Abstract
Objective
Neurotrophin receptor signaling has become increasingly recognized as an important factor in the development and progression of a variety of malignancies. In order to analyze the potential contribution of neurotrophin signaling to lymphoma cell survival, we investigated the role of a neurotrophin axis in promoting survival and proliferation of Non-Hodgkin Lymphoma (NHL) cells.
Methods
The role of neurotrophins in the survival and proliferation of NHL cells was determined by exposing cells to the Trk specific inhibitor, K252a, and then performing 3H-thymidine incorporation and Annexin-V/propidium iodide staining. The involvement of NF-κB in this process was studied using western blot, EMSA and immunofluorescence assays.
Results
Here, we demonstrate that both primary NHL cells and DLBCL cell lines express Trk receptors and their neurotrophin ligands. Furthermore, these cells are sensitive to the Trk-specific inhibitor, K252a, as evidenced by inhibition of proliferation and/or the induction of apoptosis. Analysis of the mechanism into the effects of K252a revealed that, in the OCI-LY3 cell line, K252a induced a subnuclear distribution of NF-κB resulting in the sequestration of RelA in the nucleolus, thereby inhibiting NF-κB-dependent gene transcription. This results in the loss of IL-6 production; a known survival promoting signal for OCI-LY3, as well as many primary DLBCLs.
Conclusion
Thus, Trk receptors represent a novel therapeutic target for the treatment of NHL.
Introduction
Non-Hodgkin Lymphoma (NHL) is a diverse group of malignancies, which develops from lymphoid tissues. Based on a WHO classification system, more than 30 different subtypes have been identified [1]. The most common type of NHL is Diffuse Large B-cell Lymphoma (DLBCL). This type accounts for 30-40% of all lymphomas [1]. Follicular lymphoma is the second most common; accounting for 20-30% [2]. The success of treatment is dependent upon several factors including, but not limited to, disease stage at diagnosis, site of involvement and genetic features of the lymphoma. Besides being the most common NHL, DLBCL also accounts for 80% of aggressive lymphomas. It is heterogenous in nature, and has been subdivided into two major groups: germinal center variant (GC) and activated B-cell variant (ABC) [3]. As their names suggest, the GC subtype was classified as such due to a gene expression pattern that most closely resembles a normal germinal center B-cell, whereas the ABC subtype most resembles an in vitro activated peripheral blood B cell [3]. Interestingly, the GC subtype has a far better prognosis than its counterpart; 60% 5 year survival rate versus 35%. However, in light of the fact that a large percentage of patients present with stage IV disease [4] and disease variability renders some unresponsive to conventional chemotherapy, it is important to identify new targets for the development of additional therapeutic options.
Neurotrophins are a family of molecules, which have become increasingly important in the development and progression of a variety of malignancies, including prostate cancer and neuroblastoma [5, 6]. The neurotrophin family consists of Nerve Growth Factor (NGF), Brain Derived Neurotrophic Factor (BDNF), Neurotrophin-3 (NT-3) and Neurotrophin-4/5 (NT-4/5). The receptors for these molecules are receptor tyrosine kinases, known as tropomyosin receptor kinases (Trk). These include TrkA, TrkB and TrkC, of which TrkA was first identified as an oncogene fused to the tropomyosin gene in colon carcinoma [7]. Signaling by neurotrophins through Trk receptors has been most extensively studied in the context of nervous system development, survival and plasticity. Within the immune system, Trk and neurotrophin expression has been detected on both T and B lymphocytes [8, 9]. In B cells, autocrine NGF signaling appears to be essential for the survival of memory B cells [10]. Furthermore, NGF can rescue B cells from anti-IgM induced apoptosis [11]. The survival promoting effects of neurotrophins are a result of the activation of specific signaling pathways within cells. Neurotrophin binding to Trk receptors results in receptor dimerization leading to subsequent kinase activation. Activation of mitogen activated protein kinase (MAPK), PI3K/AKT and nuclear factor κB (NF-κB) signaling cascades all contribute to maintenance of cell survival by neurotrophins. NF-κB activation, in particular, has been implicated in lymphoma development and progression [12]. Hodgkin/Reed-Steinberg cells possess constitutive activation of NF-κB, which is essential for their survival and proliferation [13]. Furthermore, the ABC subtype of DLBCL has been demonstrated to maintain strong, constitutive NF-κB activation that is required for their survival [14]. In contrast, the GC subtype does not appear to depend upon NF-κB for survival [14]. Thus, given the ability of neurotrophins to induce NF-κB activity, and the fact that many B cell malignancies express Trk receptors and neurotrophins, this is a potential pathway, which lymphoma cells may exploit for survival. Therefore, manipulation of Trk receptor activation has potential therapeutic implications for tumors expressing these proteins.
Herein, we describe our experiments to evaluate Trk signaling in lymphoma and normal B cells and find that the Trk-neurotrophin axis appears as a novel therapeutic target for NHL.
MATERIALS AND METHODS
Patient Material
Cryopreserved single-cell suspension of tumor specimens, derived from 13 different patients that were clinically diagnosed with NHL (including follicular, diffuse large B-cell, and small lymphocytic lymphoma), were obtained from the lymphoma specimen bank at the University of Rochester Medical Center, under an Institutional Review Board (IRB)-approved protocol. At the time of tissue collection, the Strong Memorial Hospital Pathology Laboratory at the University of Rochester had conducted flow cytometric analyses of these specimens with staining for various antigens. A lymphocyte gate was chosen by using low to high forward scatter to include large lymphoid events and low side scatter to exclude nonlymphoid events. Gating for lymphocytes, which encompasses over 94% of total cells, showed presence of monoclonal B cell population (in a range of 63% to 78%) in most of the patient materials. During these preliminary analyses the viability of cells remained high (>97%).
Cell Lines and Primary B cells
The diffuse large B-cell lymphoma lines, OCI-LY3, OCI-LY10, OCI-LY7, SUDHL6, and OCI-LY19 (kind gift from Dr. Lou Staudt); as well as Burkitt’s lymphoma cell lines, Ramos, Raji, Daudi and multiple myeloma cell line, RPMI-8226, were cultured in RPMI 1640 (Invitrogen, Carlsbad, CA) supplemented with 10% heat inactivated fetal bovine serum (FBS), 100U/mL penicillin and 100 μg/mL streptomycin, 2mM L-glutamine and 5×10-5M 2-mercaptoethanol. The various cancer cells and transformed cell lines, MCF7 (human breast adenocarcinoma), MDA-MB231 (human breast adenocarcinoma), SKBR3 (human breast adenocarcinoma), 293T (human embryonic kidney), CHME5 (human microglia); and THP-1 cells (human acute monocytic leukemia), were maintained in Dulbecco’s modified Eagle medium (DMEM, Invitrogen, Carlsbad, CA) containing 10% FBS, 2 mM glutamine, and antibiotics; and RPMI 1640 respectively.
Normal B cells were isolated from peripheral blood of healthy donors by using a protocol that was approved by the University of Rochester Institutional Review Board. Briefly, peripheral blood mononuclear cells (PBMC) were isolated from blood after centrifugation at 933 × g for 8 min. B lymphocytes were then isolated by positive selection with anti-CD19 CD19 magnetic beads (Dynal Invitrogen, Carlsbad, CA), and cultured in RPMI1640 medium supplemented with 10% fetal bovine serum for 18-24 hours prior to use. This procedure resulted in >98% CD19-positive cells as determined by flow-cytometric analyses using FITC-conjugated anti-CD19 antibody. Flow cytometric analyses using FITC-conjugated anti-CD69 antibody confirmed the cells were not activated during isolation.
