRES-LacZ-Neo cassette via homologous recombination in ES cells (Figure 1A). Correct targeting of the Pept1 locus in ES clones was determined in both directions by PCR of the genomic DNA (Figure 1B). Heterozygous male and female mice (F1 generation) were mated to produce wild-type and Pept1 null mouse (F2 generation). The targeted Pept1 allele was detected in these offspring by PCR analysis of genomic DNA isolated from tail biopsies (Figure 1C).
Figure 1.
Schematic of the genomic Pept1 locus, targeting vector and mutated allele (A). The endogenous locus is depicted by thick solid lines or filled boxes. The small open box within exon 3 of the wild-type locus (top) represents a 25 bp region that was replaced with a 6.9 kb IRES-LacZ-Neo cassette. The IRES-LacZ-Neo locus is depicted in the open box (middle), and the 5' and 3' probes utilized for PCR of the homologous recombinants are represented by solid arrows (bottom). Genomic DNA from the recombinant ES cell line was assayed for homologous recombination by PCR in both the 5' and 3' directions (B). The figure represents the design and results of three separate primers in the neomycin insert paired with gene-specific primers. Lane 1 is a 1.0 kB ladder; lanes 2, 3 and 4 are the PCR product using neo 1, 2 and 3 primers, respectively, paired with