Abstract
The mitogen-activated protein kinase (MAPK) and cyclic adenosine monophosphate (cAMP) signal transduction pathways have critical roles in the consolidation of hippocampus-dependent memory. We found that extracellular regulated kinase 1/2 MAPK phosphorylation and cAMP underwent a circadian oscillation in the hippocampus that was paralleled by changes in Ras activity and the phosphorylation of MAPK kinase and cAMP response element-binding protein (CREB). The nadir of this activation cycle corresponded with severe deficits in hippocampus-dependent fear conditioning under both light-dark and free-running conditions. Circadian oscillations in cAMP and MAPK activity were absent in memory-deficient transgenic mice that lacked Ca2+-stimulated adenylyl cyclases. Furthermore, physiological and pharmacological interference with oscillations in MAPK phosphorylation after the cellular memory consolidation period impaired the persistence of hippocampus-dependent memory. These data suggest that the persistence of long-term memories may depend on reactivation of the cAMP/MAPK/CREB transcriptional pathway in the hippocampus during the circadian cycle.
There is considerable interest in the molecular mechanisms underlying the persistence of long-term memory (LTM). Several signal transduction pathways, including the extracellular regulated kinase (Erk) 1/2 MAPK and cAMP signaling pathways are strongly implicated in memory consolidation (for reviews, see refs. 1-3). Activation of MAPK is necessary for amygdala- and hippocampus-dependent LTM consolidation4-6. In addition, the reconsolidation of some amygdala- and hippocampus-dependent memories require MAPK activity7,8. It is hypothesized that MAPK activation may be required for memory consolidation to stimulate the expression of a family of genes regulated through the CREB/CRE transcriptional pathway (for reviews, see refs. 9-11) and that memory consolidation may also depend on increased translation mediated by MAPK12.
The role of cAMP signaling in LTM has been demonstrated in numerous species including rodents. For example, the amount of cAMP in the hippocampus increases following inhibitory avoidance training in rats, and inhibition of protein kinase A (PKA) activity substantially impairs consolidation of the task13. The discovery that Ca2+-activated adenylyl cyclases are required for hippocampus-dependent LTM14,15 and the observation that PKA is activated in a subpopulation of CA1 pyramidal neurons following contextual memory training16 provide evidence that cAMP increases are required for LTM. Studies showing that genetic reductions in PKA activity impair memory also support the hypothesis that cAMP signaling is critical for LTM17-19. Although these studies contribute to an understanding of memory, mechanisms for the persistence of LTM have not been elucidated. Because training-induced activation of cAMP and MAPK during memory formation is transient and de novo transcription and translation result in the synthesis of synaptic proteins with half lives measured in hours or, at most, weeks, how are LTMs sustained over months or years?
We considered the possibility that the biochemical pathways underlying memory may be reactivated repeatedly to sustain the levels of protein that are required for the persistence of memory. This general hypothesis is supported by evidence that repeated rounds of NMDA receptor synthesis are required for memory consolidation and maintenance20. Furthermore, persistence of memory for a one-trial associative learning task depends on protein synthesis and brain-derived neurotrophic factor 12 h after training, suggesting that memory persistence depends on recurrent rounds of consolidation21. Electro-physiological evidence supporting this idea comes from a series of experiments showing that the same sequence of synaptic firing that occurs during training for a spatial memory task is repeated during sleep22. Although the process of replay is extremely rapid, there is evidence that learning creates a homeostatic need for sleep and that post-training slow-wave sleep episodes improve memory performance compared with an equivalent post-training period spent in the waking state23-25.
Evidence from several studies demonstrates that circadian rhythm affects memory consolidation. Circadian phase-shifting following training interferes with the retention of the hippocampus-dependent Morris water maze task26 and also causes retrograde amnesia for hippocampus-dependent passive avoidance memory27. Furthermore, lesions of the suprachiasmatic nucleus (SCN) decrease hippocampus-dependent LTM in rodents28. Aplysia also undergo circadian oscillations in long-term sensitization29. There are disparate results regarding the effect of circadian rhythm on hippocampusand amygdala-dependent memory tasks, yet there is substantial evidence that memory consolidation in various species depends on circadian rhythm26,27,30-35.
We report that MAPK activity and cAMP underwent a circadian oscillation that is lost in a transgenic mouse strain lacking Ca2+-stimulated adenylyl cyclases. Furthermore, disruption of the oscillation in MAPK activity in the hippocampus by subjecting mice to constant light conditions or by direct pharmacological interference with oscillations in MAPK activity days after training interfered with memory persistence. These data suggest that circadian oscillation of MAPK activity, and not just the presence of activated MAPK in the hippocampus, may contribute to the persistence of contextual memory.
RESULTS
MAPK activity oscillates during the circadian cycle
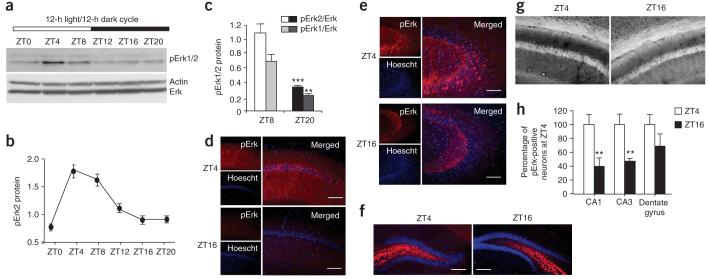
To determine whether mice entrained to a 12-h light/12-h dark (L/D) cycle have oscillations in hippocampal MAPK activity, we maintained mice under L/D conditions for 10 d and then analyzed them for phosphorylated MAPK isoforms (pErk1 and pErk2). Hippocampal tissue was taken from mice every 4 h starting at light onset (zeitgeber time = 0, ZT0). The mice were killed at ZT16 under red light to avoid robust, light-induced activation of MAPK in the SCN36. Western blot analysis showed a pronounced oscillation in MAPK phosphorylation over the 24-h period (Fig. 1a,b). To determine whether activated Erk oscillates relative to total Erk protein, we quantified pErk1/2 levels relative to Erk2 protein. Total Erk2 protein was invariant. Phosphorylation of Erk1 and Erk2 was significantly higher at ZT8 compared with ZT20 (P < 0.001; Fig. 1c). Because normalized pErk1/2 levels were not substantially different between ZT4 and ZT8, both time points were used for subsequent experiments. For the same reason, both ZT16 and ZT20 were used in L/D and free-running experiments as dark-period time points. We did not observe any circadian oscillations in Erk activity in cerebellar tissue (Supplementary Fig. 1 online), indicating either that there was no pErk oscillation in cerebellar neurons or that there was no synchronized activity in this region.
Figure 1.