Indirect Immunofluorescence Assay
Cryopreserved samples were suspended in RPMI 1640 supplemented with 10% FBS, antibiotics and other reagents as outlined. These cells (and OCI LY3 cells in other assays) were then transferred on to cover slips and fixed with 4% formaldehyde for 10 minutes, followed by thorough washing in PBS. For intracellular staining, the cell membranes were permeabilized with 0.1% Triton X-100 in PBS, whereas this step was avoided for the cells that were subjected to surface staining of the proteins. The cells were treated with 5% FBS for 60 minutes (to eliminate non-specific binding of the antibodies) followed by overnight incubation with the following primary antibodies: anti-TrkA (R & D Systems, Minneapolis, MN), anti-CD20 FITC (BD Pharmingen, San Jose, CA), anti-CD19, anti-RelA, and anti-fibrillarin (Santa Cruz Biotechnology, Santa Cruz, CA). The cells were next washed thoroughly in PBS and incubated with the appropriate Alexa Fluor (Invitrogen, Carlsbad, CA) secondary antibodies for 60 minutes. After washing in PBS, cover slips were mounted and immunofluorescence was observed by fluorescent microscopy.
Immunoblot Analysis
These assays were performed as previously described [15]. Primary antibodies consisted of either anti-pan-Trk, anti-α-tubulin, anti-RelA, anti-actin, anti-Histone H4 (Santa Cruz Biotechnology, Santa Cruz, CA); or anti-cleaved PARP and anti-cleaved caspase 3 (Cell Signaling Technology, Berverly, MA). Immunblots were developed using ECL (Amersham, Arlington Heights, IL) and exposure to X-ray film (Kodak).
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using the Roche High Pure RNA Isolation kit (Roche Applied Science, Indianapolis, IN) and the protocol provided by the manufacturer. Complementary DNA (cDNA) synthesis was performed using the Super Script III First-Strand Synthesis kit (Invitrogen, Carlsbad, CA) in which random hexamer primers were applied. Primers used were as follows: human TrkA sense: 5′-GCATCTGGAGCTCCGTGATCT-3′ and anti-sense: 5′-CTCTGCCCAGCACGTCAAGT-3′, human TrkB(gp95) sense: 5′-GTTTCATAAGATCCCACTGGATGG-3′ and anti-sense: 5′-TGCTGCTTAGCTGCCTGAGAGTTA-3′, human TrkC sense: 5′-CCCCCATTTGCGTTATATAAACC-3′ and anti-sense: 5′-CACACGTGGGGGATAGTAGACA-3′, and GAPDH sense: 5′-CGGCAAGTTCAACGGGACA-3′ and anti-sense: 5′-CCACAGCTTTCCAGAGGG-3′, human NGF sense: 5′-CTTCAGCATTCCCTTGACAC-3′ and anti-sense: 5′-AGCCTTCCTGCTGAGCACAC-3′, human NT-3 sense: 5′-GTATCTCATGGAGGATTACGTGGG-3′ and anti-sense: 5′-TGTTCTCTGAAGTCAGTGCTCGGA-3′, human BDNF sense: 5′-TACTTTGGTTGCATGAAGGCTGCC-3′ and anti-sense: 5′-ACTTGACTACTGAGCATCACCCTG-3′. Agarose (0.7%) gel electrophoresis of the PCR reactions was used to identify the PCR products.
ELISA
BDNF or NGF levels were measured in culture supernatant (pre-cleared by brief centrifugation) by using the Emax Immunoassay System (Promega, Madison, WI) per the manufacturer’s instructions. The ELISA kits have a minimum sensitivity threshold of 7.8 (BDNF) or 3.9 (NGF) pg/ml. Levels of IL-6 were also measured in culture supernatant by using ELISA kits specific to human IL-6 (eBioscience, San Diego, CA; sensitivity: 2 pg/ml).
BrdU incorporation assay
BrdU incorporation was measured using the APC BrdU Flow Kit (BD Pharmingen, San Jose, CA). Briefly, the cryopreserved samples were thawed, washed and incubated in 12-well plates (1×106 cells/ml of RPMI 1640 medium containing recombinant human IL-4 (8ng/mL; BD Bioscience, San Jose, CA)) over a layer of CD40L-expressing irradiated NIH 3T3 cells (kindly provided by Dr. M. Zand, University of Rochester Medical Center) as previously described [16]. Under these conditions, the cells were treated with either 50nM or 100nM K252a, or the corresponding membrane insoluble analog K252b (Calbiochem, Gibbstown, NJ), followed by addition of 10μM BrdU (BD Pharmingen, San Jose, CA) at 1 hour prior to the termination of treatment at 48h. At the end of the pulse, the cells were washed and surface stained with anti-CD20 FITC (BD Pharmingen, San Jose, CA). The cells were then fixed, permeabilized and exposed to DNase to expose BrdU epitopes. Incorporated BrdU was then detected utilizing an anti-BrdU APC-conjugated antibody (BD Biosciences, San Jose, CA).
Thymidine uptake assay
The OCI-LY19 and OCI-LY3 cells (8×104) were plated in 96-well A/2 flat-bottom plates containing RPMI 1640 medium supplemented with FBS and antibiotics. The cells were exposed to K252a or AG879 (Calbiochem, Gibbstown, NJ) for 24 or 48h, pulsed for 18 hours with 1 μCi/well of [3H]thymidine (DuPont NEN, Wilmington, DE), and harvested on to a 96-well filter plate. The 3H-thymidine incorporation was detected as counts per minute (CPM) using a Topcount Luminometer (PerkinElmer, Boston, MA) at each time point.
Cell viability assays
Cellular viability of DLBCL lines and normal B cells was assessed using an Annexin V-FITC staining kit (BD Pharmingen, San Jose, CA). Briefly, the cells (1×106/ml) were treated with either K252a or AG879 for 48h. After washing with cold PBS, the cells were resuspended in binding buffer and incubated with Annexin V-FITC and propidium iodide for 15 minutes. Cellular apoptosis was then analyzed by flow cytometry using a Beckton Dickson FACS Calibur.
The MTT assay was used to assess the viability of adherent cells. Briefly the cells (2 × 104 cell/well of 96 well plate) were incubated with K252a or with vehicle for 48h and the assay were initiated by adding a solution of 5mg/ml MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; 10 μl/100 μl) into the culture medium. Following 1h incubation at 37° C, the plate was centrifuged, the medium was aspirated, the cultures were allowed to dry, and 0.2 ml of DMSO was then added to each well to disrupt the cells and dissolve the formazan crystals. The absorbance was then recorded at 570 nm in a microplate reader. Cell viability was expressed as the percentage of the absorption in control cultures (=100 %).
The viability of adherent cells was also measured by using the ApoPercentage Apoptosis Assay kit (Biocolor, Westbury, NY) per the manufacturer’s instructions.
Elecrophoretic mobility shift assay (EMSA)
Nuclear extracts and electrophoretic mobility shift assays were performed from OCI-LY3 cells as described previously [15]. The double-stranded oligonucleotide probes used in EMSA were as follows: (i) NF-κB: 5′-CAACGGCAGGGGAATTCCCCTCTCCTT-3′, and (ii) OCT-1: 5′-TGTCGAATGCAAATCACTAGAA-3′.
Luciferase assay
Transient transfection in OCI-LY3 was performed using Nucleofector (Amaxa) and the methods recommended by the manufacturer. Briefly, 1×106 cells were pre-treated with indicated amounts of K252a for 4h and then transfected with 2μg of NF-κB luciferase plasmid DNA. The cells were continually incubated for 8h in the presence of K252a. Upon completion of the treatments, the cells were lysed and luciferase activity was measured using the Promega Luciferase kit (Promega, Madison, WI) and a Microplate Luminometer (Packard Instruments).
Statistical Analysis
Mean data values and the SEM were computed for each variable. All data was analyzed by one-way ANOVA followed by Bonferroni’s test for multiple comparisons. A value of p < 0.05 was designated as statistically significant.
