Abstract
Numerous pharmaceutical efforts have targeted neuronal nicotinic receptors (nAChRs) for amelioration of cognitive deficits. While α4β2 and α7 are the more prominent nAChR in brain, other heteromeric nAChR can have important impact on agonist pharmacology. ABT-089 is a pioneer nAChR agonist found to enhance cognitive function with an exceptionally low incidence of adverse effects. To further investigate the mechanism of action of ABT-089, we evaluated its function in mouse brain preparations in which we have characterized the subunit composition of native nAChR. Among α4β2*- nAChR, ABT-089 had partial agonist activity (7-23% of nicotine) and high selectivity for α4α5β2 nAChR as evidenced by loss of activity in thalamus of α5-/- mice. ABT-089 stimulated [3H]-dopamine release (57%) exceeded the activity at α4β2* nAChR, that could be explained by the activity at α6β2* nAChR The concentration-response relationship for ABT-089 stimulation of α6β2* nAChR was biphasic. EC50 and efficacy values for ABT-089, respectively, were 28 μM and 98% at the less sensitive α6β2* nAChR and 0.11 μM and 36% at the more sensitive subtype (the most sensitive target for ABT-089 identified to date). ABT-089 had essentially no agonist or antagonist activity at concentrations ≤ 300 μM at α3β4-nAChR measured by [3H]-acetylcholine release from interpeduncular nucleus. Thus, ABT-089 is a β2* nAChR ligand with demonstrable agonist activity at α4β2* and α6β2* receptors. As one form of α6β2* nAChR is sensitive to sub-μM concentrations, we propose that that this receptor in particular may contribute to the enhanced cognitive performance following low doses of ABT-089.
Keywords: nicotinic acetylcholine receptor, dopamine, thalamus, striatum, cortex, desensitization
1. Introduction
Amelioration of cognitive deficits in disorders such as attention deficit, schizophrenia, Parkinson's Disease, and Alzheimer's disease and other forms of dementia comprises an extensive research effort with a variety of potential therapeutic targets. Neuronal nicotinic receptor (nAChR) agonists and positive allosteric modulators are prominent among approaches addressed over the past decade. Some candidates have entered clinical trials, and others are on the horizon.
nAChRs are pentameric ligand-gated ion channels (LGIC) assembled from among nine known α subunits, α2-αl0, and three known β subunits, β2-β4 (α1, β1 and other non-α subunits are found in skeletal muscle nicotinic receptors). Most nAChR are heteromeric, with at least one α subtype and at least one β subtype. Some, however, are homomeric with one α subtype comprising a functional pentameric LGIC. Orthosteric ligands bind in the interface between subunits such that two different subunit faces contribute to the binding pocket. The functional LGIC apparently requires at least two α subunits. Nevertheless, the variety of nAChR that potentially could be assembled is quite complex, with alternate stoichiometry and modifier subunits influencing the functional pharmacology if not binding. For example, there is evidence that while the α5 subunit does not form functional nAChR alone or in combination with any know β subunit, it can modify the pharmacology of nAChR containing α4 and β2 (α4β2*) [1;2;2-5]. Studies with recombinant nAChR containing β2 (α4β2 and α3β2) demonstrate different agonist sensitivities depending upon stoichiometry inferred from the ratio of α:β expression [6-9].
Heteromeric α4β2*-nAChR and the homomeric α7-nAChR have attracted the most attention as therapeutic targets for nicotinic drugs. Both are widespread in the CNS, and are expressed in cortex, hippocampus and other regions important in cognitive function [10]. α7 nAChR are also found in the peripheral nervous system and in certain non-neuronal cells [11-15]. α4β2* nAChR thus far have been found almost entirely in central nervous system (CNS), with some evidence for expression in sensory ganglia [16-18]. α4β2* nAChR are the predominant the high-affinity nicotine binding sites in rodent CNS [19]. α6β2* nAChR also are highly restricted to CNS, and are of particular interest for their prominence in dopamine-containing neurons [2;20;21] and their role in nicotine self-administration [22]. Unfortunately, functional α6β2* have been notoriously difficult to express in recombinant systems without modifications that could also influence pharmacology of the construct [23-25], hampering drug discovery efforts aimed at α6β2*-nAChR.
ABT-089 is one of the first novel compounds identified as an α4β2-selective agonist [26;27]. It binds to rat brain α4β2 nAChR with a Ki of 17 nM while binding to α7 nAChR is insignificant. In cognitive behavior models, ABT-089 enhances performance in monkey delayed match-to-sample and in rat Morris Water Maze with deficit induced by surgical or pharmacologic lesion [28]. ABT-089 acts like an α4β2-nAChR partial agonist to stimulate [3H]-dopamine ([3H]DA) release from rat striatal slices [27]. However, for heterologously expressed α4β2 and α7 nAChR assayed electrophysiologically, ABT-089 has shown only weak (≤ 12%) efficacy compared to the 65% relative efficacy exhibited in striatal [3H]-dopamine release [29]. Thus, two questions arise. (i) Does ABT-089 discriminate between recombinant α4β2 and native α4β2* assemblies (e.g., α4α5β2), providing a tool to hone (a) our understanding of nAChR physiology and (b) the utility of recombinant nAChR screening assays. (ii) Does ABT-089 actually act through α4β2*-nAChR, or do other nAChR, potentially α6β2*, contribute to the efficacy of ABT-089 in CNS?
To address such questions, we evaluated ABT-089 activity at native mouse nAChR using thalamic synaptosomes to measure α4β2* activity, and striatal synaptosomes to measure α6β2* as well as α4β2* activity. The results indicate that ABT-089 interacts both with α6β2*- and α4β2*-nAChR, and has an unusual selectivity profile within these receptor classes. In thalamic synaptosomes, ABT-089 exhibits selectivity for α4α5β2 nAChR. In striatal synaptosomes, ABT-089 stimulates [3H]-dopamine release via α6β2*- and α4β2*-nAChR. Further, within the α6β2*-nAChR mediated response, the concentration-response curve for ABT-089 is clearly biphasic, suggesting a further selectivity between α6β2* subtypes, possibly α6β2β3 and α4α6β2β3. In interpeduncular nucleus (IPN) where α3β4*-nAChR dominate receptor mediated ACh release, ABT-089 had essentially no effect. Thus, ABT-089 is an interesting potential therapeutic and a tool compound that could help evaluate the physiological roles of β2* -nAChR subtypes.
2. Materials and Methods
2.1 Animals
Mice of the C57BL/6J strain, 60-90 days of age, used for this study were bred and maintained at the Institute for Behavioral Genetics, University of Colorado (Boulder, CO). After weaning at 25 days of age, same sex littermates were housed 5 to a cage with free access to food (Teklad Rodent Diet, Harlan, Madison, WI) and water, with a 12-hr light/dark cycle at 22°C. The mice differing in α5 genotype were originally obtained from Arthur Beaudet, Baylor University College of Medicine. These mice have subsequently been backcrossed at least 10 generations with C57BL/6J. Wild-type and null mutant mice were produced by mating heterozygous animals. Genotyping protocols have been described previously [4]. Animal care and experimental procedures were in accordance with the guidelines and approval of the Animal Care and Utilization Committee of the University of Colorado, Boulder, CO. All animal procedures were in accordance with the guidelines of the National Institutes of Health.
