Abstract
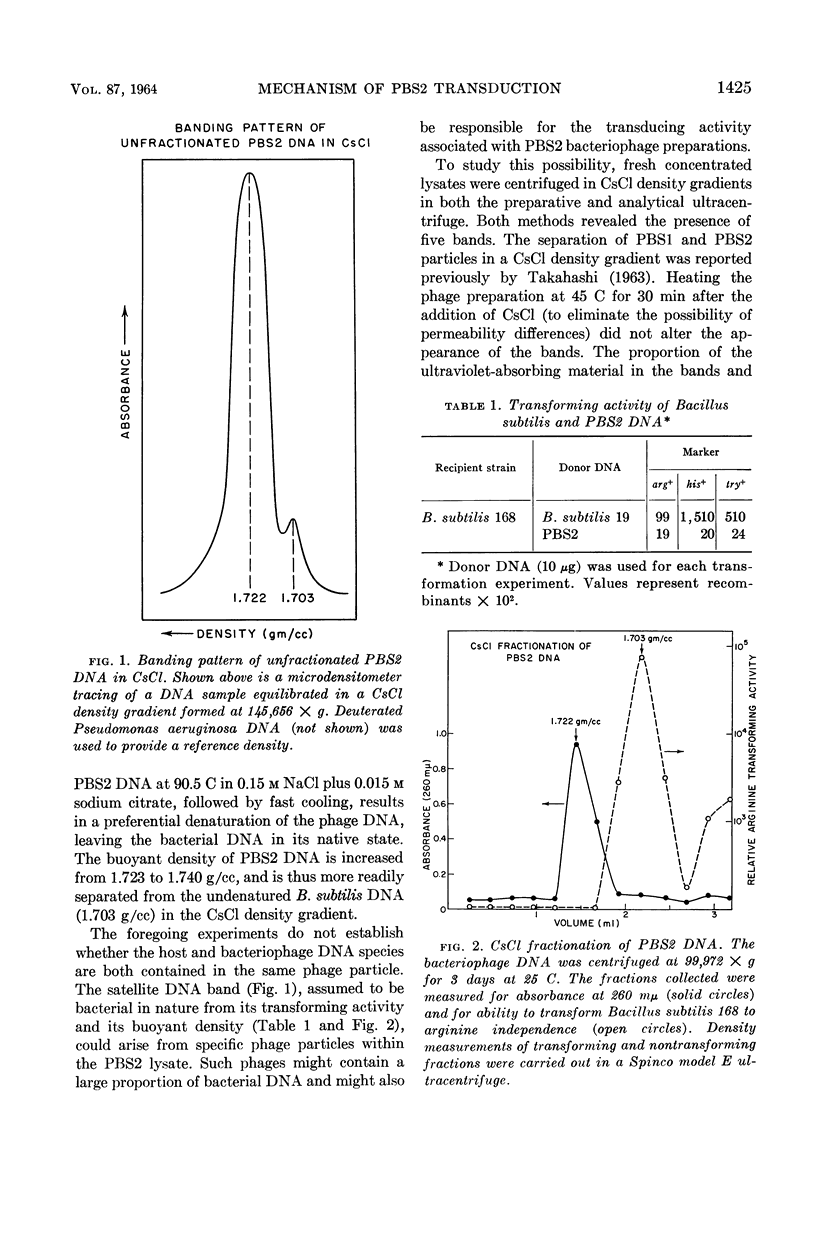
Mahler, I. (Brandeis University, Waltham, Mass.), M. Cahoon, and J. Marmur. Bacillus subtilis deoxyribonucleic acid transfer in PBS2 transduction. J. Bacteriol. 87:1423–1428. 1964.—Lysates of the general transducing bacteriophage PBS2 grown on Bacillus subtilis SB19 were fractionated by preparative CsCl density-gradient centrifugation. Five distinct and separate bands which varied in their ability to transduce three nutritional markers were obtained by this procedure. Deoxyribonucleic acid (DNA) samples prepared from unfractionated lysates and from each of the separate bands were examined by analytical CsCl density-gradient centrifugation. In addition to a major band (density, 1.723 g/cc) identified as PBS2 DNA, a satellite band of lighter density was detectable in the nucleic acids obtained from whole lysates and those bands that possessed transducing activity. The biological activity of the purified nucleic acids, determined by transformation experiments, was found to reside in the light satellite band (1.703 g/cc) characteristic of B. subtilis DNA. In view of the correlation between general transducing ability of phage particles and the presence of bacterial DNA which appears not to be physically associated with the phage DNA, it is suggested that transduction by bacteriophage PBS2 does not depend upon areas of homology between the phage genome and the host chromosome but resides in specific particles which have incorporated segments of bacterial DNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. N., Luria S. E. TRANSDUCTION BY BACTERIOPHAGE P1: ABNORMAL PHAGE FUNCTION OF THE TRANSDUCING PARTICLES. Proc Natl Acad Sci U S A. 1958 Jun;44(6):590–594. doi: 10.1073/pnas.44.6.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL A. Transduction and segregation in Escherichia coli K12. Virology. 1957 Oct;4(2):366–384. doi: 10.1016/0042-6822(57)90070-3. [DOI] [PubMed] [Google Scholar]
- COWIE D. B., MCCARTHY B. J. HOMOLOGY BETWEEN BACTERIOPHAGE LAMBDA DNA AND E. COLI DNA. Proc Natl Acad Sci U S A. 1963 Sep;50:537–543. doi: 10.1073/pnas.50.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER G., ZICHICHI M. L., WEIGLE J. A mutation affecting the DNA content of bacteriophage lambda and its lysogenizing properties. J Mol Biol. 1961 Aug;3:399–408. doi: 10.1016/s0022-2836(61)80053-3. [DOI] [PubMed] [Google Scholar]