RESULTS
Primary non-hodgkin lymphoma cells express TrkA
Since Trk expression has also been identified on normal, as well as certain transformed B cells [9, 17, 18], we sought to determine whether B cells derived from primary NHL tumor specimens also express Trk receptors. To do this, we collected single-cell suspensions of lymphoid tissues from 13 NHL patients. Initial flow cytometric and immunohistochemical analyses of these samples revealed an enriched B cell population (nearly 63 to 78% cells were either CD19 or CD20 positive) with varied predominance of kappa or lambda light chain expression. These cells were subjected to the indirect immunofluorescence assays in which we used antibodies specific to either CD19 or CD20 and to the extracellular domain of TrkA. This assay revealed positive expression of TrkA on CD19-, as well as CD20-, positive cells from NHL patients (Figure 1A; representative data are shown, but similar results were obtained in all 13 samples), whereas isotype control antibodies did not produce any detectable staining of the cells. The staining pattern observed is likely due to the known accumulation of Trk and CD19 in lipid rafts of the plasma membrane [19-21]. Among these specimens, 3 were consistent with DLBCL (these were 06TLN0090, 07TLN0154 and 06TPL0027 respectively), which is in concordance with a previous study of pathologic lymphoid tissue that demonstrated strong TrkA immunoreactivity in DLBCL [22].
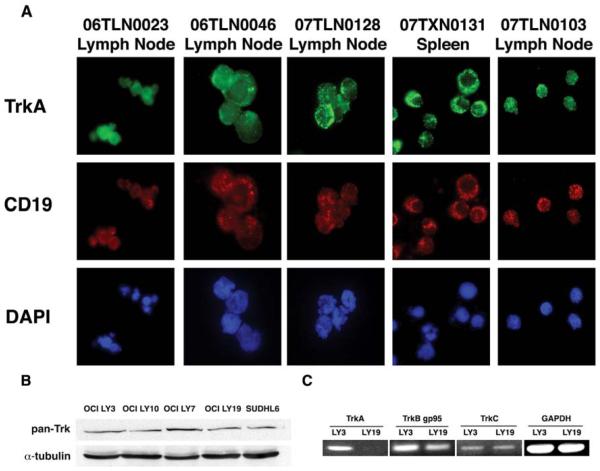
Figure 1.
B cells from primary Non-Hodgkin Lymphomas express TrkA. (A) A panel of single cell suspensions derived from patient biopsies of NHL were adhered to glass slides via cytospin. Immunofluorescence was performed using antibodies specific for CD19 (to identify B cells) and surface TrkA. Each patient’s cells expressed similar levels of TrkA on the surface of B cells derived from NHL tumors (TrkA: green, CD19: red and DAPI: blue). (B) DLBCL cell lines express Trk. The DLBCL cell lines were analyzed via immunoblot for expression of Trk receptors using a pan-Trk antibody, which recognizes TrkA, TrkB and TrkC. As shown, all cell lines exhibit pan-Trk expression. (C) The specific Trk receptors expressed was determined by RT-PCR in OCI-LY3 and OCI-LY19.
DLBCL cell lines express Trk protein and mRNA
We also analyzed Trk expression in DLBCL cell lines that were derived from primary tumors. These include OCI-LY3, OCI-LY19, OCI-LY10, OCI-LY7 and SUDHL6. Using a pan-Trk antibody, which recognizes conserved regions in TrkA, TrkB and TrkC, we performed immunoblot analysis on cell lysates from these cell lines. Our results show that all DLBCL cell lines tested express Trk protein (Figure 1B). In order to delineate the specific Trk receptors being expressed, we selected one ABC cell line (OCI-LY3) and one GC cell line (OCI-LY19) to perform reverse transcriptase PCR for individual Trk receptors. As shown in Figure 1C, OCI-LY3 contained mRNA for all 3 Trk receptor types, whereas OCI-LY19 was positive for only TrkB and TrkC mRNA. Interestingly, our RT-PCR analyses revealed expression of the truncated form of TrkB (gp95), but not full-length TrkB (gp145), in both the cell lines.
Primary NHL and DLBCL cell lines produce neurotrophin
Next, we investigated whether the cells derived from primary NHL tumors or DLBCL cell lines also produced neurotrophins. To do this, we isolated total RNA from a panel of primary NHL cells, maintained in culture for 3 days, and performed RT-PCR using primers specific for transcripts of NGF, BDNF and NT-3. We found that 8 of 8 NHL specimens tested produced NGF and NT-3 mRNA, whereas only 1 patient sample exhibited BDNF mRNA (Figure 2A). The production of NGF protein was confirmed in 06TSP0095 (5-6.5pg/mL) and 07TXN0054 (2-4pg/mL) via ELISA (data not shown). Because primary tumor samples contain a mixed population of cells, we then performed the same analysis on DLBCL cell lines, which represent a homogeneous population of malignant B cells. Both OCI-LY3 and OCI-LY19 produced mRNA for NGF, BDNF and NT-3 (Figure 2B). GAPDH was used as a control as in Figure 1C (data not shown). These results were further confirmed by ELISA, in which LY3 produced 16-26pg/mL of NT-3, and LY19 produced 26-40pg/mL of NT-3. Levels of NGF and BDNF in these cell lines, as well as in other OCI lines LY10 and LY7, were below the range of detection for this assay (data not shown).
Figure 2.
Primary Non-Hodgkin Lymphoma and DLBCL cell lines produce neurotrophins. (A) RT-PCR analysis was performed on NHL patient biopsies using primers specific to NGF, BDNF, NT-3 and GAPDH transcripts. (B) A similar analysis as in (A) was performed on OCI-LY3 and OCI-LY19.
Pharmacologic inhibition of Trk receptors inhibits proliferation of primary NHL cells and DLBCL cell lines
The presence of both Trk receptors and neurotrophin suggest the possibility of an autocrine mechanism of neurotrophin signaling in B cell lymphoma. Thus, we undertook experiments using the Trk tyrosine kinase inhibitor, K252a, to determine the role of Trk receptors in promoting proliferation of primary NHL cells and DLBCL cell lines. First, we suspended primary NHL cells in RPMI 1640 medium that was supplemented with recombinant human IL-4 (rhIL-4; 8ng/mL). These cells were then placed on a monolayer of CD40L-expressing NIH 3T3 cells as previously described [16] and exposed to BrdU for 1h. Cell proliferation was then measured by flow cytometry. The combination of CD40L and rhIL-4 provided an environment stimulatory for proliferation of primary NHL cells. This approach was chosen due to the high level of basal cell death in cultures maintained in RPMI 1640 medium alone (data not shown). As shown in Figure 3A and 3B, two patient samples (07TLN0154 and 06TLN0090) contained approximately 5% of the CD20-positive cells that were in S-phase (these cells were positive for BrdU incorporation). However, the number of CD20-positive cells in S-phase was dramatically reduced in cultures that were pretreated with increasing amounts of K252a. The third patient sample (06TPL0027) contained a relatively high percentage (over 40%) of CD20-positive cells in S-phase that was minimally reduced following K252a treatment (Figure 3C). These results were consistent with the initial analyses of NHL specimens in which overall proliferation rate was determined to be 15% by staining of the nuclear antigen Ki-67 (this rate varies from 10% to 40% focally; these analyses were performed by the Strong Memorial Hospital Pathology Laboratory). It is important to note that K252b, which has a much reduced membrane solubility as compared to K252a, exerted no effect on BrdU incorporation. This effect of K252a was also observed in tumor cells (07TLN0154) pulsed with BrdU for 16 hours (data not shown).
Figure 3.
K252a inhibits proliferation of primary DLBCL cells. (A-C) Cells obtained from patients diagnosed with DLBCL were cultured in the presence of K252a (100nM) over CD40L-expressing NIH 3T3 cells in combination with rhIL-4 for 48 hours. The percentage of cells in S-phase was determined after a 1h pulse with BrdU. BrdU incorporation of CD20 positive cells was measured by flow cytometry. Shown are results from 3 patients. K252a results in a substantial decrease in the percentage of cells in S-phase, as compared to DMSO and K252b (100nM) controls.
In order to further confirm our results, we exposed OCI-LY3 and OCI-LY19 cell lines to increasing concentrations of K252a or AG879 (this reagent is also known to block Trk receptors at less than 10μM concentration [23]) for either 24h or 48h. Here, we added 1μCi of 3H-thymidine to the cultures 18h prior to the end of the treatment. Analysis of incorporated thymidine into DNA revealed a reduction in proliferation of both OCI-LY3 and OCI-LY19 cells by 24 hours (Figure 4A and 4C, left panel). This effect was even more dramatic by 48 hours (Figure 4B and 4D, right panel). Together, these results suggest that an autocrine neurotrophin signaling mechanism is in effect in both primary NHL cells and DLBCL cell lines, which stimulates cell proliferation.
