Abstract
Background and Purpose
Predictive models of outcome after ischemic stroke have incorporated acute diffusion-weighted MRI (DWI) information with mixed results. We hypothesized that serial measurements of DWI infarct volume would be predictive of functional outcome after ischemic stroke.
Methods
The prospective Acute Stroke Accurate Prediction (ASAP) Study included a prespecified serial imaging subgroup who underwent DWI studies at baseline (<24 hours after symptom onset) and Day 5 (±2 days). DWI infarct volumes were calculated using the Analyze software (Rochester, Minn). Clinical outcomes were assessed at 3 months. Univariate and multivariable regression analysis was performed to assess the relationship between change in DWI lesion volume and excellent neurological outcome (modified Rankin Scale 0, 1, and Barthel Index ≥95).
Results
In total, 169 cases from the ASAP study had serial DWI scans with a measurable lesion at baseline, follow-up, or both. The median baseline National Institutes of Health Stroke Scale score was 6 (interquartile range, 3 to 13). For each 10 cm3 of growth in DWI infarct volume, the OR for achieving an excellent outcome by modified Rankin Scale was 0.52 (95% CI, 0.38 to 0.71) and for the Barthel Index was 0.64 (95% CI, 0.51 to 0.79). Adjusting for clinically important covariates, the OR for an excellent modified Rankin Scale outcome was 0.57 (95% CI, 0.37 to 0.88) and excellent Barthel Index outcome was 0.75 (95% CI, 0.56 to 1.01).
Conclusions
Based on these data, the likelihood of achieving an excellent neurological outcome diminishes substantially with growth in DWI infarct volume in the first 5 days after ischemic stroke of mild to moderate severity.
Keywords: cerebral ischemia, diffusion-weighted imaging, prognosis, stroke outcome
Functional outcomes after ischemic stroke are variable. Accurate estimates of prognosis can inform patients and families about the likelihood of recovery after stroke. In addition, prognostic estimates can assist with anticipation of rehabilitation needs and may also be useful in clinical trials for selection of potential responders, risk stratification, and to estimate outcomes for patients lost to follow-up.1 Clinical variables such as age and National Institutes of Health Stroke Scale score have consistently been associated with outcome after ischemic stroke,2–5 but the usefulness of neuroimaging to predict outcomes has not been definitively established.
Recently, interest has focused on combining clinical and neuroimaging data to develop multivariable risk adjustment models of stroke outcome. Such models may provide greater discriminatory power to predict clinical outcomes than clinical or neuroimaging information alone. Diffusion-weighted MRI (DWI) is able to demonstrate areas of cerebral infarction within hours of symptom onset.6 Studies of early DWI to predict recovery have resulted in different conclusions. Some have found a strong association between DWI and outcome,7,8 whereas others have not supported a strong relation.9,10
Previously, we combined commonly collected clinical variables and baseline DWI lesion volume data in an acute ischemic stroke prediction model to determine whether baseline DWI improves the prediction of 3-month outcome. The data suggested that DWI did not have a clinically significant effect on outcome prediction.11 In the hours to days after stroke, infarct volume is dynamic and influenced by a number of biological factors.12,13 In some cases, these changes are likely to have clinical significance14 that single measurements of infarct volume may not fully capture. The results of our prior investigation of single DWI lesion volume measurements and observations regarding the dynamic nature of cerebral infarction provided the rationale for this study.
The purpose of this study was to estimate the frequency and magnitude of change in DWI infarct volume after stroke and to determine the relationship between change in DWI lesion volume and functional outcome after stroke. Preliminary evidence of a relationship between change in stroke volume and functional outcome would justify the additional time and expense of serial MRI. The study hypothesis was that patients with evidence of significant early infarct growth as measured on serial DWI studies would be less likely to achieve excellent neurological outcomes at 90 days.