gene-specific primers; lane 5 is a DNA sample control and lane 6 is the positive control. Genotyping results via PCR from mouse tail biopsies (C). The schematic demonstrates the PCR primer strategy to detect the endogenous (213 bp) or targeted (403 bp) allele. The reaction “M” for each sample includes the neomycin primer (NEO) and gene-specific primers (GS), and detects the endogenous (E) and targeted (T) alleles. The reaction “E” contains only the GS primers and detects only the endogenous allele.
Tissue Expression of Proton-Coupled Oligopeptide Transporters
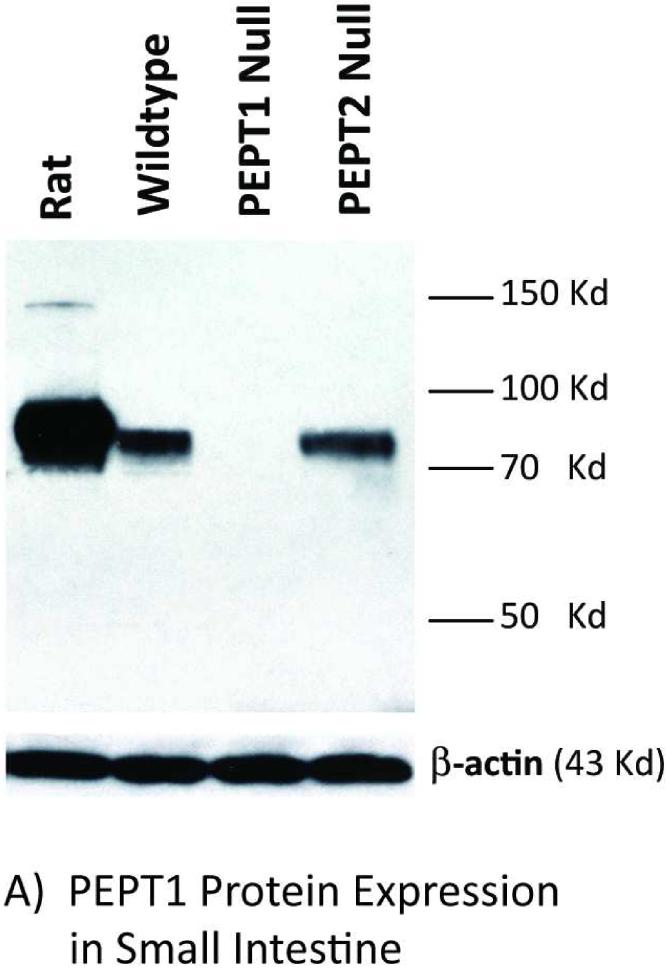
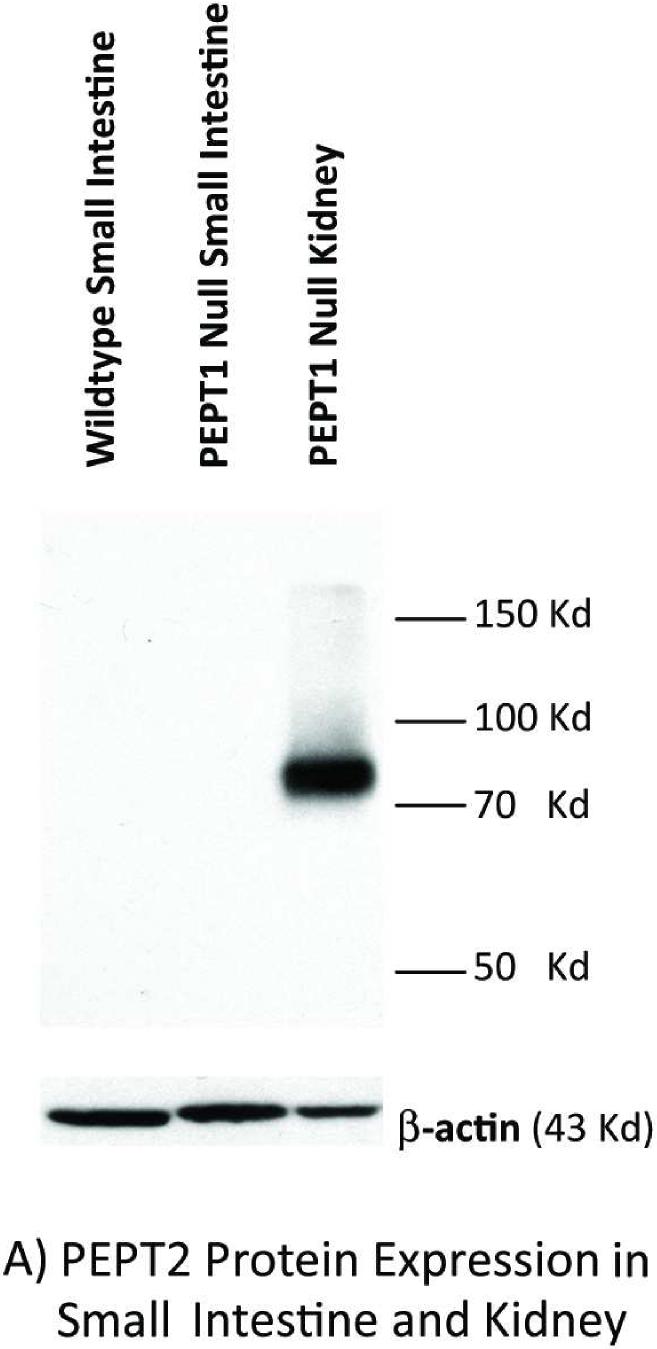
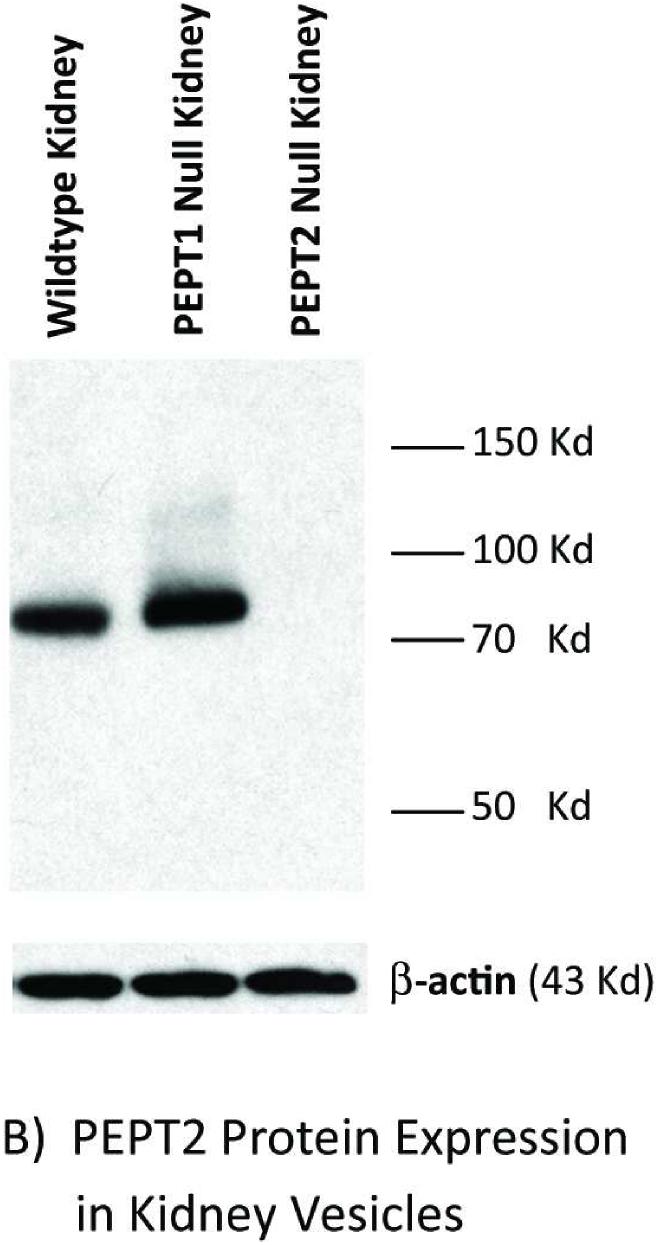
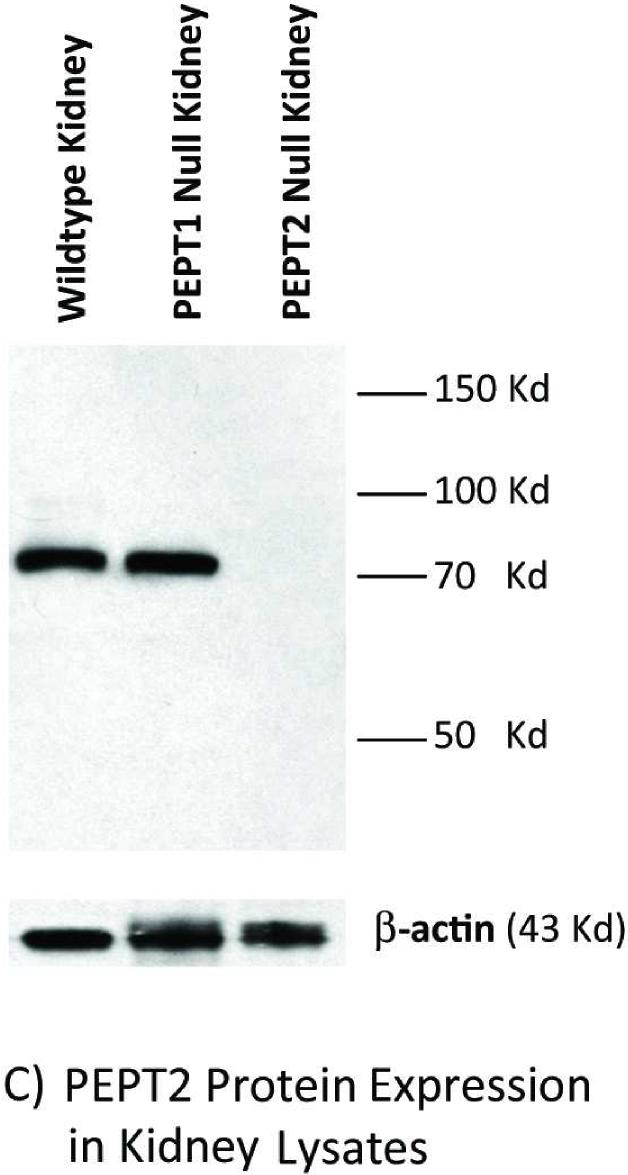
Immunoblot analyses of intestinal and kidney membranes were performed to probe whether aberrant levels of PEPT2 protein appear in the intestine of Pept1 null mice, or if PEPT2 protein levels in the kidney differ between genotypes. As expected, PEPT1 protein was expressed in the brush border membranes of small intestine (Figure 2A) and kidney (Figure 2B) of rat, wild-type and Pept2 null mice. Moreover, there was a complete absence of this immunoreactive protein in the tissues of Pept1-deficient animals. On the other hand, PEPT2 protein was absent from the brush border membranes