Abstract
Metastasis continues to be the leading cause of mortality for patients with cancer. Several years ago, it became clear that chemokines and their receptors could control the tumor progress. CXCR3 has now been identified in many cancers including osteosarcoma and CXCR3 ligands were expressed by lungs that are the primary sites to which this tumor metastasize. This study tested the hypothesis that disruption of the CXCR3/CXCR3 ligands complexes could lead to a decrease in lungs metastasis. The experimental design involved the use of the CXCR3 antagonist, AMG487 and 2 murine models of osteosarcoma lung metastases. After tail vein injection of osteosarcoma cells, mice that were systematically treated with AMG487 according to preventive or curative protocols had a significant reduction in metastatic disease. Treatment of osteosarcoma cells in vitro with AMG487 led to decreased migration, decreased matrix metalloproteinase activity, decreased proliferation/survival and increased caspase-independent death. Taken together, our results support the hypothesis that CXCR3 and their ligands intervene in the initial dissemination of the osteosarcoma cells to the lungs and stimulate the growth and expansion of the metastatic foci in later stages. Moreover, these studies indicate that targeting CXCR3 may specifically inhibit tumor metastasis without adversely affecting antitumoral host response.
Keywords: chemokine receptors, osteosarcoma, lung metastasis, animal models
Most cancer deaths result from metastases rather than from primary tumor growth. Osteosarcoma, the most common primary bone tumor in children, is highly metastatic with lungs as the main site of metastasis. Understanding the mechanism involved in the metastatic spread of osteosarcoma to the lungs is essential to uncover new therapeutic targets.1
Chemokines are a superfamily of small secreted molecules that exert their activity by binding to G-protein-coupled receptors. About 50 ligands and 20 receptors are currently identified in human or mouse. Several years ago, it became clear that chemokines and receptors regulate the migration of numerous cells, and this raised the possibility that chemokines could also control the migration of tumor cells in the body. Moreover, emerging evidence suggests that chemokines and receptors could regulate other fundamental steps of tumor development such as proliferation/survival, apoptosis and angiogenesis process.2 Cancer cells were found to express chemokine receptors in a nonrandom manner, in this way indicating that several chemokine/receptor pairs could control tumor progress.3
Regarding the direct role of chemokines in tumor metastasis, tumor cell expression of chemokine receptors is associated with more aggressive disease and poorer prognosis in several malignancies.4-6 Most notably, a CXCR4-CXCL12 gradient was described to drive CXCR4-expressing tumor cells to a specific site where its ligand, CXCL12, is abundantly expressed.7,8 Moreover, CXCR4 expressed by tumor cells may also interact with CXCL12 to facilitate tumor growth.9 Similar to CXCR4, CXCR3 has now been identified in a variety of malignant cells6,10-15 including osteosarcoma cells.6 To date, 3 recent reports demonstrated that CXCR3 was able to control the metastases of melanoma and colon cancer into lymph nodes, as well as those of breast cancer to lungs where the CXCR3 ligands, CXCL9, CXCL10 or CXCL11, are expressed.16 CXCR3 has also been described in human osteosarcoma,6 nevertheless its role has not been elucidated yet.
Taken together, these findings led us to explore if the interactions between CXCR3 and its ligands are critical components for the metastatic progression of osteosarcoma. To test this hypothesis, experimental metastasis models of osteosarcoma as well as a specific small molecular weight antagonist of CXCR3 and AMG48716,17 were used. Our findings show that the inhibition of the CXCR3/chemokines pathway decreases the metastatic growth of osteosarcoma within the lung and that the receptors/ligands interactions can directly promote survival/growth- and invasion-related processes within the tumor cells.
Material and methods
Tumor cell lines
K7M2 (mouse osteosarcoma),18 SAOS-2 and CAL72 (both human osteosarcomas) and B16F10 (mouse melanoma) cell lines were obtained from American Type Culture Collection (LGC Promochem, Molsheim, France). SAOS-LM7 cell line (human osteosarcoma) was from Anderson Cancer Center, Houston, TX.19 All cell lines were maintained in DMEM (K7M2, B16F10) or MEM (SAOS-LM7) or RPMI (CAL72) supplemented with nonessential amino acids, sodium pyruvate (excepted CAL72), L-glutamine and 10% FCS.
Cells used for in vivo and in vitro studies were verified to be negative for Mycoplasma species using the Mycoplasma plus PCR primer set (Stratagene, La Jolla, CA).
Mice
Female BALB/c and athymic nude mice, 6- to 8-weeks old, were purchased from Harlan (Gannat, France). All of the procedures involving laboratory animals and their care were conducted in accordance with institutional guidelines under Veterinary supervision.
Calcium mobilization assay
Calcium mobilization in response to chemokines was performed using the calcium sensitive dye Fluo-4 AM (Molecular Probes, Eugene, OR) according to the manufacturer’s instructions. Various concentrations of CXCR3 ligands (between 10 and 500 ng/ml) (R&D systems, Abington, UK) were added to the cells and calcium mobilization was assessed using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA). Calcimycin (2 μmol/L) (Sigma-Aldrich, St Quentin Fallavier, France) served as the positive controls.
Receptor internalization
Cells were incubated in FCS-containing DMEM or MEM medium with CXCL11 (500 ng/mL) for 1 hr at 37°C. At the end of the incubations, cells were used either for analysis of CXCR3 cell surface expression or for chemotaxis.
Western blot analysis
Cell lysates from serum-cultured or 2 days serum-deprivated K7M2 or SAOS-LM7 cells were prepared as previously described.20 Briefly, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) resolved samples were transferred onto Immobilon-P membranes (Millipore, Bedford, MA). Immunoblots were first incubated with rabbit anti-CXCR3 polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA) then with secondary horseradish peroxidase (HRP)-conjugated goat anti-rabbit (Dako, Coppenhagen, Denmark) and antibody-bound proteins were detected using Enhanced ChemoLuminescence kit (Amersham, Arlington Heights, IL).
TaqMan real-time PCR experiments
Total RNA from mouse healthy lungs was extracted using RNeasy kit (Qiagen, Courtaboeuf, France) and transcribed into cDNA using the Superscript III enzyme (Invitrogen, Cergy Pontoise, France). Real-time PCR was performed in an ABI PRISM 7000 and carried out using human TaqMan® gene expression assays (Applied Biosystem, Courtaboeuf, France). Cycle parameters were 50°C for 2 min, 95°C for 10 min followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Relative levels of mRNA expression are presented as ΔCT, which shows an inverse correlation with absolute mRNA levels. ΔCT values obtained by subtracting CT control (GAPDH) from CT target gene (mouse CXCL9, CXCL10 or CXCL11) measured in the same RNA preparation.
Flow cytometry
For surface receptor staining, cells were incubated with 2.5 μg/mL CD16/CD32 antibody (BD Pharmingen, Le pont-de Claix, France), for 20 min at 4°C. Cells were stained with PE-labeled rat anti-mouse/IgG2a CXCR3 monoclonal antibody (R&D Systems) or with an isotype-matched control antibody (R&D Systems) for 30 min at 4°C. Cells were analyzed using a FACScan flow cytometer.
AMG487 formulation
AMG487 was kindly provided by Amgen (South San Francisco, CA). The in vitro formulation of AMG487 was prepared as a 10 mmol/L stock with DMSO. Tumor cells growing in culture were washed and refed with fresh culture medium containing 1 μmol/L AMG487 or DMSO vehicle. After 18 hr at 37°C, the tumor cells were washed and processed as usual for injection into mice. The in vivo formulation of AMG487 was prepared in 20% of hydroxypropyl-ß-cyclodextrin (Sigma-Aldrich) as previously described16 and used to s.c. treat mice twice daily at 5 mg/kg.
