Abstract
Aims
To estimate the cost-effectiveness of three behavioral interventions provided to enhance hepatitis A virus (HAV) and hepatitis B virus (HBV) joint vaccination (HAV/HBV) compliance among homeless persons living in Los Angeles County.
Scope
A cost-effectiveness analysis (CEA) based on data from a randomized trial where the costs and compliance data from the trial are incorporated into two Markov models, simulating the natural history of acute and chronic hepatitis infection, following HAV/HBV vaccination. Conclusions: Reductions in HBV-related disease is cost-effective to society and is associated with substantial improvements in quality of life.
Keywords: Cost-Effectiveness Analysis, Hepatitis, Homeless
1. Introduction
Over the past decade, a number of evaluations have been conducted to determine the costs and benefits of hepatitis B virus (HBV) vaccination. The majority of cost-effectiveness analyses (CEAs), conducted in countries where the prevalence of HBV disease is low, have concluded that universal vaccination of either infants or adolescents, in tandem with vaccination of high-risk groups (men who have sex with other men, injection drug users, persons with clotting factor disorders, homeless persons, and health care workers in contact with blood products), is cost-saving for society.1 In 1991, the Advisory Committee on Immunization Practices (ACIP) recommended that universal childhood hepatitis B vaccination be provided to all children in the United States (U.S.).2 In 1999, the ACIP recommended that routine hepatitis A vaccination be provided to children living in areas with hepatitis A rates of greater than 20 cases per 100,000: California was identified as one of the eleven states fulfilling this requirement.3 Universal hepatitis A virus (HAV) vaccination of children, adolescents, and high-risk groups has generally been determined to be cost-effective both in highly endemic areas of the U.S., and nationally.4–8
Despite the availability of a joint HAV/HBV vaccine, and the fact that HAV/HBV vaccination has been shown to be cost-effective, HAV/HBV infection remains a health problem, especially among high-risk groups. 1, 9, 10 The prevalence of HAV ranges from 27%11 to 30%12, and the prevalence of HBV ranges from 22%13 to 30.8%14, among homeless groups. Homeless persons are vulnerable to infectious diseases because they often live in environments which lack proper sanitation and are consequently exposed to pathogens. 15 Suboptimal patient compliance with the three-dose HAV/HBV vaccination series is one factor implicated in the on-going persistence of HAV/HBV infections in the U.S.16 Lack of access to health care services is another factor underlying the persistence of HAV/HBV infection among homeless populations.15
In the setting of high-risk adults, the compliance rate for all three doses of HBV vaccination ranges from 13%10 to 63%.17 Poor compliance has been associated with low perceived susceptibility, limited knowledge about HBV infection and vaccination, and the myths associated with HBV-vaccine-related side effects.18 Some studies, designed to improve vaccination compliance in the setting of high-risk groups, have shown that interventions providing added services such as a needle exchange program19 or telephonic follow-up reminders, 20 resulted in greater HBV completion rates.
There is limited information with respect to compliance with medical regimens such as HBV vaccination, especially in the setting of homeless adults. Moreover, there have been no economic evaluations of the cost-effectiveness of HBV vaccination programs designed to enhance compliance among homeless persons. Given the high economic costs of HAV/HBV disease, 1 programs that are able to enhance vaccination compliance and consequently reduce disease could greatly reduce the burden of HAV/HBV infection in the U.S. A randomized clinical trial provided the reference parameters used in this CEA. The purpose of this analysis is to construct a model to determine whether behavioral intervention in conjunction with HBV vaccination is cost-effective.
2. Method
2.1 General Overview
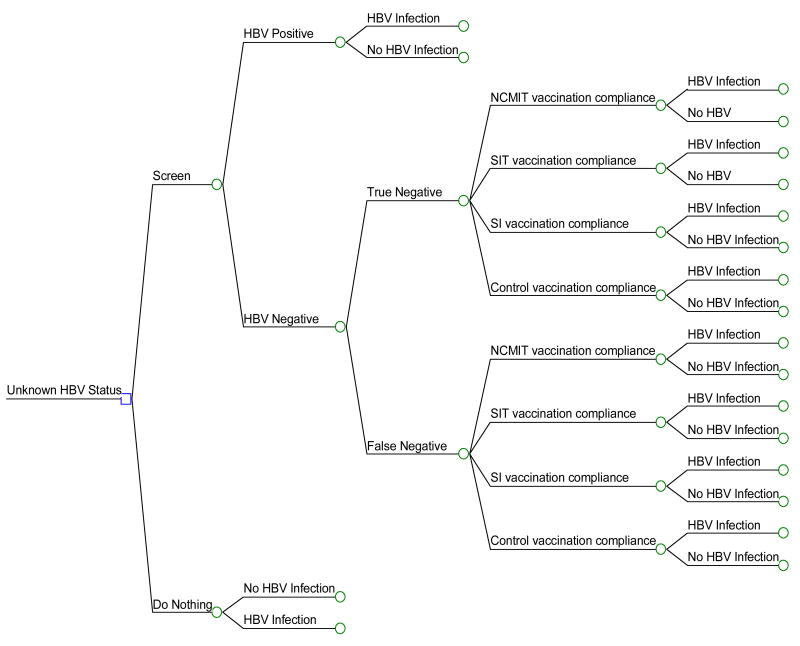
Using decision analysis software21, we built an HBV and an HAV model. An on-going randomized clinical trial (studying the effects of three behavioral interventions designed to promote HAV/HBV compliance among homeless persons (n=865) was used to inform the model. Since HBV vaccination is delivered in three vaccination doses, we chose to focus this analysis on the impact of the three interventions in terms of HBV compliance (Figure 1).