Figure 4.
K252a inhibits proliferation of DLBCL cell lines. (A-D) OCI-LY3 and OCI-LY19 were cultured for 24 and 48h in the presence of K252a (100nM and 200nM), AG879 (5μM and 10μM) and DMSO. Proliferation was measured using 3H-thymidine uptake analysis. Both LY3 and LY19 exhibited a significant reduction in proliferation in response to K252a as compared to DMSO control. Results shown are mean +/- SEM of 3 trials. # equals p<0.05, ## equals p<0.01, ### equals p<0.001.
We also tested whether K252a is capable of blocking tyrosine kinase activity of Trk receptors in OCI-LY3. To do this, the serum was removed from cultures of OCI-LY3. One hour later, the cells were treated with K252a (100nM) for 15 minutes and then acutely (5 minutes) exposed to exogenous NGF (200ng/ml) to activate Trk receptors. Trk molecules were then immunoprecipitated from cellular lysates by addition of pan-Trk antibodies followed by protein-A-agarose beads. The immune-complexes were resolved by SDS-PAGE and Trk phosphorylation was assessed by immunoblot analysis. As expected, addition of NGF resulted in an increase in tyrosine phosphorylation of Trk, which was blocked by pre-treatment with K252a (data not shown). These results support the notion that K252a inhibits Trk receptor signaling in OCI-LY3 cells.
Inhibitors of Trk receptor induces apoptosis in DLBCL cells
To test if Trk receptors promote cellular survival, we exposed OCI-LY3 and OCI-LY19 to K252a or AG879 for 48 hours. The cellular apoptosis was then measured by conducting Annexin V and propidium iodide staining followed by flow cytometric analysis. As shown in Figure 5A, OCI-LY3 underwent dose-dependent apoptosis after exposure to K252a or AG879, reaching up to approximately 70% total Annexin V positive cells at 200nM of K252a. Notably, the poorly membrane permeable Trk inhibitor, K252b, did not affect the survival of OCI-LY3 (data not shown). Interestingly, neither K252a nor AG879 resulted in apoptosis of OCI-LY19 (Figure 5B), thereby suggesting that K252a’s effects on LY19 are limited to inhibition of proliferation. We performed additional experiments in which we found that OCI-LY19 cells possess spontaneous caspase 9 activity, whereas OCI-LY3 cells do not (data not shown). Under these conditions OCI-LY19 cells survive, presumably due to high expression and activity of XIAP in these cells [24]. Therefore, we speculate that the resistance of OCI-LY19 to the toxic effects of K252a might be due to the abnormal activity of certain apoptosis-related proteins in these cells.
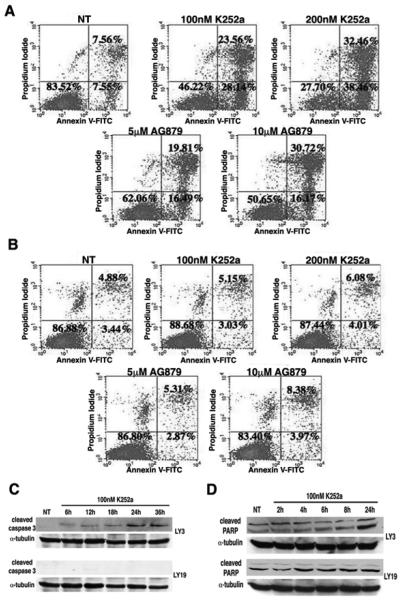
Figure 5.
K252a and AG879 induce apoptosis in OCI-LY3. (A) OCI-LY3 treated with 100nM and 200nM K252a for 48 hours were analyzed for apoptosis by flow cytometry using Annexin V and propidium iodide as indicators of cell death. Both K252a and AG879 dose-dependently increased apoptosis of LY3 as compared to DMSO control. (B) A similar analysis as in (A) was performed on OCI-LY19. Neither K252a nor AG879 had an effect on survival of LY19. (C) K252a induces expression of cleaved caspase 3 in OCI-LY3. OCI-LY3 and OCI-LY19 were exposed to 100nM K252a for the indicated time points. Immunoblot analysis using antibodies against cleaved caspase 3 show increased cleaved caspase 3 levels in OCI-LY3 upon exposure to K252a. No effect is seen in OCI-LY19. (D) K252 induces cleavage of PARP in OCI-LY3. OCI-LY3 and OCI-LY19 were exposed to K252a (100nM) for the indicated time points. Cell lysates were collected and immunoblot was performed using anti-cleaved PARP. OCI-LY3 demonstrated an increase in cleaved PARP by 24h, whereas OCI-LY19 possessed constitutively cleaved PARP levels.
Additional features of cellular apoptosis were examined in K252a-treated DLBCL cells by performing immunoblot analyses. Our results revealed an activation of caspase 3 (as indicated by appearance of cleaved caspase 3 immunoreactive bands) in OCI-LY3 cells as early as 6h of exposure to 100nM K252a, which peaked at 24h and remained elevated until 36h of treatment (Figure 5C, upper panel). This effect of K252a was not observed in OCI-LY19 cells (Figure 5C, lower panel). As activation of caspases results in the cleavage of downstream substrates, we next examined levels of cleaved Poly (ADP-Ribose) Polymerase (PARP) in K252a treated cells. Elevated levels of cleaved PARP were also detected in OCI-LY3 cells following 24h of exposure to K252a (Figure 5D, upper panel). In contrast, K252a treatment failed to induce changes in cleaved PARP levels in OCI-LY19 cells (Figure 5D, lower panel).
Normal B cells express Trk, neurotrophin and are sensitive to K252a
Given the ability of K252a to inhibit proliferation and survival of malignant B cells, we next wanted to examine its effects on normal B cells isolated from healthy donors. First, we sought to verify Trk receptor expression in resting, as well as activated, normal human B cells. To do this, CD19 positive B cells were isolated from 3 healthy donors and exposed to various immunologic stimuli for a defined period. In this case, the flow cytometric analyses of cells treated with CpG plus anti-IgM exhibited a characteristic phenotype of activated B-cells (Figure 6A). Following the treatment, whole-cell lysates were subjected to immunoblot analyses. As shown in Figure 6B, varying levels of Trk receptor were detected in resting, as well as activated B cells. Second, the culture supernatants were collected from the very same experiments and the levels of both NGF and BDNF were analyzed by ELISA. Our results indicate that resting and activated B cells produce varying levels of BDNF in the range of 18-26 pg/ml (BDNF) and values below the detection limit of NGF. However, both NGF and BDNF production in normal human B cells has been demonstrated in previous reports [10, 25].
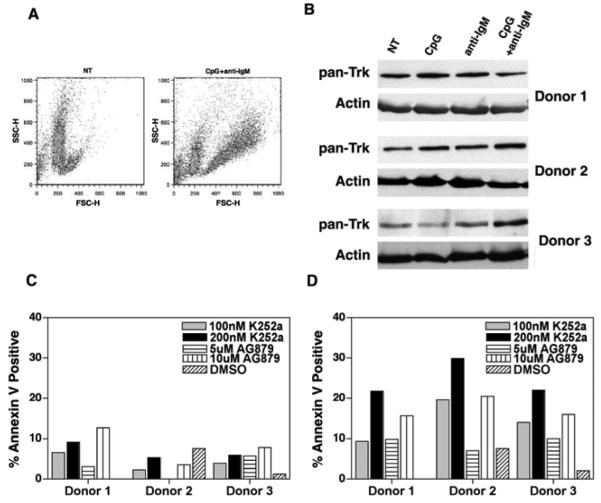
Figure 6.
