Abstract
Objective
Offspring of patients with type 2 diabetes (OPDs) exhibits endothelial dysfunction (ED) associated with a chronic inflammatory state. N-3 polyunsaturated fatty acids (n-3 PUFA) may have antioxidant and anti-inflammatory properties that are beneficial for cardiovascular and metabolic health. Therefore, in the present study, we tested the hypothesis that dietary supplementation with fish oil rich in n-3 PUFA may improve ED in otherwise healthy OPDs.
Methods and design
A double-blind, placebo-controlled trial was conducted with 50 OPDs. Participants were randomized to treatment with either placebo or n-3 PUFA (2 g/day) for 12 weeks. Before and after treatment we evaluated endothelial function (using flow-mediated dilation (FMD) of the brachial artery), circulating inflammatory markers (adiponectin, TNF-α, and high sensitivity-CRP), and insulin resistance (QUICKI).
Results
No significant changes were observed in study outcomes in subjects treated with placebo. By contrast, when compared with baseline values, subjects treated with n-3 PUFA had significant improvement in FMD (9.1 ± 5.8% vs. 11.7 ± 4.4%, p = 0.02) that was accompanied by decreased plasma triglycerides (117 ± 73 mg/dl vs. 86 ± 44 mg/dl, p = 0.001) and TNF-α levels (8.9 ± 2.3 pg/ml vs. 6.8 ± 2.7 pg/ml, p = 0.001), and a trend towards increased plasma adiponectin levels (7.8 ± 4.5 μg/ml vs. 9.5 ± 5.1 μg/ml, p = 0.09). When data were analyzed by multiple regression analysis, decreased TNF-α after treatment with n-3 PUFA predicted increased FMD.
Conclusion
Dietary supplementation with n-3 PUFA significantly improved endothelial function and reduced pro-inflammatory markers in OPDs. Thus, fish oil consumption may have beneficial cardiovascular and metabolic health effects in otherwise healthy subjects predisposed to diabetes and its vascular complications.
Keywords: Fish oil, Type 2 diabetes, Endothelial function, TNF-alpha, Inflammation
1. Introduction
Endothelial dysfunction (ED) is a major contributor to the pathogenesis of cardiovascular disease (CVD) [1,2]. ED is frequently observed in normoglycemic offspring of patients with type 2 diabetes (OPDs) [3]. Indeed, several clinical studies demonstrate that OPDs have impaired endothelium-mediated vasodilation that may be linked to underlying cardiovascular risk factors including insulin resistance and increased circulating pro-inflammatory markers [4]. We previously reported that OPDs, characterized by cardiometabolic abnormalities, have concomitant ED [5–7]. Moreover, several clinical trials demonstrate that therapy with antioxidant or antidiabetic agents ameliorates insulin resistance and the pro-inflammatory state in OPDs [8–10]. However, only few studies have tested effects of pharmacological interventions to improve impaired endothelial vascular reactivity of OPDs [11].
Evidence from both animal and human studies suggests that n-3 polyunsaturated fatty acids (n-3 PUFA) including eicosapentaenoic (EPA) and docosahexaenoic (DHA) acid have anti-inflammatory properties [12]. Moreover, dietary supplementation with fish oil rich in n-3 PUFA positively impacts vascular function and reduces CVD in the setting of atherosclerosis [13]. Therefore, in the present study, we tested the hypothesis that dietary supplementation with fish oil rich in n-3 PUFA may improve ED by normalizing lipid abnormalities and decreasing the pro-inflammatory state in OPDs that is suggested by elevated levels of circulating pro-inflammatory cytokines and other biomarkers [4]. To investigate this hypothesis we carried out a randomized double-blind, placebo-controlled clinical trial in 50 OPDs for 12 weeks.
2. Materials and methods
2.1. Subjects
We studied 50 healthy subjects (age 29.9 ± 6.2 years, 25 men and 25 women) with ≥1 parent with a diagnosis of type 2 diabetes (DM2). Subjects were recruited and completed all phases of the study at the Clinical Centre for Atherosclerosis at the University of Rome “Tor Vergata”.
All of the participating women reported regular menstrual cycles without oral contraceptives therapy. In female subjects, all experiments were performed during the first week of the menstrual cycle. None of the study’s participants were taking any medication (including aspirin or vitamin supplements) at the time of the study. Exclusion criteria included history or evidence of hypertension (blood pressure (BP) >140/90 mmHg), diabetes mellitus according to the American Diabetes Association criteria [14], CVD, peripheral vascular disease, or coagulopathy. The study protocol was approved by the University of Tor Vergata Institutional Review Board and all participants submitted written informed consent for all procedures.
2.2. Study design
This was a prospective, placebo-controlled, randomized, double-blind, parallel-group trial designed to compare effects of daily intake of 2 g of n-3 PUFA vs. placebo over 12 weeks. We evaluated effects of the treatments at the beginning (day 0) and end (day 84) of the study in two parallel groups of healthy OPDs. None of the participants were taking medications or other supplements that are known to influence endothelial function, metabolic parameters, or markers of inflammation. Each participant was asked to take one capsule twice daily for 84 days. Each capsule contained either 1000 mg of n-3 PUFA (≥85% ethylic esters of EPA + DHA in a ratio of 0.9–1.5:1) or 1000 mg of refined olive oil (placebo). Compliance with treatment was assessed by capsule counts at the end of the study.
