Abstract
Study Design
Randomized, placebo-controlled trial
Objective
To evaluate the efficacy of epsilon aminocaproic acid (EACA) to reduce the number of red-cell (RBC) transfusions in adult patients undergoing major spinal surgery.
Summary of Background Data
Reconstructive spinal surgery is associated with significant blood loss. The number of studies evaluating the efficacy of EACA in adult patients undergoing spinal surgery remains scarce and limited.
Methods
EACA (100 mg/kg) or placebo was administered to 182 adult patients after the induction of anesthesia followed by an infusion that was continued for 8 hours postoperatively. Primary end-points included total allogeneic RBC transfusions through postoperative day (POD) 8 and postoperative allogeneic plus autologus RBC transfusions through POD 8.
Results
Mean total allogeneic RBC transfusions were not statistically different between the groups (5.9 units EACA versus 6.9 units placebo; P=0.17). Mean postoperative RBC transfusions in the EACA group was less (2.0 units versus 2.8 units placebo; P=0.03). There was no significant difference in mean estimated intraoperative EBL (2938 cc EACA vs. 3273 cc placebo; P=0.32). Mean intensive care unit length of stay was decreased (EACA 1.8 days versus 2.8 days placebo; P=0.04). The incidence of thromboembolic complications was similar (2.2% EACA vs 6.6% placebo; P=0.15).
Conclusions
The difference in total allogeneic RBC transfusions between the groups was not statistically significant. EACA was associated with a 30% (0.8 units) reduction in postoperative RBC transfusions and a one-day reduction in ICU LOS, without an increased incidence of thromboembolic events. EACA may be considered for patients undergoing major spinal surgery. Larger studies are needed to evaluate the relationship between EACA and total RBC requirements.
Introduction
Orthopedic surgery, particularly reconstructive spinal surgeries, can be associated with significant blood loss during both the intraoperative and postoperative period. As a result, patients often receive multiple red-cell (RBC) transfusions. Despite potential benefit, RBCs may be associated with morbidity such as transmission of infectious diseases, immunosuppression, acute lung injury, transfusion reactions, and graft-vs-host reactions. (1–2) In addition, RBCs are expensive when one considers the direct costs of administering blood and the indirect costs of increased morbidity. (3) Studies that identify effective strategies to reduce patient exposure to RBCs are paramount.
Epsilon-aminocaproic acid (EACA) is a synthetic anti-fibrinolytic that acts by competitively inhibiting plasminogen activation and thereby blocking dissolution of fibrin clots. EACA is less expensive and may be associated with fewer thrombotic complications compared to other antifibrinolytic agents. (4) While EACA has been shown to decrease RBC transfusions in various surgical patient populations (5), including pediatric patients undergoing reconstructive surgery for scoliosis (6), the number of studies evaluating the efficacy of EACA in adult patients undergoing spinal surgery remains scarce and limited (7–10). The objective of this study was to evaluate the efficacy of EACA to reduce RBC transfusions in adults undergoing major spinal surgery.
Materials and Methods
General Description
We performed a prospective, randomized, double-masked, placebo-controlled trial at an academic medical center between February 2001 and February 2006 (ClinicalTrials.gov number, NCT00320619). Our local internal review board approved the study and all patients provided written informed consent. An independent interdisciplinary data and safety monitoring board (DSMB) reviewed un-blinded data at 26%, 47%, and 75% of planned enrollment to evaluate efficacy (or futility) and potential complications.
Study Population
All adult patients with a diagnosis of scoliosis, kyphosis, kyphoscoliosis, pseudoarthrosis, spinal stenosis, or spondylothesis scheduled to undergo elective spinal surgery by one of four participating orthopedic surgeons were evaluated for eligibility. Eligible procedures included anterior spinal fusion, posterior spinal fusion, anterior-posterior spinal fusion, or osteotomy. This population was chosen because our preliminary data demonstrated that these patients were more likely to require blood transfusion (94% vs 78%) compared to patients undergoing spinal surgery for other reasons. Exclusion criteria include: patients under 16 years of age, pregnancy, renal failure requiring dialysis, urgent/emergency surgery, or a known bleeding diathesis. Bleeding diathesis was defined as a documented history of an inherited bleeding disorder (hemophilia, von Willebrand’s disease), a prothrombin time ratio > 1.5 seconds, or a documented previous arterial or venous thrombosis within 1 year prior to surgery.
Randomization was performed 24–72 hours prior to the day of surgery to allow time for study drug preparation. Randomization was carried out by our investigational pharmacy using a computer-generated block sequence of 10 patients and stratified by surgeon. All study personnel, patients and care-providers were blinded to treatment allocation. To evaluate the adequacy of blinding, we surveyed 15 anesthesiologists and 15 intensive care unit nurses regarding their ability to determine treatment allocation.
Study Design
The study drug (EACA, 100 mg/kg) or an identical-appearing placebo was administered in the operating room immediately after the induction of anesthesia followed by an infusion (EACA 10 mg / kg / hr or placebo) that was continued for 8 hours postoperatively. At our institution, 68% of RBC transfusions occur within this time frame. Our hematology specialist (PMN) affirmed this dosing and regimen scheme. The infusion dose of EACA was reduced by 25% for patients with a creatinine clearance <30 cc/min.
Preoperative autologous RBC donation was performed at the discretion of the patient and surgeon. The anesthetic plan and need for transfusions were determined by the anesthesiologist and surgeon. Because a mandated uniform transfusion policy was not acceptable at our institution, we stratified randomization by surgeon. RBC transfusion was not recommended if the hemoglobin concentration was 8 gm/dl or more, except for patients >60 yrs of age or patients with pre-existing heart or lung disease, in which case, a target hemoglobin of 10 gm/dl was recommended. All RBC units were pre-storage leukoreduced. The surgeon determined the use of intraoperative blood scavenging techniques. Estimates of intraoperative blood loss were determined by the anesthesiologist.
All patients were postoperatively admitted to the admitted to the ICU and co-managed by a critical care physician lead interdisciplinary team. The need for post-operative RBC transfusion was determined jointly by the critical care physician and surgeon in the ICU and by the surgeon after ICU discharge. Shed blood in the postoperative period was not routinely re-administered. Administration of postoperative deep venous thrombosis/pulmonary embolism (DVT/PE) prophylaxis was at the discretion of the surgeon and not influenced by participation in this study. Decisions regarding discharge from the ICU and the hospital were determined jointly by the critical care physician and surgeon.
