Abstract
Objective
Clozapine, a dibenzodiazepine antipsychotic, is the most effective medication for treatment resistant schizophrenia. However, its use has been limited by the high risk of neutropenia. In children, the rate of neutropenia is higher when compared to adults. We decided to explore the use of lithium to manage neutropenia in childhood-onset schizophrenia (COS) through a systematic audit of COS cases.
Methods
Medical records were reviewed for patients with childhood-onset schizophrenia (COS) who had been treated with the combination of clozapine and lithium carbonate.
Results
Seven patients were found to have been treated with both clozapine and lithium. After initiation of lithium, ANC increased significantly in six out of seven subjects by 29% to 106% with a mean of 66%. In addition, six out of seven subjects continued use of both clozapine and lithium for over 2 years (range: 2.0 to 7.2 years) and do not have immediate plans for discontinuation of either medications.
Conclusions
Our study bolsters support for the use of lithium in the management of neutropenia in children treated with clozapine. Although the coadministration of lithium and clozapine appears effective in the management of neutropenia, it is not without its risks and clinicians must be diligent in their joint use of these medications.
Introduction
Clozapine is a dibenzodiazepine antipsychotic with a distinct clinical profile that is uniquely effective in treatment-resistant schizophrenia(Elkis, 2007). Multiple studies have demonstrated that clozapine remains the most effective medication for treatment-resistant schizophrenia in adults (Kane, 1992, McEvoy et al., 2006). In addition, clozapine has lower rates of extrapyramidal side effects and risk of tardive dyskinesia compared to many conventional and atypical antipsychotics (Essali et al., 2009).
As with adults, clozapine remains the last resort medication for childhood onset schizophrenia (COS) (Kumra et al., 2007). Childhood-onset schizophrenia, defined as the onset of psychosis before the thirteenth birthday, is a rare form of the disorder which is clinically and neurobiologically continuous with the adult-onset disorder (Nicolson and Rapoport, 1999). COS is distinguished from other psychotic illnesses by its chronic, non-episodic course and the ability to compromise many intellectual and social functions (Nicolson et al., 2000). Since 1990, the Child Psychiatry Branch at the National Institute of Mental Health (NIMH) in Bethesda, MD has been conducting an ongoing study of COS. When compared to adult-onset schizophrenia (AOS), COS is often associated with more severe symptomatology and chronic disability (Nicolson et al., 2000, Kranzler et al., 2006). Despite the advent of multiple first and second generation antipsychotics, many children and adolescents with COS require long term treatment (Kranzler et al., 2005).
While clozapine has been used successfully in children with treatment-resistant psychoses and schizophrenia (Sporn et al., 2003, Lieberman, 1998, Gogtay and Rapoport, 2008, Sporn et al., 2007), its use has been limited by a high incidence of neutropenia and agranulocytosis (Lieberman, 1998, Munro et al., 1999). Due to the incidence of neutropenia and agranulocytosis, the Food and Drug Administration has mandated any patient on clozapine must receive regular blood monitoring including total white blood count (WBC) and absolute neutrophil count (ANC). The minimal acceptable WBC and ANC for ongoing clozapine treatment are 3,500/mm3 and 2,000/mm3 respectively. After initiation of clozapine, such testing must occur on a weekly basis for the first six months. After six months of continuous uninterrupted therapy with clozapine, monitoring can be extended to every two weeks. After twelve months of continuous uninterrupted therapy with clozapine, patients may undergo monthly monitoring.
Several reports indicate that a lower age is an independent risk factor for clozapine-induced neutropenia and agranulocytosis (Alvir et al., 1993, Usiskin et al., 2000). A large retrospective chart review of 172 children on clozapine found a 13% incidence of neutropenia over the course of one year (Gerbino-Rosen et al., 2005). In contrast, the incidence of neutropenia in adult patients receiving clozapine is thought to be around 3% (Atkin et al., 1996, Kanaan and Kerwin, 2006). Other studies have demonstrated that both children and adolescents have a lower mean resting ANC relative to adults. (Timmons et al., 2006). This increased risk for neutropenia may lead to significant morbidity and mortality in children with psychotic illnesses (Hummer et al., 1994, Dunk et al., 2006).
