Abstract
New achiral sulfamide, phosphoric triamide, and thiophosphoric triamide compounds have been synthesized. Their activity as hydrogen bond catalysts for the Friedel-Crafts and Baylis-Hillman reactions compares favorably with that of a known and active thiourea catalyst. The new compounds were also studied by X-ray crystallography and their solid state structures are described.
Hydrogen-bonding is a ubiquitous force in nature. Not until relatively recently have chemists begun to implement this force in catalysis, but already with extraordinary success.1 The majority of hydrogen bonding (HB) catalysts incorporates the thiourea functional group.2 Advantages of the thiourea include air and water stability, ease of synthesis and modification, and activity toward a wide range of substrates.
Despite their success, thiourea catalysts suffer from some significant disadvantages. First, they have relatively weak activity, frequently necessitating high catalyst loading and/or long reaction times to achieve a satisfactory yield. A more active HB catalyst would be desirable. A second concern for the thiourea functionality is its sensitivity to heat. At elevated temperatures (>75 °C), some thiourea catalysts have been reported to decompose.3 Although the majority of HB catalyzed reactions are run at or below room temperature, a more thermally robust catalyst could allow for a more versatile catalyst system.
Several non-thiourea HB catalysts have been developed as potential alternatives. These can be categorized into neutral and protonated catalysts. Notable examples of neutral compounds include those based on squaramide,4 sulfonamide,5 and urea-N-sulfoxide6 structures. Protonated catalysts include those based on guanidinium,7 quinolinium thioamide,8 and ammonium9 structures. While the latter catalysts tend to activate electrophiles more strongly than the former, due to their increased acidity, they are incompatible with basic functionality. This serves to limit substrate scope and places restrictions on catalyst design. Based on this consideration, we focused on improved neutral thiourea alternatives. Our aim was to develop new neutral molecular designs that display improved catalytic activity over the thiourea motif. We focus in this communication on the relative catalytic efficiency of these structural motifs.
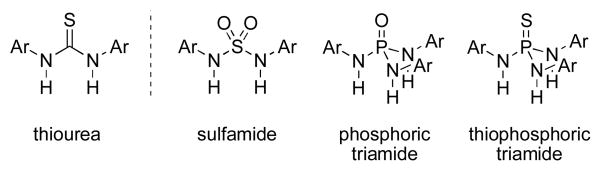
We identified three motifs as promising candidates: i) sulfamides, ii) phosphoric triamides, and iii) thiophosphoric triamides. All of the structures, like thioureas, join two or more amide-like groups to a single electron-withdrawing atom (Figure 1). As such, they were expected to exhibit a similar mode of activity toward common substrates. Replacement of the thiocarbonyl group with sulfone, phosphorus oxide, or phosphorus sulfide tethers however was anticipated to give these compounds modified, perhaps improved, binding properties. In addition, the phosphoric triamide and thiophosphoric triamide would hold a potential kinetic binding advantage as a consequence of a third active hydrogen bond. All three structures exhibit exceptional hydrogen bonding ability in the solid state,10 yet to our knowledge none have been utilized as HB catalysts.
Figure 1.
The sulfamide, phosphoric triamide, and thiophosphoric triamide structures.
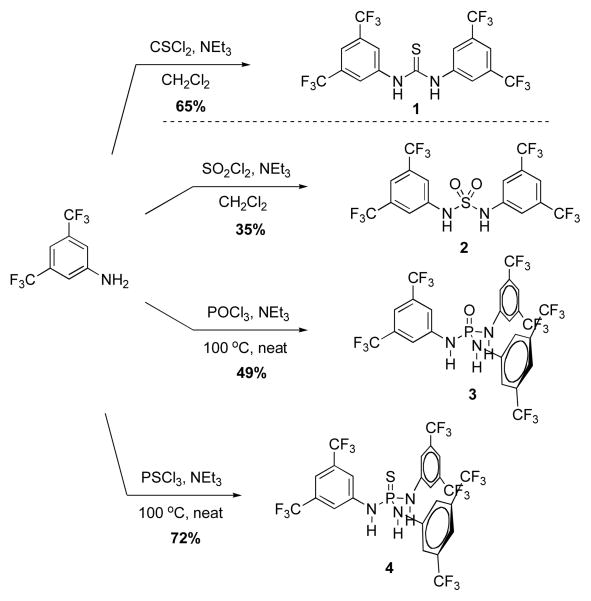
For this study, the thiourea catalyst 1 designed by Schreiner and coworkers2a was used for comparison purposes. This catalyst represents one of the most active and versatile thiourea catalysts known. The corresponding sulfamide 2, phosphoric triamide 3, and thiophosphoric triamide 4 were synthesized as shown in Scheme 1. Sulfamide 2 was prepared using a procedure developed for thiourea 1, in which sulfuryl chloride was treated with 3,5-bis(trifluoromethyl)aniline in the presence of triethylamine. A modified procedure, with elevated temperature in the absence of solvent, was necessary to synthesize phosphoric triamide 3 and thiophosphoric triamide 4. The three new structures 2–4 would allow a direct comparison between the thiourea, sulfamide, phosphoric triamide, and thiophosphoric triamide groups as HB catalysts.
