Preface
The natural killer (NK)-cell immunological synapse is the dynamic interface formed between an NK cell and its target cell. Formation of the NK-cell immunological synapse involves several distinct stages, beginning with the initiation of contact with a target cell and culminating in the directed delivery of lytic granule contents to lyse the target cell. Progression through the individual stages is methodical and underlies the precision with which NK cells select and kill susceptible target cells (including virally infected cells and cancerous cells) that they encounter during their routine surveillance of the body.
Introduction
Natural killer (NK) cells are lymphocytes of the innate immune system that provide effector functions after the ligation of germline-encoded receptors. In humans, NK cells are poised to deliver a response immediately after recognizing specific signals of stress, ‘danger’ or foreign origin. They were originally described as having rapid cell-mediated cytotoxicity. However, they are now also known to promote slower receptor-mediated apoptosis, provide contact-dependent costimulation and efficiently produce soluble mediators including cytokines. NK cells therefore participate in the defence against infections, the regulation of immune responses and the surveillance of stressed or cancerous cells (reviewed in1). Although these functions are not unique to NK cells, they use NK-cell specific receptor systems, and the ability to mediate effector functions rapidly without the need to develop further is a key distinguishing feature of mature NK cells and cytotoxic T lymphocytes (CTLs), both of which are efficient at mediating cytotoxicity.
NK-cell cytotoxicity involves the secretion of cytolytic effector molecules from specialized organelles known as lytic granules. Much of what is known about this process is derived from CTLs due to an abundance of studies and derivative concepts that have often served as starting points for investigations of NK cells. NK cells, however, have important distinctions and thus the process in the two cell types should not be assumed identical. The lytic granules are preformed in resting human NK cells but not in CTLs. As a result, the cytolytic process in NK cells needs to be well regulated, and this may involve additional or enhanced mechanisms for controlling secretion of lytic granule contents in NK cells compared with CTLs. Indeed, direct comparisons between NK cells and CTLs have identified important kinetic and mechanistic differences2.
The induction of many NK-cell effector functions, including cytotoxicity, requires that the NK cell contact its target cell. This ensures precise targeting of the cytolytic process to a single diseased cell within a tissue without affecting its neighbouring cells. Events occurring on interaction between a cytolytic cell and its target cell have been well studied. They include the delivery of cytolytic effector molecules to and the secretion of these molecules at the interface formed between the cytotoxic cell and its target cell through a process known as directed secretion.
Our understanding of directed secretion for cytotoxicity has been advanced by the concept of the immunological synapse. The immunological synapse was originally defined in the late 1990s3, 4 as the crucial junction between a T cell and an antigen-presenting cell (APC) at which T-cell receptors (TCRs) interact with MHC molecules. Subsequent studies extended these observations and identified relevant immunological synapses between different types of immune cells, as well as between immune cells and non-immune cells. Thus, an immunological synapse can be defined as the intentional arrangement of molecules in an immune cell at the interface with another cell. Numerous molecules have been identified as participating in the immunological synapse, including receptors, signalling molecules, cytoskeletal elements and cellular organelles. Some studies suggest that these molecules accumulate in distinct regions within an activating immunological synapse to form a supramolecular activation cluster (SMAC), which may be segregated into peripheral (pSMAC) and central (cSMAC) zones. However, the purpose of this molecular patterning is debated as function typically attributed to the SMAC can be induced in its absence under some circumstances5. Furthermore, single molecule and kinetic studies of the immunological synapse have shown that, in some settings, the engagement of individual receptors6 or the involvement of microclusters of cell-surface and signalling molecules can support cell activation without the need for a mature synapse7, 8. Therefore, the importance of larger scale accumulations and distinct segregation of molecules at the synapse is currently being re-evaluated.
Although debate regarding the role of the immunological synapse in enabling immune responses continues, several of its potential functions seem relevant and worth considering (BOX 1). Many of these were originally assigned to the immunological synapse between a T helper cell and APC, but they also apply to the NK-cell synapse. Certain aspects, however, are more relevant to NK-cells, such as directed secretion of lytic granules for cytotoxicity.
Lytic granules are hybrid organelles that are specialized for secretion of the lytic effector molecules that reside within them (BOX 2). For an NK cell to mediate cytotoxicity, lytic granules are emptied onto a target cell at a prototypical mature lytic synapse (Figure 1). In the mature synapse, filamentous (F)-actin and adhesion receptors accumulate and are thought to form a ring in the pSMAC through which perforin and other lytic granule contents are secreted. Domains within the lytic synapse containing specific signalling molecules or secretory machinery have been described in CTLs9, but may not always be required for CTL-mediated cytotoxicity6. Similar molecule distribution patterns have been observed in NK cells, but can be inconsistent10–12 and many aspects of NK cell synapse organization have not been elucidated. Nevertheless, LGs are large organelles and must traverse dense F-actin networks at the synapse, and actin reorganization is required for their release. Synapse formation, therefore, has a specific function in NK cells to enable cytotoxic function.
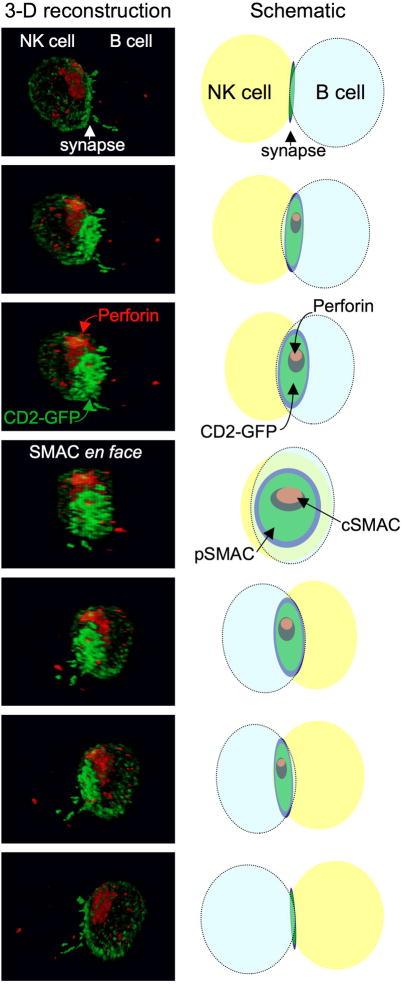
Figure 1. The prototypical mature NK-cell lytic synapse.
The mature natural killer (NK)-cell lytic synapse is defined by the formation of a supramolecular activation cluster (SMAC) at the interface between the NK-cell and target cell to which lytic granules polarize. The prototypical version of this synapse contains a central SMAC (cSMAC) that includes a secretory domain through which lytic granules may traverse. The series of images show a YTS human NK cell expressing a CD2-GFP (green fluorescent protein) fusion protein making contact with an EBV-transfomed B-cell line. The cell-cell conjugates were fixed, permeabilized and evaluated for the presence of perforin (red) using a monoclonal antibody. Perforin is contained within lytic granues and is presented as a surrogate for them, while CD2 patterning under normal conditions parallels F-actin at the mature synapse.12 Serial images were obtained using a confocal microscope through the z-axis and the three-dimensional cell volume reconstructed in silico. The individual images on the left from top to bottom demonstrate a 180-degree rotation around the z-axis (Scale bar=5μM) The schematic on the right displays the position of the NK cell (yellow) and B cell (blue), CD2 at the SMAC (green) and perforin at the SMAC (red) in each image. Also see an interactive version in online Supplementary Figure 1.
In this Review, the specific steps leading to the formation and function of the NK-cell lytic synapse are considered. Recent evidence has defined a sequence of events that has some surprising linearity and a series of important checkpoints. Appreciation of these will help to better define and control NK-cell cytotoxic ability.
Stages of the NK-cell lytic synapse
The formation of a mature and functional NK-cell lytic synapse can be divided into a series of discrete stages — the recognition and initiation stage, the effector stage and the termination stage (Figure 2). Each of the stages can be further subdivided into multiple steps. Some of these have been defined in a linear sequence with clear prerequisites and thus occur in series and not in parallel. Together, these processes enable the delivery of lytic granules to the synapse followed by their close association with the NK-cell membrane to which they can fuse and release their contents onto the target cell. Because lytic granules exist in resting NK cells before activation, each stage must be controlled to prevent accidental release of cytotoxic mediators and to enable rapid directed secretion at the appropriate moment.
Figure 2. Model for sequential stages in the formation and function of the NK-cell lytic synapse.
It is proposed that the formation of a functional natural killer (NK)-cell lytic synapse can be divided into three main stages — recognition, effector and termination stages — that are each subdivided into multiple steps. Important steps proposed to occur in the recognition stage include adhesion and initial activation signaling. In the effector stage key steps include actin reorganization, receptor clustering, MTOC and lytic granule polarization, and lytic granule fusion with the plasma membrane. In the termination stage crucial steps are proposed to include a period of inactivity and detachment. The specific time required to progress through the various stages varies and is likely to be a feature of the given target cell as well as the activation state of the NK cell. The linearity of connections between certain steps is supported by experimental data as outlined in the text, such as a requirement for actin reorganization for MTOC polarization. In others, however, linearity is inferred and is presented as a proposed model. The inhibitory synapse (Box 3) has been shown to halt progression of the lytic synapse by interfering with the late recognition stage and early effector stage – specifically activation signaling, actin reorganization and receptor clustering steps.
Recognition and initiation stages
The initial stage in the formation of a lytic synapse includes establishing a close association between the NK cell and target cell, initial signaling and adherence of the NK cell to its target cell (Figure 2). These steps are in large part theoretical, but are suggested by certain experimental evidence. In the first step, the NK cell either intentionally or accidentally encounters its potential target cell. This can be in response to chemotactic signals, enabling their localization within the organism at sites where NK cell effector functions are required (reviewed elsewhere13). The first true contact between an NK cell and its target is a cellular association akin to tethering. For this, the NK cell localizes to the site of, and would establish interactions with the target cell. Although studies of the NK-cell synapse have not firmly established the exact molecules, they may include the selectin family members, as has been shown in in vivo studies of NK cell localization14. They may also include the recognition of sialyl Lewis X by CD2 expressed by NK cells15, as CD2 has been shown to accumulate rapidly at the developing NK-cell synapse12. Identification of the molecules involved in initial contact between an NK cell and its target cell is challenging, as in vitro approaches used to study the immunological synapse may reduce the requirements for this particular step. The early interactions, however, could then result in longer lasting association leading to an initial adhesion. These events are likely to contribute to NK cell activation as receptors potentially engaged at this point may participate in activation signaling, such as CD216.