Figure 1. Truncated Hepatitis B (HBV) Decision Tree.
HBV = Hepatitis B virus; NCMIT = Nurse Case Management with Incentives and Tracking; SIT = Standard Incentives plus Tracking; SI = Standard plus Incentives.
As mentioned previously, HAV vaccination has been routinely recommended in Los Angeles County since 1999, however HAV infection continues to be a problem among homeless persons: Moreover, although some of the people living in Los Angeles County might have received HAV vaccination prior to 1999, homeless adults often lack access to prevention health services.15 Consequently, we provided HAV/HBV vaccination because we believed that joint vaccination would provide value-added product, compared with HBV vaccination alone. We chose not to evaluate the cost-effectiveness of HAV vaccination in our model because HAV vaccination has not been shown to be cost-effective when provided alone.22 However, it has been suggested that although the cost-effectiveness of nationwide routine hepatitis A immunization has not been established, it becomes cost-effective when out-of-cohort herd immunity is considered.3
Health benefits were measured in terms of life-years and quality-adjusted life-years (QALYs), and total program costs for each strategy were computed. The societal perspective (including direct and indirect costs) recommended by the Panel on Cost-Effectiveness in Health and Medicine, was used to guide the analysis.23–26 Based on the actual subject compliance rates, we estimated the health and economic outcomes of HBV vaccination under competing strategies.
2.2 Competing Strategies
The competing strategies included: (1) nurse case management plus incentives and tracking (NCMIT), (2) standard management plus incentives and tracking (SIT), or (3) standard management plus incentives only (SI). All three programs included monetary incentives plus the delivery of three HAV/HBV vaccinations (Twinrix™, delivered at baseline, 1-month and 6-months). Both the NCMIT and SIT programs also included subject tracking where a member of the research team went out into the community to locate subjects. The NCMIT program included enhanced education about hepatitis A and B, and risk reduction counseling in conjunction with incentives and tracking.
2.3 Subject Characteristics
The sample (n=865) included homeless adults living in homeless shelters in Los Angeles, California. All subjects were between the ages of 18 and 65 (mean age of 42 years), and were proven to be HBV-negative by serological testing. Table 1 describes the sociodemographic characteristics of the subjects.
Table 1.
Sociodemographic Characteristics of Subjects from Randomized Trial
| Sample of Homeless Adults n=865 | ||
|---|---|---|
| Mean | Standard Deviation | |
| Age | 42.30 | 9.02 |
| Education | 12.06 | 1.84 |
| Number | Percent | |
| Race | ||
| African-American | 597 | 69.01 |
| White | 127 | 14.70 |
| Hispanic/Latino | 120 | 13.90 |
| Other | 21 | 2.40 |
| Marital Status | ||
| Never Married | 482 | 55.70 |
| Married | 75 | 8.70 |
| D/W/S | 308 | 35.6 |
D/W/S = divorced, widowed, separated. Subjects recruited from 20 homeless shelters across Los Angeles.
2.4 Overview of Model
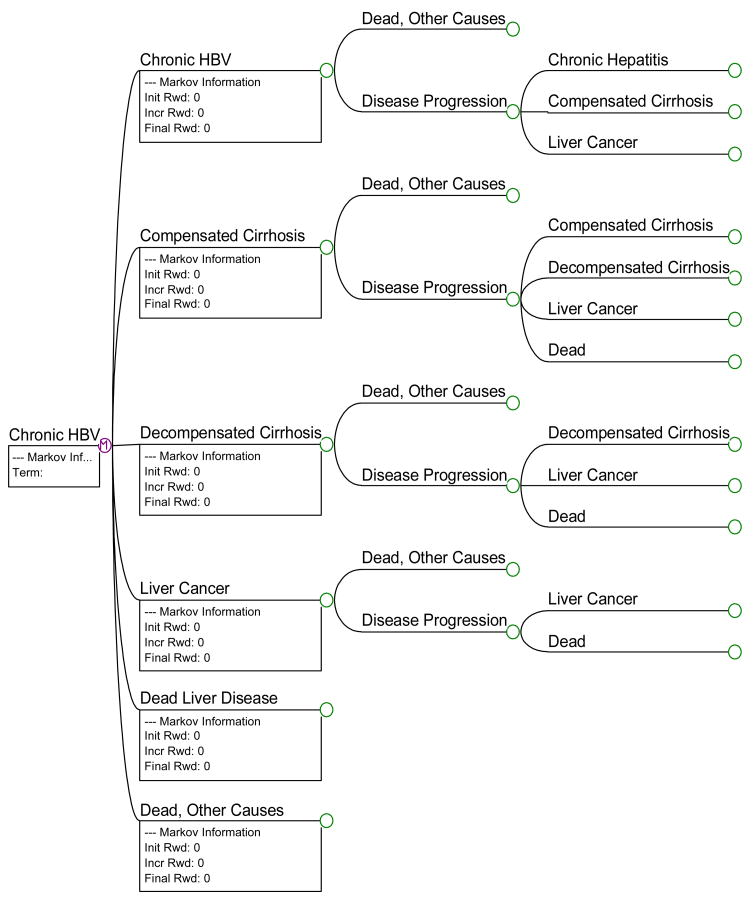
Our model began with the decision to screen and vaccinate, or to do nothing. The “do-nothing” option was a hypothetical group, included into the model for comparison alone. The screen-and-vaccinate arms represented the actual randomized trial upon which the CEA is based. Once the subjects were screened, found to be HBV negative, they were randomized into one of three groups: NCMIT, SIT or SI. A hypothetical group, the control group, served as the referent case. The control group received vaccination but no incentives or tracking. The subjects were followed over the course of a lifetime. The age of the base case subject was 40 years and the model was run over a 39-year duration (there is currently no specific life table estimate for this demographic group, our estimate is based on U.S. life table estimate for the general population27). A Markov model was used to simulate disease progression in the HBV model. This model estimated the life expectancy, quality-adjusted life expectancy and total lifetime costs associated with different sequellae in the natural progression of chronic HBV infection.