2.3. Endothelial function test
Assessment of endothelial function was conducted in the fasting state using a standardized procedure at approximately the same point in time and before all of the scheduled metabolic tests. All studies were performed in the morning in a quiet room with a temperature of about 22 °C. Participants were asked to refrain from drinking alcohol or beverages containing caffeine for at least 24 h before the test, and all study subjects fasted for at least 10 h before the study day. Endothelium-dependent and -independent vasodilator functions were assessed as previously reported [5–7]. Briefly, subjects lay supine on a bed and were allowed to rest for at least 10 min. Then, the left brachial artery was visualized from a distance of 2–15 cm proximal to the antecubital fossa by using a high-resolution ultrasound (ATL HDI 3000, with a 7.5-MHz linear array transducer, Philips Medical Systems, Best, Netherlands). After baseline images and flow measurements were obtained, a pressure cuff applied on the upper arm was inflated to 200–250 mmHg for 5 min. Blood flow was measured during the 15 s following cuff release, and arterial images that measured diameter were acquired between 60 and 90 s after cuff deflation. Flow-mediated dilation (FMD) was calculated as the increase in post-stimulus diameter as a percentage of the baseline diameter. After at least a 15-min rest, endothelium-independent vasomotor responsiveness was assessed by acquiring images and flow measurements before and after administration of 0.4 mg sublingual nitroglycerin. Blood flow and images for arterial diameter were recorded between 3 and 4 min after nitroglycerin administration. The nitroglycerin-mediated dilation (NMD) was calculated as the increase in post-stimulus diameter as a percentage of the baseline diameter. For both FMD and NMD, arterial diameter was measured from the anterior to the posterior endothelial–lumen interface at the end diastole, coincident with the R wave on the electrocardiogram. Images were then coded and analyzed by an investigator blinded to the image sequence and to the treatment arm of subjects.
2.4. Laboratory methods
After at least 10 h of fasting (at the first and last study day), weight, height, and waist circumference measurements were obtained. Body mass index (BMI) was calculated as weight (kg) divided by height (m2). BP was measured in subjects in a sitting position after a 5-min rest using a mercury sphygmomanometer. The average of three measurements was used to calculate systolic and diastolic BPs. Blood samples were obtained (in the fasting state) from all patients and multiple aliquots of serum were stored at −80 °C until analysis. Samples were divided in aliquots that were frozen and thawed only once. The following circulating markers were evaluated: serum high sensitivity C reactive protein (hs-CRP; nephelometric assay, Dade-Behring, Liederbach, Germany); CD40L levels (ELISA; Bender MedSystem, Vienna, Austria); V-CAM, I-CAM, IL-6, E-selectin, and TNF-α levels (ELISA; Bender MedSystem); adiponectin was measured by ELISA using a high sensitivity commercial kit for total adiponectin (Alpco Diagnostics, USA).
2.5. Metabolic evaluation
After endothelial function testing, each subject underwent a standard OGTT. Seventy-five grams of glucose was given orally and venous blood samples were drawn at 0, 30, 60, 90, and 120 min after glucose ingestion for determination of blood glucose and plasma insulin concentrations. A surrogate index of insulin sensitivity (quantitative insulin sensitivity check index [QUICKI] [15] was calculated as described.
2.6. Statistics
Data for each of the study outcome variables were checked for normality of distribution where appropriate using the Kolmogorov–Smirnof test. Quantitative variables were expressed as mean ± S.D. if normally distributed, and as median (with interquartile range) if non-normally distributed. Categorical variables were presented as numbers. For normally distributed data, unpaired Student’s t-tests were used to compare each of the reported study outcomes for NGT and IGT subjects where appropriate. Moreover, when appropriate, analysis of covariance was used to further evaluate differences in the study outcome parameters of interest. For variables with non-normal distributions, the non-parametric Mann–Whitney test was used to evaluate whether differences in study outcomes were statistically significant. In the study treatment arms, a paired t-test or Wilcoxon test was used as appropriate to analyze the effect of n-3 PUFA or placebo on study parameters that were evaluated. Correlations were evaluated by univariate and stepwise forward multivariate regression analysis. A p-value of less than 0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS for Windows (version 14.0).
3. Experimental results
Results from OGTT identified 33 subjects with normal glucose tolerance (NGT; 2-h post-load glucose level <7.8 mmol/L), and 17 subjects with impaired glucose tolerance (IGT; 2-h post-load glucose level between 7.8 and 11.1 mmol/L). After participants were randomized to placebo or n-3 PUFA treatment, the subject distributions according to OGTT results were as follows: PUFA–NGT (n = 16), PUFA–IGT (n = 10) and placebo–NGT (n = 17), placebo–IGT (n = 7).
3.1. Baseline characteristics
Table 1 reports anthropometric and biochemical features of study subjects from the entire cohort as well as from subgroup representations according to OGTT results (IGT vs. NGT subjects). Subjects with IGT were older, with higher BMI and plasma triglyceride levels. Moreover, subjects with IGT had elevated fasting insulin levels with relative insulin resistance (assessed by QUICKI) when compared with NGT individuals. FMD was significantly lower in IGT when compared with NGT subjects. However, no significant differences were observed in NMD when NGT and IGT subjects were compared.
Table 1.