A next step in the formation of the lytic synapse is firm adhesion, which is facilitated by receptor–ligand interactions of higher affinity. The integrin family of adhesion molecules is important, and in particular the integrins lymphocyte function-associated antigen 1 (LFA1; CD11a/CD18) and MAC1 (CD11b/CD18) expressed by NK cells have been well studied. These integrins cluster rapidly at the NK-cell synapse following initiation12, 17, 18, but they probably function in adhesion prior to their rearrangement to the synapse and participate in signaling19, even in resting NK cells20). Integrin signalling can fully activate some NK cells20 and partially activate others21. As early events, however, integrin signalsmay be less likely to commit a NK cell to cytotoxicity although still physiologically relevant in promoting maturation of the synapse. Owing to the diffuse cell-surface distribution of more-potent NK-cell activating receptors such as the natural cytotoxicity receptor family, under physiological conditions, subsets of these receptors are also likely to be engaged at this stage by ligands on the target cell. These interactions provide additional signals that complement early NK-cell activation. The function of these activating receptors however is probably distinct from that of TCRs at the CTL immunological synapse. In favour of this, TCRs couple with ten immunoreceptor tyrosine-based activation motifs (ITAMs), whereas NK-cell activating receptors couple with less. So, the ligation of individual TCRs could theoretically provide more potent signals for synapse formation and function compared with NK-cell activating receptors. This may contribute to the observation of rapid minimal triggering of TCRs leading to CTL activation6, and may not apply to NK cells.
Importantly, these initial steps in the formation of the NK-cell lytic synapse probably occur before molecular patterning or polarization is evident and are completed quickly. Whether the NK cell progresses to molecular reorganization at the synapse seems to depend on the level of signals through inhibitory receptors, such as KIRs (killer-cell immunoglobulin-like receptors), which can establish a so-called inhibitory synapse (Box 3). Such regulation ensures that NK cells effectively carry out their surveillance function, by leaving most cells undisturbed while being poised to destroy those that are diseased. The NK-cell inhibitory synapse is especially elegant in that it directly interferes with the ability of the lytic synapse to progress past the initiation stage22–24.
Effector stages
After target-cell recognition and synapse initiation has occurred, and in the absence of overriding inhibitory signals, reorganization of the immunological synapse can proceed. The key steps of the effector stage can allow: formation of a stable NK-cell–target-cell interface that has a ‘cleft’ into which cytolytic molecules are secreted; recruitment of lytic granules from throughout the cell to the synapse; clearance of a conduit in the NK-cell cortex through which lytic granules could be directed to the cell membrane; and fusion of the lytic-granule membrane with plasma membrane for release of lytic-granule contents. The exact sequence of events is debatable, but several observations support some linearity.
An initial stage in the commitment to lytic-synapse formation is actin reorganization. This involves the formation of F-actin networks from the cellular pool of monomeric G-actin — a step that was first observed in polarized NK cells in 1983 using fluorescently labelled phalloidin (which binds junctions between approximated actin subunits)25. F-actin reorganization at the NK-cell synapse occurs downstream of activating-receptor-induced VAV1 activity19, 26 and depends on Wiskott-Aldrich syndrome protein (WASP)27 a known part of the protein unit that promotes F-actin branching. Accordingly, in the absence of WASP, or in the presence of actin inhibitors, F-actin accumulation at the synapse and NK-cell cytotoxicity are reduced12, 27–29. The WASP-dependent reorganization of F-actin at this stage is also important for characteristic changes in NK-cell shape that occur soon after synapse formation2,12, 27, 29 (J.S.O., unpublished observations).
The events occurring at the same time as actin reorganization have not yet been fully elucidated, but they include receptor clustering, lipid-raft aggregation, further activation signalling and lytic-granule redistribution. Although many receptors might be present within the synapse at the initial stages, owing to their diffuse distribution in the plasma membrane, some accumulate rapidly after cell conjugation, including those that are important in both adhesion and triggering of cytotoxicity. Of these, CD11a, CD11b and CD2 do not seem to cluster in the absence of actin reorganization12 thus providing some evidence for linearity in synapse formation. The mechanisms underlying actin-dependent receptor clustering in NK cells are unknown, but the ezrin, radixin and moesin (ERM) family of proteins are present in the NK-cell lytic synapse23, 30, 31 and are known to function in lateral receptor motility in other cell types32–35. A related issue is that of lipid rafts. Whether these membrane domains represent discrete entities or more fluid collections of molecules, they have been shown to aggregate at the NK-cell lytic synapse (reviewed in36) and their integrity is likely required for cytotoxicity as demonstrated using the somewhat nonspecific cholesterol-sequestering drug β-methylcyclcodextrin37. The existence and function of lipid rafts, unfortunately, has been defined using indirect methods such as cell membrane cholera toxin binding, detergent resistant biochemical fractionation and cholesterol sequestration and thus remains controversial. Similar to receptor clustering, however, lipid-raft aggregation at the NK-cell synapse depends on actin polymerization23 additionally supporting a linear progression through the early effector steps of synapse formation.
It has been shown that receptor clustering at the NK-cell lytic synapse is important for the generation of robust signalling in live NK cells38. In T cells, TCR signalling stems from microclusters of TCR molecules that move from the pSMAC into the cSMAC as activation proceeds39. Although functional microclusters have been identified in NK cells, these have only been investigated at the supramolecular inhibitory cluster (SMIC)40. It is unclear whether this level of signaling organization of the pSMAC and cSMAC identified in T cells can be similarly applied to NK cells.
Another requirement for effector function of the NK-cell lytic synapse is the polarization of lytic granules to the synapse. This begins with movement of the granules along the microtubules to the microtubule-organizing centre (MTOC). As the minus ends of microtubules are present at the MTOC, lytic granules require a minus-ended directed motor such as dynein to move along microtubules. The exact motor used, however, has not been defined. While lytic granules aggregate around the MTOC, the MTOC also begins to polarize towards the immunological synapse. Signals required for MTOC polarization include ERK (extracellular-signal-regulated kinase) phosphorylation, VAV1 activation and PYK2 (protein tyrosine kinase 2) activity in NK cells26, 41, 42. In T cells MTOC polarization requires CDC42 (cell-division cycle 42)43 and activation of a signalling platform comprising ZAP70 (ζ-chain-associated protein kinase of 70 kDa), SLP76 (SH2-domain-containing leukocyte protein of 76 kDa) and LAT (linker for activation of T cells)44, as well as the function of the formins Diaphanous-1 and Formin-like-145. The molecular motors used for propulsion or force-generating steps required to pull the MTOC to the NK-cell synapse, however, have not been defined. The CDC42 interacting protein 4 (CIP4), which interacts with tubulin, CDC42 and WASP, localizes to the MTOC after immunological synapse formation and is required for complete MTOC polarization46. Unlike CDC4247 in T cells or VAV1 in NK cells26, however, reducing CIP4 function does not impair actin reorganization in NK cells46.
Although the mechanism underlying CIP4 function is not known, it may participate actively in MTOC motility or may help to anchor the MTOC at the NK-cell synapse by interacting with WASP or CDC42. Polarization of the MTOC in NK cells is also likely to depend on the appropriate insertion of the plus ends of microtubules into the accumulated F-actin at the synapse, which in T cells has been shown to be mediated through ADAP (adhesion- and degranulation-promoting adaptor protein)48 and could function to apply force to the MTOC as the synapse reorganizes. In NK cells, the integrity and reorganization of the F-actin network is required for MTOC and lytic-granule polarization to the synapse, as blockade of actin function prevents such polarization2, 12, 26, thus further defining a linear series of events in synapse formation. Requirements for cytokine secretion at the synapse, are less well established but have been shown in T cells to require WASP49 and can occur as both directed and multi-directional50. The cellular mechanisms underlying cytokine secretion, however, have important distinctions from secretion of lytic granule contents as the separation of the two is likely critical for enabling specificity in immune responses.
Although actin function is required for MTOC and lytic granule polarization, a discrete region of the F-actin network needs to be disassembled to create a conduit through which the granules gain access to the plasma membrane. Although the function of these conduits remains hypothetical, large channels of 1–4μ in diameter, are often observed in the cSMAC of a prototypical NK-cell lytic synapse12, 18, 51, 52. Theoretically, these conduits, could also be smaller channels just large enough to allow the ~500nm diameter lytic granules through. The mechanism responsible for this targeted F-actin disassembly is unknown, but it seems to be independent of MTOC polarization, as actin clearance occurs normally in NK cells treated with microtubule depolymerizing agents12. Thus this step occurs before MTOC polarization in the sequence leading to synapse maturation. Once the MTOC has become polarized to the synapse, there are several ways in which the lytic granules might proceed through the conduit in the cortical F-actin. Originally, lytic granules, once they had been delivered near to the synapse by the MTOC, were thought to traffic to the plasma membrane using plus-ended microtubule motors. Consistent with this, lytic granules have been shown in vitro to undergo plus-ended microtubule motility using kinesin53. Recently however, the MTOC in CTLs was shown to associate closely with the synapse and directly deliver the lytic granules, thereby without the need for additional plus-ended motility of the granules along individual microtubules54. The transit of lytic granules or even the MTOC together with the granules through clearances in the F-actin network in NK cells, however, may require additional motor functions. Myosin-II is a candidate protein for this function. NK cells in which myosin-II function is inhibited biochemically or its expression is downregulated by small interfering RNA form a mature lytic synapse with polarized MTOC and lytic granules, but do not degranulate52. Lytic granule approximation to the membrane after polarization is thus another important linear step in synapse maturation.
Once the lytic granules have traversed the F-actin and can interact with the plasma membrane, there are most likely several final steps in the effector stage of NK-cell lytic synapse formation. These include concepts defined in neurological synapses, and identified in CTLs, but in NK cells are predicted to inlcude the docking of lytic granules to the synapse and their priming for membrane fusion. After priming, the membrane of the lytic granules could fuse with the plasma membrane, causing the release of granule contents into the cleft formed between the two interacting cells. Although all of these events occur within the confined space of the synapse, individual steps can be visualized using electron microscopy55 and fluorescent imaging techniques such as total-internal reflection fluorescence microscopy56.