Study End-Points
The primary end-point, total allogeneic RBC transfusions, was defined as the number of allogeneic RBC units transfused per patient during the intraoperative period through postoperative day (POD) 8. At our institution, 98% of transfusions are administered by POD 8. A second primary endpoint, postoperative RBC transfusions, was added on September 15, 2003 (approximately 30% patients enrolled) based on a DSMB recommendation. Postoperative RBC transfusions, defined as the number of allogeneic plus autologous RBC units transfused post-surgery through POD 8, was added for two reasons; first, the initial primary endpoint did not account for autologous transfusions, possibly introducing bias. Second, we hypothesized that EACA may be more effective for preventing postoperative compared to intraoperative bleeding; hence, our second primary outcome included only the postoperative period.
We evaluated potential thrombotic complications of EACA (DVT, PE, transient ischemic attack, cerebral infarction, myocardial infarction, and renal failure) through POD 8. Thrombotic complications were determined based on documentation and treatment by the patient’s care team. Mandated screening was not employed due to concerns for additional cost and risk to the patient. Acute renal failure was defined as a 2-fold increase in serum creatinine during hospitalization or a new requirement for hemodialysis.
We collected data regarding potential infectious complications (wound infection, nosocomial pneumonia, central venous line infection, and urinary tract infection as defined by our Hospital Epidemiology and Infection Control department), need for reoperation due to bleeding and/or thrombosis, and in-hospital mortality. To evaluate the economic impact of EACA, we measured ICU length of stay (LOS), hospital LOS and costs of care. Costs of care included total hospital charges, excluding professional fees, and were obtained from discharge data.
Statistical Analysis
Based upon pilot data, we calculated that 182 patients would be required to detect a 30% reduction in allogeneic blood transfusions in the EACA group compared to the placebo group, assuming 90% power, two-tailed alpha of 5%, and a mean requirement of 7 (SD = 4) allogeneic blood units in the placebo group. In the absence of EACA studies in patients undergoing orthopedic surgery when this study was designed, we chose a 30% reduction in total allogeneic RBC transfusions based on the results of clinical trials evaluating other anti-fibrinolytics. A subsequent systematic review (11) supports this assumption and concluded that as compared with placebo, the use of another commonly used anti-fibrinolytic, aprotinin reduces the need for blood transfusion by 30%. Statistical analyses were based on the intention-to-treat principle and involved all patients who underwent randomization.
To evaluate the adequacy of blinding we calculated a kappa statistic to compare the chance-corrected proportional agreement between physician and nurse guesses and treatment allocation. The primary end-points were evaluated using a two-sample t-test with unequal variances. Linear regression was used to assess whether clinically important covariates (autologous donation status) or covariates in the bivariate analysis with a p value ≤ 0.10 (preoperative chronic renal disease, preoperative aspirin use, preoperative diagnosis of pseudoarthrosis, and intraoperative RBC salvage use) confounded the association of EACA and blood units transfused.
Secondary end-points were evaluated using the Mann-Whitney rank-sum test or two-sample t-test with unequal variances as appropriate. Potential thrombotic and infectious complications were compared using the Fisher’s exact test. All tests were two-tailed with the critical region defined as alpha of 0.05. All statistical analysis was performed using Stata statistical software (StataCorp. 2004, Release 8.2, College Station, TX).
Results
Study Population
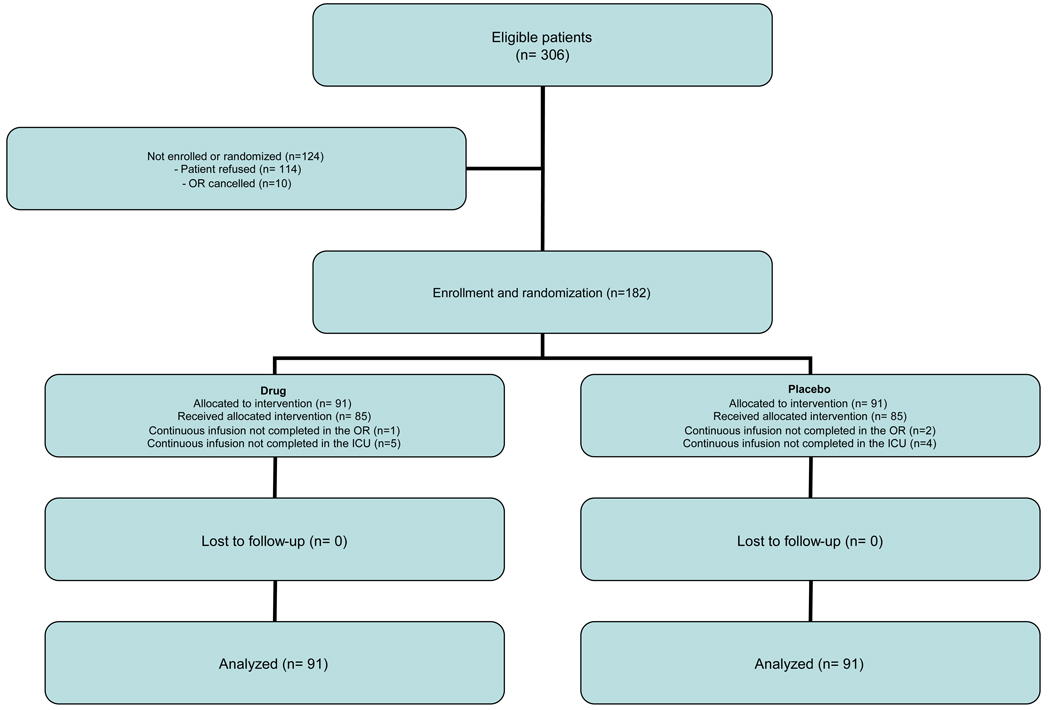
We identified 306 eligible patients, of whom 182 were enrolled and randomly assigned; 91 patients to receive EACA and 91 patients to receive placebo (Figure 1). Overall, 12 patients (6.6%) experienced a protocol violation: 6 patients (6.6%) in the EACA group and 6 (6.6%) in the placebo group. There was no relationship (kappa=0.06; p=0.72) between the clinician’s guess and the solution content.