2.5 Natural History
The natural history for the model projected the lifetime burden of illness caused by HBV infection (Table 2). This natural history model was constructed as a decision tree with Markov models embedded wherever chronic HBV infection developed. We obtained base-case probability estimates for the natural history of acute HBV from published studies documenting the likelihood of HBV-related events among high-risk groups.14, 28 The base-case estimates used in our HAV model were taken from published estimates of HAV-related events among high-risk groups.29,30 The base-case probabilities used for chronic HBV infection were taken from a systematic review of multiple studies addressing the natural history of chronic HBV infection.31
Table 2.
Base Case Probability Estimates
| Variable | Base-Case Estimate (Range in Sensitivity Analysis) |
|---|---|
| Natural History Variables HAV (Reference) | % |
| Probability of HAV/high risk populations (22) | 27 (20–50) |
| Probability of asymptomatic HAV among infected (23) | 85 (70–90) |
| Probability of symptomatic HAV among infected (23) | 15 (10–30) |
| Hospitalization among symptomatic cases (23) | 18 (10–30) |
| Hospitalized with acute liver failure (23) | 1.0 (0.5–1.0) |
| Fatality among symptomatic cases (23) | 1.0 (0.5–1.0) |
| Natural History Variables Acute HBV | |
| Probability of HBV/High risk populations (11) | 30.8 (25–35) |
| Probability of subclinical HBV (21) | 65.7 (50–75) |
| Probability of clinical HBV (21) | 34 (25–40) |
| Probability of fulminant HBV (21) | 0.3 (0.1–0.5) |
| Subclinical HBV with no sequellae (21) | 92 (85–95) |
| Subclinical HBV progression to chronic HBV (21) | 2.0 (1–3) |
| Clinical HBV progression to chronic HBV (21) | 8.0 (5–10) |
| Fulminant HBV with no sequellae (21) | 96 (94–98) |
| Fulminant HBV progression to chronic HBV (21) | 4.0 (4–5) |
| Fatality among fulminant HBV cases (21) | 78 (75–80) |
| Natural History Variables Chronic HBV | |
| Probability of HBeAg-negative chronic HBV (24) | 55 (0–100) |
| Probability of HBeAg-positive chronic HBV (24) | 45 (0–100) |
| Annual rate of progression to hepatocellular cancer (24) | 1.5 (0–10) |
| Annual rate of progression to compensated cirrhosis (HBeAg−) (24) | 4.6 (0.5–15) |
| Annual rate of progression to compensated cirrhosis (HbeAg+) (24) | 3.0 (0.5–11) |
| Annual rate of mortality in compensated cirrhosis (24) | 4.9 (2–14) |
| Annual rate of progression compensated to decompensated cirrhosis (24) | 7.3 (3.5–10) |
| Annual rate of mortality in decompensated cirrhosis (24) | 19 (6–25) |
| Annual rate of progression from cirrhosis to hepatocellular cancer (24) | 3.4 (1–12) |
| Annual rate of mortality in hepatocellular cancer (24) | 43.3 (20–60) |
| Annual probability of a liver transplant in decompensated cirrhosis (24) | 25 (0–40) |
| Annual probability of a liver transplant in hepatocellular cancer (24) | 30 (0–40) |
| Annual rate of mortality after liver transplantation (24) | 6.9 (2–12) |
| Utility Estimates | |
| Utility of asymptomatic HAV (23) | 1.0 (0.9–1.0) |
| Utility of symptomatic HAV/39 days illness (23) | .57 (.45–.65) |
| Utility of asymptomatic HBV (23) | 1.0 (0.9–1.0) |
| Utility of symptomatic HBV/39 days illness (23) | .57 (.45–.65) |
| Utility of chronic HBV with no cirrhosis (24) | .99 (0.8–1.0) |
| Utility of compensated cirrhosis (24) | .80 (0.7–0.9) |
| Utility of decompensated cirrhosis (24) | .60 (0.5–0.7) |
| Utility of liver transplantation (24) | .86 (0.7–0.9) |
| Utility of hepatocellular cancer (24) | .73 (0.5–0.8) |
2.6 Disease Transmission
Secondary transmission of infection was not factored into the model because it is possible to avoid the very complex process of calculating the difficult-to-capture secondary transmission costs if interventions are found to be more effective and less costly (dominant) than alternatives.24
2.7 Immunity after HBV Infection and/or Vaccination
Subjects who recovered from acute HBV infection were considered to be naturally immune to acute hepatitis and, consequently, chronic HBV could never develop in this group.16 It has been estimated that 0.5% of those fully vaccinated against HAV/HBV infection lose immunity during each of years 1–10 following vaccination, and that 1% lose immunity annually, thereafter.7, 30 Nonetheless, HBV booster doses are currently not recommended.32 Therefore, the impact of loss of immunity was not factored into either model.
2.8 Utilities
Utilities from published studies were used to estimate outcome in both models (Table 2). These studies had used various utility elicitation methods including time-trade-off utilities elicited from patients, utilities from the Health Utilities Index (based on community preferences using the standard gamble method), and values taken from transformations of the Quality of Well-Being Scale.30, 31 All of the utilities were discounted at a rate of 3%.