of small intestine (Figure 3A), and showed only a minor increase of expression in brush border membranes of kidney (Figure 3B) or whole tissue lysates (Figure 3C) of wild-type and Pept1 null mice.
Figure 2.
PEPT1 protein expression in brush border membrane vesicles of small intestine (A) and kidney (B). Apical membranes were pooled from six 8-week old mice or a single adult rat and subjected to 7.5% SDS-PAGE (50 μg of protein for intestine of both species; 40 μg of protein for rat kidney and 100 μg of protein for mouse kidney).
Figure 3.
PEPT2 protein expression in brush border membrane vesicles of mouse small intestine (A) and kidney (A or B), and whole tissue lysates of mouse kidney (C). Apical membranes and lysates were pooled from six 8-week old mice and subjected to 7.5% SDS-PAGE (20 μg of protein for mouse intestine and kidney vesicles; 90 μg of protein for mouse kidney lysates).
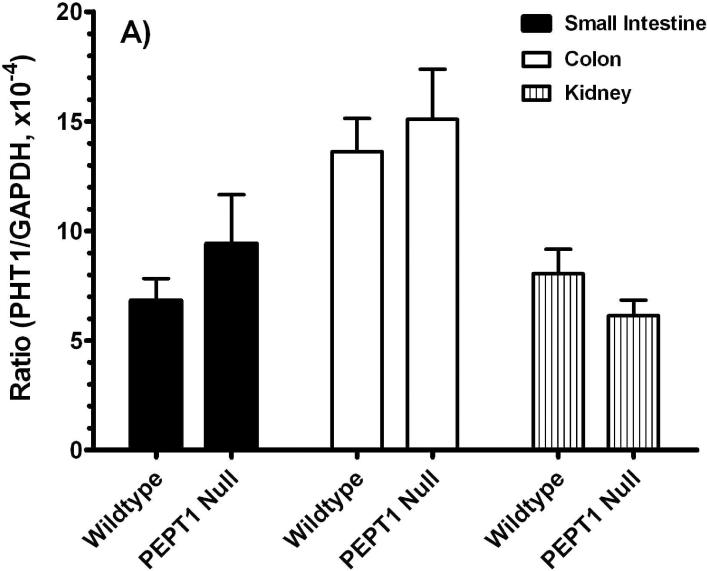
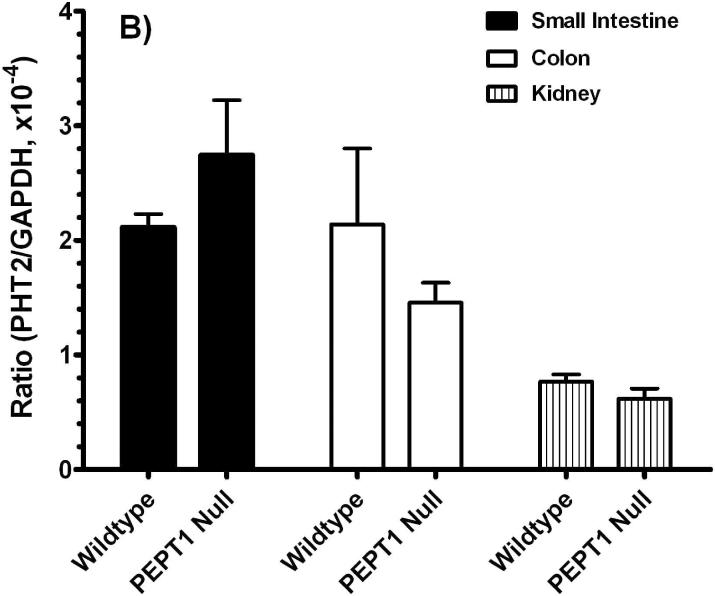
Given the lack of available antisera, real-time PCR studies (i.e., GAPDH-normalized) were performed for PHT1 and PHT2. As shown in Figure 4, there were no significant differences for PHT1 or PHT2 transcripts (Figures 4A or 4B, respectively) in the small intestine, large intestine and kidney of Pept1-deficient and competent mice.
