Abstract
Background
d-limonene is an unsaturated volatile organic chemical found in cleaning products, air fresheners and soaps. It is oxidized by ozone to secondary organic aerosols consisting of aldehydes, acids, oxidants and fine and ultra fine particles. The lung irritant effects of these limonene ozone reaction products (LOP) were investigated.
Methods
Female F344 rats (2 mo and 18 mo) were exposed for 3 h to air or LOP formed by reacting 6 ppm d-limonene and 0.8 ppm ozone. BAL fluid, lung tissue and cells were analyzed 0 h and 20 h later.
Results
Inhalation of LOP increased TNF-α, cycloxygenase-2, and superoxide dismutase in alveolar macrophages (AM) and Type II cells. Responses of older animals were attenuated when compared to younger animals. LOP also decreased p38 MAP kinase in AM from both younger and older animals. In contrast, while LOP increased p44/42 MAP kinase in AM from younger rats, expression decreased in AM and Type II cells from older animals. NF-κB and C/EBP activity also increased in AM from younger animals following LOP exposure but decreased or was unaffected in Type II cells. Whereas in younger animals LOP caused endothelial cell hypertrophy, perivascular and pleural edema and thickening of alveolar septal walls, in lungs from older animals, patchy accumulation of fluid within septal walls in alveolar sacs and subtle pleural edema were noted.
Conclusions
LOP are pulmonary irritants inducing distinct inflammatory responses in younger and older animals. This may contribute to the differential sensitivity of these populations to pulmonary irritants.
Keywords: LOP, lung, aging, PM, TNF-α, SOD, MAP kinase, transcription factor
Introduction
Epidemiological studies indicate that the elderly (≥ 65 yr old) are generally more susceptible to the health effects of air pollution (Donaldson et al., 2001; Pope et al., 2002; USEPA, 2004). Air pollutants found indoors are of particular concern since on average people spend 85-90% of their time indoors (USEPA, 1996), and this may be especially important among older individuals. Many household products are sources of volatile organic compounds (VOCs), an important class of air pollutants that are typically found in concentrations several fold higher indoors when compared to outdoors (Wallace, 1991). It has been suggested that the elderly may have a higher exposure to VOCs due to more frequent cleaning activities in their residences, including nursing homes (USEPA, 1996). Unsaturated VOCs, such as the terpenes, limonene and pinene, are found in many common household products including cleansers and air fresheners. Ozone, infiltrating from outdoors or generated directly indoors by electrostatic air cleaners, printers and photocopiers, reacts rapidly with terpenes to form secondary organic aerosols (SOA) containing formaldehyde, free radical species and fine and ultrafine organic particles (Wainman et al., 2000; Weschler, 2000). The health effects of exposure to SOA are unclear. Whereas, we found that short-term exposure of young healthy volunteers to SOA generated by the reaction of ozone with a mixture of VOCs including terpenes caused no changes in lung function or nasal irritation/inflammation (Laumbach et al., 2005), Wolkoff and colleagues (Wolkoff et al., 2000) reported respiratory and mucus membrane irritation from terpene-ozone SOA. The health effects of SOA have not been investigated in elderly humans or animals.
Following inhalation of smog or nitrogen dioxide, greater structural injury is observed in the lungs of older when compared to younger animals (Bils et al., 1967; Cabral-Anderson et al., 1977; Bowes et al., 1989; van Bree et al., 2000). Older animals are also more sensitive to the pulmonary and systemic effects of various types of inhaled gases and particulate matter (PM) (Elder et al., 2000; Oberdorster et al., 2000; Zelikoff et al., 2003; Elder et al., 2004; Nadziejko et al., 2004; Tankersley et al., 2004; Kasagi et al., 2006). Although pre-existing diseases may be important in the increased sensitivity of the elderly to inhaled air pollutants, specific mechanisms underlying their enhanced responsiveness are unknown. AM represent the first line of cellular defense in the lung. These cells play an important role in the inflammatory response to inhaled substances, and their function has been reported to be altered in the elderly (Plackett et al., 2004; Gomez et al., 2005). Antioxidant defenses are also known to be impaired in the elderly (Heffner, 1991; Nel et al., 1998; Dhalla et al., 2000; Lang et al., 2002). With increasing age, levels of antioxidants and the capacity of oxidative injury to stimulate release of antioxidants declines (Lykkesfeldt et al., 1999; Squier, 2001; Thomas et al., 2001; Servais et al., 2005). Thus alterations in macrophage inflammatory activity and antioxidant defense may be important factors contributing to age-related increases in the toxicity of inhaled PM and this was investigated in the present studies. We found that inhalation of SOA containing LOP by younger adult rats resulted in increased expression of the inflammatory proteins, tumor necrosis factor-alpha (TNFα) and cyclooxygenase-2 (COX-2), as well as the antioxidant enzyme, Cu/Zn superoxide anion dismutase (SOD) in the lung. This was associated with activation of the p44/42 MAP kinase signaling pathway and transcription factors, NF-κB and C/EBP in macrophages. Although similar responses were also observed in elderly rats exposed to LOP, they were significantly attenuated. These data demonstrate that the response to LOP is age dependent. Age related differences in the sensitivity to inhaled LOP may be due to impaired activity of signaling molecules and transcription factors that regulate macrophage activity and antioxidant defenses in the lung.
Methods
Aerosol generation and characterization
The aerosol generation and characterization system is shown in Fig. 1. Organic aerosols were formed by gas-phase reaction of d-limonene and ozone in a Teflon flow tube reactor. Animals were exposed to the resulting aerosol in a Plexiglas whole body exposure chamber immediately downstream (W 20.3 cm × L 41cm × H 17.8 cm). Ozone (0.8 ppm) was generated from oxygen gas via an ultraviolet light generator (Orec Corp., Phoenix, AZ). d-limonene vapor was generated by bubbling compressed purified (charcoal and HEPA filtered) air through a 250 ml glass reservoir containing liquid d-limonene (>97%, Sigma-Aldrich, Allentown, PA). The d-limonene concentration was adjusted to 6 ppm by dilution with partially humidified air (dry mixed with humidified air; membrane humidifier; Perma Pure Inc., Toms River, NJ). Ozone and d-limonene were mixed in the flow tube reactor and then introduced into the chamber. Particle number size distribution, ozone concentration, temperature and relative humidity (RH) were measured continuously (1 min resolution) in the exposure chamber (ozone = 0.79 ± 0.05 ppm; temperature = 25.5 ± 1.9 °C; RH = 21.4 ± 5.3 %; SOA = 2242 ± 183.1 μg/m3). Integrated particle mass measurements were also performed. The temperature remained stable over the exposure period (data not shown). RH was largely dependent on animal activity. Thus RH was high while the animals were active and lower when animals were sleeping. Exposures were conducted between 10-30% RH and 22-28 °C (RH/Temp data logger: HOHO Pro, Onset, Burne, MA; monitor: model Testo 625, Hotek Technologies, Tacoma, WA).
Figure 1.
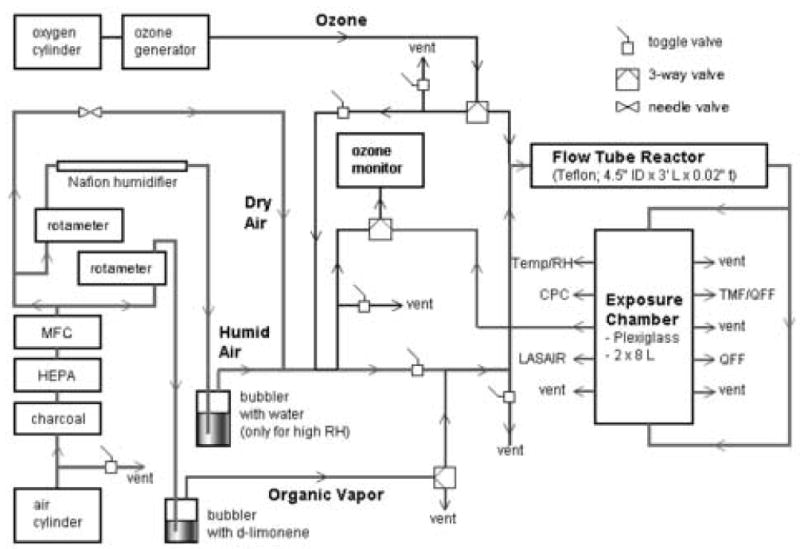
Flow diagram of aerosol generation and animal exposure system. Mixture of ozone and d-limonene generates a secondary organic aerosol at fixed relative humidity (RH) in the flow tube reactor. Particle size, mass, number, ozone concentration, temperature and RH were monitored in the exposure chamber.