2.2. Materials
Radioisotopes 86RbCl (4-10 Ci/mg), [3H]-dopamine (32.7 Ci/mmol) and [3H]-choline (66.7 Ci/mmol) as well as scintillation cocktail (Optiphase Supramix) were purchased from PerkinElmer Life and Analytical Sciences, Shelton, CT. Nicotine tartrate, NaCl, KCl, CaCl2, MgSO4, K2HPO4, bovine serum albumin, pargyline, nomifensine, ascorbic acid, tetrodotoxin, dihydro-β-erythroidine dihydrochloride (DHβE) and glucose were obtained from Sigma Chemical Company, St. Louis, MO. Sucrose was obtained from Fisher Chemical Co., Pittsburgh, PA. HEPES and HEPES, sodium salt were products of BDH Chemicals, obtained through VWR International, West Chester, PA. CsCl was purchased from RPI, Arlington Heights, IL.
ABT-089 [2-methyl-3(2-(S)pyrrodidinylmethoxy)pyridine dihydrochoride] was prepared by Abbott Laboratories.
α-ConotoxinMII was obtained from J. Michael Mcintosh, University of Utah, Salt Lake City, UT.
2.3 Synaptosomal preparation
Regions of interest were dissected from fresh mouse brains and homogenized in ice-cold isotonic sucrose (0.32 M) buffered with HEPES (5 mM, pH 7.5). The suspension was centrifuged at 12,000 × g for 20 min and the pellet resuspended in the uptake buffer appropriate for each assay [2;30;31] and used immediately. Protein content of the resuspended samples was not measured since signals were normalized to baseline release or efflux as described below.
2.4 [3H]-Dopamine uptake and release
Release methods of [2;4] were used. Briefly, the crude synaptosomal pellet from striatal tissue was resuspended in dopamine uptake buffer (NaCl, 128 mM; KCl, 2.4 mM; CaCl2, 3.2 mM; MgSO4, 1.2 mM; KH2PO4, 1.2 mM; HEPES, 25 mM; pH 7.5; glucose, 10 mM; ascorbic acid, 1 mM; pargyline, 0.01 mM) at 1.6 ml/tissue from one mouse. Synaptosomes were incubated at 37°C for 10 min before addition of [3H]-dopamine at 100 nM, (1 μCi for every 0.2 ml) and the incubation continued for another 5 min. Subsequently, aliquots of the suspension (80 μl) were distributed onto filters and perfused at room temperature with uptake buffer containing 0.1% BSA, nomifensine (1 μM) and atropine (1 μM) at 0.7 ml/min for 10 min before stimulation with agonist for 20 s. Selected aliquots were perfused with α-CtxMII (50nM) for the last 5 min of the wash period, immediately before stimulation. Fractions (∼0.1 ml) were collected every 10s into 96-well plates using a Gilson F204 fraction collector (Middleton WI) for 3 min after the 10 min washout. After addition of 0.15 ml of Optiphase SuperMix scintillation cocktail, radioactivity was determined in a 1450 MicroBeta Trilux counter (Perkin Elmer Life Sciences – Wallac Oy, Turku, Finland).
2.5 [3H]-ACh uptake and release
Release methods of Grady et al. [31] were followed with minor modifications. Briefly, the crude synaptosomal pellet from IPN tissue was resuspended in choline uptake buffer (NaCl, 128 mM; KCl, 2.4 mM; CaCl2, 3.2 mM; MgSO4, 1.2 mM; KH2PO4, 1.2 mM; HEPES, 25 mM; pH 7.5; glucose, 10 mM; 0.1% BSA) at 0.1 ml/mouse. After the addition of [3H]-choline at 0.25 nM (2 μCi/0.1 ml), the suspension was incubated at 37°C for 30 min. Then, aliquots (20 μl) were distributed onto filters on the perfusion system at room temperature and perfused for 10 min at 0.7 ml/min with choline uptake buffer containing atropine (1 μM) before stimulation by agonist for 20 s. Collection of fractions and determination of radioactivity were as for [3H]-dopamine release.
ABT-089 was tested as a potential antagonist of [3H]-ACh release by examining the effects of several concentrations of ABT-089 on the [3H]-ACh evoked by stimulation with 100 μM L-nicotine. For these studies, ABT-089 and nicotine (30 μM) were added simultaneously. Stimulation time was 20 s.
2.6 86Rb+efflux
Nicotine-stimulated 86Rb+ efflux from synaptosomes was investigated using the methods of Marks et al. [30;32] with minor modifications. Briefly, crude synaptosomes prepared from thalamus, cortex or striatum were resuspended in uptake buffer (NaCl, 140 mM; KCl, 1.5 mM; CaCl2, 2 mM; MgSO4, 1 mM; HEPES, 25 mM; pH 7.5; glucose, 20 mM) (350 μl/mouse thalamus, 800 μl/mouse cortex, 150 μl/mouse striatum). Aliquots (25 μl) of the suspension were added to 10μl of uptake buffer containing 4 μCi 86Rb+ and incubated at room temperature for 30 min. The whole sample was then collected onto filter paper (Type AE, Gelman, Ann Arbor, MI) and transferred to the perfusion apparatus, perfused with buffer (NaCl, 135 mM;CsCl, 5 mM; KCl, 1.5 mM; CaCl2, 2 mM; MgSO4, 1 mM; HEPES, 25 mM; pH 7.5; glucose, 20 mM; tetrodotoxin, 50 nM; atropine 1 μM; BSA 0.1%) at 2.5 ml/min for 5 min before data collection began. Stimulation by agonist was for 5s. Effluent was pumped through a 200 μl Cherenkov cell in a β-Ram HPLC detector (IN/US Systems, Tampa, FL) to continuously monitor radioactivity.
The effect of exposure to ABT-089 on a subsequent response to stimulation by nicotine was performed as described previously [33-35]. The concentration effect curve was constructed by exposing thalamic synaptosomes to one of several concentrations of ABT-089 (0.03 μM to 3 μM) for 10 min prior to a 1-min stimulation with 10 μM L-nicotine. The time course for the onset of desensitization was determined by exposing the synaptosomes to 1 μM ABT-089 for varying times (0.5 min to 12 min) prior to stimulation by 10 μM L-nicotine. The time course for recovery from desensitization was determined by exposing the synaptosomes to 1 μM ABT-089 for 10 min followed by perfusion with drug-free buffer for varying times (0.5 min to 12 min) before stimulation for 1 min with 10 μM nicotine.