MAPK activity in the hippocampus shows circadian oscillations. Mice maintained on a 12-h light/12-h dark cycle for at least 10 d before experimentation were killed every 4 h. During the dark cycle, mice were killed under red light. (a) Pooled hippocampal extracts were evaluated by western blot analysis for pErk1/2 expression. (b) pErk2, normalized to actin, expression in the hippocampus during the 24-h cycle is shown. Error bars represent s.e.m. (n = 3 mice per time point). (c) pErk1 and pErk2, normalized to total Erk protein, at ZT8 and ZT20. *** P < 0.001, ** P < 0.01, t test. Error bars represent s.e.m. (n = 5 mice per time point). (d-h) MAPK activity underwent circadian oscillations in hippocampal areas CA1 and CA3. pErk expression is shown in area CA1 neurons (20×, d), CA3 neurons (20×, e) and granule cells of the dentate gyrus (10×, f). Scale bars represent 125 µm in d,e and 250 µm in f. 3,3′-diaminobenzidine staining showed an increase in pErk-positive cells in area CA1 at ZT4 compared with ZT16 (g). Quantification of pErk-positive cell bodies expressed as percent of pErk-positive cells at ZT4 in CA1, CA3 and the dentate gyrus (h). Error bars represent s.e.m. (n = 6-8 mice per time point).
Immunohistochemistry and immunofluorescence staining of hippocampal slices was carried out on mice at ZT4 and ZT16 to determine which regions of the hippocampus show circadian variations in MAPK activity. We observed prominent 24-h variations in pErk staining in the pyramidal layer of areas CA1 and CA3 (Fig. 1d,e) but not in the granule cells of the dentate gyrus (Fig. 1f). Similar patterns in staining were observed using two immunohistochemical staining techniques (Fig. 1g and Supplementary Methods online). Quantification of cell bodies positive for pErk revealed differences between ZT4 and ZT16 in areas CA1 and CA3, but not in the dentate gyrus (Fig. 1h).
Because circadian variations in MAPK phosphorylation were observed in the hippocampus, we analyzed hippocampal tissue for activation of the upstream kinase of MAPK, MAPK kinase (pMEK1/2), as well as CREB phosphorylation at Ser133. Phosphorylation of CREB at Ser133 is required for MAPK stimulation of CRE-mediated transcription. Hippocampal lobes taken from mice every 4 h throughout the circadian cycle were analyzed for MEK1/2 activity using a phosphoserine 217/221 specific antibody. MEK1/2 activity also showed significant 24-h variations (Fig. 2a) when normalized to both actin (pMEK1/2 actin ZT8, 0.68 ± 0.05; ZT20, 0.17 ± 0.01; P < 0.01, ANOVA) and MEK1 protein (P = 0.016; Fig. 2b). CREB activation, monitored by changes in phosphorylation at Ser133, was also significantly higher during the day than during the night (Supplementary Fig. 2 online).
Figure 2.
Oscillations in the MAPK pathway occur upstream of MAPK and show oscillations that are free running. (a) pMEK1/2 protein expression in hippocampal tissue during the circadian cycle. (b) Quantification of pMEK1/2 at ZT8 and ZT20, normalized to MEK1 protein expression. * P = 0.016, Mann Whitney. Error bars represent s.e.m. (n = 5 mice per time point). (c) Actograms of voluntary mouse movement during D/D (free-running) conditions show a circadian oscillation of approximately 23.3 h. (d) pErk1/2 expression in the hippocampus oscillates even when mice are in free-running conditions. (e) pErk2 expression, normalized to total Erk protein, in the hippocampus during D/D conditions. * P < 0.05, Mann Whitney. Error bars represent s.e.m. (n = 4-5 mice per time point).
To verify that the L/D oscillations in MAPK activity are truly circadian, we tested whether or not they persisted under free-running conditions. Mice were entrained to an L/D cycle for 10 d and then placed in complete darkness (D/D) for 5 d before being analyzed for oscillations in hippocampal MAPK activity. Actograms generated from monitoring voluntary activity of mice in D/D indicate that C57/BL6 mice have a circadian oscillation of slightly less than 24 h. As expected, locomotion drifted slightly under free-running conditions; however, rhythms were maintained (Fig. 2c). On the sixth subjective day in complete darkness, we removed hippocampal tissue at circadian times (CT) 0, 4, 8, 12, 16 and 20 and analyzed it for pErk by western blot (Fig. 2d). SCN lobes were taken from the same mice and pooled for analysis (data not shown). As previously reported36, MAPK activity in the SCN showed circadian variations under free-running conditions (SCN pErk2/Erk CT4, 3.83; CT16, 1.47). Oscillations in MAPK activity were also maintained in the hippocampus under D/D conditions (Fig. 2e).
Training and testing at ZT4 results in better memory
Because the MAPK pathway is required for the consolidation of hippocampus-dependent fear memory, we asked whether there is a correlation between the oscillations of MAPK activity in the hippocampus and memory formation. We analyzed contextual fear memory, as this task has a well-established dependence on MAPK activation4,6,37,38. Regardless of zeitgeber time, all contextual fear training and testing sessions were carried out under dim red light (1-2 lx), (see Supplementary Methods). The purpose of training under dim light was to avoid variations in the lighting during contextual training. Studies using open-field locomotion in a novel environment show that locomotion it is not dependent on the endogenous circadian rhythmicity of the mouse, although it varies according to illumination conditions39. Although light at 1-2 lx spares the SCN of robust MAPK phosphorylation36, it is sufficient for the maintenance of vision. Mice placed in a water maze were able to efficiently navigate to a platform under these dim-light conditions (data not shown).
Mice were entrained to an L/D cycle for 10 d and then trained at ZT4 or ZT16 for contextual fear. Following training, mice were tested at one of two diurnal times (ZT4 or ZT16) 24, 36 or 48 h after training (Fig. 3a). Scoring for contextual fear was carried out both manually and electronically. An investigator that was unaware of the training time manually scored freezing behavior (Fig. 3b). Mice tested 24 and 48 h following dark-period training showed similar deficits to the mice tested 36 h after dark training, indicating that enhanced memory in the ZT4-trained and ZT4-tested group was not merely the result of a ‘time-stamping’ effect. Furthermore, mice that were trained at ZT4 and tested 48 h later were unimpaired in contextual fear, indicating that memory decay between 24 and 36 h was not the cause of the deficits that we observed in the ZT4/ZT16 and ZT16/ZT4 trained and tested groups. Because deficits in contextual memory occurred during periods of low hippocampal pErk expression, we also tested the performance of mice that were trained and tested at an alternative zeitgeber time when Erk activation is low. Mice trained at ZT0 (starting at ZT23.5) also had reduced levels of freezing compared with mice trained at ZT4, underscoring the relationship between high levels of hippocampal pErk expression and efficient consolidation of hippocampus-dependent memory (Fig. 3c).
Figure 3.