Models of osteosarcoma lung metastases
As previously described, intravenous injection of murine K7M221 and human SAOS-LM7 cells,19 respectively into BALB/c or nude mice formed lung metastases.
To prevent CXCR3-dependent metastasis formation in the lungs, a preventive treatment to antagonize CXCR316 was performed by incubating the cells with 1 μmol/L of AMG487 or vehicle for 18 hr in vitro before their injection into mice. Concomitantly, the mice were treated twice daily with subcutaneous injections of therapeutic doses of 5 mg/kg of AMG487 or vehicle from Day –1 to Day +3 for the K7M2 model and from Day –1 to Day +5 for the SAOS-LM7 model.
To treat the mice bearing lung metastases, a curative protocol was achieved by treating the mice twice daily with subcutaneous injections of 5 mg/kg of AMG487 or vehicle from Day +5 to Day +12 for the K7M2 model and both from Day +5 to Day +15 and from Day +30 to Day +40 for the SAOS-LM7 model.
Fifteen days after the injection of the K7M2 cells or 10 weeks after the injection of the SAOS-LM7 cells, mice were killed and the lungs were insufflated with India ink dye,22 then harvested by thoracic and tracheal dissection.
The extent of tumor development was assessed by recording the number of tumor nodules visible on the pleural surface (K7M2 model) or by recording the volume of all tumor nodules (cumulative volume) in the SAOS-LM7 model.
Histopathological analysis
The lungs were harvested at necropsy and injected with 10% neutral buffered formalin. The fixed samples were then embedded in paraffin and subjected to serial sections. Paraffin sections (5 μm) were stained with hematoxylin/eosin and analyzed for the presence of metastases by light microscopy.
In vitro chemotaxis assay
Chemotactic responses of osteosarcoma cells were evaluated by using 24-well chemotaxis chambers and polyethylene terephthalate inserts with 8-μm pores (Becton Dickinson, San Jose, CA). Cells (5 × 104) pretreated 30 min or not with 1 μmol/L AMG487, 5 μmol/L GM6001 (Millipore, St Quentin en Yvelines, France) or vehicle were placed in the upper well and various concentrations of recombinant human or murine CXCL9, CXCL10 and CXCL11 were added to the lower wells. The positive control chemoattractant was 1% FCS and the negative control chemoattractant was base medium supplemented with 0.1% bovine serum albumin and 0.1% insulin-transferrin-selenium supplement (Invitrogen) (BSA/ITS medium). After 18 hr incubation at 37°C, the nonmigrated cells were removed from the upper well and the migrated cells that were collected on the lower side of the insert were stained using crystal violet dye and numerated. At least, 3 sets of experiments were performed for each set.
Cell viability assay
Cells pretreated (30 min) or not with 1 μmol/L AMG487 or vehicle were incubated in six triplicate for 72 hr in base medium containing 10% FCS or 0.1% BSA with or without various concentrations of recombinant human or murine CXCL9, CXCL10 or CXCL11 in the presence or in the absence of 20 μmol/L Q-VD-OPH (MP Biomedical, Illkirch, France), an irreversible caspase inhibitor.23
Viable cells were counted using the trypan blue dye exclusion method. At least, 3 sets of experiments were performed for each set.
Caspase activity measurements
After stimulation, cells were lysed as previously described.24 Briefly, cellular extracts were then incubated for various periods of time at 37°C in a 96-well plate with 0.2 mmol/L Ac-DEVD-AMC (ALEXIS Biochemicals, Illkirch, France) as substrate of caspase-3/7. Caspase activity was measured at 460 nm in the presence or not of 1 μmol/L of Ac-DEVD-CHO (ALEXIS Biochemicals). Caspase activity is expressed as U/mg of protein, where 1 U is defined as the amount of enzyme cleaving 1.0 nmol of substrate per min.
Zymography
At the end of chemotaxis assays, conditioned media were collected, centrifugated 5 min at 1,000g to eliminate cellular fragments and analyzed for matrix metalloproteinase (MMP) production as previously described.25
Statistical analysis
Results are expressed as mean ± SEM and analyzed using the unpaired Student’s t test to evaluate the statistical significance of the difference between 2 groups and the one-way analysis of variance (ANOVA) was performed for multiple groups.
Results
CXCR3 expression and its regulation in metastatic osteosarcoma cell lines
Mouse K7M2 and human SAOS-LM7 cells were used in experimental osteosarcoma lung metastasis models to investigate the hypothesis that CXCR3 and its ligands would intervene in metastatic process. Therefore, we first determined the expression of CXCR3 in these cell lines by Western blot analysis. As shown in Figure 1a, CXCR3 expression was observed on the K7M2 and on the SAOS-LM7 and also on 2 other osteosarcoma cell lines, CAL72 and SAOS-2. The membranous expression of CXCR3 on K7M2 cells was then clarified by fluorescence-activated cell sorter (FACS) analysis (Fig. 1b).
Figure 1.
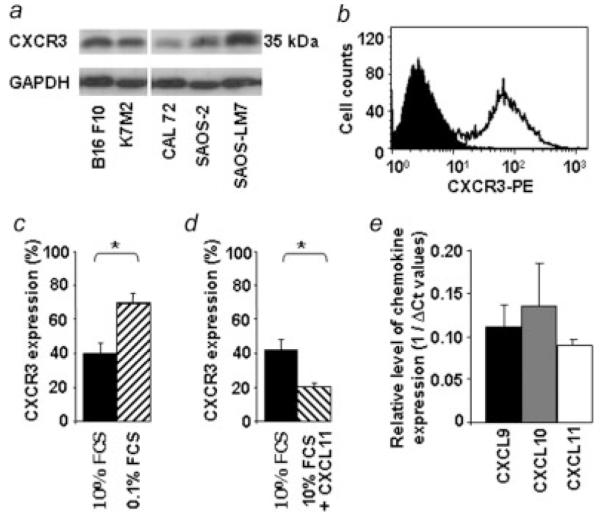
Expression of CXCR3 on mouse and human osteosarcoma cell lines. (a) Western blot analysis of CXCR3 expression in mouse K7M2 and human CAL 72, SAOS-2, SAOS-LM7 osteosarcoma cells. Human B16F10 melanoma was used as a positive control of CXCR3 whereas glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for control of equal loading. (b–d) Flow cytometry detection of CXCR3. K7M2 cells were stained for surface CXCR3 and analyzed by flow cytometry. (b) Filled histogram represents cells stained with the isotype-matched control antibody. Open histogram shows cells stained with an anti-CXCR3 antibody. (c–d) Data reported as the percentage of total staining positive for CXCR3: (c) K7M2 cells were cultured for 48 hr in medium containing 0.1% FCS (black bar) or 10% FCS (hatched bar). (d) K7M2 cells grown in 10% FCS were either not exposed (black bar) or exposed for 1 hr (hatched bar) to 500 ng/mL murine CXCL11. (Student’s t test; (p < 0.05). (e) Expression of CXCR3 ligands in mouse healthy lungs. Relative levels of expression are determined by quantitative RT-PCR analysis using GAPDH as normalizing gene. (n = 3 mice).
Tumor cells very often encounter stress conditions, imposed by hypoxia, immune activities or nutrient deprivation.26 To gain insight into the regulation of CXCR3 in osteosarcoma cells under stress conditions, the effects of serum deprivation on receptor expression were determined. The results shown in Figure 1c indicate that under serum-deprivation conditions, CXCR3 expression levels were potently up-regulated by 1.9-fold rising to 73% of CXCR3-positive cells (Fig. 1c).