2.7 Synaptosomal function data analysis
All synaptosomal function assays were calculated as counts exceeding basal release determined from samples immediately preceding and following stimulation [4;31;32]. Stimulated release was normalized to baseline to give units of release as a fraction of baseline. Fractions significantly over baseline for each perfusion were summed. EC50 values were calculated by fitting the data to the Hill equation, or two Michaelis-Menten equations when data were biphasic, and IC50 values from the inhibition equation (release=Ro/(1+[A]/IC5o, where Ro=uninhibited release and [A] is the concentration of ABT-089) using the non-linear least squares algorithm in SigmaPlot 5.0 (Jandel Scientific, San Rafael, CA). Error terms for these values were generated from the curve fits. Standard errors for ratios or percentages were estimated using Taylor's expansion (generally: semz2 =(dz/dx)2*semx2+(dz/dy)2*semz2 so for z=x/y; semz2 = 1/y2)*semx2+(x2/y4)*semy2) Student's t-test was used to evaluate differences between responses to agonists, differences in responses between brain regions, and the effect of α5 gene deletion.
3. Results
3.1 86Rb+ Efflux
3.1.1 Stimulation of 86 Rb+ Efflux
nAChR-mediated 86 Rb+ efflux in mouse thalamus and cortex is predominantly mediated by α4β2*-nAChR [32;34;35]. Several effects of ABT-089 on this response were measured.
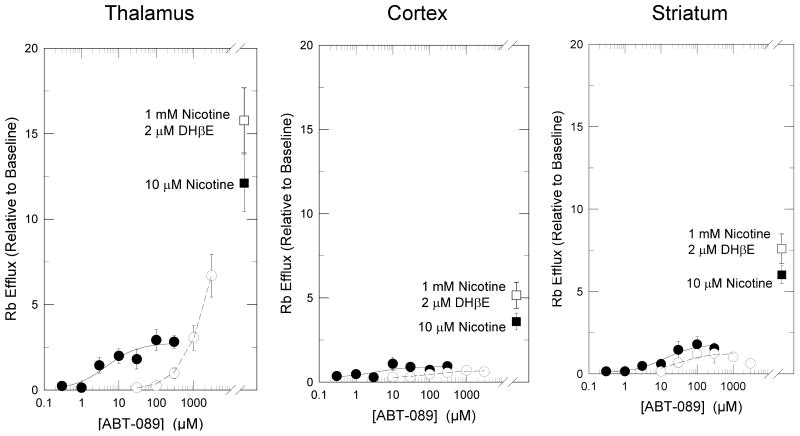
Concentration-effect curves were constructed for the stimulation of 86Rb+ efflux from mouse thalamic, cortical and striatal synaptosomes in the absence or presence of 2 μM DHβE to determine total efflux and DHβE-resistant efflux [32]. These curves are shown in Figure 1. ABT-089 elicited a concentration-dependent stimulation of both DHβE-sensitive and -resistant 86Rb+ efflux that differed among the regions. The EC50 for stimulation of DHβE-sensitive 86Rb+ efflux from thalamus was 3.5±2.1 μM with a maximal efflux that was 20.3±4.0% of the response elicited by 10 μM nicotine. Total DHβE-sensitive 86Rb+ efflux stimulated by 10 μM nicotine from striatal and cortical synaptosomes was significantly less than that from thalamic synaptosomes (48±8% and 30±6% of thalamus, respectively; P<0.05 for both regions, t-test). 86Rb+ efflux stimulated by ABT-089 in striatum and cortex was even lower than that elicited by nicotine. The EC50 for stimulation of DHβE-sensitive 86Rb+ efflux from striatum was 2.0±1.2 μM with a maximal efflux that was 11.1±1.8% of the response elicited by 10 μM nicotine. The EC50 for stimulation of DHβE-sensitive 86Rb+ efflux from cortex was 5.9±1.4 μM with a maximal efflux that is 11.4±5.7% of the response elicited by 10 μM nicotine.
Figure 1.
Concentration-Effect Curves of Activation of 86Rb+ Efflux by ABT-089. Mouse thalamic, striatal and cortical synaptosomes were stimulated with indicated concentrations of ABT-089 for 5 sec either in the absence (●) or presence (○) of 2 μM DHβE. Each point represents mean±SEM from five separate experiments. Curves are nonlinear fits of the data to the Michaelis-Menten equation. Stimulation elicited by 10 μM nicotine or 1000 μM nicotine plus 2 μM DHβE are shown for reference.
While the EC50 values for ABT-089 did not differ among the regions for the DHβE-sensitive responses, apparent pattern of activation observed for DHβE-resistant 86Rb+ efflux did differ. In thalamus DHβE-resistant 86Rb+ efflux was also stimulated by ABT-089, but concentrations required to elicit this response were relatively high: there was no measurable response with 100 μM ABT-089 but significant activity was observed following stimulation with 1000 μM and 3000 μM. Even at these high concentrations, the response was not as high as that observed with nicotine and was not yet maximal. In contrast, DHβE-resistant 86Rb+ efflux elicited by ABT-089 in striatum and cortex and was low (16.7±3.0% and 8.7±1.9 of the response to nicotine, respectively), but appeared to be saturable with EC50 values of 27±16 μM and 100±65 μM, respectively.
Inasmuch as ABT-089 was less efficacious in stimulating 86Rb+ efflux in cortex and striatum than in thalamus, it is possible that the α4β2*-nAChR subtypes mediating responses in these regions differed from that in thalamus. It has previously been shown that deletion of the α5 nAChR subunit markedly reduced 86Rb+ efflux with high sensitivity to stimulation by ACh in thalamus but had less effect in striatum and no significant effect in cortex [5]. Therefore, the effect of α5 nAChR gene deletion on ABT-stimulated 86Rb+ efflux was evaluated and is shown in Figure 2. Consistent with the results of Brown et al. [5], deletion of the α5 subunit reduced the maximal nicotine-stimulated 86Rb+ efflux by 35±11% (from 9.89±1.26 Units to 6.42±0.77 Units, P<0.05, t-test). Maximal 86Rb+ efflux stimulated by ABT-089 was nearly eliminated in α5-/- mice (reduced from a maximal signal 25±4% that of nicotine in α5+/+ mice to 3±1% in α5-/- mice). This 88±4% reduction in ABT-089 stimulated 86Rb+ efflux following deletion of the α5 subunit is significantly greater than the 35±11% reduction in nicotine stimulated 86Rb+ efflux following deletion of the α5 subunit (P<0.05, t-test).
Figure 2.
Effect of Deletion of the α5 nAChR Subunit on 86Rb+ Efflux Stimulated by ABT-089. Concentration-effect curves for stimulation of 86Rb+ efflux by ABT-089 were measured in thalamic synaptosomes prepared from α5+/+ (●, wild-type) and α5-/- (○, null mutant) mice. Each point represents the mean±SEM of five separate experiments. Responses are presented as the percentage relative the efflux stimulated by 10 μM nicotine in wild-type mice. The curve is a fit of the wild-type data to the Michaelis-Menten equation. Data for the null-mutant could not be fit.