Although most of these steps remain hypothetical in NK cells insights from CTLs are instructive. Herelytic granule docking to the synapse requires members of the RAB family of small GTPases, which are important regulators of vesicle trafficking and compartmentalization (reviewed in57). RAB proteins undergo post-translational prenylation to gain hydrophobicity, which allows them to link to a cargo to and facilitate interaction with a target membrane. RAB27a perfoms this function in docking lytic granules in CTLs58. Furthermore, active RAB27a can interact with key effector molecules, which enable vesicle priming for fusion with the plasma membrane. Priming refers to the process of preparing the lytic granule for a functional ability to fuse with the inner leaflet of the NK cell membrane at the synapse. In NK cells, MUNC13-4 has been shown to interact with RAB27a59 and is probably responsible for priming. Interestingly, MUNC13-4 can be derived from distinct vesicles, through a process of regulated fusion with developing lytic granules, thereby suggesting the existence of an independent step in and introducing an opportunity to regulate this stage of synapse formation. Probable targets of lytic-granule-associated MUNC13-4 are members of the SNARE (soluble N-ethylmaleimide-sensitive-factor accessory-protein receptor) family. SNARE proteins act in a coordinated manner to facilitate the fusion of two distinct membranes (reviewed in60). SNARE proteins present on vesicles are termed v-SNAREs and those present on target membranes are termed t-SNAREs; an interaction between v-SNAREs and t-SNAREs is requisite for membrane fusion. Although direct interactions between Munc13-4 and SNAREs have not been shown in NK cells, homologous proteins in other cell types have been shown interact to enable fusion61, 62. The only SNARE components required for lytic function in NK cells identified so far are the t-SNAREs VAMP7 (vesicle-associated membrane protein 7)63 and syntaxin-1164,65. The v-SNARE syntaxin-7 is present on lytic-granule membranes in NK cells and is likely to participate66. The regulation of SNAREs and other proteins of these late effector stages will probably prove to be crucial in the fine-tuning of the NK-cell lytic synapse.
Termination stages
Termination stages of the NK-cell lytic synapse refer to those that occur after the lytic-granule contents have been secreted. Their sequence is presently hypothetical, but include a period of inactivity and down modulation of the accumulated activating receptors followed by NK-cell detachment from the target cell and recycling of cytolytic capacity (Figure 2). The cleft formed at the lytic synapse between the NK and target cell creates a protected pocket of up to 55 nm deep that is present 45 minutes after conjugation31. Although it is unclear how long this cleft is stable for, it probably remains intact after the granules have been released during a period of relative inactivity. The cleft and this delay in progression to subsequent stages may serve to increase the concentration of the lytic effector molecules exposed to the target cell while protecting neighbouring cells from exposure to these damaging molecules, which is likely to give the lytic synapse a specific purpose in vivo After this time, the synapse function is downmodulated, which in T cells is achieved by TCR internalization via the cSMAC39, 67 and includes localized TCR ubiquitylation, suggesting that the receptors are targeted for degradation68. In NK cells, downregulation of the activating receptor NKG2D from the synapse has been observed under some circumstances69 and may also occur for other receptors, such as 2B4 and NKp4670, 71, although the mechanisms responsible for and purpose of downregulation of NK-cell activating receptors may be different to those underlying TCR downmodulation. Receptor downregulation at the NK-cell synapse could potentially happen at any point after signal generation is complete, but the persistence of certain activating receptors at the SMAC at late times after conjugation12, 51 suggests that it may be a relatively late event. Once the NK cell has carried out its cytolytic function, it can detach from the target cell and restore its ability to kill another susceptible cell. The physical process of detachment has been evaluated in the context of the NK-cell inhibitory synapse72 but not the lytic synapse. At the inhibitory synapse, detachment may result from reduced integrity of interactions between the F-actin cortex and the plasma membrane through dephosphorylation of ERM protein targets23. Although this may also participate in the lytic synapse it has not yet been defined in NK cells. There may, however, be additional active detachment processes with similarity to those defined at the T-cell synapse, such as an involvement of protein kinase Cθ (PKCθ) in promoting synapse destabilization73. Indeed, PKCθ has been found to localize to the lytic synapse in NK cells18. After detachment, the NK cell can restore its cytotoxic potential by generating new lytic granules and re-expressing any downmodulated activating receptors. Early functional studies74 suggested that some NK cells retain the capacity to form a second synapse immediately after dissipation of the first, and more recent work defines the serial killing ability of activated NK cells75. Other NK cells, as well as those completing serial kills will need to actively renew their functional capacity; this might occur rapidly (within a few hours) given the known kinetics of lytic-granule refilling as defined in CTLs76. The signals initiating the process of recycling NK-cell cytolytic capacity are unknown but might be derived from those involved in cytotoxic function at the NK-cell lytic synapse. Interestingly, ligation of the activating receptor NKp30 induces rapid activation of nuclear factor-κB (NF-κB)77, which has been shown to serve as a transcription factor for expression of the lytic granule component perforin78. The physical process of detachment itself might also provide a signal for recycling in NK cells, although this remains hypothetical.
Human genetic diseases affecting the NK-cell immunological synapse
Primary immunodeficiency diseases are characterized by genetic aberrations that impair immunological function, defence or both. Several of these diseases affect NK cells (reviewed in79, 80) and an informative subset are characterized by a specific blockade in the stages leading to the formation of a functional lytic synapse (Table 1). To date, none of the diseases in this subset has been defined as impairing NK cells in isolation and are expected to affect all cytotoxic lymphocytes. So, insight into how the lytic synapse is formed in cells from patients with these diseases has been gained mostly from T-cell studies. For those considered here, however, the functional defect has at least been established in patient NK cells. Although cytotoxic lymphocytes have crucial roles in host defence and immune regulation, there are likely to be specific contributions of NK-cell deficiency to the clinical phenotypes.
Table I.
Defects of the lytic synapse in natural killer cells
| Disease | Gene | Protein | HLH phenotype | Effect on the lytic synapse | Step affected† | References§ |
|---|---|---|---|---|---|---|
| LAD-I* | ITGB2 | CD18 | No | Decreased conjugation with target cells | 3–5 | 87, 88 |
| WAS | WASP | WASP | Some | Decreased F-actin reorganization and integrin redistribution | 6–10 | 12, 27, 91 |
| CHS | LYST | Lysosomal trafficking regulator | Yes | Inability to generate normal lytic granules for trafficking to the synapse. | 6–10 | 98–103 |
| HPS2 | ADTB3A | AP3 | Yes | Inappropriate formation of lytic granules and movement along microtubules | 6–10 | 106, 107 |
| GS2 | RAB27A | RAB27a | Yes | Lytic granules move to the synapse but remain with microtubules | 14–15 | 106, 107 |
| FHL3 | UNC13D | Munc13-4 | Yes | Lytic granules move to the synapse but fail to dock and thus do not obtain an intimate association with the NK-cell plasma membrane. | 16–17 | 85 |
| FHL4 | STX11 | Syntaxin-11 | Yes | Lytic granules polarize to and dock at the NK-cell plasma membrane, but fail to fuse. | 18 | 64, 65, 114 |
Abbreviations: LAD-I leukocyte adhesion deficiency type I, WAS – Wiskott-Aldrich syndrome, CHS – Chediak-Higashi syndrome, HPS2 - Hermansky-Pudlak syndrome type-II, GS2 – Griscelli syndrome type-II, FHL - Familial erythrophagocytic lymphohistiocytosis
Step affected refers to the progress in formation of the NK-cell lytic synapse as depicted in Figure 2.
References directly relevant to NK cells are provided, others indirectly related to NK cells are provided in the text.
Most of these diseases can result in haemophagocytic lymphohistiocytosis (HLH)81. HLH represents an inappropriately robust immune response to infection (typically with herpesviruses), which results in a persistent systemic inflammatory syndrome. This leads to the physiological symptoms of septic shock, but is also associated with the pathological finding of haematophagocytosis (the ingestion of red blood cells by phagocytes). The defect in cytotoxic lymphocytes is believed to contribute to this phenotype, as the infected and other activated cells that promote inflammation cannot be eliminated (Figure 3). NK cells may be most relevant to the HLH phenotype, given their localization to marginal zones in lymphoid organs after viral infection, their innate function early in the course of infection82 and their inherent ability to eliminate hyperactivated macrophages83. The defective NK cells in these diseases, although unable to eliminate infected cells by direct cytotoxicity can still carry out other functions, including the production of cytokines and stimulation of inflammatory responses. This further underscores distinct requirements for secretion of cytokines and lytic granule contents at the synapse. Nevertheless, the inability of NK cells to eliminate infected cells early in the infection (before T cells would be expanded) may be an important cause of HLH.
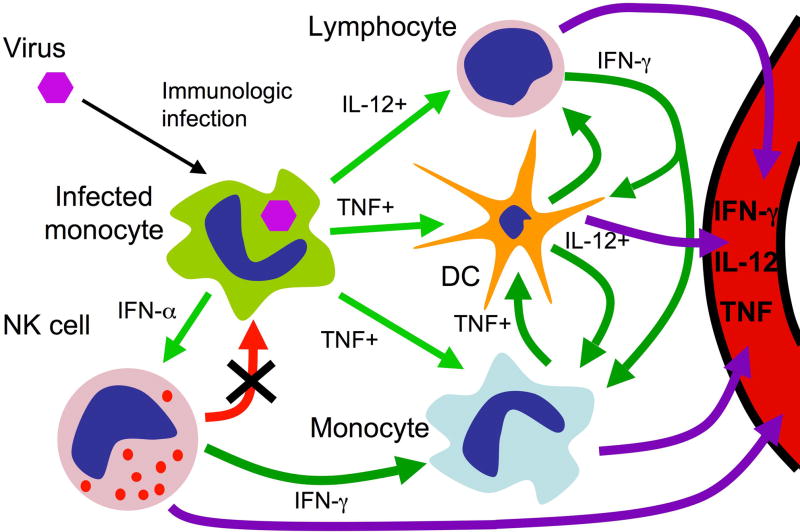
Figure 3. Proposed mechanism of HLH immunopathogenesis due to defective NK-cell lytic synpase function.
Pathogenic viral infection in a normal individual leads to the production of pro-inflammatory factors, such as tumour-necrosis factor (TNF), interferon-α (IFNα) and interleukin-12 (IL-12) by the infected cell (light green arrows). This induces relevant responses from uninfected cells including dendritic cells, monocytes, natural killer (NK) cells and other lymphocytes. These cells produce additional factors (dark green arrows) to further activate the induced cells and elicit the responses of others. Once induced, NK-cell cytotoxicity can help to rapidly eliminate the infected cell (thick red arrow) and serves to prevent further immune-cell activation induced by the infected cell. NK cell cytotoxicity can also eliminate other non-infected, activated monocytes and dendritic cells to provide additional and critical immunoregulatory function (thin red arrows). These processes have the potential to remain localized and focused to sites of infection. In an individual with impaired NK-cell cytotoxicity but a normal capacity for NK-cell activation, the NK-cell lytic synapse directed against the infected cell does not eliminate it (x). So, the inflammatory response continues unabated, leads to further activation of uninfected cells, and further pro-inflammatory activity from the induced cells. Although the infection may be localized, and the initial responding cells localized to the infection, the response amplifies and leads to an uncontrolled systemic inflammatory syndrome (purple arrows). The inflammatory response may control the viral replication, but without eliminating the source the inflammation itself may not be containable.