The mean residual ozone in the exposure chamber was 0.062 ppm (photometric ozone analyzer, model 1008-AH, Dasabi Environmental Corp, Glendale, CA). Residual d-limonene was not measured. Assuming the reaction of d-limonene and ozone is pseudo-first order (d-limonene levels were much higher than ozone levels), and using the reactor residence time and the rate constant for d-limonene oxidation by ozone (5.1×10-6 ppb-1 s-1 at 23 °C) (Atkinson et al., 1990) the predicted ozone concentration at the exposure chamber entrance is 0.046 ppm. This is comparable to the measured concentration of 0.062 ppm. Applying a comparable loss rate for d-limonene, 5.2 ppm d-limonene is predicted at the entrance of the exposure chamber.
Particle number concentrations in the exposure chamber increased immediately after the initiation of the reaction and reached steady state within 10-30 min (TSI 7610 condensation particle counter, CPC, >0.01 μm in diameter, Shoreview, MN). As shown in Fig. 2, the organic aerosol size distribution contained both “ultrafine” and “fine” size modes. Ultrafine particles (0.01-0.1 μm in diameter) dominated the particle number distribution, whereas accumulation mode particles (0.1-1.0 μm in diameter) dominated the particle mass distribution (Optical particle counter: LASAIR 1002, Particle Measuring System, Boulder, CO, 8 size channels, 0.1-2.0 μm in diameter. Ultrafine particle concentrations were determined by the difference between the CPC and LASAIR. Dilution was used to bring concentrations within the operating range of the LASAIR and CPC).
Figure 2.
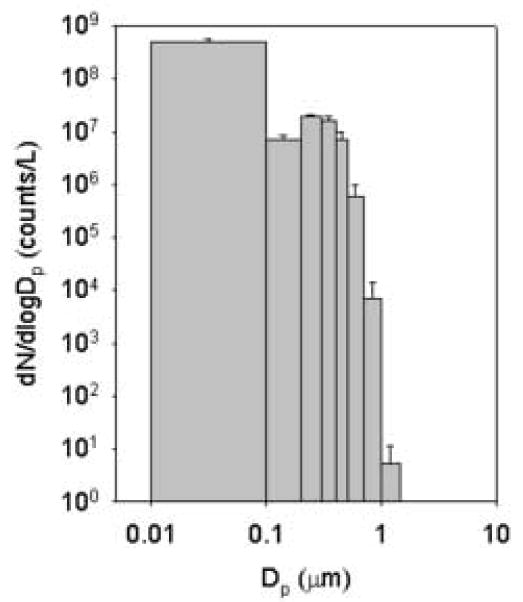
Particle number size distribution in the exposure chamber. Particle number from 0.1- 2 μm in diameter by LASAIR optical particle counter; particle number from 0.01-0.1 μm in diameter determined by difference between the condensation particle counter (CPC) and LASAIR. Each bar is the mean ± SD (n=13).
Animals
Female specific pathogen-free F344 rats (2 mo and 18 mo) were purchased from the National Institute of Aging (MD). Animals were housed in microisolator cages and maintained on sterile food and pyrogen-free water ad libitum. All animals received humane care in compliance with the institution's guidelines, as outlined in the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health. Animals were exposed to ultra-pure air (control) or LOP for 3 h and sacrificed immediately (0 h) or 20 h post exposure (PE). Four sets each of younger and older animals were used for each exposure.
Cell Isolation
Animals were euthanized by ip injection of Nembutal (250 mg/kg). The lungs were perfused with 50 ml of warm (37°C) HBSS containing 25 mM HEPES, 0.5 mM EGTA, and 4.4 mM NaHCO3, pH 7.3, followed by perfusion with Ca+2/Mg+2 -free HBSS containing 22 mM HEPES, 4.2 mM NaHCO3, pH 7.3 at a rate of 22 ml/min. AM were isolated from perfused lungs by bronchoalveolar lavage (BAL) 5-6 times with HBSS. Cells were washed 4 times with HBSS containing 2% FBS. Cell viability was 98% as determined by trypan blue dye exclusion, and cell purity greater than 97% as assessed morphologically after Giemsa staining. Type II alveolar epithelial cells were isolated from lavaged lungs as previously described (Sunil et al., 2002). Briefly, after washing twice with 10 ml of buffer (140 mM NaCl, 5 mM KCl, 2.5 mM Na2HPO4, 1.3 mM MgSO4.7H2O, 2 mM CaCl2,10 mM HEPES, pH 7.4), 30 ml of elastase (4.2 U/ml, Worthington Biochemicals, NJ) was infused into the lungs using a 30 cc syringe. The tissue was then incubated at 37°C for 20 min, the trachea and major bronchi removed, the lungs minced in the presence of 4 ml DNAse (1 μg/ml), and then digested for 5 min at 37°C with 10 ml of elastase. The reaction was stopped by the addition of 5 ml FBS. The tissue was then sequentially filtered through 220, 60 and 15 μm nylon mesh. Cells were collected, washed and purified by negative selection (1 h, 37°C) on IgG coated plates. Non-adherent cells were collected and washed with DMEM containing 10% FBS. Cell purity, assessed by modified Papinicolou staining, was 95% and viability, determined by trypan blue dye exclusion, was greater than 98%.
Measurement of BAL protein
Total protein was quantified in the BAL fluid recovered from the first lung lavage using the BCA Protein Assay kit (Pierce Biotechnologies Inc., Rockford, IL) with bovine serum albumin as the standard.
Immunohistochemistry
Lungs were perfused with buffer, removed, fixed in 3% paraformaldehyde in PBS for 4 h on ice, transferred to 50% ethanol and paraffin embedded. Tissue sections (6 μm) were deparaffinized and incubated overnight at 4°C with goat or rabbit IgG, anti-TNF-α (2 μg/ml), anti-COX-2 (2 μg/ml, Santa Cruz Biotechnology), or anti-Cu/Zn SOD (2.5 μg/ml, Stressgen Biotechnologies Inc., San Diego, CA) antibodies, followed by 30 min incubation with biotinylated secondary antibody (Vector Labs, Burlingame, CA). Binding was visualized using a Vectastain Elite ABC kit (Vector Labs). Some sections were stained with H & E to assess lung structure.
Western Blotting
Cytoplasmic extracts of cells were prepared using Nuclear and Cytoplasmic Extraction Reagents (NE-PER), (Pierce, Rockford, IL). Proteins (15-25 μg) were fractionated on 10%-12.5% SDS polyacrylamide gels and transferred to nitrocellulose membranes. Non-specific binding was blocked by incubation of the blot for 30 min at room temperature with 5% non-fat dry milk in Enhanced Chemi-Luminescence (ECL) buffer (Amersham Life Sciences, Arlington Heights, IL). Blots were incubated overnight at 4°C with antibodies to p38, p44/42 or JNK mitogen activated protein kinase (1:1000, Cell Signaling Technology, Beverly, MA) prepared in ECL buffer with 1% milk. After 4 washes in ECL buffer, blots were incubated with anti-rabbit horse radish peroxidase (HRP)-conjugated secondary antibody (1:2000) for 30 min at room temperature and the bands visualized using an ECL detection system (Amersham).
Electrophoretic Mobility Shift Assays (EMSA)
Assays were performed using nuclear extracts as described previously (Sunil et al., 2002) with some modifications. Binding reactions were carried out at room temperature for 20 min in a total volume of 10-15 μl containing 10 μg of nuclear extracts, 3-6 μl of 5× gel shift binding buffer (20% glycerol, 5mM MgCl2, 2.5 mM EDTA, 2.5 mM DTT, 250 mM NaCl, 50 mM Tris-HCl, pH 7.5), 2 μg poly dI-dC and 3 × 104 cpm/μl γ[32P]ATP (3000 Ci/mmol at 10 mCi/ml) labeled NF-κB (5′-AGT TGA GGG GAC TTT CCC AGG-3′), C/EBP (5′-TGC AGA TTG CGC AAT CTG CA-3′) or CREB (5′-AGA GAT TGC CTG ACG TCA GAG AGC TAG-3′) consensus oligonucleotide (Santa Cruz Biotechnology, CA). Protein-DNA complexes were separated on 5-7% non-denaturing polyacrylamide gels run at 250V in 0.5× TBE and visualized after the gels were dried and autographed. For competitor reactions, 50-fold excess of unlabeled NF-κB, C/EBP or CREB oligonucleotide was added to the mixture prior to the addition of the labeled probe.