Normal B cells express Trk, neurotrophin and are sensitive to the effects of K252a. (A) Flow cytometric analysis of resting cells (NT) and cells stimulated with CpG plus anti-IgM reveal a characteristic forward and side scatter pattern that is consistent with cell activation. (B) Cell lysates of normal B cells obtained from 3 healthy donors were immunoblotted with antibodies against pan-Trk and actin. As shown, resting cells (labeled as NT) express Trk receptors. Upon activation with the signals CpG and CpG plus anti-IgM, cells maintain Trk expression. (C and D) Resting normal human B cells (first panel) and activated B cells (second panel) were exposed to K252a (100nM and 200nM) and AG879 (5μM and 10μM) for 48h. Apoptosis was measured by flow cytometry using Annexin V and propidium iodide. Resting cells exhibited very low levels of cell death in the presence of both K252 and AG879, as compared to DMSO. In contrast, levels of apoptosis increased in activated cells exposed to drugs.
Finally, to explore whether pharmacologic inhibition of Trk induces apoptosis in normal B cells (resting and activated B cells), the cells were treated with K252a or AG879 for 48 hours. Apoptosis was then assessed by using flow cytometric evaluation of Annexin V and propidium iodide staining. Interestingly, K252a or AG879 treatment induced low levels of apoptosis in resting B cells (Figure 6C, left panel); this effect of K252a or AG879 was enhanced in B cells that were pre-exposed to CpG plus anti-IgM (Figure 6D, right panel).
K252a inhibits NF-κB activity in OCI-LY3 cells
Because previous studies have implicated constitutive activation of NF-κB in promoting survival of OCI-LY3 (ABC subtype of DLBCL), and to understand better how K252a might influence survival of DLBCL cells, we examined the effect of K252a on NF-κB activation in OCI-LY3 cells. To test whether K252a influences NF-κB activation, we analyzed all three of the main events of NF-κB signaling in OCI-LY3. Briefly, the cells were treated with K252a and nuclear extracts were prepared at various time intervals. The lysates (nuclear and cytoplasmic) were first subjected to immunoblot analyses in which RelA -specific antibodies were used. A strong RelA band was detected in the lysates of untreated OCI-LY3 cells. The levels of RelA were not changed in the nucleus nor in the cytosol, following addition of K252a, indicating marked failure of K252a in blocking nuclear translocation of RelA (Figure 7A). Next, we used same nuclear lysates to conduct EMSA in which NF-κB- and Oct-1-specific, radiolabeled, double-stranded oligonucleotide probes were employed. A high level of NF-κB-specific DNA binding activity was observed in untreated OCI-LY3 cells. Whereas, addition of K252a failed to inhibit the overall level of NF-κB-specific DNA binding activity (densitometric analyses revealed that the modest alterations in DNA binding were not statistically significant) (Figure 7B). Similarly, the Oct-1-specific DNA binding activity remained unaltered following the addition of K252a for equivalent duration.
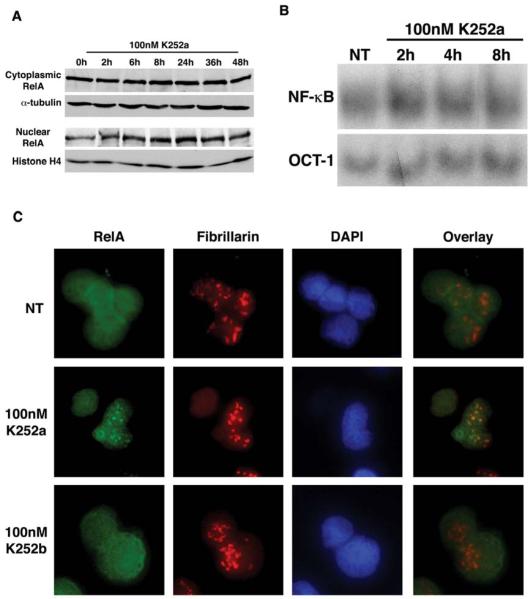
Figure 7.
K252a inhibits NF-κB-dependent transcription, which is associated with nucleolar sequestration of RelA. (A) The nuclear translocation of RelA was analyzed through immunblot analysis of cytoplasmic and nuclear extracts of OCI-LY3. K252a (100nM) induces a modest increase in RelA levels in the nucleus. (B) OCI-LY3 cells were treated with 100nM K252a for the indicated time points, and nuclear lysates were analyzed by EMSA. K252a induces a modest increase in NF-κB DNA binding activity (C) RelA localization in K252a and K252b treated OCI-LY3 cells was analyzed by indirect immunofluorescence assay using RelA and fibrillarin specific antibodies. K252a results in a redistribution of RelA, which co-localizes with the nucleolar marker, fibrillarin.
Nuclear translocation of NF-κB can be best studied by microscopic examination of intact cells. Therefore, we treated the OCI-LY3 cells with K252a or K252b (100 nM each) for 16h. The cells were then fixed, permeabilized and immunocytochemically stained with RelA antibodies. As expected, the untreated cells contain high levels of RelA accumulated in the nucleoplasm (Figure 7C; as determined by the RelA immunoreactivity that overlaps with DAPI staining). Interestingly, following incubation of the cells with K252a, but not K252b, RelA molecules became compartmentalized into distinct nuclear bodies. Since the eukaryotic nucleus contains a number of well-defined entities, including splicing speckles, Cajal bodies, chromatin domains and nucleoli [26], we argued that RelA was compartmentalized within the nucleolus. This was verified by immunocytochemical analysis demonstrating that K252a-induced nuclear RelA colocalizes with the nucleolar protein fibrillarin (Figure 7C).
Next, we tested whether K252a inhibited NF-κB-dependent transcription in OCI-LY3 cells. The cells were transiently transfected with a reporter plasmid in which the luciferase gene was placed under the control of a minimal promoter containing NF-κB responsive elements [27]. Recipient cells were then exposed to varying amounts of K252a or 100nM K252b (control). Twelve hours later, the cells were disrupted and luciferase activity was measured. As shown in Figure 8A, K252a dose-dependently inhibited transcription of luciferase, indicating the inhibition of endogenous NF-κB. This effect was not observed in K252b-treated cells. We posit that the inhibition of NF-κB activity by K252a might be due to compartmentalization of RelA into nucleolar bodies.
Figure 8.
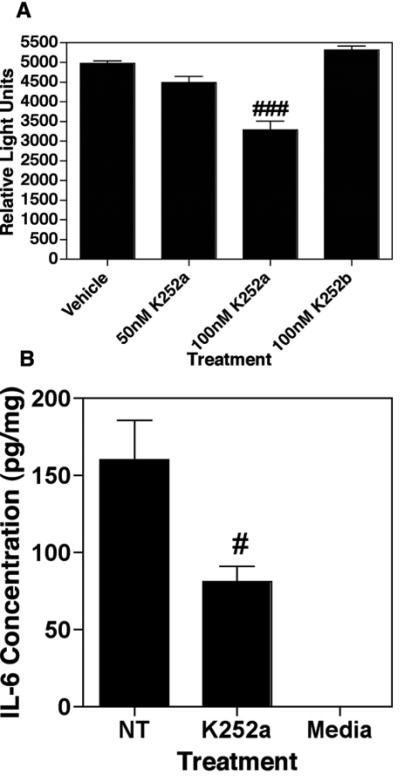
K252a treatment results in inhibition of NF-κB transcriptional activity and IL-6 production in OCI-LY3. (A) NF-κB transcriptional activity was analyzed in a luciferase assay of K252a treated OCI-LY3. Exposure of these cells to K252a leads to an inhibition of NF-κB-dependent transcriptional activity. Results are mean +/- SEM of 3 observations. ### equals p<0.001. (B) K252a treatment inhibits IL-6 production in OCI-LY3. Cells were treated with 100nM K252a for 24h followed by ELISA analysis of supernatants for IL-6. Results are mean +/- SEM of 3 observations. # equals p<0.05.
Since, the expression of IL-6 is known to be regulated by NF-κB [28, 29], we tested whether K252a also blocks production of IL-6, in a manner that might be important for the survival of OCI-LY3 cells [30]. The cells were exposed to K252a (100 nM) for 24h and the culture supernatants were collected for measuring IL-6 levels by ELISA. Our data reveal that K252a inhibits IL-6 production in OCI-LY3 cells (Figure 8B). It is important to note that IL-6 levels were measured based on total amount of protein so as to minimize the possibility that the observed effect was due to cell death.