There have been a variety of methods used in the treatment of clozapine related neutropenia including use of granulocyte colony-stimulating factor (Chengappa et al., 1996, Kanaan and Kerwin, 2006, Raison et al., 1994) and lithium carbonate (Boshes et al., 2001, Adityanjee, 1995, Bender et al., 2004). However, the chronic use of granulocyte colony-stimulating factor has not been well studied. In addition, injections of the factor are expensive, often painful, and must be conducted daily (Sporn et al., 2003). A preferable option is lithium carbonate. While lithium does have potential side effects, studies have demonstrated that it can cause a reversible leukocytosis and bolster neutrophil counts in both children and adults (Boshes et al., 2001, Esposito et al., 2005, Bender et al., 2004). Lithium increases ANC and total WBC count both acutely and chronically although the magnitude of effect has not been clearly determined (Lapierre and Stewart, 1980, Small et al., 2003). Previous work has supported the idea that effect is not dose related although a study by Blier and colleagues found that a lithium level of at least 0.4 may be required (Lapierre and Stewart, 1980, Blier et al., 1998). The mechanism by which lithium increases in WBC and ANC is unclear but may involve demargination (Small et al., 2003) ), stimulation of granulocyte-macrophage colony-stimulating factor (GM-CSF) (Ozdemir et al., 1994) and stimulation of cytokines (Phiel and Klein, 2001). Further justification for use of lithium in psychiatric patients is related to the medication's beneficial effects on aggression, suicidality, and overall mood.
Literature regarding the adjunctive use of clozapine and lithium in children or adolescents is sprase. As a result, we decided to explore the management of neutropenia and concomitant use of lithium through a systematic audit of COS cases treated with lithium to counter the neutropenia. The long-term use of the dual clozapine-lithium combination was evaluated along with the question of whether lithium treatment can be successfully terminated after stabilization. Following our findings, we provide guidelines for the long term management of neutropenia during clozapine treatment.
Methods
Subjects
Potential subjects from our COS cohort were assessed according to the Diagnostic and statistical manual of mental disorders, DSM-IV-TR (American Psychiatric Association. and American Psychiatric Association. Task Force on DSM-IV., 2000). Eligibility was contingent upon satisfaction of stringent diagnostic criteria including but not limited to a required age range of 6-18 years, onset of psychosis before age thirteen, pre-psychotic I.Q. > 70, and absence of serious medical conditions. Those who met preliminary criteria for the study were brought in for a thorough screening by physicians and mental health professionals on the research team.
COS subjects who participated were admitted to the Pediatric Behavioral Health Unit of the NIMH for hospitalization. When deemed necessary and appropriate, patients underwent a complete medication taper and washout before starting clozapine. Patients were carefully monitored during hospitalization including weekly blood levels. Of the cohort, eighty-four patients received clozapine out of whom twelve developed neutropenia following initiation of clozapine. Neutropenic levels were indicated by an ANC of ≤ 2,000/mm3.
Data Acquisition
Medical records were reviewed for the seven patients who had been treated with the combination of clozapine and lithium carbonate in our study using the NIH Clinical Center's computerized Clinical Research Information System (CRIS) as well as hard copies of archived patient records. Hematologic data, progress notes, and discharge summaries were reviewed for acquisition of the following information: clozapine dose ranges during hospitalization, clozapine dose prior to first neutropenic detection, lithium dose ranges, adverse events, post-lithium ANC levels, relapses while on lithium or following rechallenge (if applicable), and medication regimen at follow-up and/or currently. Updates from primary guardians were obtained by phone when certain data were unavailable.
Statistical Analysis
A student t-test of unequal variance was conducted comparing patients' ANC values of subjects before and after lithium initiation. All reported p values are one tailed.