Besides, chemokine receptor expression was shown to be downregulated on exposure to high concentrations of their ligands, usually as a result of ligand-induced internalization.27 In this way, K7M2 osteosarcoma cells exposed for 1 hr to 500 ng/mL of recombinant CXCL11 showed a decrease of 52.5% in CXCR3 expression (Fig. 1d).
Next, we verified the ability of the mouse healthy lungs to produce CXCR3 ligands as previously described.16 Analyses by quantitative RT-PCR done on mouse CXCL9, CXCL10 and CXCL11 indicated that all 3 chemokines were expressed by healthy lung tissues (Fig. 1e).
Calcium mobilization in osteosarcoma cells on CXCR3 ligand stimulation
To determine whether CXCR3 was functional on osteosarcoma cells, we asked if ligand stimulation would mobilize intracellular calcium which is one of the earliest biochemical events taking place in response to chemokines.28 Then, osteosarcoma cells were subjected to various concentrations of chemokines. All the CXCR3 ligands induced calcium mobilization in both cells with similar dose-response curves indicating no significant differences in magnitude (data not shown). Figure 2 shows intracellular calcium increase in K7M2 cells (panel A) and SAOS-LM7 cells (panel B) in response to CXCL9 (100 ng/mL), CXCL10 or CXCL11 (50 ng/mL).
Figure 2.
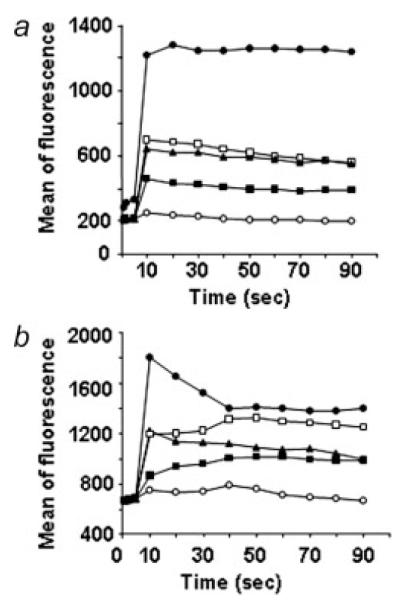
Intracellular calcium increase on exposure to CXCR3 ligands in K7M2 and SAOS-LM7 cells. K7M2 (a) and SAOS-LM7 (b) osteosarcoma cells were loaded with Fluo-4 AM and exposed to 100 ng/mL CXCL9 (▴) and 50 ng/mL CXCL10 (∎) or CXCL11 (◻). Calcimycin (●) served as positive control agonist, whereas the addition of base medium alone (○) served as the negative control. Calcium mobilization is defined as increased fluorescent intensity of cells.
CXCR3-mediated inhibition of experimental lung metastases
Systemic CXCR3 antagonism was first evaluated in mice bearing experimental lung metastases. At Day 0, K7M2 cells were injected into mice. Mice were then treated twice daily on Days +5 to +12 with AMG487 as described in Material and Methods section.
As depicted visually in Figure 3a, AMG487 treatment inhibits experimental lung metastases. AMG487-treated mice exhibited a 47% reduction in the number of tumor nodules visible on the pleural surface compared to vehicle-treated mice (77 versus 146 nodules, Fig. 3b). These data were confirmed with the histological analysis of the lungs showing less tumor foci and of smaller size in the AMG487-treated group than in the untreated group (Fig. 3c).
Figure 3.
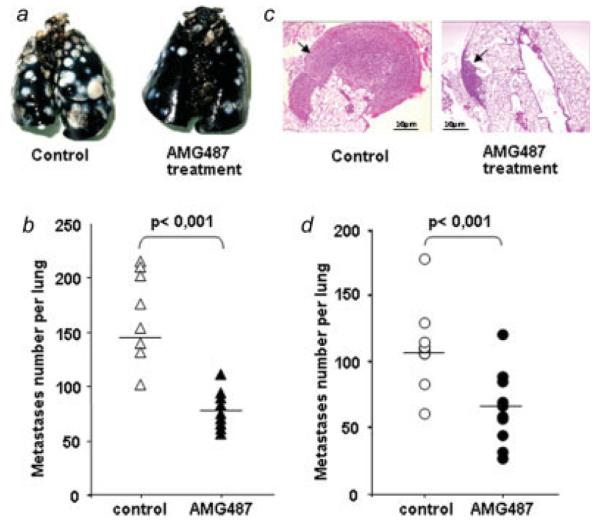
Inhibition of K7M2 metastasis to lungs by treatment with the CXCR3 antagonist AMG487. (a–c) CXCR3 antagonism was evaluated in order to treat the mice bearing lung metastases. Mice were injected with K7M2 cells into the tail vein before receiving subcutaneous injections of AMG487 or vehicle as described in Material and Methods section. Fifteen days later, mice were sacrificed for lung examination. (a) Representative gross photographs of India ink-stained lungs derived from control (left) or AMG487-treated (right). (b) The extent of tumor development was assessed by recording the number of pulmonary metastases. (c) Representative histological image taken of lungs harvested from control or AMG487-treated mice (hematoxylin/eosin staining, original magnification: ×40). Arrow indicates tumor nodule. Scale bars, 10 μm. (d) CXCR3 antagonism was evaluated in order to prevent lung metastases. Preventive AMG487 treatment consisted in treating both the K7M2 cells, in vitro before their injection into animals, and the mice with AMG487 or vehicle as described in Material and Methods section. The extent of tumor development was assessed by recording the number of pulmonary metastases. (b and d) Each dot represents the number of nodules per mouse. Horizontal bars indicate the median value in each group. The Student’s t test was used for statistical analysis (n = 12 mice per group). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The effects of systemic CXCR3 antagonism were then evaluated in the SAOS-LM7 model. Mice were subjected to AMG487 treatment on Days +5 to +15 and on Days +30 to +40 post-transplantation. As shown in Figure 4a, AMG487 treatment potently inhibits the experimental lung metastases of human osteosarcoma. AMG487-treated mice exhibited a 94% reduction in the cumulative volume of lung nodules compared with vehicle-treated mice (16 mm3 versus 1 mm3, Fig. 4b). In the same way, the histological analysis of lungs showed a drastic reduction in the number and in the size of lung nodules in AMG487-treated mice compared with untreated mice (Fig. 4c).
Figure 4.