Figure 4.
Real time-PCR analyses for PHT1 (A) and PHT2 (B) transcripts in the small intestine, colon and kidney of wild-type and Pept1 null mice (n=6, mean±SE).
Initial Phenotypic Analysis
No pathological phenotype was observed between gender-matched wild-type and Pept1-deficient mice. In this regard, Pept1 null animals were found to be viable, fertile, grew to normal size, body weight and organ weight, and had no significant differences in serum clinical chemistry (Table 1). Moreover, histological examination excluded any genotype-related abnormalities of major organs (including the kidneys, and small and large intestines).
Table 1.
Serum clinical chemistry of Pept1-/-, Pept1+/+, and C57BL6 Mice
| Parameter | Pept1-/- | Pept1+/+ | C57BL/6 |
|---|---|---|---|
| Body Weight, 7 wk (g) | 24.4 ± 1.5 | 23.4 ± 4.6 | 24.1 ± 2.7 |
| Serum | |||
| Sodium (mmol/liter) | 139 ± 3 | 141 ± 2 | 142 ± 1 |
| Potassium (mmol/liter) | 5.9 ± 0.7 | 6.2 ± 0.6 | 5.0 ± 0.3 |
| Chloride (mmol/liter) | 100 ± 2 | 102 ± 3 | 103 ± 32 |
| Glucose (mg/dl) | 245 ± 56 | 239 ± 38 | 264 ± 8 |
| Bicarbonate (mg/dl)) | 17.7 ± 1.5 | 17.8 ± 1.1 | 18.3 ± 1.5 |
| Calcium (mg/dl) | 9.9 ± 0.3 | 10.2 ± 0.3 | 9.8 ± 0.1 |
| Bilirubin (mg/dl) | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
| Urea Nitrogen (mg/dl) | 23.4 ± 4.6 | 24.1 ± 2.7 | 23.2 ± 4.1 |
| Alkaline Phosphatase (units/liter) | 174 ± 34 | 175 ± 30 | 196 ± 25 |
| Alanine Transaminase (units/liter) | 33.3 ± 18.9 | 48.5 ± 10.8 | 35.0 ± 18.6 |
| Aspartate Aminotransferase (units/liter) | 95.9 ± 44.4 | 139 ± 55 | 98.8 ± 74.2 |
| Protein (g/dl) | 4.9 ± 0.2 | 5.0 ± 0.1 | 4.8 ± 0.3 |
| Albumin (g/dl) | 1.3 ± 0.1 | 1.4 ± 0.0 | 1.4 ± 0.3 |
Results are mean ± SD (n=4-6 for Pept1-/- F2, Pept1+/+ F2, and C57BL/6 mice).
In Vitro Intestinal Uptake, In Situ Intestinal Perfusion and In Vivo Pharmacokinetic Studies
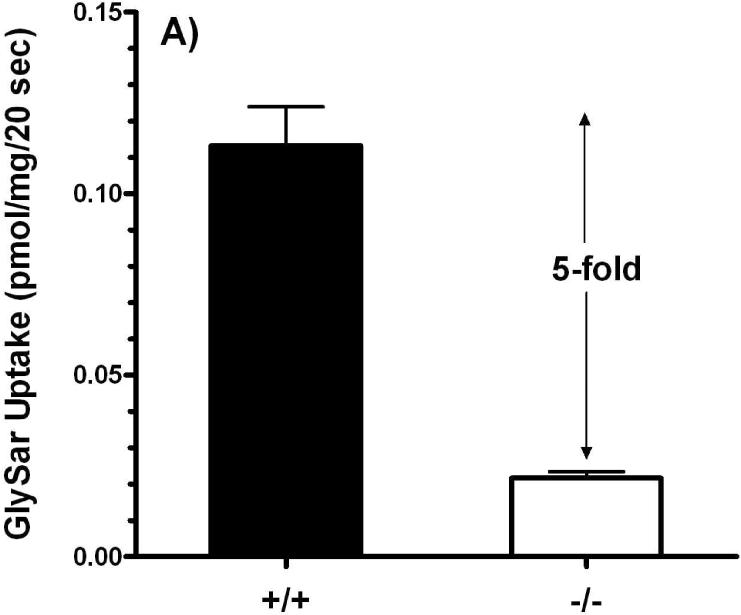
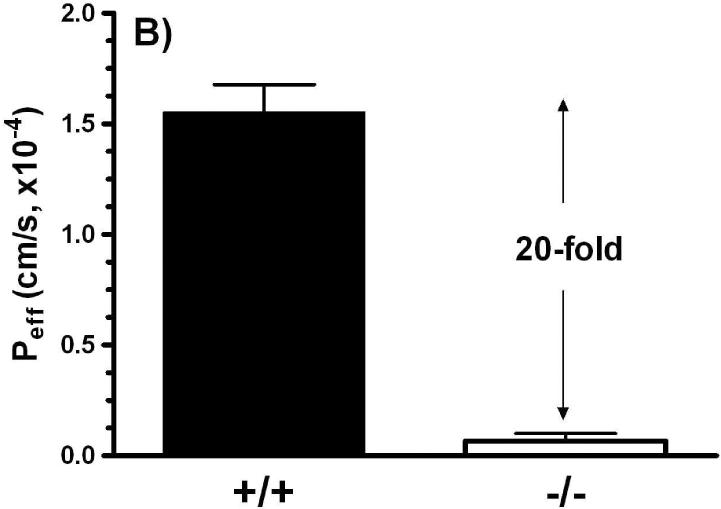
Studies in everted jejunal rings showed that GlySar uptake in Pept1 null mice was only 22% of that observed in wild-type mice (Figure 5A). While in vitro intestinal uptake studies have certain advantages (in terms of simplicity, speed, and ability to study axial differences in uptake), they are limited by their lack of an intact blood supply. As a result, we also performed in situ jejunal perfusions of GlySar. As shown in Figure 5B, the effective permeability of GlySar in Pept1-deficient mice was only 5% of the value observed in wild-type animals.