Figure 1.
Screening and Enrollment of Study Patients.
The patient characteristics, surgeon distribution, and surgical procedure types in the two groups were similar at enrollment (Table 1). There were more patients with a preoperative diagnosis of pseudoarthrosis in the EACA group (29.7%) compared to the placebo (17.6%) Overall 33 patients (18.1%) pre-donated autologous blood. The mean baseline hemoglobin (SD) was lower in patients that pre-donated autologous blood compared to those that did not pre-donate (12.0 g/dl and 13.6 g/dl, respectively, p<0.001).
Table 1.
Baseline Characteristics*
| EACA (N = 91) |
Placebo (N = 91) |
P value | |
|---|---|---|---|
|
Patient Characteristics | |||
| Age - yrs | 55.5 ± 14.0 | 55.4 ± 15.5 | 0.96 |
| Female - no. (%) | 65 (71) | 62 (68) | 0.63 |
| White - no. (%) | 87 (96) | 82 (91) | 0.23 |
| Weight (kg) | 76.0 ± 19.0 | 75.0 ± 19.5 | 0.79 |
| ASA classification - no. (%) | |||
| 1 | 2 (2.2) | 3 (3.3) | 0.65 |
| 2 | 44 (48.3) | 43 (47.2) | 0.88 |
| 3 | 44 (48.4) | 43 (47.3) | 0.88 |
| 4 | 1 (1.1) | 2 (2.2) | 0.56 |
|
Medical History - no. (%) | |||
| Hypertension | 38 (41.8) | 33 (36.3) | 0.45 |
| Diabetes mellitus | 7 (7.7) | 9 (9.9) | 0.60 |
| Tobacco use | 34 (37.4) | 40 (44.0) | 0.37 |
| Chronic renal disease (Cr >1.2) | 3 (3.3) | 0 | 0.08 |
| Liver Disease | 6 (6.6) | 9 (9.9) | 0.42 |
| Chronic obstructive pulmonary disease | 5 (5.5) | 6 (6.6) | 0.76 |
| Any malignancy | 3 (3.3) | 4 (4.4) | 0.70 |
| Metastases from solid tumor | 3 (3.3) | 3 (3.3) | 1.0 |
| Stroke or TIA | 6 (6.6) | 10 (11.0) | 0.30 |
| Deep venous thrombosis | 4 (4.4) | 9 (9.9) | 0.15 |
| Pulmonary embolism | 4 (4.4) | 3 (3.3) | 0.70 |
| Prior myocardial infarction | 4 (4.4) | 3 (3.3) | 0.70 |
|
Preoperative Medications - no. (%) | |||
| Aspirin | 0 | 3 (3.3) | 0.08 |
| Heparin- SubQ | 0 | 1 (1.1) | 0.32 |
| NSAIDS | 1 (1.1) | 3 (3.3) | 0.32 |
| LMWH | 0 | 1 (1.1) | 0.32 |
| Steroids | 0 | 1 (1.1) | 0.32 |
| Warfarin | 0 | 1 (1.1) | 0.32 |
| Cox-2 inhibitors | 2 (2.2) | 3 (3.3) | 0.65 |
| Erythropoietin | 0 | 2 (2.2) | 0.16 |
|
Surgical Diagnosis - no. (%) | |||
| Scoliosis | 38 (41.8) | 44 (48.3) | 0.37 |
| Kyphoscoliosis | 35 (38.5) | 40 (44.0) | 0.58 |
| Pseudoarthrosis | 27 (29.7) | 16 (17.6) | 0.05 |
| Stenosis | 18 (19.8) | 22 (24.2) | 0.47 |
| Spondylothesis | 17 (18.7) | 14 (15.4) | 0.55 |
|
Surgeon - no. (%) | |||
| 1 | 34 (37.4) | 35 (38.5) | 0.88 |
| 2 | 34 (37.4) | 34 (37.4) | 1.0 |
| 3 | 20 (22.0) | 21 (23.1) | 0.86 |
| 4 | 3 (3.3) | 1 (1.1) | 0.31 |
|
Surgical Procedure - no. (%) | |||
| Anterior spinal fusion | 25 (27.5) | 28 (30.8) | 0.63 |
| Posterior spinal fusion (0–6 levels) | 27 (29.7) | 23 (25.3) | 0.50 |
| Posterior spinal fusion (>6 levels) | 52 (57.1) | 57 (62.6) | 0.45 |
| Anterior-posterior spinal fusion | 16 (17.6) | 18 (19.8) | 0.70 |
| Osteotomy | 29 (31.9) | 23 (25.3) | 0.33 |
|
Autologous donation | |||
| Patients donating - no. (%) | 13 (14.3) | 20 (22.0) | 0.18 |
| Number units per patient | 3.2 ± 1.4 | 3.5 ± 1.8 | 0.60 |
plus-minus values are mean ± SD. TIA is transient ischemic attack. NSAIDS are non-steroidal anti-inflammatory medications. LMWH is low molecular weight heparin. The baseline characteristics of patients, medical co-morbidities, preoperative medication use, surgical diagnosis, surgical attending, surgical procedure type, percent of patients pre-donating autologous blood, and the mean number of pre-donated autologous units in the two groups were similar at enrollment.
There was no significant difference between the groups in the anesthetic technique used (93.4% balanced anesthetic, 6.6% propofol and remifentanyl), use of normovolemic hemodilution (0.5%), deliberate hypotension (5.5%), RBC salvage (90%), or lowest OR hemoglobin (9.2 g/dl and 9.0 g/dl, respectively). The mean (SD) duration of surgery was similar among patients in the two groups (418 minutes and 412 minutes, respectively).
Primary End-Points
Compared to placebo, patients in the EACA group received 1 unit less of total allogeneic RBC (mean 5.9 and 6.9 units, respectively; p = 0.18), though this was not statistically significant (Table 2 and Figure 2). Patients in the EACA group received 0.8 units less postoperative RBCs compared to placebo (mean 2.0 and 2.8, respectively, p=0.03) (Figure 3). These results were similar when we adjusted for autologous donation status, preoperative chronic renal disease, aspirin use, diagnosis of pseudoarthrosis, or intraoperative RBC salvage.
