Abstract
Human immunodeficiency virus type 1 (HIV-1) envelope (Env) binding induces proapoptotic signals in CD4+ T cells without a requirement of infection. Defective virus particles, which represent the majority of HIV-1, usually contain a functional Env and therefore represent a potentially significant cause of such CD4+-T-cell loss. We reasoned that an HIV-1 inhibitor that prohibits Env-host cell interactions could block the destructive effects of defective particles. HIV-1 attachment inhibitors (AIs), which potently inhibit Env-CD4 binding and subsequent downstream effects of Env, display low-nanomolar antiapoptotic potency and prevent CD4+-T-cell depletion from mixed lymphocyte cultures, also with low-nanomolar potency. Specific Env amino acid changes that confer resistance to AI antientry activity eliminate AI antiapoptotic effects. We observed that CD4+-T-cell destruction is specific for CXCR4-utilizing HIV-1 strains and that the fusion blocker enfuvirtide inhibits Env-mediated CD4+-T-cell killing but is substantially less potent than AIs. These observations, in conjunction with observed antiapoptotic activities of soluble CD4 and the CXCR4 blocker AMD3100, suggest that this AI activity functions through a mechanism common to AI antientry activity, e.g., prevention of Env conformation changes necessary for specific interactions with cellular factors that facilitate viral entry. Our study suggests that AIs, in addition to having potent antientry activity, could contribute to immune system homeostasis in individuals infected with HIV-1 that can engage CXCR4, thereby mitigating the increased risk of adverse clinical events observed in such individuals on current antiretroviral regimens.
CD4+-T-cell levels decrease in patients throughout the course of human immunodeficiency virus type 1 (HIV-1) infection (12, 13, 16, 31). Eventually this decline leads to the disruption of effective immune responses, numerous opportunistic infections, AIDS-defining illnesses, and death (10, 41). Antiretroviral therapy (ART) has been successful in mitigating these effects (15). However, even among ART responders, those who harbor HIV-1 that engages CXCR4 have an increased risk of adverse clinical outcome, including an increased incidence of both AIDS-related and non-AIDS-related diseases (1, 25, 33). This pattern suggests a need for an antiretroviral strategy that blocks the harmful effects of viruses that can signal through CXCR4. A source of these effects is CXCR4-mediated signal transduction induced by Env interactions, which could provide an explanation for the observed correlation between the presence of CXCR4-utilizing HIV-1 and accelerated disease progression (35, 38). Recent studies indicate that up to 18% of asymptomatic ART-naive and 47% of asymptomatic ART-experienced individuals harbor HIV-1 that exploits CXCR4 for cell entry (6, 22, 34, 54), suggesting that this interaction is pertinent throughout the course of infection in many individuals.
Clearly, the high level of ongoing HIV-1 production is a primary contributor to the loss of CD4+ T cells, and it has been estimated that the rate of production of HIV-1 RNA approximates the rate of CD4+-T-cell destruction (17, 52). Replication-competent HIV-1 is cytotoxic to these cells, and it is thought that the viral proteins Vpr, Tat, and Nef (in addition to Env) contribute to this effect (11, 26, 45, 53). However, the infectious titer of HIV-1 is low in comparison to viral genome levels (7, 36), suggesting that the effects of replication-competent HIV-1 alone cannot account for the rate of CD4+-T-cell loss. Consequently, replication-defective virus likely contributes substantially to this effect. Such particles usually contain a functional Env, which can act as a signaling partner for a variety of lymphocyte surface factors, thereby influencing the physiology of HIV-uninfected (bystander) cells (4, 24, 28, 38, 47). The relevance of bystander killing to primate lentivirus-mediated disease progression is supported by observations of simian immunodeficiency virus-infected sooty mangabeys. These monkeys display substantially lower levels of bystander killing than are observed in HIV-infected humans (39, 40). This characteristic is thought to contribute to their capacity to carry high viral loads (comparable to HIV-1 loads found in humans with progressive AIDS) while sustaining an asymptomatic state of infection. Moreover, by maintaining consistently high CD4+-T-cell counts sooty mangabeys seem to avoid the potential consequences of immune dysfunction encountered by HIV-infected humans.
Based on this information, it would seem that an HIV-1 inhibitor that prohibits Env-host cell interactions and Env-mediated CD4+-T-cell destruction would increase the likelihood of a favorable clinical outcome. Fuzion (enfuvirtide) and maraviroc are FDA-approved drugs that inhibit the HIV-cell fusion process or block the HIV coreceptor CCR5, respectively (2, 20, 23, 29, 43). In addition, attachment inhibitors (AIs) are antiretrovirals currently in development that target HIV-1 Env-mediated cell entry. AIs operate by potently inhibiting Env interactions with CD4, meaning that they operate upstream of CCR5 engagement or the virus-cell fusion process (14, 18, 27, 49, 50). In this study we tested whether these different types of HIV-1 entry inhibitors could prevent Env-induced cytopathicity and reveal that only AIs provide low-nanomolar protection against this effect, which was not unexpected since it seems to be driven through CXCR4 engagement.
MATERIALS AND METHODS
Construction of pVLPNL4-3 and pVLP/Env.
