Abstract
Experimental animal studies have demonstrated that exposure to some phthalates may be associated with altered endocrine function and adverse effects on male reproductive development and function, but human studies are limited. In the present study, urine and serum samples were collected from 425 men recruited through a US infertility clinic. Urinary concentrations of mono(2-ethylhexyl) phthalate (MEHP), the hydrolytic metabolite of di(2-ethylhexyl) phthalate (DEHP), and other phthalate monoester metabolites were measured, along with serum levels of testosterone, estradiol, SHBG, FSH, LH, inhibin B and prolactin. Two oxidized urinary metabolites of DEHP were also measured in urine from 221 of the men. In multiple regression models adjusted for potential confounders, MEHP was inversely associated with testosterone, estradiol, and free androgen index (FAI). An interquartile range increase in MEHP was associated with 4% (95%CI −7% to −1%) and 7% (95%CI −11% to −2%) declines in testosterone and estradiol, respectively, relative to the population median hormone levels. There was limited evidence for effect modification of the inverse association between MEHP and FAI by the proportion of DEHP metabolites in the urine measured as MEHP (MEHP%), which is considered a phenotypic marker of less efficient metabolism of DEHP to its oxidized metabolites. Finally, the ratio of testosterone to estradiol was positively associated with MEHP (p-value=0.07) and MEHP% (p-value=0.007), suggesting potential relationships with aromatase suppression. In conclusion, these results suggest that urinary metabolites of DEHP are inversely associated with circulating steroid hormone levels in adult men. However, additional research is needed to confirm these findings.
Keywords: androgen, biomarker, endocrine, environment, human, estrogen, male reproduction, testosterone
INTRODUCTION
There is concern for adverse human health risks resulting from exposure to environmental endocrine-disrupting compounds (EDCs). A number of recent studies from several countries have suggested secular declining trends in testosterone levels among males (Travison et al. 2007; Andersson et al. 2006; Perheentupa et al. 2006) that may coincide with increased use and human exposure to EDCs. In men, altered hormone levels from environmental or occupational exposures may be associated with or lead to declined reproductive capacity or possibly increased risk of testicular or prostate cancer (Toft et al. 2004; Pfleiger-Bruss et al. 2004; Fleming et al. 1999). Certain environmental chemicals may cause altered hormone levels through a number of biological mechanisms alone or in combination, ranging from effects on hormone receptors to effects on hormone synthesis, secretion or metabolism. While the health impacts of sub-clinical alterations in circulating hormone levels remain unclear, a limited but growing body of evidence exists for these changes to be associated with environmental and occupational exposure to commonly used chemicals.
Phthalates have a wide range of industrial and commercial uses resulting in widespread human exposure through various pathways. High molecular weight phthalates (e.g., di(2-ethylhexyl) phthalate [DEHP], diisononyl phthalate [DiNP], di(n-octyl) phthalate [DnOP]), are primarily used as plasticizers in the manufacture of flexible vinyl which, in turn, is used in consumer products, flooring and wall coverings, food contact applications, and medical devices (ATSDR 2002; David et al. 2001). Low molecular weight phthalates (e.g., diethyl phthalate [DEP] and dibutyl phthalate [DBP]) can be used in personal-care products (e.g., perfumes, lotions, cosmetics), as solvents and plasticizers for cellulose acetate, and in making lacquers, varnishes, and coatings, including those used to provide timed releases in some pharmaceuticals (David et al. 2001; ATSDR 1995; 2001). The Centers for Disease Control and Prevention’s (CDC) Third National Report on Human Exposure to Environmental Chemicals showed that the majority of males in the United States have detectable concentrations of several phthalate monoesters in urine (monoethyl phthalate (MEP), mono(2-ethylhexyl) phthalate (MEHP), monobutyl phthalate (MBP), and monobenzyl phthalate (MBzP)), demonstrating that exposure to the parent diester compounds is common among the general population (CDC 2005). Two oxidized metabolites of DEHP, mono(2-ethyl-5-hydroxylhexyl) phthalate (MEHHP) and mono(2-ethyl-5-oxohexyl) phthalate (MEOHP) were present in most subjects at urinary concentrations higher than those of MEHP, the hydrolytic metabolite of DEHP (CDC 2005).
While experimental studies have demonstrated anti-androgenic activity and male reproductive toxicity in relation to several phthalates (Ge et al. 2007; Foster 2006), only limited human studies have investigated associations between exposure to phthalates and circulating hormone levels. A relationship between phthalates and hormone levels in infants has been reported. Within a Danish/Finnish cohort on cryptorchidism, Main and coworkers (2006) analyzed breast milk samples for phthalate metabolites and measured reproductive serum hormone levels in 3-month old breastfeeding boys. The authors reported positive associations between MEP, monomethyl phthalate (MMP), and mono(n-butyl) phthalate (MBP) with LH:free testosterone ratio, which is a measure of Leydig cell function. There were also positive associations between MEP, MBP and SHBG and between monoisononyl phthalate (MiNP) and LH, and an inverse association between MBP and free testosterone. One US study among male infants found inverse associations between urinary MBP, MBzP, MEP and monoisobutyl phthalate (MiBP) and anogenital distance, which is thought to be a sensitive marker for androgen activity (Swan et al. 2005).
Among a group of adult Chinese workers producing PVC flooring with high exposure to DEHP and DBP, urinary concentrations of metabolites of these phthalates were inversely associated with free testosterone levels (Pan et al. 2006). In a study of 295 men from a Massachusetts infertility clinic, we previously reported a suggestive (though not statistically significant) inverse association between urinary MEHP and testosterone, along with a statistically significant positive association between urinary MBP(a urinary metabolite of DBP) and inhibin B (p<0.05), and a statistically significant inverse association between urinary MBzP and FSH (Duty et al. 2005). However, the significant results for MBP and MBzP and hormone levels were in patterns inconsistent with our study hypotheses. Finally, a study of 234 young Swedish men found an inverse association between urinary MEP (the main DEP metabolite) and LH but no association between MEP, MEHP, or other phthalate metabolites in urine and FSH, testosterone, estradiol, or inhibin B (Jonsson et al. 2005).
The present study extends our previous analysis (Duty et al. 2005) by including a much larger sample size, by expanding the range of hormones measured to include estradiol, prolactin, and testosterone:LH ratio (a measure of Leydig cell function), and by expanding the phthalate metabolites that were measured by also including two oxidized DEHP metabolites: MEHHP and MEOHP.
METHODS
Subjects were recruited from an ongoing study on the relationship between environmental agents and male reproductive health. They were men who were partners in subfertile couples seeking treatment from the Vincent Burnham Andrology lab at Massachusetts General Hospital in Boston between January 2000 and May 2004. The study was approved by the Human Studies Institutional Review Boards of the Massachusetts General Hospital, Harvard School of Public Health, the Centers for Disease Control and Prevention, and the University of Michigan. After the study procedures were explained and all questions answered, subjects signed an informed consent. Men between the ages of 18 to 55 years without post-vasectomy status who presented to the Andrology Laboratory were eligible to participate. Of those approached, approximately 65% consented. Most men that declined to participate in the study cited lack of time on the day of their clinic visit as the reason for not participating.