Results
We identified 84 patients who had received clozapine during hospitalization. It was determined that 12 of these patients met criteria for neutropenia. Seven patients (five male and two female) had received both clozapine and lithium. Complete data for the remaining five patients was not available. The median age of these subjects was 13.4 years (range: 6.7 to 14.8 years). A range of ethnicities were represented in our sample including African American, Caucasian, Hispanic, and Middle-Eastern. Two of these subjects (subjects 1 and 3) had previous clozapine-induced neutropenia which had required termination of the medication. The remaining five subjects did not receive clozapine prior to their hospitalization at the NIMH.
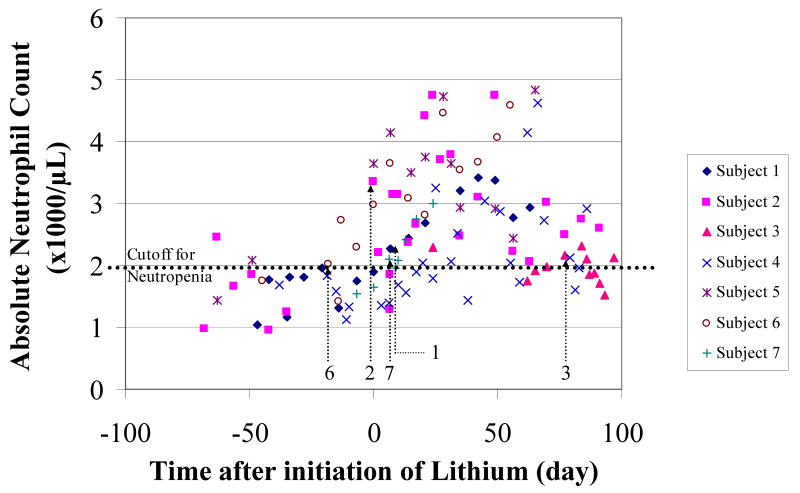
The duration of neutropenia was highly variable, ranging from 3-58 days (median = 14 days). (See Tables 1 and 2 and Figure 1). Before initiation of lithium, the median ANC ranged from 1.46 to 2.02 ×103/μL, and the nadir ANC ranged from 0.95 to 1.74 ×103/μL. The stable dosage of lithium carbonate ranged from 450 to 1500 mg per day. In this study, we were able to determine the exact dates of initiation of lithium carbonate and clozapine for five subjects. Lithium was initiated before clozapine escalation in three cases; in one case, lithium was started on the same day; in another case, lithium was started after clozapine was initiated. After initiation of lithium, the median ANC increased significantly in six out of seven subjects by 29 – 106% with a mean of 66%. The range of final clozapine doses ranged from 450 to 1500 mg/day. Six out of seven subjects have continued to use both clozapine and lithium carbonate for over two years (range: 2.0 to 7.2 years) and do not have immediate plans for discontinuation of either medication. None of the subjects have had a major relapse of neutropenia that required discontinuation of clozapine after discharge from the NIMH. Similarly, no subject discontinued combined treatment due to adverse events.
Table 1.
Summary of demographic and previous treatment data for subjects who received clozapine and lithium.
| Subject Number | Gender | Age at time of first neutropenia (years) | Ethnicity | Previous Clozapine use before admission to NIMH | Dosage of Clozapine at time of first neutropenia (mg/day) | Duration of break in Clozapine treatment following first neutropenia (weeks) |
|---|---|---|---|---|---|---|
| 1 | M | 13.4 | African American | Yes | 32.5 | 7.6 |
| 2 | F | 13.3 | African American | No | N/A | N/A |
| 3 | M | 9.8 | Caucasian | Yes | 150 | 36 |
| 4 | M | 6.7 | Hispanic | No | N/A | N/A |
| 5 | M | 14.8 | African American | No | N/A | N/A |
| 6 | F | 14.8 | Middle Eastern | No | N/A | N/A |
| 7 | M | 13.4 | African American / Caucasian | ** | N/A | N/A |
Not available.
N/A: Not applicable
Table 2.