To create pVLPNL4-3, pNL4-3 DNA obtained from the NIH AIDS Research and Reference Reagent Program (Rockville, MD) was subjected to a series of molecular biology-based manipulations following the indicated manufacturers' protocols. Initially, the vector was digested with the restriction enzymes AgeI and EcoRI (New England Biolabs, Beverly, MA). A fragment of the expected size was isolated and purified using Qiaquick gel extraction and PCR purification kits (Qiagen, Valencia, CA), respectively. The purified fragment was then treated with T4 DNA polymerase and T4 DNA ligase (New England Biolabs) and used to transform XL-10 Gold Ultracompetent Escherichia coli cells (Stratagene, La Jolla, CA). Plasmid DNA from individual colonies was isolated using a Qiaprep Spin miniprep kit (Qiagen) and screened with the restriction enzyme StuI (New England Biolabs). Clones displaying the expected digestion pattern were subjected to fluorescent sequencing (Applied Biosystems, Foster City, CA) using HIV-1-specific primers. A clone that contained the desired sequence that included a 2,256-nucleotide deletion that removed the 3′-half of the reverse transcriptase gene, the entirety of the integrase gene and vif, and the 5′ of vpr without disrupting gag, the protease gene, or env was utilized for subsequent experiments (pVLPNL4-3). To our knowledge there is no reported evidence that any of the sequences deleted from the virus-like particles (VLPs) play a role in viral entry. To engineer pVLP/Env-, pVLPNL4-3 DNA was subjected to site-directed mutagenesis using the QuikChange site-directed mutagenesis kit (Stratagene) and the primers 5′-GTACCTGTGTGGAAGTAAGCAACCACCACTC-3′ and 5′-GAGTGGTGGTTGCTTACTTCCACACAGGTAC-3′, which converted Glu43 of env to a stop codon (TAA). Individual clones were screened and sequenced as described above to identify a plasmid with the desired sequence to be utilized in subsequent experiments.
Purified VLP production.
To produce HIV-1 particles, pVLPNL4-3 DNA was transfected into 293T cells (ATCC, Manassas, VA) using Lipofectamine Plus reagents (Invitrogen, Carlsbad, CA) diluted in OptiMEM medium (Gibco BRL, Carlsbad, CA) and following the manufacturer's recommendations. Alternatively, these reagents were used to cotransfect pVLP/Env- DNA with pcDNA vectors expressing distinct Env sequences. In either case culture supernatants were harvested 48 h posttransfection and passed through a 0.45-μm filter (Corning, Corning, NY) to remove cellular debris. For its use in subsequent experiments, VLPs in filtered culture supernatants were purified by ultracentrifugation through a 20% sucrose cushion.
Characterization of the VLP.
The quantity of Env in purified VLP preparations was determined using Western blotting in which denatured particles were electrophoresed (NuPAGE; 4 to 12% bis-Tris; Invitrogen) under reducing conditions. For these experiments purified monomeric gp120 (JRFL) served as the standard. Env protein was probed with 2G12 (Polymun Scientific, Vienna, Austria), a human primary antibody directed against a carbohydrate moiety on the gp120 surface. A peroxidase labeled F(ab′)2 anti-human secondary antibody (Jackson Immunodiagnostics, Avondale, PA) was used for detection with the ECL Plus kit (GE Healthcare, Anaheim, CA) following the manufacturer's recommendations. Images were captured using a Storm 860 PhosphorImager (GE Healthcare).
To ensure that the HIV-1 particles produced for this study were not replication competent, 1 × 106 copies of the HIV-1-permissive T-cell line MT-2 (NIH AIDS Research and Reference Reagent Program) were treated overnight with 1 × 106 particles of VLPNL4-3. The concentration of p24Gag in purified VLP preparations was used to calculate the concentration of HIV-1 particles, by factoring the established copy number of this protein/particle (1,500) and its molecular weight (5). Based on these parameters a conversion factor of 15,000 VLPs/pg of p24Gag was employed to determine VLP concentration. After VLP treatment, cells were washed extensively to remove residual input particles in the supernatant and were then incubated in RPMI 1640 (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT). On a weekly basis (for 5 weeks) a sample of the culture supernatant was harvested and tested for the presence of HIV-1 protein (p24Gag enzyme-linked immunosorbent assay; Perkin-Elmer Life Sciences, Waltham, MA). To ensure that purified particles were coated with functional Env, 1 × 106 MOLT4 cells (NIH AIDS Research and Reference Reagent Program) were treated with 1 × 106 counts of VLP and assayed microscopically for syncitium formation 48 h posttreatment.
CD4+-T-cell apoptosis.
Peripheral blood mononuclear cells (PBMCs) were purified from blood obtained from HIV-uninfected individuals by centrifugation through a Ficoll gradient (Histopaque 1077; Sigma-Aldrich) and were treated with 4 μg/ml phytohemagglutinin P (Sigma-Aldrich) and 10 U/ml interleukin-2 (Roche, Basel, Switzerland) for 24 h. The CD4+ T cells were then isolated using a CD4+-T-cell positive isolation kit (Dynal, Carlsbad, CA). A total of 20,000 of these cells were aliquoted into wells of 96-well collagen-coated tissue culture plates (Biocoat, Bedford MA) and exposed overnight to 2 × 106 VLPs (or a series of twofold dilutions). At 48 h poststimulation the cells were then washed and their nuclei were stained with Hoechst (Molecular Probes, Carlsbad, CA) and fixed with paraformaldehyde (Alfa Aesar, Ward Hill, MA). The sizes of the nuclei of individual cells was determined using an AssayScan (Cellomics, Pittsburgh, PA); the data were analyzed using Bioapplication software (Cellomics). For these and subsequent experiments a threshold nuclear size of 5.5 pixels was used to designate shrunken nuclei. This standard was established by determining the effects of a 10 nM concentration of the apoptosis inducer staurosporine Streptomyces (CalBiochem, San Diego, CA). The 48-h stimulation/VLP treatment time frame provided an optimal window of shrunken nuclei over background and therefore was employed in these studies. For experiments designed to determine the potency of compound antiapoptotic activity, a series of threefold dilutions of specific HIV-1 entry inhibitors (50) (synthesized at Bristol Myers Squibb) or sCD4 (46) (Jackson Immunodiagnostics), diluted in a final concentration of 0.4% dimethyl sulfoxide (DMSO), were added at the time of VLP treatment to assess their effects on nuclear shrinkage. Fifty percent effective concentrations (EC50s) were determined using XL-Fit software (Microsoft, Redmond, WA). For these calculations 100% AI protection was based on the prevalence of apoptotic CD4+ T cells in untreated cultures (with 0.4% DMSO), and 0% AI protection was based on the prevalence of apoptotic CD4+ T cells in cultures exposed to VLPNL4-3 alone.