Figure 5.
Intestinal uptake (A) of 4 μM [3H]GlySar into everted jejunal rings of wild-type (+/+) and Pept1 null (-/-) mice. Studies were performed in pH 6.0 buffer (n=3, mean±SE; p<0.001). Effective permeability (B) of 10 μM [3H]GlySar during jejunal perfusions of wild-type and Pept1 null mice. Studies were performed in pH 6.5 buffer (n=6, mean±SE; p<0.001).
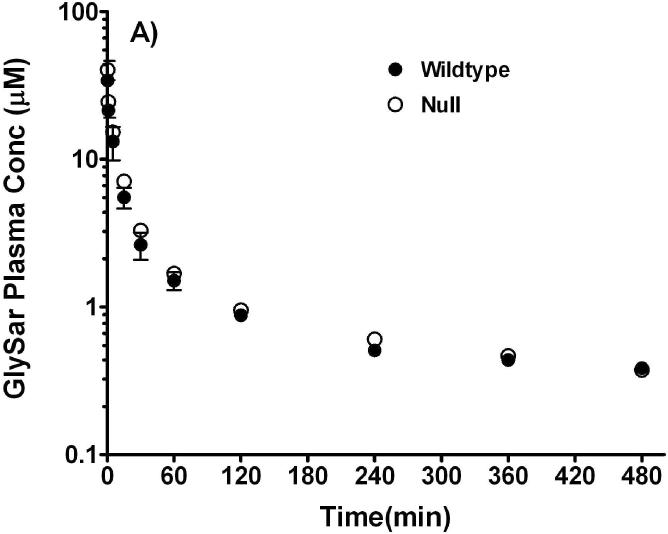
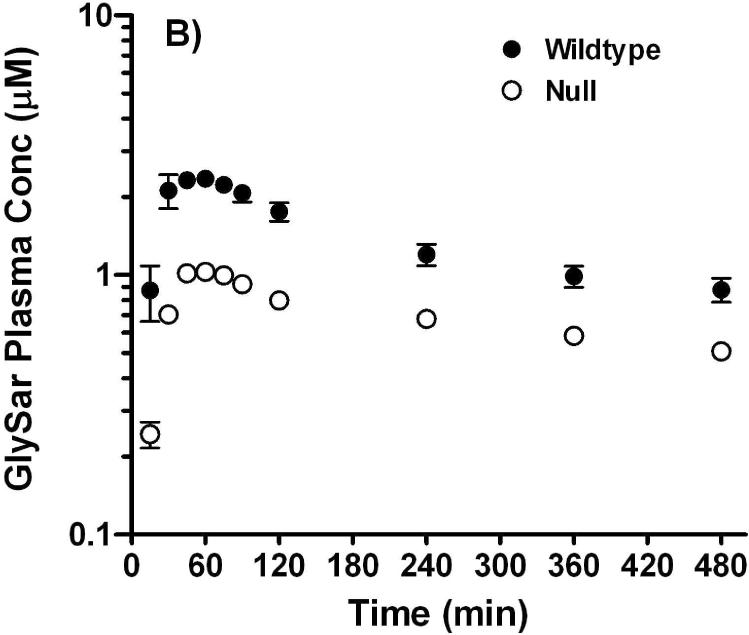
Studies were performed after oral and intravenous routes of administration to probe whether or not disruption of the Pept1 gene would affect the in vivo systemic kinetics and oral availability of a model dipeptide. As shown in Figure 6A, plasma concentrations of GlySar were initially about 40-50 μM after intravenous dosing and declined rapidly over time. The two curves were almost superimposable and there were no pharmacokinetic differences between wild-type and Pept1 null animals. In particular, the clearance of GlySar differed by only 7% between the two genotypes (i.e., 0.17±0.01 and 0.16±0.01 ml/min; wild-type and null mice, respectively). In contrast, Pept1-deficient mice had substantially reduced peak concentrations of GlySar (i.e., 2.5±0.1 and 1.0±0.1 μM; wild-type and null mice, respectively; p<0.001) and areas under the curve (i.e., 641±50 and 322±10 μM·min over 8 hr; wild-type and null mice, respectively; p<0.001) after gastric lavage as compared to Pept1-competent animals (Figure 6B). These findings confirm our previous results using in vitro and in situ methods, and suggest strongly that Pept1 deletion can significantly reduce the in vivo oral absorption of dipeptides.