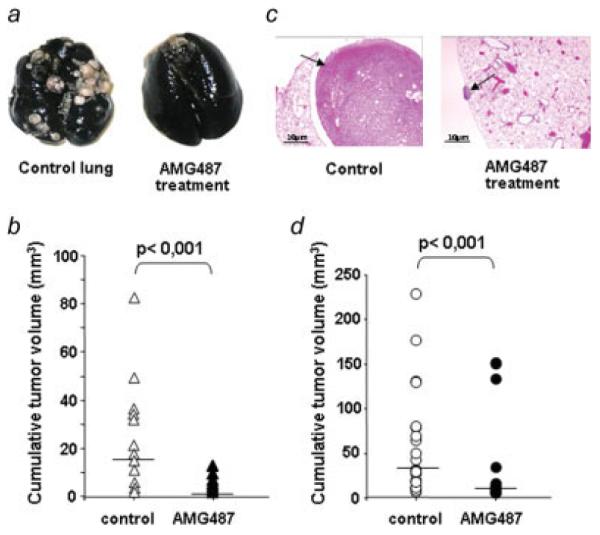
Inhibition of SAOS-LM7 metastasis to lungs by AMG487 treatment. (a, b and c) CXCR3 antagonism was evaluated to treat the mice bearing lung metastases. Mice were injected with SAOS-LM7cells into the tail vein before receiving subcutaneous injections of AMG487 or vehicle as described in Material and Methods section. Ten weeks later, mice were killed for lung examination. (a) Representative gross photographs of India Ink-stained lungs derived from control (left) or AMG487-treated (right). (b) The extent of tumor development was assessed by measuring the cumulative tumor volume in the lung. (c) Representative histological image taken of lungs harvested from control or AMG487-treated mice (hematoxylin/eosin staining, original magnification: ×40). Arrow indicates tumor nodule. Scale bars, 10 μm. (d) CXCR3 antagonism was evaluated in order to prevent lung metastases. Preventive AMG487 treatment consisted in treating both the SAOS-LM7 cells, in vitro before their injection into animals, and the mice with AMG487 or vehicle as described in Material and Methods section. The extent of tumor development was assessed by measuring the cumulative tumor volume in the lung. (b and d) Each dot represents the cumulative tumor volume per mouse. Horizontal bars indicate the median value in each group. The Student’s t test was used for statistical analysis (n = 12 mice per group). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The contribution of CXCR3 was then evaluated in early stages of metastasis formation. K7M2 and SAOS-LM7 cells were in vitro pretreated with AMG487 before injection into mice. To reinforce the blockade of CXCR3 at the time of osteosarcoma cell implantation within the lungs in both models, mice were also treated twice daily from Day −1 to Day +3 for K7M2 model (Fig. 3d) and from Day −1 to Day +5 for SAOS-LM7 model (Fig. 4d) with AMG487.
Fifteen days after the injection of the K7M2 cells, AMG487-treated mice developed fewer metastases than untreated mice. The number of lung nodules was reduced by 40% in mice treated with AMG487 compared with control mice (66 versus 110 nodules, Fig. 3d).
The extent of lung metastases was also inhibited in the model of human osteosarcoma. Indeed, when the lungs were observed 10 weeks after cell injection, AMG487-treated group exhibited a 78% reduction in the cumulative volume of lung nodules compared with vehicle-treated mice (32 mm3 versus 7 mm3, Fig. 4d).
Taken together, these data highly suggest that CXCR3/chemokines interactions could contribute to the spread and to the development of osteosarcoma cells to the lungs.
CXCR3-induced migration in metastatic osteosarcoma cell lines
In an effort to understand the mechanism by which CXCR3 inhibition could decrease lung metastases, several of the steps that are believed to be necessary for successful metastasis, including migration, survival/proliferation, apoptosis and MMPs expression/activation were examined in vitro.
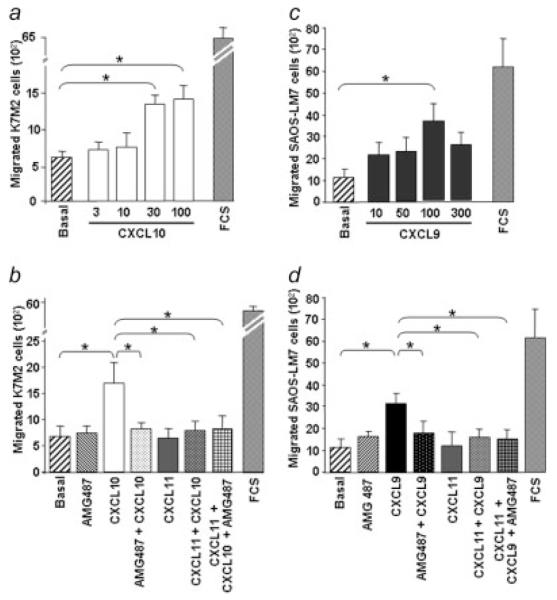
To assess the ability of CXCR3 ligands to control migration process, cells placed into modified Boyden chambers were subjected to various concentrations of CXCL9, CXCL10 or CXCL11 in BSA/ITS medium (Fig. 5). The K7M2 cells migrated in a dosedependent manner in response to CXCL10 (Fig. 5a) compared with BSA/ITS medium alone (basal lane), whereas CXCL9 and CXCL11 were not effective at stimulating migration (data not shown). The peak response of CXCL10 occurred at 30-100 ng/mL (Fig. 5a).
Figure 5.
CXCR3-induced migration of osteosarcoma cells. Osteosarcoma cells incubated in BSA/ITS medium were placed in the upper well of a migration chamber and assayed for chemotaxis in response to the indicated concentration (in ng/mL) of CXCR3 ligands, respectively CXCL10 for K7M2 cells (a) and CXCL9 for SAOS-LM7 cells (c). The chemotactic responses of K7M2 cells to CXCL10 (30 ng/mL) (b) and of SAOS-LM7 to CXCL9 (100 ng/mL) (d) were probed in the absence or in the presence of the CXCR3 antagonist AMG487 or/and in the condition where CXCR3 was down-regulated by a CXCL11-pretreatment at 500 ng/mL for 1 hr. Results represent the mean ± SEM of 3 determinations. FCS served as the positive control of migration. (ANOVA test; *p < 0.05).
Next, we analyzed if CXCR3 antagonism was able to affect CXCL10-induced migration of the K7M2 cells by performing the assay in the presence of AMG487. As shown in Figure 5b, the CXCR3 antagonist significantly inhibited the migratory responses of the K7M2 cells to CXCL10.
To confirm the CXCR3-specificity of this chemotactic response, K7M2 cells were CXCL11-pretreated before being subjected to AMG487 and/or CXCL10 treatments (Fig. 5b). In these conditions allowing the down-regulation of CXCR3, CXCL10 failed to induce K7M2 migration and, as expected, AMG487 lacked effect, in this way confirming its specific action on CXCR3.
The role of CXCR3 was further investigated on the migratory response of the human osteosarcoma cells. SAOS-LM7 cells migrated in a dose-dependent manner to CXCL9 with a peak response at 50-100 ng/mL (Fig. 5c) and AMG487 significantly inhibited this migratory response (Fig. 5d). CXCL10 and CXCL11 were not able to induce SAOS-LM7 cell migration (data not shown). Moreover, CXCR3 specificity of the CXCL9-induced migration was confirmed by the experiments where CXCR3 was down-regulated by CXCL11 treatment. In these conditions, CXCL9 had no effect on SAOS-LM7 cell migration (Fig. 5d).
CXCR3-mediated activation of MMPs in osteosarcoma cells
Because they are capable of degrading almost all extracellular matrix macromolecules in the tissues, MMPs are believed to play important role in the processes of cancer invasion and metastases.29,30 We observed that CXCL10-induced migration of K7M2 cells was abrogated in the presence of GM6001 (data not shown), a broad-spectrum MMP inhibitor,31 suggesting that those enzymatic activities were mandatory during this process.
Gelatin zymography was then performed to evaluate MMP activities in the conditioned medium obtained from K7M2 cells subjected to migration in the absence or presence of CXCL10. In the absence of chemokine, both latent proforms and active forms of MMP-2 (respectively 72 kDa and 59-62 kDa) and only latent proforms of MMP-9 (92 kDa) were detected in the supernatants of K7M2 cells (Fig. 6). After 16 hr in the presence of CXCL10, we observed the apparition of the active form of MMP-9 and an increase of both MMP-9 and MMP-2 proforms and MMP-2 active form. Interestingly, AMG487 and GM6001, which blocked CXCL10-induced K7M2 cell migration, abrogated CXCL10-induced MMP-2 and MMP-9 active forms (Fig. 6).