Scheme 1.
Synthesis of sulfamide 2, phosphoric triamide 3, and thiophosphoric triamide 4.
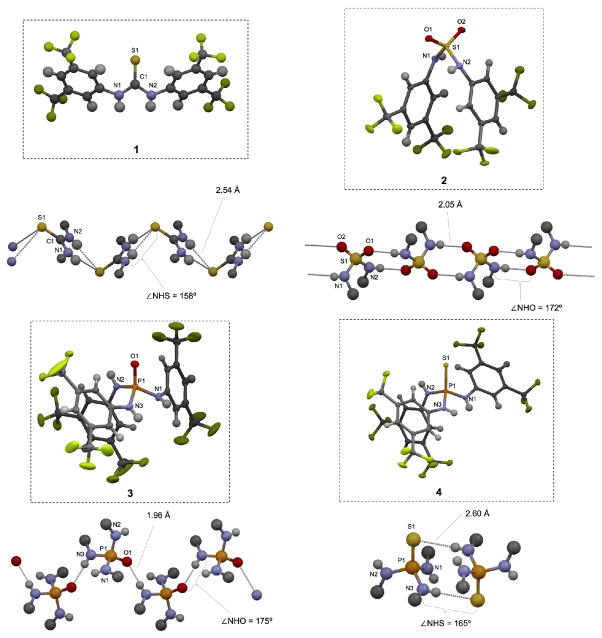
The target molecules 2–4 were isolated as crystalline solids, and X-ray crystal structures were solved for each one (see Figure 2). The solid state structure of thiourea 1 has been previously solved,11 and was used for comparison. In all cases, the compounds formed three-dimensional hydrogen bonding networks. Thiourea 1 exists almost completely flat in the solid state, forming linear hydrogen bonding chains involving double hydrogen bonding typical of ureas and thioureas.12 Hydrogen bonds were 2.54 Å in length, measured from the hydrogen atom to the acceptor atom, with a 158° NHS angle. In contrast to the thiourea’s flat structure, sulfamide 2 adopted a folded configuration in the crystal. It formed linear intermolecular hydrogen bonded chains with two 2.05 Å hydrogen bonds between each pair of neighboring molecules, each of which had an NHO angle of 172°. Phosphoric triamide 3 adopted a partially folded conformation, with two parallel N-H bonds. It also formed linear chains, but with only one 1.96 Å hydrogen bond between each pair of neighboring molecules with an NHO angle of 175°. Thiophosphoric triamide 4 adopted a similar conformation to that of 3, but its extended crystal structure consisted of isolated, loosely associated dimers with weaker 2.60 Å hydrogen bonds at NHS angles of 165°. Compared to thiourea 1, each of the new compounds 2–4 displayed a similar ability to donate organized and directional hydrogen bonds, suggesting a potential for catalytic activity.
Figure 2.
Solid state structures of compounds 1, 2, 3, and 4. Packing behavior and hydrogen bonding networks are shown with selected bond distances and angles highlighted.