Contextual fear memory formation is dependent on zeitgeber time. (a) Mice were trained for contextual fear conditioning at either ZT4 or ZT16 and tested 24, 36 or 48 h later. Six mouse groups were used: those trained at ZT4 and tested 24 h later at ZT4 (4:4 (24)), those trained at ZT16 and tested 24 h later at ZT16 (16:16 (24)), those trained at ZT4 and tested 36 h later at ZT16 (4:16 (36)), those trained at ZT16 and tested 36 h later at ZT4 (16:4 (36)), and those groups trained at ZT4 or ZT16 and tested 48 h later (4:4 (48); 16:16 (48)). (b) Mice were tested for freezing behavior. *** P < 0.001, ** P < 0.01, ANOVA, Bonferroni's multiple comparison test for post hoc analysis. Error bars represent s.e.m. (n = 10-18 mice per group). (c) Freezing percentage of mice trained at ZT4 or ZT0 and tested 24 h after training. * P < 0.015, Mann Whitney. Error bars represent s.e.m. (n = 6 mice per time point).
To test for differences in the baseline movement patterns of mice trained at ZT4 versus mice trained at ZT16, we measured movement patterns of the mice before shock delivery (Supplementary Fig. 3 online). Day- and night-trained mice showed no differences in movement. Although nocturnal mice are generally less active during the day, this was obviously overcome by the novelty of the chamber.
Deficits in contextual fear are maintained in D/D conditions
As oscillations in phosphorylated MAPK persisted during free-running conditions, we suspected that deficits in contextual memory might also occur under free-running conditions when mice were trained during the subjective night. Indeed, we observed severe deficits when mice were trained and tested during the subjective night compared with those trained and tested during the subjective day. The movement times of CT16-trained mice during training and testing sessions were similar (Fig. 4a,b), indicating that this group of mice did not attain robust context-dependent learning. The manual scoring of freezing percentage during testing was reflective of the computer scoring; mice that were trained at CT4 had a significant increase in testing freezing percentage over those trained at CT16 (P = 0.018; Fig. 4c). As in L/D conditions, baseline movements of the mice in the novel context during free-running conditions were equivalent at the two circadian times (data not shown).
Figure 4.
Contextual fear memory is impaired when mice are trained during the subjective night. Mice were maintained in constant darkness for 6 d before being trained under red light for contextual fear conditioning. Mice were trained at CT4 or CT16 and tested 24 h later at CT4 or CT16, respectively. (a) The movement time (MT) during testing is plotted for each mouse (open circles indicate training movement time and filled circles represent testing movement time). (b,c) Mice trained at CT4 moved less (b) and had a higher freezing percentage (c) during testing compared with the mice trained at CT16. * P < 0.05, Mann Whitney. Error bars represent s.e.m. (n = 10 mice per group). (d) Freezing percentage of mice trained during the light period and tested 1 h later for short-term memory (ZT8). *** P ≤ 0.0001, Mann Whitney. Error bars represent s.e.m. (n = 10). (e) Freezing percentage of mice trained during the dark cycle and tested 1 h later (ZT20). ** P < 0.01, Mann Whitney. Error bars represent s.e.m. (n = 5).
To decipher whether the behavioral deficits were attributable to insufficient memory acquisition or impaired consolidation, we tested whether mice could acquire and express contextual fear behavior during both the day and night. L/D-entrained mice were trained for contextual fear at ZT8 or ZT20 under 1-2 lx red light and tested 1 h after training for short-term contextual memory. Mice trained during the day showed strong contextual memory (Fig. 4d). Mice trained during the dark period were also able to acquire contextual memory (Fig. 4e). Both groups of mice had an increase in freezing percentage compared with the training session that was reflected as a decrease in movement time (movement time: ZT8 training, 54.24 ± 1.31; ZT8 testing, 40.15 ± 2.53; P < 0.001, Mann Whitney, error bars represent s.e.m; ZT20 training, 54.39 ± 1.37; ZT20 testing, 32.87 ± 3.64; P = 0.016, Mann Whitney, error bars represent s.e.m.). This indicates that mice can acquire and retrieve short-term contextual memories regardless of zeitgeber time.
Hippocampal cAMP is elevated during the day
Because of the relationship between cAMP and MAPK signaling in neurons following training for contextual memory16, we measured the amount of cAMP in the hippocampus of wild-type mice throughout the L/D cycle. Hippocampal cAMP was higher during the day than at night (cAMP in pmol per mg of protein; ZT0, 28.56 ± 1.82; ZT4, 34.12 ± 3.44; ZT8, 34.64 ± 9.32; ZT12, 24.20 ± 1.96; ZT16, 20.52 ± 1.83; ZT20, 18.46 ± 1.00; Fig. 5a). The decrease in cAMP at ZT20, compared with ZT8, was averaged from independent experiments using different sets of mice (Fig. 5b).
Figure 5.
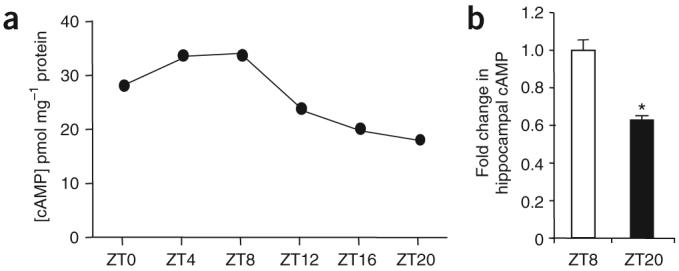
cAMP in the hippocampus is higher during the day than at night. (a) Hippocampal cAMP from tissues excised at ZT0, 4, 8, 12, 16 and 20. Each data point is the average of quadruplicate determinations derived from three pooled hippocampi obtained from separate mice. Error bars represent s.e.m. (b) The relative decrease in cAMP at ZT20 compared with ZT8 from independent experiments. * P < 0.05, t test. Error bars represent s.e.m. (n = 3-4 mice per time point per experiment).
Absolute values of cAMP levels in wild-type mice and mice lacking Adcy1 and Adcy8 (AC1 and AC8, respectively; DKO mice) were lower, on average, in DKO mice at ZT8 compared with wild-type mice (cAMP in pmol per mg of protein; wild type ZT8. 40.27 ± 5.625; DKO ZT8. 28.38 ± 5.33). This decrease is probably the result of the absence of the calcium-sensitive adenylyl cyclases, although several other adenylyl cyclases are expressed in the hippocampus. cAMP levels at ZT20 were similar between wild-type and DKO mice (cAMP in pmol per mg of protein; wild type ZT20, 24.18 ± 5.72; DKO ZT20, 22.89 ± 5.46).