Figure 6.
Plasma concentration vs. time curves of [14C]GlySar after intravenous (A) and gastric gavage (B) doses of dipeptide (5 nmol/g body weight) in wild-type and Pept1 null mice (n=3-4, mean±SE).
Discussion
Proteins originating from the diet and from gastrointestinal secretions are digested by several gastric and pancreatic proteases, which are further acted upon by a cadre of peptidases in the brush border membrane of intestinal epithelium.21 These protein digestion products then enter the enterocyte, primarily as di- and tripeptides as opposed to free amino acids. Once inside the cell, cytoplasmic peptidases act on the di-/tripeptides so that the majority of protein digestion products actually enter the portal vein in the form of amino acids. Although the basolateral efflux of amino acids occurs via different amino acid transporters, hydrolysis-resistant peptides (and peptide-like drugs) can exit the cell by a basolateral peptide-transporting system that has yet to be identified. The nature of the peptide-transporter(s) at the apical surface of intestinal epithelial cells was revealed once PEPT1 was cloned from a rabbit intestinal cDNA library22 and shown to be of high capacity and low affinity (Km values in mM range) for di- and tripeptides.1,4 This transporter has subsequently been cloned in a number of mammalian species including human23 and mouse.24 Immunolocalization studies demonstrated that PEPT1 was expressed in the apical membrane of enterocytes in the small intestine (i.e., duodenum, jejunum and ileum) of both species but not in the colon.5,6 While there is no evidence for PEPT2 in mammalian intestinal epithelia, this protein has been reported in neuromuscular layers of the gastrointestinal tract, and in enteric glial cells and tissue-resident macrophages.7 PHT1 and PHT2 transcripts have been reported in both human and rat intestinal tissue segments.8 Moreover, immunohistochemical analyses have indicated that PHT1 is expressed in the villous epithelium of small intestine,25 although its subcellular localization remains to be elucidated.
It is generally believed that PEPT1 is the primary (if not sole) transport protein responsible for handling the tremendous variety of di/tripeptides encountered in a physiological setting and the many peptide-like drugs absorbed in a pharmacological setting. However, some experimental findings in intact tissue preparations have suggested that more than one type of peptide carrier may exist.26 It is also possible that peptide-like drugs are absorbed by multiple transporters. For example, using a rat intestinal perfusion technique, it was suggested that, in addition to the proton-coupled oligopeptide transporter (presumably PEPT1), organic anion and organic cation transporters were involved in the small intestinal uptake of valacyclovir.27 Regardless, it is clear that the role and relative importance of PEPT1 in the intestine (and other tissues) will not be elucidated by in vitro, nonphysiologic studies that lack blood flow as well as appropriate residence times at the critical sites of transport.
The in vivo role of PEPT1 in protein nutrition was initially investigated using a deletion mutant of Caenorhabditis elegans.28 In doing so, it was found that abolition of the Pept1 ortholog in C. elegans (i.e., PEP-2) resulted in a severely retarded development as well as reduced progeny and body size. These findings clearly identify intestinal peptide absorption as a key process in body protein homeostasis and contend that rodent models lacking the Pept1 gene are sorely needed to determine whether or not these findings from the nematode are reproducible in higher organisms.
In the present study, a Pept1-deficient mouse model was successfully generated by targeted gene disruption in embryonic stem cells. The PCR analyses confirmed that this mutant mouse strain did not produce the gene to encode Pept1. Moreover, the immunoblots demonstrated convincingly that PEPT1 protein was absent from the intestine and kidney of Pept1-null mice, while being retained in the same tissues of wild-type mice. As expected, the high-affinity peptide transporter PEPT2 was absent from the intestine but retained in the kidney of Pept1- deficient animals. With the loss of PEPT1 in the intestine, the jejunal uptake and permeability of GlySar was drastically reduced during the in vitro and in situ studies, respectively, when comparing wild-type and Pept1-null mice. These studies were subsequently confirmed by in vivo experiments in which the oral absorption of GlySar in Pept1-deficient animals was one-half that of Pept1-competent animals. In contrast, Pept1 deletion had no effect on the systemic exposure of GlySar following intravenous administration.
Because of the lack of apparent biological effects, it is possible that other POT family members may be upregulated as a compensatory response to Pept1 gene deletion. However, immunoblot analyses revealed that PEPT2 protein was not aberrantly expressed in the small intestine of Pept1 null mice, and that the change of PEPT2 expression in kidney was unremarkable. Moreover, the real-time PCR studies demonstrated that PHT1 and PHT2 mRNA were not significantly different in the small intestine, colon and kidney of wild-type and Pept1 null animals. Thus, an adaptive response by related peptide transporters is very unlikely to explain the lack of a prominent phenotype in Pept1 null mice. Alternatively, if the brush border membrane enzymes (e.g., peptidases) were upregulated along with amino acid transporters (to handle the increased load), then PEPT1 would not be necessary for the maintenance of protein nutrition. Notwithstanding this uncertainty, our studies have demonstrated definitely that a transport defect of dipeptide absorption occurs in the small intestine. By challenging our transgenic mice in subsequent studies, it is possible that unique, pathological phenotypes will be revealed. This scenario was certainly exemplified when Mdr1(-/-) mice died suddenly after a chance encounter with the anthelmintic pesticide ivermectin,29 when Bcrp1(-/-) mice developed phototoxic ear lesions when being fed a diet containing alfalfa,30 and when Pept2(-/-) mice developed neurotoxicity during chronic dosing of 5-aminolevulinic acid.31
The quantitative relevance of PEPT1 between genotypes is quite dramatic when comparing the everted ring uptakes and single-pass perfusions of GlySar in jejunal tissue. Based on the in vitro intestinal uptake studies, PEPT1 accounts for about 80% of the total uptake process. Likewise, results form the in situ perfusion studies suggest that PEPT1 accounts for about 95% of the total uptake process. The in vivo findings, although to a lesser extent, corroborate the in vitro and in situ results, and support a major role for PEPT1 in the intestinal absorption of GlySar (and presumably other di- and tripeptides). Paracellular transport was not different between wild-type and Pept1 null mice, as suggested by perfusion studies with mannitol (data not shown), thereby excluding this mechanism as a possible factor in explaining any differences in uptake between the genotypes.