2.9 Costs
The base-case model took the societal perspective, to allow for comparison with other CEAs.24 All vaccination-related program costs were valued by micro-costing out the actual costs from the randomized trial (Table 3). These costs included travel to clinic visits, nursing time, subject tracking, incentives, serostatus testing, and HAV/HBV vaccinations. Work loss costs were considered to be factored into the denominator QALY calculations and, to avoid double-counting, were not included in the numerator.24 Costs associated with vaccination side effects were not included into either model, because the side effects of Twinrix™ are both local and infrequent.33
Table 3.
Base-Case Cost Estimates
| Costs | Base-Case Estimates in $ Adjusted to Year 2006*(Range in Sensitivity Analysis) |
|---|---|
| Acute HAV disease-related | |
| Acute HAV/no hospitalization (23) | 341.58 (300–500) |
| Acute HAV/hospitalization but no liver failure (23) | 9,711.24 (8,000–10,000) |
| Acute HAV/hospitalization with liver failure (23) | 26,678.43 (25,000–27,000) |
| Acute HBV disease-related high-risk groups | |
| Acute subclinical HBV (21) | 10.20 (5–25) |
| Acute clinical HBV (21) | 1,195.00 (900–1,300) |
| Fulminant HBV (21) | 20,611 (18,000–22,000) |
| Chronic HBV disease-related | |
| Chronic HBV (28) | 792.96 (600–900) |
| Compensated cirrhosis (28) | 288.63 (178–378) |
| Decompensated cirrhosis (28) | 11,917 (10,000–14,000) |
| Hepatocellular cancer (28) | 7,834 (6,000–8000) |
| Liver transplant (28) | 90,014 (85,000–95,000) |
| Direct vaccine-related | |
| Nursing time | 33.90 |
| Tracking | 116.00 |
| Initial blood draw | 8.00 |
| Initial screening incentive | 2.00 |
| Two-week return incentive | 10.00 |
| First vaccination incentive | 10.00 |
| Second vaccination incentive | 15.00 |
| Third vaccination incentive | 25.00 |
| Baseline serostatus test analysis | 50.00 |
| HAV/HBV vaccination series | 150.00 |
| Transportation to and from clinic | 12.00 |
| Total costs per group | |
| Nurse case management plus incentives and tracking (NCMIT) | 431.90 (400–800) |
| Standard plus incentives and tracking (SIT) | 425.00 (400–800) |
| Standard plus incentive | 315.00 (300–600) |
| Control, no incentives or tracking | 241.90 (200–500) |
Values for direct medical costs of health care associated with the development of acute HAV and HBV and chronic HBV were derived from published reports.27, 29, 34 The direct medical costs of HAV and HBV were associated with time spent in each transition and absorbing state (excluding death). All costs were discounted at 3% per year. All costs were reported in 2005 U.S. dollars.
2.10 Sensitivity Analysis
All parameters were assigned ranges based on clinically plausible values, taken from published studies. Sensitivity analyses were performed to test the robustness of the results by changing each variable across its range of feasible values (including vaccination 2 and 3 compliance rates). The impact of changing the inputs for each variable in terms of costs and QALY outcomes was calculated. Costs were doubled and halved to determine whether this had any impact in terms of overall cost-effectiveness comparisons among the groups. A tornado analysis was performed to test the influence of all the variables on the model results, and the most influential variables were rank-ordered. A Monte Carlo analysis performed (10,000 simulations) across a range of willingness-to-pay thresholds was done to compare vaccination strategies.
2.11 Key Model Assumptions
The subjects in the study received their first vaccination dose upon randomization. Therefore, we assumed that compliance with the first dose of HAV/HBV vaccine was 100% for each program, including the control group. The compliance rates for doses two and three were computed from the actual completion rates per program. Compliance rates for the second and third doses of HBV vaccination vary, in the setting of high-risk adults.35 Compliance rates for the third dose of HBV vaccine have been found to be as low as 13%10 whereas compliance for the second and third doses of HBV have been reported at 77% and 63%, respectively.17 Bloom28 published a landmark CEA using compliance rates of 20% for the first and second doses and 33% for the third dose of HBV vaccine.
Vaccination success rates used in this analysis were based on the results of clinical trials of the bivalent vaccine.36–38 These data demonstrated that hepatitis B virus protection is conferred at rates of 29.7%, 77.4%, and 98.2%, after one, two, and three doses, respectively.
The key outcome measures included the costs and the number of quality-adjusted life years (QALYs) lived in each intervention strategy. We assumed no impairment in quantity of life with acute hepatitis A.4 and no impairment in quantity of life with acute hepatitis B. 31 A quality-adjusted utility of 0.57 was used for the 39 days of symptomatic HAV30 and a utility of 0.6739 was used for the 39 days of symptomatic HBV30; we used the utility weights provided by Kanwal et al.31 for chronic active HBV, cirrhosis, posttransplant survival, and hepatocellular carcinoma. Utility losses were not calculated for asymptomatic infections including chronic HBV carriage. 40 The rest of the assumptions made in this CEA are summarized in Table 4.
Table 4.