Baseline clinical, anthropometric and inflammatory data of all subjects and divided according to the oral glucose tolerance test result (OGTT) (NGT vs. IGT). Values are expressed as mean ± S.D. or median (interquartile range).
| Overall cohort | Subjects divided according to OGTT |
||||
|---|---|---|---|---|---|
| n = 50 | NGT (n = 33) | IGT (N = 17) | p | p* | |
| Age (years) | 31.1 ± 5.8 | 27.6 ± 6.6 | 34.3 ± 4.1 | <0.05 | NA |
| Sex (f/m) | 25/25 | 15/18 | 10/7 | n.s. | NA |
| BMI (kg/m2) | 26.2 ± 4.3 | 24.1 ± 4.3 | 29.4 ± 4.5 | 0.01 | NA |
| Systolic pressure (mmHg) | 115.1 ± 11.2 | 114.6 ± 13.8 | 116.9 ± 10.1 | 0.45 | 0.432 |
| Diastolic pressure (mmHg) | 76.9 ± 8.5 | 75.1 ± 6.1 | 78.1 ± 10.8 | 0.40 | 0.297 |
| Fasting glucose (mg/dl) | 86.3 ± 10.3 | 81.8 ± 10.9 | 92.1 ± 10.1 | 0.04 | 0.065 |
| Fasting insulin (mU/ml) | 11.2 ± 6.3 | 8.9 ± 4.1 | 14.9 ± 7.4 | 0.01 | 0.009 |
| QUICKI | 0.35 ± 0.03 | 0.36 ± 0.03 | 0.33 ± 0.03 | 0.01 | 0.010 |
| Total cholesterol (mg/dl) | 191.8 ± 31.9 | 180.3 ± 38.0 | 207.6 ± 25.4 | 0.03 | 0.078 |
| HDL cholesterol (mg/dl) | 55.1 ± 11.9 | 55.9 ± 12.7 | 53.9 ± 11.6 | 0.10 | 0.06 |
| LDL cholesterol (mg/dl) | 121.7 ± 29.1 | 113.6 ± 31.5 | 136.7 ± 25.4 | 0.02 | 0.08 |
| Triglycerides (mg/dl) | 117.7 ± 72.9 | 88.9 ± 40.6 | 141.7 ± 76.5 | 0.01 | 0.01 |
| CD40L (ng/ml) | 7.9 (13.6-2.5) | 7.1 (11.1-2.5) | 9.3 (14.2-3.5) | 0.20 | NP |
| Adiponectin (μg/ml) | 9.2 ± 4.2 | 11.6 ± 4.9 | 5.3 ± 2.7 | 0.03 | 0.06 |
| IL-6 (pg/ml) | 1.35 (2.67–0.23) | 1.33 (2.56–0.23) | 1.39 (2.76–0.42) | 0.80 | NP |
| V-CAM (ng/ml) | 985 ± 332 | 970 ± 350 | 1010 ± 323 | 0.56 | 0.60 |
| I-CAM (ng/ml) | 281 ± 75 | 261 ± 67 | 306 ± 60 | 0.32 | 0.39 |
| E-selectin (ng/ml) | 28.8 (37.1–24.9) | 28.1 (33.8–24.9) | 31.3 (37.4–24.9) | 0.87 | NP |
| Hs-CRP (mg/l) | 1.2 (3.2-0.6) | 1.0 (2.1-0.6) | 1.5 (3.2-0.5) | 0.10 | NP |
| FMD (%) | 7.9 ± 4.0 | 9.9 ± 4.6 | 5.2 ± 3.6 | 0.02 | 0.04 |
| NMD (%) | 13.1 ± 6.0 | 15.9 ± 5.1 | 10.4 ± 6.4 | 0.08 | 0.09 |
| TNF-α (pg/ml) | 7.8 ± 3.2 | 7.3 ± 3.8 | 8.6 ± 2.0 | 0.10 | 0.10 |
Hs-CRP: high sensitivity C reactive protein; QUICKI: quantitative insulin sensitivity check index; FMD: flow mediated dilation; NMD: nitroglycerin-mediated dilation; TNF-α: tumor necrosis factor-α. p: values for differences between the two groups in univariate analysis. p*: values after adjusting for age, sex and BMI as covariates in multivariate analysis. NA: not applicable. NP: not performed.
3.2. Effects of treatment with n-3 PUFA
All subjects completed the study and the capsule counts at the end of the study revealed excellent compliance with the treatments (93.7%).
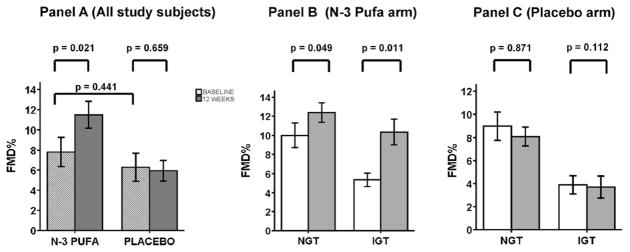
Table 2 presents baseline metabolic and inflammatory characteristics of study subjects divided according to the two arms of the study (n-3 PUFA or placebo). Table 2 also shows the values after 12 weeks of treatment and the absolute mean change (Δ) of selected variables of interest associated with n-3 PUFA or placebo treatment. No significant differences were found between baseline anthropometric, metabolic and inflammatory characteristics of n-3 PUFA and placebo treatment groups. No significant changes in metabolic or circulating inflammatory markers were observed when results before and after placebo treatment were compared. By contrast, subjects who received n-3 PUFA had significantly lower plasma triglyceride levels following treatment when compared with baseline levels. They also showed both decreases in plasma triglycerides levels and increases of FMD percent improvement (Fig. 1, Panel A). Interestingly, while both NGT and IGT subjects who received n-3 PUFA had comparable decreases in plasma triglycerides, a greater percent improvement of FMD was observed in the IGT group when compared with the NGT group (Fig. 1, Panel B). We also performed a comparison between the Δ of variables of interest (values after treatment minus baseline values) associated with n-3 PUFA and placebo treatment. Significant differences in Δ between n-3 PUFA vs. placebo groups were observed for TNF-α, triglycerides, and FMD (p < 0.05 for TNF-α and p < 0.01 for both triglycerides and FMD, Table 2).