Materials and Methods
Study Population and Clinical Variables
The subjects included in this serial imaging study were participants in the Acute Stroke Accurate Prediction (ASAP) study. ASAP was a prospective, observational study designed to provide a validation data set for a previously developed predictive model of outcome after ischemic stroke.11Consecutive patients with acute ischemic stroke were enrolled at the University of Virginia over a 5-year period. Eligible subjects were ≥18 years old, received a clinical diagnosis of stroke with symptom onset ≤24 hours prior, did not have a contraindication to brain MRI, did not receive experimental therapy for the ictal event, and did not have pre-existing neurological disease that would confound clinical assessment. All patients underwent baseline clinical examination and brain DWI within 24 hours of symptom onset and each had 90-day clinical follow-up.
The ASAP protocol prespecified an early outcome substudy that included subjects who agreed to undergo follow-up DWI and clinical assessment at hospital discharge or Day 5, whichever was sooner. All ASAP subjects were invited to participate in the early outcome substudy. Positive DWI lesions were defined as hyperintensity on the DWI sequence in a clinically relevant area with a corresponding area of hypoattenuation on the apparent diffusion coefficient sequence. Subjects from the early outcome substudy with complete clinical information and any of the following imaging patterns were included in this ASAP serial imaging study: (1) positive DWI lesion at baseline and follow-up (discharge or Day 5); (2) negative DWI lesion at baseline but positive follow-up scan; or (3) positive DWI lesion at baseline but negative at follow-up. The ASAP serial imaging study eligibility criteria were used to select patients with radiographically confirmed stroke in whom at least one measurement of DWI infarct volume could be made to assess for early change. All subjects were enrolled under the University of Virginia Institutional Review Board-approved protocol and informed consent was obtained in all cases.
Baseline demographic and clinical information were prospectively captured. The National Institutes of Health Stroke Scale score was used to measure clinical stroke severity at the time of initial assessment.15 Functional outcomes after stroke were measured by the modified Rankin Scale (mRS)16 and the Barthel Index (BI)17 at the 3-month follow-up visit. Scores of ≤1 on the mRS and 95 or 100 on the BI were considered to indicate an excellent outcome.
Brain MRI
DWI was performed according to routine clinical practice on a 1.5-Tesla echoplanar scanner (Siemens Vision MRI). Axial DWI images were obtained with the following parameters: echo time 99.1/TA 30/b=1000 s/mm2, where the b value is the diffusion gradient strength (eg, b1000). Field of view was 240×240. All sequences were obtained with 7-mm section thickness.
DWI infarct volumes were measured by one of 2 readers blinded to clinical outcome using computer-assisted volumetric software (Analyze software 6.1; Biomedical Imaging Resource, Rochester, Minn). A pen-trace method was used to outline the region of interest (ie, area of acute stroke) on each axial image and the total volume was reported in cubic centimeters. In cases of multifocal infarction, the sum of the individual volumes was used. Change in DWI infarct volume was calculated by subtracting the baseline DWI infarct volume from the follow-up DWI infarct volume according to the following formula:
Change in infarct volume as calculated by this formula was termed “growth” and categorized into one of 4 mutually exclusive clinically relevant categories chosen before analysis of the data: (1) <0 cm3 (eg, follow-up infarct volume smaller than baseline); (2) >0 to 10 cm3; (3) >10 to 20 cm3; and (4) >20 cm3.
Statistical Analysis
All analyses were performed using SAS software Version 9.1 (SAS Institute, Cary, NC). Median and interquartile range (IQR) were used to summarize continuous and ordinal variables. Frequency counts and percentages were used to summarize categorical variables. χ2 test statistics and logistic regression analysis were used to assess the univariate relationship between the magnitude of DWI infarct growth and excellent neurological outcome for each outcome scale. Multivariable logistic regression techniques were used to examine the association between magnitude of DWI infarct growth and excellent neurological outcome with adjustment for possible confounding covariates. The covariates included in the model were previously demonstrated to have strong relations with excellent recovery and/or devastating outcome11,18 and included age, National Institutes of Health Stroke Scale score, time from symptom onset to DWI scan, baseline DWI lesion volume, history of disability, history of diabetes, and treatment with recombinant tissue-type plasminogen activator. The predictors age, National Institutes of Health Stroke Scale score, time to DWI, and baseline DWI lesion volume were used as continuous variables in the model. DWI infarct growth was used as a categorical variable. History of disability, history of diabetes, and recombinant tissue-type plasminogen activator treatment were dichotomized (present/absent). Model performance was measured by area under the receiver operating characteristics curve for discrimination. The prespecified threshold for excellent model performance was an area under the receiver operating characteristics curve ≥0.80. Statistical significance was defined at the α=0.05 level.