Phthalate Metabolites in Urine
A single spot urine sample was collected from each subject on the day of their clinic visit in a sterile specimen cup prescreened for phthalate metabolites. Phthalate metabolites were measured in urine because of potential sample contamination from the parent diester and because the metabolites, as opposed to the parent diesters, are believed to be the active toxicants (Li et al. 1998; Peck and Albro 1982). The analytical approach for the analysis of the urinary phthalate monoester metabolites (i.e., MEHP, MBP, MBzP, MEP) and two oxidized metabolites of DEHP (i.e., MEHHP and MEOHP) involved enzymatic deconjugation of the metabolites from their glucuronidated form, solid-phase extraction, separation with high performance liquid chromatography, and detection by isotope-dilution tandem mass spectrometry (Blount et al. 2000; Silva et al. 2004a; 2003). Detection limits were in the low nanogram per milliliter range and varied slightly depending on the analytical method used (Blount et al. 2000; Silva et al. 2004a; 2003) for each phthalate metabolite (MEP, 1.00 to 1.21 ng/ml; MBP, 0.60 to 1.07 ng/ml; MBzP, 0.47 to 1.0 ng/ml; MEHP, 0.87 to 1.20 ng/ml; MEHHP, 0.95 to 1.60 ng/ml; MEOHP, 1.07 to 1.20 ng/ml). Isotopically-labeled internal standards and conjugated internal standards were used to increase precision of measurements. Along with the unknown samples, each analytical run included calibration standards, reagent blanks, and quality control materials of high and low concentration to monitor for accuracy and precision. Analysts at the Centers for Disease Control and Prevention (CDC), Atlanta, Georgia, U.S.A., were blind to all information concerning subjects. Urinary phthalate metabolite concentrations were adjusted for urine dilution by specific gravity (SG) using the following formula: Pc = P[(1.024 − 1)/SG − 1)], where Pc is the SG-adjusted phthalate metabolite concentration (ng/ml), P is the observed phthalate metabolite concentration, and SG is the specific gravity of the urine sample. SG was measured using a handheld refractometer (National Instrument Company, Inc., Baltimore, MD, USA).
We calculated (in nanomoles per milliliter) the sum of DEHP metabolites that were measured (i.e., MEHP, MEHHP and MEOHP). These values were also used to calculate the percent of these DEHP metabolites excreted as MEHP. We refer to this as MEHP% and consider it a phenotypic marker of the proportion of DEHP metabolized to and excreted in the urine as MEHP (Hauser et al 2006; 2007; Meeker et al. 2007). The greater the %MEHP, the larger the percentage of DEHP excreted as MEHP relative to the excretion of the two oxidized metabolites. To calculate MEHP%, we converted MEHP, MEHHP and MEOHP concentrations to nanomoles per milliliter, divided MEHP concentrations by the sum of concentrations of MEOHP, MEHHP and MEHP, and multiplied by 100.
Serum hormones
One non-fasting blood sample was drawn between the hours of 9 am and 4 pm on the same day that the semen sample was collected. Semen analysis procedures and relationships between semen parameters and phthalate metabolites were described in a previous report (Hauser et al. 2006). Blood samples were centrifuged and the resulting serum was stored at −80 °C until analysis. Testosterone was measured directly using the Coat-A-Count RIA kit (Diagnostics Products, Los Angeles, CA, USA), which has an interassay and intraassay coefficient of variation (CV) of 12% and 10%, respectively with a sensitivity of 4 ng/dL (0.139 nmol/L). The free androgen index (FAI) was calculated as the molar ratio of total testosterone to sex hormone binding globulin (SHBG). SHBG was measured using a fully automated system (Immulite: DPC, Inc., Los Angeles, CA, USA) which uses a solid-phase two-site chemiluminescent enzyme immunometric assay and has an interassay CV of less than 8%. Inhibin B was measured using a commercially available, double antibody, enzyme-linked immunosorbent assay (Oxford Bioinnovation, Oxford, UK) with interassay and intraassay CVs of 20% and 8%, respectively, limit of detection (LOD) of 15.6 pg/mL and a functional sensitivity (20% CV) of 50 pg/mL. Serum luteinizing hormone (LH), follicle stimulating hormone (FSH), estradiol, and prolactin concentrations were determined by microparticle enzyme immunoassay using an automated Abbott AxSYM system (Abbott Laboratories, Chicago, IL, USA). The Second International Reference Preparation (WHO 71/223) was used as the reference standard. The assay sensitivity for LH and FSH were 1.2 international units per liter (IU/L) and 1.1 IU/L, respectively. The intraassay CVs for LH and FSH are less than 5% and less than 3%, respectively, with interassay CVs for both hormones of less than 9%. The testosterone:LH ratio, a measure of Leydig cell function, was calculated by dividing testosterone (nmol/L) by LH (IU/L). The assay sensitivity for estradiol and prolactin were 20 pg/mL and 0.6 ng/mL, respectively. For estradiol the within-run coefficient of variation (CV) was between 3% and 11%, and the total CV was between 5% and 15%. For prolactin the within-run CV was ≤3% and the total CV was ≤6%.
Statistical analysis
Data analysis was performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). Descriptive statistics on subject demographics were tabulated, along with the distributions of phthalate metabolite concentrations and reproductive hormones. For phthalate metabolite concentrations or hormone levels below the limit of detection (LOD), an imputed value equal to one-half the LOD was used. In preliminary data analysis, hormone and phthalate metabolite concentrations were stratified by demographic categories to investigate the potential for confounding. Multivariable linear regression was used to explore relationships between urinary phthalate metabolite and hormone concentrations. Concentrations of inhibin B, testosterone and estradiol closely approximated normality and were used in statistical models untransformed, while the distribution of FSH, LH, SHBG, FAI, T:LH ratio, and prolactin were skewed left and transformed by the natural logarithm for statistical analyses. SG-adjusted phthalate metabolite concentrations were also ln-transformed. Inclusion of covariates was based on statistical and biologic considerations (Kleinbaum et al. 1998). Age and BMI were modeled as a continuous variable, smoking status was dichotomized by current smoker versus never smoked or former smoker, and race was categorized into four groups: white, African American, Hispanic, and other. Previous exam for infertility (yes or no), prior ability to impregnate a partner (yes or no), and timing of blood/urine samples by season (winter vs. spring, summer or fall) and time of day (9:00 am – 12:59 pm vs. 1:00 pm – 4:00 pm) were considered for inclusion in the models as dichotomous variables. To improve interpretability, the regression coefficients were back transformed and expressed as a change in the dependent variable (i.e., hormone levels) for an interquartile range (IQR) increase in phthalate metabolite concentrations.