Other primary immunodeficiency diseases that impair cytolytic-cell function but do not disrupt the formation of the NK-cell synapse can also result in HLH. For example, HLH occurs in patients with a mutation in the gene encoding perforin84 and in this setting is characterized by the normal secretion of lytic granules that are unable to mediate cytotoxicity due to the absence of perforin85. The diseases that provide specific insight into the NK-cell lytic synapse are considered in two groups. Diseases in the first group affect steps involved with the recognition stage, or activation steps of the effector stage of the synapse. Diseases in the second group affect steps in lytic granule traffic to the synapse within the effector stage.
Diseases affecting recognition or activation steps of the NK-cell lytic synapse
Leukocyte adhesion deficiency type I (LAD-I) results from a defect in the CD18 (β-integrin) component of leukocyte integrin heterodimers86. Thus, LAD-I leukocytes do not appropriately adhere to inflamed or activated cells and cannot localize effectively to tissues and sites of inflammation. This leads to increased numbers of leukocytes in the blood and susceptibility to infectious diseases. Because early steps in NK-cell synapse formation — adhesion and activation signalling — depend on integrins, NK cells from patients with LAD-I do not adhere to their target cells, resulting in defective cytotoxicity20, 87–89. LAD-I is also distinguished from other diseases discussed here because it does not lead to HLH. This is presumably because NK cells from LAD-I patients do not form and are not activated through the synapse to produce cytokines.
Wiskott-Aldrich syndrome (WAS) results from a haematopoietic-cell-specific defect in actin reorganization and cell signalling due to WASP deficiency (reviewed in90). Patients lacking WASP expression or expressing abnormal WASP have NK cells with decreased cytolytic capacity27, 91. Clinically, patients with WAS are susceptible to herpesviruses92 and can develop HLH93–95, thereby demonstrating the functional relevance of WASP deficiency for the NK-cell lytic synapse. Formation of the lytic synapse is abnormal in NK cells from WAS patients and includes decreased F-actin accumulation and adhesion-receptor clustering at the synapse12, 27, 91. This underscores a requirement for WASP in early effector stages of the NK-cell lytic synapse. Importantly, exposure to interleukin-2 in vitro reverses the defect in WASP-deficient NK cells by restoring lytic function and F-actin accumulation at the synapse91, 96. This demonstrates the existence of potential alternative pathways of actin polymerization in NK cells. At baseline, however, WASP is crucial for lytic synapse function but not early steps in synapse formation, thereby potentially enabling some pro-inflammatory functions to result in an occasional HLH phenotype.
Diseases affecting lytic granule traffic to the NK-cell lytic synapse
Chediak-Higashi syndrome (CHS) and Hermansky-Pudlak syndrome type II (HPS2) both affect the normal formation of lytic granules and lead to the presence of “giant” lytic granules. Both are also associated with albinism, which is caused by aberrant functioning of melanocytes, which pigment skin via secretion of melanosomes (an equivalent of lytic granules). CHS and HPS2 are similar in that they represent a failure in generation of the NK-cell lytic synapse at the end of the effector stages, as the abnormal lytic granules will not migrate along the microtubules to the MTOC. CHS results from a mutation in the LYST gene, which encodes the lysosomal trafficking regulator (reviewed in97). Most patients with CHS ultimately experience an “accelerated phase” of the disease, which is an infection-induced HLH. The mechanics of NK-cell cytotoxicity and the NK-cell lytic synapse in CHS have not been studied in depth in recent years. Nevertheless, older studies identified defective cytolytic activity in patient NK cells despite normal adhesion to target cells98–102. NK cells from CHS patients contain abnormal giant lytic granules103, and studies in CTLs indicate that these giant granules arise from the fusion of individual lytic granules 104. HPS2 is caused by a mutation in the ATD3BA gene (reviewed in105), which encodes adaptor protein 3 (AP3) and, unlike CHS, HPS2 is associated with excessive bleeding due to an absence of the platelet storage pool and ensuing abnormal platelet aggregation. HPS2 patients exhibit defective NK-cell cytotoxicity106, 107 and the HLH phenotype107. AP3 is required for the appropriate sorting of molecules from the Golgi into lytic granules. Similar to patients with CHS, CTLs from patients with HPS2 have enlarged granules that fail to move along microtubules108. Griscelli syndrome type II (GS2) is a third syndrome that combines albinism and immunodeficiency and is also associated with an accelerated phase HLH phenotype. It is distinct from CHS and HPS2, in that patients have a broader range of hypopigmentation. GS2 is caused by mutation in the RAB27A gene, which encodes the RAB27A small GTPase109. NK-cell cytotoxicity is decreased in patients with GS2, but not necessarily absent110, 111, and can be reversed by culturing the NK cells in the presence of IL-2111. So, the mechanism underlying the defect of the NK-cell lytic synapse in GS2 patients is also distinct from that in CHS and HPS2 patients. Studies performed in mouse RAB27A-deficient CTLs show that lytic granules migrate and polarize towards but fail to dock at the synapse58. Specifically, they were separate from the membrane at the level of resolution in confocal microscopy. The reversal of the defect by IL-2, however, suggests there may be some redundancy in RAB27A function. Interestingly, RAB27B is capable of substituting for deficient RAB27A function under certain circumstances112 and may provide a means for activation-induced augmented lytic granule traffic.
Familial erythrophagocytic lymphohistiocytosis (FHL) types 3 and 4 are similar to GS2, but are not associated with albinism demonstrating that the affected genes are not essential in melanocytes. FHL3 is caused by mutation in the UNC13D gene, which encodes MUNC13-4. Initially defined in CTLs, the lytic granules in MUNC13-4-deficient cells polarize towards the synapse and dock at the plasma membrane, but do not fuse113. Defective cytolytic activity and decreased granule fusion with the plasma membrane have also been observed in MUNC13-4-deficient NK cells85. As explained above, MUNC13-4 interacts with RAB27A and primes the lytic granules for SNARE-mediated fusion with the NK-cell plasma membrane at the lytic synapse.
FHL4 is caused by mutations in the STX11 gene, which encodes syntaxin-11114. Although NK cells from patients harboring a STX11 mutation are defective in cytotoxic activity, it was initially thought that syntaxin-11 conferred a costimulatory role in promoting susceptibility of monocytes to NK-cell cytotoxicity. Subsequent studies however indicated a direct role for syntaxin-11 in NK-cell degranulation64, 65. As described above, syntaxin-11 is a t-SNARE in NK cells and presumably interacts with respective v-SNAREs to enable lytic granule fusion. Importantly, the polarization of lytic granules to the synapse in NK cells from FHL4 patients, without subsequent degranulation, has been directly observed65. FHL4 patients have diverse clinical phenotypes, with some showing late onset of disease115, which implies that syntaxin-11 mutations are hypomorphic or that there is some redundancy of the protein for enabling lytic granule fusion. In support of some level of redundancy, stimulation of NK cells from FHL4 patients with IL-2 can restore degranulation65, suggesting an activation-induced synthesis of proteins that complement deficient syntaxin-11 function.
Concluding remarks
The NK-cell lytic synapse is formed in a series of stages that are required for cytolytic function. These include recognition/initiation, effector and termination stages that together enable the precise delivery of lytic-granule contents onto a susceptible target cell. Experimental investigations have defined some sequence to the individual steps, but have also raised many new and unanswered questions. First, what governs the access of lytic granules from the NK cell cytoplasm to the plasma membrane? A prerequisite conduit through the actin cortex at the synapse is likely a crucial element of cytotoxic cells, which may be generated by a mechanism as elegant as that of cytotoxicity itself. A second question relates to the regulation of the individual steps in synapse formation. Each step may have its own specific signal requirements, or may more simply represent a gradient emanating from strong initial signals. The former would enable the ideal fine-tuning of cytotoxic host defence.
There are important similarities of the NK-cell lytic synapse to the immunological synapse in T cells, however, there are also several notable actual and potential distinctions. These may relate to the fact that NK cells are armed for cytotoxicity in the resting state and rely on a balance of signals from inhibitory and activating receptors to function in immunosurveillance. Thus it is likely that studies of synapse regulation in NK cells will highlight mechanisms that control progression through the steps of lytic synapse formation. Some of these may be augmented, or even specific to NK cells to provide exacting control of the innate immune response in the face of less antigen specificity compared to the adaptive immune response. Identifying these mechanisms spatially and in real time as they relate to the synapse will provide valuable insight into the cellular control of the NK-cell cytolytic process.
Our understanding of the mechanisms of NK-cell lytic synapse formation and function has also been obtained from the study of rare diseases that affect these processes. Dissection of the HLH phenotype, in particular, has enhanced understanding of the discrete stages in the formation of the NK-cell lytic synapse. Although a limited number of genes have been ascribed to the HLH phenotypes, there are many patients with defective NK-cell function and HLH for which the genetic defects are unknown. It is likely that further study of these individuals will uncover additional proteins involved in the progression of NK-cell lytic-synapse formation.
Box 1: Proposed functions of the immunological synapse
There are numerous functions ascribed to the immunological synapse, many of which are relevant to natural killer (NK) cells.
Ligand recognition
The immunological synapse creates a critical zone at which ligands may be recognized with extraordinary precision amidst complex surroundings of non-relevant interactions.
Signal amplification and integration
Whether in large clusters at the central supramolecular activation cluster (cSMAC) or in discrete microclusters located in the periphery, important signalling molecules localize with receptors at the synapse. Innovative studies in NK cells have demonstrated function at these sites40 as well as functional integration among signals11.
Costimulation
NK-cell expression of critical costimulatory ligands such as CD40L116 and OX40L117 can enable their direct presentation to adaptive immune cells for facilitating responses. NK cells can form dual synapses in vivo with other innate cells and T cells118. The innate cell presumably induces NK-cell costimulatory ligands, which are then recognized by adjoined T cells.
Cytotoxicity
Precise targeting of cytotoxicity achieved through the immunological synapse seals the interaction between NK cell and target cell31 and can protect neighbouring cells from damage.
Directed secretion
Arrangement of molecules at the synapse can create a conduit through which cellular components can be secreted9. The synapse can facilitate linkage to intracellular transport machinery to allow focused secretion of small and large components including lytic granules21 and cytokines119.
Multi-driectional secretion
Activation signals from the synapse can induce secretion from within an NK cell, but at sites away from the synapse21, 50. This might be useful after recognizing opsonizing IgG through NK cell FcγRIII, combating extracellular infections, and promoting local inflammation.
