Abstract
We report the in vitro activities of trioxaquines against larval and adult-stage schistosomes at 5 and 50 μg/ml, respectively. Such activities are equivalent to that of praziquantel, the major and rather unique drug currently used for the treatment of schistosomiasis. In this range of concentrations, artemisinin derivatives (artesunate and artemether) have no activity.
Schistosoma mansoni is a flatworm responsible for a chronic human disease called schistosomiasis or bilharziosis. Schistosomiasis ranks just after malaria in terms of parasite-induced human morbidity and mortality, with more than 200 million people infected (4, 7). Because a vaccine is not yet available (10), chemotherapy is the only method of schistosomiasis control. This control is dependent mainly on the use of praziquantel. This drug is active against all human Schistosoma species after a single oral dose without severe side effects. However, the prospect of having a single drug available for a disease affecting 200 million people is quite alarming, especially if both laboratory and field observations leave little room for doubt that resistance to praziquantel has emerged or will soon emerge (1, 6). Consequently, the development of new drugs is ongoing (8, 13, 15).
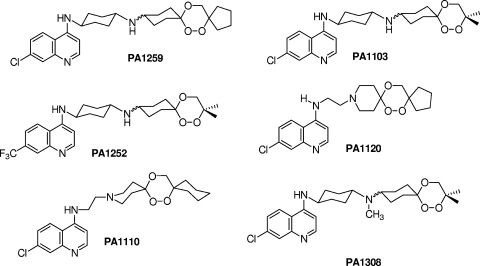
Hemoglobin metabolism is a common feature of schistosomiasis and malaria. Host hemoglobin is ingested by worms and degraded to amino acids in the cecum of parasites, and the generated free heme is eliminated as hemozoin. Hemozoin is a disposal product shared by Plasmodium and Schistosoma (12). Recent studies have shown that antimalarial artemisinin derivatives, which are heme-alkylating agents (14), and synthetic trioxolanes also display significant activity against several Schistosoma species (18, 19). Our first attempt to obtain antischistosomal drugs based on new hybrid compounds, trioxaquantels, which are based on 1,2,4-trioxanes linked to praziquantel, did not provide the expected activity (9). For these reasons, we decided to evaluate the activities of trioxaquines against Schistosoma mansoni (Fig. 1). Trioxaquines are antimalarial hybrid molecules containing two pharmacophores (a 1,2,4-trioxane and a 4-aminoquinoline) with a dual mode of action (heme alkylation with the trioxane entity, heme stacking with the aminoquinoline moiety, and inhibition of hemozoin formation) (2, 5, 11). All different trioxaquines evaluated in this study have shown activities against chloroquine-resistant Plasmodium falciparum strains. For this reason, it was interesting to compare the activities against plasmodia with the antischistosomal activities.
FIG. 1.
Formulas of evaluated trioxaquines.
The host-parasite system used was an albino variety of Biomphalaria glabrata from Brazil and a strain of Schistosoma mansoni, also from Brazil, maintained in Swiss OF1 mice (Charles River, L'Arbresle, France). Methods for mollusk and mouse infections and for parasite recovery were previously described (3). Each mouse was percutaneously infected using either 120 or 400 parasite cercariae. Mice exposed to 400 cercariae were sacrificed at 21 days after infection for larval recovery (i.e., schistosomula), while mice exposed to 120 cercariae were sacrificed at 49 days after infection for adult recovery. Adults or larvae, freshly recovered, were washed and placed in RPMI 1640 medium (supplemented with l-glutamine and HEPES [25 mM]) and stored in an incubator chamber at 37°C. Ten to twenty larvae or adults were placed in a 24-well or 6-well Falcon plate containing 3 ml or 1 ml of RPMI 1640 medium (supplemented with l-glutamine and HEPES [25 mM]), respectively. The S. mansoni cultures were then incubated with each compound at a final concentration of 5 or 50 μg/ml for larvae or adults, respectively. The compounds tested were praziquantel (Sigma-Aldrich), artemether (Rhône-Poulenc Rohrer Doma, Antony, France), artesunate (Sanofi-Aventis, Toulouse, France), and the trioxaquines PA1110 and PA1120 (J. Cazelles, F. Cosledan, B. Meunier, and A. Pellet, 2 June 2005, World Intellectual Property Organization, publication no. WO/2005/049619) and PA1252, PA1259, PA1103, and PA1308 (B. Meunier, F. Cosledan, and A. Pellet, 21 December 2007, World Intellectual Property Organization, publication no. WO/2007/144487) (all trioxaquines were prepared by Palumed). Each compound was first dissolved in dimethyl sulfoxide (DMSO) to give a mother solution at 100 mg/ml. The entire range of dilutions was carried out in DMSO except the last dilution, performed in RPMI 1640 complemented with 2.17% Tween 80 in order to obtain a final RPMI/Tween 80/DMSO dilution ratio of 1,000/0.95/3.8 (vol/vol/vol). These dilutions were added to wells that contained parasites. Control worms were treated with RPMI-Tween 80-DMSO (1,000/0.95/3.8 [vol/vol/vol]) without drug. Each test was performed in duplicate. Parasites were subsequently observed for body contractility and movement at the indicated times. Parasites showing no body contractions during a 30-s observation were considered dead (no worm started to move again after 30 s without motor activity). Kaplan-Meier survival analyses followed by pairwise log-rank tests were used to compare survival data.