Summary of current treatment data for subjects who received clozapine and lithium carbonate.
| Subject number | Nadir neutrophil count on first exposure (×103/μL) | Duration of first neutropenia (days) | Range of stable dosage of Clozapine (mg/day) | Range of dosage of Lithium Carbonate used in Clozapine escalation (mg/day) | Duration between initiation of Lithium and initiation of Clozapine (days) | Median ANC before initiation of Lithium | Median ANC after initiation of Lithium | p value# | Current Lithium Carbonate dosage (mg/day) | Duration of Lithium Carbonate Use (years) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.05 | 54 | 350-400 | 450-900 | 7 | 1.76 | 2.77 | 7.3×10-6 | N/A | 0.2 |
| 2 | 0.95 | 5 | 350 | 900 | 0 | 1.46 | 2.75 | 0.00025 | 900 (450 bid) | 2.5 |
| 3 | 1.26 | 3 | 150-450 | 825-1350 | 84 | 1.97 | 2.06 | 0.14 | 500 (250 bid) | 7.2 |
| 4 | 1.12 | 58 | 300-400 | 900-1500 | N/A | 1.59 | 2.05 | 0.00053 | N/A | 6.2 |
| 5 | 1.44 | 14 | 400 | 600-1500 | N/A | 1.77 | 3.64 | 0.0027 | N/A | 3.3 |
| 6 | 1.41 | 27 | 350 | 600 | -18 | 2.02 | 3.64 | 0.00019 | 700 (350 bid) | 2.4 |
| 7 | 1.54 | 13 | 175-500 | 1050-1500 | 6 | 1.54 | 2.11 | 0.0022 | 1650 (900+750) | 2.0 |
Student t-test comparing ANC values before and after lithium carbonate initiation ANC = Absolute neutrophil count
Figure 1.
Absolute neutrophil count versus time after initiation of lithium carbonate treatment. Arrows indicate time of initiation of clozapine.
Discussion
This case series, to our knowledge, is the first evaluation of neutropenia in the COS population. The 14.3% incidence of neutropenia in our cohort appears to be significantly higher than in AOS. (Dunk et al., 2006). This supports the hypothesis that younger patients treated with clozapine may be more susceptible to neutropenia. (Porter and Mohamed, 2006).
Our study bolsters support for the efficacy of lithium and clozapine coadministration in children. This is similar to the adult population in which previous work suggests the combination of clozapine and lithium is safe and effective (Alvir et al., 1993, Blier et al., 1998, Silverstone, 1998, Adityanjee, 1995). Most cases reported in this study were successful in that patients tolerated coadministration without development or relapse of neutropenia. This includes two patients who had a history of clozapine-induced neutropenia prior to rechallenge with lithium. Thus, lithium appears to have a protective effect within the context of prevention and potential treatment of neutropenia.
Once initiated, the combination of clozapine and lithium was continued by most patients. As noted in the results, the requisite daily dosage needed for prevention of neutropenia was dependent on the individual and ranged from 450 mg to 1500 mg. Most individuals required gradual increases in lithium dose over time. The duration of combined treatment ranged from 0.2 – 7.2 years. Six out of seven subjects remained on combined treatment for at least two years following discharge from the NIMH. As noted previously, no subject undergoing combined treatment had an episode of neutropenia that required discontinuation of clozapine. One patient, subject 3, had his lithium discontinued six months after onset of combined lithium-clozapine treatment. However, he had a recurrence of neutropenia requiring termination of clozapine six weeks after lithium discontinuation. This suggests that patients may need to remain on the combined treatment for at least two years before considering a taper of lithium.
There are several limitations to our study. First, our case series was limited by the small sample of children with combined treatment. Results from a larger study may have strengthened our findings. Secondly, blood levels for lithium were not consistently available and consequently could not be related to treatment efficacy. Thirdly, some data regarding a patient's medical history were acquired through phone interviews with patients' guardians. As a result, some families could not provide exact dates regarding termination of clozapine and/or lithium or whether there was concurrent use of other medications that would increase the risk of neutropenia (eg: valproate).