AC1 and AC8 are required for oscillations in MAPK activity
Calmodulin-stimulated adenylyl cyclase activity is required for LTM, but for not short-term memory15. To determine whether the circadian oscillations in cAMP and MAPK activity depend on AC1 and AC8, we analyzed DKO mice for MAPK and cAMP activity in the hippocampus at ZT8 and ZT20 (Fig. 6a). DKO mice showed normal circadian rhythm under both L/D and D/D conditions and readily entrained to changes in the lighting cycle (Supplementary Fig. 4 online). MAPK activity in the hippocampus of DKO mice did not change during the circadian cycle (Fig. 6a,b). In fact, the basal levels were elevated somewhat compared with wild-type mice. There was also no variation in cAMP in the hippocampus of these mice (Fig. 6c). These data indicate that the oscillations in MAPK and cAMP in the hippocampus depend on Ca2+-stimulated adenylyl cyclase activity.
Figure 6.

DKO mice do not have diurnal oscillations in pErk activity or cAMP in the hippocampus and are deficient in long-term contextual memory. (a) pErk1/2, actin and total Erk proteins in pooled wild-type (WT) and DKO hippocampal tissue taken at ZT8 or ZT20. (b) Quantification of pErk relative to Erk expression in DKO mice. P = 0.6600, t test. Error bars represent s.e.m. (n = 10 for each genotype, 5 per time point). (c) Quantification of the fold change in cAMP accumulation in DKO hippocampus at ZT8 and ZT20. P = 0.13, t test. Error bars represent s.e.m. (error derived from four experiments, three hippocampal lobes pooled per time point per experiment). N.S., not significant. (d) Freezing percentage of DKO mice trained at ZT4 or ZT16 and tested 24 h later compared with wild-type mice trained and tested at ZT4. ** P < 0.01, ANOVA, Bonferroni's multiple comparison test for post hoc analysis. Error bars represent s.e.m. (n = 6-8 mice per group).
Because previously published memory deficits in DKO mice were determined using different training conditions, we tested whether DKO mice had contextual memory deficits when trained at different zeitgeber times under dim red light. DKO mice had severe deficits in contextual fear memory when tested 24 h after training at both ZT4 and ZT16 (Fig. 6d).
Adenylyl cyclase and Ras activities rise during the day
To determine whether changes in adenylyl cyclase activity contribute to oscillations in cAMP, we measured Ca2+-stimulated adenylyl cyclase activity in the hippocampus of wild-type mice at ZT8 and ZT20 (Supplementary Methods). Indeed, Ca2+-stimulated adenylyl cyclase activity was higher at ZT8 compared with ZT20 (Fig. 7a). Ca2+-stimulated adenylyl cyclase activity at ZT20 averaged 86.27 ± 3.51% of that at ZT8 over several independent experiments (P = 0.017, t test; Fig. 7b). This data supports the hypothesis that the circadian oscillation of cAMP in the hippocampus is the result of Ca2+-stimulated adenylyl cyclase activity. That oscillations may depend on circadian variations in adenylyl cyclase activity is consistent with the observation that cAMP oscillations in the hippocampus are lost in DKO mice.
Figure 7.
Ca2+-stimulated adenylyl cyclase activity and Ras activity in the hippocampus peak during the day. (a) Ca2+-stimulated adenylyl cyclase activity in the hippocampus of wild-type mice was determined at ZT8 and ZT20. (b) Ca2+-stimulated adenylyl cyclase activity at ZT20 expressed as a percentage of the activity at ZT8. Data are pooled from independent experiments. * P < 0.05; t test. Error bars represent s.d. in a and s.e.m. in b. (c) Western analysis of Ras activity showing pan (H, N and K) GTP-bound Ras protein in the hippocampus at ZT0, ZT4, ZT8, ZT12, ZT16 and ZT20 and actin protein in supernatant fractions from the corresponding pull-downs. (d,e) Quantification of GTP-Ras expression (d), normalized to actin protein, and the average decrease in Ras activity at ZT20 compared with ZT8 (e) from independent experiments. ** P < 0.01, t test. Error bars represent s.e.m.
As cAMP can stimulate MAPK through Ras, we measured Ras activity in the hippocampus of wild-type mice every 4 h during the L/D zeitgeber cycle. Using the Raf-1 Ras-binding domain (RBD), we isolated active GTP-bound Ras from hippocampal preparations (Fig. 7c). RBD-bound Ras protein was analyzed by western blot using a pan-Ras antibody that detects N-, K- and H-Ras proteins. Ras activity was greater during the day than at night (Fig. 7d,e). Because Ras showed a circadian variation coincident with cAMP and MAPK activity, we conclude that the oscillation of MAPK activity may be driven by cAMP stimulation of Ras activity.
Disruption of pErk oscillations impairs memory persistence
As exposure to constant light impairs circadian rhythms, we wondered whether L/L conditions would be sufficient to uncouple oscillations in hippocampal MAPK phosphorylation. The advantage of this approach over using an SCN lesion is that it maintains neuronal connectivity while still disturbing rhythms in both locomotion and gene expression in the SCN40,41. We observed that exposure of mice to L/L conditions (approximately 300 lx) for several days resulted in severe disruptions in locomotion rhythms. The τ of each mouse monitored for locomotion increased by an average of 1.11 h d−1, resulting in a substantial shift of locomotion rhythms while in L/L conditions, following Aschoff's rule42. We found that exposure to constant light was sufficient to disrupt pErk oscillations in the hippocampus, underscoring the notion that hippocampal pErk oscillations are under tight circadian control (Supplementary Fig. 5 online). We used L/L conditions to test whether disruptions in circadian oscillations interfered with hippocampus-dependent memory persistence. Mice were exposed to L/L or maintained in L/D for 10 d following training for context, but were then allowed to re-entrain to the L/D cycle. Exposure to L/L conditions after training for contextual fear resulted in a decrease in contextual memory persistence (Supplementary Fig. 6 online).
We also tested whether pharmacological interference of MAPK oscillations affected memory persistence. Mice trained for contextual fear were bilaterally infused with vehicle or the MEK1/2 inhibitor UO126 at ZT0 and ZT4, starting 44 h post-training and persisting for an entire week. The week of infusions was followed by a 1-week recovery period (Fig. 8a). Hippocampal tissue surrounding the cannulae sites of UO126-infused mice showed a decrease in hippocampal pErk at ZT8 following two injections with UO126 compared with vehicle-infused hippocampi (Fig. 8b,c). UO126-infused mice tested 1 week after infusions had deficits in contextual fear compared with vehicle-infused mice (Fig. 8d). The freezing levels in both groups showed higher deviation from the mean compared with contextual fear experiments in which few or no injections were given, perhaps as a result of stress that repeated infusions might cause the mice during this circadian time. To address this deviation, mice were divided into two groups on the basis of freezing percentage. Mice that showed ≥25% freezing (a percentage exceeding that permitted for training levels) were considered to be moderate-to-strong learners. When compared with UO126-infused mice, vehicle-infused mice had a significantly higher percentage of moderate-to-strong learners (vehicle versus UO125: 53.8% to 6.3% and 7 out of 13 versus 1 out of 16, respectively; P = 0.0097, Fisher's exact test; Fig. 8e). We considered the possibility that infusions of UO126 during the day might, in effect, cause a lesion of the hippocampus, and thereby render the ability to retain contextual fear impossible. To control for normal hippocampal function, we trained mice in the hippocampus-dependent passive avoidance task and tested them 24 h later. Vehicle- and UO126-treated mice were able to learn the task and had comparable training and testing step-through latencies when given the opportunity to traverse to the dark side (Supplementary Fig. 7 online).