Table 2.
Effect of n-3 PUFA and placebo supplementation on biochemical parameters. Values are expressed as mean ± S.D. or median (interquartile range).
|
n = 26 subjects (n-3 PUFA) |
n = 24 subjects (PLACEBO) |
Δ1 | Δ2 | p | p* | p° | p§ | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 weeks | Baseline | 12 weeks | |||||||
| BMI (kg/m2) | 26.1 ± 5.9 | 25.8 ± 5.5 | 25.8 ± 4.6 | 25.7 ± 4.7 | −0.5 ± 1.7 | −0.1 ± 1.8 | 0.24 | 0.24 | 0.89 | 0.25 |
| SBP (mmHg) | 114.2 ± 14.5 | 113.3 ± 11.2 | 116.7 ± 10.8 | 115.2 ± 9.8 | −1.0 ± 11.7 | −1.5 ± 10.9 | 0.40 | 0.77 | 0.29 | 0.86 |
| DBP (mmHg) | 75.2 ± 8.5 | 74.8 ± 8.4 | 77.1 ± 7.8 | 78.3 ± 7.2 | −0.4 ± 10.2 | 1.2 ± 11.8 | 0.11 | 0.85 | 0.43 | 0.57 |
| FG (mg/dl) | 89.9 ± 10.8 | 90.9 ± 9.5 | 80.0 ± 10.4 | 82.9 ± 9.4 | 1.0 ± 5.7 | 2.9 ± 6.9 | 0.09 | 0.33 | 0.12 | 0.31 |
| FI (mU/ml) | 10.4 ± 6.2 | 11.4 ± 6.3 | 11.3 ± 5.9 | 12.2 ± 5.3 | 1.0 ± 3.7 | 0.9 ± 4.0 | 0.33 | 0.18 | 0.12 | 0.90 |
| QUICKI | 0.33 ± 0.02 | 0.33 ± 0.02 | 0.34 ± 0.03 | 0.34 ± 0.02 | 0.00 ± 0.01 | 0.00 ± 0.02 | 0.31 | 0.16 | 0.11 | 0.87 |
| Tot CE (mg/dl) | 184.9 ± 40.68 | 192.1 ± 39.3 | 193.5 ± 31.6 | 193.7 ± 33.0 | 7.2 ± 16.5 | 0.2 ± 15.4 | 0.28 | 0.10 | 0.94 | 0.09 |
| LDL CE (mg/dl) | 120.6 ± 39.1 | 123.5 ± 27.7 | 121.4 ± 20.7 | 121.6 ± 21.3 | 2.8 ± 17.1 | 0.2 ± 12.4 | 0.80 | 0.41 | 0.73 | 0.60 |
| HDL CE (mg/dl) | 57.7 ± 14.6 | 60.2 ± 18.2 | 52.6 ± 8.8 | 51.8 ± 7.5 | 2.5 ± 9.6 | −0.8 ± 7.1 | 0.61 | 0.20 | 0.08 | 0.10 |
| TG (mg/dl) | 116.8 ± 72.6 | 86.2 ± 43.6 | 99.9 ± 38.5 | 97.8 ± 37.8 | −30.6 ± 40.0 | −2.1 ± 28.9 | 0.10 | <0.01 | 0.57 | <0.01 |
| CD40L (ng/ml) | 8.1 (4.2–12.4) | 8.5 (3.9–11.3) | 8.6 (4.2–11.9) | 8.8 (4.8–11.6) | 0.2 ± 10.2 | −0.1 ± 9.4 | 0.38 | 0.93 | 0.07 | 0.91 |
| Adiponectin (μg/ml) | 7.8 ± 4.5 | 9.5 ± 5.1 | 10.6 ± 5.4 | 8.4 ± 4.7 | 1.7 ± 3.1 | −2.2 ± 3.8 | 0.09 | 0.09 | 0.12 | 0.07 |
| IL-6 (pg/ml) | 1.2 (0.7–2.4) | 1.5 (0.1–2.9) | 1.4 (0.3–2.8) | 1.5 (0.3–3.4) | 0.3 ± 3.0 | 0.2 ± 3.4 | 0.51 | 0.86 | 0.65 | 0.82 |
| V-CAM (ng/ml) | 987 ± 298 | 923 ± 321 | 1012 ± 307 | 999 ± 284 | −64 ± 205 | −13 ± 167 | 0.50 | 0.30 | 0.65 | 0.92 |
| I-CAM (ng/ml) | 278 ± 68 | 261 ± 78 | 289 ± 77 | 281 ± 71 | −18 ± 41 | −8 ± 49 | 0.68 | 0.80 | 0.88 | 0.83 |
| E-selectin (ng/ml) | 25.3 (20.1–27.4) | 24.8 (21.6–30.1) | 28.4 (22.5–34.1) | 26.3 (19.2–37.1) | −0.7 ± 9.3 | −1.9 ± 14.2 | 0.32 | 0.66 | 0.65 | 0.56 |
| Hs-CRP (mg/l) | 1.2 (0.5–2.0) | 1.4 (0.7–2.5) | 1.6 (0.7–2.3) | 2.0 (0.6–3.1) | 0.2 ± 1.9 | 0.5 ± 2.7 | 0.20 | 0.62 | 0.43 | 0.35 |
| TNF-α (pg/ml) | 8.9 ±2.3 | 6.8 ± 2.7 | 7.6 ± 3.7 | 6.6 ± 2.7 | −2.1 ± 2.5 | −1.0 ± 2.1 | 0.13 | <0.01 | 0.76 | 0.05 |
| FMD (%) | 7.9 ± 3.8 | 11.7 ± 4.4 | 6.1 ± 3.6 | 5.9 ± 3.9 | 3.8 ± 2.9 | −0.2 ± 3.8 | 0.44 | 0.02 | 0.66 | <0.01 |
| NMD (%) | 14.5 ± 5.2 | 15.2 ± 6.8 | 13.1 ± 4.0 | 13.9 ± 4.3 | 0.7 ± 5.7 | 0.8 ± 5.4 | 0.69 | 0.47 | 0.58 | 0.43 |