Results
Effects of exposure to LOP on inflammatory mediator and antioxidant expression in the lung
In our first series of studies, we compared the effects of inhaled LOP on production of inflammatory mediators in the lungs of younger and older rats. Initially, we focused on the inflammatory protein TNF-α, and the enzyme COX-2, which catalyzes the production of prostaglandins. TNF-α and COX-2 were not detected in the lungs of air-exposed animals (Figs. 3 and 4). Inhalation of LOP caused a rapid increase in expression of both of these proteins in the lung which persisted for at least 20 h. This was evident in AM, as well as in Type II alveolar epithelial cells. LOP-induced expression of TNF-α and COX-2 was greater in younger when compared to older animals.
Figure 3.
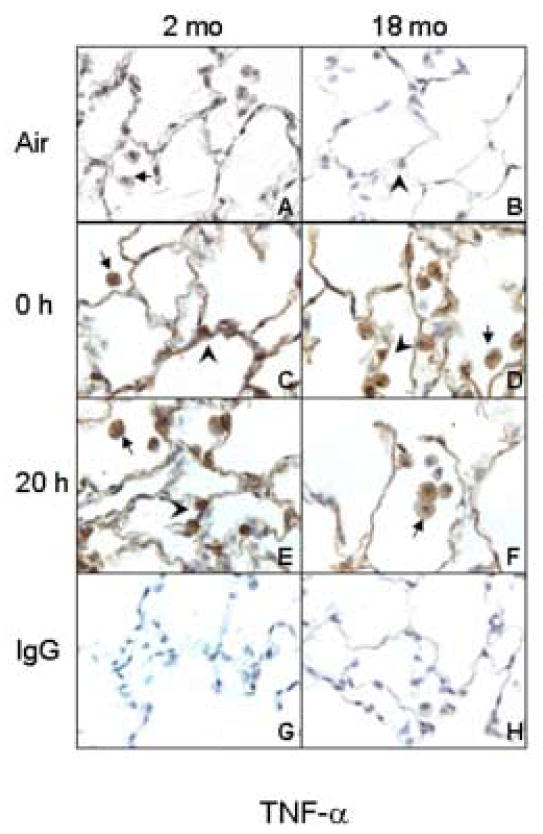
Effects of inhalation of LOP on TNF-α expression. Lung sections (6 μm) were prepared from younger (2 mo) and older (18 mo) rats immediately (0 h) and 20 h after exposure to air (panels A and B) or LOP (panels C-F). Sections were stained with antibody to TNF-α (panels A-F) or IgG (panels G and H). One of three similar experiments is presented. Brown staining is indicative of TNF-α expression. Arrows, alveolar macrophages; Arrowheads, Type II cells.
Figure 4.
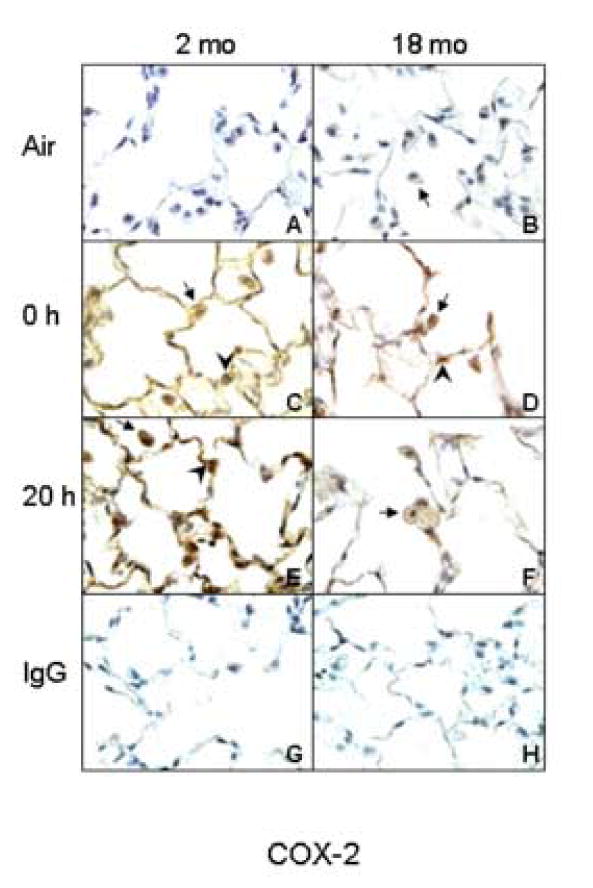
Effects of inhalation of LOP on COX-2 expression. Lung sections (6 μm) were prepared from younger (2 mo) and older (18 mo) rats immediately (0 h) and 20 h after exposure to air (panels A and B) or LOP (panels C-F). Sections were stained with antibody to COX-2 (panels A-F) or IgG (panels G and H). One of three similar experiments is presented. Brown staining is indicative of COX-2 expression. Arrows, alveolar macrophages; Arrowheads, Type II cells.
In the lung, Cu/Zn SOD is considered the first line of antioxidant defense (Kinnula et al., 2003; Rahman et al., 2006). As observed with TNF-α and COX-2, Cu/Zn SOD was not detected in lungs of air-exposed younger or older animals (Fig. 5). Exposure of rats to LOP caused a rapid and time-related increase in Cu/Zn SOD expression which was evident immediately after exposure. LOP-induced expression of Cu/Zn SOD was greater in younger when compared to older animals.
Figure 5.
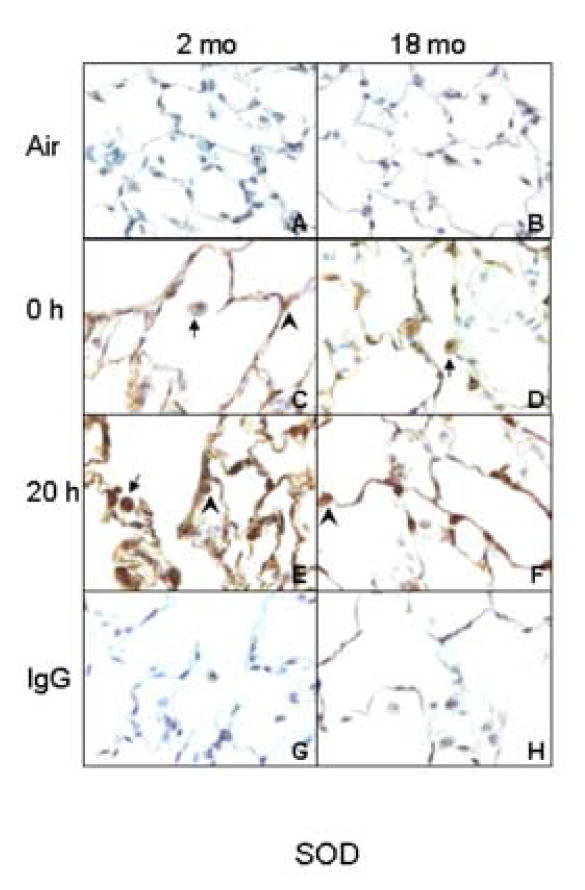
Effects of inhalation of LOP on Cu/Zn SOD expression. Lung sections (6 μm) were prepared from younger (2 mo) and older (18 mo) rats immediately (0 h) and 20 h after exposure to air (panels A and B) or LOP (panels C-F). Sections were stained with antibody to Cu/Zn SOD (panels A-F) or IgG (panels G and H). One of three similar experiments is presented. Brown staining is indicative of Cu/Zn SOD expression. Arrows, alveolar macrophages; Arrowheads, Type II cells.
Effects of exposure to LOP on MAP kinase expression in the lung
MAP kinases such as p38, p44/42 (ERK) and JNK are upstream regulators of inflammatory and antioxidant gene expression (Rahman, 2002; Lopez-Neblina et al., 2006). In our next series of experiments, we determined if LOP-induced increases in inflammatory proteins was associated with alterations in expression of MAP kinases. AM from both younger and older animals were found to constitutively express p38 MAP kinase protein (Fig. 6, top panel). Following inhalation of LOP, a rapid and transient decrease in p38 MAP kinase expression was observed, which returned to control levels by 20 h post exposure in both groups. p44/p42 MAP kinase proteins were also detected in AM from air-exposed younger and older animals (Fig. 6, top panel). Inhalation of LOP resulted in a delayed increase in p44/42 MAP kinase expression in AM from younger animals, which was evident at 20 h. In contrast, an immediate and persistent decrease in expression of this protein was noted in AM from older rats.