Results
Serial Imaging Study Population
In total, 209 subjects were enrolled in the ASAP early outcome substudy and underwent follow-up brain MRI. Of these, 169 subjects with a measurable DWI lesion at baseline, follow-up, or both and 3-month clinical outcome assessment were included in the serial imaging study population. Reasons for exclusion were the presence of negative baseline and follow-up DWI imaging (n=25), diagnosis of transient ischemic attack (n=10), and missing Day 5 clinical information (n=5). The baseline characteristics and 90-day outcomes of the serial imaging study population are shown in Table 1.
Table 1.
Patient Characteristics
| ASAP Serial Imaging Study (n=169) | |
|---|---|
| Baseline characteristics | |
| Median age, years (IQR) | 68 (56–78) |
| Median NIHSS score (IQR) | 6 (3–13) |
| Median DWI volume, cm3 (IQR) | 4.6 (1.0–20.7) |
| Median time to DWI, hours (IQR) | 14.4 (9.8–20.2) |
| Female sex | 81 (48%) |
| Diabetes mellitus | 58 (34%) |
| Previous disability | 29 (17%) |
| Tissue plasminogen activator treatment | 30 (18%) |
| Follow-up characteristics | |
| Median DWI volume (IQR) | 7.8 (1.6–39.5) |
| Median time to DWI, days (IQR) | 3.6 (2.9–4.5) |
| 3-month outcome | |
| Death | 14 (8%) |
| mRS ≤ (excellent) | 91 (54%) |
| BI 95 or 100 (excellent) | 104 (62%) |
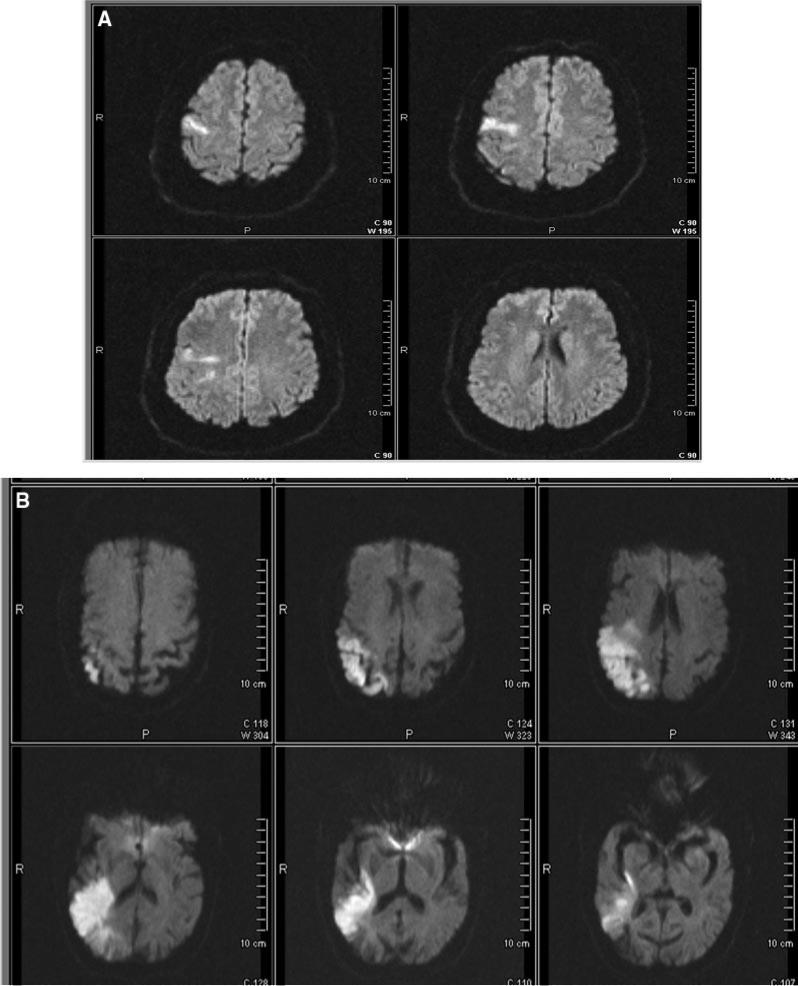
There were no significant differences in age, gender, stroke severity, time to DWI, or functional outcomes for the subjects included in the serial imaging study (n=169) compared with the overall early outcome population (n=209). The median baseline volume was 4.6 cm3 (IQR 1.0 to 20.7) and follow-up volume was 7.8 cm3 (IQR 1.6 to 39.5). Two representative DWI infarct volumes as measured by the Analyze software are shown in the Figure.