Figure 8.
Infusion of MEK inhibitors into the hippocampus during the circadian peak, but not during the trough of MAPK activation impairs LTM. (a) Mice trained for contextual fear were infused with vehicle (Veh) or UO126 (UO) at ZT0 and ZT4, starting 2 d after training and lasting for an entire week thereafter. (b,c) UO126 administration during the day reduced pErk in the hippocampal areas infused compared with vehicle-infused controls. * P = 0.0159, Mann Whitney. Error bars represent s.e.m. (n = 5). (d) UO mice (n = 16) had reduced freezing during testing compared with the Veh group (n = 13). * P < 0.05, t test. Error bars represent s.e.m. (e) The percentage of weak and nonlearners between Veh and UO groups was compared by Fisher's exact test (P < 0.01). (f) Mice trained for contextual fear conditioning were infused with vehicle or UO126 at ZT12 and ZT16 starting 36 h after training and lasting for an entire week thereafter. (g) UO mice (n = 8) had normal freezing during testing compared to the Veh group (n = 9). P = 0.669, t test. Error bars represent s.e.m.
Although daytime infusions test the dependence of memory persistence on peak levels of pErk in the hippocampus, we tested whether UO126 affected memory persistence when administered at the trough of MAPK activity in the hippocampus (Fig. 8f). Mice were trained for contextual fear and then infused with vehicle or UO126 at ZT12 and ZT16 for seven consecutive nights, starting 36 h after training. Following infusions, mice were given a 1-week recovery period and then tested for contextual fear at ZT4. Memory was not impaired in mice exposed to UO126 at ZT12 and ZT16 compared to vehicle-infused mice (Fig. 8g). Notably, the freezing percentages of night-infused mice were considerably higher across all groups (compare vehicle in Fig. 8d,g). Whether diurnal stress hormone release, interruption in sleep or some other physiological activity induced this circadian effect of infusions remains unclear. Hippocampal tissue surrounding the cannulae sites of mice infused with UO126 at ZT12 did not show a further reduction in pErk protein 1 h after infusion compared with the baseline pErk protein already present at the circadian nadir in vehicle-infused control mice (Supplementary Fig. 8 online).
Finally, we tested whether increases in cAMP signaling at night, the typical nadir in MAPK activity, interfered with memory persistence. Mice infused 36 h after training with forskolin at both ZT12 and ZT16 for seven consecutive nights had impaired memory for contextual fear (Supplementary Fig. 9 online), suggesting that it is the oscillation of the cAMP/MAPK pathway that is required for normal persistence and not merely the presence of diurnal MAPK activity.
DISCUSSION
Hippocampal MAPK signaling shows circadian oscillations
Recently, there has been renewed interest in circadian influences on synaptic plasticity. The objective of our study was to determine whether cAMP or MAPK activity in the hippocampus undergoes circadian oscillations. We discovered that MAPK activity peaks during the day, which is usually the inactive period for these mice. Furthermore, oscillations in MAPK activity are circadian and persist under free-running conditions. Our data also show that MEK activity, as well as CREB phosphorylation, have similar circadian oscillations.
Hippocampal oscillations in MAPK activity are coincident with oscillations in cAMP and Ras activity in the hippocampus, suggesting that changes in cAMP may regulate MAPK activity during the circadian cycle. Although cAMP can impinge on MAPK phosphorylation through Ras or Rap signaling, the coincident Ras activation suggests that cAMP activation of MAPK may be mediated through a Ras guanine nucleotide exchange factor (Epac)-mediated Ras activation43. Notably, oscillations in MAPK activity and cAMP are lost in mice that lack calmodulin-stimulated adenylyl cyclase activity, suggesting that cAMP signals generated by these enzymes may regulate the circadian oscillation of MAPK activity.
The nadir in MAPK phosphorylation during the circadian cycle coincides with severe deficits in contextual fear memory processing. Short-term memory testing indicated that mice were able to acquire and express contextual fear conditioning at both peak and trough periods of hippocampal MAPK activity, suggesting that the consolidation process is deficient in mice trained during the night or subjective night. Unlike LTM formation, short-term memory formation does not depend on MAPK activity. Consequently, we hypothesize that the low levels of MAPK activity in the hippocampus during the dark period may impair memory consolidation and stabilization.
Ablation of MAPK rhythms impairs memory persistence
Our data demonstrate that ablation of hippocampal pErk oscillations by deletion of the calcium-sensitive adenylyl cyclases, exposure to constant light or pharmacological interference all result in deficits in contextual memory formation and persistence. DKO mice had deficits in context memory persistence at 8 d as well as deficits in passive avoidance training at 24 h15. Furthermore, although wild-type mice maintained in L/D conditions had strong contextual fear memory even at 2 weeks after fear training, mice with L/L-induced disruptions in hippocampal pErk expression showed impairments in memory persistence. Blocking diurnal phosphorylation of MAPK in vivo by UO126 demonstrated that a reduction in the peak of MAPK activity impairs memory persistence. Conversely, the activation of the MAPK pathway during the endogenous nadir by infusions of forskolin also impaired memory persistence, whereas UO126 had no effect on memory when administered at night. Notably, although MAPK phosphorylation was indeed present in the hippocampi of DKO and L/L-exposed wild-type mice, it appears to be completely uncoupled from circadian regulation. That this state interferes with memory persistence, as does direct pharmacological inhibition of MAPK activity at its peak or upregulation of MAPK phosphorylation at its trough, leads us to conclude that the oscillations, not merely the presence of phosphorylated MAPK, are required for normal memory maintenance.
It has yet to be determined whether the expression of clock genes contributes to the maintenance of pErk oscillations in the hippocampus. We found robust levels of both Per1 and Clock expression in areas CA1, CA3 and the dentate gyrus of the hippocampus (data not shown), but how these clock genes may relate to hippocampal synaptic plasticity or memory persistence is still unknown.
Physiological implications of MAPK pathway oscillations
Oscillations of MAPK activity in the hippocampus may affect a number of physiological processes, as MAPK regulates transcription, translation, ion channel activity and neuronal survival (for reviews, see refs. 2,44,45). Because of its central role in memory consolidation, we hypothesize that oscillations of MAPK in the hippocampus may contribute to the persistence of hippocampus-dependent memories. For example, repeated rounds of MAPK activation may stimulate multiple cycles of transcription and translation necessary for memory stabilization and persistence. Perhaps renewed synaptic strength, mediated by repeated rounds of circadian-controlled MAPK activation, is one method by which ‘tagged synapses’ maintain a potentiated state. In freely behaving rodents, NMDA receptor turnover is approximately 5 d46. That de novo protein synthesis is required for memory maintenance is supported by the fact that temporary inhibition of the NMDA receptor subunit NR1 expression, even months after training, causes disruption of remote memory maintenance20.