Model Assumptions
| Key Model Assumptions | Literature Citations |
|---|---|
| 1. All cases of asymptomatic infection incur no costs | Jacobs [30] |
| 2. The immunoassay sensitivity and specificity for detecting HAV and HBV is 98% for both infections. | Saab [41] |
| 3. HBV prevalence among homeless adults in Los Angeles is 30.8% | Gelberg [14] |
| 4. HAV prevalence among homeless adults among high-risk persons is 30% | CDC [42] |
| 5. For each program, vaccination compliance is 100% for the first HAV/HBV vaccination | All subjects are vaccinated after randomization. |
| 6. Vaccination compliance in the control group is 20% for the second and 33% for the third dose. | Bloom [28] |
| 7. HAV/HBV infection confers lifelong immunity. | Bloom [28] |
| 8. Costs of booster doses and the impact of loss of immunity is not factored into the model. | Jacobs [30]; [32] |
| 9. HAV infection and acute HBV infection do not alter survival | WHO [43,44] |
| 10. None of the subjects will receive pharmacological treatment for chronic HBV | Sleisenger [45] |
| 11. A $14,000/QALY and a $50,000/QALY are the established thresholds for the analysis | [6,30,40] |
| 12. HAV protection (antibodies to HAV) is achieved in 93.6% of vaccines after one dose, 99% after two doses and 99.9% after three doses. | [6,30,40] |
| 13. HBV protection (antibodies to HBsAg) is achieved in 29.7% of vaccines after one dose, 77.4% after two doses, and 98.2% after three doses. | [6,30,40] |
HAV= hepatitis A virus infection; HBV= hepatitis B virus infection
2.12 Model Validation
Prior to analyzing the decision trees with respect to the cost-effectiveness of each strategy, the validity of providing vaccination in the setting of homeless adults was tested. This was done by looking at the provision of vaccination (by any strategy) versus “do-nothing.” The do-nothing options for each model were structured to reflect the annual prevalence of HAV and HBV infection among homeless adults. Figures 1 and 2 present truncated versions of the HBV model and the HBV Markov model, respectively.
Figure 2.
Truncated Markov Model for Chronic Hepatitis B Infection
3.0. Results
3.1 Reference Case
The reference case model incorporated a wide range of estimates guiding relevant clinical probabilities in the natural disease progression of hepatitis B infection. The hepatitis A infection model is not presented in this analysis because our model did not demonstrate HAV vaccination under any strategy to be cost-effective. In the HBV model, the mean costs for the SI, SIT and NCMIT groups were $611.30, $669.80, and $676.00, respectively; the mean costs for the control group (using a hypothetical group of subjects) were estimated to be $2153.30.
3.2 Incremental Analysis of SI, SIT, NCMIT and Control
The purpose of the CEA was to evaluate whether there was any difference in cost and clinical outcomes when comparing three different vaccination strategies. (The control group was set up as a hypothetical cohort who received only HAV/HBV vaccination, no incentives or tracking). The NCMIT option was the most cost-effective option, followed by the SIT, SI and control options. The NCMIT dominated (was less costly and more effective) than the SIT group, the SIT group dominated the SI group, and the SI group dominated the control group (Table 5).
Table 5.
Life-Time Costs and Benefits of HBV Vaccination: Base Case Comparison
| Strategy | Total Cost | Total Eff | Incr Cost | Incr Eff | Incr C/E |
|---|---|---|---|---|---|
| Control | $2153.3 | 10.9 | $1304.1 | −10.5 | (Dominated) |
| SI | $1039.1 | 19.5 | $189.9 | −1.9 | (Dominated) |
| SIT | $964.2 | 20.7 | $115.0 | −0.7 | (Dominated) |
| NCMIT | $849.2 | 21.3 |
SI=standard management plus incentives;
SIT=standard management plus incentives and tracking; NCMIT= nurse case management plus incentives and tracking; Control= vaccination only without incentives or tracking; Total Cost= costs of intervention minus savings from intervention; Total Eff= total effectiveness; Incr Cost= incremental cost; Cost-Eff= cost-effectiveness; Incr C/E= incremental cost-effectiveness; costs and effectiveness are discounted at the rate of 3%.
3.3 Compliance
In conjunction with cost and QALYs, vaccination compliance was another major outcome measured in this CEA. Compliance with vaccination #2 was 0.81, 0.89, and 0.93, for the SI, SIT, and NCMIT groups, respectively. (For the control group, compliance was estimated at .20 and .33 for vaccinations #2 and #3, respectively.) Compliance rates for vaccination #3 were 0.54, 0.61, and 0.67, for the SI, SIT, and NCMIT groups, respectively. The NCMIT group had the highest rates of compliance for both vaccinations #2 and #3.
Quality-adjusted life years (QALYs) were used to evaluate effectiveness. The NCMIT group was the most effective yielding 21.3 QALYs, followed by the SIT, the SI and control groups (20.7, 19.5 and 10.9 QALYs, respectively). For each QALY gained, the NCMIT cost $31.81, compared with costs per QALY of $46.63, $53.40, and $198.03 for SIT, SI and control groups, respectively.
3.4 Monte Carlo Simulation
To examine the stability of the results, we used a Monte Carlo simulation with a $50,000 threshold to generate 10,000 different study simulations by re-sampling with replacement for the study population. The cost-effectiveness ratio can be simulated by repeatedly taking draws from the multivariate distribution of the study estimates and then performing a cost-effectiveness analysis for each of those draws. 24 The simulation results showed that NCMIT was the single best strategy overall. Compared with the control group, the three HBV intervention programs showed that NCMIT, SIT and SI were cost-effective in 50%, 47%, and 41% of the simulations, respectively.
3.5 Sensitivity Analysis
Univariate sensitivity analyses of the critical probabilities used in the model were carried out. These analyses were undertaken to evaluate whether varying the values of the parameters used in the model over a plausible range would change the study results. Specific parameters analyzed are presented below.