Figure 6.
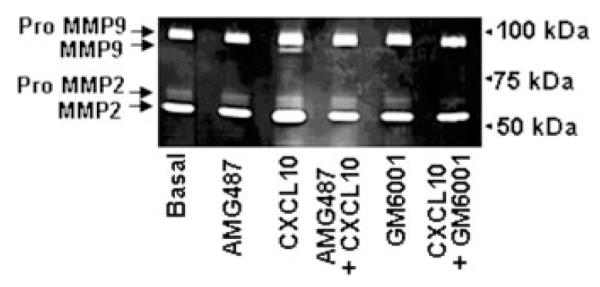
CXCR3-induced MMPs activation of osteosarcoma cells. K7M2 cells were subjected to chemotaxis assays in modified transwell chambers for 16 hr in response to CXCL10 in the presence or in the absence of AMG487 or GM6001. Then, the conditioned media were harvested and subjected to gelatin zymogram. The results shown are representative of 3 separate experiments.
CXCR3-mediated increase of osteosarcoma cell growth/survival
In addition to induction of chemotaxis and stimulation of MMPs, activation of chemokine receptors by their ligands can intervene in the development of some tumor types in stimulating cell growth and/or survival.32
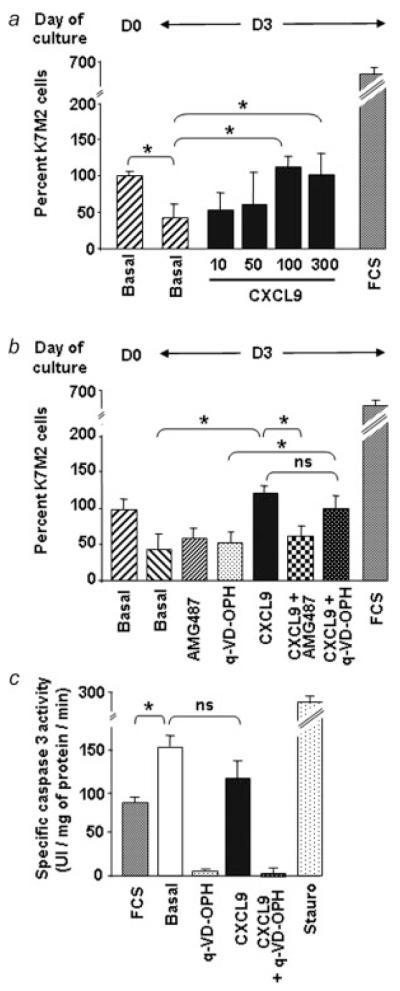
To establish if CXCR3 ligands could modulate growth/survival of osteosarcoma cells in vitro, we analyzed cell viability after 3 days of plating at low density in conditions of serum starvation in the presence or in the absence of CXCL9, CXCL10 or CXCL11. As shown in Figure 7a, K7M2 cell number decreased on serum starvation (from Day 0 to Day 3) highlighting the down-regulation of cell survival. In this serum starvation context, we observed that only CXCL9 (Fig. 7a), but neither CXCL10 nor CXCL11 (data not shown), could significantly maintain, in a dose-dependent manner, cell survival. The involvement of CXCR3 in CXCL9-induced cell survival was assessed in the presence of AMG487. In these conditions, cell survival induced by CXCL9 was abolished (Fig. 7b). Besides, CXCL9-induced survival was lost when CXCR3 was down-regulated by CXCL11 pretreatment (data not shown).
Figure 7.
CXCR3-induced survival of murine K7M2 cells. (a) K7M2 cells were placed in base medium containing 0.1% BSA and assessed for viability at this moment (D0) and after 3 days (D3) in the absence or in the presence of increasing concentrations of CXCL9. (b) Cell viability was assessed in response to a 3-day treatment with CXCL9 (100 ng/mL) alone or in the presence of AMG487 or Q-VD-OPH. In (a) and (b), the cell number at D0 was taken as 100% and FCS served as positive control of cell growth. (c) Samples from K7M2 cells incubated in medium containing 10% FCS or 0.1% BSA with or without CXCL9 and/or Q-VD-OPH were assayed for their ability to cleave the fluorogenic caspase 3/7 substrate, Ac-DEVD-AMC. Treatment with staurosporine (stauro) was used as a positive control. (ANOVA test; *p < 0.05).
One possible mechanism to explain CXCR3-mediated increase of K7M2 cell survival in the condition of serum starvation was that CXCL9 blocked cell apoptosis. To test this hypothesis, CXCL9-induced cell survival was evaluated when cells were incubated in the presence of Q-VD-OPH,23 an irreversible pan inhibitor of caspases.33 In these conditions, we failed to observe any significant change in CXCL9-induced cell survival, suggesting that CXCL9 intervene in the survival process by a caspase-independent death way (Fig. 7b).
This was confirmed by measurement of caspase activation. As shown in the Figure 7c, the serum-starved K7M2 cells (basal) exhibited significant higher caspase-3/7 activity than cells growing in the presence of serum. Nevertheless, CXCL9 failed to significantly reduce serum starvation-induced activation of caspases-3/7.
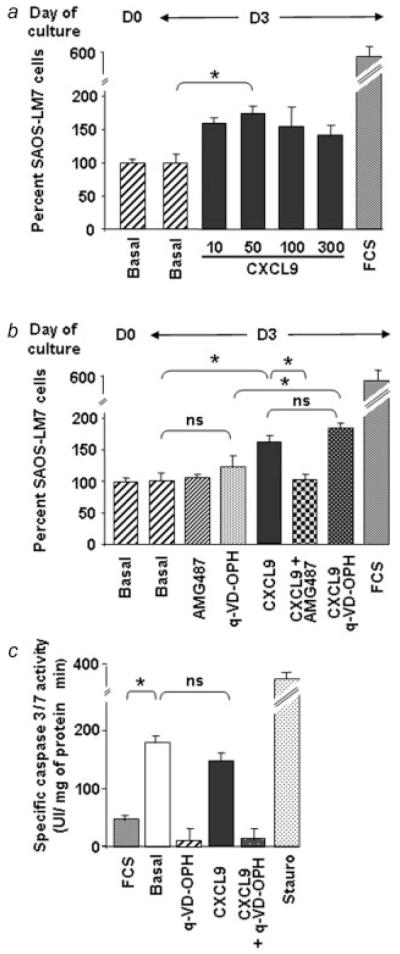
CXCR3 antagonism was further investigated in the human osteosarcoma cell line. As shown in the Figure 8a, SAOS-LM7 cell number remained constant on serum starvation. Only CXCL9 (Fig. 8a), but neither CXCL10 nor CXCL11 (data not shown), was able to induce SAOS-LM7 proliferation, which was blocked by AMG487 (Fig. 8b). Besides, blocking caspase-dependent apoptosis with Q-VD-OPH did not change the rate of CXCL9-induced SAOS-LM7 growth. Moreover, CXCL9 did not prevent caspase activation induced by serum starvation in this way confirming that CXCL9-induced cell growth was independent of the apoptotic process.
Figure 8.
CXCR3-induced growth of human SAOS-LM7 cells. (a) SAOS-LM7 cells were placed in base medium containing 0.1% BSA and assessed for viability at this moment (D0) and after 3 days (D3) without or with increasing concentrations of CXCL9. (b) Cell growth was assessed in response to a 3-day treatment with CXCL9 (100 ng/mL) alone or in the presence of AMG487 or Q-VD-OPH. In (a) and (b), the cell number at D0 was taken as 100 % and FCS served as positive control of cell growth. (c) Samples from SAOS-LM7 cells incubated in medium containing 10% FCS or 0.1 % BSA with or without CXCL9 and/or Q-VD-OPH were assayed for their ability to cleave the fluorogenic caspase 3/7 substrate, Ac-DEVD-AMC. Treatment with staurosporine (stauro) was used as a positive control. (ANOVA test; *p < 0.05).