VLP-induced CD4+-T-cell depletion and the effect of BMS-378806.
PBMCs obtained as described above were treated with 4 μg/ml PHA-P and 10 U/ml interluekin-2 for 48 h, and nonadherent cells were isolated from the cultures for further experiments. A total of 50,000 of these cells were treated with 5 × 106 copies of VLPNL4-3 or VLPJRFL. The mixture was cultured for 10 days in RPMI plus 10% FBS. To test the effect of BMS-378806 on CD4+-T-cell depletion, a series of threefold dilutions of this compound (dissolved in DMSO) were added at the time of VLPNL4-3 treatment. After incubation, the cultures were washed and stained with a fluorescein isothiocyanate (FITC)-conjugated mouse anti-CD4 antibody (clone S3.5; Molecular Probes, Carlsbad, CA); staining with a FITC-conjugated mouse immunoglobulin G2a (IgG2a) antibody (Molecular Probes) served as a control. Additional controls included untreated cells and cells treated with VLPNL4-3 (in the presence of 0.4% Gag) but no BMS-378806. The cells that received various treatments were subjected to fluorescence-activated cell sorter (FACS) analyses on an EasyCyte apparatus (Guava Technologies, Hayward, CA) to assess the prevalence of CD4+ T cells in the cultures (gated on live lymphocytes based on forward and side scatter patterns). The number of events for each histogram was derived from 3,000 cells surveyed in this manner. EC50s of BMS-378806 inhibition were determined using XL-Fit software. For these calculations 100% AI protection was based on the prevalence of CD4+ T cells in untreated cultures, and 0% AI protection was based on the prevalence of CD4+ T cells in cultures exposed to VLPNL4-3 alone.
Antientry assay.
MT-2 cells were centrifuged at 1,200 rpm in a Beckman Allegra centrifuge for 10 min. HIV-1 was added to the pellet in a volume of 0.5 ml at a multiplicity of infection of 0.01. The cell/virus mixture was resuspended in RPMI 1640 plus 10% FBS to a final density of 1.0 × 105 cells/ml and containing serial dilutions of antientry test compounds. The cultures were incubated for 5 days, and the level of HIV production and inhibition was quantified by p24 enzyme-linked immunosorbent assay (Perkin-Elmer Life Sciences) following the manufacturer's recommendations.
RESULTS
Construction and characterization of an HIV-1 VLP.
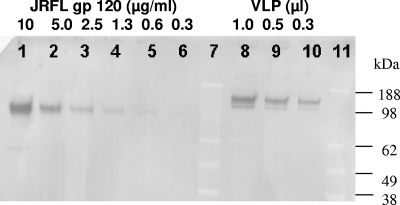
To study the effects of native trimeric HIV-1 Env on CD4+ T cells without a requirement for biosafety level 3 containment, a replication-defective VLP was engineered. To create such a particle, which retains the structure of an infectious HIV-1, a large (2,256-bp) deletion was introduced into a molecular clone containing HIV-1 NL4-3 sequences that disrupted the 3′ half of the reverse transcriptase gene, the entirety of the integrase gene and vif, and the 5′ of vpr without disrupting gag, the protease gene, or env (pVLPNL4-3). Particles produced in tissue culture were purified as described in Materials and Methods. We tested if VLPs were coated with a biologically relevant amount of envelope protein by quantifying the gp120 surface protein in purified VLP preparations by Western blotting (Fig. 1). The calculated level of Env along with p24Gag quantification was used to establish a 1-to-10 ratio of Env to p24Gag associated with purified VLPNL4-3, revealing that VLPNL4-3 preparations contained approximately 30 copies of gp120/particle, a density that is consistent with that of infectious particles (48, 58). Moreover, VLPNL4-3 induces syncitium formation in MOLT4 cell cultures (data not shown), suggesting HIV-1 Env functionality.
FIG. 1.
gp120 quantitative Western blot results. Lanes 7 and 11 are molecular mass markers (sizes are indicated at the right). Lanes 1 to 6 are serial twofold dilutions (10 to 0.3 μg/ml) of purified JRFL gp120, used to establish a standard curve. Lanes 8 to 11 are twofold dilutions (lane 8, 1 μl) of gp120 obtained from a preparation of purified VLPNL4-3.
Attachment inhibitors block VLP-induced CD4+-T-cell loss.
To expand our studies to include distinct Env sequences, a derivative of VLPNL4-3 was engineered in which Glu43 of env was mutated to a stop codon to create VLP/Env-, as described in Materials and Methods. This backbone DNA was cotransfected with the desired gp160-encoding DNAs to create HIV-1 particles with different Envs. This strategy was employed to assess whether HIV-1 particles containing Envs that utilize different coreceptors for cell entry induce CD4+-T-cell depletion, thereby allowing for the contributions of these factors to this effect to be determined. Moreover, by including strains that engage the major HIV-1 coreceptors CXCR4 and CCR5, we would also be providing information on viruses (dual tropic) that can utilize either protein for entry (55, 56). For these studies PBMCs were treated with VLP/Env-, VLPNL4-3 (CXCR4 utilizing), VLP/Env- pseudotyped with JRFL Env (VLPJRFL; CCR5 utilizing), or LAI Env (VLP/LAI, CXCR4 utilizing) (3, 32, 42). After 10 days the cultures treated with VLP/NL4-3 or VLP/LAI displayed a substantial decrease in the prevalence of CD4+ T cells in comparison to the cultures treated with VLP/Env- (data not shown). In contrast, cultures treated with VLP/JRFL maintained a CD4+-T-cell frequency that was similar to that of cultures treated with VLP/Env- (data not shown). These data suggest a requirement for CXCR4-utilizing Env for CD4+-T-cell loss, although cells not expressing this factor could also be affected through indirect mechanisms (19), which could contribute to the overall level of cell loss.