The estimated HBV prevalence was varied to determine the break-even point, the point at which vaccination would be cost-saving compared with a strategy of doing nothing. When the prevalence of HBV in the population was assumed to be .4 or higher, vaccination was cost-saving compared with do-nothing. The prevalence used in this CEA was .30; thus, although vaccination was not considered to be cost saving, it was cost-effective.
Sensitivity analyses were also carried out to examine vaccination compliance. The compliance rates for each of the intervention groups varied from 0.50 to .95. None of the analyses showed that variations in compliance rate (with respect to either vaccination #2 or #3) altered either the HAV or HBV model. The compliance rate was then altered in the control group (from 20%–50%). There was some marginal impact found with respect to altering compliance in the control group for vaccination #2; that is, when the compliance rate was increased, control group vaccination costs were reduced and control group vaccination became more cost-effective. However, despite the fact that higher compliance (ranging from .20 to .50) resulted in less cost, the outcomes of each model did not change; alteration of the compliance rate continued to show that the SI, SIT, and NCMIT models were more cost-effective compared with control.
Sensitivity analyses were also carried out with respect to the discount rate. All costs and effects used in each model were discounted at a rate of 3%. The discount rate was varied over five thresholds from 0% to 5%. As the discount rate was lowered, the interventions became less costly. However, there were no changes with respect to the basic model when the discount rate was varied.
Tornado analysis was undertaken to determine which variables had the most impact on the model results. No variables were found to influence the models to the degree that the cost-effectiveness order among the strategies changed. A Monte Carlo simulation was run to compare the different interventions across a range of willingness-to-pay thresholds. Using willingness-to-pay thresholds of $14,000 and $50,000, NCMIT was shown to be the most cost-effective option.
Finally, sensitivity analysis was conducted with respect to cost. Although the models were based on the costs incurred with each intervention strategy, vaccination costs and nursing time costs might differ from setting to setting. Doubling either vaccination costs or nursing time costs had no impact on either model: NCMIT still dominated SIT and control, and SI was the least cost-effective option. The effect of doubling all intervention costs in the HBV model was that NCMIT continued to dominate SIT, and SIT continued to dominate SI.
4.0. Discussion
In the health care field, CEAs have typically been undertaken to evaluate the costs and benefits of medical interventions. Few CEAs have actually been designed to assess the costs and benefits of interventions targeted at behavior change. To date, there have been no CEAs evaluating patient compliance in the setting of homeless adults. This is the first CEA evaluating the costs and benefits of methods of HAV/HBV prevention, designed to improve vaccination compliance, among homeless adults.
The results of the randomized trial upon which this CEA was piggybacked showed that vaccination compliance can be greatly improved among homeless adults. The results of this CEA demonstrate the impact of what happens when compliance is increased from 20% and 33% for vaccinations #2 and #3, respectively, 27 to over 93% and 67% (vaccinations #2 and #3, for the NCMIT group, respectively). The significance of greatly improved compliance can be elaborated vis-à-vis consideration of monetary gains and health gains. Higher rates of compliance are associated with greater cost-effectiveness and more quality in terms of adjusted life years.
The NCMIT group is more effective and less costly than the other groups. The NCMIT compliance rate was higher than the SIT and SI groups for both vaccinations 2 and 3. We have shown that compliance improvements in general are cost-effective and specifically, the NCMIT intervention is the intervention of choice.
4.1 Study Limitations
One of the limitations of this analysis had to do with the life table selected for the long-term analysis of chronic hepatitis B. A life table for U.S. adults 27 was used in the construction of the “death, other causes” branch of the Markov model. The randomized trial upon which the CEA was based was conducted in the setting of homeless adults. Homeless persons have been found to have age-adjusted mortality rates of 3.6 times that of non-homeless people.41 There are no life tables published for homeless adults. Consequently, estimates of life years gained may have been overestimated.
Another study limitation is the fact that this CEA was conducted in the setting of infectious disease. Disease transmission was not factored into the model because transmission models can be laborious to construct and can be foregone if “dominance” is obtained. 24 Dominance was achieved, as the NCMIT was dominant over the next best option, SIT and SIT was dominant over the next best option SI. It can be assumed that if an intervention dominates others, the effect of disease transmission will only add to the overall cost-effectiveness of the dominant option.
The final study limitation has to do with the assumption that HAV and HBV vaccination confers lifetime immunity. There is currently no recommendation for booster doses of vaccine.12,42 The efficacy of HAV/HBV vaccine is thought to be long-term.8, 30 Therefore, the costs of booster doses were not factored into the model, nor was attention given to the potential loss of vaccine protective immunity over time. Nonetheless, in the cost sensitivity analysis, the cost of vaccination was doubled, which, in effect, demonstrated that even had booster costs been included, the overall cost-effectiveness of the interventions would have remained the same.
4.2 Conclusions
The findings of this CEA are extremely important because they demonstrate that interventions designed to promote behavior change can have significant economic and health status ramifications, especially when they are applied in the setting of homeless adults. This CEA shows that improvement in vaccination compliance can be optimally achieved by a program of nurse case management with incentives and tracking (NCMIT). This program results in substantial improvement with respect to participant compliance, which, in turn, yields monetary savings (secondary to reductions in disease-related morbidity and mortality). Moreover NCMIT is associated with significant enhancement in quality-adjusted life-years.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Barbara Greengold, University of California, Los Angeles, School of Nursing Box 956917, Los Angeles, CA 90095-6917, (310) 794-4814, (310) 267-0413, Barby3ann@yahoo.com.
Adeline Nyamathi, University of California, Los Angeles, School of Nursing, Box 951702, Los Angeles, CA 90095-1702, (310) 825-8405, (310) 206-7433, anyamath@sonnet.ucla.edu.