Protein transfer
Cell-surface proteins can be transferred from the NK cell to target cell membrane through the immunological synapse51, 120–124 to induce target-cell signal generation and protect NK cells from fratricide69, 125.
Cell-fate determination
The immunological synapse polarizes cellular components in CTLs to enable unequal cell division leading to distinct cell fates126. This has not yet been defined in NK cells, but will probably be important.
Inhibition of activation
The immunological synapse affords the opportunity to specifically ligate inhibitory receptors and prevent activation (see Box 3).
Signal termination
Molecular patterns at the immunological synapse may facilitate NK cell receptor internalization and/or end their productive signalling127. This may be important in recycling or creating a refractory period and has been defined in T cells39, 67.
Box 2: NK-cell lytic granules
The lytic granules found in natural killer (NK) cells are secretory lysosomes that have characteristics of both the cellular lysosomal compartment and specialized secretory machinery (reviewed in128). They arise from the fusion of endosomes with specific secretory components from the trans-Golgi network. This results in a dual function organelle, that is, one specialized for destructive activities due to its lysosomal properties and secretory function. Important regulators of granule maturation are adaptor protein 3 (AP-3) and lysosomal trafficking regulator (LYST) protein, which regulate the addition of lysosomal proteins from the Golgi and lysosomal fusion, respectively. Components that are sorted into the lytic granules include granzymes A and B, which are transported from the trans-Golgi network by the mannose-6-phosphate receptor, as well as FAS ligand, granulysin and perforin. Mannose-6-phosphate receptor-independent sorting mechanisms exist and are likely also relevant to NK cell lytic granule development129.
The genesis of the secretory lysosome in NK cells is a multistep process and is similar to that in cytotoxic T cells. There are, however, likely to be important differences between the processes in the two cell types as the generation of the lytic granules in T cells is only induced after cell activation. By contrast, secretory lysosomes are preformed and maintained in resting human NK cells130. In addition, NK cells contain various related organelles with distinct morphology, including mature lytic granules, lytic granule precursors, multivesicular bodies and late endosomes (see figure – red arrowheads denote lytic granules and their precursory forms). These can polarize to the immunological synapse (as shown) where their contents can be secreted into a cleft formed between NK and target cell.

Box 3: The inhibitory immunological synapse in NK cells
An important structure besides the lytic synapse that can form between an NK cell and another cell is the inhibitory synapse17. Because resting NK cells contain lytic granules and express germline-encoded activating receptors, a robust mechanism for restraining the formation of a lytic synapse is essential to avoid inadvertent cytolysis. NK cells express a family of inhibitory receptors that recognize determinants of self and prevent the lysis of healthy cells. This inhibitory activity is mediated through the formation of an inhibitory synapse. Here inhibitory receptors such as the killer-cell immunoglobulin-like receptors (KIRs), which contain long cytoplasmic tails with immunoreceptor tyrosine-based inhibitory motifs (ITIMs), engage their ligands and induce the activity of phosphatases such as SHP1 (SH2-domain-containing protein tyrosine phosphatase 1)40, 131. The clustering of KIRs and associated components at the inhibitory synapse has been termed the supramolecular inhibitory cluster (SMIC)17. The SMIC is distinguished from the NK-cell supramolecular activation cluster (SMAC) in that it excludes lipid raft membrane domains37, 132–134, does not accumulate substantial F-actin31, and includes inhibitory signalling molecules such as SHP110, 18, 131, 134. The function of the SMIC in preventing SMAC formation is achieved by preventing actin reorganization22, 23, blocking the recruitment of activating receptors24, 135 and promoting detachment from a target cell72. Mechanistically, this is likely due to the dephosphorylation of key molecules involved in F-actin dynamics including VAV123, 136 and ezrin-radixin-moesin proteins 23. As a result the NK cell synapse is likely a key site for signal integration between inhibitory and activating receptors11.
The three-dimensional organization of the SMIC has been described to include an early recruitment of KIRs to the centre of the SMIC, which then migrate to the periphery, although several molecular patterns for the SMIC have been reported10, 17, 18. The figure provides aschematic of the inhibitory synapse in which NK cells expressing KIR2DL1 are paired with target cells expressing the cognate ligand HLA-Cw4 (Left). The SMIC is characterized by clustered KIR2DL1 on the NK cell and HLA-Cw4 on the target cell. F-actin does not accumulate at the NK cell SMIC and the MTOC is not polarized. KIR2DL1 expressing NK cells do not form a SMIC with non-cognate HLA-Cw3-expressing target cells and neither accumulate at the synapse (Right). Here a SMAC is formed as F-actin accumulates in the NK cell at the synapse and the MTOC is polarized.
Supplementary Material
Online Supplementary information S1 (Figure). Interactive version of a prototypical mature NK cell lytic synapse. The mature NK cell lytic synapse in Figure 1 is provided in an interactive format. The image shows a human NK cell (YTS) expressing CD2-GFP (green fluorescent protein) conjugated to an EBV-transformed B cell (721.221). NK cells were conjugated to 721.221 cells, fixed, permeabilized and stained with a perforin-specific monoclonal antibody (red) to visualize the lytic granules. Serial images were obtained using a confocal microscope through the z-axis and the three-dimensional cell volume reconstructed in silico and QTVR output generated with complete rotation around the z-axis generated using Improvision Volocity visualization software. The accumulated GFP fluorescence indicates the supramolecular activation cluster (SMAC) formed at the synpase between the NK cell and the target cell. Notably, a region in the centre of the SMAC that is devoid of GFP is consistent with the presence of a cSMAC and delineates a channel through which the secretion of lytic granule contents is likely to occur. Although there is an accumulation of perforin internal to the SMAC (consistent with the lytic granules surrounding the microtubule-organizing centre (MTOC)), perforin can be seen coming from this region to the cSMAC. This is consistent with the secretory domain of the cytolytic immunological synapse.
Acknowledgments
This work was supported by NIH grant AI-067946 and a faculty development award from the Education Research Trust of the American Academy of Allergy, Asthma and Immunology. The author thanks Dr. Janis Burkhardt for her constructive comments The author would also like to apologize to the authors of the many relevant works that were not cited.
Glossary
- Danger signal
Agents that alert the immune system to danger and thereby promote the generation of immune responses. Danger signals can be associated with microbial invaders (exogenous danger signals), but can also be produced or expressed by damaged cells (endogenous danger signals)
- Microclusters
Discrete collection of molecules at the immunological synapse capable of moving and generating signals. They are smaller than and exclusive of the central supramolecular activation cluster (cSMAC), which can represent a coalescence of multiple microclusters
- Lipid rafts
Liquid-ordered membrane domains that are enriched in sphingolipidsand cholesterol, and also contain glyophosphatidylinositol-anchored receptors. They can provide ordered structure to the lipid bilayer and have the ability to include or exclude specific signalling molecules and complexes. There is some degree of controversy regarding lipid rafts given that most techniques for their investigation are indirect and include cell-membrane binding of cholera toxin, cholesterol sequestration using drugs, and cell fractionation based upon detergent sensitivity
- microtubule-organizing centre
(MTOC). A structure that is found in all plant and animal cells, from which microtubules radiate. The two most important types of MTOCs are the basal bodies that are associated with cilia and the centrosome, which is composed of γ-tubulin-ring complexes for microtubule nucleation
- NKG2D
(Natural-killer group 2, member D). A primary activating receptor encoded by the NK-cell gene complex and expressed by all mature NK cells. It recognizes distinct families of ligands that are generally expressed only by infected, stressed or transformed cells
- Perforin
A component of cytolytic granules that participates in the permeabilization of plasma membranes, allowing granzymes and other cytotoxic components to enter, or be taken up by, target cells
Footnotes
Related Web Sites
John Coligan Laboratory: http://www3.niaid.nih.gov/labs/aboutlabs/lig/receptorCellBiologySection/coligan.htm
Dan Davis Laboratory: http://www.dandavislab.co.uk/
Bo Dupont Laboratory: http://www.mskcc.org/mskcc/html/10966.cfm
Eric Long Laboratory: http://www3.niaid.nih.gov/labs/aboutlabs/lig/molecularCellularImmunologySection/
Jordan Orange Laboratory: www.orangelab.org
Jack Strominger Laboratory: http://www.mcb.harvard.edu/Faculty/Strominger.html
Carsten Watzl Laboratory: http://www.carstenwatzl.com/
References
- 1.Orange JS, Ballas ZK. Natural killer cells in human health and disease. Clin Immunol. 2006;118:1–10. doi: 10.1016/j.clim.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Wulfing C, Purtic B, Klem J, Schatzle JD. Stepwise cytoskeletal polarization as a series of checkpoints in innate but not adaptive cytolytic killing. Proc Natl Acad Sci U S A. 2003;100:7767–72. doi: 10.1073/pnas.1336920100. Introduction of steps in NK cell immunological synapse formation centered around the cytoskeleton (see also #12) with critical comparisons to T cells underscoring differences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grakoui A, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–7. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 4.Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–6. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- 5.Davis DM, Dustin ML. What is the importance of the immunological synapse? Trends Immunol. 2004;25:323–7. doi: 10.1016/j.it.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Purbhoo MA, Irvine DJ, Huppa JB, Davis MM. T cell killing does not require the formation of a stable mature immunological synapse. Nat Immunol. 2004;5:524–30. doi: 10.1038/ni1058. Important challenge to the functional organization of lytic immunological synapse in CTLs. [DOI] [PubMed] [Google Scholar]