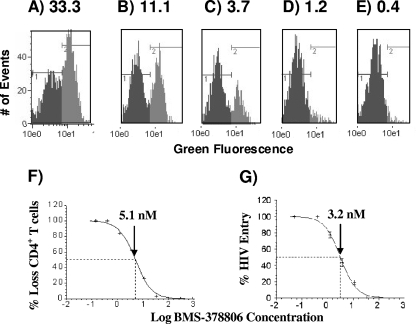
To assess the capacity of HIV-1 attachment inhibitors to prevent VLP-induced CD4+-T-cell depletion, PBMCs were isolated and treated with VLPNL4-3 and the previously described AI BMS-378806 (27). FACS analyses revealed that this compound prevented CD4+-T-cell depletion (Fig. 2A to E) with an estimated EC50 in the low-nanomolar range (Fig. 2F), meaning that an AI can potently inhibit Env-mediated CD4+-T-cell depletion induced by replication-defective HIV-1 particles. A similar antientry potency was observed for this compound (Fig. 2G), which is consistent with previously reported antientry potency observed using infectious whole HIV-1 coated with a variety of Envs that utilize CCR5 and/or CXCR4 for cell entry (27).
FIG. 2.
Potency of BMS-378806 against CD4+-T-cell loss. (A to E) FACS histograms of PBMCs treated with VLPNL4-3 and a series of threefold dilutions of BMS-378806: 33.3 nM (A), 11.1 nM (B), 3.7 nM (C), 1.2 nM (D), or 0.4 nM (E). The area in black represents the CD4-negative population, and the area in gray indicates the CD4-positive population. (F) EC50 curve based on the data from panels A to E. The arrow indicates the estimated concentration of compound (5.1 nM) required to protect against the loss of 50% of the CD4+ T cells. (G) EC50 curve based on data from the anti-HIV entry assay described in Materials and Methods. The arrow indicates the estimated concentration of compound (3.2 nM) required to inhibit 50% of HIV entry.
Attachment inhibitors block VLP-induced apoptosis of CD4+ T cells.
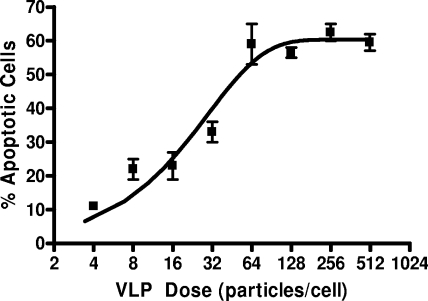
To further investigate Env-mediated CD4+-T-cell loss, a high-throughput assay was developed that employed a marker of apoptosis within VLP-exposed cells. Nuclear shrinkage is a hallmark of cellular apoptosis via both intrinsic and extrinsic mechanisms and therefore was utilized for our studies (30, 37, 44). To test this system, cells were treated with twofold dilutions of VLPNL4-3, and the extent of nuclei shrinkage was determined. This approach established VLPNL4-3 dose dependence for the induction of apoptosis of CD4+-T-cell cultures (Fig. 3) reminiscent of previous observations with chemically inactivated viral particles (8). As we saw for the CD4+-T-cell depletion studies, this effect was specific for VLPs expressing Envs that utilize CXCR4 for cell entry; it was not observed for VLPs expressing CCR5-utilizing Envs, such as JRFL and ADA (data not shown) (21).
FIG. 3.
Dose response for VLPNL4-3-induced CD4+-T-cell apoptosis. The calculation of input VLP dose for this curve was based on 15,000 particles/pg of p24Gag.
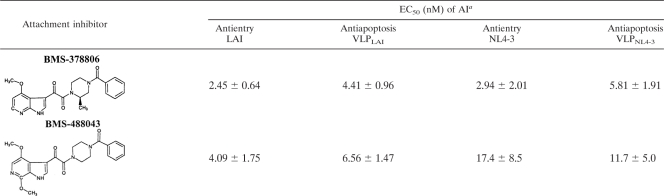
To test the capability of AIs to prevent HIV-1 Env-induced CD4+-T-cell apoptosis, VLP4-3-treated cultures were exposed to a series of threefold dilutions of BMS-378806 or BMS-488043. In these experiments both compounds displayed potent antiapoptosis activities, with EC50s in the low-nanomolar range (Table 1). For both, EC90 values were observed (data not shown) that were three- to fivefold higher than their EC50s for both antiapoptosis and antientry activities (Table 1). Moreover, for both compounds VLPLAI was more sensitive to AI antiapoptosis activity than VLPNL4-3, which parallels their AI sensitivities in antientry assays (Table 1). From these data we surmise that the antientry and antiapoptotic activities of AIs likely operate through a common mechanism.
TABLE 1.
Antientry and antiapoptotic potencies of HIV-1 attachment inhibitors against distinct envelope sequences
Values represent the means and standard deviations from three independent experiments.
Previous accumulated evidence supports a model in which AI binding to a cavity at the bottom of the gp120 CD4 binding pocket underlies its antientry activity; this includes the observation that mutations in this pocket (S375I and M426L) confer high-level resistance to BMS-488043 antientry activity (18). To further substantiate a common mechanism theory for the two AI activities in question, these mutations were engineered into the LAI Env; the recombinant Envs were pseudotyped onto an HIV-1 core particle and tested for their sensitivities to BMS-488043 antientry and antiapoptosis activities. These experiments revealed that like the parental LAI Env, the mutant Envs retained their capacity to induce apoptosis (data not shown). However, they were insensitive to BMS-488043 antientry and antiapoptotic activities (Table 2), consistent with a common AI antientry and antiapoptotic activity mechanism model.