Table 2.
Primary Transfusion End-Points*
| EACA (n = 91) |
Placebo (n = 91) |
P Value | |
|---|---|---|---|
| Total allogeneic RBC transfusions | |||
| Mean | 5.9 ± 4.7 | 6.9 ± 5.4 | 0.18 |
| Median | 5 (2,8) | 6 (3,10) | 0.19 |
| Postoperative RBC transfusions | |||
| Mean | 2.0 ± 1.8 | 2.8 ± 2.8 | 0.03 |
| Median | 2 (0,3) | 3 (1,4) | 0.04 |
Total allogeneic RBC transfusion is defined as the number of allogeneic RBC units transfused per patient during the intraoperative period through postoperative day (POD) 8. Postoperative RBC transfusion is defined as the number of allogeneic and autologous RBC units transfused per patient post-surgery through POD 8. P values for means determined by two-sample t-test with unequal variances; median values by Mann-Whitney rank-sum test. Plus-minus values are mean ± SD. Median expressed as median (interquartile range). When excluding RBC transfusions in the intraoperative period, the mean number of allogeneic plus autologous RBC transfusions during the postoperative period (post-surgery through POD 8) in the EACA group was significantly less than the mean in the placebo group.
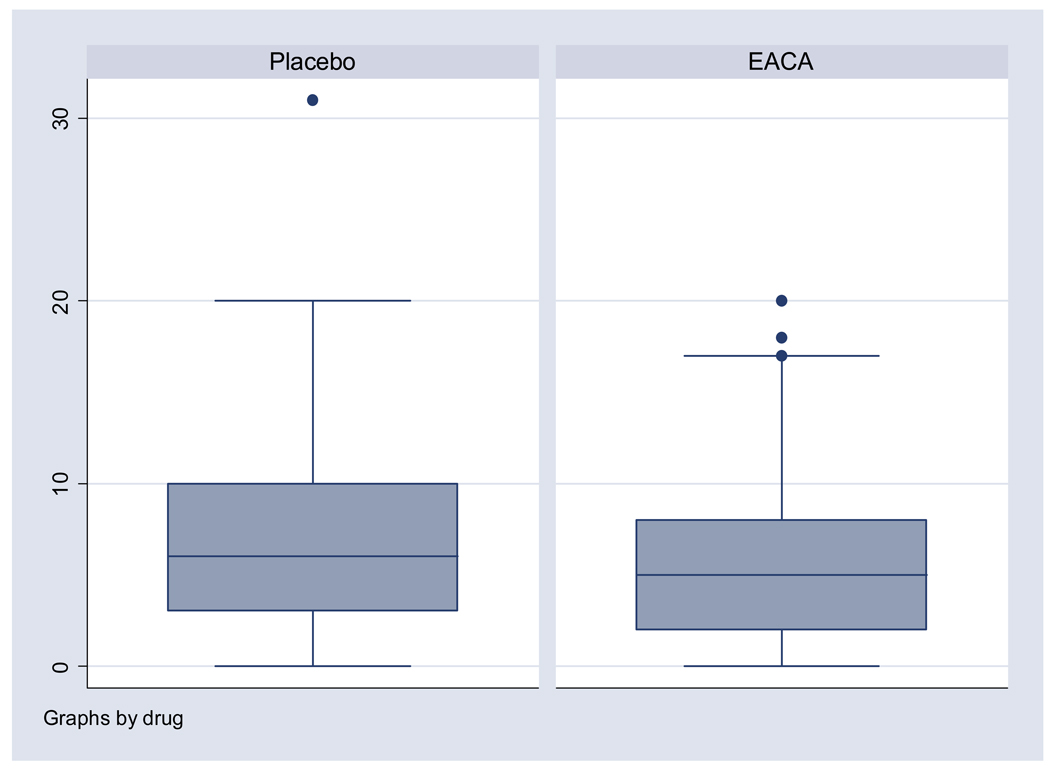
Figure 2. Total allogeneic RBC transfusions.
The box-plot represents the distribution of allogeneic RBC units transfused per patient during intraoperative period through postoperative day 8. The box represents the 25th to 75th percentile and the center line in the box represents the 50th percentile of units transfused. The bars above and below the box represent the range of units transfused. Mean and median total allogeneic RBC transfusions was only slightly decreased in the EACA group compared to the placebo group relative to the decrease in the upper range of the number of units transfused
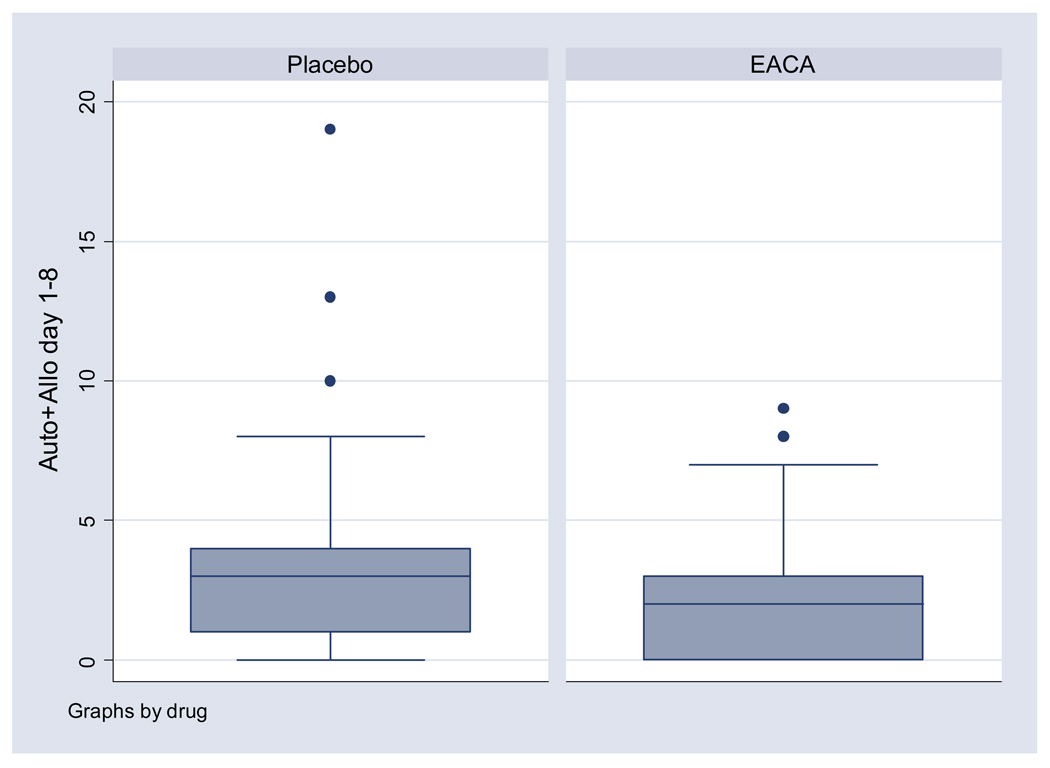
Figure 3. Postoperative RBC transfusions.