Gerald Kominski, University of California, Los Angeles, School of Public Health, Box 951772, Los Angeles, CA 90095-1772.
Dorothy Wiley, University of California, Los Angeles, School of Nursing, Box 956919, Los Angeles, CA 90095-6919.
Mary Ann Lewis, University of California, Los Angeles, School of Nursing, Box 956919, Los Angeles, CA 90095-6919.
Felicia Hodge, University of California, Los Angeles, Schools of Public Health and Nursing, Box 956919, Los Angeles, CA 90095-6919.
Mendel Singer, Case Western Reserve University Department of Epidemiology and Biostatistics, Wood Bldg. WG-69, Cleveland, OH 44106.
Brennan Spiegel, University of California, Los Angeles, School of Medicine, Box 951792, Los Angeles, CA 90095-1792.
References
- 1.Beutels P. Economic evaluations of hepatitis B immunization: A global review of recent studies (1994–2000) Health Economics. 2001;10:751–74. doi: 10.1002/hec.625. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Hepatitis B virsu: A comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination: Recommendations of the Immunization Practices Advisory Committee (ACIP) MMWR. 1991;40(RR13):1–9. [PubMed] [Google Scholar]
- 3.Committee on Infectious Diseases. Hepatitis A vaccine recommendations. American Academy of Pediatrics. 2007;120(1):189–99. doi: 10.1542/peds.2007-1088. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor JB, Imperiale TF, Singer ME. Cost-effectiveness analysis of hepatitis A vaccination strategies for adults. Hepatology. 1999;30(4):1077–81. doi: 10.1002/hep.510300422. [DOI] [PubMed] [Google Scholar]
- 5.Das A. An economic analysis of different strategies of immunization against hepatitis A virus in developed countries. Hepatology. 1998;29(2):548–52. doi: 10.1002/hep.510290225. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs RJ, Greenberg DP, Koff RS, Saab S, Meyerhoff AS. Regional variation in the cost effectiveness of childhood hepatitis A immunization. Pediatr Infect Dis J. 2003;22:904–14. doi: 10.1097/01.inf.0000091295.53969.6a. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs RJ, Rosenthal P, Meyerhoff AS. Cost-effectiveness of hepatitis A/B versus hepatitis B vaccination for U.S. prison inmates. Vaccine. 2004;22:1241–8. doi: 10.1016/j.vaccine.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Meyerhoff AS, Jacobs RJ, Margolis HS, Coleman PJ. Cost-effectiveness of childhood hepatitis A vaccination in the USA. Antivir Ther. 2003;5(1):10. [Google Scholar]
- 9.Rosenthal P. Cost-effectiveness of hepatitis A vaccination in children, adolescents, and adults. Hepatology. 2003;37(1):44–51. doi: 10.1053/jhep.2003.50016. [DOI] [PubMed] [Google Scholar]
- 10.Lamagni TL, Davison KL, Hope VD, Luutu JW, Newham JA, Parry JV. Poor hepatitis B vaccine coverage in injecting drug users: England. Commun Dis Public Health. 1999;2(3):174–7. [PubMed] [Google Scholar]
- 11.Ochnio JJ, Patrick D, Ho M, Talling DN, Dobson SR. Past infection with hepatitis A virus among Vancouver street youth, injection drug users and men who have sex with men: implications for vaccination programs. CMAJ. 2001;165:293–7. [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Disease burden from viral hepatitis A, B, and C in the United States. 2004 [Google Scholar]
- 13.Beech BM, Myers L, Beech DJ. Hepatitis B and C infections among homeless adolescents. Family and Community Health. 2002;25(2):28–37. doi: 10.1097/00003727-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Gelberg L, Robertson MJ, Leake B, et al. Hepatitis B among homeless and other impoverished U.S. military veterans in residential care in Los Angeles. Public Health. 2001;115:286–91. doi: 10.1038/sj/ph/1900783. [DOI] [PubMed] [Google Scholar]
- 15.Badiaga S, Raoult D, Brouqui P. Preventing and controlling emerging and reemerging transmissible diseases in the homeless. Emerging Infectious Diseases. 2008;14(9):1353–9. doi: 10.3201/eid1409.082042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fendrick AM, Lee JH, LaBarge C, Glick HA. Clinical and economic impact of a combination Haemophilus influenzae and hepatitis B vaccine. Arch Pediatr Adolesc Med. 1999;153:126–36. doi: 10.1001/archpedi.153.2.126. [DOI] [PubMed] [Google Scholar]
- 17.Moses S, Mestery K, Kaita KD, Minuk GY. Viral hepatitis in a Canadian street-involved population. Canadian Journal of Public Health. 2002;93(2):123–8. doi: 10.1007/BF03404552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Remis RS, Dufour A, Alary M, et al. Association of hepatitis B virus infection with other sexually transmitted infections in homosexual men. American Journal of Public Health. 2000;90:1570–4. doi: 10.2105/ajph.90.10.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Des Jarlais DC, Fisher DG, Clark-Newman J, et al. Providing hepatitis B vaccination to injection drug users: referral to health clinics vs. on-site vaccination at a syringe exchange program American Journal of Public Health. 2001;91:1791–2. doi: 10.2105/ajph.91.11.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sellors J, Pickard L, Mahony JB, et al. Understanding and enhancing compliance with the second dose of hepatitis B vaccine: A cohort analysis and a randomized controlled trial. Canadian Medical Association. 1997;157:143–8. [PMC free article] [PubMed] [Google Scholar]
- 21.TreeAge Pro. TreeAge Software, Inc; Williamstown, Massachusetts: 2005. [Google Scholar]
- 22.Rein DB, Weinbaum CM. The cost-effectiveness of using hepatitis A/B combined vaccine versus hepatitis B vaccine alone for high-risk heterosexuals. Vaccine. 2008;26:5331–3. doi: 10.1016/j.vaccine.2008.07.089. [DOI] [PubMed] [Google Scholar]
- 23.Weinstein M, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276(15):1253–8. [PubMed] [Google Scholar]
- 24.Gold MR. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 25.Gold MR, Franks P, McCoy KI, Fryback DG. Toward consistency in cost-utility analyses using national measures to create condition-specific values. Medical Care. 1998;36(6):778–92. doi: 10.1097/00005650-199806000-00002. [DOI] [PubMed] [Google Scholar]
- 26.Muennig PA. Designing cost-effectiveness analyses in medicine and health care. New York City: Jossey-Bass; 2002. [Google Scholar]
- 27.National Vital Statistics Center. U.S. life tables, 2003. National Vital Statistics Report 2006;54(14):Table 4–5.