TABLE 2.
Antientry and antiapoptotic potencies of BMS-488043 against wild-type LAI or the indicated derivative mutations
| AI activity | EC50 (nM)a potency for: |
||
|---|---|---|---|
| WTb | S375I | M426L | |
| Antientry | 4.1 ± 1.2 | >5,000 | >5,000 |
| Antiapoptosis | 5.2 ± 0.7 | >5,000 | >5,000 |
Values represent the means and standard deviations from three independent experiments.
WT, wild type.
Evidence for the importance of AI interactions with the gp120/CD4 binding pocket for its antientry behavior also stems from the observation that sCD4 acts as an AI competitor (27). Therefore, to further investigate the role of the CD4 binding pocket in AI antiapoptosis properties, the capacity of sCD4 to prevent VLP-mediated CD4+-T-cell apoptosis was tested. We observed that when added simultaneously with VLPNL4-3 sCD4 is a potent inhibitor of Env-mediated cellular apoptosis (Table 3). However, when added 4 hours after VLPNL4-3, sCD4 did not provide such protection (data not shown), indicating that interactions with the CD4 binding pocket are essential for both AI antientry and antiapoptotic properties, again supporting a common mechanism model for the two activities.
TABLE 3.
Antientry and antiapoptotic potencies of HIV-1 entry inhibitors
| Inhibitor | EC50 (nM)a for: |
|
|---|---|---|
| Antientry (NL4-3) | Antiapoptosis (VLPNL4-3) | |
| sCD4 | 12.4 ± 6.3 | 98.1 ± 10.2 |
| AMD3100 | 10.8 ± 2.2 | 8.8 ± 1.4 |
| Enfuvirtide | 22.4 ± 4.5 | 548 ± 32.6 |
Values represent the means and standard deviations from three independent experiments.
To further characterize Env-mediated apoptosis, the ability of distinct HIV-1 entry inhibitors to block this effect was assessed. These experiments showed that the CXCR4 inhibitor AMD-3100 (4) acted as a low-nanomolar inhibitor of this activity, a potency similar to its antientry activity (Table 3), further supporting a role for CXCR4 in CD4+-T-cell killing. We also observed that the fusion inhibitor enfuvirtide (29) displayed mid-nanomolar antiapoptosis activity, which was substantially higher than its antientry activity in our systems (Table 3) (9).
DISCUSSION
In this report we demonstrate through both CD4+-T-cell depletion and apoptosis assays that HIV-1 AIs are highly efficient in prohibiting HIV-1 Env apoptotic effects associated with bystander killing of CD4+ T cells. In our studies the destructive effects of Env were confined to sequences that can interact with CXCR4, which is consistent with previous observations (19). Moreover, our data lead us to the conclusion that AI antiapoptotic activity operates by blocking mechanisms required for viral entry of CXCR4-utilizing strains. The relative antiapoptotic potencies of BMS-378806 and BMS-488043 parallel their antientry potencies, and both display greater antiapoptosis potencies against the LAI compared to the NL-4-3 Env, as is the case for their antientry effects (Table 1). Furthermore, mutations (S375I and M426L) that confer resistance to the antientry activities of BMS-488043 also confer resistance to its antiapoptotic activities (Table 3), and time of addition experiments with sCD4 demonstrate that blocking CD4-Env interactions inhibits VLP-mediated apoptosis (Table 3). Taken together these observations are consistent with a mechanism (underlying both antientry and antiapoptotic activities) in which AI binding to the gp120/CD4 binding site prohibits conformational changes required for interactions between the viral Env and CXCR4 and the resultant downstream effects.
Interestingly, in contrast to AIs, the FDA-approved fusion inhibitor enfuvirtide displayed low but detectable antiapoptotic activity (EC50, 548 nM ∼ 24-fold lower than its antientry activity) (Table 3). It has been shown that sensitivity to enfuvirtide can be influenced by V3 loop sequences in gp120 that dictate coreceptor usage (57). Consequently, enfuvirtide binding may produce allosteric effects on gp120, thereby inhibiting CXCR4 binding, which could explain the observed weak inhibitory property of this drug for CXCR4-mediated CD4+-T-cell apoptosis. However, additional studies have described varied levels of bystander killing protection provided by enfuvirtide (2, 20), suggesting that its effect could be dictated by experimental conditions and might therefore require further examination. As expected, the CXCR4 blocker AMD3100 did provide potent protection against this effect. Due to toxicity issues, this compound is no longer in development, although CXCR4 is being pursued as a potential antiretroviral target (23). Since maraviroc has no effect on HIV-CXCR4 interactions, we would not expect this FDA-approved inhibitor to affect bystander killing through the mechanisms described here.
In summary, we conclude that AI antiapoptotic activity against Env-mediated CD4+-T-cell loss operates through a mechanism common with its antientry activity, i.e., preventing CD4-induced conformational changes that facilitate gp120-CXCR4 interactions. Since HIV-1 virions that interact with CXCR4 can be found in a substantial portion of the asymptomatic population and are associated with an increased risk of adverse clinical events (51), this class of inhibitor is particularly well-suited to limit the incidence of AIDS, particularly in resource-limited settings in which treatment is likely to be initiated at a low CD4+-T-cell count. Thus, our studies suggest that blocking HIV-1 attachment could provide infected patients with a secondary benefit beyond direct antientry effects (1, 25, 33).
Footnotes
Published ahead of print on 31 August 2009.