Although our data demonstrate that these oscillations are circadian in nature, we have not controlled for the influence of circadian activities such as sleep. Notably, many studies suggest that post-training memory processing during sleep or quiet wake is important for the consolidation and integrity of hippocampal long-term memories22,25,47-50; however, the molecular mechanisms for this apparent stabilization are still unclear.
In conclusion, the cAMP/MAPK/CREB signaling pathway undergoes circadian oscillations in the hippocampus that correlate with the ability of mice to consolidate and maintain contextual memory. This is consistent with the hypothesis that reactivation of this signaling pathway may be important for the persistence of contextual memories.
METHODS
Mice
Adult, male C57BL/6 mice were used for most experiments (see Supplementary Methods for additional information). Experiments were carried out in accordance with the Institutional Animal Care and Use Committee's recommendations at the University of Washington.
Circadian time courses
Mice were housed in a 12-h light/12-h dark cycle for at least 10 d before time courses. For oscillation experiments, mice were killed every 4 h during one 24-h period. In dark lighting conditions mice were killed under 1-2 lx, provided by a Kodak GBX-2 red light with a safelight filter. Lux was measured with a light meter (VWR).
Western blot analysis
Isolated tissue was flash frozen in liquid nitrogen and then homogenized twice in homogenization buffer (10 mM Tris base, pH 7.5, 100 mM NaCl, 1 mM EDTA, 10 mM NaF, 1mM Na3VO4, 1mM PMSF, and a protease inhibitor tablet containing a mixture of pancreas extract and inhibitors of papain, trypsin, chymotrypsin and thermolysin (Roche)) for 10 s with a 60-s interval on ice. An equal volume of boiling 6× SDS load dye (300 mM Tris HCl, pH 6.8, 6% β-mercaptoethanol, 12% SDS, 0.6% bromophenol blue (wt/vol), 60% (vol/vol) glycerol, and 12 mM EDTA) was added to homogenates and the solutions were boiled for 5 min. Samples were loaded onto a 12.5% Tris-HCl (vol/vol) polyacrylamide gel (13.3 cm × 8.7 cm × 1.0 mm thick, BioRad). Proteins were transferred to a PVDF membrane (Millipore) and the membrane was blocked with 5% milk (wt/vol) in phosphate-buffered saline (PBS) with 0.05% Tween-20 (vol/vol). For western blot analysis, we used rabbit polyclonal antibody to phospho-p44/42 MAPK (Thr202/Tyr204, 1:1,000, Cell Signaling), mouse monoclonal antibody to phospho-CREB (Ser133, 1:500, Upstate), rabbit polyclonal antibody to phospho-MEK1/2 (Ser217/221, 1:1,000, Cell Signaling), rabbit polyclonal antibody to MEK1 (1:1,000, Upstate), mouse monoclonal antibody to pan-Erk (MAPK, 1:2,000, BD Transduction Laboratories) and mouse antibody to actin (1:5,000, Chemicon International). Blots were probed with one of the following antibodies (1:10,000 dilution): alkaline phosphatase-conjugated goat antibody to mouse IgG (Sigma), alkaline phosphate-conjugated goat antibody to rabbit IgG (Sigma), horseradish peroxidase-conjugated goat antibody to rabbit (MP Biomedicals), horseradish peroxidase-conjugated sheep antibody to mouse (MP Biomedicals). Immunoreactivity was developed with either CDP-Star western alkaline phosphatase detection system (Tropix) or ECL detection reagent (Amersham Biosciences). The western blots shown are representative experiments; pErk1/2 oscillations, as well as peak and trough time points, were analyzed in a minimum of three independent experiments. When necessary, blots were stripped with 25 mM Tris, pH 8, 250 mM NaCl, 1% SDS, and 100 mM β-mercaptoethanol at 60 °C for 30 min (see Supplementary Methods for western blot quantification).
Immunofluorescence staining
Immunofluorescence staining of pErk was carried out as described previously16, with a few exceptions. Mice were killed under dim red light, and their brains were removed and sliced quickly by vibratome in chilled PBS containing the phosphatase inhibitors NaF (50 mM) and activated Na3VO4 (1 mM, pH 10). We fixed 800-µm sections by immersion in 4% PFA (wt/vol) for 7 h and then cryoprotected them in 30% sucrose (wt/vol) in PBS overnight at 4 °C in the presence of inhibitors. Brain sections were sliced into 30-µm sections by cryostat or a sliding, freezing microtome. Fluorescent images of pErk-positive cells in area CA1 were obtained on an Olympus FV-1000 confocal microscope using a 20× objective and sequential line scanning. The channels that we used were adjusted to avoid saturation and the exposure conditions were kept constant during image capture. Random single-plane images were captured from each region by an investigator that was blind to the condition to focus the largest number of cell bodies in the randomly selected objective field. Thresholded images were quantified in ImageJ (US National Institutes of Health) to quantify pErk-positive cells. Cell body counts were made from 6-8 mice per condition. The numbers of pErk-positive cells in each region were averaged between 2-8 separate sections per mouse and from regions that fell within −1.46 and −2.30 to bregma. Cell body counts from mice killed at ZT16 are expressed as a percentage of those killed at ZT4.
Actogram acquisition
Mice were individually housed in rat cages (19 × 10 × 8 inches) for circadian voluntary activity data acquisition. This was carried out with the use of QA-4 activity input modules coupled to infrared motion detectors. Data were acquired using VitalView Data Acquisition System (Mini Mitter, version 4.1) and were transferred to the ActiView Biological Rhythm Analysis program (Mini Mitter, version 1.2) for actogram generation. The actograms that are shown are representative locomotion plots for a group of mice in the corresponding L/D, D/D or L/L conditions.
Contextual fear conditioning during L/D and free-running (D/D and L/L) conditions
For experiments in free-running conditions, L/D-entrained mice were placed into D/D conditions for 5 d. Before training, mice were allowed 2 min to adjust to dim red light conditions, regardless of zeitgeber time. Following training for contextual fear training (Supplementary Methods), mice were returned to their home cages and placed in the lighting conditions from which they came (L/D or D/D). For L/L circadian experiments, training and testing for both L/D- and L/L-shifted mice were carried out under standard white fluorescent light. Mice were switched to L/L conditions (≈300 lx) or else maintained in L/D cycles 24 h following context training. After 7-10 d in L/L, mice were returned to L/D conditions for 1 week to re-entrain to the L/D cycle (Supplementary Figs. 5 and 6).