Discussion
Metastases are the cause of 90% of cancer deaths.34,35 Therefore, 2 major goals of cancer research are to identify biological factors that predispose to metastases and, to develop new approaches to prevent or cure metastases. Recently, chemokine receptors have emerged as key mediators in metastatic potential and site-specific spread of cancer cells, their cognate ligands being expressed by the target organs.
Among these receptors, CXCR3 has been identified in a variety of malignant cells, including osteosarcoma cells.6 Moreover, CXCR3 receptors seem even to be one of the main receptors expressed in malignant osteoblasts.6 Nevertheless, no study had still reported their role in the development of primary bone tumor or its lung metastases.
This study tested the hypothesis that disruption of the CXCR3/CXCR3 ligands axis could lead to a decrease in lung metastasis from osteosarcoma. The experimental design was based on the use of 2 murine models of osteosarcoma lung metastasis using the mouse K7M2 or the human SAOS-LM7 cells. First, we have high-lighted that CXCR3 receptors are expressed in K7M2 and SAOS-LM7 osteosarcoma cell lines and that they are functional, since each CXCR3 ligands, CXCL9, CXCL10 and CXCL11, were able to mobilize intracellular calcium.
Then, the in vivo relevance of CXCR3 expression for metastases was assessed in both murine models using the CXCR3 antagonist AMG487 according to preventive or curative protocols. With the aim of investigating whether CXCR3 could intervene in the growth of lung metastases, a curative AMG487 treatment was achieved by treating subcutaneously the tumor-bearing mouse. Interestingly, systemic antagonism of CXCR3 strongly reduces the tumor expansion within lungs of K7M2- and SAOS-LM7–inoculated mice. Thus, CXCR3 appears to be a key factor in the progression of osteosarcoma metastases in the lung. In addition to already published studies that only tested the involvement of CXCR3 in cancer progression by performing CXCR3 inhibition at the time of cancer cell implantation into mice, our results provide the first evidence of the therapeutic potential of CXCR3-blocking strategies on pre-existing metastases.
As a next step, a preventive treatment with the CXCR3 antagonist was administrated to osteosarcoma cells before their inoculation into AMG487-pretreated mice. We observed that preventive AMG487 treatment significantly reduced metastasis formation, thus pointing to a role of CXCR3 in the early stages of metastasis formation of osteosarcoma cells within the lungs.
To gain insights in the role played by CXCR3 in osteosarcoma lung metastases, we looked for the cell functions under control of this receptor in vitro. Our experiments show that the migration, the MMPs activation and the proliferation/survival processes are significantly enhanced by the CXCR3 ligands unlike the caspase-independent death which are considerably reduced. Interestingly, all these effects are prevented by CXCR3 antagonism.
Concerning the MMPs, it is interesting to note that CXCL10 is able to control 2 enzymes, namely MMP-2 and MMP-9 which have been correlated with the process of tumor cell invasion and metastasis36-38 We showed that CXCL10, via its interactions through CXCR3, is involved in regulating the production and/or activities of MMP-2 and MMP-9 in osteosarcoma cells. Our data suggest that CXCL10 could control the transcription of MMP-9, since its regulation has been essentially attributed to mRNA levels. Consisting with this, through the several possible intracellular signal cascades,39 CXCL10 could activate transcription factors such as activator protein-1 (AP-1) or nuclear factor kappa B (NF-kB) which have been reported to mediate MMP2 activation in many conditions including cancer.40,41 Besides, because MMP-2 is almost totally regulated at the level of its activation which is critically dependent on a processing by membrane type-MMPs (MT-MMPs, in particular MT1-MMP) in association with tissue inhibitors of metalloproteinases (TIMP-2),42,43 we can suggest that CXCL10 could exert its effect by stimulating MT-MMPs and/or modulating expression level of TIMP-2.
Taken together, in vitro and in vivo results support the hypothesis that CXCR3 and their ligands not only help the initial dissemination of the osteosarcoma cells to the lungs but also stimulate the growth and expansion of the metastatic foci in later stages. Nevertheless, even in osteosarcoma progress, CXCR3 is not the sole player. Indeed, in all cancers, a complex network of chemokines and their receptors influences the development of the primary tumors and their metastatic foci.39 Notably, the CXCR4/CXCL12 pair which was found to be involved in numerous malignancy44 was recently shown to be also required in osteosarcoma.8 Thereby, we can not exclude that, as reported in plasmacytoid dendritic cells,45 even if all CXCR3 ligands are not able to induce osteosarcoma cell chemotaxis, they could nevertheless control the migration of osteosarcoma cells via the CXCL12/CXCR4 axis. Indeed, it was demonstrated that despite expression of very high levels of CXCR3, plasmacytoid dendritic cells did not respond efficiently to CXCR3 ligands. However, they migrated in response to CXCL12 and, CXCR3 ligands synergized with CXCL12 to induce plasmacytoid dendritic cell migration. This synergy reflected a sensitizing effect of CXCR3 ligands which independently of a gradient and chemoattraction decrease the threshold of sensitivity to CXCL12.
The biological role of these chemokines/receptors networks has two aspects in these processes; the influence of chemokines on the metastatic potential and site-specific spread of tumor cells,3 as discussed above, and the role of chemokines/receptors in controlling leukocyte infiltration in tumor. Concerning CXCR3 ligands, it has been demonstrated that CXCL9, CXCL10 and CXCL11 exert antitumor activities by inducing immune-stimulating and angiostatic effects46,47 Notably, CXCR3 has been reproducibly found on monocytes,48 dendritic cells,49 B cells, natural killer (NK) cells, memory and activated CD4 and CD8 lymphocytes,50,51 which contribute to mount host antitumoral response by killing tumor cells directly or by secreting some antitumoral cytokines. In this way, in an experimental lymphoma model and in the context of chemokine-transduced tumor cells, CXCL10 expression has been shown to promote a tumor-suppressive activity by improving the recruitment of CXCR3-positive NK cells.52
Consistent with this, we were expecting that neutralizing all interactions between CXCR3 and its ligands by systemic antagonism could promote metastasis. However, the extent of tumor development was never significantly enhanced in the AMG487-treated mice compared with the corresponding control group. Similar results were obtained when AMG487 was systematically administered in metastatic breast cancer.16
Moreover, this apparent paradox corroborates studies demonstrating that tumor-infiltrating leukocytes actually do not react against the tumor cells.53 Our data suggest that inflammatory cells and chemokines found in tumors are more likely to contribute to tumor growth, progression and immunosuppression than they are to mount an effective host antitumor response. Nevertheless, we can not exclude the possibility that the host antitumor response is mediated, at least in part, by CXCR3-null leukocytes in particular CXCR3-null NK cells since the predominant NK cell subset in the peripheral blood or lungs has been described to be negative for CXCR3.54
Finally, another possibility to explain the lack of effect of systemic CXCR3 antagonism on antitumoral host response could be that the concentration of AMG487 required to inhibit host-CXCR3 may be higher than the concentration necessary to inhibit tumor CXCR3.