In addition to its role in the intestinal absorption and renal reabsorption of peptide-bound amino acids generated from the diet, PEPT1 has significance in the pharmacology and pharmacokinetics of several therapeutic agents, and as a target for drug delivery strategies.32 Moreover, PEPT1 has recently been implicated in inflammatory bowel disease, as a potential target in the development of anti-inflammatory therapies, and as a gateway to the innate immune response.9 It is our view that Pept1 null mice will offer scientists and clinicians an unparalleled opportunity to examine the role, relevance, and regulation of this peptide transporter under a variety of physiologic, pharmacologic and pathophysiologic conditions.
Acknowledgment
This work was supported in part by grant R01 GM035498 (to DES), Deltagen and Lilly Research Laboratories.
References
- (1).Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Arch. 2004;447:610–618. doi: 10.1007/s00424-003-1101-4. [DOI] [PubMed] [Google Scholar]
- (2).Herrera-Ruiz D, Knipp GT. Current perspectives on established and putative mammalian oligopeptide transporters. J. Pharm. Sci. 2003;92:691–714. doi: 10.1002/jps.10303. [DOI] [PubMed] [Google Scholar]
- (3).Rubio-Aliaga I, Daniel H. Mammalian peptide transporters as targets for drug delivery. Trends Pharmacol. Sci. 2002;23:434–440. doi: 10.1016/s0165-6147(02)02072-2. [DOI] [PubMed] [Google Scholar]
- (4).Daniel H, Rubio-Aliaga I. An update on renal peptide transporters. Am. J. Physiol. 2003;284:F885–F892. doi: 10.1152/ajprenal.00123.2002. [DOI] [PubMed] [Google Scholar]
- (5).Walker D, Thwaites DT, Simmons NL, Gilbert HJ, Hirst BH. Substrate upregulation of the human small intestinal peptide transporter, hPepT1. J. Physiol. 1998;507:697–706. doi: 10.1111/j.1469-7793.1998.697bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Groneberg DA, Döring F, Eynott PR, Fischer A, Daniel H. Intestinal peptide transport: Ex vivo uptake studies and localization of peptide carrier PEPT1. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G697–G704. doi: 10.1152/ajpgi.2001.281.3.G697. [DOI] [PubMed] [Google Scholar]
- (7).Rühl A, Hoppe S, Frey I, Daniel H, Schemann M. Functional expression of the peptide transporter PEPT2 in the mammalian enteric nervous system. J. Comp. Neurol. 2005;490:1–11. doi: 10.1002/cne.20617. [DOI] [PubMed] [Google Scholar]
- (8).Herrera-Ruiz D, Wang Q, Gudmundsson OS, Cook TJ, Smith RL, Faria TN, Knipp GT. Spatial expression patterns of peptide transporters in the human and rat gastrointestinal tracts, Caco-2 in vitro cell culture model, and multiple human tissues. AAPS PharmSci. 2001;3(1):E9. doi: 10.1208/ps030109. PMID: 11741260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Charrier L, Merlin D. The oligopeptide transporter hPepT1: Gateway to the innate immune response. Lab. Invest. 2006;86:538–546. doi: 10.1038/labinvest.3700423. [DOI] [PubMed] [Google Scholar]
- (10).Dalmasso G, Charrier-Hisamuddin L, Nguyen HTT, Yan Y, Sitaraman S, Merlin D. PepT1-mediated tripeptide KPV uptake reduces intestinal inflammation. Gastroenterology. 2008;134:166–178. doi: 10.1053/j.gastro.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O'Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- (12).Akarawut W, Lin C-J, Smith DE. Noncompetitive inhibition of glycylsarcosine transport by quinapril in rabbit renal brush border membrane vesicles: Effect on high-affinity peptide transporter. J. Pharmacol. Exp. Ther. 1998;287:684–690. [PubMed] [Google Scholar]
- (13).Ganapathy V, Mendicino JF, Leibach FH. Transport of glycyl-L-proline into intestinal and renal brush border vesicles from rabbit. J. Biol. Chem. 1981;256:118–124. [PubMed] [Google Scholar]
- (14).Shen H, Smith DE, Yang T, Huang YG, Schnermann JB, Brosius FC., 3rd. Localization of PEPT1 and PEPT2 proton-coupled oligopeptide transporter mRNA and protein in rat kidney. Am. J. Physiol. 1999;276:F658–F665. doi: 10.1152/ajprenal.1999.276.5.F658. [DOI] [PubMed] [Google Scholar]
- (15).Shen H, Smith DE, Keep RF, Brosius FC., 3rd. Immunolocalization of the proton-coupled oligopeptide transporter PEPT2 in developing rat brain. Mol. Pharm. 2004;1:248–256. doi: 10.1021/mp049944b. [DOI] [PubMed] [Google Scholar]