3.1.2 Desensitization of 86Rb+ efflux
Prolonged exposure of nAChR to agonists desensitizes the receptors [36]. Desensitization has also been measured for nAChR-mediated 86Rb+ efflux in mouse brain [33;37].
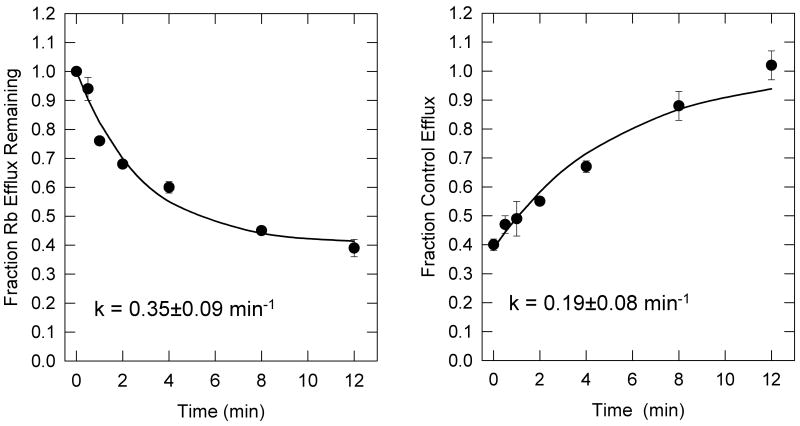
The rate of onset and recovery from desensitization elicited by 1 μM ABT-089 were measured and these results are shown in Figure 3. Exposure of thalamic synaptosomes to ABT-089 resulted in a time-dependent reduction of activity of nicotine-stimulated 86Rb+ efflux leading to a steady state level of desensitization. The apparent rate constant under these conditions was 0.35 ± 0.09 min-1 and the response was reduced by 60%. Nicotine-stimulated 86Rb+ efflux was fully recovered from desensitization induced by a 10-min exposure to 1 μM ABT-089 by a 12 min washout in drug-free buffer. The apparent rate constant for reversal of desensitization was 0.19±0.08min-1.
Figure 3.
Time Courses for the Onset and Recovery of ABT-089 Induced Desensitization. The time course for the onset of desensitization by exposure to 1 μM ABT-089 is shown in panel A. Thalamic synaptosomes were exposed to 1 μM ABT-089 for the indicated times before stimulation with 10 μM nicotine for 1 min. Each point represents the mean ±SEM of three separate experiments. The curve is the least squares curve fit of the data to an exponential decay with residual activity. The time course for recovery from desensitization elicited by exposure to 1 μM ABT-089 is shown in Panel B. Synaptosomes were perfused 1 μM ABT-089 for 10 min after which they were perfused with drug free buffer before stimulation with 10 μM nicotine for 1 min. Each point represents the mean±SEM of data from three separate experiments. The curve represents the exponential recovery of activity starting at a non-zero point. The values for the rate constants calculated from the curve fits are listed in the figure.
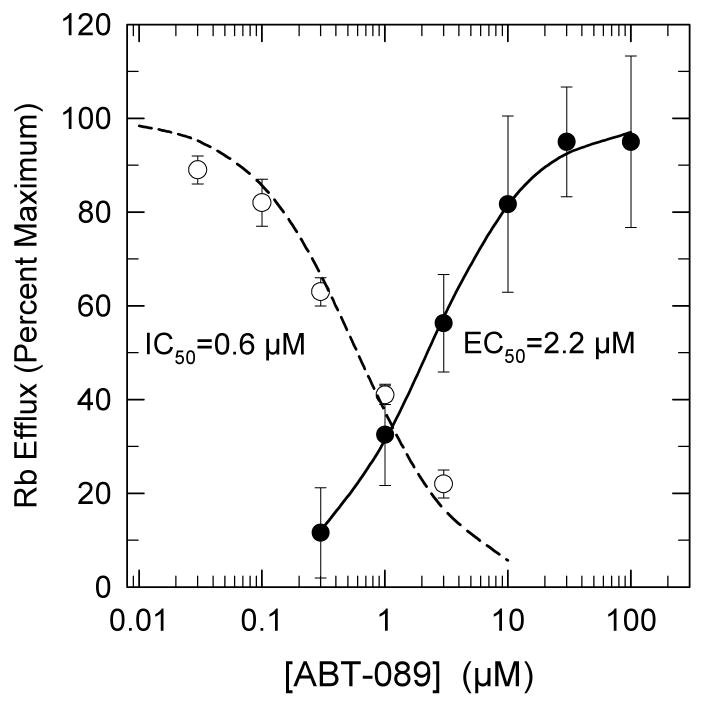
The concentration dependence for the desensitization of nicotine-stimulated 86Rb+ efflux from thalamic synaptosomes was measured and the results are shown in Figure 4. A concentration-dependent decrease in nicotine-stimulated 86Rb+ efflux was observed following a 10-min exposure to ABT-089 for which an IC50 value of 0.68±0.16 μM was determined. This value is consistent with that calculated from the ratio of the rates of recovery and onset of desensitization (0.19min-1/0.35min-1*[ABT-089]-1 = 0.54 μM). The concentration-effect curve for ABT-089 activation of 86Rb+ efflux determined under similar conditions (a 1-min stimulation) is also shown in Figure 4. The EC50 value of 2.2±0.1 μM is only 3-fold higher than the IC50 value for desensitization. Consquently, the desensitization and activation curves for ABT-089 effects on 86Rb+ efflux show considerable overlap in the range of ABT-089 concentrations between 0.3 μM and 3 μM.
Figure 4.
Concentration-Effect Curves for Desensitization and Stimulation of 86Rb+ Efflux by ABT-089. To measure desensitization, thalamic synaptosomes were perfused with the indicated concentrations of ABT-089 for 10 min prior to stimulation by exposure to 10 μM nicotine (○). Each point represents the mean±SEM from three separate experiments. The line was obtained by the non-linear curve fit of the data to the equation: Ea = E0/(1+A/IC50), where Ea is the 86Rb+ efflux stimulated with 10 μM nicotine after treatment with [ABT-089]. E0 is the response to10 μM nicotine without ABT-089 treatment. A is the concentration of ABT-089 to which the samples have been exposed and IC50 is the effective inhibition constant. Stimulation of 86Rb+ efflux (●) by a 1-min exposure to the indicated concentrations of ABT-089 was determined. Each point represents the mean±SEM from six separate experiments. The line is a fit of the data to the Michaelis-Menten equation. For the desensitization experiments the response elicited by 10 μM nicotine is set at 100%, while for the stimulation experiments the maximal ABT-089 elicited efflux is set at 100%. Maximal efflux rate for ABT-089 was 32±4% that elicited by stimulation with 10 μM nicotine.