For MEHP, we also included MEHHP, MEOHP or MEHP% in the models to explore evidence of whether individual differences in DEHP metabolism alter susceptibility to MEHP. We also explored the possibility of effect modification of MEHP-hormone associations by MEHP%. Our hypothesis is that the concentrations of MEHHP (or MEOHP) and/or MEHP% may represent phenotypic markers for efficient or inefficient metabolism of DEHP to its oxidized metabolites (Meeker et al. 2007; Hauser et al. 2007). In secondary sensitivity analyses, the multivariable models were rerun after excluding men with highly concentrated or highly dilute urine samples (SG above 1.03 or below 1.01) (Teass et al. 1998), and rerun among the strata of men with sperm concentration, motility and morphology all above reference values (Hauser et al. 2006). Finally, we assessed non-linear relationships between phthalate metabolite concentrations and hormones by regressing the hormones on tertiles or quintiles of phthalate metabolites. We also assessed whether phthalate metabolite tertiles or quintiles predicted low steroid hormone levels, where the odds of being in the lowest quartile for testosterone, estradiol or FAI were calculated using multiple logistic regression.
RESULTS
A total of 484 men had phthalate metabolite concentrations measured in urine, and 439 of these men also had hormone levels measured in serum. An additional 14 subjects taking hormone medications (e.g., propecia, finasteride, cabergoline, clomid, GnRH, testosterone, or prednisone taper) were excluded from the present analysis. Among the remaining subjects (n=425), most were white (85%) and had never smoked (72%). The mean (SD) age and BMI were 36 (5.3) years and 28 (4.5), respectively. Distributions of SG-adjusted urinary phthalate metabolite concentrations are presented in Table 1 and serum hormone levels in Table 2. Among the 425 urine samples, MEP was detected in 100 percent of the samples, while MBP and MBzP were detected in over 97 and 94 percent of the samples, respectively. Eighty-three percent of samples had detectable concentrations of MEHP. The sample size for MEOHP and MEHHP was 221 because analytical methods for the quantification of these analytes were not available at the onset of the study. Over ninety-five percent of these samples had detectable concentrations of MEHHP and MEOHP. Spearman correlations between MEHP and MEHHP or MEOHP concentrations were 0.75 and 0.71, respectively.
Table 1.
Distribution of SG-adjusted Phthalate Metabolites in Urine (ng/mL)
| Phthalate Metabolitea | N | Geometric Mean |
Selected Percentiles | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 10th | 25th | 50th | 75th | 90th | 95th | Max | |||
| MEP | 425 | 179 | 30.2 | 59.9 | 153 | 518 | 1,376 | 2,269 | 11,370 |
| MBP | 425 | 17.1 | 5.06 | 10.6 | 17.7 | 32.7 | 50.8 | 69.9 | 14,460 |
| MBzP | 425 | 7.73 | 2.27 | 4.20 | 8.20 | 15.9 | 24.9 | 40.6 | 540 |
| MEHP | 425 | 8.22 | 1.04 | 3.18 | 7.89 | 20.7 | 64.3 | 122 | 876 |
| MEHHP | 221 | 55.6 | 13.2 | 23.1 | 47.0 | 105 | 272 | 784 | 4,806 |
| MEOHP | 221 | 36.2 | 8.35 | 15.4 | 32.2 | 61.9 | 193 | 446 | 3,063 |
| Sum DEHP (nmol/mL) | 221 | 0.36 | 0.09 | 0.16 | 0.31 | 0.67 | 2.09 | 4.53 | 29.4 |
| MEHP% (%) | 221 | 9.39 | 3.46 | 5.76 | 10.3 | 16.8 | 24.3 | 30.7 | 61.5 |
Table 2.
Distribution of reproductive hormones in serum (N = 425).
| Hormone | Geometric Mean | Selected Percentiles | ||||||
|---|---|---|---|---|---|---|---|---|
| 5th | 10th | 25th | 50th | 75th | 90th | 95th | ||
| FSH (IU/L) | 8.03 | 3.67 | 4.33 | 5.67 | 7.51 | 10.5 | 15.7 | 22.7 |
| LH (IU/L) | 10.1 | 4.90 | 5.76 | 7.34 | 9.90 | 13.6 | 17.2 | 20.4 |
| Inhibin B (pg/mL) | 166a | 61.6 | 81.6 | 118 | 160 | 198 | 262 | 283 |
| Testosterone (ng/dL) | 420a | 223 | 254 | 324 | 408 | 492 | 609 | 665 |
| T:LH ratio | 39.4 | 17.0 | 22.0 | 30.5 | 40.9 | 54.5 | 70.0 | 78.8 |
| SHBG (nmol/mL) | 25.8 | 12.5 | 15.3 | 10.1 | 26.0 | 33.7 | 44.0 | 49.3 |
| Free Androgen Index | 0.53 | 0.32 | 0.35 | 0.43 | 0.52 | 0.67 | 0.84 | 0.95 |
| Estradiol (pg/mL) | 29.2a | <10 | <10 | 23.0 | 30.0 | 36.0 | 45.0 | 49.0 |
| Prolactin (ng/mL) | 11.6 | 5.67 | 6.53 | 8.30 | 11.5 | 15.8 | 21.4 | 25.6 |
Arithmetic mean
FAI and estradiol were both inversely associated with age (Spearman correlation coefficients = −0.2 and −0.1, respectively; both p-values <0.05), but positively associated with BMI (Spearman coefficients = 0.2 and 0.1, respectively; p-values<0.05). BMI was inversely associated with inhibin B, testosterone, and SHBG, but positively associated with MBzP and MEOHP (all p-values <0.05). Current smokers had lower median prolactin levels (9.2 ng/mL) than never smokers (11.7 ng/mL). Current smokers also had higher median concentrations of MEP but lower concentrations of MEHHP and MEOHP compared to never smokers. Median inhibin B was higher in samples collected in the morning compared to samples collected in the afternoon (166 versus 155 pg/mL), but morning samples had lower median estradiol (29 versus 30 pg/mL) and prolactin (9.9 versus 12.7 ng/mL) compared to afternoon samples. As previously observed (Silva et al. 2004b), median MEHP concentration was also higher among men whose urine sample was collected in the afternoon (9.0 ng/mL) versus men providing the urine sample in the morning (6.9 ng/mL). Finally, urine samples collected in the winter had median inhibin B concentrations that were lower than those collected in spring, summer or fall (143 versus 168 pg/mL), but a median FAI that was slightly higher (0.55 vs. 0.52). Samples collected in the winter also had slightly higher concentrations of MEHHP and MEOHP, but also a higher MEHP%, than samples collected in spring, summer or fall.