Relative sensitivity of other cancer cells to K252a
To extend our findings to other cancer types, we analyzed expression of Trk and neurotrophins (NGF and BDNF) in cell lines derived from breast cancer (MCF-7, MDA MB231, SKBR3), Burkitt’s lymphoma (Ramos, Raji, Daudi), and multiple myeloma (RPMI-8226). These analyses were also performed in transformed cell lines derived from monocytes (THP-1), embryonic kidney (293T) and microglia (CHME-5). As depicted in Table II, each cell line possessed varying levels of Trk and neurotrophin expression. Interestingly, when we exposed the cells to K252a (100nM), there was a positive correlation between elevated Trk and neurotrophin expression with sensitivity to K252a. These results further support our conclusions that the cytotoxic effects of K252a may involve disruption of neurotrophin signaling through Trk receptors.
Table II. Sensitivity of Cell Lines to K252a.
A panel of cancer cell lines was analyzed for Trk expression via immunoblot, neurotrophin expression by ELISA, and cell death after exposure to 100nM K252a or 10μM AG879 as indicated. Suspension cells were analyzed for apoptosis by flow cytomety using Annexin V and propidium iodide staining. Apoptosis in adherent cells was determined by MTT assay and ApoPercentage Assay. ‘+’ indicates low level of Trk or neurotrophin expression, ‘++’ indicates intermediate expression levels and ‘+++’ indicates high expression levels. NA indicates assay not performed because it was not appropriate for designated cell type. ND indicated not determined due to variability in results. +( indicates sensitivity to K252a and AG879.
| Cell Line | Trk Expression | NGF | BDNF | MTT | ApoPercentage | Annexin V |
|---|---|---|---|---|---|---|
| 293T | ++ | + | - | - | +ψ | NA |
| CHME5 | + | +++ | - | +ψ | ND | NA |
| MCF-7 | ++ | + | - | +ψ | +ψ | NA |
| MDA MB231 | + | - | + | - | - | NA |
| THP-1 | +++ | - | + | NA | NA | +ψ |
| Ramos | ++ | - | - | NA | NA | - |
| Raji | + | - | - | NA | NA | - |
| Daudi | ++ | - | - | NA | NA | - |
| RPMI-8226 | + | - | - | NA | NA | - |
DISCUSSION
Overall, our study provides strong evidence for the existence of a survival and proliferation promoting autocrine neurotrophin loop in Non-Hodgkin Lymphoma. The results presented in this study point to the potential of K252a as a novel therapeutic agent for Non-Hodgkins Lymphoma. While the Trk blocker, CEP-701, has been investigated in clinical trials for the treatment of prostate and pancreatic cancer, the results have yet to show promise. An obstacle for the use of CEP-701 is its ability to bind to serum proteins, thereby limiting the amount of free drug for tumor penetration, as well as the limited ability of the drug to penetrate a tumor [31]. Therefore, new Trk blockers are needed as alternatives. As of yet, there have been no clinical trials utilizing K252a as a therapy for any type of cancer. This is likely due to the limited availability of the drug as it is derived from Nocardiopsis sp, and the only source is microbial fermentation. However, our results clearly demonstrate the potential for this drug in NHL therapy.
We demonstrate the presence of a functional neurotrophin signaling loop in primary DLBCL cells by using the Trk-specific blocker, K252a. This alkaloid-like compound was originally characterized as an inhibitor of protein kinase C (IC50 being 32.9 nM; [32]). However, Tapley P. et al [33] has performed in vitro assays to show that K252a potently blocks tyrosine kinase activity of NGF receptor TrkA and other isoforms (TrkB and TrkC) at much lower IC50 (3 nM). In addition, K252a was found to have no effects (even at micromolar concentrations) on other tyrosine protein kinases such as the receptors for EGF and PDGF and the products of the v-src and v-fms oncogenes. The inhibitory actions of K252a on NGF-induced Trk activity were also been confirmed in PC12-cells treated with 200nM of K252a [34]. In these cell-based assays, Berg M.M. et al showed that the effects of K252a (200nM) does not include similar biological responses mimicked by other growth factors (such as EGF and bFGF) in PC-12 cells. Numerous studies thereafter have used nanomolar quantities of K252a as a selective inhibitor of neurotrophin signaling [35-40].
Our data indicate that K252a dose-dependently reduces the number of CD20-positive B cells in S-phase. This result is supported by previous work demonstrating that NGF has the ability to induce cells to enter S-phase [41] while K252a has been shown to reduce the number of malignant cells in S-phase thereby resulting in an accumulation of cells in G0/G1 [42, 43]. Similarly, in the DLBCL cell line, OCI-LY19, addition of K252a or an alternate Trk tyrosine kinase blocker, AG879, resulted in a dose-dependent decrease in proliferation within 24 hours. Such anti-proliferative effects of K252a are observed in previous studies where prostate and lung cancer cells were treated with this drug [44, 45]. Although our studies do not address the mechanism by which K252a inhibits cell proliferation, we speculate that K252a might induce cell cycle arrest in DLBCL cells through linked events, such as hypophosphorylation of retinoblastoma protein, upregulation of p21 and decreased Cdc2 and Cdc25c activity, similar to the one shown in K252a-treated glioma cells [46].
Even more attractive is K252a’s ability to induce apoptosis through subnuclear redistribution of RelA, as shown in the present study. Given the ability of most chemotherapeutic agents to induce cell death in normal cells in addition to malignant cells, it would be ideal to identify a therapeutic target, which is toxic to cancerous cells while keeping healthy cells intact. In this context, it is noteworthy that the exposure of resting B cells to K252a resulted in limited cell death in the 3 donors tested, while activated B cells appear to be more sensitive to K252a. Based on the degree of cell death incurred by K252a-exposed OCI-LY3 cells, it is highly likely that the malignant B cells would prove more sensitive to K252a than their normal counterpart, which is an enticing feature for a potential therapy.
Nucleolar targeting of RelA is a novel mechanism of NF-κB regulation, yet not entirely surprising as NF-κB is known to be regulated on several levels including phosphorylation, acetylation, sumoylation, ubiquitination as well as dimer exchange. Furthermore, NF-κB regulating proteins, including NIK and ARF, are controlled through nucleolar sequestration as well [47, 48]. A more detailed analysis of this pathway showed that such relocalization of RelA was mediated by p38-mediated inactivation of the cyclin D1/cdk4 complex [49]. Additionally, direct inhibition of cdk4 is sufficient for nucleolar targeting of RelA [50]. Given the ability of NGF withdrawal to induce activation of p38 and subsequent apoptosis in both neurons and B cells [51, 52], it is likely that K252a also produces the same effect on cancer cells. The inhibition of NF-κB is an appealing therapeutic option for a variety of cancers, however, complete pathway inhibition is very toxic to healthy cells of the body. Velcade, a current NF-κB blocker being used as a chemotherapeutic agent, is proving to be successful likely due to its indirect blockade of NF-κB (as a proteasome inhibitor). Therefore, K252a may show similar promise because it essentially leaves all components of the NF-κB pathway intact.
In summary, the results we have presented outline a novel therapeutic for the treatment of Non-Hodgkin Lymphoma, which may be extended to a variety of other cancers.
Table I. Primary NHL Immunophenotypes.