In practice, clinicians are often wary about the use of clozapine given its higher rate of side effects and adverse events. This concern is of particular importance in children who may be more susceptible to neutropenia versus adults. However, clozapine remains the most effective medication, and at times the last resort medication for treatment refractory cases of COS (Sporn et al., 2007). With this in mind, it is clear that any potential strategies that can be used to minimize the dangerous side effect of neutropenia will be of great benefit to clinicians and patients. Our study suggests that the combined use of clozapine and lithium in the prevention and treatment of neutropenia is safe and effective. In light of this, clinicians should consider combining lithium with clozapine under the following circumstances: 1) a patient's baseline ANC is marginally greater than 2,000 mm3 2) clozapine induction leads to a significant decrease in ANC (1,000 mm3-2,000 mm3) 3) a potential rechallenge option for subjects who failed an initial clozapine trial. As clozapine is not FDA approved for COS, clinicians must pay particular attention to adhere to dosage guidelines and requisite monitoring of blood counts and plasma levels.
Since long term use of lithium can be associated with serious health risks (eg: kidney damage, weight gain, sedation, tremor, etc), the potential to taper or discontinue the medicine may be important. As noted previously, review of the literature supports the idea that ANC is related to age and younger children are more susceptible to neutropenia. With this in mind, patients who develop neutropenia and are treated with lithium may benefit from a potential discontinuation of lithium after a period of two years. Lithium could be tapered slowly over the course of 8-12 weeks. However, serial laboratory evaluations including serum lithium levels, BUN, creatinine, as well as thyroid monitoring (TSH/T4) are necessary while lithium is being used. Stringent follow-up with an outpatient psychiatrist would also be crucial. During this process, clinicians should have a low threshold for restarting or maintaining lithium if concerns about neutropenia reemerge.
Conclusions
In conclusion, our study recommends use of lithium to help prevent and manage neutropenia in COS. This is important in the context of limited treatment options for children and adolescents with treatment refractory schizophrenia. Although the coadministration of lithium and clozapine appears effective in the management of neutropenia, it is not without risks and clinicians must be diligent in their joint utilization of the medications.
refer
- Adityanjee Modification of clozapine-induced leukopenia and neutropenia with lithium carbonate. Am J Psychiatry. 1995;152:648–9. doi: 10.1176/ajp.152.4.648. [DOI] [PubMed] [Google Scholar]
- Alvir JM, Lieberman JA, Safferman AZ, Schwimmer JL, SCHAAF JA. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329:162–7. doi: 10.1056/NEJM199307153290303. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association & American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. Task Force on DSM-IV. [Google Scholar]
- Atkin K, Kendall F, Gould D, Freeman H, Liberman J, O'sullivan D. Neutropenia and agranulocytosis in patients receiving clozapine in the UK and Ireland. Br J Psychiatry. 1996;169:483–8. doi: 10.1192/bjp.169.4.483. [DOI] [PubMed] [Google Scholar]
- Bender S, Linka T, Wolstein J, Gehendges S, Paulus HJ, Schall U, Gastpar M. Safety and efficacy of combined clozapine-lithium pharmacotherapy. Int J Neuropsychopharmacol. 2004;7:59–63. doi: 10.1017/S1461145703003870. [DOI] [PubMed] [Google Scholar]