All linear regression results in Table 3 were adjusted for age, BMI, smoking and time of day blood/urine samples were collected. Crude regression results (not shown) were similar to the adjusted results presented in Table 3, though the relationship between MEHP and FAI became somewhat less statistically significant in the adjusted model (p-value = 0.05 in crude model, 0.1 in adjusted model). In adjusted models (Table 3), there was an inverse association between SG-adjusted urinary MEHP concentration and serum testosterone levels, where an IQR increase in MEHP was associated with a 14.9 ng/dL decrease in testosterone (95% confidence interval (CI) −27.5 to −2.30 ng/dL; p-value = 0.02). For the median level of testosterone (408 ng/dL), this represents a 3.7% (−6.8% to −0.5%) decrease in testosterone for an IQR increase in MEHP (IQR 3.18 to 20.7 ng/dL). SG-adjusted MEHP was also inversely associated with estradiol, where an IQR increase in MEHP was associated with a 6.8% decline (95% CI −11.2% to −2.4%) in estradiol relative to the population median estradiol level (30 pg/mL).
Table 3.
Adjusteda regression coefficients (95% confidence intervals) for change in hormones associated with an interquartile range (IQR) increase in SG-adjusted urinary phthalate metabolite concentrations. N=425b
| Phthalate Metabolitec | FSHd,f | LHd,f | Inhibin Be | Testosteronee | T:LH ratiod,f |
|---|---|---|---|---|---|
| MEP | 0.98 (0.91, 1.06) | 0.98 (0.91, 1.04) | 0.73 (−9.99, 11.4) | 8.87 (−7.18, 24.9) | 1.03 (0.95, 1.11) |
| MBP | 1.02 (0.97, 1.08) | 1.01 (0.97, 1.06) | 1.34 (−5.98, 8.66) | −4.65 (−15.7, 6.33) | 0.99 (0.94, 1.04) |
| MBzP | 0.98 (0.92, 1.04) | 1.00 (0.95, 1.05) | 1.81 (−6.54, 10.2) | 4.58 (−7.91, 17.0) | 1.02 (0.97, 1.09) |
| MEHP | 1.03 (0.97, 1.10) | 0.97 (0.92, 1.02) | −1.32 (−9.82, 7.18) | −14.9 (−27.5, −2.30)** | 0.99 (0.93, 1.05) |
| MEHHPb | 0.97 (0.89, 1.05) | 0.98 (0.91, 1.06) | −2.45 (−15.9, 11.0) | −9.33 (−25.3, 6.60) | 1.01 (0.93, 1.08) |
| MEOHPb | 0.96 (0.90, 1.04) | 0.99 (0.93, 1.06) | −1.22 (−13.4, 10.9) | −8.88 (−23.4, 5.59) | 1.00 (0.94, 1.07) |
| Sum DEHPb | 0.98 (0.92, 1.04) | 0.99 (0.94, 1.04) | −1.53 (−11.2, 8.07) | −6.35 (−17.8, 5.10) | 1.00 (0.95, 1.06) |
| MEHP%b | 1.01 (0.92, 1.11) | 0.98 (0.90, 1.06) | 6.88 (−7.78, 21.5) | 7.13 (−10.3, 24.6) | 1.00 (0.99, 1.01) |
| Phthalate Metabolitec | SHBGd,f | FAId,f | Estradiole | T:E2 ratio | Prolactind,f |
|---|---|---|---|---|---|
| MEP | 0.97 (0.91, 1.02) | 1.04 (0.99, 1.09) | 0.71 (−0.97, 2.40) | 1.00 (0.92, 1.07) | 0.97 (0.91, 1.03) |
| MBP | 1.02 (0.98, 1.06) | 0.98 (0.94, 1.01) | −0.47 (−1.62, 0.68) | 1.01 (0.96, 1.07) | 1.00 (0.96, 1.04) |
| MBzP | 1.00 (0.95, 1.04) | 1.03 (0.99, 1.07) | −0.21 (−1.53, 1.09) | 1.04 (0.98, 1.11) | 1.01 (0.96, 1.06) |
| MEHP | 0.99 (0.94, 1.03) | 0.98 (0.96, 1.00)* | −2.04 (−3.37, −0.73)** | 1.06 (1.00, 1.12)* | 1.00 (0.95, 1.05) |
| MEHHPb | 1.04 (0.98, 1.11) | 0.95 (0.90, 1.00)** | −1.32 (−2.98, 0.33) | 1.03 (0.95, 1.13) | 1.01 (0.94, 1.08) |
| MEOHPb | 1.05 (0.99, 1.11)* | 0.95 (0.91, 0.99)** | −1.09 (−2.60, 0.42) | 1.02 (0.95, 1.11) | 1.01 (0.95, 1.08) |
| Sum DEHPb | 1.03 (0.99, 1..08) | 0.96 (0.93, 1.00)** | −1.03 (−1.15, 0.16)* | 1.03 (0.97, 1.10) | 1.03 (0.97, 1.10) |
| MEHP%b | 0.96 (0.90, 1.02) | 1.05 (0.99, 1.11)* | −2.01 (−3.81, −0.21)** | 1.14 (1.04, 1.25)** | 0.95 (0.88, 1.03) |
Adjusted for age, BMI, current smoking, season, and time of day blood sample was collected. Testosterone and estradiol were additionally adjusted for SHBG.
N=221 for MEHHP, MEOHP, sum of DEHP metabolites, and MEHP%.
In all models ln-transformations of urinary phthalate metabolite concentrations were used.
Ln-transformations of FSH, LH, SHBG, FAI, T:LH, T:E2 and prolactin were used. Inhibin B, testosterone, and estradiol were modeled untransformed.
Coefficient represents the change in hormone level for an IQR change in phthalate metabolite concentration after back-transformation of the phthalate metabolite concentrations. For an IQR change in phthalate metabolite concentration, a coefficient equal to 0 indicates no change in hormone level, a coefficient < 0 indicates a decrease in hormone level, and a coefficient > 0 indicates an increase in hormone level.
Coefficient represents a multiplicative change in hormone level for an IQR change in phthalate metabolite concentration after back-transformation of both hormone and phthalate metabolite concentrations. For an IQR change in phthalate metabolite concentration, a coefficient equal to 1.0 indicates no change in hormone level, a coefficient < 1.0 indicates a multiplicative decrease in hormone level, and a coefficient > 1.0 indicates a multiplicative increase in hormone level.
p≤0.1
p<0.05
In sensitivity analyses, effect estimates from the multivariable models were similar when men with SG outside the acceptable range were excluded (N=343, results not shown). The effect estimates were also similar when limiting the analyses to men with above reference (‘normal’) semen quality measures (data not shown). This provided limited evidence that the relationships between MEHP and reproductive hormones among men with semen parameters above reference values are similar to those among men with below reference semen parameters. This suggests that the associations reported may be generalizable to men with ’normal’ fertility, recognizing however that even men with above reference semen parameters may be infertile.