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; FG: fasting glucose; FI: fasting insulin; QUICKI: quantitative insulin sensitivity check index; FMD: flow mediated dilation; NMD: nitroglycerin-mediated dilation; TNF-α: tumor necrosis factor-α. Tot CE: total cholesterol; LDL CE: low density lipoprotein cholesterol; HDL CE: high density lipoprotein cholesterol; TG: triglycerides; CD40L: CD40 ligand; IL-6: interleukin-6; V-CAM: vascular cell adhesion molecule; I-CAM: intercellular adhesion molecule; Hs-CRP: high sensitivity C reactive protein. Δ1: absolute mean change of variables of interest associated with n-3 PUFA treatment. Δ2: absolute mean change of variables of interest associated with PLACEBO treatment. p values for differences between baseline values of the two groups. p* values for differences between baseline and 12 weeks variables associated with n-3 PUFA treatment. p° values for differences between baseline and 12 weeks variables associated with PLACEBO treatment. p§ values for differences between Δ1 and Δ2.
Fig. 1.
Change in flow mediated dilation (FMD) in OPDs after n-3 PUFA or placebo treatment. Panel A: baseline FMD (hatched bars) and FMD after 12 weeks (dark grey bars) of n-3 PUFA or placebo. Panel B: white bars represent baseline FMD; grey bars represents FMD after 12 weeks of n-3 PUFA. Panel C: white bars represent baseline FMD; grey bars represents FMD after 12 weeks of placebo. NGT: normal glucose tolerance; IGT: impaired glucose tolerance. Bar graphs show data as mean ± S.E.M.
3.3. Correlation analyses
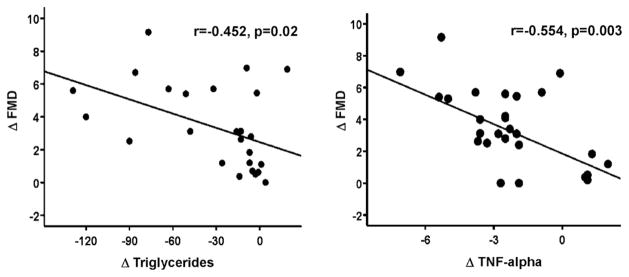
We evaluated correlations among the absolute mean change (Δ) of selected variables of interest associated with n-3 PUFA treatment after adjustment for age, gender, and BMI at baseline. In the group receiving n-3 PUFA, increase in FMD was associated with decreased plasma triglycerides (r = −0.452, p = 0.02) and TNF-α levels (r = −0.554, p = 0.003) (Fig. 2).
Fig. 2.
Correlations between changes in FMD and changes in triglyceride or TNF-α levels after 12 weeks of therapy with n-3 PUFA.
To identify parameters linked to effects of n-3 PUFA to improve endothelial function, a multiple regression model was used in which Δ BMI, Δ triglycerides, Δ TNF-α, age, and gender were considered as independent variables with Δ FMD as the dependent variable. Changes in the independent variables included in this model explained about 30% of the improvement in FMD (adjusted R2 = 0.292; F = 10.15, d.f. = 4.65, p = 0.003). Interestingly, using this analysis, changes in TNF-α levels emerged as a unique independent and significant predictor of increased FMD after n-3 PUFA treatment (β coefficient = −0.485, p = 0.025).
4. Discussion
We demonstrate that oral supplementation with fish oil rich in n-3 PUFA improves endothelial function in OPDs with or without IGT. N-3 PUFA treatment of OPDs is also associated with a significant lowering of elevated circulating TNF-α levels consistent with improvement in a chronic pro-inflammatory state. Chronic metabolic diseases such as obesity and diabetes are often associated with persistent elevations in circulating levels of pro-inflammatory cytokines such as TNF-α and other biomarkers that can be reduced by interventions that target the inflammatory component of these diseases. Therefore, a chronic pro-inflammatory state is often considered to contribute importantly to the pathophysiology of diabetes, obesity, and its cardiovascular complications [33].