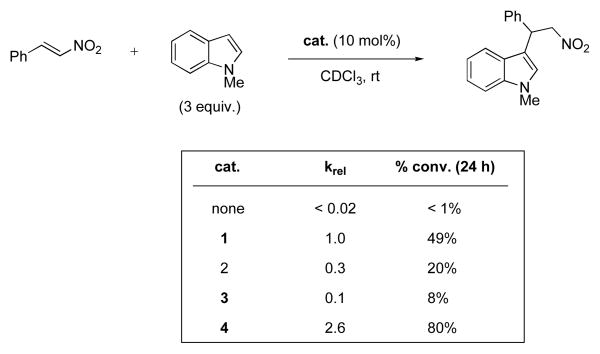
A study to assess catalytic activity was next initiated. The Friedel-Crafts reaction between N-methyl indole and β-nitrostyrene was chosen as the first test reaction to compare the catalytic activity of compounds 1–4. Thioureas have previously been shown to be competent catalysts for this transformation.13 With a threefold excess of indole starting material, pseudo-first order kinetics were observed, and the relative rate constants were measured for each catalyst. The results are summarized in Scheme 3. All new compounds 2–4 exhibited some catalytic activity for the chosen reaction, placing them in the category of HB catalysts for the first time. Furthermore, thiophosphoric triamide 4 displayed a 2.6 fold increase in activity compared to thiourea 1.
Scheme 3.
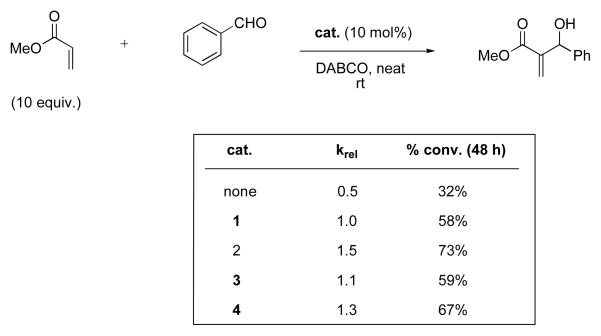
Catalyst comparison for the Baylis-Hillman reaction of methyl acrylate with benzaldehyde.
The Baylis-Hillman reaction between methyl acrylate and benzaldehyde was also examined with catalysts 1–4. As in the previous reaction, thioureas have been used successfully to catalyze this transformation.14 Following literature protocol,15 the reactions were carried out neat at room temperature with 1,4-diazabicyclo[2.2.2]octane (DABCO) as cocatalyst (Scheme 3). With a tenfold excess of methyl acrylate compared to benzaldehyde, this reaction also exhibited pseudo-first order behavior, so relative rate constants were calculated. As in the first example, all catalysts 2–4 showed activity, but this time all were equal to or slightly more effective than reference catalyst 1. Interestingly, sulfamide 2 catalyzed the reaction at the fastest rate, 50% faster than thiourea 1.
In summary, three new compounds 2–4 were synthesized as candidates for HB catalysts. The compounds exhibit extensive hydrogen bonding in the solid state. Each of the three compound types showed activity as HB catalysts. Furthermore, modest improvements (1.5–2.6 fold) were observed for selected Friedel-Crafts and Bayliss-Hillman reactions when compared to the corresponding thiourea catalyst. In consideration of the relatively slow rates for many known HB catalysts, the modest rate enhancements exhibited by the sulfamides, phosphoric triamides, and thiophosphoric triamides represent fertile territory for the development of new, more efficient HB catalyst designs.
Future work will involve asymmetric catalyst development. The fact that catalysts 2–4 adopt three-dimensional structures as opposed to the thiourea’s preferred flat structure is expected to aid in creating compounds with unique chiral space. In the case of phosphoric triamides and thiophosphoric triamides, the phosphorus atom itself can serve as a chiral center. Work in this project is ongoing.
Supplementary Material
Experimental methods and selected 1H and 13C NMR data.
Scheme 2.
Catalyst comparison for the Friedel-Crafts reaction of N-methyl indole with β-nitrostyrene.