Figure 6.
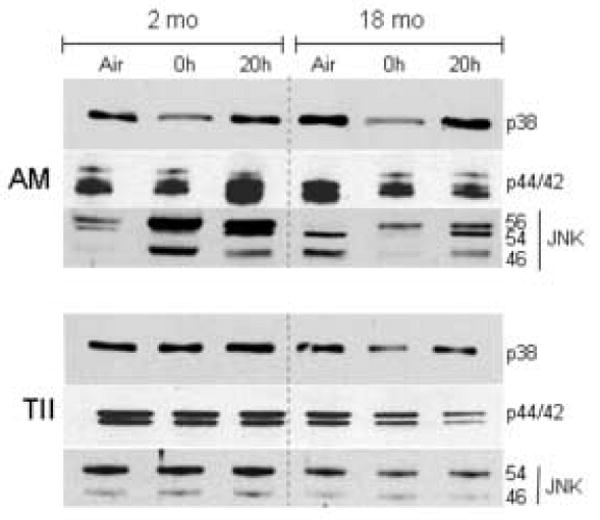
Effects of inhalation of LOP on MAP kinase expression in alveolar macrophages (AM) and Type II cells (TII). Cells were isolated from younger (2 mo) and older (18 mo) animals immediately (0 h) and 20 h after exposure to air or LOP. Cytoplasmic extracts were prepared and analyzed for p38, p44/42 and JNK MAP kinase expression by western blotting. One representative gel from three separate experiments is shown.
The JNK kinase antibody detects 3 proteins of molecular weight 56, 54 and 46 kDa which are activated by distinct stimuli (Chan et al., 1997). AM from younger animals were found to constitutively express the p56 and p54 isoforms of JNK, whereas cells from older rats constitutively expressed the p54 and p46 isoforms (Fig. 6, top panel). Treatment of younger animals with LOP caused a significant increase in expression of all three isoforms of JNK which was observed in AM collected immediately and 20 h post exposure. In older animals, LOP caused a rapid and persistent increase in the p56 JNK isoform, but a transient decrease in the p54 and p46 isoforms.
We also analyzed expression of MAP kinases in Type II alveolar epithelial cells isolated from air and LOP-exposed animals. As observed in AM, Type II cells from both younger and older animals exposed to air control constitutively expressed p38 and p44/42 MAP kinase proteins (Fig. 6, bottom panel). Although levels of p38 were unaffected by LOP exposure in the younger animals, a decrease in both p38 and p44/42 MAPK proteins was observed in cells from older rats. Interestingly, in contrast to AM, in Type II cells, only the p54 and p46 isoforms of JNK kinase were detected. Type II cells isolated from older rats constitutively expressed lower levels of p54 JNK (Fig. 6, bottom panel). While exposure to LOP did not significantly alter expression of p54 JNK in younger animals, a sustained decrease in expression was noted in Type II cells from older animals.
Effects of exposure to LOP on transcription factor activity
Inflammatory and anti-oxidant genes are regulated by transcription factors including NF-κB, C/EBP and CREB (Castranova, 2004; Rahman et al., 2005; Barnes, 2006; Park et al., 2006). We next compared the effects of LOP inhalation on transcription factor activity in AM and Type II cells from younger and older animals. Constitutive DNA binding activity of NF-κB, C/EBP and CREB was noted in AM from both younger and older animals (Fig. 7, left panels). Greater activity was detected in AM from older rats. While exposure to LOP caused a transient increase in NF-κB and C/EBP DNA binding activity in younger animals, there was no significant effect on CREB DNA binding activity. In older rats, exposure to LOP had no effect on the activity of any of these transcription factors in AM. Binding was decreased or eliminated by using 50-fold excess of the NF-κB, C/EBP or CREB unlabeled competitor oligonucleotide, demonstrating the specificity of the probe.
Figure 7.
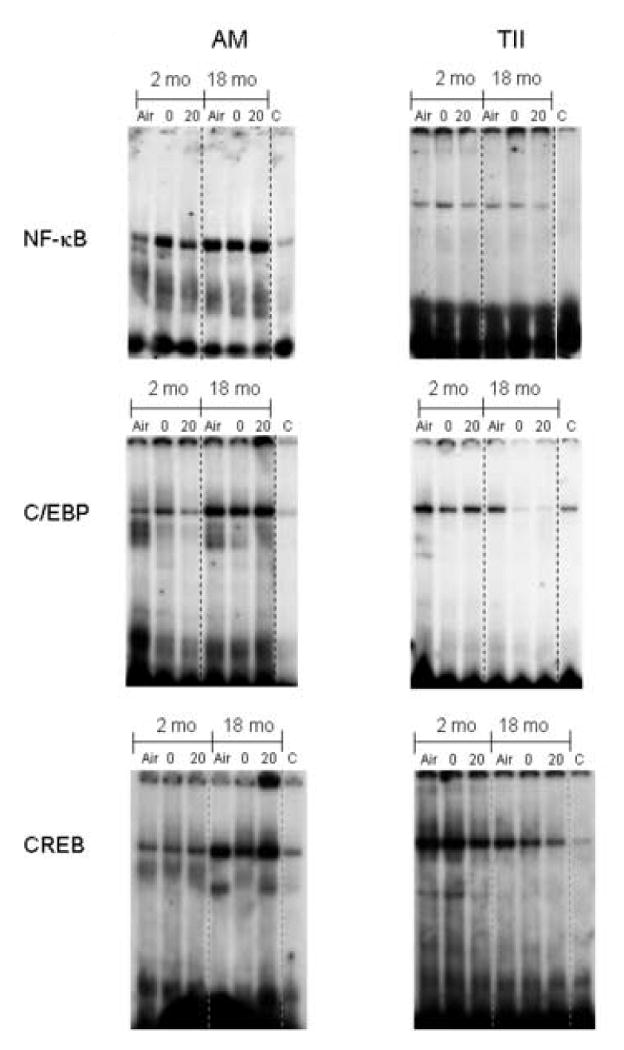
Effects of inhalation of LOP on NF-κB, C/EBP and CREB nuclear binding activity. Alveolar macrophages (AM) and Type II cells (TII) were isolated from younger (2 mo) and older (18 mo) rats immediately (0) and 20 h (20) after exposure to air or LOP. Nuclear extracts were prepared and analyzed for NF-κB, C/EBP and CREB binding activity by EMSA. For supershift analysis, extracts prepared immediately after exposure of older rats to LOP (18 mo/ 20 h) were pre-incubated with 50-fold excess of unlabeled specific competitor probes (C). One representative of three gels is shown.
Constitutive NF-κB, C/EBP and CREB DNA binding activity was also detected in Type II cells from both younger and older rats (Fig. 7, right panels). Exposure to LOP had no significant effect on NF-κB DNA binding activity in younger or older rats. In contrast to AM, Type II cells from younger animals exhibited greater C/EBP and CREB DNA binding activity than cells from older rats (Fig. 7). Whereas exposure to LOP had no major effect on C/EBP in younger animals, a significant decrease was noted in Type II cells from older rats. LOP also significantly decreased CREB DNA binding activity in Type II cells from both younger and older animals.
Effects of LOP on BAL protein and cell number
Protein accumulation in BAL fluid is an indication of altered epithelial cell permeability and a measure of injury in the alveolar regions of the lung (Bhalla, 1999). This is typically associated with increased numbers of inflammatory cells in the tissue. In further studies we determined if inhalation of LOP caused lung injury and inflammatory cell accumulation in the lung. Low levels of protein were detected in BAL fluid from both younger and older animals exposed to air control (Fig. 8, upper panel). Exposure of animals to LOP had no significant effect on BAL protein levels in either younger or older rats. Similarly, LOP had no major effect on the number or type of cells recovered in BAL fluid. Thus in both younger and older rats, greater than 97% of BAL cells were identified as AM and the number and percentage of these cells did not change after LOP exposure (Fig 8, middle panel and data not shown). In contrast to AM, significantly fewer Type II cells were recovered from the older animals when compared to the younger animals (Fig. 8, bottom panel). However, this was unaffected by exposure to LOP.
Figure 8.