We chose to treat our subjects with n-3 PUFA for 12 weeks because previous studies suggest that this may be sufficient to observe improvement in FMD [17]. The daily dose of 2 g n-3 PUFA was chosen because doses of n-3 PUFA ranging from 1 to 2 g/day used in previous studies do not adversely affect glucose homeostasis [30]. It has been reported that higher doses of n-3 PUFA may worsen glucose homeostasis in patients with DM2 [31]. Moreover, in DM2 patients, treatment with ~2 g n-3 PUFA reduced platelet aggregation [32]. Since our study subjects are at higher risk for DM2 and its cardiovascular complications due to genetic factors, we chose to exclude doses of n-3 PUFA greater than 2 g/day to avoid further complicating interpretation of our results with respect to metabolic and cardiovascular outcomes. We previously reported that OPDs of diabetic patients have metabolic and inflammatory abnormalities [5–7]. This is accompanied by ED, an early pathogenic condition that predisposes to atherosclerosis and cardiovascular complications. Moreover, a recent report of the Framingham Offspring Study identified ED as an independent risk factor for the development of diabetes [16]. Thus, improvement of ED mediated by n-3 PUFA supplementation that we observed in subjects predisposed to development of diabetes is intriguing and may have clinical relevance.
Anti-inflammatory actions of n-3 PUFA supplementation is observed in several trials demonstrating health benefits in patients with systemic lupus erythematosus, rheumatoid arthritis, psoriasis, asthma, inflammatory bowel disorders, and overt diabetes. These diseases are accompanied by increased oxidative stress and high circulating levels of pro-inflammatory cytokines in general and TNF-α in particular. TNF-α acts primarily in an autocrine/paracrine fashion [21,22]. Nevertheless, in many animal and human studies, circulating TNF-α levels are elevated in obese/metabolic syndrome models [23]. It has been suggested that the metabolic- or obesity-related elevation of circulating TNF-α may be caused by increased activity of TNF-α converting enzyme (TACE) [24], a transmembrane sheddase/metalloproteinase which cleaves pro-TNF-α into its active form at the membrane level.
Of note, in our study subjects, n-3 PUFA intake substantially decreased circulating levels of TNF-α. Moreover, a multivariate regression model demonstrated that decrease in TNF-α levels after n-3 PUFA supplementation was a significant strong predictor, explaining about 30% of the improvement in FMD. Subtle decreases in IL-6 and hs-CRP were also found. In addition, we observed that n-3 PUFA treatment resulted in trends towards lowering of markers of endothelial activation including V-CAM, I-CAM and E-selectin (although these did not reach statistical significance). Thus, our results documenting beneficial effects of n-3 PUFA in our study subjects are consistent with results recently reported in other populations with immunological diseases including lupus that are also characterized by altered endothelial function and increased levels of oxidative stress markers [22]. It is possible that with a larger sample size, significant effects on these other markers may become evident as well. Alternatively, decreased expression of endothelial adhesion molecules and dampening production of inflammatory cytokines may not be the sole mechanism underlying improvement in endothelial function with n-3 PUFA treatment. Indeed, additional mechanisms may be involved in FMD improvement including changes in circulating free fatty acid (FFA) levels [18]. Unfortunately, we were not able to directly assess FFA levels in our study because the amount of blood obtained from our study subjects was insufficient. Other limitations of our study include the relatively small sample size. In addition, the use of a single dose of n-3 PUFA did not allow us to evaluate possible dose-dependent effects. It is important to consider that our study cohort is composed of relatively young, and otherwise healthy people subjected to a limited course of therapy. This may explain, in part, why large changes in many circulating markers of inflammation were not detectable in our study. Consistent with this notion, effects of n-3 PUFA were more evident in subjects with IGT who had greater BMI and TNF-α levels at the baseline than subjects with NGT. Decreased TNF-α levels in response to n-3 PUFA therapy were not accompanied by reduced insulin levels or improved insulin sensitivity. These results are consistent with previous studies where reduction of TNF-α levels using infusion of anti-TNF-α monoclonal antibodies (infliximab) failed to improve insulin sensitivity [27]. As might be expected for young, clinically healthy individuals, none of our study participants were using medications or supplements that are known to substantially affect endothelial function or pro-inflammatory markers. In our study of OPDs, n-3 PUFA supplementation did not determine any significant change in blood pressure. By contrast, Yosefy et al. describe a significant reduction in systolic and diastolic blood pressure in patients with obesity DM2 treated with high doses of n-3 PUFA for 13 days [29]. These differences may be explained by the facts that our study subjects are younger without frank DM2 and hypertension, and are only mildly overweight.
Interestingly, effects of n-3 PUFA intake to decrease circulating TNF-α levels have been described previously [19–20]. N-3 PUFA intake may mediate membrane stabilization [25] through incorporation of n-3 PUFA into membranes that leads to increased eicosanoid membrane concentration resulting in increased membrane fluidity that may modulate several membrane protein complexes by influencing ion channel function or by negatively modulating voltage-gated sodium channels [26]. Although our study does not directly address pathophysiological mechanisms by which n-3 PUFA may decrease circulating TNF-α levels, it is possible that n-3 PUFA oral supplementation, through altering lipid membrane composition, acts as a modulator of TNF-α synthesis through targeting TACE activity.
One intriguing novel finding of the present study is the ability of n-3 PUFA supplementation to simultaneously improve endothelial function and reduce TNF-α levels. These observations are consistent with recent findings from our group that the TNF-α-neutralizing effect of infliximab is associated with increased endothelial function evaluated by intra-arterial infusion of acetylcholine in subjects with metabolic syndrome, and in particular those with increased insulin resistance [28].
It is well known that dietary supplementation with fish oil rich in n-3 PUFA positively impacts vascular function [13]. In fact, dietary omega-3 fatty acids decrease the risk of CVD. Both epidemiologic and interventional studies have demonstrated beneficial effects of n-3 PUFA on many CVD end points, including all CVD defined as all coronary artery disease, fatal and nonfatal myocardial infarction, sudden cardiac death, stroke and all-cause mortality. Much of this evidence comes from studies with fish oil interventions [34]. The supplementation of n-3 PUFA also provides a small beneficial advantage in terms of mortality and admission to hospital for cardiovascular reasons in patients with heart failure [35]. Supplementation with n-3 PUFA also reduces the progression of increases in carotid intima–media thickness [36] Therefore, findings from our present study may help to explain mechanisms underlying the protective role of n-3 PUFA in the progression of CVD related to atherosclerosis.