- 7.Yokosuka T, et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–62. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 8.Mossman KD, Campi G, Groves JT, Dustin ML. Altered TCR signaling from geometrically repatterned immunological synapses. Science. 2005;310:1191–3. doi: 10.1126/science.1119238. [DOI] [PubMed] [Google Scholar]
- 9.Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–61. doi: 10.1016/s1074-7613(01)00234-5. Original definition of the lytic immunological synapse and its organization in CTL (to be contrasted with references 17 and 18 for NK cells) [DOI] [PubMed] [Google Scholar]
- 10.Vyas YM, Maniar H, Dupont B. Cutting edge: differential segregation of the SRC homology 2-containing protein tyrosine phosphatase-1 within the early NK cell immune synapse distinguishes noncytolytic from cytolytic interactions. J Immunol. 2002;168:3150–4. doi: 10.4049/jimmunol.168.7.3150. [DOI] [PubMed] [Google Scholar]
- 11.Almeida CR, Davis DM. Segregation of HLA-C from ICAM-1 at NK cell immune synapses is controlled by its cell surface density. J Immunol. 2006;177:6904–10. doi: 10.4049/jimmunol.177.10.6904. [DOI] [PubMed] [Google Scholar]
- 12.Orange JS, et al. The mature activating natural killer cell immunologic synapse is formed in distinct stages. Proc Natl Acad Sci U S A. 2003;100:14151–6. doi: 10.1073/pnas.1835830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gregoire C, et al. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–82. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Kawashima H, Lowe JB, Lanier LL, Fukuda M. Suppression of tumor formation in lymph nodes by L-selectin-mediated natural killer cell recruitment. J Exp Med. 2005;202:1679–89. doi: 10.1084/jem.20051473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warren HS, Altin JG, Waldron JC, Kinnear BF, Parish CR. A carbohydrate structure associated with CD15 (Lewis x) on myeloid cells is a novel ligand for human CD2. J Immunol. 1996;156:2866–73. [PubMed] [Google Scholar]
- 16.Inoue H, et al. Lipid rafts as the signaling scaffold for NK cell activation: tyrosine phosphorylation and association of LAT with phosphatidylinositol 3-kinase and phospholipase C-gamma following CD2 stimulation. Eur J Immunol. 2002;32:2188–98. doi: 10.1002/1521-4141(200208)32:8<2188::AID-IMMU2188>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 17.Davis DM, et al. The human natural killer cell immune synapse. Proc Natl Acad Sci U S A. 1999;96:15062–7. doi: 10.1073/pnas.96.26.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vyas YM, et al. Spatial organization of signal transduction molecules in the NK cell immune synapses during MHC class I-regulated noncytolytic and cytolytic interactions. J Immunol. 2001;167:4358–67. doi: 10.4049/jimmunol.167.8.4358. [DOI] [PubMed] [Google Scholar]
- 19.Riteau B, Barber DF, Long EO. Vav1 phosphorylation is induced by beta2 integrin engagement on natural killer cells upstream of actin cytoskeleton and lipid raft reorganization. J Exp Med. 2003;198:469–74. doi: 10.1084/jem.20021995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barber DF, Faure M, Long EO. LFA-1 contributes an early signal for NK cell cytotoxicity. J Immunol. 2004;173:3653–9. doi: 10.4049/jimmunol.173.6.3653. [DOI] [PubMed] [Google Scholar]
- 21.Bryceson YT, March ME, Barber DF, Ljunggren HG, Long EO. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J Exp Med. 2005;202:1001–12. doi: 10.1084/jem.20051143. Uses a insect cell target expressing human NK cell ligands (along with #20) to define that some NK cell activation receptors induce lytic granule polarization torward the immunological synapse, while others can induce degranulation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dietrich J, Cella M, Colonna M. Ig-like transcript 2 (ILT2)/leukocyte Ig-like receptor 1 (LIR1) inhibits TCR signaling and actin cytoskeleton reorganization. J Immunol. 2001;166:2514–21. doi: 10.4049/jimmunol.166.4.2514. [DOI] [PubMed] [Google Scholar]
- 23.Masilamani M, Nguyen C, Kabat J, Borrego F, Coligan JE. CD94/NKG2A Inhibits NK Cell Activation by Disrupting the Actin Network at the Immunological Synapse. J Immunol. 2006;177:3590–6. doi: 10.4049/jimmunol.177.6.3590. [DOI] [PubMed] [Google Scholar]
- 24.Endt J, et al. Inhibitory receptor signals suppress ligation-induced recruitment of NKG2D to GM1-rich membrane domains at the human NK cell immune synapse. J Immunol. 2007;178:5606–11. doi: 10.4049/jimmunol.178.9.5606. [DOI] [PubMed] [Google Scholar]
- 25.Carpen O, Virtanen I, Lehto VP, Saksela E. Polarization of NK cell cytoskeleton upon conjugation with sensitive target cells. J Immunol. 1983;131:2695–8. An early and original description of the NK cell immunological synapse long before the immunological synapse was defined as a biological concept (see also reference #55) [PubMed] [Google Scholar]
- 26.Graham DB, et al. Vav1 controls DAP10-mediated natural cytotoxicity by regulating actin and microtubule dynamics. J Immunol. 2006;177:2349–55. doi: 10.4049/jimmunol.177.4.2349. [DOI] [PubMed] [Google Scholar]
- 27.Orange JS, et al. Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci U S A. 2002;99:11351–6. doi: 10.1073/pnas.162376099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz P, Zaytoun AM, Lee JH., Jr Mechanisms of human cell-mediated cytotoxicity. III. Dependence of natural killing on microtubule and microfilament integrity. J Immunol. 1982;129:2816–25. [PubMed] [Google Scholar]
- 29.Gismondi A, et al. Cutting edge: functional role for proline-rich tyrosine kinase 2 in NK cell-mediated natural cytotoxicity. J Immunol. 2000;164:2272–6. doi: 10.4049/jimmunol.164.5.2272. [DOI] [PubMed] [Google Scholar]
- 30.Ramoni C, et al. Differential expression and distribution of ezrin, radixin and moesin in human natural killer cells. Eur J Immunol. 2002;32:3059–65. doi: 10.1002/1521-4141(200211)32:11<3059::AID-IMMU3059>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 31.McCann FE, et al. The size of the synaptic cleft and distinct distributions of filamentous actin, ezrin, CD43, and CD45 at activating and inhibitory human NK cell immune synapses. J Immunol. 2003;170:2862–70. doi: 10.4049/jimmunol.170.6.2862. Definition of the protected ‘cleft’ at the NK cell immunological synapse into which lytic granule contents can be secreted. [DOI] [PubMed] [Google Scholar]
- 32.Helander TS, et al. ICAM-2 redistributed by ezrin as a target for killer cells. Nature. 1996;382:265–8. doi: 10.1038/382265a0. [DOI] [PubMed] [Google Scholar]
- 33.Allenspach EJ, et al. ERM-dependent movement of CD43 defines a novel protein complex distal to the immunological synapse. Immunity. 2001;15:739–50. doi: 10.1016/s1074-7613(01)00224-2. [DOI] [PubMed] [Google Scholar]
- 34.Delon J, Kaibuchi K, Germain RN. Exclusion of CD43 from the immunological synapse is mediated by phosphorylation-regulated relocation of the cytoskeletal adaptor moesin. Immunity. 2001;15:691–701. doi: 10.1016/s1074-7613(01)00231-x. [DOI] [PubMed] [Google Scholar]
- 35.Ilani T, Khanna C, Zhou M, Veenstra TD, Bretscher A. Immune synapse formation requires ZAP-70 recruitment by ezrin and CD43 removal by moesin. J Cell Biol. 2007;179:733–46. doi: 10.1083/jcb.200707199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taner SB, et al. Control of immune responses by trafficking cell surface proteins, vesicles and lipid rafts to and from the immunological synapse. Traffic. 2004;5:651–61. doi: 10.1111/j.1600-0854.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 37.Lou Z, Jevremovic D, Billadeau DD, Leibson PJ. A balance between positive and negative signals in cytotoxic lymphocytes regulates the polarization of lipid rafts during the development of cell-mediated killing. J Exp Med. 2000;191:347–54. doi: 10.1084/jem.191.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giurisato E, et al. Phosphatidylinositol 3-kinase activation is required to form the NKG2D immunological synapse. Mol Cell Biol. 2007;27:8583–99. doi: 10.1128/MCB.01477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25:117–27. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Treanor B, et al. Microclusters of inhibitory killer immunoglobulin-like receptor signaling at natural killer cell immunological synapses. J Cell Biol. 2006;174:153–61. doi: 10.1083/jcb.200601108. Demonstrates the existance and function of microclusters for signalling at the NK cell immunological synapse using an innovative fluorescence resonance energy transfer approach. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen X, et al. CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc Natl Acad Sci U S A. 2006;103:10346–51. doi: 10.1073/pnas.0604236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sancho D, et al. The tyrosine kinase PYK-2/RAFTK regulates natural killer (NK) cell cytotoxic response, and is translocated and activated upon specific target cell recognition and killing. J Cell Biol. 2000;149:1249–62. doi: 10.1083/jcb.149.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stowers L, Yelon D, Berg LJ, Chant J. Regulation of the polarization of T cells toward antigen-presenting cells by Ras-related GTPase CDC42. Proc Natl Acad Sci U S A. 1995;92:5027–31. doi: 10.1073/pnas.92.11.5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowin-Kropf B, Shapiro VS, Weiss A. Cytoskeletal polarization of T cells is regulated by an immunoreceptor tyrosine-based activation motif-dependent mechanism. J Cell Biol. 1998;140:861–71. doi: 10.1083/jcb.140.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez TS, et al. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity. 2007;26:177–90. doi: 10.1016/j.immuni.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Banerjee PP, et al. Cdc42-interacting protein-4 functionally links actin and microtubule networks at the cytolytic NK cell immunological synapse. J Exp Med. 2007;204:2305–20. doi: 10.1084/jem.20061893. Separates actin reorganization at the lytic NK immunological synapse from microtubule organizing center polarization by defining CIP4 as a linking protein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tskvitaria-Fuller I, et al. Specific patterns of Cdc42 activity are related to distinct elements of T cell polarization. J Immunol. 2006;177:1708–20. doi: 10.4049/jimmunol.177.3.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Combs J, et al. Recruitment of dynein to the Jurkat immunological synapse. Proc Natl Acad Sci U S A. 2006;103:14883–8. doi: 10.1073/pnas.0600914103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morales-Tirado V, et al. Cutting edge: selective requirement for the Wiskott-Aldrich syndrome protein in cytokine, but not chemokine, secretion by CD4+ T cells. J Immunol. 2004;173:726–30. doi: 10.4049/jimmunol.173.2.726. [DOI] [PubMed] [Google Scholar]