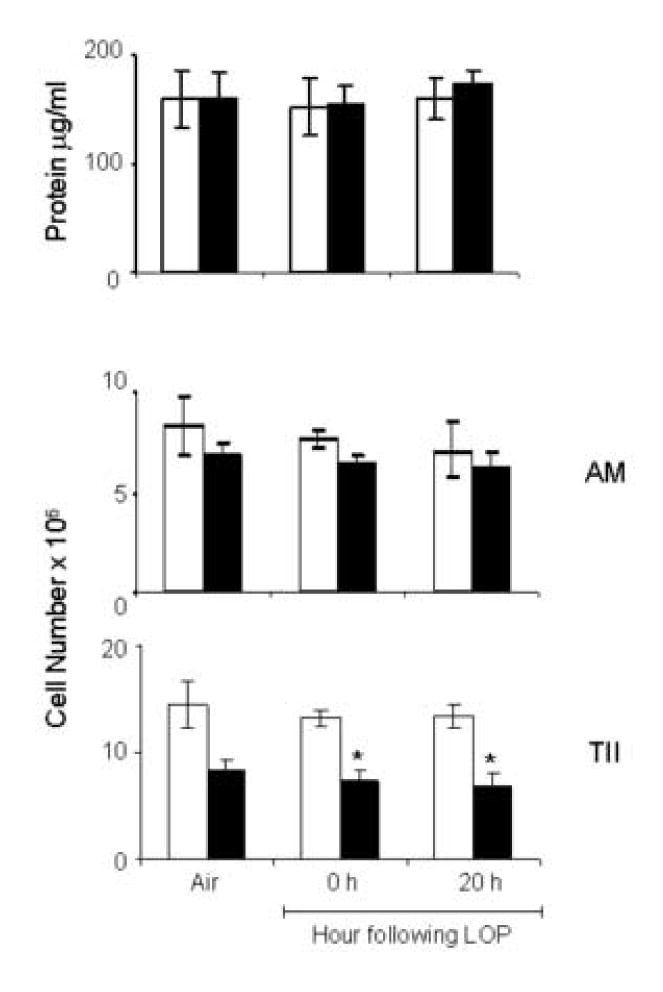
Effects of LOP on BAL protein and cells. BAL fluid was collected immediately (0 h) or 20 h after exposure of younger (open bars) and older (closed bars) animals to air or LOP. Upper panel: Protein in BAL. Each bar is the average ± SEM of triplicate samples from three groups of animals (n=3/group). Lower panel: Number of viable alveolar macrophages (AM) and Type II alveolar cells (TII) recovered from the lung. Each bar is the average ± SE (n=3). *Significantly different (p<0.05), from younger animals (One way ANOVA).
We also analyzed lung sections histologically after LOP exposure. Although no major differences were noted in lung histology between air-exposed younger and older rats (Fig. 9), the response of the rats to LOP was distinct. Thus, while in younger animals, exposure to LOP caused endothelial cell hypertrophy, increased perivascular and pleural edema, and thickening of alveolar septal walls (Fig 9, left panel), in older animals, patchy accumulation of fluid within septal walls in alveolar sacs and subtle pleural edema was noted (Fig. 9, right panel).
Figure 9.
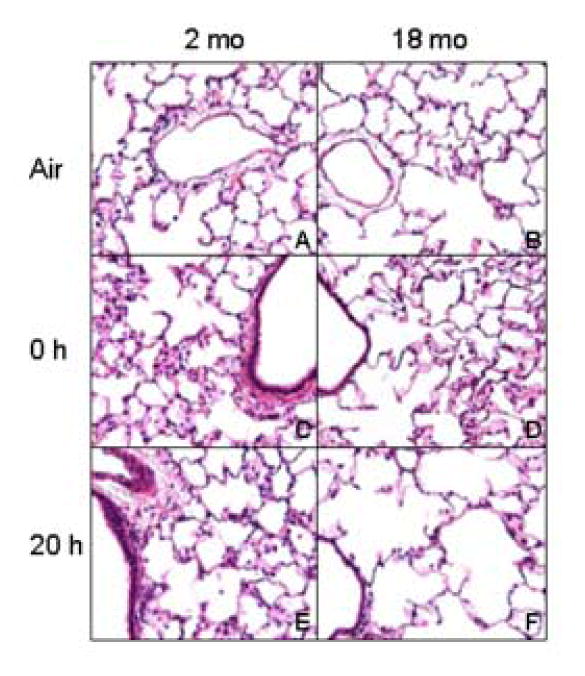
Effect of LOP on lung histology. Lung sections (6 μm) prepared from younger (2 mo) and older (18 mo) rats immediately (0 h) and 20 h after exposure to air (panels A and B) or LOP (panels C-F) were stained with H & E, Magnification, 40×.
Discussion
Alterations in the production of inflammatory mediators and antioxidants, as well as pulmonary epithelial-endothelial barrier dysfunction have been suggested as potential factors mediating increases in the susceptibility of the elderly to air pollutants (Tankersley et al., 2003). Consistent with this idea, the present studies demonstrate that the pulmonary inflammatory response to LOP at levels similar to those found in indoor environments after cleaning (Singer et al., 2006), was significantly attenuated in older when compared to younger rats. This may lead to impaired ability to repair damaged tissue and to protect against infectious agents, thus contributing in part, to increased morbidity and mortality in the elderly.
Cytokines such as TNF-α and eicosanoids have been reported to be generated in the lung following exposure to gaseous and particulate air pollutants (Mundandhara et al., 2006). These mediators play an important role in initiating tissue repair after injury (Wong et al., 1996). We found that acute exposure of younger and older rats to inhaled LOP resulted in a rapid and persistent induction of TNF-α and COX-2 in lung macrophages and Type II cells. However, expression of these proteins was reduced in older, when compared to younger rats. This suggests an impaired ability of geriatric animals to mount a robust pulmonary inflammatory response. These findings are in accord with previous reports of decrements in TNF-α production by AM from elderly rats exposed to silica or in isolated cells treated with LPS (Corsini et al., 1999; Corsini et al., 2003; Corsini et al., 2004). We have previously reported increases in TNF-α production by AM following exposure of animals to fine PM plus peroxides (Morio et al., 2001), and others have demonstrated PM-induced upregulation of TNF-α, as well as COX-2 expression, in macrophages and epithelial cells (van Eeden et al., 2001; Monn et al., 2003; Ishii et al., 2004; Becker et al., 2005). These findings suggest that TNF-α and COX-2 are sensitive markers of exposure to inhaled PM.
Induction of antioxidant enzymes within the lung is critical for limiting oxidant-induced damage. Of particular interest is Cu/Zn SOD which is one of the first antioxidant enzymes to be expressed in tissues undergoing oxidative stress (Tsan, 1993, 1997; Kinnula et al., 2003). The present studies show that LOP exposure caused a rapid induction of SOD in the lung. The fact that there are lower levels of SOD in the lung of older when compared to younger animals after LOP exposure, indicates that antioxidant defenses are also impaired in these animals. Similar deficits in SOD expression have been noted in lungs of elderly rats exposed to PM2.5 or diesel exhaust particles (Liu et al., 2005; Sugimoto et al., 2005). Age-dependent decreases in antioxidant enzyme activity following LOP may be due to post-translational chemical modifications of SOD (Machado et al., 1991; Augustyniak et al., 2004). In contrast to our findings with LOP, rats exposed to ozone or oxygen exhibit either increases (Gumuslu et al., 2001; Servais et al., 2005) or no changes in lung SOD (Gomi et al., 2002). Differences between these findings and ours may be due to the distinct exposures and exposure conditions, and/or to the type of SOD analyzed in the studies.
Genes involved in production of inflammatory mediators such as TNF-α and eicosanoids, and antioxidants like SOD, are regulated, in part, by upstream signaling molecules including members of the MAP kinase family (Rahman, 2002; Lopez-Neblina et al., 2006). We found that AM from both younger and older rats constitutively expressed p38 and p44/42 MAP kinases and JNK proteins, suggesting that these cells are primed to respond to inhaled irritants. However, differences in expression were noted between younger and older animals, as well as in their responses to LOP. In general, cells from older animals constitutively expressed lower levels of these signaling proteins when compared to cells from younger animals. Whereas LOP exposure resulted in increased p44/42 and JNK MAP kinase expression in AM from younger rats, in older animals, expression of these proteins decreased. Exposure to LOP also transiently decreased p38 MAP kinase expression in AM from both younger and older animals. Similar decreases in MAP kinase protein expression have been reported previously in macrophages from older animals following exposure to LPS (Ding et al., 1994; Boehmer et al., 2004; Boehmer et al., 2005). Reduced levels of MAP kinases may account for decreased LOP-induced expression of inflammatory mediators by AM observed in the older animals in the present studies.