Figure.
A, DWI demonstrating a right frontal acute ischemic stroke (bright region) with a volume measurement of 3 cm3. B, DWI demonstrating a right parietotemporal ischemic stroke (bright region) with a volume measurement of 40 cm3.
Brain MRI and Functional Outcomes
Overall, 85% of subjects had measurable infarct growth between baseline and follow-up DWI studies. Twenty-eight percent of subjects had growth of 10 cm3 or more on follow-up scans. The proportion of subjects within each category of DWI lesion growth was as follows: <0 cm3, 15%; >0 to 10 cm3, 56%; >10 to 20 cm3, 8%; and >20 cm3, 21%.
The frequency of excellent outcomes in relation to category of DWI growth is shown in Table 2. χ2 analysis demonstrated a significant association between magnitude of DWI infarct growth and 90-day functional outcome as measured by the mRS (P<0.0001) and BI (P<0.0001). The proportion of patients with either shrinkage of stroke volume (growth <0 cm3) or negligible growth (<10 cm3) on follow-up scans that achieved an excellent outcome by mRS or BI was similar (approximately 70%). The proportion of patients with excellent outcome diminished with greater magnitudes of early DWI infarct growth (>10 cm3), suggesting a “dose–response” relationship between magnitude of infarct growth and outcome.
Table 2.
DWI Growth and Outcome by mRS and BI
| mRS |
BI |
||||
|---|---|---|---|---|---|
| Growth Category | Excellent Outcome, no. | Not Excellent Outcome, no. | Growth Category | Excellent Outcome, no. | Not Excellent Outcome, no. |
| <0 cm3 | 17 | 8 | <0 cm3 | 18 | 7 |
| >0–10 cm3 | 63 | 32 | >0–10 cm3 | 69 | 26 |
| >10–20 cm3 | 8 | 6 | >10–20 cm3 | 9 | 5 |
| >20 cm3 | 3 | 32 | >20 cm3 | 8 | 27 |
Univariate logistic regression analysis found that for each 10 cm3 of growth in DWI infarct volume, the OR for achieving an excellent outcome by mRS was 0.52 (95% CI, 0.38 to 0.71) and for BI was 0.64 (95% CI, 0.51 to 0.79). These results were highly significant for both outcomes (P=0.0001). The univariate logistic regression model had an area under the receiver operating characteristics curve of 0.74 for the mRS outcome and 0.73 for the BI outcome.
Adjusting for potential confounding covariates in the multivariable model resulted in an OR of 0.57 (95% CI, 0.37 to 0.88) for excellent mRS outcome and 0.75 (95% CI, 0.56 to 1.01) for excellent BI outcome. The adjusted association between DWI growth and mRS excellent outcome remained significant (P=0.01). For BI excellent outcome, the multivariable association did not reach statistical significance (P=0.056), but only slightly missed the critical value. The multivariable model had an area under the receiver operating characteristics curve of 0.86 for the mRS outcome and 0.91 for the BI outcome suggesting excellent discrimination.
