Abstract
New cells, including neurons, arise in several brain regions during puberty in rats. Sex differences in pubertal addition of cells coincide with adult sexual dimorphisms: for each region, the sex that gains more cells during puberty has a larger volume in adulthood. Removing gonadal hormones before puberty eliminates these sex differences, indicating that gonadal steroids direct the addition of new cells during puberty to maintain and accentuate sexual dimorphisms in the adult brain.
Keywords: sexual differentiation, sexual dimorphism, postnatal neurogenesis, puberty, gonadal hormones, AVPV, SDN, amygdala
The establishment of structural sexual dimorphisms in the nervous system leads to life-long sex differences in physiology, learning and memory, social interactions, responses to stress and injury, and risk for disease1. Sexual differentiation of the nervous system is primarily attributable to actions of testosterone and its metabolites early in development2. It has been presumed that once established perinatally, sexual dimorphisms in cell number are passively maintained throughout life. Here we challenge this view by providing evidence that sexual dimorphisms are actively maintained, and that pubertal hormones contribute to the postnatal preservation of sexual dimorphisms via sex-specific modulation of new cells added to sexually dimorphic brain regions.
Male and female rats received injections of the cell birthdate marker bromo-deoxyuridine (BrdU) on three consecutive days at one of three postnatal ages corresponding to pre-puberty, early puberty, and mid-puberty (Supplementary Methods online). Brains were collected twenty days later, when all subjects were young adults. We analyzed BrdU-immunoreactive cells in three sexually dimorphic brain nuclei: the anteroventral periventricular nucleus of the hypothalamus (AVPV), the sexually dimorphic nucleus of the preoptic area (SDN), and the medial amygdala (Me) (Fig. 1). The AVPV is larger in females, while the SDN and Me are larger in males. BrdU-labeled cells in these regions were frequently observed as closely apposed pairs, indicating that they may have divided in situ, as suggested by Kokoeva et al3. Quantification of BrdU-labeled cells revealed sex differences independent of the age at which BrdU was administered. The number of AVPV BrdU-labeled cells was higher in females than in males (Fig. 1a; F1,27=32.69, p<0.0001). In contrast, the number of BrdU-labeled cells in SDN (Fig. 1b; F1,27=27.45, p<0.0001) and Me (Fig 1c; F1,30=28.59, p<0.0001) was higher in males than in females. These sex differences in BrdU-labeled cell number correlated with sex differences in regional volume determined from analysis of Nissl-stained sections, i.e., AVPV volume was greater in females whereas SDN and Me volumes were greater in males (Supplemental Fig 1 online).
Figure 1.
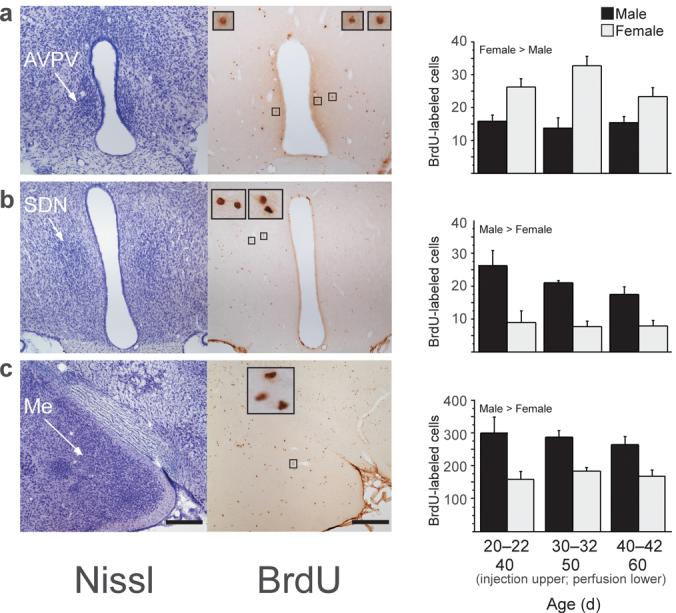
New cells are added during puberty to the AVPV (a), SDN (b), and Me (c) in male and female rats. Left photomicrographs are of thionin-stained sections and right photomicrographs are of BrdU-labeled cells in nearby sections from the same animal. Insets show BrdU-labeled cells framed in small boxes at 10x higher magnification. Subjects received a daily injection of 300 mg/kg BrdU on three consecutive days on either 20-22, 30-32, or 40-42 days of age (n=6-8/age and sex). BrdU is incorporated into DNA during the S phase of the cell cycle and can be later visualized to identify cells replicating at the time of BrdU administration. Brain tissue was collected 20 days after the first BrdU injection, on 40, 50, or 60 days of age, respectively. All protocols involving animals were approved by the Michigan State University Institutional Animal Care and Use Committee. Quantitative analyses of BrdU-labeled cells revealed that during puberty, significantly more cells were added to AVPV (a) in females than in males, while significantly more cells were added to SDN (b) and Me (c) in males than in females. Data are means ± SEM. Scale bars: 250 μm in lower magnification images.