Type II cells from both younger and older animals, also constitutively expressed p38, p44/42 and JNK proteins indicating that these cells, like AM, are primed to respond to pulmonary irritants. Moreover, following exposure to LOP, Type II cells from older rats expressed lower levels of each of the MAP kinase proteins when compared to younger animals. This suggests that Type II cells participate in the pulmonary response to LOP, and that MAP kinase and JNK signaling pathways are impaired in these cells in elderly rats. Similar age-associated decreases in p44/42 MAP kinase protein have been observed in rat intestine (Gentili et al., 2000; Pardo et al., 2004), hepatocytes (Ikeyama et al., 2002; Li et al., 2003), and fibroblasts (Park et al., 2000). Our observations that MAP kinase expression is reduced in older when compared to younger rats is consistent with our findings with TNF-α, COX-2 and SOD expression, and suggest that these signaling molecules are important in regulating LOP-induced expression of inflammatory mediators and antioxidants in the lung.
Transcription factors such as NF-κB, C/EBP and CREB regulate the expression of inflammatory and antioxidant genes downstream of MAP kinases (Pourazar et al., 2005; Wang et al., 2005; Dong et al., 2006; Kim et al., 2006; Tsatsanis et al., 2006). We found that DNA binding activity of NF-κB and C/EBP, but not CREB, increased in AM following exposure to LOP. However this was only observed in cells from younger rats. We also noted significantly greater constitutive NF-κB, C/EBP and CREB DNA binding activity in AM from older animals. Moreover, this was not altered by exposure to LOP. These data suggest that activation of these transcription factors is not required for AM responsiveness to inhaled irritants in elderly animals. Alternatively, other transcription factors may play a role in LOP-induced inflammatory and antioxidant responses in these animals. For example, in A549 cells, supernatants from AM exposed to PM caused an increase in AP-1 and SP-1 activity (Jimenez et al., 2002; Ishii et al., 2004). In contrast to our findings, decreased CREB activity has been observed in older rats following carrageenan-induced lung inflammation (Corsini et al., 2005). Differences between these findings and ours may reflect irritant-specific pulmonary responses. Reduced constitutive NF-κB, C/EBP and CREB binding activity was also noted in Type II cells from older when compared to younger animals. Moreover, LOP reduced C/EBP and CREB activity, but only in the older animals. These results are in accord with our findings that expression of TNF-α, COX-2 and SOD, as well as MAP kinases was reduced in Type II cells from older animals. These results suggest that the effects of LOP on transcription factor activity depends on age, as well as the responding cell type.
A characteristic feature of lung injury is the accumulation of protein and inflammatory cells in BAL fluid (Bhalla, 1999). Despite our findings of altered structural integrity in the lungs following LOP exposure, we did not detect increases in BAL protein or inflammatory cell number. This is consistent with our previously reported lack of spirometric or nasal inflammatory changes in young adults (Laumbach et al., 2005). It may be that more sensitive methods are required to identify biochemical markers of lung injury in rodents and humans. Interestingly, significantly fewer Type II epithelial cells were isolated from the lung of older when compared to younger rats which is consistent with previous reports (Pinkerton et al., 1982). The significance of this deficit is unknown but it may result in reduced surfactant production (Betsuyaku et al., 2004) and increased sensitivity to pulmonary irritants.
The present studies demonstrate that inhalation exposure of rats to a SOA, potentially generated in indoor environments, resulted in increased inflammatory mediator and antioxidant expression in lung macrophages and Type II cells. These findings indicate that these aerosols may be a possible health concern. Of particular interest was our observation that the pulmonary response to LOP was significantly attenuated in the older animals. These findings suggest a potential mechanism that may in part, be important in the heightened responsiveness of the elderly to air pollutants. Further studies are in progress to determine if reduced activation of MAP kinases and/or altered transcription factor activity plays a role in impaired inflammatory and antioxidant responses in elderly animals.
Acknowledgments
Dr. Sherritta Ridgely, Assistant Clinical Veterinarian, Rutgers University; Dr. Charles Weschler, Adjunct Professor, University of Medicine and Dentistry of New Jersey, RWJ Medical School.
Grant Support: ALA-NJ, US EPA, ARO55073 and NIH ES04738, ES005022, ESO03520 and GM034310
Abbreviations
- LOP
limonene ozone reaction products
- TNF-α
tumor necrosis factor-alpha
- COX-2
cyclooxygenase -2
- SOD
superoxide dismutase
- AM
alveolar macrophage
- VOC
volatile organic compound
- SOA
secondary organic aerosols
- PM
particulate matter
- BAL
bronchoalveolar lavage
Footnotes
Conflict of Interest: There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atkinson R, Hasegawa D, Aschmann S. Rate constants for the gas-phase reactions of ozone with a series of monoterpenes and related compounds at 296 +/- 2K. Int J Chem Kin. 1990;22:871–887. [Google Scholar]
- Augustyniak A, Skrzydlewska E. Antioxidative abilities during aging. Postepy Hig Med Dosw (Online) 2004;58:194–201. [PubMed] [Google Scholar]
- Barnes PJ. Transcription factors in airway diseases. Lab Invest. 2006;86:867–872. doi: 10.1038/labinvest.3700456. [DOI] [PubMed] [Google Scholar]
- Becker S, Mundandhara S, Devlin RB, Madden M. Regulation of cytokine production in human alveolar macrophages and airway epithelial cells in response to ambient air pollution particles: Further mechanistic studies. Toxicol Appl Pharmacol. 2005;207:269–275. doi: 10.1016/j.taap.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Betsuyaku T, Kuroki Y, Nagai K, Nasuhara Y, Nishimura M. Effects of ageing and smoking on SP-A and SP-D levels in bronchoalveolar lavage fluid. Eur Respir J. 2004;24:964–970. doi: 10.1183/09031936.04.00064004. [DOI] [PubMed] [Google Scholar]
- Bhalla DK. Ozone-induced lung inflammation and mucosal barrier disruption: toxicology, mechanisms, and implications. J Toxicol Environ Health B Crit Rev. 1999;2:31–86. doi: 10.1080/109374099281232. [DOI] [PubMed] [Google Scholar]
- Bils RF, Romanovsky JC. Ultrastructural alterations of alveolar tissue of mice. II. Synthetic photochemical smog. Arch Environ Health. 1967;14:844–858. doi: 10.1080/00039896.1967.10664851. [DOI] [PubMed] [Google Scholar]
- Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. J Leukoc Biol. 2004;75:342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- Boehmer ED, Meehan MJ, Cutro BT, Kovacs EJ. Aging negatively skews macrophage TLR2- and TLR4-mediated pro-inflammatory responses without affecting the IL-2-stimulated pathway. Mech Ageing Dev. 2005;126:1305–1313. doi: 10.1016/j.mad.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Bowes SM, 3rd, Laube BL, Links JM, Frank R. Regional deposition of inhaled fog droplets: preliminary observations. Environ Health Perspect. 1989;79:151–157. doi: 10.1289/ehp.8979151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral-Anderson LJ, Evans MJ, Freeman G. Effects of NO2 on the lungs of rats. I. Morphology. Exp Mol Pathol. 1977;27:353–365. doi: 10.1016/0014-4800(77)90006-5. [DOI] [PubMed] [Google Scholar]