- Blier P, Slater S, Measham T, Koch M, Wiviott G. Lithium and clozapine-induced neutropenia/agranulocytosis. Int Clin Psychopharmacol. 1998;13:137–40. doi: 10.1097/00004850-199805000-00008. [DOI] [PubMed] [Google Scholar]
- Boshes RA, Manschreck TC, Desrosiers J, Candela S, Hanrahan-Boshes M. Initiation of clozapine therapy in a patient with preexisting leukopenia: a discussion of the rationale of current treatment options. Ann Clin Psychiatry. 2001;13:233–7. doi: 10.1023/a:1014681418969. [DOI] [PubMed] [Google Scholar]
- Chengappa KN, Gopalani A, Haught MK, Mcchesney K, Baker RW, Schooler NR. The treatment of clozapine-associated agranulocytosis with granulocyte colony-stimulating factor (G-CSF) Psychopharmacol Bull. 1996;32:111–21. [PubMed] [Google Scholar]
- Dunk LR, Annan LJ, Andrews CD. Rechallenge with clozapine following leucopenia or neutropenia during previous therapy. Br J Psychiatry. 2006;188:255–63. doi: 10.1192/bjp.188.3.255. [DOI] [PubMed] [Google Scholar]
- Elkis H. Treatment-resistant schizophrenia. Psychiatr Clin North Am. 2007;30:511–33. doi: 10.1016/j.psc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Esposito D, Rouillon F, Limosin F. Continuing clozapine treatment despite neutropenia. Eur J Clin Pharmacol. 2005;60:759–64. doi: 10.1007/s00228-004-0835-z. [DOI] [PubMed] [Google Scholar]
- Essali A, AL-Haj Haasan N, Li C, Rathbone J. Clozapine versus typical neuroleptic medication for schizophrenia. Cochrane Database Syst Rev. 2009:CD000059. doi: 10.1002/14651858.CD000059.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbino-rosen G, Roofeh D, Tompkins DA, Feryo D, Nusser L, Kranzler H, Napolitano B, Frederickson A, Henderson I, Rhinewine J, Kumra S. Hematological adverse events in clozapine-treated children and adolescents. J Am Acad Child Adolesc Psychiatry. 2005;44:1024–31. doi: 10.1097/01.chi.0000171904.23947.54. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Rapoport J. Clozapine use in children and adolescents. Expert Opin Pharmacother. 2008;9:459–65. doi: 10.1517/14656566.9.3.459. [DOI] [PubMed] [Google Scholar]
- Hummer M, Kurz M, Barnas C, Saria A, Fleischhacker WW. Clozapine-induced transient white blood count disorders. J Clin Psychiatry. 1994;55:429–32. [PubMed] [Google Scholar]
- Kanaan RA, Kerwin RW. Lithium and clozapine rechallenge: a retrospective case analysis. J Clin Psychiatry. 2006;67:756–60. doi: 10.4088/jcp.v67n0509. [DOI] [PubMed] [Google Scholar]
- Kane JM. Clinical efficacy of clozapine in treatment-refractory schizophrenia: an overview. Br J Psychiatry Suppl. 1992:41–5. [PubMed] [Google Scholar]
- Kranzler H, Roofeh D, Gerbino-Rosen G, Dombrowski C, Mcmeniman M, Dethomas C, Frederickson A, Nusser L, Bienstock MD, Fisch GS, Kumra S. Clozapine: its impact on aggressive behavior among children and adolescents with schizophrenia. J Am Acad Child Adolesc Psychiatry. 2005;44:55–63. doi: 10.1097/01.chi.0000145371.23122.5a. [DOI] [PubMed] [Google Scholar]
- Kranzler HN, Kester HM, Gerbino-Rosen G, Henderson IN, Youngerman J, Beauzile G, Ditkowsky K, Kumra S. Treatment-refractory schizophrenia in children and adolescents: an update on clozapine and other pharmacologic interventions. Child Adolesc Psychiatr Clin N Am. 2006;15:135–59. doi: 10.1016/j.chc.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Kumra S, Kranzler H, Gerbino-Rosen G, Kester HM, Dethomas C, Kafantaris V, Correll CU, Kane JM. Clozapine and “High-Dose” Olanzapine in Refractory Early-Onset Schizophrenia: A 12-Week Randomized and Double-Blind Comparison. Biol Psychiatry. 2007 doi: 10.1016/j.biopsych.2007.04.043. [DOI] [PubMed] [Google Scholar]