In summary, this study supports the hypothesis that osteosarcoma cells express CXCR3 to facilitate the development of metastases at distant sites where are expressed CXCR3 ligands. In addition, our study shows that CXCR3 antagonism can be a therapeutic tool to suppress or prevent lung osteosarcoma metastases. The current studies and those of Walser et al.16 indicate that targeting CXCR3 may specifically inhibit tumor metastasis without adversely affecting antitumoral host response. Further studies are required to elucidate the relevant mechanism and to optimize this therapeutic approach.
Acknowledgements
We thank Violette Breittmayer for the excellent technical assistance and the MD Anderson Cancer Center of the University of Texas for providing SAOS-LM7 cell line. TJ Sullivan, TL Collins, MG Johnson and JC Medina were from Amgen Inc.
Grant sponsor: Institut National de la Santé et de la Recherche Médicale; Grant sponsor: Association pour la Recherche sur le Cancer; Grant number: 4034; Grant sponsor: Cancéropole PACA; Grant number: ACI 42259.
Abbreviations
- BSA
bovine serum albumine
- FACS
fluorescence-activated cell sorting
- FCS
fetal calf serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- ITS
insulin-transferrin-selenium
- MMP
matrix metalloproteinase
- PBS
phosphate buffer saline
- sc
subcutaneous
References
- 1.Bruland OS, Pihl A. On the current management of osteosarcoma. A critical evaluation and a proposal for a modified treatment strategy. Eur J Cancer. 1997;33:1725–31. doi: 10.1016/s0959-8049(97)00252-9. [DOI] [PubMed] [Google Scholar]
- 2.Murphy PM. Chemokines and the molecular basis of cancer metastasis. N Engl J Med. 2001;345:833–5. doi: 10.1056/NEJM200109133451113. [DOI] [PubMed] [Google Scholar]
- 3.Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14:171–9. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Makishima H, Ito T, Asano N, Nakazawa H, Shimodaira S, Kamijo Y, Nakazawa Y, Suzuki T, Kobayashi H, Kiyosawa K, Ishida F. Significance of chemokine receptor expression in aggressive NK cell leukemia. Leukemia. 2005;19:1169–74. doi: 10.1038/sj.leu.2403732. [DOI] [PubMed] [Google Scholar]
- 5.Zafiropoulos A, Crikas N, Passam AM, Spandidos DA. Significant involvement of CCR2-64I and CXCL12-3a in the development of sporadic breast cancer. J Med Genet. 2004;41:e59. doi: 10.1136/jmg.2003.013649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laverdiere C, Hoang BH, Yang R, Sowers R, Qin J, Meyers PA, Huvos AG, Healey JH, Gorlick R. Messenger RNA expression levels of CXCR4 correlate with metastatic behavior and outcome in patients with osteosarcoma. Clin Cancer Res. 2005;11:2561–7. doi: 10.1158/1078-0432.CCR-04-1089. [DOI] [PubMed] [Google Scholar]
- 7.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 8.Kim SY, Lee CH, Midura BV, Yeung C, Mendoza A, Hong SH, Ren L, Wong D, Korz W, Merzouk A, Salari H, Zhang H, et al. Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the development of murine pulmonary metastases. Clin Exp Metastasis. 2008;25:201–11. doi: 10.1007/s10585-007-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, Luker GD. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–12. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 10.Kawada K, Sonoshita M, Sakashita H, Takabayashi A, Yamaoka Y, Manabe T, Inaba K, Minato N, Oshima M, Taketo MM. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 2004;64:4010–7. doi: 10.1158/0008-5472.CAN-03-1757. [DOI] [PubMed] [Google Scholar]
- 11.Robledo MM, Bartolome RA, Longo N, Rodriguez-Frade JM, Mellado M, Longo I, van Muijen GN, Sanchez-Mateos P, Teixido J. Expression of functional chemokine receptors CXCR3 and CXCR4 on human melanoma cells. J Biol Chem. 2001;276:45098–105. doi: 10.1074/jbc.M106912200. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg-Bittman L, Neumark E, Sagi-Assif O, Azenshtein E, Meshel T, Witz IP, Ben-Baruch A. The expression of the chemokine receptor CXCR3 and its ligand. CXCL10, in human breast adenocarcinoma cell lines. Immunol Lett. 2004;92:171–8. doi: 10.1016/j.imlet.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Engl T, Relja B, Blumenberg C, Muller I, Ringel EM, Beecken WD, Jonas D, Blaheta RA. Prostate tumor CXC-chemokine profile correlates with cell adhesion to endothelium and extracellular matrix. Life. Sci. 2006;78:1784–93. doi: 10.1016/j.lfs.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Goldberg-Bittman L, Sagi-Assif O, Meshel T, Nevo I, Levy-Nissenbaum O, Yron I, Witz IP, Ben-Baruch A. Cellular characteristics of neuroblastoma cells: regulation by the ELR–CXC chemokine CXCL10 and expression of a CXCR3-like receptor. Cytokine. 2005;29:105–17. doi: 10.1016/j.cyto.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Jones D, Benjamin RJ, Shahsafaei A, Dorfman DM. The chemokine receptor CXCR3 is expressed in a subset of B-cell lymphomas and is a marker of B-cell chronic lymphocytic leukemia. Blood. 2000;95:627–32. [PubMed] [Google Scholar]
- 16.Walser TC, Rifat S, Ma X, Kundu N, Ward C, Goloubeva O, Johnson MG, Medina JC, Collins TL, Fulton AM. Antagonism of CXCR3 inhibits lung metastasis in a murine model of metastatic breast cancer. Cancer Res. 2006;66:7701–7. doi: 10.1158/0008-5472.CAN-06-0709. [DOI] [PubMed] [Google Scholar]
- 17.Wijtmans M, Verzijl D, Leurs R, de Esch IJ, Smit MJ. Towards small-molecule CXCR3 ligands with clinical potential. Chem Med Chem. 2008;3:861–72. doi: 10.1002/cmdc.200700365. [DOI] [PubMed] [Google Scholar]
- 18.Khanna C, Prehn J, Yeung C, Caylor J, Tsokos M, Helman L. An orthotopic model of murine osteosarcoma with clonally related variants differing in pulmonary metastatic potential. Clin Exp Metastasis. 2000;18:261–71. doi: 10.1023/a:1006767007547. [DOI] [PubMed] [Google Scholar]
- 19.Jia SF, Worth LL, Kleinerman ES. A nude mouse model of human osteosarcoma lung metastases for evaluating new therapeutic strategies. Clin Exp Metastasis. 1999;17:501–6. doi: 10.1023/a:1006623001465. [DOI] [PubMed] [Google Scholar]
- 20.Rezzonico R, Schmid-Alliana A, Romey G, Bourget-Ponzio I, Breuil V, Breittmayer V, Tartare-Deckert S, Rossi B, Schmid-Antomarchi H. Prostaglandin E2 induces interaction between hSlo potassium channel and Syk tyrosine kinase in osteosarcoma cells. J Bone Miner Res. 2002;17:869–78. doi: 10.1359/jbmr.2002.17.5.869. [DOI] [PubMed] [Google Scholar]
- 21.Khanna C, Khan J, Nguyen P, Prehn J, Caylor J, Yeung C, Trepel J, Meltzer P, Helman L. Metastasis-associated differences in gene expression in a murine model of osteosarcoma. Cancer Res. 2001;61:3750–9. [PubMed] [Google Scholar]
- 22.Williams TM, Medina F, Badano I, Hazan RB, Hutchinson J, Muller WJ, Chopra NG, Scherer PE, Pestell RG, Lisanti MP. Caveolin-1 gene disruption promotes mammary tumorigenesis and dramatically enhances lung metastasis in vivo. Role of Cav-1 in cell invasiveness and matrix metalloproteinase (MMP-2/9) secretion. J Biol Chem. 2004;279:51630–46. doi: 10.1074/jbc.M409214200. [DOI] [PubMed] [Google Scholar]