- Castranova V. Signaling pathways controlling the production of inflammatory mediators in response to crystalline silica exposure: role of reactive oxygen/nitrogen species. Free Radic Biol Med. 2004;37:916–925. doi: 10.1016/j.freeradbiomed.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Chan ED, Winston BW, Jarpe MB, Wynes MW, Riches DW. Preferential activation of the p46 isoform of JNK/SAPK in mouse macrophages by TNF alpha. Proc Natl Acad Sci U S A. 1997;94:13169–13174. doi: 10.1073/pnas.94.24.13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsini E, Battaini F, Lucchi L, Marinovich M, Racchi M, Govoni S, Galli CL. A defective protein kinase C anchoring system underlying age-associated impairment in TNF-alpha production in rat macrophages. J Immunol. 1999;163:3468–3473. [PubMed] [Google Scholar]
- Corsini E, Di Paola R, Viviani B, Genovese T, Mazzon E, Lucchi L, Marinovich M, Galli CL, Cuzzocrea S. Increased carrageenan-induced acute lung inflammation in old rats. Immunology. 2005;115:253–261. doi: 10.1111/j.1365-2567.2005.02148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsini E, Giani A, Lucchi L, Peano S, Viviani B, Galli CL, Marinovich M. Resistance to acute silicosis in senescent rats: role of alveolar macrophages. Chem Res Toxicol. 2003;16:1520–1527. doi: 10.1021/tx034139+. [DOI] [PubMed] [Google Scholar]
- Corsini E, Giani A, Peano S, Marinovich M, Galli CL. Resistance to silica-induced lung fibrosis in senescent rats: role of alveolar macrophages and tumor necrosis factor-alpha (TNF) Mech Ageing Dev. 2004;125:145–146. doi: 10.1016/j.mad.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- Ding A, Hwang S, Schwab R. Effect of aging on murine macrophages. Diminished response to IFN-gamma for enhanced oxidative metabolism. J Immunol. 1994;153:2146–2152. [PubMed] [Google Scholar]
- Donaldson K, Stone V, Seaton A, MacNee W. Ambient particle inhalation and the cardiovascular system: potential mechanisms. Environ Health Perspect. 2001;109 4:523–527. doi: 10.1289/ehp.01109s4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Liu Y, Du M, Wang Q, Yu CT, Fan X. P38 mitogen-activated protein kinase inhibition attenuates pulmonary inflammatory response in a rat cardiopulmonary bypass model. Eur J Cardiothorac Surg. 2006;30:77–84. doi: 10.1016/j.ejcts.2006.02.040. [DOI] [PubMed] [Google Scholar]
- Elder AC, Gelein R, Azadniv M, Frampton M, Finkelstein J, Oberdorster G. Systemic effects of inhaled ultrafine particles in two compromised, aged rat strains. Inhal Toxicol. 2004;16:461–471. doi: 10.1080/08958370490439669. [DOI] [PubMed] [Google Scholar]
- Elder AC, Gelein R, Finkelstein JN, Cox C, Oberdorster G. Pulmonary inflammatory response to inhaled ultrafine particles is modified by age, ozone exposure, and bacterial toxin. Inhal Toxicol. 2000;12 4:227–246. doi: 10.1080/089583700750019585. [DOI] [PubMed] [Google Scholar]
- Gentili C, de Boland AR. Age-related decline in mitogen-activated protein kinase phosphorylation in PTH-stimulated rat enterocytes. Exp Gerontol. 2000;35:1003–1015. doi: 10.1016/s0531-5565(00)00133-9. [DOI] [PubMed] [Google Scholar]
- Gomez CR, Boehmer ED, Kovacs EJ. The aging innate immune system. Curr Opin Immunol. 2005;17:457–462. doi: 10.1016/j.coi.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Gomi F, Matsuo M. Effects of 60% oxygen inhalation on the survival and antioxidant enzyme activities of young and old rats. Mech Ageing Dev. 2002;123:1295–1304. doi: 10.1016/s0047-6374(02)00038-6. [DOI] [PubMed] [Google Scholar]
- Gumuslu S, Bilmen S, Korgun DK, Yargicoglu P, Agar A. Age-related changes in antioxidant enzyme activities and lipid peroxidation in lungs of control and sulfur dioxide exposed rats. Free Radic Res. 2001;34:621–627. doi: 10.1080/10715760100300511. [DOI] [PubMed] [Google Scholar]
- Heffner JE, Repine JE. Antioxidants in the lung. In: Crystal RG, West JB, editors. The Lung. Raven Press; New York: 1991. pp. 1811–1820. [Google Scholar]
- Ikeyama S, Kokkonen G, Shack S, Wang XT, Holbrook NJ. Loss in oxidative stress tolerance with aging linked to reduced extracellular signal-regulated kinase and Akt kinase activities. Faseb J. 2002;16:114–116. doi: 10.1096/fj.01-0409fje. [DOI] [PubMed] [Google Scholar]
- Ishii H, Fujii T, Hogg JC, Hayashi S, Mukae H, Vincent R, van Eeden SF. Contribution of IL-1 beta and TNF-alpha to the initiation of the peripheral lung response to atmospheric particulates (PM10) Am J Physiol Lung Cell Mol Physiol. 2004;287:L176–183. doi: 10.1152/ajplung.00290.2003. [DOI] [PubMed] [Google Scholar]
- Jimenez LA, Drost EM, Gilmour PS, Rahman I, Antonicelli F, Ritchie H, MacNee W, Donaldson K. PM(10)-exposed macrophages stimulate a proinflammatory response in lung epithelial cells via TNF-alpha. Am J Physiol Lung Cell Mol Physiol. 2002;282:L237–248. doi: 10.1152/ajplung.00024.2001. [DOI] [PubMed] [Google Scholar]
- Kasagi S, Seyama K, Mori H, Souma S, Sato T, Akiyoshi T, Suganuma H, Fukuchi Y. Tomato juice prevents senescence-accelerated mouse P1 strain from developing emphysema induced by chronic exposure to tobacco smoke. Am J Physiol Lung Cell Mol Physiol. 2006;290:L396–404. doi: 10.1152/ajplung.00483.2004. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee HS, Chong YH, Kang JL. p38 Mitogen-activated protein kinase up-regulates LPS-induced NF-kappaB activation in the development of lung injury and RAW 264.7 macrophages. Toxicology. 2006;225:36–47. doi: 10.1016/j.tox.2006.04.053. [DOI] [PubMed] [Google Scholar]
- Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- Lang JD, McArdle PJ, O'Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest. 2002;122:314S–320S. doi: 10.1378/chest.122.6_suppl.314s. [DOI] [PubMed] [Google Scholar]
- Laumbach RJ, Fiedler N, Gardner CR, Laskin DL, Fan ZH, Zhang J, Weschler CJ, Lioy PJ, Devlin RB, Ohman-Strickland P, Kelly-McNeil K, Kipen HM. Nasal effects of a mixture of volatile organic compounds and their ozone oxidation products. J Occup Environ Med. 2005;47:1182–1189. doi: 10.1097/01.jom.0000183338.95778.f0. [DOI] [PubMed] [Google Scholar]
- Li J, Holbrook NJ. Common mechanisms for declines in oxidative stress tolerance and proliferation with aging. Free Radic Biol Med. 2003;35:292–299. doi: 10.1016/s0891-5849(03)00308-3. [DOI] [PubMed] [Google Scholar]
- Liu X, Meng Z. Effects of airborne fine particulate matter on antioxidant capacity and lipid peroxidation in multiple organs of rats. Inhal Toxicol. 2005;17:467–473. doi: 10.1080/08958370590964467. [DOI] [PubMed] [Google Scholar]
- Lopez-Neblina F, Toledo-Pereyra LH. Phosphoregulation of signal transduction pathways in ischemia and reperfusion. J Surg Res. 2006;134:292–299. doi: 10.1016/j.jss.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt J, Ames BN. Ascorbic acid recycling in rat hepatocytes as measurement of antioxidant capacity: decline with age. Methods Enzymol. 1999;299:83–88. doi: 10.1016/s0076-6879(99)99011-0. [DOI] [PubMed] [Google Scholar]
- Machado A, Ayala A, Gordillo E, Revilla E, Santa Maria C. Relationship between enzymatic activity loss and post-translational protein modification in aging. Arch Gerontol Geriatr. 1991;12:187–197. doi: 10.1016/0167-4943(91)90027-n. [DOI] [PubMed] [Google Scholar]
- Monn C, Naef R, Koller T. Reactions of macrophages exposed to particles <10 microm. Environ Res. 2003;91:35–44. doi: 10.1016/s0013-9351(02)00021-x. [DOI] [PubMed] [Google Scholar]
- Morio LA, Hooper KA, Brittingham J, Li TH, Gordon RE, Turpin BJ, Laskin DL. Tissue injury following inhalation of fine particulate matter and hydrogen peroxide is associated with altered production of inflammatory mediators and antioxidants by alveolar macrophages. Toxicol Appl Pharmacol. 2001;177:188–199. doi: 10.1006/taap.2001.9316. [DOI] [PubMed] [Google Scholar]
- Mundandhara SD, Becker S, Madden MC. Effects of diesel exhaust particles on human alveolar macrophage ability to secrete inflammatory mediators in response to lipopolysaccharide. Toxicol In Vitro. 2006;20:614–624. doi: 10.1016/j.tiv.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Nadziejko C, Fang K, Narciso S, Zhong M, Su WC, Gordon T, Nadas A, Chen LC. Effect of particulate and gaseous pollutants on spontaneous arrhythmias in aged rats. Inhal Toxicol. 2004;16:373–380. doi: 10.1080/08958370490439533. [DOI] [PubMed] [Google Scholar]