Measurement of cAMP
Following decapitation, hippocampal lobes were removed from mice and flash frozen in liquid nitrogen. We used the ELISA-based cAMP Biotrak Enzymeimmunoassay System protocol (Amersham Biosciences) with some minor deviations. Hippocampal tissue (3-4 lobes per time point per experiment and from different mice) was homogenized in 500 µl of ice cold 0.1 M TrisHCl buffer containing 1 mM EDTA and 1 mM of the PDE inhibitor IBMX. Ice-cold ethanol (99%/1% 1N HCl, vol/vol) was added to the cell suspension to give a final mixture of 65% ethanol (vol/vol). Homogenates were spun for 2 min at a speed of 1,000 g at 4 °C. Resulting supernatants were evaporated in a heat block at 70 °C and precipitates were resuspended in 500 µl of assay buffer. Competition binding was carried out according to the Enzymeimmunoassay system instructions. Extracts were diluted 1:100 to achieve concentrations in the linear range of the assay. cAMP levels were normalized to starting protein concentrations from each time point.
Ras activity assay
We homogenized hippocampal tissues excised from mice at ZT0, ZT4, ZT8, ZT12, ZT16 and ZT20 in magnesium lysis buffer (25 mM HEPES, pH 7.5, 150 mM NaCl, 1% Igepal CA-630, 10% glycerol, 25 mM NaF, 10 mM MgCl2, 1 mM EDTA, 1 mM activated Na3VO4, pH 10, and one half of a protease inhibitor tablet per 10 ml buffer (Roche)). Hippocampal lysates (60 µg) from each time point were incubated with 50 µl of Raf-1 RBD protein fused to agarose beads (Upstate Signaling Solutions) at 4 °C for 1 h. Beads were pelleted by pulsation and the resulting supernatant was saved for western analysis. Beads were washed three times with 500 µl of magnesium lysis buffer and the final bead pellet was boiled with an equal volume of 6× SDS buffer for 5 min. After a brief pulsation, samples were analyzed by western blot using a monoclonal antibody to Ras (Upstate) at a dilution of 1:1,000. Amounts of GTP-bound Ras were normalized to actin protein present in control lysates lacking RBD-bead complexes.
Drug infusions
UO126 was prepared as described previously7 with minor deviations. Forskolin (Calbiochem) and UO126 were dissolved in 35% DMSO (vol/vol), 65% saline (vol/vol) to concentrations of 0.5 mM and 5 mM, respectively. Mice were bilaterally implanted with cannulae (Plastics One) using the coordinates (from bregma) −1.65 mm anterior/posterior, 1.5 mm medial/lateral and −1.5 mm dorsal/ventral. Mice were infused via polyethylene tubing with an automated syringe pump (World Precision Instruments) at a rate of 0.25 µl min−1.
Statistical analysis
Unless otherwise stated, reported values are averages for the group ± s.e.m. In general, significance was analyzed between two groups by the use of two-tailed parametric Student's t tests or the nonparametric Mann Whitney t tests for small sample groups, significance was set at P < 0.05. Normality was tested using D'Agostino Pearson omnibus test. Multiple group experiments were analyzed by one-way or Kruskal-Wallis ANOVA analysis, using Dunn's or Bonferroni's multiple comparison test for post hoc analysis. Unless otherwise specified, n refers to the biological, not technical, replicate for a specific condition. For experiments using only pooled hippocampal tissues for analysis (cAMP ELISA measurements, membrane adenylyl cyclase assays and Ras activity assays) quantification and statistical analyses were carried out and are reported on data from several independent experiments. One animal's behavior was omitted as it reached ± 3 s.d. away from the mean for the UO126-infused group in Fig. 8d. Fisher's exact test analysis yielded significant results with or without this data point removed, as did a Wilcoxin test for paired movement time between training and testing, whereas a t test on freezing percentage did not.
ACKNOWLEDGMENTS
We thank H. de la Iglesia for valuable advice concerning some of the circadian procedures. We also would like to thank several members of the Storm lab for insightful discussions and critical readings of this manuscript. This work was supported by a grant from the US National Institutes of Health (NS 20498), a predoctoral Ruth L. Kirschstein US National Institutes of Health Research Award (1 F31 MH075489-01A1) to K.L.E.-M. and a Korea Research Foundation Grant for Young Scientists to S.H. (KRF-2005-213-C00036).
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
Supplementary Material
References
- 1.Impey S, Obrietan K, Storm DR. Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron. 1999;23:11–14. doi: 10.1016/s0896-6273(00)80747-3. [DOI] [PubMed] [Google Scholar]
- 2.Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr. Opin. Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Storm DR. Calmodulin-regulated adenylyl cyclases: cross-talk and plasticity in the central nervous system. Mol. Pharmacol. 2003;63:463–468. doi: 10.1124/mol.63.3.463. [DOI] [PubMed] [Google Scholar]
- 4.Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat. Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 5.Schafe GE, et al. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of Pavlovian fear conditioning. J. Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Athos J, Impey S, Pineda VV, Chen X, Storm DR. Hippocampal CRE-mediated gene expression is required for contextual memory formation. Nat. Neurosci. 2002;5:1119–1120. doi: 10.1038/nn951. [DOI] [PubMed] [Google Scholar]
- 7.Duvarci S, Nader K, LeDoux JE. Activation of extracellular signal-regulated kinase-mitogen-activated protein kinase cascade in the amygdala is required for memory reconsolidation of auditory fear conditioning. Eur. J. Neurosci. 2005;21:283–289. doi: 10.1111/j.1460-9568.2004.03824.x. [DOI] [PubMed] [Google Scholar]
- 8.Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J. Neurosci. 2003;23:5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu. Rev. Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson GD, Storm DR. Why calcium-stimulated adenylyl cyclases? Physiology (Bethesda) 2004;19:271–276. doi: 10.1152/physiol.00010.2004. [DOI] [PubMed] [Google Scholar]
- 11.Scott R, Bourtchuladze R, Gossweiler S, Dubnau J, Tully T. CREB and the discovery of cognitive enhancers. J. Mol. Neurosci. 2002;19:171–177. doi: 10.1007/s12031-002-0029-z. [DOI] [PubMed] [Google Scholar]
- 12.Kelleher RJ, III, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 13.Bernabeu R, et al. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc. Natl. Acad. Sci. USA. 1997;94:7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu ZL, et al. Altered behavior and long-term potentiation in type I adenylyl cyclase mutant mice. Proc. Natl. Acad. Sci. USA. 1995;92:220–224. doi: 10.1073/pnas.92.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong ST, et al. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- 16.Sindreu CB, Scheiner ZS, Storm DR. Ca2+-stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron. 2007;53:79–89. doi: 10.1016/j.neuron.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang YY, et al. A genetic test of the effects of mutations in PKA on mossy fiber LTP and its relation to spatial and contextual learning. Cell. 1995;83:1211–1222. doi: 10.1016/0092-8674(95)90146-9. [DOI] [PubMed] [Google Scholar]