- (16).Stewart BH, Chan OH, Jezyk N, Fleisher D. Discrimination between drug candidates using models for evaluation of intestinal absorption. Adv. Drug Deliv. Rev. 1997;23:27–45. [Google Scholar]
- (17).Adachi Y, Suzuki H, Sugiyama Y. Quantitative evaluation of the function of small intestinal P-glycoprotein: Comparative studies between in situ and in vitro. Pharm. Res. 2003;20:1163–1169. doi: 10.1023/a:1025088628787. [DOI] [PubMed] [Google Scholar]
- (18).Komiya I, Park JY, Kamani A, Ho NFH, Higuchi WI. Quantitative mechanistic studies in simultaneous fluid flow and intestinal absorption using steroids as model solutes. Int. J. Pharm. 1980;4:249–262. [Google Scholar]
- (19).Kou JH, Fleisher D, Amidon GL. Calculation of the aqueous diffusion layer resistance for absorption in a tube: Application to intestinal membrane permeability determination. Pharm. Res. 1991;8:298–305. doi: 10.1023/a:1015829128646. [DOI] [PubMed] [Google Scholar]
- (20).Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. Marcel Dekker Inc.; New York: 1982. pp. 409–417. [Google Scholar]
- (21).Ganapathy V, Gupta N, Martindale RG. Protein digestion and absorption. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. 4th ed. Elsevier; Burlington: 2006. pp. 1667–1692. [Google Scholar]
- (22).Fei Y-J, Kanai Y, Nussberger S, Ganapathy V, Leibach FH, Romero MF, Singh SK, Boron WF, Hediger MA. Expression cloning of a mammalian proton-coupled oligopeptide transporter. Nature. 1994;368:563–566. doi: 10.1038/368563a0. [DOI] [PubMed] [Google Scholar]
- (23).Liang R, Fei Y-J, Prasad PD, Ramamoorthy S, Han H, Yang-Feng TL, Hediger MA, Ganapathy V, Leibach FH. Human intestinal H+/peptide cotransporter: Cloning, functional expression, and chromosomal localization. J. Biol. Chem. 1995;270:6456–6463. doi: 10.1074/jbc.270.12.6456. [DOI] [PubMed] [Google Scholar]
- (24).Fei Y-J, Sugawara M, Liu JC, Li HW, Ganapathy V, Ganapathy ME, Leibach FH. cDNA structure, genomic organization, and promoter analysis of the mouse intestinal peptide transporter PEPT1. Biochim Biophys Acta. 2000;1492:145–154. doi: 10.1016/s0167-4781(00)00101-9. [DOI] [PubMed] [Google Scholar]
- (25).Bhardwaj RK, Herrera-Ruiz D, Eltoukhy N, Saad M, Knipp GT. The functional evaluation of human peptide/histidine transporter 1 (hPHT1) in transiently transfected COS-7 cells. Eur. J. Pharm. Sci. 2006;27:533–542. doi: 10.1016/j.ejps.2005.09.014. [DOI] [PubMed] [Google Scholar]
- (26).Daniel H. Molecular and integrative physiology of intestinal peptide transport. Ann Rev Physiol. 2004;66:361–384. doi: 10.1146/annurev.physiol.66.032102.144149. [DOI] [PubMed] [Google Scholar]
- (27).Sinko PJ, Balimane PV. Carrier-mediated intestinal absorption of valacyclovir, the L-valyl ester prodrug of acyclovir: 1. Interactions with peptides, organic anions and organic cations in rats. Biopharm Drug Dispos. 1998;19:209–217. doi: 10.1002/(sici)1099-081x(199805)19:4<209::aid-bdd93>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- (28).Meissner B, Boll M, Daniel H, Baumeister R. Deletion of the intestinal peptide transporter affects insulin and TOR signaling in Caenorhabditis elegans. J. Biol. Chem. 2004;279:36739–36745. doi: 10.1074/jbc.M403415200. [DOI] [PubMed] [Google Scholar]
- (29).Schinkel AH, Smit JJM, van Tellingen O, Beijnen JH, Wagenaar E, van Deemter L, Mol CAAM, van der Valk MA, Robanus-Maandag EC, te Riele HPJ, Berns AJM, Borst P. Disruption of the mouse mdr1a p-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- (30).Jonker JW, Buitelaar M, Wagenaar E, van der Valk MA, Scheffer GL, Scheper RJ, Plosch T, Kuipers F, Elferink RF, Rosing H, Beijnen JH, Schinkel AH. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15649–15654. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Hu Y, Shen H, Keep RF, Smith DE. Peptide transporter 2 (PEPT2) expression in brain protects against 5-aminolevulinic acid neurotoxicity. J. Neurochem. 2007;103:2058–2065. doi: 10.1111/j.1471-4159.2007.04905.x. [DOI] [PubMed] [Google Scholar]
- (32).Brandsch M, Knütter I, Bosse-Doenecke E. Pharmaceutical and pharmacological importance of peptide transporters. J. Pharm. Pharmacol. 2008;60:543–585. doi: 10.1211/jpp.60.5.0002. [DOI] [PubMed] [Google Scholar]