3.2 [3H]-ACh Release
nAChR-mediated [3H]-ACh release from mouse IPN synaptosomes is mediated by α3β4*-nAChR [31]. The ability of ABT-089 to stimulate or inhibit [3H]-ACh release was tested.
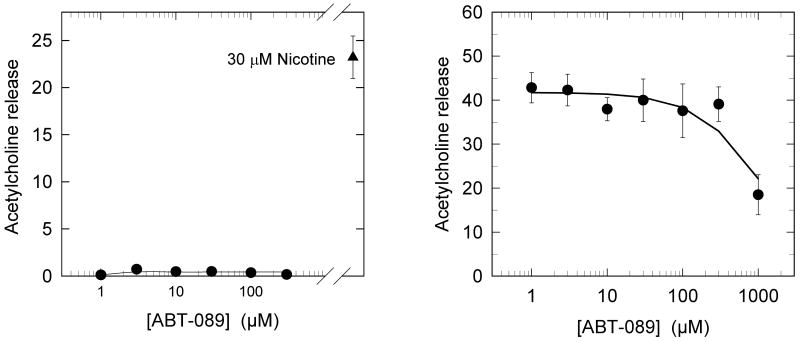
The concentration-effect curve for stimulation of [3H]-ACh release from mouse IPN synaptosomes is shown in Figure 5a. No significant stimulation was observed for ABT-089 concentrations between 1 μM and 300 μM indicating no agonist activity in this concentration range. Subsequently the inhibitory activity of ABT-089 on nicotine-stimulated [3H]-ACh release from mouse IPN synaptosomes was tested. No inhibition of [3H]-ACh release stimulated by 30 μM nicotine was observed for simultaneous exposure of the synaptosomes to nicotine and concentrations of ABT-089 between 1 μM and 300 μM. However, inhibition was observed when the concentration of ABT-089 was increased to 1000 μM.
Figure 5.
ABT-089 Evaluated as Agonist or Antagonist for nAChR-Mediated [3H]ACh Release from IPN Synaptosomes. Panel A presents data for [3H]ACh release elicited by the indicated concentrations of ABT-089. There was no detectable response. Panel B presents data for the [3H]ACh release elicited by 100 μM nicotine in the presence of the indicated concentrations of ABT-089. Each point represents the mean±SEM of three separate experiments.
3.3 [3H]-Dopamine Release
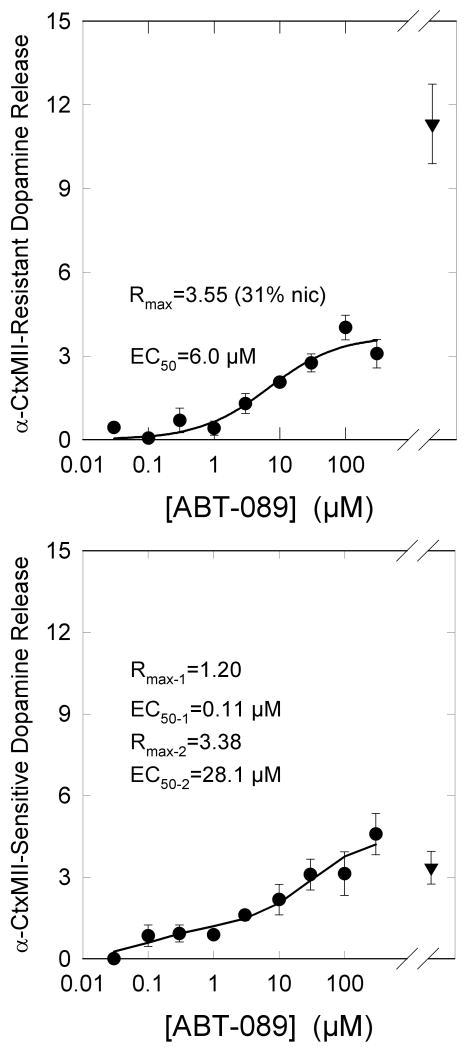
[3H]-Dopamine release from mouse striatal synaptosomes is mediated by several nAChR subtypes [2;3;30]. These subtypes are comprised of two major classes: one class that is sensitive to inhibition by α-CtxMII and is mediated by α6β2*-nAChR and a second class that is not inhibited by α-CtxMII and is mediated by α4β2*-nAChR. Each of these classes is heterogeneous with the predominant subtypes that are sensitive to α-CtxMII being α6α4β2β3- and α6β2β3-nAChR, while the predominant subtypes that are resistant of αCtxMII being α4α5β2- and α4β2-nAChR [30].
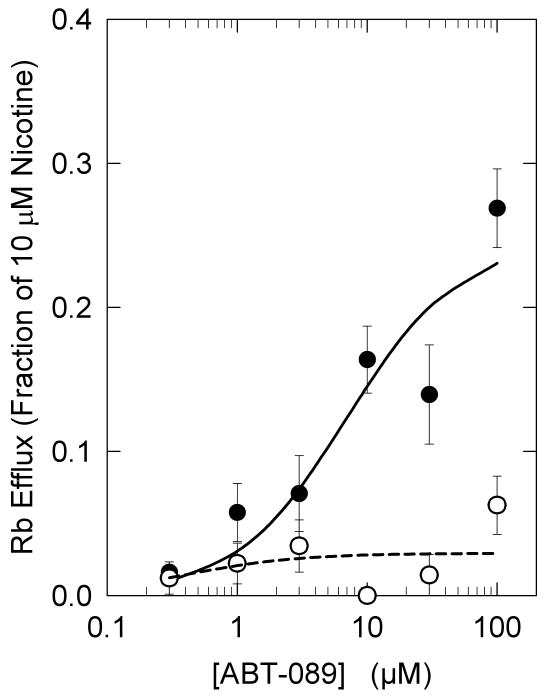
The concentration-effect curves for stimulation of α-CtxMII-resistant and –sensitive [3H]-dopamine release from mouse striatal synaptosomes are shown in Figure 6. ABT-089 is a partial agonist for the α-CtxMII-resistant component. Maximal release elicited by ABT-089 was 31±5% that of nicotine with an EC50 of 6.1±2.4 μM. In contrast, the maximal release of [3H]-dopamine that is sensitive to inhibition by α-CtxMII exceeds that elicited by nicotine. Furthermore, the concentration-effect curve for ABT-089 stimulated, α-CtxMII-sensitive [3H]-dopamine is biphasic. An EC50 value of 0.11±0.14 μM was calculated for the higher affinity component, while an EC50 value of 28.1±20.1 μM was calculated for the lower affinity component. Maximal [3H]-dopamine release stimulated for the higher and lower affinity components were 36±16% and 98±25% yielding a total α-CtxMII-sensitive release 134±33% of that elicited by the α-CtxMII-sensitive component of nicotine stimulated [3H]-dopamine release.
Figure 6.