When the analysis was limited to the subset of men with oxidized DEHP metabolite measures (n=221), there was no evidence of strengthened associations between MEHP and hormone levels when additionally adjusting for MEHHP, MEOHP, or MEHP% as we previously reported for sperm DNA damage and thyroid hormone levels (Hauser et al. 2007; Meeker et al. 2007). However, both MEHHP and MEOHP were inversely associated with FAI when modeled without MEHP. For both metabolites an IQR increase was associated with a 5% decline in FAI (Table 3). Likewise, the sum of measured DEHP metabolites was associated with a statistically significant 4% decline in FAI. There was also an inverse association between MEHP% and estradiol. Because MEHP% was associated with estradiol but not testosterone, an additional secondary analysis involved exploring associations with the ratio of testosterone to estradiol (T:E2; a measure of aromatase activity). In this analysis MEHP% was positively associated with T:E2 ratio, where an IQR increase in MEHP% was associated with a 14% increase in T:E2 (95% CI 4%, 25%; p-value = 0.007). There was also a suggestive positive association between MEHP and T:E2 in the full dataset (n=425), where an IQR increase in MEHP was associated with a 6% increase in T:E2 (95% CI −0.4%, 12%; p-value = 0.07).
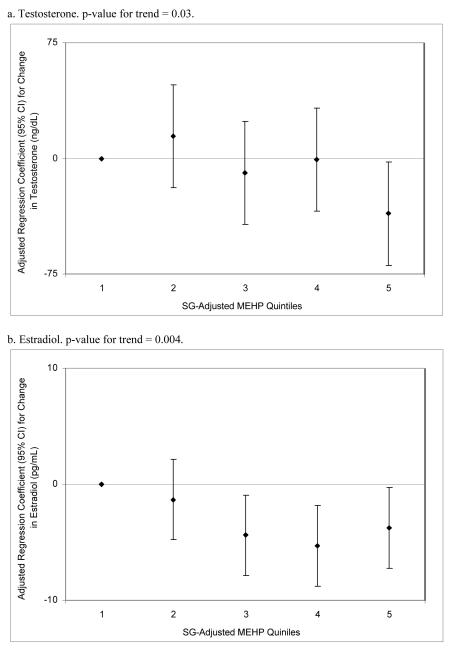
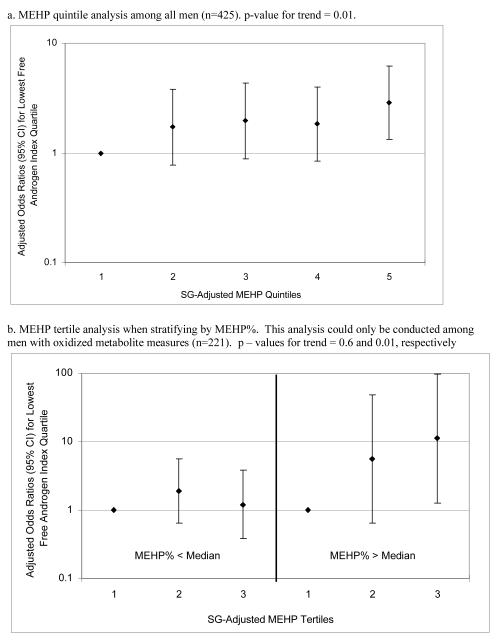
To assess the robustness of the associations between phthalate metabolite concentrations and steroid hormones, and potential non-linear relationships, we divided SG-adjusted phthalate concentrations into categories. In quintile analysis among the full cohort (n=425) there were significant but non-monotonic trends in the associations between MEHP and testosterone and estradiol (Figure 1a and 1b). There was a sharp decline in the regression coefficient for change in testosterone among the highest MEHP quintile, whereas the regression coefficients for declined estradiol among increasing quintiles of MEHP appeared to plateau at quintile 4. The lack of a monotonic trend could perhaps be a result of unequal bin widths stemming from a skewed-right distribution, where the occurrence of exposure misclassification may be more likely in the lower quintiles where cutoff values are closer together. Conversely, there was no trend in FAI in relation to MEHP quintiles (results not shown; p-value for trend = 0.33). However, in multiple logistic regression for low FAI, there was a significant increasing trend in the adjusted odds of being in the lowest FAI quartile among increasing MEHP quintiles (p-value for trend = 0.01; Figure 2a). When limiting this analysis to the 221 men with oxidized metabolite measures the trend became weaker (p-value for trend = 0.13 among MEHP tertiles; tertiles were used instead of quintiles due to the smaller sample size). However, there was evidence of effect modification by MEHP% in this relationship, as an MEHP-FAI trend was present among men with MEHP% above the median but not among men with MEHP% below the median (Figure 2b). The odds ratio for low FAI among men in the highest MEHP tertile and with high MEHP% was 11.2 (95% CI 1.3, 97.1). There was no clear evidence of effect modification by MEHP% in the other MEHP-hormone relationships, but the number of men with DEHP oxidized metabolite measures (n=221), and thus a calculable MEHP%, limited statistical power for detecting interactions. Among the full 425 men there were also increased adjusted odds for low (lowest quartile) testosterone and estradiol among increasing MEHP quintiles (results not shown; p-values for trend = 0.04 and 0.02, respectively).
Figure 1.
Adjusted (adjusted for age, BMI, smoking, season, and time of day) regression coefficients for a change in hormone level associated with increasing quintiles of SG-adjusted MEHP (n=425).
Figure 2.
Adjusted (adjusted for age, BMI, smoking, season, and time of day) odds ratios for having FAI level in the lowest quartile with increasing SG-adjusted MEHP categories.
DISCUSSION
In the present study, urinary MEHP concentrations were inversely associated with circulating testosterone and estradiol levels in adult men recruited through an infertility clinic. We also found evidence for inverse associations between MEHP, MEHHP, MEOHP, sum of measured DEHP metabolites and FAI, and a positive association between MEHP% and T:E2 ratio. We previously reported associations between MBP and inhibin B, and between MBzP and FSH, among a smaller and overlapping group of men in our study (Duty et al. 2005). However, in the present analysis we observed no relationships among MEP, MBP or MBzP with any of the measured hormones.
Only a limited number of studies have investigated the relationship between human phthalate exposure and circulating reproductive hormone levels or hormone indicators (Pan et al. 2006; Jonsson et al. 2005; Main et al. 2006; Swan et al. 2005), and only two other previous studies have been conducted among men (Pan et al. 2006; Jonsson et al. 2005). Pan et al. (2006) studied 74 workers in a Chinese factory exposed to high levels of DEHP and DBP in the production of unfoamed PVC, and 64 unexposed workers. The authors reported inverse associations between urinary MBP and MEHP concentrations and free testosterone among the workers, but no relationship between the phthalate metabolites and estradiol. Interestingly, although MEHP concentrations in the present study among non-occupationally exposed men were several orders of magnitude lower than those measured in the Chinese workers with occupational exposure (Pan et al. 2006), the evidence for decreased testosterone in relation to DEHP and/or MEHP was consistent between the two studies.