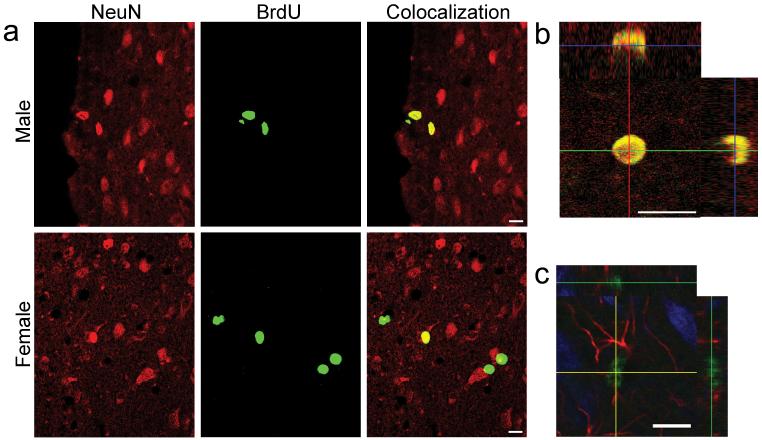
To determine the characteristics of BrdU-labeled cells, we labeled sections with BrdU and either neuron-specific nuclear protein (NeuN), a marker of mature neurons, or glial fibrillary acidic protein (GFAP), a marker of astrocytic glial cells, and assessed colocalization of proteins using confocal microscopy (Supplemental Methods online). Over half of the BrdU-labeled cells in the AVPV (Fig. 2a,b) also expressed NeuN, and none expressed GFAP. Approximately a quarter of BrdU-labeled cells in Me of both female and male rats also expressed NeuN, and an even greater number of BrdU-labeled cells in Me expressed GFAP (Fig 2c). BrdU-labeled cells in SDN expressed neither NeuN nor GFAP at detectable levels. In all of the regions, cells that did not colocalize NeuN or GFAP may be slated for apoptosis or for later differentiation in the event of functional demand. Alternatively, they may remain undifferentiated to support local neuronal or glial function in some manner.
Figure 2.
Many BrdU-labeled cells in the AVPV are mature neurons, not astrocytes. Many BrdU-labeled cells in Me are astrocytes; some are neurons. a: Confocal images of cells in male and female rat AVPV. Sections were processed for double-label BrdU (green) and the mature neuron marker NeuN (red); colocalization is yellow. b: Orthogonal views of confocal images verify colocalization of BrdU and NeuN in the female AVPV of a neuron that was born on P30-32. c: Confocal image of cells in rat Me. Section was processed for triple-label BrdU (green), NeuN (blue), and the astrocytic glial marker GFAP (red). Orthogonal views verify colocalization of BrdU, which is in the nucleus, and GFAP, which is in astrocytic processes. Scale bars: 10 μm.
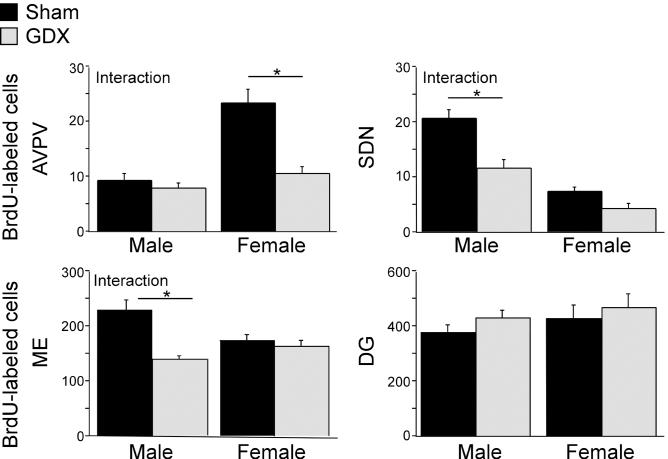
To assess whether pubertal hormones modulate cell addition to sexually dimorphic brain regions, we gonadectomized male and female rats before puberty, injected them with BrdU for three consecutive days during early puberty, and collected brains three weeks later (Supplemental Methods online). Gonadectomy interacted with sex to influence the number of BrdU-labeled cells in all three sexually dimorphic regions of interest. In the female-biased AVPV, prepubertal gonadectomy decreased BrdU-labeled cells in females but not males (Fig. 3; F1,24=12.90, p<0.0015, post hoc Fisher tests between groups). Prepubertal gonadectomy also resulted in a significant decrease in adult AVPV volume and neuron number only in females [Supplemental Fig 2 online]. Conversely, in the male-biased SDN and Me, gonadectomy decreased BrdU-labeled cells in males but not females (Fig 3; SDN F1,26=5.13, p<0.032; Me F1,27=10.24, p<0.004). The effect of male gonadectomy on SDN and Me BrdU-labeled cells was paralleled by a significant decrease in volume of the adult SDN, but not the Me (Supplemental Fig. 2 online). In contrast, neither sex (F1,26=1.12, p=0.3) nor prepubertal gonadectomy (F1,26=1.39, p=0.25) affected the number of BrdU-labeled cells in the dentate gyrus (Fig 3), which is not overtly larger in either sex. Gonadal steroids may influence the pubertal addition of new cells and maintenance of sex differences by brain region-specific modulation of either cell proliferation and differentiation4-6 or survival7.