In conclusion, our findings suggest that dietary supplementation with n-3 PUFA significantly improves ED in OPDs and this may be mediated, in part, by lowering TNF-α levels.
These results may have important implications for identifying populations that can derive substantial benefits from early lifestyle interventions including diet and exercise. In addition, our results may lead to the development of novel therapeutic strategies in OPDs, particularly in subjects with IGT who may be more predisposed to DM2 and its vascular complications.
Acknowledgments
We thank Miss Leela Thaiparambil for technical assistance.
Funding: This manuscript was funded in part by research grants: Italian Ministero della Salute RS 2003 (to Drs. Tremoli and D. Lauro) and Italian Ministero dell’Università e della Ricerca Scientifica/Tecnologica (to Drs. Mannarino and D. Lauro).
Footnotes
Conflict of interest
No conflict of interest, potentially prejudicing the impartiality of the research reported, concerns this manuscript.
References
- 1.Kathiresan S, Gona P, Larson MG, et al. Cross-sectional relations of multiple biomarkers from distinct biological pathways to brachial artery endothelial function. Circulation. 2006;113:938–45. doi: 10.1161/CIRCULATIONAHA.105.580233. [DOI] [PubMed] [Google Scholar]
- 2.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–7. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 3.Balletshofer BM, Rittig K, Enderle MD, et al. Endothelial dysfunction is detectable in young normotensive first-degree relatives of subjects with type 2 diabetes in association with insulin resistance. Circulation. 2000;101:1780–4. doi: 10.1161/01.cir.101.15.1780. [DOI] [PubMed] [Google Scholar]
- 4.Goldfine AB, Beckman JA, Betensky RA, et al. Family history of diabetes is a major determinant of endothelial function. J Am Coll Cardiol. 2006;47(12):2456–61. doi: 10.1016/j.jacc.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 5.Tesauro M, Rizza S, Iantorno M, et al. Vascular, metabolic, and inflammatory abnormalities in normoglycemic offspring of patients with type 2 diabetes mellitus. Metabolism. 2007;56(3):413–9. doi: 10.1016/j.metabol.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Iellamo F, Tesauro M, Rizza S, et al. Concomitant impairment in endothelial function and neural cardiovascular regulation in offspring of type 2 diabetic subjects. Hypertension. 2006;48(3):418–23. doi: 10.1161/01.HYP.0000234648.62994.ab. [DOI] [PubMed] [Google Scholar]
- 7.Scuteri A, Tesauro M, Rizza S, et al. Endothelial function and arterial stiffness in normotensive normoglycemic first-degree relatives of diabetic patients are independent of the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2008;18(5):349–56. doi: 10.1016/j.numecd.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 8.Osei K, Gaillard T, Kaplow J, Bullock M, Schuster D. Effects of rosiglitazone on plasma adiponectin, insulin sensitivity, and insulin secretion in high-risk African Americans with impaired glucose tolerance test and type 2 diabetes. Metabolism. 2004;53(12):1552–7. doi: 10.1016/j.metabol.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Levin K, Hother-Nielsen O, Henriksen JE, Beck-Nielsen H. Effects of troglitazone in young first-degree relatives of patients with type 2 diabetes. Diabetes Care. 2004;27(1):148–54. doi: 10.2337/diacare.27.1.148. [DOI] [PubMed] [Google Scholar]
- 10.McSorley PT, Bell PM, Young IS, et al. Endothelial function, insulin action and cardiovascular risk factors in young healthy adult offspring of parents with Type 2 diabetes: effect of vitamin E in a randomized double-blind, controlled clinical trial. Diabet Med. 2005;22(6):703–10. doi: 10.1111/j.1464-5491.2005.01506.x. [DOI] [PubMed] [Google Scholar]
- 11.De Aguiar LG, Bahia LR, Villela N, et al. Metformin improves endothelial vascular reactivity in first-degree relatives of type 2 diabetic patients with metabolic syndrome and normal glucose tolerance. Diabetes Care. 2006;29(5):1083–9. doi: 10.2337/diacare.2951083. [DOI] [PubMed] [Google Scholar]
- 12.Rallidis LS, Paschos G, Liakos GK, et al. Dietary α-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. Atherosclerosis. 2003;167:237–42. doi: 10.1016/s0021-9150(02)00427-6. [DOI] [PubMed] [Google Scholar]
- 13.Goodfellow J, Bellamy MF, Ramsey MW, Jones JHC, Lewis MJ. Dietary supplementation with marine omega-3 fatty acids improve systemic large artery endothelial function in subjects with hypercholesterolemia. JACC. 2000;35(2):265–70. doi: 10.1016/s0735-1097(99)00548-3. [DOI] [PubMed] [Google Scholar]
- 14.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2003;26(Suppl 1):S5–20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 15.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 16.Meigs JB, O’donnell CJ, Tofler GH, et al. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: the Framingham Offspring Study. Diabetes. 2006;55(2):530–7. doi: 10.2337/diabetes.55.02.06.db05-1041. [DOI] [PubMed] [Google Scholar]