Conversely, the findings in the present study were inconsistent with those reported in the other study of non-occupationally exposed Swedish men by Jonsson and colleagues (2005). In that study no associations between urinary MEHP and testosterone or estradiol were observed. It is possible that the discrepancies in findings between studies may be explained by differences in study design or laboratory methods. For example, there were large differences in the ages of the study populations and the methods of recruitment. The Swedish study population consisted of young men (median age 18 years, range 18-21 years) undergoing a medical examination before military service. Since approximately 95 percent of young men in Sweden undergo the conscript examination, these young men reflected the general population of young Swedish males. In contrast, in the present study the median age was 35.5 years and ranged from 22 to 54 years. However, it is unclear whether men presenting to an infertility clinic are more ‘susceptible’ to exposure to reproductive toxicants than men from the general population or whether older men are more ‘susceptible’ to reproductive toxicants because of an age related response. Other differences between the two studies include major differences in participation rates (14% in the Swedish study and 65% in the US study) and differences in the analytical methods used to measure urinary phthalate metabolites, where the method in the present study was more sensitive than that used in the Swedish study.
Consistent with our findings, animal and in vitro studies have demonstrated that several phthalates, including DEHP or its metabolites, are endocrine disrupting chemicals that possess anti-androgenic activity and reduce testosterone and estradiol levels (ATSDR 2002). The mechanisms by which these alterations are occurring are not fully established. Recent evidence suggests phthalates may inhibit expression of genes or proteins related to steroidogenesis, such as steroidogenic acute regulated protein (StAR), peripheral benzodiazepine receptor (PBR), P450 side chain cleavage (P450scc), and peroxisome proliferator activated receptors (PPAR) in Leydig cells (Borch et al. 2006). However, this and most other studies of DEHP or MEHP anti-androgenic activity that have been conducted to date were performed in rat fetal testis and thus may not reflect what occurs in adult humans. Additional research is needed on reproductive health effects from phthalate exposures in adulthood, and the involvement of alternative mechanisms such as hepatic SHBG transcription activity and altered pituitary function should be considered.
We found limited evidence for interaction by MEHP% of the inverse association between MEHP and FAI. In multiple logistic models for having FAI in the lowest quartile, the association was much stronger among men with high MEHP%. This was consistent with our a priori hypothesis that a high percentage of MEHP (a bioactive hydrolytic metabolite of DEHP) to the sum of all measured DEHP metabolites reflects increased susceptibility to DEHP exposure due to less efficient oxidation and excretion of DEHP and/or MEHP. However, we did not find evidence for modification of the other observed associations by MEHP%, though this may be due to the small number of men with oxidized DEHP metabolites measured (n=221). The possibility of effect modification by MEHP% should be tested in future epidemiological studies with larger sample sizes.
Another secondary finding from the present study was a suggestive positive association between T:E2 ratio and MEHP, and a strong positive association between T:E2 ratio and MEHP%. A larger T:E2 ratio is a marker for reduced aromatase activity. Consistent with these findings, animal and in vitro studies have demonstrated that DEHP and/or MEHP can suppress aromatase in a human adrenocortical carcinoma cell line, in cultured rat ovarian granulosa cells, and in the brain and testes of young male rats (Noda et al. 2007; Andrade et al. 2006; Kim et al. 2003; Lovekamp and Davis 2001; Davis et al. 1994). Although suggested as a potential treatment for certain cases of male infertility (Liu and Handelsman 2003; Schiff et al. 2007), aromatase suppression could be associated with adverse effects on male reproduction (Carreau et al. 2006). A potential alternative explanation for an increased T:E2 ratio in relation to DEHP and/or its metabolites in the present study could be through an increase in estradiol metabolism through PPARα-dependent mechanisms (Corton et al. 1997).
Recent experimental evidence has suggested that estradiol plays an important role in spermatogenesis and male reproduction, and that estradiol is a potent inhibitor of male germ cell death (Pentikainen et al. 2000; Hess et al. 1997). We also recently reported strong inverse associations between estradiol levels and human sperm DNA damage (Meeker et al. 2008). Thus, it is possible that a decrease in estradiol levels and/or aromatase activity may explain our previous observation of a significant increase in sperm DNA damage associated with increased urinary MEHP when accounting for oxidized DEHP metabolites (Hauser et al. 2007). Alternatively, the significant positive association between MEHP% and T:E2 ratio may reflect relationships between aromatase and enzymes involved in efficient DEHP or MEHP metabolism. Future research is needed to help explain these findings.
A potential limitation of the present study is the measurement of urinary phthalate metabolites and serum hormones at a single point in time. Phthalates are rapidly metabolized and excreted, and metabolite concentrations in urine only reflect exposure in the preceding 1 or 2 days. Several studies have explored temporal variability of urinary phthalate metabolites, where high within-individual variability has been reported over the course of several days to months (Fromme et al. 2007; Hoppin et al. 2002; Teitelbaum et al. 2008). Nevertheless, we demonstrated that a single sample may adequately predict average monoester concentrations over a 3-month period in adult men (Hauser et al. 2004). Serum hormone levels may also vary within an individual over time, but a single measure has been shown to provide a reliable measure in population studies (Bjornerem et al. 2006; Schrader et al. 1993; Vermeulen and Verdonck 1992; Bain et al. 1988). In addition, requiring multiple blood samples from participants may result in a reduced participation rate and lower statistical power.
Concentrations of (unadjusted) urinary phthalate metabolites measured in the present study were compared to those among U.S. males measured as part of the 2003-2004 National Health and Nutrition Examination Survey (NHANES) (CDC 2008). Metabolite distributions were generally similar between the present study and the national data, although in the present study concentrations of MEP, MBP and MBzP were slightly lower, and concentrations of MEHP, MEHHP and MEOHP were somewhat higher. For example, the median and 95th percentile values for MEHP (unadjusted for specific gravity) in the present study were 6.0 and 112 ng/ml, respectively, compared to 2.2 and 33.3 ng/ml in males from NHANES 2003-2004.
In conclusion, the present study found that urinary DEHP metabolites, at levels that are representative of those found among the general US population, may be associated with altered steroid hormone levels and perhaps aromatase activity. Additional work is needed to confirm these findings and determine clinical implications of subclinical alterations in hormone levels in adult men following exposure to environmental EDCs.
Acknowledgements
This work was supported by grants ES009718 and ES00002 from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH). The authors gratefully acknowledge research nurses Jennifer Ford and Myra Keller at MGH, the technical assistance of Manori Silva, Jack Reidy, Ella Samandar and Jim Preau (CDC, Atlanta, GA) in measuring the urinary concentrations of phthalate metabolites, and Janna Frelich in data management.
Work supported by grants ES009718 and ES00002 from the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH).