The box-plot represents the distribution of allogeneic and autologous RBC units transfused per patient during the postoperative period (post-surgery through postoperative day 8). The box represents the 25th to 75th percentile and the center line in the box represents the 50th percentile of units transfused. The bars above and below the box represent the range of units transfused. Mean and median postoperative RBC transfusions was only slightly decreased in the EACA group compared to the placebo group relative to the decrease in the upper range of the number of units transfused.
Secondary End-Points
There was no significant difference between the EACA and placebo groups in terms of intraoperative estimated-blood loss (EBL), post-surgery through POD 1 EBL, proportion receiving an allogeneic RBC transfusion (93% and 93%, respectively), number of allogeneic RBC transfusions, or number of autologous RBC transfusions (Table 3).
Table 3.
Secondary End-Points: Perioperative blood loss, transfusion and fluid requirements*
| EACA (n = 91) |
Placebo (n = 91) |
P Value | |
|---|---|---|---|
|
Estimated blood loss | |||
| Intraoperative | |||
| Mean | 2938 ± 2315 | 3273 ± 2195 | 0.32 |
| Median | 2200 (1000,4000) | 2700 (1600,4400) | 0.12 |
| Post-surgery - POD 1 | |||
| Mean | 3265 ± 2416 | 3695 ± 2341 | 0.23 |
| Median | 2440 (1300,4605) | 3020 (2095,4625) | 0.08 |
| Allogeneic RBC units transfused per patient | |||
| Intraoperative | 3.9 ± 3.7 | 4.2 ± 3.6 | 0.63 |
| Post-surgery - POD 2 | 1.4 ± 1.6 | 1.9 ± 2.6 | 0.13 |
| POD 3 – POD 8 | 0.6 ± 1.0 | 0.9 ± 1.2 | 0.12 |
|
Autologous RBC units transfused per patient | |||
| Intraoperative | 0.4 ± 1.1 | 0.6 ± 1.3 | 0.31 |
| Post-surgery - POD 2 | 0.0 ± 0.1 | 0.0 ± 0.1 | 1.0 |
| POD 3 – POD 8 | 0.0 ± 0.2 | 0 ± 0 | 0.32 |
| Intraop - POD 8 | 0.4 ± 1.1 | 0.6 ± 1.4 | 0.27 |
|
Allogeneic and autologous RBC units transfused per patient | |||
| Intraoperative | 4.3 ± 4.0 | 4.8 ± 3.8 | 0.43 |
| Post-surgery - POD2 | 1.4 ± 1.6 | 1.9 ± 2.6 | 0.13 |
| POD 3 – POD 8 | 0.6 ± 1.0 | 0.9 ± 1.2 | 0.09 |
| Intraop - POD 8 | 6.4 ± 4.9 | 7.6 ± 5.5 | 0.12 |
|
Fresh Frozen Plasma units transfused per patient | |||
| Intraoperative | 2.7 ± 3.9 | 2.6 ± 3.4 | 0.95 |
| Post-surgery - POD 2 | 0.1 ± 0.6 | 0.9 ± 4.0 | 0.10 |
| POD 3 – POD 8 | 0.0 ± 0.2 | 0.0 ± 0.2 | 1.00 |
| Intraop - POD 8 | 2.8 ± 3.9 | 3.5 ± 6.0 | 0.37 |
|
Platelet units transfused per patient | |||
| Intraoperative | 1.1 ± 3.1 | 1.3 ± 3.2 | 0.62 |
| Post-surgery - POD 2 | 0.1 ± 0.7 | 0.6 ± 2.9 | 0.12 |
| POD 3 – POD 8 | 0 | 0 | -- |
| Intraop - POD 8 | 1.2 ± 3.1 | 1.2 ± 4.8 | 0.23 |
|
Cryoprecipitate units transfused per patient | |||
| Intraoperative | 0 | 0 | -- |
| Post-surgery - POD 2 | 0 | 0.0 ± 0.2 | 0.32 |
| POD 3 – POD 8 | 0 | 0 | -- |
| Intraop - POD 8 | 0 | 0.0 ± 0.2 | 0.32 |
|
All blood product units transfused per patient | |||
| Intraoperative | 8.1 ± 10.0 | 8.7 ± 9.3 | 0.65 |
| Post-surgery - POD 2 | 1.6 ± 2.2 | 3.4 ± 8.8 | 0.07 |
| POD 3 – POD 8 | 0.7 ± 1.1 | 0.9 ± 1.2 | 0.11 |
| Intraop - POD 8 | 10.4 ± 10.8 | 13.0 ± 14.9 | 0.17 |
|
Isotonic crystalloid administered per patient | |||
| Intraoperative | 6408 ± 3430 | 6348 ± 2729 | 0.90 |
| Post-surgery - POD 1 | 8041 ± 3651 | 8394 ± 3107 | 0.48 |
|
3% saline administered per patient | |||
| Intraoperative | 220 ± 437 | 387 ± 1128 | 0.19 |
| Post-surgery - POD 1 | 285 ± 531 | 453 ± 1158 | 0.21 |
|
Colloid (Hespan, albumin) administered per patient | |||
| Intraoperative | 186 ± 408 | 100 ± 252 | 0.12 |
| Post-surgery - POD 1 | 186 ± 408 | 100 ± 252 | 0.12 |
P values determined by two-sample t-test with unequal variances Mann-Whitney rank-sum test as appropriate. Plus-minus values are mean ± SD. Median expressed as median (IQR). POD is postoperative day. There was no significant difference in the mean or median estimated intraoperative blood loss between the two groups. The mean estimated blood loss from drain output post-surgery through POD 1 was similar between the two groups, however, median estimated blood loss from drain output post-surgery through POD 1 tended to be less in the EACA group. There were no significant differences between groups when transfusion during various intervals (intraoperative period only, post-surgery through POD 2 only, or POD 3 through POD 8 only) was evaluated. There were no significant differences between groups in the volume of isotonic crystalloid, 3% normal saline, or colloid administered.