ABT-089 Stimulation of α-CtxMII-Resistant and α-CtxMII-Sensitive [3H]-Dopamine Release. [3H]-Dopamine release was measured in the absence or presence of 50 nM α-CtxMII as an α6β2* nAChR antagonist. α-CtxMII resistant release is that persisting in the presence of the toxin and α-CtxMII sensitive release was calculated as the difference in response in the absence and presence of the toxin. Each point represents the mean±SEM of five separate experiments. Results for the αCtxMII-resistant response were fit to the Michaelis-Menten equation (Panel A), while those for α-CtxMII resistant release were analyzed as a biphasic curve (Panel B). Values for the parameters are shown. [3H]-Dopamine release elicited by exposure to 10 μM nicotine is provided for reference.
4. Discussion
Prior studies in our laboratory have demonstrated that in the mouse thalamic synaptosomal 86Rb+ flux assay, α4β2* nAChR subtypes mediate virtually all nAChR responses [30;32;35]. Here, we found that ABT-089 stimulated 86Rb+ flux in a DHβE-sensitive manner with an EC50 value of 2.5 μM and maximal response of 20.33% relative to nicotine. These values are similar to those reported by Anderson et al. [29] for [3H]-dopamine release from rat prefrontal cortex where again the response is thought to be predominantly α4β2*-mediated. In the presence of DHβE (2 μM), only very high concentrations of ABT-089 (≥ 300 μM) stimulated 86Rb+ flux in thalamic synaptosomes. This may be due to activation of receptors other than α4β2* nAChR, or simply to displacement of the competitive inhibitor DHβE. However, previous studies suggest that activation of 86Rb+ efflux by high concentrations of nicotinic agonists is mediated by α4β2-nAChR with alternate α/β stoichiometry [35]. It should be noted that the responses to the higher concentrations of ABT-089 in striatum and cortex were small, but did appear to be saturable. Whether this difference in response between these regions and thalamus reflects differences in receptor expression has not been resolved.
In cortical and striatal synaptosomes, the relative efficacy of ABT-089 in stimulating DHβE-sensitive 86Rb+ efflux was lower than that observed in thalamus. This result suggests that the α4β2*-nAChR subtype mediating 86Rb+ efflux stimulated with relatively low concentrations of ABT-089 differs among these regions. Indeed, it has previously been reported that deletion of the α5 subunit elicited a larger decrease in efflux in thalamus than in the other regions [5], suggesting that ABT-089 may act through α4α5β2-nAChR selectively compared to α4β2 nAChR. This hypothesis is supported by the observation that deletion of the α5 subunit reduced thalamic 86Rb+ efflux stimulated by ABT-089 by 90%. In contrast, the response to nicotine was reduced by only 36% following deletion of the α5 subunit, a value similar to that reported previously [5].
Prolonged exposure to nAChR agonists desensitizes the receptors [36 for review]. This property is also exhibited following prolonged exposure to relatively low concentrations of ABT-089 as illustrated in Figures 3 and 4. The onset and recovery from desensitization elicited by exposure to these concentrations is relatively slow and is concentration dependent. It is important to note, however, that there is a substantial overlap of the concentration effect curves for desensitization and activation of 86Rb+ efflux for ABT-089 indicating that this agonist exhibits significant residual activity. That is, there is a relatively wide concentration range where α4β2*-nAChR activity persists in which the receptors are not completely desensitized or fully activated. Similar patterns of residual activation have been observed for other partial agonists including cytisine, nornicotine and D-nicotine [33]. The concentration of ABT-089 required to elicit 50% desensitization is significantly higher that the Ki for binding to this receptor subtype [27]. A similar dichotomy between these two measures has been observed previously and is not unique to ABT-089 [33].
ABT-089 also was more efficacious in stimulating [3H]-dopamine release in slices prepared from rat striatum (65% that of nicotine) than from slices prepared from prefrontal cortex (32% that of nicotine) [29]. Similarly using mouse synaptosomes, we found that ABT-089 stimulated total [3H]-dopamine release with a relative efficacy of 57% in striatum compared to 20% in thalamic 86Rb+ flux mediated by α4β2*-nAChR. Dopamine neurons express a relatively high proportion of α6β2*-nAChR compared to other neurons [2;30] and the relative importance of the α6β2*-nAChR is illustrated by the ability of αCtx MII to inhibit a significant fraction of nAChR-mediated [3H]-dopamine release. Consequently, activity of ABT-089 at α6β2*-nAChR potentially could explain the higher efficacy observed in eliciting [3H]-dopamine release in striatum compared to its efficacy in eliciting 86Rb+ efflux from thalamus, cortex or striatum. To test whether ABT-089 was indeed more efficacious at α6β2*-nAChR, we measured the stimulation of [3H]-dopamine release from mouse striatal synaptosomes in the absence and presence of the α6β2*-nAChR antagonist α-CtxMII. In our previous studies using αCtxMII inhibition and evaluating the effects of nAChR gene deletion, we have shown that αCtxMII-resistant [3H]-dopamine release elicited by nicotinic receptor stimulation is mediated predominantly by α4β2*- and α4α5β2-nAChR, while αCtxMII-sensitive [3H]-dopamine release elicited by nicotine receptor stimulation is mediated predominantly by α6β2β3- and α4α6β2β3-nAChR [2;30]. In the presence of αCtxMII, ABT-089 stimulated [3H]-dopamine release with an EC50 of 6 μM and efficacy of 31% relative to nicotine, values similar to those determined for α4β2-nAChR mediated thalamic 86Rb+ flux. In contrast, the αCtxMII-sensitive [3H]-dopamine release stimulated by ABT-089 exhibited a biphasic concentration-response relationship in which ABT-089 was particularly potent at one component (0.11 μM EC50) and particularly efficacious (98% relative to αCtxMII sensitive stimulation by nicotine) at the other component. Overall, the efficacy of ABT-089 at αCtxMII-sensitive α6β2*-nAChR was 134% of nicotine. This observation potentially explains the greater efficacy of ABT-089 in striatal [3H]-dopamine release assays compared to α4β2-nAChR mediated 86Rb+ efflux and recombinant α4β2-nAChR assays [29;38]. Further, the high-sensitivity component for ABT-089, with an EC50 of 0.11 μM, appears to correlate with the potency of ABT-089 to enhance cognitive function [26]. Whether this high-sensitivity component represents α6β2β3- or α4α6β2β3-nAChR is not yet known with certainty. However, given that ABT-089 binds to α4β2*-nAChR sites with a Ki of 17 nM [27] and is able to activate α4β2* nAChR, we propose that the [3H]-dopamine release with high sensitivity to ABT-089 is mediated by α4α6β2β3-nAChR.
The selectivity of ABT-089 was further evaluated using [3H]-ACh release from IPN synaptosomes. This nAChR response has been shown to be mediated predominantly by α3β4*-nAChR [31;39]. Here, ABT-089 elicited essentially no response. Furthermore, ABT-089 did not significantly inhibit the response to nicotine, except at a very high concentration (1,000 μM ABT-089). Thus, ABT-089 exhibits very little interaction with mouse native α3β4* nAChR, consistent with its selectivity against human α3β4-nAChR [27;38] and its benign adverse effect profile in preclinical and clinical studies [26;40].
