Abstract
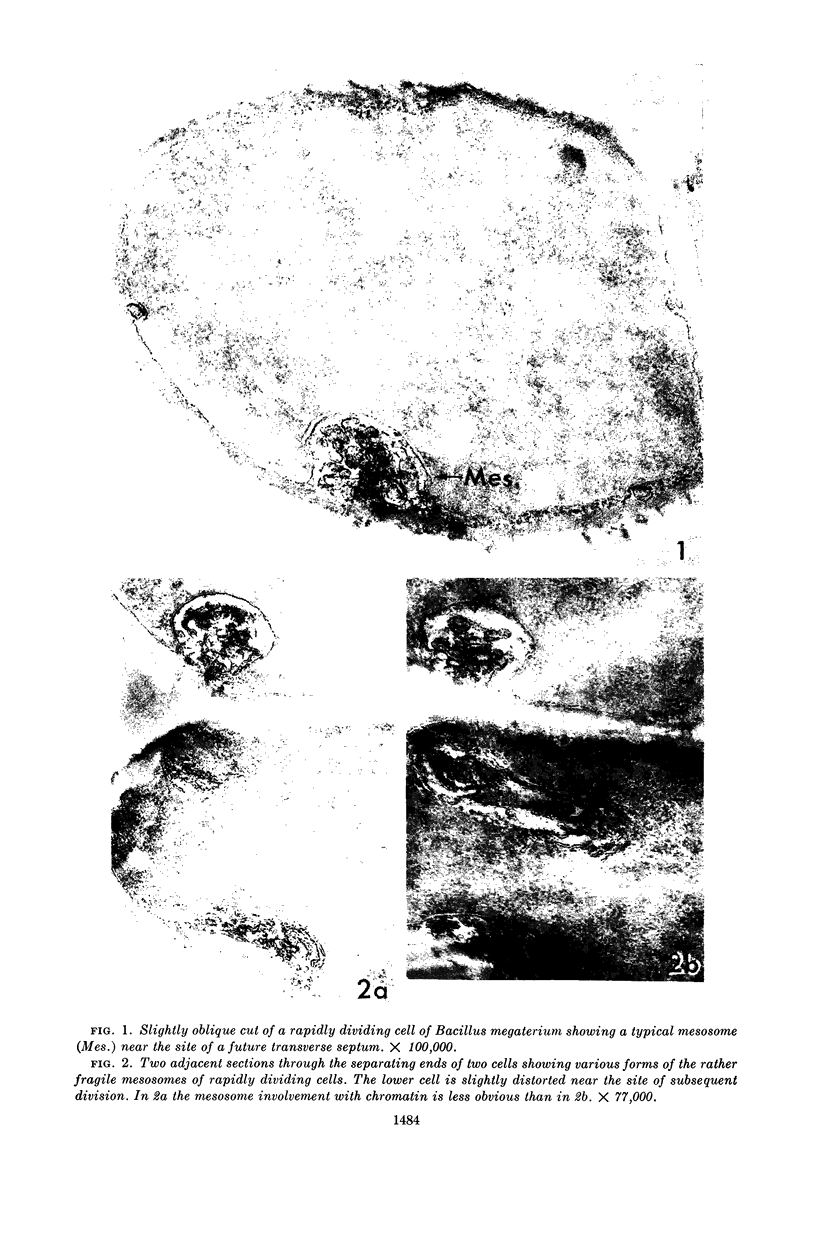
Fitz-James, Philip (University of Western Ontario, London, Canada). Fate of the mesosomes of Bacillus megaterium during protoplasting. J. Bacteriol. 87:1483–1491. 1964.—When cells of Bacillus megaterium growing rapidly in peptone medium are treated with sucrose buffer prior to protoplasting, their membranous organelles or mesosomes are extruded as a collection of vesicles into the wall-membrane interspace. On subsequent protoplast formation with lysozyme digestion, the mesosome remnants become dispersed. If glucose is added to the peptone, a slightly more persistent mesosome can be seen and photographed by phase microscopy during lysozyme digestion, either as a wall-attached granule or as a protoplast tag. In thin section, mesosomes are found as small, variably dispersed aggregates of vesicles and wall-like material. Complete protoplasts are usually free from internal mesosomes but may contain twists or everted pockets of membrane on their periphery. By lysozyme digestion of partially fixed cells, the empty mesosome pockets in the membrane can be demonstrated in rod-shaped protoplasts.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FITZ-JAMES P. C. POLYRIBOSOMES IN PROTOPLASTS OF BACILLUS MEGATERIUM. Can J Microbiol. 1964 Feb;10:92–94. doi: 10.1139/m64-014. [DOI] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Studies on the morphology and nucleic acid content of protoplasts of Bacillus megaterium. J Bacteriol. 1958 Apr;75(4):369–382. doi: 10.1128/jb.75.4.369-382.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P. Electron microscopy of Bacillus megaterium undergoing isolation of its nuclear bodies. J Bacteriol. 1964 May;87(5):1202–1210. doi: 10.1128/jb.87.5.1202-1210.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTON M. R., CHAPMAN J. A. Isolation of the membranemesosome structures from Micrococcus lysodeikticus. J Ultrastruct Res. 1962 Jun;6:489–498. doi: 10.1016/s0022-5320(62)80004-5. [DOI] [PubMed] [Google Scholar]
- VAN ITERSON W. Some features of a remarkable organelle in Bacillus subtilis. J Biophys Biochem Cytol. 1961 Jan;9:183–192. doi: 10.1083/jcb.9.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]