Overall, 97 patients (53.4%) received FFP, 30 patients (16.5%) received platelets, and 1 patient (0.5%) received cryoprecipitate. There was no difference between the two groups in terms of crystalloid, colloid, or other blood product resuscitation.
Laboratory data
Patients in the EACA group had lower hemoglobin values at ICU admission (10.7 ± 1.5 g/dl and 11.1 ± 1.6 g/dl, respectively, p=0.06), prothrombin time on POD 1 (12.0 ± 1.0 seconds and 12.4 ± 1.3 seconds, respectively, p=0.02), and D-Dimer values (3.9 ± 8.6 ng/ml and 7.0 ± 9.3 ng/ml, respectively, p=0.03) compared to the placebo group (Table 4).
Table 4.
Laboratory studies*
| EACA (n = 91) |
Placebo (n = 91) |
P Value | |
|---|---|---|---|
| Hemoglobin - g/dl | |||
| Preoperative | 13.3 ± 1.6 | 13.2 ± 1.4 | 0.59 |
| ICU admission | 10.7 ± 1.5 | 11.1 ± 1.6 | 0.06 |
| POD 1 | 10.2 ± 1.3 | 10.2 ± 1.3 | 0.60 |
| POD 8 | 11.2 ± 1.3 | 10.9 ± 1.1 | 0.41 |
| Serum creatinine - mg/dl | |||
| Preoperative | 0.8 ± 0.3 | 0.8 ± 0.2 | 0.89 |
| POD 1 | 0.6 ± 0.2 | 0.6 ± 0.3 | 0.85 |
| POD 8 | 0.1 ± 0.3 | 0.1 ± 0.4 | 0.98 |
| Platelet count - ×103/dl | |||
| Preoperative | 262 ± 76 | 277 ± 80 | 0.19 |
| POD 1 | 141 ± 55 | 139 ± 54 | 0.88 |
| Prothrombin time - seconds | |||
| Preoperative | 10.7 ± 0.9 | 10.9 ± 0.8 | 0.10 |
| POD 1 | 12.0 ± 1.0 | 12.4 ± 1.3 | 0.02 |
| Partial thromboplastin time - seconds | |||
| Preoperative | 28.4 ± 2.9 | 28.0 ± 3.0 | 0.40 |
| POD 1 | 28.2 ± 6.3 | 28.0 ± 4.3 | 0.86 |
| D-dimer - ng/ml | |||
| Preoperative | 2.1 ± 2.4 | 1.8 ± 1.3 | 0.31 |
| POD 1 | 3.9 ± 8.6 | 7.0 ± 9.3 | 0.03 |
P values determined by two-sample t-test with unequal variances Mann-Whitney rank-sum test as appropriate. Plus-minus values are mean ± SD. POD is postoperative day. ICU is intensive care unit. Hemoglobin values at ICU admission tended to be lower in the EACA group compared to the placebo group. Prothrombin time and DDimer values on POD 1 were statistically less in the EACA group compared to the placebo group.
Complications
Overall, five patients (2.7%) developed an infectious complication, eight (4.4%) developed a thrombotic complication, and one died (placebo group), likely due to a hospital-acquired pneumonia (Table 5). There was no difference in complications between the two groups.
Table 5.
Complications and Outcomes*
| EACA (n = 91) |
Placebo (n = 91) |
P Value | |
|---|---|---|---|
| Potential complications of transfusion - no. (%) | |||
| Wound infection | 0 | 0 | -- |
| Nosocomial pneumonia | 1 (1.1) | 2 (2.2) | 0.56 |
| Central venous line infection | 0 | 0 | -- |
| Urinary tract infection | 1 (1.1) | 1 (1.1) | 1.00 |
| Any infectious complication | 2 (2.2) | 3 (3.3) | 0.65 |
| Potential complications of EACA - no. (%) | |||
| Deep venous thrombosis | 0 | 2 (2.2) | 0.16 |
| Cerebral infarction/ TIA | 0 | 1 (1.1) | 0.32 |
| Myocardial infarction | 0 | 0 | -- |
| Pulmonary embolism | 1 (1.1) | 3 (3.3) | 0.31 |
| Acute renal failure | 1 (1.1) | 1 (1.1) | 1.00 |
| Any thrombotic complication | 2 (2.2) | 6 (6.6) | 0.15 |
| Potential surgical complications - no. (%) | |||
| Reoperation due to bleeding | 0 | 2 (2.2) | 0.16 |
| Outcomes - no. (%) | |||
| In-hospital mortality | 0 | 1 (1.1) | 0.32 |
| ICU length of stay - days | 1.8 ± 1.6 | 2.8 ± 4.6 | 0.04 |
| Hospital length of stay - days | 8.5 ± 3.9 | 9.5 ± 8.6 | 0.32 |
| Total hospital charges - dollars | 62,344 ± 27,497 | 68,670 ± 32,141 | 0.16 |
P values determined by Fisher’s exact test, two-sample t-test with unequal variances or Mann-Whitney rank-sum test as appropriate. Plus-minus values are mean ± SD. TIA is transient ischemic attack. ICU is intensive care unit. There were no differences in the incidence of clinically identified infections or thromboembolic complications. ICU LOS in the EACA group was significantly less, however, in-hospital LOS and charges were similar between the groups.
Economic end-points
Intensive care unit LOS in the EACA group was one-day less compared to placebo (mean of 1.8 days and 2.8 days, respectively, p=0.04) (Table 5). There were no significant differences in hospital LOS or charges between the groups.