- 18.Brandon EP, et al. Hippocampal long-term depression and depotentiation are defective in mice carrying a targeted disruption of the gene encoding the RI beta subunit of cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA. 1995;92:8851–8855. doi: 10.1073/pnas.92.19.8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abel T, et al. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 20.Cui Z, et al. Inducible and reversible NR1 knockout reveals crucial role of the NMDA receptor in preserving remote memories in the brain. Neuron. 2004;41:781–793. doi: 10.1016/s0896-6273(04)00072-8. [DOI] [PubMed] [Google Scholar]
- 21.Bekinschtein P, et al. Persistence of long-term memory storage requires a late protein synthesis- and BDNF-dependent phase in the hippocampus. Neuron. 2007;53:261–277. doi: 10.1016/j.neuron.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Lee AK, Wilson MA. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron. 2002;36:1183–1194. doi: 10.1016/s0896-6273(02)01096-6. [DOI] [PubMed] [Google Scholar]
- 23.Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn. Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gais S, Plihal W, Wagner U, Born J. Early sleep triggers memory for early visual discrimination skills. Nat. Neurosci. 2000;3:1335–1339. doi: 10.1038/81881. [DOI] [PubMed] [Google Scholar]
- 25.Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430:78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- 26.Devan BD, et al. Circadian phase-shifted rats show normal acquisition, but impaired long-term retention of place information in the water task. Neurobiol. Learn. Mem. 2001;75:51–62. doi: 10.1006/nlme.1999.3957. [DOI] [PubMed] [Google Scholar]
- 27.Tapp WN, Holloway FA. Phase shifting circadian rhythms produces retrograde amnesia. Science. 1981;211:1056–1058. doi: 10.1126/science.7193351. [DOI] [PubMed] [Google Scholar]
- 28.Stephan FK, Kovacevic NS. Multiple retention deficit in passive avoidance in rats is eliminated by suprachiasmatic lesions. Behav. Biol. 1978;22:456–462. doi: 10.1016/s0091-6773(78)92565-8. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez RI, Lyons LC, Levenson J, Khabour O, Eskin A. Circadian modulation of long-term sensitization in Aplysia. Proc. Natl. Acad. Sci. USA. 2003;100:14415–14420. doi: 10.1073/pnas.2336172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leirer VO, Tanke ED, Morrow DG. Time of day and naturalistic prospective memory. Exp. Aging Res. 1994;20:127–134. doi: 10.1080/03610739408253958. [DOI] [PubMed] [Google Scholar]
- 31.Maury P, Queinnec Y. Influence of time of 24-hour day on depth of processing in recall memory. Br. J. Psychol. 1992;83:249–260. doi: 10.1111/j.2044-8295.1992.tb02439.x. [DOI] [PubMed] [Google Scholar]
- 32.Folkard S, Wever RA, Wildgruber CM. Multi-oscillatory control of circadian rhythms in human performance. Nature. 1983;305:223–226. doi: 10.1038/305223a0. [DOI] [PubMed] [Google Scholar]
- 33.Chaudhury D, Colwell CS. Circadian modulation of learning and memory in fearconditioned mice. Behav. Brain Res. 2002;133:95–108. doi: 10.1016/s0166-4328(01)00471-5. [DOI] [PubMed] [Google Scholar]
- 34.Hauber W, Bareiss A. Facilitative effects of an adenosine A1/A2 receptor blockade on spatial memory performance of rats: selective enhancement of reference memory retention during the light period. Behav. Brain Res. 2001;118:43–52. doi: 10.1016/s0166-4328(00)00307-7. [DOI] [PubMed] [Google Scholar]
- 35.Holloway FA, Wansley RA. Multiple retention deficits at periodic intervals after active and passive avoidance learning. Behav. Biol. 1973;9:1–14. doi: 10.1016/s0091-6773(73)80164-6. [DOI] [PubMed] [Google Scholar]
- 36.Obrietan K, Impey S, Storm DR. Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nat. Neurosci. 1998;1:693–700. doi: 10.1038/3695. [DOI] [PubMed] [Google Scholar]
- 37.Selcher JC, Atkins CM, Trzaskos JM, Paylor R, Sweatt JD. A necessity for MAP kinase activation in mammalian spatial learning. Learn. Mem. 1999;6:478–490. doi: 10.1101/lm.6.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shalin SC, et al. Neuronal MEK is important for normal fear conditioning in mice. J. Neurosci. Res. 2004;75:760–770. doi: 10.1002/jnr.20052. [DOI] [PubMed] [Google Scholar]
- 39.Valentinuzzi VS, et al. Locomotor response to an open field during C57BL/6J active and inactive phases: differences dependent on conditions of illumination. Physiol. Behav. 2000;69:269–275. doi: 10.1016/s0031-9384(00)00219-5. [DOI] [PubMed] [Google Scholar]
- 40.Granados-Fuentes D, Prolo LM, Abraham U, Herzog ED. The suprachiasmatic nucleus entrains, but does not sustain, circadian rhythmicity in the olfactory bulb. J. Neurosci. 2004;24:615–619. doi: 10.1523/JNEUROSCI.4002-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat. Neurosci. 2005;8:267–269. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- 42.Aschoff J. Circadian rhythms: influences of internal and external factors on the period measured in constant conditions. Z. Tierpsychol. 1979;49:225–249. doi: 10.1111/j.1439-0310.1979.tb00290.x. [DOI] [PubMed] [Google Scholar]
- 43.Keiper M, et al. Epac- and Ca2+-controlled activation of Ras and extracellular signal-regulated kinases by Gs-coupled receptors. J. Biol. Chem. 2004;279:46497–46508. doi: 10.1074/jbc.M403604200. [DOI] [PubMed] [Google Scholar]
- 44.Sharma SK, Carew TJ. The roles of MAPK cascades in synaptic plasticity and memory in Aplysia: facilitatory effects and inhibitory constraints. Learn. Mem. 2004;11:373–378. doi: 10.1101/lm.81104. [DOI] [PubMed] [Google Scholar]
- 45.Hetman M, Gozdz A. Role of extracellular signal-regulated kinases 1 and 2 in neuronal survival. Eur. J. Biochem. 2004;271:2050–2055. doi: 10.1111/j.1432-1033.2004.04133.x. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu E, Tang YP, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290:1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- 47.Peigneux P, et al. Are spatial memories strengthened in the human hippocampus during slow wave sleep? Neuron. 2004;44:535–545. doi: 10.1016/j.neuron.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Mednick S, Nakayama K, Stickgold R. Sleep-dependent learning: a nap is as good as a night. Nat. Neurosci. 2003;6:697–698. doi: 10.1038/nn1078. [DOI] [PubMed] [Google Scholar]
- 49.Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- 50.Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–424. doi: 10.1177/1073858406292647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.