- LANNI F. Genetic significance of microbial DNA composition. Perspect Biol Med. 1960;3:418–432. doi: 10.1353/pbm.1960.0043. [DOI] [PubMed] [Google Scholar]
- MAHLER I., NEUMANN I. M., MARMUR J. Studies of genetic-units controlling arginine biosynthesis in Bacillus subtilis. Biochim Biophys Acta. 1963 May 28;72:69–79. [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- MARMUR J., FALKOW S., MANDEL M. NEW APPROACHES TO BACTERIAL TAXONOMY. Annu Rev Microbiol. 1963;17:329–372. doi: 10.1146/annurev.mi.17.100163.001553. [DOI] [PubMed] [Google Scholar]
- OKUBO S., STODOLSKY M., BOTT K., STRAUSS B. SEPARATION OF THE TRANSFORMING AND VIRAL DEOXYRIBONUCLEIC ACIDS OF A TRANSDUCING BACTERIOPHAGE OF BACILLUS SUBTILIS. Proc Natl Acad Sci U S A. 1963 Oct;50:679–686. doi: 10.1073/pnas.50.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. The formation of hybrid DNA molecules and their use in studies of DNA homologies. J Mol Biol. 1961 Oct;3:595–617. doi: 10.1016/s0022-2836(61)80024-7. [DOI] [PubMed] [Google Scholar]
- STARLINGER P. Uber einen Defekt des transduzierenden Salmonella-Phagen P22. Z Naturforsch B. 1958 Aug;13B(8):489–493. [PubMed] [Google Scholar]
- TAKAHASHI I., MARMUR J. Glucosylated DNA from a transducing phage for Bacillus subtilis. Biochem Biophys Res Commun. 1963 Feb 18;10:289–292. doi: 10.1016/0006-291x(63)90526-6. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI I., MARMUR J. Replacement of thymidylic acid by deoxyuridylic acid in the deoxyribonucleic acid of a transducing phage for Bacillus subtilis. Nature. 1963 Feb 23;197:794–795. doi: 10.1038/197794a0. [DOI] [PubMed] [Google Scholar]
- TAKAHASHI I. Transducing phages for Bacillus subtilis. J Gen Microbiol. 1963 May;31:211–217. doi: 10.1099/00221287-31-2-211. [DOI] [PubMed] [Google Scholar]
- TING R. C. The specific gravity of transducing particles of bacteriophage P1. Virology. 1962 Feb;16:115–121. doi: 10.1016/0042-6822(62)90286-6. [DOI] [PubMed] [Google Scholar]
- WEIGLE J. Densities of transducing lambda bacteriophages. J Mol Biol. 1961 Aug;3:393–398. doi: 10.1016/s0022-2836(61)80052-1. [DOI] [PubMed] [Google Scholar]