Acknowledgments
This work was supported by a grant from the NIH. H. Y. is grateful for support from the University Research Opportunities Program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.(a) Pihko PM. Angew Chem Int Ed. 2004;43:2062–2064. doi: 10.1002/anie.200301732. [DOI] [PubMed] [Google Scholar]; (b) Taylor MS, Jacobsen EN. Angew Chem Int Ed. 2006;45:1520–1543. doi: 10.1002/anie.200503132. [DOI] [PubMed] [Google Scholar]; (c) Akiyama T, Itoh J, Fuchibe K. Adv Synth Catal. 2006;348:999–1010. [Google Scholar]; (d) Doyle AG, Jacobsen EN. Chem Rev. 2007;107:5713–5743. doi: 10.1021/cr068373r. [DOI] [PubMed] [Google Scholar]
- 2.(a) Schreiner PR. Chem Soc Rev. 2003;32:289–296. doi: 10.1039/b107298f. [DOI] [PubMed] [Google Scholar]; (b) Takemoto Y. Org Biomol Chem. 2005;3:4299–4306. doi: 10.1039/b511216h. [DOI] [PubMed] [Google Scholar]; (c) Connon SJ. Chem Eur J. 2006;12:5418–5427. doi: 10.1002/chem.200501076. [DOI] [PubMed] [Google Scholar]
- 3.(a) Curran DP, Kuo LH. Tetrahedron Lett. 1995;36:6647–6650. [Google Scholar]; (b) Vakulya B, Varga S, Csmpai A, Sos T. Org Lett. 2005;7:1967–1969. doi: 10.1021/ol050431s. [DOI] [PubMed] [Google Scholar]
- 4.Malerich JP, Hagihara K, Rawal VH. J Am Chem Soc. 2008;130:14416–14417. doi: 10.1021/ja805693p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Oh SH, Rho HS, Lee JW, Lee JE, Youk SH, Chin J, Song CE. Angew Chem Int Ed. 2008;47:7872–7875. doi: 10.1002/anie.200801636. [DOI] [PubMed] [Google Scholar]; (b) Luo J, Xu LW, Hay RAS, Lu Y. Org Lett. 2009;11:437–440. doi: 10.1021/ol802486m. [DOI] [PubMed] [Google Scholar]
- 6.Robak MT, Trincado M, Ellman JA. J Am Chem Soc. 2007;129:15110–15111. doi: 10.1021/ja075653v. [DOI] [PubMed] [Google Scholar]
- 7.(a) Corey EJ, Grogan MJ. Org Lett. 1999;1:157–160. doi: 10.1021/ol990623l. [DOI] [PubMed] [Google Scholar]; (b) Sohtome Y, Hashimoto Y, Nagasawa K. Adv Synth Catal. 2005;347:1643–1648. [Google Scholar]; (c) Terada M, Nakano M, Ube H. J Am Chem Soc. 2006;128:16044–16045. doi: 10.1021/ja066808m. [DOI] [PubMed] [Google Scholar]; (d) Uyeda C, Jacobsen EN. J Am Chem Soc. 2008;130:9228–9229. doi: 10.1021/ja803370x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganesh M, Seidel D. J Am Chem Soc. 2008;130:16464–16465. doi: 10.1021/ja8063292. [DOI] [PubMed] [Google Scholar]
- 9.Huang J, Corey EJ. Org Lett. 2004;6:5027–5029. doi: 10.1021/ol047698w. [DOI] [PubMed] [Google Scholar]
- 10.(a) Atwood JL, Cowley AH, Hunter WE, Mehrotra SK. Inorg Chem. 1982;21:435–437. [Google Scholar]; (b) Chivers T, Krahn M, Schatte G, Parvez M. Inorg Chem. 2003;42:3994–4005. doi: 10.1021/ic034151p. [DOI] [PubMed] [Google Scholar]
- 11.Kotke M, Schreiner PR. Tetrahedron. 2006;62:434–439. [Google Scholar]
- 12.Etter MC, Urbanczyk-Lipkowska Z, Zia-Ebrahimi M, Panunto TW. J Am Chem Soc. 1990;112:8415–8426. [Google Scholar]
- 13.Dessole G, Herrera RP, Ricci A. Synlett. 2004:2374–2378. [Google Scholar]
- 14.(a) Sohtome Y, Tanatani A, Hashimoto Y, Nagasawa K. Tetrahedron Letters. 2004;45:5589–5592. [Google Scholar]; (b) Wang J, Li H, Yu X, Zu L, Wang W. Org Lett. 2005;7:4293–4296. doi: 10.1021/ol051822+. [DOI] [PubMed] [Google Scholar]
- 15.Maher DJ, Connon SJ. Tetrahedron Lett. 2004;45:1301–1305. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental methods and selected 1H and 13C NMR data.