REFERENCES
- 1.Baker, J. V., G. Peng, J. Rapkin, D. I. Abrams, M. J. Silverberg, R. D. MacArthur, W. P. Cavert, W. K. Henry, and J. D. Neaton. 2008. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS 22:841-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barretina, J., J. Blanco, M. Armand-Ugon, A. Gutierrez, B. Clotet, and J. A. Este. 2003. Anti-HIV-1 activity of enfuvirtide (T-20) by inhibition of bystander cell death. Antivir. Ther. 8:155-161. [PubMed] [Google Scholar]
- 3.Berkowitz, R. D., S. Alexander, C. Bare, V. Linquist-Stepps, M. Bogan, M. E. Moreno, L. Gibson, E. D. Wieder, J. Kosek, C. A. Stoddart, and J. M. McCune. 1998. CCR5- and CXCR4-utilizing strains of human immunodeficiency virus type 1 exhibit differential tropism and pathogenesis in vivo. J. Virol. 72:10108-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blanco, J., J. Barretina, G. Henson, G. Bridger, E. De Clercq, B. Clotet, and J. A. Este. 2000. The CXCR4 antagonist AMD3100 efficiently inhibits cell-surface-expressed human immunodeficiency virus type 1 envelope-induced apoptosis. Antimicrob. Agents Chemother. 44:51-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briggs, J. A., M. C. Johnson, M. N. Simon, S. D. Fuller, and V. M. Vogt. 2006. Cryo-electron microscopy reveals conserved and divergent features of gag packing in immature particles of Rous sarcoma virus and human immunodeficiency virus. J. Mol. Biol. 355:157-168. [DOI] [PubMed] [Google Scholar]
- 6.Brumme, Z. L., J. Goodrich, H. B. Mayer, C. J. Brumme, B. M. Henrick, B. Wynhoven, J. J. Asselin, P. K. Cheung, R. S. Hogg, J. S. Montaner, and P. R. Harrigan. 2005. Molecular and clinical epidemiology of CXCR4-using HIV-1 in a large population of antiretroviral-naive individuals. J. Infect. Dis. 192:466-474. [DOI] [PubMed] [Google Scholar]
- 7.Dimitrov, D. S., R. L. Willey, H. Sato, L. J. Chang, R. Blumenthal, and M. A. Martin. 1993. Quantitation of human immunodeficiency virus type 1 infection kinetics. J. Virol. 67:2182-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esser, M. T., J. W. Bess, Jr., K. Suryanarayana, E. Chertova, D. Marti, M. Carrington, L. O. Arthur, and J. D. Lifson. 2001. Partial activation and induction of apoptosis in CD4+ and CD8+ T lymphocytes by conformationally authentic noninfectious human immunodeficiency virus type 1. J. Virol. 75:1152-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Este, J. A., and A. Telenti. 2007. HIV entry inhibitors. Lancet 370:81-88. [DOI] [PubMed] [Google Scholar]
- 10.Fauci, A. S., S. M. Schnittman, G. Poli, S. Koenig, and G. Pantaleo. 1991. NIH conference. Immunopathogenic mechanisms in human immunodeficiency virus (HIV) infection. Ann. Intern. Med. 114:678-693. [DOI] [PubMed] [Google Scholar]
- 11.Geleziunnas, R., W. Xu, K. Takeda, H. Ichijo, and W. C. Greene. 2001. HIV-1 Nef inhibits ASK-1 dependent death signaling providing a potential mechanism for protecting the infected host cell. Nature 410:834-838. [DOI] [PubMed] [Google Scholar]
- 12.Geng, E. H., and S. G. Deeks. 2009. CD4+ T cell recovery with antiretroviral therapy: more than the sum of the parts. Clin. Infect. Dis. 48:362-364. [DOI] [PubMed] [Google Scholar]
- 13.Grossman, Z., M. Meier-Schellersheim, A. E. Sousa, R. M. Victorino, and W. E. Paul. 2002. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat. Med. 8:319-323. [DOI] [PubMed] [Google Scholar]
- 14.Guo, Q., H. T. Ho, I. Dicker, L. Fan, N. Zhou, J. Friborg, T. Wang, B. V. McAuliffe, H. G. Wang, R. E. Rose, H. Fang, H. T. Scarnati, D. R. Langley, N. A. Meanwell, R. Abraham, R. J. Colonno, and P. F. Lin. 2003. Biochemical and genetic characterizations of a novel human immunodeficiency virus type 1 inhibitor that blocks gp120-CD4 interactions. J. Virol. 77:10528-10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammer, S. M., J. J. Eron, Jr., P. Reiss, R. T. Schooley, M. A. Thompson, S. Walmsley, P. Cahn, M. A. Fischl, J. M. Gatell, M. S. Hirsch, D. M. Jacobsen, J. S. Montaner, D. D. Richman, P. G. Yeni, and P. A. Volberding. 2008. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society—USA panel. JAMA 300:555-570. [DOI] [PubMed] [Google Scholar]
- 16.Hazenberg, M. D., D. Hamann, H. Schuitemaker, and F. Miedema. 2000. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat. Immunol. 1:285-289. [DOI] [PubMed] [Google Scholar]
- 17.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 18.Ho, H. T., L. Fan, B. Nowicka-Sans, B. McAuliffe, C. B. Li, G. Yamanaka, N. Zhou, H. Fang, I. Dicker, R. Dalterio, Y. F. Gong, T. Wang, Z. Yin, Y. Ueda, J. Matiskella, J. Kadow, P. Clapham, J. Robinson, R. Colonno, and P. F. Lin. 2006. Envelope conformational changes induced by human immunodeficiency virus type 1 attachment inhibitors prevent CD4 binding and downstream entry events. J. Virol. 80:4017-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holm, G. H., and D. Gabuzda. 2005. Distinct mechanisms of CD4+ and CD8+ T-cell activation and bystander apoptosis induced by human immunodeficiency virus type 1 virions. J. Virol. 79:6299-6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holm, G. H., C. Zhang, P. R. Gorry, K. Peden, D. Schols, E. De Clercq, and D. Gabuzda. 2004. Apoptosis of bystander T cells induced by human immunodeficiency virus type 1 with increased envelope/receptor affinity and coreceptor binding site exposure. J. Virol. 78:4541-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hung, C. S., S. Pontow, and L. Ratner. 1999. Relationship between productive HIV-1 infection of macrophages and CCR5 utilization. Virology 264:278-288. [DOI] [PubMed] [Google Scholar]