Discussion
The ability of stroke volume measurements to add clinically significant information to predictive models of stroke outcome has yet to be definitively established. Single measures of infarct volume used in prior studies9,10 may not reflect the true biology of cerebral infarction and do not capture the clinical significance of changes in volume. In this study, measuring the early growth in DWI infarct volume with serial brain MRI revealed a significant association between magnitude of DWI infarct growth and 3-month functional outcomes. Our prospective results from a large ischemic stroke population confirm a similar relationship first reported by Beaulieu et al in a small study of 21 patients.14
The mechanisms underlying early DWI lesion growth are likely multifactorial. Presumably, a progressive increase in DWI lesion volume reflects expansion of the primary ischemic injury through increasing cytotoxic edema, infarction of tissue at the periphery of the lesion, or both.14 Although we were not able to assess such a relationship in this study, stroke subtype likely influences early lesion growth. Collateral flow patterns, systemic blood pressure, reperfusion, and excitotoxicity after large vessel occlusions influence early volume change. Infarction due to penetrating artery disease may be less susceptible to early changes in volume because the affected tissue is not able to recruit robust collateral flow from neighboring vascular territories.
The reduced sensitivity of the BI to changes in disability compared with the mRS and other measures of functional outcome likely explain the difference in statistical significance derived from multivariable analyses using the mRS (P=0.01) and BI (P=0.056). The BI is susceptible to a ceiling effect whereby the scale loses discriminatory power at higher levels of functioning.19 The ASAP population of predominantly mild to moderate strokes had a higher proportion of patients achieve an excellent neurological outcome as measured by the BI (62%) compared with the mRS (54%). The proportion of patients with the greatest increase in DWI growth (>20 cm3) who did not achieve an excellent outcome by mRS was greater (32 of 35) compared with the BI (27 of 35). Additionally, a greater proportion of subjects achieved an excellent BI outcome despite having the greatest magnitude of infarct growth (>20 cm3).
The rationale for using an absolute difference to calculate infarct growth in this study was 2-fold. The median baseline and follow-up infarct volumes were small (4.6 cm3 and 7.8 cm3, respectively) and calculation of relative changes would have resulted in disproportionately large percentage changes for a given absolute change in volume. In clinical practice, large percentage differences (ie, >200%) can be difficult to conceptualize. Second, categorization of infarct growth by 10-cm3 increments provides a unit of change that is simple to visually appreciate. In practice, one can easily estimate if a change in stroke volume of more than 10 cm3 has occurred by visual inspection of serial scans without the use of sophisticated volumetric software or the need for cumbersome calculations. The absolute volume change threshold for the volume change categories was chosen based on prior data that suggest 10 cm3 of incremental change is generously outside the range of measurement error.18 Infarct growth of this magnitude is likely to have biological significance. Although some relevant information may be lost through categorization of infarct growth, the strata are small enough to minimize differences within categories.
This study has several important limitations. The ASAP serial imaging study was a subgroup analysis from the larger ASAP study and as such may have been affected by selection bias. The most common reason for exclusion from the substudy was the absence of an appreciable baseline DWI abnormality. It is possible that subjects with normal DWI had areas of infarction beyond the resolution of the technique or false-negative studies.20 More likely is the possibility of focal neurological deficits caused by transient ischemic attack or nonischemic etiologies such as migraine or seizures. Although differences in some unmeasured characteristics between subjects enrolled in the substudy and the larger ASAP population may have existed, the lack of significant differences in baseline characteristics reduces the probability of bias significantly affecting these results.
The preponderance of subjects with strokes of mild to moderate severity and relatively small infarct volumes constrain the generalizability of these results. The higher clinical acuity and care requirements of severely affected patients may have reduced the priority of enrolling such patients into an observational research protocol. Additionally, those patients with milder deficits were likely more suitable to undergo serial imaging studies required for enrollment into the early outcome substudy. We cannot make valid inferences from these data regarding those with more severe deficits at stroke onset and it is possible that the relationship between change in infarct volume and functional outcome might be different in those with larger strokes compared with those with smaller strokes. Conversely, the limited range of stroke severity may have reduced the power to detect a stronger multivariable adjusted relationship between stroke volume growth and outcome, particularly for the BI outcome.
DWI infarct volume was measured by one of 2 expert readers for each subject. Intra- and interrater reliability data as quantitative measures of reproducibility were beyond the scope of this study. However, a recent study demonstrated that a single expert reader can provide consistent and repeatable measurements of DWI infarct volume using computerized software.18 The interrater percent difference was <5% for DWI for a subset of patients in the same study, which minimizes concerns related to measurement error when 2 readers are used to measure stroke volume.