Discussion
In this study of patients undergoing major spinal surgery, the difference in the total number of allogeneic RBC transfusions between the EACA and placebo groups (5.9 units and 6.9 units, respectively) was not statistically significant. There was a 30% (0.8 unit) reduction in mean postoperative RBC transfusions and a one-day reduction in ICU LOS among patients receiving EACA compared to placebo.
Concerns of patients and clinicians regarding the safety of transfused blood have generated enthusiasm for the use of technologies intended to reduce the use of allogeneic blood. To reduce RBC transfusion exposure several pharmacological agents have been used, including the anti-fibrinolytic drugs aprotinin, transexamic acid and EACA. A recent systematic review of 211 RCTs and over 20,000 patients concluded that anti-fibrinolytic drugs provide worthwhile reductions in the need for allogeneic RBC transfusion. (5) Nevertheless, the authors appropriately concluded that additional studies are needed to evaluate the efficacy and safety of EACA in non-cardiac surgery.
This is the largest RCT to date to evaluate the effect of EACA on RBC transfusions in adults undergoing spinal surgery. Prior studies evaluating the efficacy of EACA in this population have produced conflicting results. (7–10) In addition, several of these studies have significant limitations that may jeopardize the validity of their results. For example, of the three randomized trials that have evaluated the effect of EACA on RBC transfusion requirements in patients undergoing spinal surgery (7–9), one evaluated pediatric scoliosis patients and concluded that EACA was an effective method to significantly reduce perioperative blood loss and autologous transfusion requirements, however none of these patients received an allogeneic transfusion. (9) Two studies (7–8) concluded that EACA did not reduce transfusion exposure; however these studies included fewer patients than our study. The remaining study (10) used a historical control and the observed benefit of EACA may have been due to improvements in surgical treatment, patient selection, or changes in criteria for transfusion between the treatment periods. Furthermore, studies that lack randomization may overestimate treatment effects. (12)
The clinical significance of a 0.8 unit reduction in postoperative RBC transfusions remains unclear. Importantly, studies suggest there is a dose-dependent relationship between the number of transfusions and potential risks associated with transfusion. (13) Furthermore, the magnitude of postoperative RBC transfusion reduction in our study is consistent with other commonly used antifbrinolytic agents. For example, the use of aprotinin or transexamic acid is associated with a 30% reduction in blood transfusion and prevents approximately 1 RBC transfusion per surgery. (11)
We recognize several potential limitations of this study. First, although patients in the EACA group received approximately 1 less total allogeneic RBC transfusion compared to patients in the placebo group (5.9 units vs 6.9 units, respectively), this was not statistically significant. We believe this was because significant variation in transfusion requirements (SD 4.7 units EACA and 5.4 units placebo group) decreased the precision of our effect estimate (wider 95% CI) and limited our ability to make inferences about the impact of EACA on total RBC transfusion requirements. Based on this variability, we would have needed approximately 1088 patients to detect a one-unit reduction in total RBC transfusions.
The second limitation was the lack of a uniform transfusion policy, which may have contributed to variation in transfusion practice. To minimize this potential impact, we stratified randomization by surgeon and blinded providers to treatment allocation. Nevertheless, increased variation would likely make it more difficult to identify a difference in transfusions between the two groups. For example, the absence of a transfusion protocol among 78 RCTs evaluating aprotinin decreased the precision of the effect estimate. (5) Finally, lack of a uniform transfusion policy likely represents customary practice at most hospitals and therefore, increases the generalizability of our results.
Third, it is unclear why EACA may have lead to a reduction in ICU LOS and we are unable to exclude other reasons. Fourth, this study was conducted at one hospital in select high risk patients that required six to seven RBC transfusions, which may decrease the generalizability of our results. Finally, while the use of EACA in our study was not associated with an increased incidence of adverse events, we acknowledge that this study was not powered to detect rare, though potentially important complications associated with EACA.
Conclusions
In this study of adults undergoing major spinal surgery, the difference in total allogeneic RBC transfusions between the groups was not statistically significant. There was a 30% (0.8 unit) reduction in postoperative RBC transfusions and a one-day reduction in ICU LOS among patients in the EACA group, without an increased incidence of thromboembolic events. EACA may be considered for patients undergoing major spinal surgery. Larger studies are needed to evaluate the relationship between EACA and total RBC requirements.
Key Points (3–5)
In this masked placebo-controlled trial 182 adult patients undergoing major spinal surgery were randomized to receive epsilon aminocaproic acid (EACA) or placebo.
The difference in total allogeneic RBC transfusions between the groups (5.9 units and 6.9 units respectively) was not statistically significant.
EACA was associated with a 30% (0.8 unit) reduction in postoperative RBC transfusions and a one-day reduction in ICU LOS.
EACA was not associated with an increased incidence of thromboembolic complications.
Acknowledgments
This work was supported a grant (K23HL70058-01) from the NIH, National Heart, Lung and Blood Institute and the Johns Hopkins GCRC. This work was presented as a poster during the 55th Annual Meeting of the Association for University Anesthesiologists, Durham, North Carolina, May 15–18, 2008
The authors would like to thank the members of the data safety and monitoring board, Lawrence J. Appel, MD, MPH (chair), Professor of Medicine, Epidemiology and International Health, The Johns Hopkins Medical Institutions, Baltimore, MD, USA; Kurtis Campbell, MD, Assistant Professor, Department of Surgery, The Johns Hopkins University, Baltimore, MD, USA; Noah Cohen, VMD, MPH, PhD, DACVIM, Associate Professor, Texas A&M University, TX, USA; Karen E. King, MD, Assistant Professor, Pathology and Oncology, The Johns Hopkins University, Baltimore, MD, USA; Elizabeth Garrett-Mayer, PhD, Associate Professor of Biostatistics, Bioinformatics, and Epidemiology, Medical University of South Carolina, Charleston, SC, USA; Alvaro Muñoz, PhD, Professor of Epidemiology, Bloomberg School of Public Health, The Johns Hopkins University, Baltimore, MD, USA.
Contributor Information
Sean M. Berenholtz, Departments of Anesthesiology / Critical Care Medicine and Surgery, The Johns Hopkins University School of Medicine.