Figure 3.
The effect of prepubertal gonadectomy (GDX) on the number of BrdU-labeled cells depends on sex and brain region. Male and female rats were gonadectomized or sham gonadectomized at 20 days of age (n=8/sex and treatment). A daily injection of BrdU was given on 30-32 days of age and brain tissue was collected at 50 days of age. Prepubertal GDX significantly decreased the number of BrdU-labeled cells in female but not male AVPV (interaction between sex and treatment). Prepubertal GDX decreased the number of BrdU-labeled cells in male but not female SDN and Me (interaction between sex and treatment). Prepubertal GDX did not affect BrdU-labeled cells in the dentate gyrus (DG) of either males or females. Data are presented as means ± SEM. Asterisks indicate p<0.05 (post hoc Fisher test between groups).
These data provide strong evidence that sex-biased, hormone-modulated, and brain region-specific addition of new cells is an active mechanism for maintaining, or in some cases, establishing, structural and functional sexual dimorphisms in the face of brain remodeling during adolescence8. This mechanism may be widespread, as both the volume and number of cells increase during puberty in the female-biased rat locus coeruleus9 and the male-biased human bed nucleus of the stria terminalis10. Addition of new cells may also be related to functional sex differences that emerge during puberty. For example, the rat AVPV controls the preovulatory surge of luteinizing hormone11, an estrogen-dependent event that emerges during puberty only in females in parallel with development of sex differences in AVPV volume12-13. Likewise, sex differences in structure and function of the human amygdala and in several mental illnesses, such as eating disorders, depression, and schizophrenia, arise during adolescent development14-15. An intriguing question remains whether sex-biased addition and survival of new cells in sexually dimorphic brain regions continues beyond the pubertal period. If so, this could contribute to a dynamic maintenance of functional sexual dimorphisms throughout adulthood to preserve the potential for adaptation to new experiences and functional demands (e.g., pregnancy, parental behavior). In summary, new cells are added to the rat brain during puberty, and sex differences in the number of newly added cells correspond to sex differences in adult volume in each region. Furthermore, pubertal gonadal hormones influence the addition of new cells, presumably by promoting cell genesis and/or survival in sexually dimorphic structures in a sex-specific manner. Thus, hormone-modulated addition of cells to sexually dimorphic neural structures after the perinatal period is an active mechanism for maintaining functional sex differences during adolescence and into adulthood.
Supplementary Material
Acknowledgments
The technical support of Jane Venier and Lisa Rogers is gratefully acknowledged. We thank Dr. Melissa Holmes for her technical and intellectual insight during protocol development. This work was supported by R01 MH-068764 (CLS), F32 MH-068975 (JLZ), F31 MH-070125 (KMS), the Michigan State University Foundation (CLS, EIA), R01 MH-062588 (LLDC), and the Schmitt Foundation (BHL).
References
- 1.Cahill L. Nat. Rev. Neuroscience. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 2.De Vries GJ, Simerly RB. In: Hormones, Brain, and Behavior. Pfaff DW, Etgen AM, Fahrbach SE, Rubin RT, editors. Academic Press; San Diego: 2002. pp. 137–191. [Google Scholar]
- 3.Kokoeva MV, Yin H, Flier JS. J. Comp. Neurol. 2007;505:209–220. doi: 10.1002/cne.21492. [DOI] [PubMed] [Google Scholar]
- 4.Fowler CD, Johnson F, Wang Z. J. Comp. Neurol. 2005;489:166–179. doi: 10.1002/cne.20638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanapat P, Hastings NB, Reeves AJ, Gould E. J. Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galea LAM. Brain Res. Rev. 2008;57:332–341. doi: 10.1016/j.brainresrev.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Forger NG. Neuroscience. 2006;138:929–938. doi: 10.1016/j.neuroscience.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Sisk CL, Zehr JL. Frontiers in Neuroendocrinology. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Pinos H, et al. Brain Res. Bull. 2001;56:73–78. doi: 10.1016/s0361-9230(01)00540-8. [DOI] [PubMed] [Google Scholar]
- 10.Chung WCJ, De Vries GJ, Swaab DF. J. Neurosci. 2002;22:1027–1033. doi: 10.1523/JNEUROSCI.22-03-01027.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen SL, Ottem EN, Carpenter CD. Biol. Reprod. 2003;69:1771–1778. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman GE, Le WW, Schulterbrandt T, Legan SJ. Brain Res. 2005;1054:116–124. doi: 10.1016/j.brainres.2005.06.082. [DOI] [PubMed] [Google Scholar]
- 13.Davis EC, Shryne JE, Gorski RA. Neuroendocrinology. 1996;63:142–148. doi: 10.1159/000126950. [DOI] [PubMed] [Google Scholar]
- 14.Lenroot RK, Giedd JN. Neurosci. Biobehav. Rev. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Killgore WDS, Yurgelun-Todd DA. Perceptual and Motor Skills. 2004;99:371–391. doi: 10.2466/pms.99.2.371-391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.