Finally, it is possible that other baseline variables may be associated with clinical outcome at 3 months. Due to the small size of our sample, we were able to adjust for only 7 clinically relevant baseline variables. These variables are recognized to be strongly associated with outcome; however, variables with weaker associates may have been excluded.
Despite these limitations, this study has several important strengths. The prospective enrollment of a large number of consecutive patients with ischemic stroke from a broad stroke population limited biases associated with alternate study designs (ie, retrospective). The large number of patients with protocol-specified follow-up DWI imaging afforded a unique opportunity to study changes in DWI infarct volume after stroke. Previous reports of serial neuroimaging performed after stroke have been largely retrospective and included small numbers of patients. Additionally, collaboration with experienced neuroradiologists and the use of an advanced software package to accurately measure stroke volume enhance the internal validity of the results.
The results of this study provide evidence of a clinically significant relationship between infarct growth as measured by DWI and poststroke functional outcomes. This observation should promote further investigation of the usefulness of change in infarct volume to improve the predictive accuracy of models of stroke outcome. The threshold at which change in stroke volume is biologically significant and the optimal timing of follow-up imaging have yet to be determined. Use of evolving neuroimaging technology may further our understanding of factors relevant to early infarct growth and clinical outcomes. Including the full spectrum of stroke severity in future studies will also help to improve the generalizability of future results.
Summary
These results confirm prior observations that DWI lesions are dynamic in the early hours to days after acute ischemic stroke. The significant association between early change in DWI infarct volume after stroke and functional outcome support the conclusion that the likelihood of achieving an excellent outcome diminishes substantially with growth in DWI lesion volume (>10 cm3) in the first 5 days after stroke of mild to moderate severity. If these results are replicated in larger data sets that include a broader range of stroke severity, the incorporation of measures of change in early DWI lesion volume may improve the prognostic accuracy of stroke predictive models.
Acknowledgments
We dedicate this manuscript to Douglas P. Wagner, PhD, who died unexpectedly weeks before the completion of this project. We acknowledge Jacyln Van-Wingerden whose collaboration on this project was critical to its successful completion.
Sources of Funding
The ASAP Study and this research were funded by the National Institutes of Health–National Institute of Neurological Disorders and Stroke (K23NS02168). K.C.J. and D.P.W. received support from this grant.
Footnotes
This work was presented in part at the 33rd International Stroke Conference, 2008, New Orleans, La, February 21, 2008.
Disclosures
None.
References
- 1.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Henon H, Godefroy O, Leys D, Mounier-Vehier F, Lucas C, Rondepierre P, Duhamel A, Pruvo JP. Early predictors of death and disability after acute cerebral ischemic event. Stroke. 1995;26:392–398. doi: 10.1161/01.str.26.3.392. [DOI] [PubMed] [Google Scholar]
- 3.Adams HP, Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, Woolson RF, Hansen MD. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: a report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST). Neurology. 1999;53:126–131. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- 4.Johnston KC, Connors AF, Jr, Wagner DP, Knaus WA, Wang X, Haley EC., Jr A predictive risk model for outcomes of ischemic stroke. Stroke. 2000;31:448–455. doi: 10.1161/01.str.31.2.448. [DOI] [PubMed] [Google Scholar]
- 5.Weimar C, Konig IR, Kraywinkel K, Ziegler A, Diener HC. Age and National Institutes of Health Stroke Scale score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. Stroke. 2004;35:158–162. doi: 10.1161/01.STR.0000106761.94985.8B. [DOI] [PubMed] [Google Scholar]