In sum, these studies confirm that ABT-089 is a partial agonist at α4β2* nAChR, extend that to demonstrate selectivity for α4α5β2 in contrast to α4β2, and further identify α6β2* nAChR as a prominent target in ABT-089 stimulation of dopamine nerve terminals. Indeed, one α6β2* nAChR subtype is particularly sensitive to ABT-089 with an EC50 of 0.11 μM. This, for the first time, points to a nAChR subtype with in vitro sensitivity comparable to the in vivo behavioral determinations. We suggest that α6β2β3*-nAChR subtypes, which regulate a subset of nAChR stimulated dopamine release, may be important targets contributing to the reported efficacy of ABT-089 in cognitive studies [26;28;40]. Further studies using, for example, α4 knockout animals could identify this α6β2* subtype, helping to clarify the physiological roles of these nAChR, and potentially leading to the discovery of novel agonists with yet-greater selectivity and efficacy for these nAChR and cognitive enhancement.
Acknowledgments
Supported in part by a grant from Abbott Laboratories and grants DA003194 and DA015663 to MJM from the National Institute on Drug Abuse.
Footnotes
Disclosure: Murali Gopalakrishnan and Clark A. Briggs are employees of Abbott Laboratories which has developed ABT-089, the object of the current study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kuryatov A, Onksen J, Lindstrom JM. Roles of Accessory Subunits in α4β2* Nicotinic Receptors. Mol Pharmacol. 2008;74:132–43. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- 2.Salminen O, Drapeau J, Mcintosh M, Collins AC, Marks MJ, Grady SR. Pharmacology of α–conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol. 2007;71:1563–71. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- 3.Champtiaux N, Changeux JP. Knockout and knockin mice to investigate the role of nicotinic receptors in the central nervous system. Prog Brain Res. 2004;145:235–51. doi: 10.1016/s0079-6123(03)45016-4. [DOI] [PubMed] [Google Scholar]
- 4.Salminen O, Murphy KL, Mcintosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit Composition and Pharmacology of Two Classes of Striatal Presynaptic Nicotinic Acetylcholine Receptors Mediating Dopamine Release in Mice. Mol Pharmacol. 2004;65:1526–35. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- 5.Brown RWB, Collins AC, Lindstrom JM, Whiteaker P. Nicotinic α5 subunit deletion locally reduces high-affinity agonist activation without altering nicotinic receptor numbers. J Neurochem. 2007;103:204–15. doi: 10.1111/j.1471-4159.2007.04700.x. [DOI] [PubMed] [Google Scholar]
- 6.Briggs CA, Gubbins EJ, Marks MJ, Putman CB, Thimmapaya R, Meyer MD, Surowy CS. Untranslated region dependent exclusive expression of high-sensitivity subforms of α4β2 and α3β2 nicotinic acetylcholine receptors. Mol Pharmacol. 2006;70:227–40. doi: 10.1124/mol.105.020198. [DOI] [PubMed] [Google Scholar]
- 7.Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of a4β2 nicotinic acetylcholine receptors. Mol Pharmacol. 2003;63:332–41. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y, Nelson ME, Kuryatov A, Choi C, Cooper J, Lindstrom J. Human α4β2 acetylcholine receptors formed from linked subunits. J Neurosci. 2003;23:9004–15. doi: 10.1523/JNEUROSCI.23-27-09004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zwart R, Vijverberg HPM. Four pharmacologically distinct subtypes of α4β2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol Pharmacol. 1998;54:1124–31. [PubMed] [Google Scholar]
- 10.Tribollet E, Bertrand D, Marguerat A, Raggenbass M. Comparative distribution of nicotinic receptor subtypes during development, adulthood and aging: an autoradiographic study in the rat brain. Neurosci. 2004;124:405–20. doi: 10.1016/j.neuroscience.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Wang NS, Orr-Urtreger A, Korczyn AD. The role of neuronal nicotinic acetylcholine receptor subunits in autonomic ganglia: lessons from knockout mice. Prog Neurobiol. 2002;68:341–60. doi: 10.1016/s0301-0082(02)00106-5. Review. [DOI] [PubMed] [Google Scholar]
- 12.Arredondo J, Hall LL, Ndoye A, Nguyen VT, Chernyavsky AI, Bercovich D, Orr-Urtreger A, Beaudet AL, Grando SA. Central role of fibroblast α3 nicotinic acetylcholine receptor in mediating cutaneous effects of nicotine. Lab Invest. 2003;83:207–25. doi: 10.1097/01.lab.0000053917.46614.12. [DOI] [PubMed] [Google Scholar]
- 13.Li XW, Wang H. Non-neuronal nicotinic α7 receptor, a new endothelial target for revascularization. Life Sci. 2006;78:1863–70. doi: 10.1016/j.lfs.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 14.Heeschen C, Weis M, Aicher A, Dimmeler S, Cooke JP. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J Clin Invest. 2002;110:527–36. doi: 10.1172/JCI14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawashima K, Fujii T. Extraneuronal cholinergic system in lymphocytes. Pharmacol Ther. 2000;86:29–48. doi: 10.1016/s0163-7258(99)00071-6. Review. [DOI] [PubMed] [Google Scholar]
- 16.Mao D, Yasuda RP, Fan H, Wolfe BB, Kellar KJ. Heterogeneity of Nicotinic Cholinergic Receptors in Rat Superior Cervical and Nodose Ganglia. Mol Pharmacol. 2006;70:1693–9. doi: 10.1124/mol.106.027458. [DOI] [PubMed] [Google Scholar]
- 17.Flores CM, Decamp RM, Kilo S, Rogers SW, Hargreaves KM. Neuronal nicotinic receptor expression in sensory neurons of the rat trigeminal ganglion - demonstration of α3β4, a novel subtype in the mammalian nervous system. J Neurosci. 1996;16:7892–901. doi: 10.1523/JNEUROSCI.16-24-07892.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Genzen JR, Van Cleve W, McGehee DS. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J Neurophysiol. 2001;86:1773–82. doi: 10.1152/jn.2001.86.4.1773. [DOI] [PubMed] [Google Scholar]