Footnotes
DISCLAIMER The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
REFERENCES
- Andersson AM, Jensen TK, Juul A, Petersen JH, Jorgensen T, Skakkebaek NE. Secular decline in male testosterone and sex hormone binding globulin serum levels in Danish population surveys. J Clin Endocrinol Metab. 2007;92(12):4696–705. doi: 10.1210/jc.2006-2633. [DOI] [PubMed] [Google Scholar]
- Andrade AJ, Grande SW, Talsness CE, Grote K, Chahoud I. A dose-response study following in utero and lactational exposure to di-(2-ethylhexyl)-phthalate (DEHP): non-monotonic dose-response and low dose effects on rat brain aromatase activity. Toxicology. 2006;227(3):185–92. doi: 10.1016/j.tox.2006.07.022. [DOI] [PubMed] [Google Scholar]
- ATSDR . Toxicological Profile for Diethyl phthalate (DEP) Agency for Toxic Substances and Disease Registry; Atlanta, GA: 1995. [PubMed] [Google Scholar]
- ATSDR . Toxicological Profile for Di-n-butyl phthalate (DBP) Agency for Toxic Substances and Disease Registry; Atlanta, GA: 2001. [PubMed] [Google Scholar]
- ATSDR . Toxicological Profile for Di(2-ethylhexyl)phthalate (DEHP) Agency for Toxic Substances and Disease Registry; Atlanta, GA: 2002. [PubMed] [Google Scholar]
- Bain J, Langevin R, D’Costa M, Sanders RM, Hucker S. Serum pituitary and steroid hormone levels in the adult male: one value is as good as the mean of three. Fertil Steril. 1988;49(1):123–6. doi: 10.1016/s0015-0282(16)59662-9. [DOI] [PubMed] [Google Scholar]
- Bjornerem A, Straume B, Oian P, Berntsen GK. Seasonal variation of estradiol, follicle stimulating hormone, and dehydroepiandrosterone sulfate in women and men. J Clin Endocrinol Metab. 2006;91(10):3798–802. doi: 10.1210/jc.2006-0866. [DOI] [PubMed] [Google Scholar]
- Blount BC, Milgram KE, Silva MJ, Malek NA, Reidy JA, Needham LL, Brock JW. Quantitative detection of eight phthalate metabolites in human urine using HPLC-APCI-MS/MS. Anal Chem. 2000;72(17):4127–34. doi: 10.1021/ac000422r. [DOI] [PubMed] [Google Scholar]
- Borch J, Metzdorff SB, Vinggaard AM, Brokken L, Dalgaard M. Mechanisms underlying the anti-androgenic effects of diethylhexyl phthalate in fetal rat testis. Toxicology. 2006;223(12):144–55. doi: 10.1016/j.tox.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Carreau S, Delalande C, Silandre D, Bourguiba S, Lambard S. Mol Cell Endocrinol. 12. Vol. 246. 2006. Aromatase and estrogen receptors in male reproduction; pp. 65–8. [DOI] [PubMed] [Google Scholar]
- CDC . Third National Report on Human Exposure to Environmental Chemicals. Centers for Disease Control and Prevention; Washington, DC: 2005. [Google Scholar]
- CDC . NHANES 2003-2004 Laboratory Files, Lab 24: Urinary Phthalates. US Centers for Disease Control and Prevention; Atlanta, GA: [accessed June 27, 2008]. 2008. http://www.cdc.gov/nchs/about/major/nhanes/nhanes2003-2004/lab03_04.htm. [Google Scholar]
- Corton JC, Bocos C, Moreno ES, Merritt A, Cattley RC, Gustafsson JA. Peroxisome proliferators alter the expression of estrogen-metabolizing enzymes. Biochimie. 1997;79(23):151–62. doi: 10.1016/s0300-9084(97)81508-8. [DOI] [PubMed] [Google Scholar]
- David RM, McKee RH, Butala JH, Barter RA, Kayser M. Esters of aromatic mono-, di-, and tricarboxylic acids, aromatic diacids, and di-, tri-, or polyalcohols. In: Bingham E, Cohrssen B, Powell CH, editors. Patty’s Toxicology. John Wiley and Sons; New York: 2001. pp. 635–932. [Google Scholar]
- Davis BJ, Weaver R, Gaines LJ, Heindel JJ. Mono-(2-ethylhexyl) phthalate suppresses estradiol production independent of FSH-cAMP stimulation in rat granulosa cells. Toxicol Appl Pharmacol. 1994;128(2):224–8. doi: 10.1006/taap.1994.1201. [DOI] [PubMed] [Google Scholar]
- Duty SM, Calafat AM, Silva MJ, Ryan L, Hauser R. Phthalate exposure and reproductive hormones in adult men. Hum Reprod. 2005;20(3):604–10. doi: 10.1093/humrep/deh656. [DOI] [PubMed] [Google Scholar]
- Fleming LE, Bean JA, Rudolph M, Hamilton K. Cancer incidence in a cohort of licensed pesticide applicators in Florida. J Occup Environ Med. 1999;41(4):279–88. doi: 10.1097/00043764-199904000-00010. [DOI] [PubMed] [Google Scholar]
- Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2006;29(1):140–7. doi: 10.1111/j.1365-2605.2005.00563.x. discussion 181-5. [DOI] [PubMed] [Google Scholar]
- Fromme H, Bolte G, Koch HM, Angerer J, Boehmer S, Drexler H, Mayer R, Liebl B. Occurrence and daily variation of phthalate metabolites in the urine of an adult population. Int J Hyg Environ Health. 2007;210(1):21–33. doi: 10.1016/j.ijheh.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Ge RS, Chen GR, Dong Q, Akingbemi B, Sottas CM, Santos M, Sealfon SC, Bernard DJ, Hardy MP. Biphasic effects of postnatal exposure to diethylhexylphthalate on the timing of puberty in male rats. J Androl. 2007;28(4):513–20. doi: 10.2164/jandrol.106.001909. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Park S, Silva MJ, Calafat AM. Temporal variability of urinary phthalate metabolite levels in men of reproductive age. Environ Health Perspect. 2004;112(17):1734–40. doi: 10.1289/ehp.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Duty S, Silva MJ, Calafat AM. Altered semen quality in relation to urinary concentrations of phthalate monoester and oxidative metabolites. Epidemiology. 2006;17(6):682–91. doi: 10.1097/01.ede.0000235996.89953.d7. [DOI] [PubMed] [Google Scholar]
- Hauser R, Meeker JD, Singh NP, Silva MJ, Ryan L, Duty S, Calafat AM. DNA damage in human sperm is related to urinary levels of phthalate monoester and oxidative metabolites. Hum Reprod. 2007;22(3):688–95. doi: 10.1093/humrep/del428. [DOI] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB. A role for oestrogens in the male reproductive system. Nature. 1997;390(6659):509–12. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppin JA, Brock JW, Davis BJ, Baird DD. Reproducibility of urinary phthalate metabolites in first morning urine samples. Environ Health Perspect. 2002;110(5):515–8. doi: 10.1289/ehp.02110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson BA, Richthoff J, Rylander L, Giwercman A, Hagmar L. Urinary phthalate metabolites and biomarkers of reproductive function in young men. Epidemiology. 2005;16(4):487–93. doi: 10.1097/01.ede.0000164555.19041.01. [DOI] [PubMed] [Google Scholar]