- 17.Schiano V, Laurenzano E, Revetti G, et al. Omega-3 polyunsaturated fatty acid in peripheral arterial disease: effect on lipid pattern, disease severity, inflammation profile, and endothelial function. Clin Nutr. 2008;27:241–7. doi: 10.1016/j.clnu.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Tsitouras PD, Gucciardo F, Salbe AD, Heward C, Haeman SM. High omega-3 intake improves insulin sensitivity and reduces CRP and IL-6, but does not affect other endocrine axes in healthy older adults. Horm Metab Res. 2008;40:199–205. doi: 10.1055/s-2008-1046759. [DOI] [PubMed] [Google Scholar]
- 19.Lo CJ, Chiu KC, Fu M, Lo R, Shelton S. Fish oil decreases macrophage tumor necrosis factor gene transcription by altering the NFκB activity. J Surg Res. 1999;82:216–22. doi: 10.1006/jsre.1998.5524. [DOI] [PubMed] [Google Scholar]
- 20.Novak TE, Babcock TA, Jho DH, Helton WS, Espat NJ. NF-κB inhibition by ω-3 fatty acids modulates LPS-stimulated macrophage TNF-α transcription. Am J Physiol. 2003;284:L84–9. doi: 10.1152/ajplung.00077.2002. [DOI] [PubMed] [Google Scholar]
- 21.Hotamisligil GS. Role of endoplasmic reticulum stress and c-Jun NH2-terminal kinase pathways in inflammation and origin of obesity and diabetes. Diabetes. 2005;54:S73–8. doi: 10.2337/diabetes.54.suppl_2.s73. [DOI] [PubMed] [Google Scholar]
- 22.Wright SA, O’Prey FM, McHenry MT, et al. A randomised interventional trial of omega-3-polyunsaturated fatty acids on endothelial function and disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2008;67(6):841–8. doi: 10.1136/ard.2007.077156. [DOI] [PubMed] [Google Scholar]
- 23.Kathryn E, Wellen G, Hotamisligil Obesity-induced inflammatory changes in adipose-tissue. J Clin Invest. 2003;112:1785–8. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Federici M, Hribal ML, Menghini R, et al. Timp3 deficiency in insulin receptor-haploinsufficient mice promotes diabetes and vascular inflammation via increased TNF-alpha. J Clin Invest. 2005;115:3494–505. doi: 10.1172/JCI26052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salem N, Jr, Wegher B, Mena P, Uauy R. Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc Natl Acad Sci. 1996;93(1):49–54. doi: 10.1073/pnas.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stillwell W, Wassall SR. Docosahexaenoic acid: membrane properties of a unique fatty acid. Chem Phys Lipids. 2003;126(1):1–27. doi: 10.1016/s0009-3084(03)00101-4. [DOI] [PubMed] [Google Scholar]
- 27.Di Rocco P, Manco M, Rosa G, Greco AV, Mingrone G. Lowered tumor necrosis factor receptors, but not increased insulin sensitivity, with infliximab. Obes Res. 2004;12:734–9. doi: 10.1038/oby.2004.86. [DOI] [PubMed] [Google Scholar]
- 28.Tesauro M, Schinzari F, Rovella V, et al. TNF-{alpha} antagonism improves vasodilation during hyperinsulinemia in metabolic syndrome. Diabetes Care. 2008;31(7):1439–41. doi: 10.2337/dc08-0219. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yosefy C, Viskoper JR, Laszt A, et al. The effect of fish oil on hypertension, plasma lipids and hemostasis in hypertensive, obese, dyslipidemic patients with and without diabetes mellitus. Prostaglandins Leukot Essent Fatty Acids. 1999;61(2):83–7. doi: 10.1054/plef.1999.0075. [DOI] [PubMed] [Google Scholar]
- 30.Sirtori CR, Crepaldi G, Manzato E, et al. One-year treatment with ethyl esters of n-3 fatty acids in patients with hypertriglyceridemia and glucose intolerance: reduced triglyceridemia, total cholesterol and increased HDL-C without glycemic alteration. Atherosclerosis. 1998;137:419–27. doi: 10.1016/s0021-9150(97)00298-0. [DOI] [PubMed] [Google Scholar]
- 31.Dunstan DW, Mori TA, Puddey IB, et al. The independent and combined effects of aerobic exercise and dietary fish intake on serum lipids and glycemic control in NIDDM. A randomized controlled study. Diabetes Care. 1997;20:913–21. doi: 10.2337/diacare.20.6.913. [DOI] [PubMed] [Google Scholar]
- 32.Axelrod L, Camuso J, Williams E, et al. Effects of a small quantity of omega-3 fatty acids on cardiovascular risk factors in NIDDM: a randomized, prospective, double-blind, controlled study. Diabetes Care. 1994;17:37–44. doi: 10.2337/diacare.17.1.37. [DOI] [PubMed] [Google Scholar]
- 33.Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14(3–4):222–31. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Psota TL, Gebauer SK, Kris-Etherton P. Dietary omega-3 fatty acid intake and cardiovascular risk. Am J Cardiol. 2006;21(98 4A):3i–18i. doi: 10.1016/j.amjcard.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 35.Investigators. Gissi HF, Tavazzi L, Maggioni AP, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1195–6. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 36.Mita T, Watada H, Ogihara T, et al. Eicosapentaenoic acid reduces the progression of carotid intima–media thickness in patients with type 2 diabetes. Atherosclerosis. 2007;191(1):162–7. doi: 10.1016/j.atherosclerosis.2006.03.005. [DOI] [PubMed] [Google Scholar]