Julius Cuong Pham, Departments of Anesthesiology / Critical Care Medicine and Emergency Medicine The Johns Hopkins University School of Medicine.
Elizabeth Garrett-Mayer, Associate Professor of Biostatistics, Bioinformatics, and Epidemiology Medical University of South Carolina.
Christine W Atchison, Department of Anesthesiology / Critical Care Medicine, The Johns Hopkins University School of Medicine.
John P. Kostuik, Department of Orthopaedic Surgery, The Johns Hopkins University School of Medicine.
David B. Cohen, Department of Orthopaedic Surgery, The Johns Hopkins University School of Medicine.
Shantanu Nundy, Johns Hopkins University School of Medicine.
Todd Dorman, Departments of Anesthesiology / Critical Care Medicine, The Johns Hopkins University School of Medicine.
Paul M. Ness, Departments of Pathology and Medicine, The Johns Hopkins University School of Medicine.
Michael J. Klag, Departments of Medicine and Health Policy and Management, The Johns Hopkins University Schools of Medicine and Hygiene and Public Health.
Peter J. Pronovost, Departments of Anesthesiology / Critical Care Medicine, Surgery, and Health Policy and Management, The Johns Hopkins University Schools of Medicine and Hygiene and Public Health.
Khaled M. Kebaish, Department of Orthopaedic Surgery, The Johns Hopkins University School of Medicine.
References
- 1.Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP. Transfusion Medicine. First of two parts: Blood transfusion. NEJM. 1999;340(6):438–447. doi: 10.1056/NEJM199902113400606. [DOI] [PubMed] [Google Scholar]
- 2.Blajchman MA, Vamvakas EC. The continuing risk of transfusion-transmitted infections. N Engl J Med. 2006;355:1303–1305. doi: 10.1056/NEJMp068178. [DOI] [PubMed] [Google Scholar]
- 3.Spahn DR, Casutt M. Eliminating blood transfusions: New aspects and perspectives. Anesthesiology. 2000;93(1):242–255. doi: 10.1097/00000542-200007000-00035. [DOI] [PubMed] [Google Scholar]
- 4.Mannucci PM, Levi M. Prevention and Treatment of Major Blood Loss. N Engl J Med. 2007;356:2301–2311. doi: 10.1056/NEJMra067742. [DOI] [PubMed] [Google Scholar]
- 5.Henry DA, Carless PA, Moxey AJ, O’Connell D, Stokes BJ, McClelland B, Laupacis A, Fergusson D. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2007;17(4) doi: 10.1002/14651858.CD001886.pub2. CD001886. [DOI] [PubMed] [Google Scholar]
- 6.Thompson GH, Florentino-Pineda I, Poe-Kochert C. The role of amicar in decreasing perioperative blood loss in idiopathic scoliosis. Spine. 2005 Sep 1;30(17 Suppl):S94–S99. doi: 10.1097/01.brs.0000175188.05542.a9. [DOI] [PubMed] [Google Scholar]
- 7.Urban MK, Beckman J, Gordon M, Urquhart B, Boachie-Adjei O. The efficacy of antifbrinolytics in the reduction of blood loss during complex adult reconstructive spine surgery. Spine. 2001;26(10):1152–1156. doi: 10.1097/00007632-200105150-00012. [DOI] [PubMed] [Google Scholar]
- 8.Amar D, Grant FM, Zhang H, Boland PJ, Leung DH, Healey JA. Antifibrinolytic therapy and perioperative blood loss in cancer patients undergoing major orthopedic surgery. Anesthesiology. 2003;98(2):337–342. doi: 10.1097/00000542-200302000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Florentino-Pineda I, Thompson GH, Poe-Kochert C, Huang RP, Haber LL, Blakemore LC. The effect of amicar on perioperative blood loss in idiopathic scoliosis: the results of a prospective, randomized double-blind study. Spine. 2004;29(3):233–238. doi: 10.1097/01.brs.0000109883.18015.b9. [DOI] [PubMed] [Google Scholar]
- 10.Florentino-Pineda I, Blakemore LC, Thompson GH, Poe-Kochet C, Adler P, Tripi P. The effect of epsilon-aminocaproic acid on perioperative blood loss in patients with ideopathic scoliosis undergoing posterior spinal fusion: a preliminary prospective study. Spine. 2001;26(10):1147–1151. doi: 10.1097/00007632-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 11.Henry DA, Moxey AJ, Carless PA, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2001;1 doi: 10.1002/14651858.CD001886. CD001886. [DOI] [PubMed] [Google Scholar]
- 12.Ioannidis JPA, Haidich A, Pappa M, et al. Comparison of Evidence of Treatment Effects in Randomized and Nonrandomized Studies. JAMA. 2001;286(7):821–830. doi: 10.1001/jama.286.7.821. [DOI] [PubMed] [Google Scholar]
- 13.Carson JL, Altman DG, Duff A, Noveck H, Weinstein MP, Sonnenberg FA, Hudson JI, Provenzano G;Risk of bacterial infection associated with allogeneic blood transfusion among patients undergoing hip fracture repair Transfusion 199939694–700. [DOI] [PubMed] [Google Scholar]
- 14.Mangano DT, Tudor IC, Dietzel C. The risk associated with aprotinin in cardiac surgery. N Engl J Med. 2006;354:353–365. doi: 10.1056/NEJMoa051379. [DOI] [PubMed] [Google Scholar]
- 15.Ferraris VA, Bridges CR, Anderson RP. Aprotinin in cardiac surgery. N Engl J Med. 2006;354:1953–1957. doi: 10.1056/NEJMc066081. [DOI] [PubMed] [Google Scholar]
- 16.Hiatt WR. Observational studies of drug safety — aprotinin and the absence of transparency. N Engl J Med. 2006;355:2171–2173. doi: 10.1056/NEJMp068252. [DOI] [PubMed] [Google Scholar]
- 17.Okubadejo GO, Bridwell KH, Lenke LG, et al. Aprotinin may decrease blood loss in complex adult spinal deformity surgery, but it may also increase the risk of acute renal failure. Spine. 2007;32(20):2265–2271. doi: 10.1097/BRS.0b013e31814ce9b0. [DOI] [PubMed] [Google Scholar]