- Kim HS, Saito K, Ishizuka M, Kazusaka A, Fujita S. Short period exposure to di-(2-ethylhexyl) phthalate regulates testosterone metabolism in testis of prepubertal rats. Arch Toxicol. 2003;77(8):446–51. doi: 10.1007/s00204-003-0466-7. [DOI] [PubMed] [Google Scholar]
- Kleinbaum DG, Kupper LL, Muller KE, Nizam A. Selecting the best regression equation. Applied regression analysis and other multivariate methods. 3rd ed Brooks/Cole Publishing Company; Pacific Grove, CA: 1998. [Google Scholar]
- Li LH, Jester WF, Jr., Orth JM. Effects of relatively low levels of mono-(2-ethylhexyl) phthalate on cocultured Sertoli cells and gonocytes from neonatal rats. Toxicol Appl Pharmacol. 1998;153(2):258–65. doi: 10.1006/taap.1998.8550. [DOI] [PubMed] [Google Scholar]
- Liu PY, Handelsman DJ. The present and future state of hormonal treatment for male infertility. Hum Reprod Update. 2003;9(1):9–23. doi: 10.1093/humupd/dmg002. [DOI] [PubMed] [Google Scholar]
- Lovekamp TN, Davis BJ. Mono-(2-ethylhexyl) phthalate suppresses aromatase transcript levels and estradiol production in cultured rat granulosa cells. Toxicol Appl Pharmacol. 2001;172(3):217–24. doi: 10.1006/taap.2001.9156. [DOI] [PubMed] [Google Scholar]
- Main KM, Mortensen GK, Kaleva MM, Boisen KA, Damgaard IN, Chellakooty M, Schmidt IM, Suomi AM, Virtanen HE, Petersen DV, Andersson AM, Toppari J, Skakkebaek NE. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ Health Perspect. 2006;114(2):270–6. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Calafat AM, Hauser R. Di(2-ethylhexyl) phthalate metabolites may alter thyroid hormone levels in men. Environ Health Perspect. 2007;115(7):1029–34. doi: 10.1289/ehp.9852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Singh NP, Hauser R. Serum concentrations of estradiol and free T4 are inversely correlated with sperm DNA damage in men from an infertility clinic. J Androl. 2008;29(4):379–88. doi: 10.2164/jandrol.107.004416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Ohno S, Nakajin S. Mono-(2-ethylhexyl) phthalate (MEHP) induces nuclear receptor 4A subfamily in NCI-H295R cells: a possible mechanism of aromatase suppression by MEHP. Mol Cell Endocrinol. 2007;274(12):8–18. doi: 10.1016/j.mce.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Pan G, Hanaoka T, Yoshimura M, Zhang S, Wang P, Tsukino H, Inoue K, Nakazawa H, Tsugane S, Takahashi K. Decreased serum free testosterone in workers exposed to high levels of di-n-butyl phthalate (DBP) and di-2-ethylhexyl phthalate (DEHP): a cross-sectional study in China. Environ Health Perspect. 2006;114(11):1643–8. doi: 10.1289/ehp.9016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck CC, Albro PW. Toxic potential of the plasticizer Di(2-ethylhexyl) phthalate in the context of its disposition and metabolism in primates and man. Environ Health Perspect. 1982;45:11–7. doi: 10.1289/ehp.824511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentikainen V, Erkkila K, Suomalainen L, Parvinen M, Dunkel L. Estradiol acts as a germ cell survival factor in the human testis in vitro. J Clin Endocrinol Metab. 2000;85(5):2057–67. doi: 10.1210/jcem.85.5.6600. [DOI] [PubMed] [Google Scholar]
- Perheentupa A, Laatikainen T, Vierula M, et al. Clear birth cohort effect in serum testosterone and SHBG levels in Finnish men. Endocrine Society Meeting. 2006 abstract OR22-3. [Google Scholar]
- Pflieger-Bruss S, Schuppe HC, Schill WB. The male reproductive system and its susceptibility to endocrine disrupting chemicals. Andrologia. 2004;36(6):337–45. doi: 10.1111/j.1439-0272.2004.00641.x. [DOI] [PubMed] [Google Scholar]
- Schiff JD, Ramirez ML, Bar-Chama N. Medical and surgical management male infertility. Endocrinol Metab Clin North Am. 2007;36(2):313–31. doi: 10.1016/j.ecl.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Schrader SM, Turner TW, Breitenstein MJ, Simon SD. Measuring male reproductive hormones for occupational field studies. J Occup Med. 1993;35(6):574–6. doi: 10.1097/00043764-199306000-00013. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Malek NA, Hodge CC, Reidy JA, Kato K, Barr DB, Needham LL, Brock JW. Improved quantitative detection of 11 urinary phthalate metabolites in humans using liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;789(2):393–404. doi: 10.1016/s1570-0232(03)00164-8. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Slakman AR, Reidy JA, Preau JL, Jr., Herbert AR, Samandar E, Needham LL, Calafat AM. Analysis of human urine for fifteen phthalate metabolites using automated solid-phase extraction. J Chromatogr B Analyt Technol Biomed Life Sci. 2004a;805(1):161–7. doi: 10.1016/j.jchromb.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ Health Perspect. 2004b;112(3):331–8. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113(8):1056–61. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teass AW, Biagini RE, DeBord DG, Hull RD. Application of biological monitoring methods. NIOSH Manual of Analytical Methods. National Institute for Occupational Safety and Health; Cincinnati, OH: 1998. pp. 52–62. [Google Scholar]
- Teitelbaum SL, Britton JA, Calafat AM, Ye X, Silva MJ, Reidy JA, Galvez MP, Brenner BL, Wolff MS. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ Res. 2008;106(2):257–69. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Toft G, Hagmar L, Giwercman A, Bonde JP. Epidemiological evidence on reproductive effects of persistent organochlorines in humans. Reprod Toxicol. 2004;19(1):5–26. doi: 10.1016/j.reprotox.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Travison TG, Araujo AB, O’Donnell AB, Kupelian V, McKinlay JB. A population-level decline in serum testosterone levels in American men. J Clin Endocrinol Metab. 2007;92(1):196–202. doi: 10.1210/jc.2006-1375. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Verdonck G. Representativeness of a single point plasma testosterone level for the long term hormonal milieu in men. J Clin Endocrinol Metab. 1992;74(4):939–42. doi: 10.1210/jcem.74.4.1548361. [DOI] [PubMed] [Google Scholar]