- 50.Huse M, Lillemeier BF, Kuhns MS, Chen DS, Davis MM. T cells use two directionally distinct pathways for cytokine secretion. Nat Immunol. 2006;7:247–55. doi: 10.1038/ni1304. [DOI] [PubMed] [Google Scholar]
- 51.Roda-Navarro P, et al. Dynamic redistribution of the activating 2B4/SAP complex at the cytotoxic NK cell immune synapse. J Immunol. 2004;173:3640–6. doi: 10.4049/jimmunol.173.6.3640. [DOI] [PubMed] [Google Scholar]
- 52.Andzelm MM, Chen X, Krzewski K, Orange JS, Strominger JL. Myosin IIA is required for cytolytic granule exocytosis in human NK cells. J Exp Med. 2007;204:2285–91. doi: 10.1084/jem.20071143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burkhardt JK, McIlvain JM, Jr, Sheetz MP, Argon Y. Lytic granules from cytotoxic T cells exhibit kinesin-dependent motility on microtubules in vitro. J Cell Sci. 1993;104 (Pt 1):151–62. doi: 10.1242/jcs.104.1.151. [DOI] [PubMed] [Google Scholar]
- 54.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–5. doi: 10.1038/nature05071. Defines a critical paradigm for lytic granule delivery to the immunological synapse in CTLs. [DOI] [PubMed] [Google Scholar]
- 55.Carpen O, Virtanen I, Saksela E. Ultrastructure of human natural killer cells: nature of the cytolytic contacts in relation to cellular secretion. J Immunol. 1982;128:2691–7. [PubMed] [Google Scholar]
- 56.Liu D, et al. Rapid biogenesis and sensitization of secretory lysosomes in NK cells mediated by target-cell recognition. Proc Natl Acad Sci U S A. 2005;102:123–7. doi: 10.1073/pnas.0405737102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Goody RS, Rak A, Alexandrov K. The structural and mechanistic basis for recycling of Rab proteins between membrane compartments. Cell Mol Life Sci. 2005;62:1657–70. doi: 10.1007/s00018-005-4486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stinchcombe JC, et al. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J Cell Biol. 2001;152:825–34. doi: 10.1083/jcb.152.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Menager MM, et al. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat Immunol. 2007;8:257–67. doi: 10.1038/ni1431. Demonstrates the interaction between Rab27a and Munc13-4 in NK cells which is required for lytic granule exocytosis and defines distinct compartments from which the proteins can originate suggesting an elegant regulatory mechanism. [DOI] [PubMed] [Google Scholar]
- 60.Stow JL, Manderson AP, Murray RZ. SNAREing immunity: the role of SNAREs in the immune system. Nat Rev Immunol. 2006;6:919–29. doi: 10.1038/nri1980. [DOI] [PubMed] [Google Scholar]
- 61.Dulubova I, et al. Munc18-1 binds directly to the neuronal SNARE complex. Proc Natl Acad Sci U S A. 2007;104:2697–702. doi: 10.1073/pnas.0611318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toonen RF, et al. Dissecting docking and tethering of secretory vesicles at the target membrane. Embo J. 2006;25:3725–37. doi: 10.1038/sj.emboj.7601256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marcet-Palacios M, et al. Vesicle-associated membrane protein 7 (VAMP-7) is essential for target cell killing in a natural killer cell line. Biochem Biophys Res Commun. 2008;366:617–23. doi: 10.1016/j.bbrc.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 64.Arneson LN, et al. Cutting edge: syntaxin 11 regulates lymphocyte-mediated secretion and cytotoxicity. J Immunol. 2007;179:3397–401. doi: 10.4049/jimmunol.179.6.3397. References 64 and 65 define the role of the NK cell SNARE protein syntaxin-11 in enabling degranulation using RNA interference (#64) and naturally occuring human syntaxin-11 mutant NK cells (#65) [DOI] [PubMed] [Google Scholar]
- 65.Bryceson YT, et al. Defective cytotoxic lymphocyte degranulation in syntaxin-11 deficient familial hemophagocytic lymphohistiocytosis 4 (FHL4) patients. Blood. 2007;110:1906–15. doi: 10.1182/blood-2007-02-074468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casey TM, Meade JL, Hewitt EW. Organelle proteomics: identification of the exocytic machinery associated with the natural killer cell secretory lysosome. Mol Cell Proteomics. 2007;6:767–80. doi: 10.1074/mcp.M600365-MCP200. [DOI] [PubMed] [Google Scholar]
- 67.Lee KH, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–22. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 68.Wiedemann A, et al. T-cell activation is accompanied by an ubiquitination process occurring at the immunological synapse. Immunol Lett. 2005;98:57–61. doi: 10.1016/j.imlet.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 69.McCann FE, Eissmann P, Onfelt B, Leung R, Davis DM. The activating NKG2D ligand MHC class I-related chain A transfers from target cells to NK cells in a manner that allows functional consequences. J Immunol. 2007;178:3418–26. doi: 10.4049/jimmunol.178.6.3418. [DOI] [PubMed] [Google Scholar]
- 70.Sivori S, et al. 2B4 functions as a co-receptor in human NK cell activation. Eur J Immunol. 2000;30:787–93. doi: 10.1002/1521-4141(200003)30:3<787::AID-IMMU787>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 71.Sandusky MM, Messmer B, Watzl C. Regulation of 2B4 (CD244)-mediated NK cell activation by ligand-induced receptor modulation. Eur J Immunol. 2006;36:3268–76. doi: 10.1002/eji.200636146. [DOI] [PubMed] [Google Scholar]
- 72.Burshtyn DN, Shin J, Stebbins C, Long EO. Adhesion to target cells is disrupted by the killer cell inhibitory receptor. Curr Biol. 2000;10:777–80. doi: 10.1016/s0960-9822(00)00568-6. [DOI] [PubMed] [Google Scholar]
- 73.Sims TN, et al. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–85. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 74.Ullberg M, Jondal M. Recycling and target binding capacity of human natural killer cells. J Exp Med. 1981;153:615–28. doi: 10.1084/jem.153.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhat R, Watzl C. Serial killing of tumor cells by human natural killer cells -enhancement by therapeutic antibodies. PLoS ONE. 2007;2:e326. doi: 10.1371/journal.pone.0000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Isaaz S, Baetz K, Olsen K, Podack E, Griffiths GM. Serial killing by cytotoxic T lymphocytes: T cell receptor triggers degranulation, re-filling of the lytic granules and secretion of lytic proteins via a non-granule pathway. Eur J Immunol. 1995;25:1071–9. doi: 10.1002/eji.1830250432. [DOI] [PubMed] [Google Scholar]
- 77.Pandey R, DeStephan CM, Madge LA, May MJ, Orange JS. NKp30 ligation induces rapid activation of the canonical NF-kappaB pathway in NK cells. J Immunol. 2007;179:7385–96. doi: 10.4049/jimmunol.179.11.7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou J, Zhang J, Lichtenheld MG, Meadows GG. A role for NF-kappa B activation in perforin expression of NK cells upon IL-2 receptor signaling. J Immunol. 2002;169:1319–25. doi: 10.4049/jimmunol.169.3.1319. [DOI] [PubMed] [Google Scholar]
- 79.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4:1545–58. doi: 10.1016/s1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 80.Orange JS. Human natural killer cell deficiencies. Curr Opin Allergy Clin Immunol. 2006;6:399–409. doi: 10.1097/ACI.0b013e3280106b65. [DOI] [PubMed] [Google Scholar]
- 81.Henter JI, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124–31. doi: 10.1002/pbc.21039. [DOI] [PubMed] [Google Scholar]
- 82.Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. doi: 10.1146/annurev.immunol.17.1.189. [DOI] [PubMed] [Google Scholar]
- 83.Nedvetzki S, et al. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood. 2007;109:3776–85. doi: 10.1182/blood-2006-10-052977. Important demonstration of the ability of NK cells to form lytic immunological synapses with activated macrophages thus having clear impications to the pathogenesis of HLH. [DOI] [PubMed] [Google Scholar]
- 84.Stepp SE, et al. Perforin gene defects in familial hemophagocytic lymphohistiocytosis. Science. 1999;286:1957–9. doi: 10.1126/science.286.5446.1957. [DOI] [PubMed] [Google Scholar]
- 85.Marcenaro S, et al. Analysis of Natural killer cell function in familial hemophagocytic lymphohistiocytosis (FHL). Defective CD107a surface expression heralds Munc13-4 defect and discriminates between genetic subtypes of the disease. Blood. 2006;108:2316–23. doi: 10.1182/blood-2006-04-015693. [DOI] [PubMed] [Google Scholar]
- 86.Kishimoto TK, Hollander N, Roberts TM, Anderson DC, Springer TA. Heterogeneous mutations in the beta subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause leukocyte adhesion deficiency. Cell. 1987;50:193–202. doi: 10.1016/0092-8674(87)90215-7. [DOI] [PubMed] [Google Scholar]
- 87.Krensky AM, et al. Heritable lymphocyte function-associated antigen-1 deficiency: abnormalities of cytotoxicity and proliferation associated with abnormal expression of LFA-1. J Immunol. 1985;135:3102–8. [PubMed] [Google Scholar]
- 88.Kohl S, Springer TA, Schmalstieg FC, Loo LS, Anderson DC. Defective natural killer cytotoxicity and polymorphonuclear leukocyte antibody-dependent cellular cytotoxicity in patients with LFA-1/OKM-1 deficiency. J Immunol. 1984;133:2972–8. [PubMed] [Google Scholar]
- 89.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Orange JS, Stone KD, Turvey SE, Krzewski K. Biomedicine and disease: the wiskott-aldrich syndrome. Cell Mol Life Sci. 2004;61:2361–85. doi: 10.1007/s00018-004-4086-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gismondi A, et al. Impaired natural and CD16-mediated NK cell cytotoxicity in patients with WAS and XLT: ability of IL-2 to correct NK cell functional defect. Blood. 2004;104:436–43. doi: 10.1182/blood-2003-07-2621. [DOI] [PubMed] [Google Scholar]
- 92.Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA. A multiinstitutional survey of the Wiskott-Aldrich syndrome. J Pediatr. 1994;125:876–85. doi: 10.1016/s0022-3476(05)82002-5. [DOI] [PubMed] [Google Scholar]
- 93.Snover DC, Frizzera G, Spector BD, Perry GS, Kersey JH. Wiskott-Aldrich syndrome: histopathologic findings in the lymph nodes and spleens of 15 patients. Hum Pathol. 1981;12:821–31. doi: 10.1016/s0046-8177(81)80085-8. [DOI] [PubMed] [Google Scholar]
- 94.Pasic S, Micic D, Kuzmanovic M. Epstein-Barr virus-associated haemophagocytic lymphohistiocytosis in Wiskott-Aldrich syndrome. Acta Paediatr. 2003;92:859–61. doi: 10.1080/08035250310003631. [DOI] [PubMed] [Google Scholar]
- 95.Wang IJ, et al. Epstein-Barr virus associated post-transplantation lymphoproliferative disorder with hemophagocytosis in a child with Wiskott-Aldrich syndrome. Pediatr Blood Cancer. 2005;45:340–3. doi: 10.1002/pbc.20191. [DOI] [PubMed] [Google Scholar]