Immunophenotype of representative patient samples. The Strong Memorial Hospital Pathology Laboratory performed flow cytometric and immunohistochemical analyses of patient tumor samples.
| Patient Specimens | Immunophenotype data summary |
|---|---|
| 06TLN0023 | CD19+, CD20+, CD5+, CD10-, CD23+, FMC7-, Clonal kappa+ |
| 06TLN0046 | CD19+, CD20+, CD45+, CD10+, CD23+, FMC7-, CD3-, BCL2+, Clonal lambda+ |
| 06TLN0031 | CD19+, CD20+, CD5-, CD22+, CD10+, CD23+, CD79+, FMC7+, CD38-, CD3-, BCL6+, Clonal kappa+ |
| 06TLN0090 | CD19+, CD20+, CD5-, CD10+, CD23+, CD3-, CD79a+, BCL2+, BCL6+, Clonal lambda+ |
| 07TLN0154 | Data unavailable |
| 07TLN0128 | CD19+, CD20+, CD10+, CD79a+, FMC7+, BCL2+, BCL6+, Clonal lambda+ |
| 07TLN0103 | CD19+, CD20+, CD5+, CD10+, CD45+, Clonal kappa+ |
| 06TPL0027 | CD19+, CD20+, CD5-, CD10+, CD22+, CD23-, FMC7-, Clonal kappa+ |
| 07TXN0131 | CD19+, CD20+, CD10-, CD45+, CD79a+, BCL2-, BCL6-, Light chains- |
Acknowledgements
We thank Drs. Robert Rose, Martin Zand, Lou Staudt for generously providing us with reagents. We also thank Christopher Lane and Tina Pellegrin for technical assistance. We generously thank the University of Rochester Pathology Department for patient samples and cytometry data. This work was supported by the following NIH grants: RO1 NS054578 (SBM); ES01247, DE011390 (RPP); T32 HL007152 (TGB); T32 AI49105 (LS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure
he authors have no financial conflicts of interest with the contents of this manuscript.
References
- [1].Fisher RI, Shah P. Current trends in large cell lymphoma. Leukemia. 2003;17:1948–1960. doi: 10.1038/sj.leu.2403096. [DOI] [PubMed] [Google Scholar]
- [2].Dave SS, Wright G, Tan B, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- [3].Rosenwald A, Staudt LM. Gene expression profiling of diffuse large B-cell lymphoma. Leuk Lymphoma. 2003;44(Suppl 3):S41–47. doi: 10.1080/10428190310001623775. [DOI] [PubMed] [Google Scholar]
- [4].Hunt KE, Reichard KK. Diffuse large B-cell lymphoma. Arch Pathol Lab Med. 2008;132:118–124. doi: 10.5858/2008-132-118-DLBL. [DOI] [PubMed] [Google Scholar]
- [5].Dionne CA, Camoratto AM, Jani JP, et al. Cell cycle-independent death of prostate adenocarcinoma is induced by the trk tyrosine kinase inhibitor CEP-751 (KT6587) Clin Cancer Res. 1998;4:1887–1898. [PubMed] [Google Scholar]
- [6].Brodeur GM, Nakagawara A, Yamashiro DJ, et al. Expression of TrkA, TrkB and TrkC in human neuroblastomas. J Neurooncol. 1997;31:49–55. doi: 10.1023/a:1005729329526. [DOI] [PubMed] [Google Scholar]
- [7].Martin-Zanca D, Mitra G, Long LK, Barbacid M. Molecular characterization of the human trk oncogene. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):983–992. doi: 10.1101/sqb.1986.051.01.112. [DOI] [PubMed] [Google Scholar]
- [8].Ehrhard PB, Erb P, Graumann U, Otten U. Expression of nerve growth factor and nerve growth factor receptor tyrosine kinase Trk in activated CD4-positive T-cell clones. Proc Natl Acad Sci U S A. 1993;90:10984–10988. doi: 10.1073/pnas.90.23.10984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Melamed I, Kelleher CA, Franklin RA, et al. Nerve growth factor signal transduction in human B lymphocytes is mediated by gp140trk. Eur J Immunol. 1996;26:1985–1992. doi: 10.1002/eji.1830260903. [DOI] [PubMed] [Google Scholar]
- [10].Torcia M, Bracci-Laudiero L, Lucibello M, et al. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell. 1996;85:345–356. doi: 10.1016/s0092-8674(00)81113-7. [DOI] [PubMed] [Google Scholar]
- [11].Kronfeld I, Kazimirsky G, Gelfand EW, Brodie C. NGF rescues human B lymphocytes from anti-IgM induced apoptosis by activation of PKCzeta. Eur J Immunol. 2002;32:136–143. doi: 10.1002/1521-4141(200201)32:1<136::AID-IMMU136>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- [12].Jost PJ, Ruland J. Aberrant NF-kappaB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood. 2007;109:2700–2707. doi: 10.1182/blood-2006-07-025809. [DOI] [PubMed] [Google Scholar]
- [13].Bargou RC, Emmerich F, Krappmann D, et al. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells. J Clin Invest. 1997;100:2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lam LT, Davis RE, Pierce J, et al. Small molecule inhibitors of IkappaB kinase are selectively toxic for subgroups of diffuse large B-cell lymphoma defined by gene expression profiling. Clin Cancer Res. 2005;11:28–40. [PubMed] [Google Scholar]
- [15].Sanchez JF, Sniderhan LF, Williamson AL, Fan S, Chakraborty-Sett S, Maggirwar SB. Glycogen synthase kinase 3beta-mediated apoptosis of primary cortical astrocytes involves inhibition of nuclear factor kappaB signaling. Mol Cell Biol. 2003;23:4649–4662. doi: 10.1128/MCB.23.13.4649-4662.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zand MS, Bose A, Vo T, et al. A renewable source of donor cells for repetitive monitoring of T- and B-cell alloreactivity. Am J Transplant. 2005;5:76–86. doi: 10.1111/j.1600-6143.2003.00637.x. [DOI] [PubMed] [Google Scholar]
- [17].D’Onofrio M, de Grazia U, Morrone S, et al. Expression of neurotrophin receptors in normal and malignant B lymphocytes. Eur Cytokine Netw. 2000;11:283–291. [PubMed] [Google Scholar]
- [18].Schenone A, Gill JS, Zacharias DA, Windebank AJ. Expression of high- and low-affinity neurotrophin receptors on human transformed B lymphocytes. J Neuroimmunol. 1996;64:141–149. doi: 10.1016/0165-5728(95)00162-x. [DOI] [PubMed] [Google Scholar]
- [19].Limpert AS, Karlo JC, Landreth GE. Nerve growth factor stimulates the concentration of TrkA within lipid rafts and extracellular signal-regulated kinase activation through c-Cbl-associated protein. Mol Cell Biol. 2007;27:5686–5698. doi: 10.1128/MCB.01109-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Li H, Ayer LM, Polyak MJ, et al. The CD20 calcium channel is localized to microvilli and constitutively associated with membrane rafts: antibody binding increases the affinity of the association through an epitope-dependent cross-linking-independent mechanism. J Biol Chem. 2004;279:19893–19901. doi: 10.1074/jbc.M400525200. [DOI] [PubMed] [Google Scholar]
- [21].Cherukuri A, Shoham T, Sohn HW, et al. The tetraspanin CD81 is necessary for partitioning of coligated CD19/CD21-B cell antigen receptor complexes into signaling-active lipid rafts. J Immunol. 2004;172:370–380. doi: 10.4049/jimmunol.172.1.370. [DOI] [PubMed] [Google Scholar]
- [22].Labouyrie E, Parrens M, de Mascarel A, Bloch B, Merlio JP. Distribution of NGF receptors in normal and pathologic human lymphoid tissues. J Neuroimmunol. 1997;77:161–173. doi: 10.1016/s0165-5728(97)00055-6. [DOI] [PubMed] [Google Scholar]
- [23].Ohmichi M, Pang L, Ribon V, Gazit A, Levitzki A, Saltiel AR. The tyrosine kinase inhibitor tyrphostin blocks the cellular actions of nerve growth factor. Biochemistry. 1993;32:4650–4658. doi: 10.1021/bi00068a024. [DOI] [PubMed] [Google Scholar]