- 28.Bloom BS, Hillman AL, Fendrick M, Schwartz S. A reappraisal of hepatitis B virus vaccination strategies using cost-effectiveness analysis. Annals of Internal Medicine. 1993;118(4):298–306. doi: 10.7326/0003-4819-118-4-199302150-00009. [DOI] [PubMed] [Google Scholar]
- 29.Roy E, Haley N, LeClerc R, Cedras L. Seroprevalence and risk factors for hepatitis A among Montreal street youth. Canadian Journal of Public Health. 2002;93:52–3. doi: 10.1007/BF03404418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobs RJ, Saab S, Meyerhoff AS. The cost effectiveness of hepatitis immunization for U. S. College Students. Journal of American College Health. 2003;51(6):227–36. doi: 10.1080/07448480309596355. [DOI] [PubMed] [Google Scholar]
- 31.Kanwal F, Gralnek IM, Martin P, Dulai GS, Farid M, Spiegel BR. Treatment alternatives for chronic hepatitis B virus infection: a cost-effectiveness analysis. Annals of Internal Medicine. 2005;142:821–31. doi: 10.7326/0003-4819-142-10-200505170-00007. [DOI] [PubMed] [Google Scholar]
- 32.McMahon BJ, Bruden DL, Petersen KM. Antibody levels and protection after hepatitis B vaccination: results of a 15 year follow-up. Annals of Internal Medicine. 2005;142(5):356–66. doi: 10.7326/0003-4819-142-5-200503010-00008. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Notice to readers: FDA approval for a combined hepatitis A and B Vaccine. MMWR. 2001;50:806–7. [PubMed] [Google Scholar]
- 34.Todd AL, Veenstrom DL, Ucherra H, Sullivan SD. Cost of chronic hepatitis B infection in the U. S J Clin Gastroenterol. 2004;38(3):S144–S7. doi: 10.1097/00004836-200411003-00005. [DOI] [PubMed] [Google Scholar]
- 35.Rich JD, Ching C, Lally M, Gaitanis MM, Schwartzaptel B, Charuvastra A. A review of the case for hepatitis B vaccination of high-risk adults. Am J Medicine. 2003;114:316–8. doi: 10.1016/s0002-9343(02)01560-7. [DOI] [PubMed] [Google Scholar]
- 36.Thoelen S, Van Damme P, Leentvaar-Kuypers A, Leroux-Roels G, Bruguera MPCF. The first combined vaccine against hepatitis A and B: an overview. Vaccine. 1999;17:1657–62. doi: 10.1016/s0264-410x(98)00421-6. [DOI] [PubMed] [Google Scholar]
- 37.Rendi-Wagner P, Kundi M, Steinberger H, Wiedermann G, Holzmann H, Hofer M. Antibody response to three recombinant hepatitis B vaccines: comparative evaluation of multicenter travel-clinic based experience. Vaccine. 2001;19:2055–60. doi: 10.1016/s0264-410x(00)00410-2. [DOI] [PubMed] [Google Scholar]
- 38.Davis JP. Experience with hepatitis A and B vaccines. The American Journal of Medicine. 2005;118(10A):7S–15S. doi: 10.1016/j.amjmed.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Tengs T, Wallace A. One thousand health-related quality-of-life estimates. Medical Care. 2000;38(6):583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 40.Jacobs RJ, Saab S, Meyerhoff AS, Koff RS. An economic assessment of pre-vaccination screening for hepatitis A and B. Public Health Reports. 2003;118:550–8. doi: 10.1016/S0033-3549(04)50291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saab S, Yee HF. A simple cost decision analysis model comparing two strategies for hepatitis A vaccination. Am J Med. 2000;109:241–4. doi: 10.1016/s0002-9343(00)00469-1. [DOI] [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention (CDC) Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR. 1999;48(RR12) [PubMed] [Google Scholar]
- 43.World Health Organization. Hepatitis A. In Response DoCDSa, ed; 2000.
- 44.World Health Organization. Hepatitis B. In Response DoCDSa, ed; 2000.
- 45.Feldman M, editor. Sleisenger & Fordtran's Gastrointestinal and Liver Disease. 7th Edition. St Louis: Elsevier Science; 2002. [Google Scholar]
- 46.Hibbs JR, Benner L, Klugman L, Spencer R, Macchia I. Mortality in a cohort of homeless adults in Philadelphia. New Engl J Med. 1994;331(5):304–9. doi: 10.1056/NEJM199408043310506. [DOI] [PubMed] [Google Scholar]