- 22.Hunt, P. W., P. R. Harrigan, W. Huang, M. Bates, D. W. Williamson, J. M. McCune, R. W. Price, S. S. Spudich, H. Lampiris, R. Hoh, T. Leigler, J. N. Martin, and S. G. Deeks. 2006. Prevalence of CXCR4 tropism among antiretroviral-treated HIV-1-infected patients with detectable viremia. J. Infect. Dis. 194:926-930. [DOI] [PubMed] [Google Scholar]
- 23.Kuritzkes, D. R. 2009. HIV-1 entry inhibitors: an overview. Curr. Opin. HIV AIDS 4:82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawson, V. A., K. A. Silburn, P. R. Gorry, G. Paukovic, D. F. Purcell, A. L. Greenway, and D. A. McPhee. 2004. Apoptosis induced in synchronized human immunodeficiency virus type 1-infected primary peripheral blood mononuclear cells is detected after the peak of CD4+ T-lymphocyte loss and is dependent on the tropism of the gp120 envelope glycoprotein. Virology 327:70-82. [DOI] [PubMed] [Google Scholar]
- 25.Lewden, C., G. Chene, P. Morlat, F. Raffi, M. Dupon, P. Dellamonica, J. L. Pellegrin, C. Katlama, F. Dabis, and C. Leport. 2007. HIV-infected adults with a CD4 cell count greater than 500 cells/mm3 on long-term combination antiretroviral therapy reach same mortality rates as the general population. J. Acquir. Immune Defic. Syndr. 46:72-77. [DOI] [PubMed] [Google Scholar]
- 26.Li, C. J., D. J. Freidman, C. Wang, V. Metelev, and A. B. Pardee. 1995. Induction of apoptosis in unifected lymphocytes by HIV-1 Yay protein. Science 268:429-431. [DOI] [PubMed] [Google Scholar]
- 27.Lin, P. F., W. Blair, T. Wang, T. Spicer, Q. Guo, N. Zhou, Y. F. Gong, H. G. Wang, R. Rose, G. Yamanaka, B. Robinson, C. B. Li, R. Fridell, C. Deminie, G. Demers, Z. Yang, L. Zadjura, N. Meanwell, and R. Colonno. 2003. A small molecule HIV-1 inhibitor that targets the HIV-1 envelope and inhibits CD4 receptor binding. Proc. Natl. Acad. Sci. USA 100:11013-11018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lum, J. J., D. J. Schnepple, and A. D. Badley. 2005. Acquired T-cell sensitivity to TRAIL mediated killing during HIV infection is regulated by CXCR4-gp120 interactions. AIDS 19:1125-1133. [DOI] [PubMed] [Google Scholar]
- 29.Makinson, A., and J. Reynes. 2009. The fusion inhibitor enfuvirtide in recent antiretroviral strategies. Curr. Opin. HIV AIDS 4:150-158. [DOI] [PubMed] [Google Scholar]
- 30.Martelli, A. M., M. Zweyer, R. L. Ochs, P. L. Tazzari, G. Tabellini, P. Narducci, and R. Bortul. 2001. Nuclear apoptotic changes: an overview. J. Cell. Biochem. 82:634-646. [DOI] [PubMed] [Google Scholar]
- 31.McCune, J. M. 2001. The dynamics of CD4+ T-cell depletion in HIV disease. Nature 410:974-979. [DOI] [PubMed] [Google Scholar]
- 32.McKnight, A., D. Wilkinson, G. Simmons, S. Talbot, L. Picard, M. Ahuja, M. Marsh, J. A. Hoxie, and P. R. Clapham. 1997. Inhibition of human immunodeficiency virus fusion by a monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent. J. Virol. 71:1692-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monforte, A., D. Abrams, C. Pradier, R. Weber, P. Reiss, F. Bonnet, O. Kirk, M. Law, S. De Wit, N. Friis-Moller, A. N. Phillips, C. A. Sabin, and J. D. Lundgren. 2008. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS 22:2143-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moyle, G. J., A. Wildfire, S. Mandalia, H. Mayer, J. Goodrich, J. Whitcomb, and B. G. Gazzard. 2005. Epidemiology and predictive factors for chemokine receptor use in HIV-1 infection. J. Infect. Dis. 191:866-872. [DOI] [PubMed] [Google Scholar]
- 35.Penn, M. L., J. C. Grivel, B. Schramm, M. A. Goldsmith, and L. Margolis. 1999. CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue. Proc. Natl. Acad. Sci. USA 96:663-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piatak, M., Jr., M. S. Saag, L. C. Yang, S. J. Clark, J. C. Kappes, K. C. Luk, B. H. Hahn, G. M. Shaw, and J. D. Lifson. 1993. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science 259:1749-1754. [DOI] [PubMed] [Google Scholar]
- 37.Rogalinska, M. 2002. Alterations in cell nuclei during apoptosis. Cell. Mol. Biol. Lett. 7:995-1018. [PubMed] [Google Scholar]
- 38.Roggero, R., V. Robert-Hebmann, S. Harrington, J. Roland, L. Vergne, S. Jaleco, C. Devaux, and M. Biard-Piechaczyk. 2001. Binding of human immunodeficiency virus type 1 gp120 to CXCR4 induces mitochondrial transmembrane depolarization and cytochrome c-mediated apoptosis independently of Fas signaling. J. Virol. 75:7637-7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silvestri, G. 2008. AIDS pathogenesis: a tale of two monkeys. J. Med. Primatol. 37(Suppl. 2):6-12. [DOI] [PubMed] [Google Scholar]