- 23.Caserta TM, Smith AN, Gultice AD, Reedy MA, Brown TL. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis. 2003;8:345–52. doi: 10.1023/a:1024116916932. [DOI] [PubMed] [Google Scholar]
- 24.Puissant A, Grosso S, Jacquel A, Belhacene N, Colosetti P, Cassuto JP, Auberger P. Imatinib mesylate-resistant human chronic myelogenous leukemia cell lines exhibit high sensitivity to the phytoalexin resveratrol. FASEB J. 2008;22:1894–904. doi: 10.1096/fj.07-101394. [DOI] [PubMed] [Google Scholar]
- 25.Vitale S, Schmid-Alliana A, Breuil V, Pomeranz M, Millet MA, Rossi B, Schmid-Antomarchi H. Soluble fractalkine prevents monocyte chemoattractant protein-1-induced monocyte migration via inhibition of stress-activated protein kinase 2/p38 and matrix metalloproteinase activities. J Immunol. 2004;172:585–92. doi: 10.4049/jimmunol.172.1.585. [DOI] [PubMed] [Google Scholar]
- 26.Xie K, Huang S. Regulation of cancer metastasis by stress pathways. Clin Exp Metastasis. 2003;20:31–43. doi: 10.1023/a:1022590402748. [DOI] [PubMed] [Google Scholar]
- 27.Mueller A. Internalization: what does it tell us about pharmacokinetic and pharmacodynamic properties of an antagonist? Br J Pharmacol. 2007;152:1145–6. doi: 10.1038/sj.bjp.0707521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burger JA, Burger M, Kipps TJ. Chronic lymphocytic leukemia B cells express functional CXCR4 chemokine receptors that mediate spontaneous migration beneath bone marrow stromal cells. Blood. 1999;94:3658–67. [PubMed] [Google Scholar]
- 29.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 31.Grobelny D, Poncz L, Galardy RE. Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosa elastase by peptide hydroxamic acids. Biochemistry. 1992;31:7152–4. doi: 10.1021/bi00146a017. [DOI] [PubMed] [Google Scholar]
- 32.Vlahakis SR, Villasis-Keever A, Gomez T, Vanegas M, Vlahakis N, Paya CV. G protein-coupled chemokine receptors induce both survival and apoptotic signaling pathways. J Immunol. 2002;169:5546–54. doi: 10.4049/jimmunol.169.10.5546. [DOI] [PubMed] [Google Scholar]
- 33.Green DR. Apoptosis. Death deceiver. Nature. 1998;396:629–30. doi: 10.1038/25248. [DOI] [PubMed] [Google Scholar]
- 34.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–72. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 35.Fidler IJ. Critical determinants of metastasis. Semin Cancer Biol. 2002;12:89–96. doi: 10.1006/scbi.2001.0416. [DOI] [PubMed] [Google Scholar]
- 36.Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67–8. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- 37.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–8. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 38.Fernandes T, de Angelo-Andrade LA, Morais SS, Pinto GA, Chagas CA, Maria-Engler SS, Zeferino LC. Stromal cells play a role in cervical cancer progression mediated by MMP-2 protein. Eur J Gynaecol Oncol. 2008;29:341–4. [PubMed] [Google Scholar]
- 39.O’Hayre M, Salanga CL, Handel TM, Allen SJ. Chemokines and cancer: migration, intracellular signalling and intercellular communication in the microenvironment. Biochem J. 2008;409:635–49. doi: 10.1042/BJ20071493. [DOI] [PubMed] [Google Scholar]
- 40.Xie Z, Singh M, Singh K. Differential regulation of matrix metalloproteinase-2 and -9 expression and activity in adult rat cardiac fibroblasts in response to interleukin-1beta. J Biol Chem. 2004;279:39513–9. doi: 10.1074/jbc.M405844200. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa K, Chen F, Kuang C, Chen Y. Suppression of matrix metalloproteinase-9 transcription by transforming growth factor-beta is mediated by a nuclear factor-kappa B site. Biochem J. 2004;381:413–22. doi: 10.1042/BJ20040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Itoh Y, Takamura A, Ito N, Maru Y, Sato H, Suenaga N, Aoki T, Seiki M. Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. Embo J. 2001;20:4782–93. doi: 10.1093/emboj/20.17.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strongin AY, Marmer BL, Grant GA, Goldberg GI. Plasma membrane-dependent activation of the 72-kDa type IV collagenase is prevented by complex formation with TIMP-2. J Biol Chem. 1993;268:14033–9. [PubMed] [Google Scholar]
- 44.Ben-Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp Metastasis. 2008;25:345–56. doi: 10.1007/s10585-007-9097-3. [DOI] [PubMed] [Google Scholar]
- 45.Vanbervliet B, Bendriss-Vermare N, Massacrier C, Homey B, de Bouteiller O, Briere F, Trinchieri G, Caux C. The inducible CXCR3 ligands control plasmacytoid dendritic cell responsiveness to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12. J Exp Med. 2003;198:823–30. doi: 10.1084/jem.20020437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tannenbaum CS, Tubbs R, Armstrong D, Finke JH, Bukowski RM, Hamilton TA. The CXC chemokines IP-10 and Mig are necessary for IL-12-mediated regression of the mouse RENCA tumor. J Immunol. 1998;161:927–32. [PubMed] [Google Scholar]
- 47.Hensbergen PJ, Wijnands PG, Schreurs MW, Scheper RJ, Willemze R, Tensen CP. The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8+ T lymphocytes but not inhibition of angiogenesis. J Immunother. 2005;28:343–51. doi: 10.1097/01.cji.0000165355.26795.27. [DOI] [PubMed] [Google Scholar]
- 48.Katschke KJ, Jr, Rottman JB, Ruth JH, Qin S, Wu L, LaRosa G, Ponath P, Park CC, Pope RM, Koch AE. Differential expression of chemokine receptors on peripheral blood, synovial fluid, and synovial tissue monocytes/macrophages in rheumatoid arthritis. Arthritis Rheum. 2001;44:1022–32. doi: 10.1002/1529-0131(200105)44:5<1022::AID-ANR181>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 49.Penna G, Vulcano M, Sozzani S, Adorini L. Differential migration behavior and chemokine production by myeloid and plasmacytoid dendritic cells. Hum Immunol. 2002;63:1164–71. doi: 10.1016/s0198-8859(02)00755-3. [DOI] [PubMed] [Google Scholar]
- 50.Loetscher M, Loetscher P, Brass N, Meese E, Moser B. Lymphocyte-specific chemokine receptor CXCR3: regulation, chemokine binding and gene localization. Eur J Immunol. 1998;28:3696–705. doi: 10.1002/(SICI)1521-4141(199811)28:11<3696::AID-IMMU3696>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 51.Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 52.Wendel M, Galani IE, Suri-Payer E, Cerwenka A. Natural killer cell accumulation in tumors is dependent on IFN-gamma and CXCR3 ligands. Cancer Res. 2008;68:8437–45. doi: 10.1158/0008-5472.CAN-08-1440. [DOI] [PubMed] [Google Scholar]
- 53.Ben-Baruch A. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol. 2006;16:38–52. doi: 10.1016/j.semcancer.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 54.Hayakawa Y, Smyth MJ. CD27 dissects mature NK cells into two subsets with distinct responsiveness and migratory capacity. J Immunol. 2006;176:1517–24. doi: 10.4049/jimmunol.176.3.1517. [DOI] [PubMed] [Google Scholar]