- [24].Cillessen SA, Hess CJ, Hooijberg E, et al. Inhibition of the intrinsic apoptosis pathway downstream of caspase-9 activation causes chemotherapy resistance in diffuse large B-cell lymphoma. Clin Cancer Res. 2007;13:7012–7021. doi: 10.1158/1078-0432.CCR-06-2891. [DOI] [PubMed] [Google Scholar]
- [25].Kerschensteiner M, Gallmeier E, Behrens L, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Belmont A. Dynamics of chromatin, proteins, and bodies within the cell nucleus. Curr Opin Cell Biol. 2003;15:304–310. doi: 10.1016/s0955-0674(03)00045-0. [DOI] [PubMed] [Google Scholar]
- [27].Sui Z, Sniderhan LF, Fan S, et al. Human immunodeficiency virus-encoded Tat activates glycogen synthase kinase-3beta to antagonize nuclear factor-kappaB survival pathway in neurons. Eur J Neurosci. 2006;23:2623–2634. doi: 10.1111/j.1460-9568.2006.04813.x. [DOI] [PubMed] [Google Scholar]
- [28].Gruss HJ, Ulrich D, Dower SK, Herrmann F, Brach MA. Activation of Hodgkin cells via the CD30 receptor induces autocrine secretion of interleukin-6 engaging the NF-kappabeta transcription factor. Blood. 1996;87:2443–2449. [PubMed] [Google Scholar]
- [29].Keller SA, Hernandez-Hopkins D, Vider J, et al. NF-kappaB is essential for the progression of KSHV- and EBV-infected lymphomas in vivo. Blood. 2006;107:3295–3302. doi: 10.1182/blood-2005-07-2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yee C, Biondi A, Wang XH, et al. A possible autocrine role for interleukin-6 in two lymphoma cell lines. Blood. 1989;74:798–804. [PubMed] [Google Scholar]
- [31].Collins C, Carducci MA, Eisenberger MA, et al. Preclinical and clinical studies with the multi-kinase inhibitor CEP-701 as treatment for prostate cancer demonstrate the inadequacy of PSA response as a primary endpoint. Cancer Biol Ther. 2007;6:1360–1367. doi: 10.4161/cbt.6.9.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kase H, Iwahashi K, Matsuda Y. K-252a, a potent inhibitor of protein kinase C from microbial origin. J Antibiot (Tokyo) 1986;39:1059–1065. doi: 10.7164/antibiotics.39.1059. [DOI] [PubMed] [Google Scholar]
- [33].Tapley P, Lamballe F, Barbacid M. K252a is a selective inhibitor of the tyrosine protein kinase activity of the trk family of oncogenes and neurotrophin receptors. Oncogene. 1992;7:371–381. [PubMed] [Google Scholar]
- [34].Berg MM, Sternberg DW, Parada LF, Chao MV. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J Biol Chem. 1992;267:13–16. [PubMed] [Google Scholar]
- [35].von Bartheld CS, Williams R, Lefcort F, Clary DO, Reichardt LF, Bothwell M. Retrograde transport of neurotrophins from the eye to the brain in chick embryos: roles of the p75NTR and trkB receptors. J Neurosci. 1996;16:2995–3008. doi: 10.1523/JNEUROSCI.16-09-02995.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Caldeira MV, Melo CV, Pereira DB, et al. Brain-derived neurotrophic factor regulates the expression and synaptic delivery of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J Biol Chem. 2007;282:12619–12628. doi: 10.1074/jbc.M700607200. [DOI] [PubMed] [Google Scholar]
- [37].Merighi A, Bardoni R, Salio C, et al. Presynaptic functional trkB receptors mediate the release of excitatory neurotransmitters from primary afferent terminals in lamina II (substantia gelatinosa) of postnatal rat spinal cord. Dev Neurobiol. 2008;68:457–475. doi: 10.1002/dneu.20605. [DOI] [PubMed] [Google Scholar]
- [38].Iwakura Y, Nawa H, Sora I, Chao MV. Dopamine D1 receptor-induced signaling through TrkB receptors in striatal neurons. J Biol Chem. 2008;283:15799–15806. doi: 10.1074/jbc.M801553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Bozdagi O, Rich E, Tronel S, et al. The neurotrophin-inducible gene Vgf regulates hippocampal function and behavior through a brain-derived neurotrophic factor-dependent mechanism. J Neurosci. 2008;28:9857–9869. doi: 10.1523/JNEUROSCI.3145-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nguyen N, Lee SB, Lee YS, Lee KH, Ahn JY. Neuroprotection by NGF and BDNF against neurotoxin-exerted apoptotic death in neural stem cells are mediated through Trk receptors, activating PI3-kinase and MAPK pathways. Neurochem Res. 2009;34:942–951. doi: 10.1007/s11064-008-9848-9. [DOI] [PubMed] [Google Scholar]
- [41].Cordon-Cardo C, Tapley P, Jing SQ, et al. The trk tyrosine protein kinase mediates the mitogenic properties of nerve growth factor and neurotrophin-3. Cell. 1991;66:173–183. doi: 10.1016/0092-8674(91)90149-s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Takai N, Ueda T, Nishida M, Nasu K, Narahara H. K252a is highly effective in suppressing the growth of human endometrial cancer cells, but has little effect on normal human endometrial epithelial cells. Oncol Rep. 2008;19:749–753. [PubMed] [Google Scholar]
- [43].Takai N, Ueda T, Nishida M, Nasu K, Fukuda J, Miyakawa I. K252a inhibits proliferation of ovarian cancer cells by upregulating p21WAF1. Oncol Rep. 2005;14:141–143. [PubMed] [Google Scholar]
- [44].Delsite R, Djakiew D. Anti-proliferative effect of the kinase inhibitor K252a on human prostatic carcinoma cell lines. J Androl. 1996;17:481–490. [PubMed] [Google Scholar]
- [45].Perez-Pinera P, Hernandez T, Garcia-Suarez O, et al. The Trk tyrosine kinase inhibitor K252a regulates growth of lung adenocarcinomas. Mol Cell Biochem. 2007;295:19–26. doi: 10.1007/s11010-006-9267-7. [DOI] [PubMed] [Google Scholar]
- [46].Chin LS, Murray SF, Doherty PF, Singh SK. K252a induces cell cycle arrest and apoptosis by inhibiting Cdc2 and Cdc25c. Cancer Invest. 1999;17:391–395. doi: 10.3109/07357909909021430. [DOI] [PubMed] [Google Scholar]
- [47].Rocha S, Garrett MD, Campbell KJ, Schumm K, Perkins ND. Regulation of NF-kappaB and p53 through activation of ATR and Chk1 by the ARF tumour suppressor. Embo J. 2005;24:1157–1169. doi: 10.1038/sj.emboj.7600608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Birbach A, Bailey ST, Ghosh S, Schmid JA. Cytosolic, nuclear and nucleolar localization signals determine subcellular distribution and activity of the NF-kappaB inducing kinase NIK. J Cell Sci. 2004;117:3615–3624. doi: 10.1242/jcs.01224. [DOI] [PubMed] [Google Scholar]
- [49].Thoms HC, Dunlop MG, Stark LA. p38-mediated inactivation of cyclin D1/cyclin-dependent kinase 4 stimulates nucleolar translocation of RelA and apoptosis in colorectal cancer cells. Cancer Res. 2007;67:1660–1669. doi: 10.1158/0008-5472.CAN-06-1038. [DOI] [PubMed] [Google Scholar]
- [50].Thoms HC, Dunlop MG, Stark LA. CDK4 inhibitors and apoptosis: a novel mechanism requiring nucleolar targeting of RelA. Cell Cycle. 2007;6:1293–1297. doi: 10.4161/cc.6.11.4312. [DOI] [PubMed] [Google Scholar]
- [51].Rosini P, De Chiara G, Lucibello M, Garaci E, Cozzolino F, Torcia M. NGF withdrawal induces apoptosis in CESS B cell line through p38 MAPK activation and Bcl-2 phosphorylation. Biochem Biophys Res Commun. 2000;278:753–759. doi: 10.1006/bbrc.2000.3871. [DOI] [PubMed] [Google Scholar]
- [52].Kummer JL, Rao PK, Heidenreich KA. Apoptosis induced by withdrawal of trophic factors is mediated by p38 mitogen-activated protein kinase. J Biol Chem. 1997;272:20490–20494. doi: 10.1074/jbc.272.33.20490. [DOI] [PubMed] [Google Scholar]