- 19.Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: from structure to brain function. Rev Physiol Biochem Pharmacol. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- 20.Meyer EL, Yoshikami D, Mcintosh JM. The neuronal nicotinic acetylcholine receptors α4* and α6* differentially modulate dopamine release in mouse striatal slices. J Neurochem. 2008;105:1761–9. doi: 10.1111/j.1471-4159.2008.05266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azam L, Chen Y, Leslie FM. Developmental regulation of nicotinic acetylcholine receptors within midbrain dopamine neurons. Neurosci. 2007;144:1347–60. doi: 10.1016/j.neuroscience.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pons S, Fattore L, Cossu G, Tolu S, Porcu E, Mcintosh JM, Changeux JP, Maskos U, Fratta W. Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–27. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grinevich VP, Letchworth SR, Lindenberger KA, Menager J, Mary V, Sadieva KA, Buhlman LM, Bohme GA, Pradier L, Benavides J, Lukas RJ, Bencherif M. Heterologous expression of human α6β4β3α5 nicotinic acetylcholine receptors: binding properties consistent with their natural expression require quaternary subunit assembly including the α5 subunit. J Pharmacol Exp Ther. 2005;312:619–26. doi: 10.1124/jpet.104.075069. [DOI] [PubMed] [Google Scholar]
- 24.Evans NM, Bose S, Benedetti G, Zwart R, Pearson KH, McPhie GI, Craig PJ, Benton JP, Volsen SG, Sher E, Broad LM. Expression and functional characterisation of a human chimeric nicotinic receptor with α6β4 properties. Eur J Pharmacol. 2003;466:31–9. doi: 10.1016/s0014-2999(03)01540-1. [DOI] [PubMed] [Google Scholar]
- 25.Kuryatov A, Olale F, Cooper J, Choi C, Lindstrom J. Human α 6 AChR subtypes: subunit composition, assembly, and pharmacological responses. Neuropharmacol. 2000;39:2570–90. doi: 10.1016/s0028-3908(00)00144-1. [DOI] [PubMed] [Google Scholar]
- 26.Rueter LE, Anderson DJ, Briggs CA, Donnelly-Roberts DL, Gintant GA, Gopalakrishnan M, Lin NH, Osinski MA, Reinhart GA, Buckley MJ, Martin RL, McDermott JS, Preusser LC, Seifert TR, Su Z, Cox BF, Decker MW, Sullivan JP. ABT-089: pharmacological properties of a neuronal nicotinic acetylcholine receptor agonist for the potential treatment of cognitive disorders. CNS Drug Rev. 2004;10:167–82. doi: 10.1111/j.1527-3458.2004.tb00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan JP, Donnelly-Roberts D, Briggs CA, Anderson DJ, Gopalakrishnan M, Xue IC, Piattoni-Kaplan M, Molinari E, Campbell JE, McKenna DG, Gunn DE, Lin NH, Ryther KB, He Y, Holladay MW, Wonnacott S, Williams M, Arneric SP. ABT-089 [2-methyl-3(2-(S)-pyrrolidinylmethoxy)pyridine]: I. A potent and selective cholinergic channel modulator with neuroprotective properties. J Pharmacol Exp Ther. 1997;283:235–46. [PubMed] [Google Scholar]
- 28.Decker MW, Bannon AW, Curzon P, Gunther KL, Brioni JD, Holladay MW, Lin NH, Li YH, Daanen JF, Buccafusco JJ, Prendergast MA, Jackson WJ, Arneric SP. ABT-089 [2-methyl-3(2-(s)-pyrrolidinylmethoxy)pyridine dihydrochloride]. 2. A novel cholinergic channel modulator with effects on cognitive performance in rats and monkeys. J Pharmacol Exp Ther. 1997;283:247–58. [PubMed] [Google Scholar]
- 29.Anderson David J, Malysz John, Grønlien Jens Halvard, El Kouhen Rachid, Håkerud Monika, Wetterstrand Caroline, Briggs Clark A, Gopalakrishnan Murali. Stimulation of dopamine release by nicotinic agonists correlates with high-sensitivity α4β2 potency. Biochemical Pharmacology. 2009 doi: 10.1016/j.bcp.2009.06.024. in press. [DOI] [PubMed] [Google Scholar]
- 30.Grady SR, Salminen O, Laverty DC, Whiteaker P, Mcintosh JM, Collins AC, Marks MJ. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–46. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grady SR, Meinerz NM, Cao J, Reynolds AM, Picciotto MR, Changeux JP, Mcintosh JM, Marks MJ, Collins AC. Nicotinic agonists stimulate acetylcholine release from mouse interpeduncular nucleus: a function mediated by a different nAChR than dopamine release from striatum. J Neurochem. 2001;76:258–68. doi: 10.1046/j.1471-4159.2001.00019.x. [DOI] [PubMed] [Google Scholar]
- 32.Marks MJ, Whiteaker P, Calcaterra J, Stitzel JA, Bullock AE, Grady SR, Picciotto MR, Changeux JP, Collins AC. Two pharmacologically distinct components of nicotinic receptor-mediated rubidium efflux in mouse brain require the β 2 subunit. J Pharmacol Exp Ther. 1999;289:1090–103. [PubMed] [Google Scholar]
- 33.Marks MJ, Robinson SF, Collins AC. Nicotinic agonists differ in activation and desensitization of 86Rb+ efflux from mouse thalamic synaptosomes. J Pharmacol Exp Ther. 1996;277:1383–96. [PubMed] [Google Scholar]
- 34.Marks MJ, Whiteaker P, Collins AC. Deletion of the α7, β2, or β4 Nicotinic Receptor Subunit Genes Identifies Highly Expressed Subtypes with Relatively Low Affinity for [3H]Epibatidine. Mol Pharmacol. 2006;70:947–59. doi: 10.1124/mol.106.025338. [DOI] [PubMed] [Google Scholar]
- 35.Gotti C, Moretti M, Meinerz N, Clementi F, Gaimarri A, Collins AC, Marks MJ. Partial deletion of the nicotinic cholinergic receptor α4 and β2 subunit genes changes the acetylcholine sensitivity of receptor mediated 86Rb+ efflux in cortex and thalamus and alters relative expression of α4 and β2 subunits. Mol Pharmacol. 2008;73:1796–807. doi: 10.1124/mol.108.045203. [DOI] [PubMed] [Google Scholar]
- 36.Quick MW, Lester RAJ. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002;53:457–78. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- 37.Marks MJ, Grady SR, Yang JM, Lippiello PM, Collins AC. Desensitization of nicotine-stimulated 86Rb+ efflux from mouse brain synaptosomes. J Neurochem. 1994;63:2125–35. doi: 10.1046/j.1471-4159.1994.63062125.x. [DOI] [PubMed] [Google Scholar]
- 38.Michelmore S, Croskery K, Nozulak J, Hoyer D, Longato R, Weber A, Bouhelal R, Feuerbach D. Study of the calcium dynamics of the human α4β2, α3β4 and α1β1γδ nicotinic acetylcholine receptors. Naunyn-Schmiedebergs Arch Pharmacol. 2002;366:235–45. doi: 10.1007/s00210-002-0589-z. [DOI] [PubMed] [Google Scholar]
- 39.Grady SR, Moretti M, Zoli M, Marks MJ, Zanardi A, Pucci L, Clementi F, Gotti C. Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J Neurosci. 2009;29:2272–82. doi: 10.1523/JNEUROSCI.5121-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilens TE, Decker MW. Neuronal nicotinic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: Focus on cognition. Biochem Pharmacol. 2007;74:1212–23. doi: 10.1016/j.bcp.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]