- 96.Huang W, Ochs HD, Dupont B, Vyas YM. The Wiskott-Aldrich syndrome protein regulates nuclear translocation of NFAT2 and NF-kappa B (RelA) independently of its role in filamentous actin polymerization and actin cytoskeletal rearrangement. J Immunol. 2005;174:2602–11. doi: 10.4049/jimmunol.174.5.2602. [DOI] [PubMed] [Google Scholar]
- 97.Introne W, Boissy RE, Gahl WA. Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol Genet Metab. 1999;68:283–303. doi: 10.1006/mgme.1999.2927. [DOI] [PubMed] [Google Scholar]
- 98.Roder JC, et al. Further studies of natural killer cell function in Chediak-Higashi patients. Immunology. 1982;46:555–60. [PMC free article] [PubMed] [Google Scholar]
- 99.Katz P, Zaytoun AM, Fauci AS. Deficiency of active natural killer cells in the Chediak-Higashi syndrome. Localization of the defect using a single cell cytotoxicity assay. J Clin Invest. 1982;69:1231–8. doi: 10.1172/JCI110562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Haliotis T, et al. Chediak-Higashi gene in humans I. Impairment of natural-killer function. J Exp Med. 1980;151:1039–48. doi: 10.1084/jem.151.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Targan SR, Oseas R. The “lazy” NK cells of Chediak-Higashi syndrome. J Immunol. 1983;130:2671–4. [PubMed] [Google Scholar]
- 102.Klein M, et al. Chediak-Higashi gene in humans. II. The selectivity of the defect in natural-killer and antibody-dependent cell-mediated cytotoxicity function. J Exp Med. 1980;151:1049–58. doi: 10.1084/jem.151.5.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Abo T, Roder JC, Abo W, Cooper MD, Balch CM. Natural killer (HNK-1+) cells in Chediak-Higashi patients are present in normal numbers but are abnormal in function and morphology. J Clin Invest. 1982;70:193–7. doi: 10.1172/JCI110592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stinchcombe JC, Page LJ, Griffiths GM. Secretory lysosome biogenesis in cytotoxic T lymphocytes from normal and Chediak Higashi syndrome patients. Traffic. 2000;1:435–44. doi: 10.1034/j.1600-0854.2000.010508.x. [DOI] [PubMed] [Google Scholar]
- 105.Huizing M, Anikster Y, Gahl WA. Hermansky-Pudlak syndrome and Chediak-Higashi syndrome: disorders of vesicle formation and trafficking. Thromb Haemost. 2001;86:233–45. [PubMed] [Google Scholar]
- 106.Fontana S, et al. Innate immunity defects in Hermansky-Pudlak type 2 syndrome. Blood. 2006;107:4857–64. doi: 10.1182/blood-2005-11-4398. [DOI] [PubMed] [Google Scholar]
- 107.Enders A, et al. Lethal hemophagocytic lymphohistiocytosis in Hermansky-Pudlak syndrome type II. Blood. 2006;108:81–7. doi: 10.1182/blood-2005-11-4413. [DOI] [PubMed] [Google Scholar]
- 108.Clark RH, et al. Adaptor protein 3-dependent microtubule-mediated movement of lytic granules to the immunological synapse. Nat Immunol. 2003;4:1111–20. doi: 10.1038/ni1000. [DOI] [PubMed] [Google Scholar]
- 109.Menasche G, et al. Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat Genet. 2000;25:173–6. doi: 10.1038/76024. [DOI] [PubMed] [Google Scholar]
- 110.Klein C, et al. Partial albinism with immunodeficiency (Griscelli syndrome) J Pediatr. 1994;125:886–95. doi: 10.1016/s0022-3476(05)82003-7. [DOI] [PubMed] [Google Scholar]
- 111.Plebani A, Ciravegna B, Ponte M, Mingari MC, Moretta L. Interleukin-2 mediated restoration of natural killer cell function in a patient with Griscelli syndrome. Eur J Pediatr. 2000;159:713–4. doi: 10.1007/s004310000501. [DOI] [PubMed] [Google Scholar]
- 112.Barral DC, et al. Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome. J Clin Invest. 2002;110:247–57. doi: 10.1172/JCI15058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Feldmann J, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–73. doi: 10.1016/s0092-8674(03)00855-9. Describes the critical role of Munc13-4 in priming lytic granules for membrane fusion in CTL and NK cells and defines the association of naturally occurring human mutations with HLH. [DOI] [PubMed] [Google Scholar]
- 114.zur Stadt U, et al. Linkage of familial hemophagocytic lymphohistiocytosis (FHL) type-4 to chromosome 6q24 and identification of mutations in syntaxin 11. Hum Mol Genet. 2005;14:827–34. doi: 10.1093/hmg/ddi076. [DOI] [PubMed] [Google Scholar]
- 115.Zur Stadt U, et al. Mutation spectrum in children with primary hemophagocytic lymphohistiocytosis: molecular and functional analyses of PRF1, UNC13D, STX11, and RAB27A. Hum Mutat. 2006;27:62–8. doi: 10.1002/humu.20274. [DOI] [PubMed] [Google Scholar]
- 116.Blanca IR, Bere EW, Young HA, Ortaldo JR. Human B cell activation by autologous NK cells is regulated by CD40-CD40 ligand interaction: role of memory B cells and CD5+ B cells. J Immunol. 2001;167:6132–9. doi: 10.4049/jimmunol.167.11.6132. [DOI] [PubMed] [Google Scholar]
- 117.Zingoni A, et al. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol. 2004;173:3716–24. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]
- 118.Bajenoff M, et al. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J Exp Med. 2006;203:619–31. doi: 10.1084/jem.20051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Borg C, et al. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104:3267–75. doi: 10.1182/blood-2004-01-0380. [DOI] [PubMed] [Google Scholar]
- 120.Carlin LM, Eleme K, McCann FE, Davis DM. Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. J Exp Med. 2001;194:1507–17. doi: 10.1084/jem.194.10.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tabiasco J, et al. Active trans-synaptic capture of membrane fragments by natural killer cells. Eur J Immunol. 2002;32:1502–8. doi: 10.1002/1521-4141(200205)32:5<1502::AID-IMMU1502>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 122.Vanherberghen B, et al. Human and murine inhibitory natural killer cell receptors transfer from natural killer cells to target cells. Proc Natl Acad Sci U S A. 2004;101:16873–8. doi: 10.1073/pnas.0406240101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roda-Navarro P, Vales-Gomez M, Chisholm SE, Reyburn HT. Transfer of NKG2D and MICB at the cytotoxic NK cell immune synapse correlates with a reduction in NK cell cytotoxic function. Proc Natl Acad Sci U S A. 2006;103:11258–63. doi: 10.1073/pnas.0600721103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Williams GS, et al. Membranous structures transfer cell surface proteins across NK cell immune synapses. Traffic. 2007;8:1190–204. doi: 10.1111/j.1600-0854.2007.00603.x. [DOI] [PubMed] [Google Scholar]
- 125.Roda-Navarro P, Reyburn HT. Intercellular protein transfer at the NK cell immune synapse: mechanisms and physiological significance. Faseb J. 2007;21:1636–46. doi: 10.1096/fj.06-7488rev. [DOI] [PubMed] [Google Scholar]
- 126.Chang JT, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–91. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 127.Hanna J, et al. Novel APC-like properties of human NK cells directly regulate T cell activation. J Clin Invest. 2004;114:1612–23. doi: 10.1172/JCI22787. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 128.Bossi G, Griffiths GM. CTL secretory lysosomes: biogenesis and secretion of a harmful organelle. Semin Immunol. 2005;17:87–94. doi: 10.1016/j.smim.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 129.Reczek D, et al. LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell. 2007;131:770–83. doi: 10.1016/j.cell.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 130.Zarcone D, et al. Ultrastructural analysis of human natural killer cell activation. Blood. 1987;69:1725–36. [PubMed] [Google Scholar]
- 131.Faure M, Barber DF, Takahashi SM, Jin T, Long EO. Spontaneous clustering and tyrosine phosphorylation of NK cell inhibitory receptor induced by ligand binding. J Immunol. 2003;170:6107–14. doi: 10.4049/jimmunol.170.12.6107. [DOI] [PubMed] [Google Scholar]
- 132.Fassett MS, Davis DM, Valter MM, Cohen GB, Strominger JL. Signaling at the inhibitory natural killer cell immune synapse regulates lipid raft polarization but not class I MHC clustering. Proc Natl Acad Sci U S A. 2001;98:14547–52. doi: 10.1073/pnas.211563598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sanni TB, Masilamani M, Kabat J, Coligan JE, Borrego F. Exclusion of lipid rafts and decreased mobility of CD94/NKG2A receptors at the inhibitory NK cell synapse. Mol Biol Cell. 2004;15:3210–23. doi: 10.1091/mbc.E03-11-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vyas YM, Maniar H, Lyddane CE, Sadelain M, Dupont B. Ligand binding to inhibitory killer cell Ig-like receptors induce colocalization with Src homology domain 2-containing protein tyrosine phosphatase 1 and interruption of ongoing activation signals. J Immunol. 2004;173:1571–8. doi: 10.4049/jimmunol.173.3.1571. [DOI] [PubMed] [Google Scholar]
- 135.Watzl C, Long EO. Natural killer cell inhibitory receptors block actin cytoskeleton-dependent recruitment of 2B4 (CD244) to lipid rafts. J Exp Med. 2003;197:77–85. doi: 10.1084/jem.20020427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stebbins CC, et al. Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol Cell Biol. 2003;23:6291–9. doi: 10.1128/MCB.23.17.6291-6299.2003. One of a number of important references defining mechanisms by which the inhibitory NK cell immunological synapse can interfere with steps in the progression through the lytic NK cell synapse (see also #23 and #24) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplementary information S1 (Figure). Interactive version of a prototypical mature NK cell lytic synapse. The mature NK cell lytic synapse in Figure 1 is provided in an interactive format. The image shows a human NK cell (YTS) expressing CD2-GFP (green fluorescent protein) conjugated to an EBV-transformed B cell (721.221). NK cells were conjugated to 721.221 cells, fixed, permeabilized and stained with a perforin-specific monoclonal antibody (red) to visualize the lytic granules. Serial images were obtained using a confocal microscope through the z-axis and the three-dimensional cell volume reconstructed in silico and QTVR output generated with complete rotation around the z-axis generated using Improvision Volocity visualization software. The accumulated GFP fluorescence indicates the supramolecular activation cluster (SMAC) formed at the synpase between the NK cell and the target cell. Notably, a region in the centre of the SMAC that is devoid of GFP is consistent with the presence of a cSMAC and delineates a channel through which the secretion of lytic granule contents is likely to occur. Although there is an accumulation of perforin internal to the SMAC (consistent with the lytic granules surrounding the microtubule-organizing centre (MTOC)), perforin can be seen coming from this region to the cSMAC. This is consistent with the secretory domain of the cytolytic immunological synapse.