- Nel AE, Diaz-Sanchez D, Ng D, Hiura T, Saxon A. Enhancement of allergic inflammation by the interaction between diesel exhaust particles and the immune system. J Allergy Clin Immunol. 1998;102:539–554. doi: 10.1016/s0091-6749(98)70269-6. [DOI] [PubMed] [Google Scholar]
- Oberdorster G, Finkelstein JN, Johnston C, Gelein R, Cox C, Baggs R, Elder AC. Acute pulmonary effects of ultrafine particles in rats and mice. Res Rep Health Eff Inst. 2000:5–74. disc 75-86. [PubMed] [Google Scholar]
- Pardo VG, de Boland AR. Tyrosine phosphorylation signaling dependent on 1alpha,25(OH)2-vitamin D3 in rat intestinal cells: effect of ageing. Int J Biochem Cell Biol. 2004;36:489–504. doi: 10.1016/j.biocel.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Park GY, Christman JW. Nuclear factor kappa B is a promising therapeutic target in inflammatory lung disease. Curr Drug Targets. 2006;7:661–668. doi: 10.2174/138945006777435317. [DOI] [PubMed] [Google Scholar]
- Park WY, Park JS, Cho KA, Kim DI, Ko YG, Seo JS, Park SC. Up-regulation of caveolin attenuates epidermal growth factor signaling in senescent cells. J Biol Chem. 2000;275:20847–20852. doi: 10.1074/jbc.M908162199. [DOI] [PubMed] [Google Scholar]
- Pinkerton KE, Barry BE, O'Neil JJ, Raub JA, Pratt PC, Crapo JD. Morphologic changes in the lung during the lifespan of Fischer 344 rats. Am J Anat. 1982;164:155–174. doi: 10.1002/aja.1001640206. [DOI] [PubMed] [Google Scholar]
- Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ. Aging and innate immune cells. J Leukoc Biol. 2004;76:291–299. doi: 10.1189/jlb.1103592. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. Jama. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourazar J, Mudway IS, Samet JM, Helleday R, Blomberg A, Wilson SJ, Frew AJ, Kelly FJ, Sandstrom T. Diesel exhaust activates redox-sensitive transcription factors and kinases in human airways. Am J Physiol Lung Cell Mol Physiol. 2005;289:L724–730. doi: 10.1152/ajplung.00055.2005. [DOI] [PubMed] [Google Scholar]
- Rahman I. Oxidative stress and gene transcription in asthma and chronic obstructive pulmonary disease: antioxidant therapeutic targets. Curr Drug Targets Inflamm Allergy. 2002;1:291–315. doi: 10.2174/1568010023344607. [DOI] [PubMed] [Google Scholar]
- Rahman I, Biswas SK, Jimenez LA, Torres M, Forman HJ. Glutathione, stress responses, and redox signaling in lung inflammation. Antioxid Redox Signal. 2005;7:42–59. doi: 10.1089/ars.2005.7.42. [DOI] [PubMed] [Google Scholar]
- Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533:222–239. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- Servais S, Boussouar A, Molnar A, Douki T, Pequignot JM, Favier R. Age-related sensitivity to lung oxidative stress during ozone exposure. Free Radic Res. 2005;39:305–316. doi: 10.1080/10715760400011098. [DOI] [PubMed] [Google Scholar]
- Singer BC, Coleman BK, Destaillats H, Hodgson AT, Lunden MM, Weschler C, Nazaroff WW. Indoor secondary pollutants from cleaning product and air freshener use in the presence of ozone. Atmospheric Environment. 2006;40:6696–6710. doi: 10.1021/es052198z. [DOI] [PubMed] [Google Scholar]
- Squier TC. Oxidative stress and protein aggregation during biological aging. Exp Gerontol. 2001;36:1539–1550. doi: 10.1016/s0531-5565(01)00139-5. [DOI] [PubMed] [Google Scholar]
- Sugimoto R, Kumagai Y, Nakai Y, Ishii T. 9,10-Phenanthraquinone in diesel exhaust particles downregulates Cu,Zn-SOD and HO-1 in human pulmonary epithelial cells: intracellular iron scavenger 1,10-phenanthroline affords protection against apoptosis. Free Radic Biol Med. 2005;38:388–395. doi: 10.1016/j.freeradbiomed.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Sunil VR, Connor AJ, Guo Y, Laskin JD, Laskin DL. Activation of type II alveolar epithelial cells during acute endotoxemia. Am J Physiol Lung Cell Mol Physiol. 2002;282:L872–880. doi: 10.1152/ajplung.00217.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tankersley CG, Campen M, Bierman A, Flanders SE, Broman KW, Rabold R. Particle effects on heart-rate regulation in senescent mice. Inhal Toxicol. 2004;16:381–390. doi: 10.1080/08958370490439551. [DOI] [PubMed] [Google Scholar]
- Tankersley CG, Shank JA, Flanders SE, Soutiere SE, Rabold R, Mitzner W, Wagner EM. Changes in lung permeability and lung mechanics accompany homeostatic instability in senescent mice. J Appl Physiol. 2003;95:1681–1687. doi: 10.1152/japplphysiol.00190.2003. [DOI] [PubMed] [Google Scholar]
- Thomas JA, Mallis RJ. Aging and oxidation of reactive protein sulfhydryls. Exp Gerontol. 2001;36:1519–1526. doi: 10.1016/s0531-5565(01)00137-1. [DOI] [PubMed] [Google Scholar]
- Tsan MF. Superoxide dismutase and pulmonary oxygen toxicity. Proc Soc Exp Biol Med. 1993;203:286–290. doi: 10.3181/00379727-203-43600a. [DOI] [PubMed] [Google Scholar]
- Tsan MF. Superoxide dismutase and pulmonary oxygen toxicity. Proc Soc Exp Biol Med. 1997;214:107–113. doi: 10.3181/00379727-214-44076. [DOI] [PubMed] [Google Scholar]
- Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signaling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol. 2006;38:1654–1661. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- USEPA. Air Quality Criteria for Particulate Matter. Environmental Protection Agency; NC: 1996. [Google Scholar]
- USEPA. Air quality criteria for particulate matter. U. S. Environmental Protection Agency; Research Triangle Park, NC: 2004. [Google Scholar]
- van Bree L, Rietjens I, Alink GM, Dormans J, Marra M, Rombout P. Biochemical and morphological changes in lung tissue and isolated lung cells of rats induced by short-term nitrogen dioxide exposure. Hum Exp Toxicol. 2000;19:392–401. doi: 10.1191/096032700678816151. [DOI] [PubMed] [Google Scholar]
- van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R, Hogg JC. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)) Am J Respir Crit Care Med. 2001;164:826–830. doi: 10.1164/ajrccm.164.5.2010160. [DOI] [PubMed] [Google Scholar]
- Wainman T, Zhang J, Weschler CJ, Lioy PJ. Ozone and limonene in indoor air: a source of submicron particle exposure. Environ Health Perspect. 2000;108:1139–1145. doi: 10.1289/ehp.001081139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace LA. Volatile Organic Compounds. In: Samet JM, Spengler JD, editors. Indoor Air Pollution: A Health Perspective. Johns Hopkins University Press; Baltimore, MD: 1991. pp. 252–272. [Google Scholar]
- Wang JM, Tseng JT, Chang WC. Induction of human NF-IL6beta by epidermal growth factor is mediated through the p38 signaling pathway and cAMP response element-binding protein activation in A431 cells. Mol Biol Cell. 2005;16:3365–3376. doi: 10.1091/mbc.E05-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weschler CJ. Ozone in indoor environments: concentration and chemistry. Indoor Air. 2000;10:269–288. doi: 10.1034/j.1600-0668.2000.010004269.x. [DOI] [PubMed] [Google Scholar]
- Wolkoff P, Clausen PA, Wilkins CK, Nielsen GD. Formation of strong airway irritants in terpene/ozone mixtures. Indoor Air. 2000;10:82–91. doi: 10.1034/j.1600-0668.2000.010002082.x. [DOI] [PubMed] [Google Scholar]
- Wong GH, Kaspar RL, Vehar G. Tumor necrosis factor and lymphotoxin: protection against oxidative stress through induction of MnSOD. Exs. 1996;77:321–333. doi: 10.1007/978-3-0348-9088-5_21. [DOI] [PubMed] [Google Scholar]
- Zelikoff JT, Chen LC, Cohen MD, Fang K, Gordon T, Li Y, Nadziejko C, Schlesinger RB. Effects of inhaled ambient particulate matter on pulmonary antimicrobial immune defense. Inhal Toxicol. 2003;15:131–150. doi: 10.1080/08958370304478. [DOI] [PubMed] [Google Scholar]