- 6.Warach S, Gaa J, Siewert B, Wielopolski P, Edelman RR. Acute human stroke studied by whole brain echo planar diffusion-weighted magnetic resonance imaging. Ann Neurol. 1995;37:231–241. doi: 10.1002/ana.410370214. [DOI] [PubMed] [Google Scholar]
- 7.Baird AE, Dambrosia J, Janket S, Eichbaum Q, Chaves C, Silver B, Barber PA, Parsons M, Darby D, Davis S, Caplan LR, Edelman RE, Warach S. A three-item scale for the early prediction of stroke recovery. Lancet. 2001;357:2095–2099. doi: 10.1016/s0140-6736(00)05183-7. [DOI] [PubMed] [Google Scholar]
- 8.Thijs VN, Lansberg MG, Beaulieu C, Marks MP, Moseley ME, Albers GW. Is early ischemic lesion volume on diffusion-weighted imaging an independent predictor of stroke outcome? A multivariable analysis. Stroke. 2000;31:2597–2602. doi: 10.1161/01.str.31.11.2597. [DOI] [PubMed] [Google Scholar]
- 9.Hand PJ, Wardlaw JM, Rivers CS, Armitage PA, Bastin ME, Lindley RI, Dennis MS. MR diffusion-weighted imaging and outcome prediction after ischemic stroke. Neurology. 2006;66:1159–1163. doi: 10.1212/01.wnl.0000202524.43850.81. [DOI] [PubMed] [Google Scholar]
- 10.Wardlaw JM, Keir SL, Bastin ME, Armitage PA, Rana AK. Is diffusion imaging appearance an independent predictor of outcome after ischemic stroke? Neurology. 2002;59:1381–1387. doi: 10.1212/01.wnl.0000032495.71720.c3. [DOI] [PubMed] [Google Scholar]
- 11.Johnston KC, Wagner DP, Wang XQ, Newman GC, Thijs V, Sen S, Warach S. Validation of an acute ischemic stroke model: does diffusion-weighted imaging lesion volume offer a clinically significant improvement in prediction of outcome? Stroke. 2007;38:1820–1825. doi: 10.1161/STROKEAHA.106.479154. [DOI] [PubMed] [Google Scholar]
- 12.Baird AE, Benfield A, Schlaug G, Siewert B, Lovblad KO, Edelman RR, Warach S. Enlargement of human cerebral ischemic lesion volumes measured by diffusion-weighted magnetic resonance imaging. Ann Neurol. 1997;41:581–589. doi: 10.1002/ana.410410506. [DOI] [PubMed] [Google Scholar]
- 13.Schwamm LH, Koroshetz WJ, Sorensen AG, Wang B, Copen WA, Budzik R, Rordorf G, Buonanno FS, Schaefer PW, Gonzalez RG. Time course of lesion development in patients with acute stroke: serial diffusion- and hemodynamic-weighted magnetic resonance imaging. Stroke. 1998;29:2268–2276. doi: 10.1161/01.str.29.11.2268. [DOI] [PubMed] [Google Scholar]
- 14.Beaulieu C, de Crespigny A, Tong DC, Moseley ME, Albers GW, Marks MP. Longitudinal magnetic resonance imaging study of perfusion and diffusion in stroke: evolution of lesion volume and correlation with clinical outcome. Ann Neurol. 1999;46:568–578. doi: 10.1002/1531-8249(199910)46:4<568::aid-ana4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 15.Lyden P, Lu M, Jackson C, Marler J, Kothari R, Brott T, Zivin J, NINDS tPA Stroke Trial investigators Underlying structure of the national institutes of health stroke scale: results of a factor analysis. Stroke. 1999;30:2347–2354. doi: 10.1161/01.str.30.11.2347. [DOI] [PubMed] [Google Scholar]
- 16.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Inter-observer agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 17.Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 18.Luby M, Bykowski JL, Schellinger PD, Merino JG, Warach S. Intra- and interrater reliability of ischemic lesion volume measurements on diffusion-weighted, mean transit time and fluid-attenuated inversion recovery MRI. Stroke. 2006;37:2951–2956. doi: 10.1161/01.STR.0000249416.77132.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dromerick AW, Edwards DF, Diringer MN. Sensitivity to changes in disability after stroke: a comparison of four scales useful in clinical trials. J Rehabil Res Dev. 2003;40:1–8. doi: 10.1682/jrrd.2003.01.0001. [DOI] [PubMed] [Google Scholar]
- 20.Oppenheim C, Stanescu R, Dormont D, Crozier S, Marro B, Samson Y, Rancurel G, Marsault C. False-negative diffusion-weighted MR findings in acute ischemic stroke. AJNR Am J Neuroradiol. 2000;21:1434–1440. [PMC free article] [PubMed] [Google Scholar]