- 40.Silvestri, G., D. L. Sodora, R. A. Koup, M. Paiardini, S. P. O'Neil, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18:441-452. [DOI] [PubMed] [Google Scholar]
- 41.Smith, C. L., and G. E. Stein. 2002. Viral load as a surrogate end point in HIV disease. Ann. Pharmacother. 36:280-287. [DOI] [PubMed] [Google Scholar]
- 42.Smyth, R. J., Y. Yi, A. Singh, and R. G. Collman. 1998. Determinants of entry cofactor utilization and tropism in a dual tropic human immunodeficiency virus type 1 primary isolate. J. Virol. 72:4478-4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soriano, V., A. M. Geretti, C. F. Perno, G. Fatkenheuer, D. Pillay, J. Reynes, G. Tambussi, V. Calvez, J. Alcami, and J. Rockstroh. 2008. Optimal use of maraviroc in clinical practice. AIDS 22:2231-2240. [DOI] [PubMed] [Google Scholar]
- 44.Squier, M. K., A. J. Sehnert, and J. J. Cohen. 1995. Apoptosis in leukocytes. J. Leukoc. Biol. 57:2-10. [DOI] [PubMed] [Google Scholar]
- 45.Stewart, S. A., B. Poon, J. Y. Song, and I. S. Y. Chen. 2000. Human immunodeficiency virus type 1 Vpr induces apoptosis through caspase activation. J. Virol. 74:3105-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan, N., Y. Sun, Q. Sattentau, M. Thali, D. Wu, G. Densilova, J. Gershoni, J. Robinson, J. Moore, and J. Sodroski. 1998. CD-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J. Virol. 72:4694-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vlahakis, S. R., A. Algeciras-Schimnich, G. Bou, C. J. Heppelmann, A. Villasis-Keever, R. C. Collman, and C. V. Paya. 2001. Chemokine-receptor activation by Env determines the mechanism of death in HIV-infected and uninfected T lymphocytes. J. Clin. Investig. 107:207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang, B. Z., W. Liu, S. M. Kang, M. Alam, C. Huang, L. Ye, Y. Sun, Y. Li, D. L. Kothe, P. Pushko, T. Dokland, B. F. Haynes, G. Smith, B. H. Hahn, and R. W. Compans. 2007. Incorporation of high levels of chimeric human immunodeficiency virus envelope glycoproteins into virus-like particles. J. Virol. 81:10869-10878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, H. G., R. E. Williams, and P. F. Lin. 2004. A novel class of HIV-1 inhibitors that targets the viral envelope and inhibits CD4 receptor binding. Curr. Pharm. Des. 10:1785-1793. [DOI] [PubMed] [Google Scholar]
- 50.Wang, T., Z. Zhang, O. B. Wallace, M. Deshpande, H. Fang, Z. Yang, L. M. Zadjura, D. L. Tweedie, S. Huang, F. Zhao, S. Ranadive, B. S. Robinson, Y. F. Gong, K. Ricarrdi, T. P. Spicer, C. Deminie, R. Rose, H. G. Wang, W. S. Blair, P. Y. Shi, P. F. Lin, R. J. Colonno, and N. A. Meanwell. 2003. Discovery of 4-benzoyl-1-[(4-methoxy-1H- pyrrolo[2,3-b]pyridin-3-yl)oxoacetyl]-2- (R)-methylpiperazine (BMS-378806): a novel HIV-1 attachment inhibitor that interferes with CD4-gp120 interactions. J. Med. Chem. 46:4236-4239. [DOI] [PubMed] [Google Scholar]
- 51.Waters, L., S. Mandalia, P. Randell, A. Wildfire, B. Gazzard, and G. Moyle. 2008. The impact of HIV tropism on decreases in CD4 cell count, clinical progression, and subsequent response to a first antiretroviral therapy regimen. Clin. Infect. Dis. 46:1617-1623. [DOI] [PubMed] [Google Scholar]
- 52.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, et al. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 53.Westendorp, M. O., R. Frank, C. Ochsenbauer, K. Stricker, J. Dhein, H. Walczak, K. M. Debatin, and P. H. Krammer. 1995. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497-500. [DOI] [PubMed] [Google Scholar]
- 54.Wilkin, T. J., Z. Su, D. R. Kuritzkes, M. Hughes, C. Flexner, R. Gross, E. Coakley, W. Greaves, C. Godfrey, P. R. Skolnik, J. Timpone, B. Rodriguez, and R. M. Gulick. 2007. HIV type 1 chemokine coreceptor use among antiretroviral-experienced patients screened for a clinical trial of a CCR5 inhibitor: AIDS Clinical Trial Group A5211. Clin. Infect. Dis. 44:591-595. [DOI] [PubMed] [Google Scholar]
- 55.Yao, Q., R. W. Compans, and C. Chen. 2001. HIV envelope proteins differentially utilize CXCR4 and CCR5 coreceptors for induction of apoptosis. Virology 285:128-137. [DOI] [PubMed] [Google Scholar]
- 56.Yi, Y., L. Loftin, L. Wang, S. J. Ratcliffe, J. Isaacman-Beck, and R. G. Collman. 2008. Entry coreceptor use and fusion inhibitor T20 sensitivity of dual-tropic R5X4 HIV-1 in primary macrophage infection. J. Acquir. Immune Defic. Syndr. 47:285-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan, W., S. Craig, Z. Si, M. Farzan, and J. Sodroski. 2004. CD4-induced T-20 binding to human immunodeficiency virus type 1 gp120 blocks interaction with the CXCR4 coreceptor. J. Virol. 78:5448-5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu, P., J. Liu, J. Bess, Jr., E. Chertova, J. D. Lifson, H. Grise, G. A. Ofek, K. A. Taylor, and K. H. Roux. 2006. Distribution and three-dimensional structure of AIDS virus envelope spikes. Nature 441:847-852. [DOI] [PubMed] [Google Scholar]