- Lapierre G, Stewart RB. Lithium carbonate and leukocytosis. Am J Hosp Pharm. 1980;37:1525–8. [PubMed] [Google Scholar]
- Lieberman JA. Maximizing clozapine therapy: managing side effects. J Clin Psychiatry. 1998;59 3:38–43. [PubMed] [Google Scholar]
- Mcevoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, Rosenheck RA, Swartz MS, Perkins DO, Keefe RS, Davis CE, Severe J, Hsiao JK. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163:600–10. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- Munro J, O'sullivan D, Andrews C, Arana A, Mortimer A, Kerwin R. Active monitoring of 12,760 clozapine recipients in the UK and Ireland. Beyond pharmacovigilance. Br J Psychiatry. 1999;175:576–80. doi: 10.1192/bjp.175.6.576. [DOI] [PubMed] [Google Scholar]
- Nicolson R, Lenane M, Hamburger SD, Fernandez T, Bedwell J, Rapoport JL. Lessons from childhood-onset schizophrenia. Brain Res Brain Res Rev. 2000;31:147–56. doi: 10.1016/s0165-0173(99)00032-6. [DOI] [PubMed] [Google Scholar]
- Nicolson R, Rapoport JL. Childhood-onset schizophrenia: rare but worth studying. Biol Psychiatry. 1999;46:1418–28. doi: 10.1016/s0006-3223(99)00231-0. [DOI] [PubMed] [Google Scholar]
- Ozdemir MA, Sofuoglu S, Tanrikulu G, Aldanmaz F, Esel E, Dundar S. Lithium-induced hematologic changes in patients with bipolar affective disorder. Biol Psychiatry. 1994;35:210–3. doi: 10.1016/0006-3223(94)91155-x. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Klein PS. Molecular targets of lithium action. Annu Rev Pharmacol Toxicol. 2001;41:789–813. doi: 10.1146/annurev.pharmtox.41.1.789. [DOI] [PubMed] [Google Scholar]
- Porter R, Mohamed A. Diurnal variation of neutropenia during clozapine treatment. Int J Neuropsychopharmacol. 2006;9:373–4. doi: 10.1017/S1461145705006061. [DOI] [PubMed] [Google Scholar]
- Raison CL, Guze BH, Kissell RL. Successful treatment of clozapine-induced agranulocytosis with granulocyte colony-stimulating factor. J Clin Psychopharmacol. 1994;14:285–6. doi: 10.1097/00004714-199408000-00015. [DOI] [PubMed] [Google Scholar]
- Silverstone PH. Prevention of clozapine-induced neutropenia by pretreatment with lithium. J Clin Psychopharmacol. 1998;18:86–8. doi: 10.1097/00004714-199802000-00016. [DOI] [PubMed] [Google Scholar]
- Small JG, Klapper MH, Malloy FW, Steadman TM. Tolerability and efficacy of clozapine combined with lithium in schizophrenia and schizoaffective disorder. J Clin Psychopharmacol. 2003;23:223–8. doi: 10.1097/01.jcp.0000084026.22282.5f. [DOI] [PubMed] [Google Scholar]
- Sporn A, Gogtay N, Ortiz-Aguayo R, Alfaro C, Tossell J, Lenane M, Gochman P, Rapoport JL. Clozapine-induced neutropenia in children: management with lithium carbonate. J Child Adolesc Psychopharmacol. 2003;13:401–4. doi: 10.1089/104454603322572697. [DOI] [PubMed] [Google Scholar]
- Sporn AL, Vermani A, Greenstein DK, Bobb AJ, Spencer EP, Clasen LS, Tossell JW, Stayer CC, Gochman PA, Lenane MC, Rapoport JL, Gogtay N. Clozapine Treatment of Childhood-Onset Schizophrenia: Evaluation of Effectiveness, Adverse Effects, and Long-Term Outcome. J Am Acad Child Adolesc Psychiatry. 2007;46:1349–1356. doi: 10.1097/chi.0b013e31812eed10. [DOI] [PubMed] [Google Scholar]
- Timmons BW, Tarnopolsky MA, Snider DP, Bar-Or O. Immunological changes in response to exercise: influence of age, puberty, and gender. Med Sci Sports Exerc. 2006;38:293–304. doi: 10.1249/01.mss.0000183479.90501.a0. [DOI] [PubMed] [Google Scholar]
- Usiskin SI, Nicolson R, Lenane M, Rapoport JL. Retreatment with clozapine after erythromycin-induced neutropenia. Am J Psychiatry. 2000;157:1021. doi: 10.1176/appi.ajp.157.6.1021. [DOI] [PubMed] [Google Scholar]