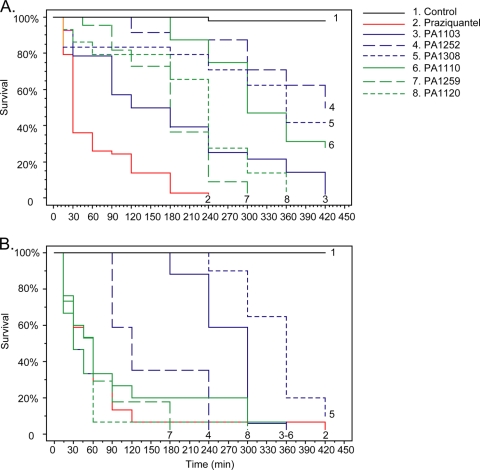
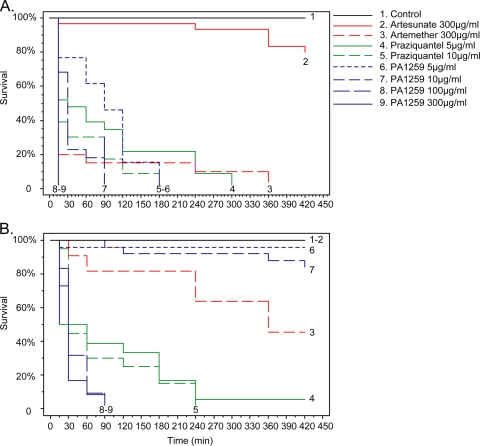
Results show that all trioxaquines present significant antischistosomal activities for both larval (Fig. 2A) and adult (Fig. 2B) stages of the parasite. Regarding the larval stage, three trioxaquines, PA1103, PA1120, and PA1259, were able to kill all schistosome larvae within 7 h of contact at a weak concentration of 5 μg/ml (Fig. 2A). Surprisingly, the trioxaquine PA1308 (a methylated derivative of PA1103; Fig. 1) was less potent than the parent compound. This result suggested that a minor chemical modification of a trioxaquine structure greatly influences the antischistosomal activity. Regarding the effects of compounds on adult worms, it should be noted that all evaluated trioxaquines, except PA1308, were able to kill adult worms within 7 h of contact in the culture medium (Fig. 2B). These trioxaquines can be divided into two groups. The first one consisted of PA1259, PA1110, and PA1120, with a fast direct effect on parasites, very similar to that of praziquantel. The second one, PA1252 and PA1103, had delayed activity but still showed a full killing effect. If we compare these different trioxaquines, it seems that the presence of a cyclopentyl group linked to the trioxane moiety of the molecule (PA1259 and PA1120) gives rise to compounds with activities against larval or adult schistosome stages similar to that of praziquantel. The compound PA1259 was the most active compound tested and was then evaluated at different concentrations and compared to artemether, artesunate, and praziquantel in the same experiment (Fig. 3). Regarding the larval stage, the PA1259 compound was able to kill parasite larvae at the same concentration as that of praziquantel (Fig. 3A). Artemether needs a 60-times-higher dose to reach the same level of activity. This could be explained by the fact that in vitro, artemether needs to interact with additional free hemin to exert a toxic effect on the parasite (16). The fact that these trioxaquines are highly active in vitro without external activation by added hemin should be considered a positive sign for the future of these molecules as potential antischistosomal drugs. The higher activity of PA1259 than of artemether was also confirmed with adult-stage parasites despite a slightly lower activity of PA1259 than of praziquantel (Fig. 3B). These data are consistent with the higher activities of trioxane-containing compounds against schistosome larval stages (17).
FIG. 2.
Survival of cultured S. mansoni larval-stage (A) or adult-stage (B) parasites treated with PA1103, PA1252, PA1308, PA1110, PA1259, PA1120, or praziquantel. Cultured larvae or adults were treated with compounds at 5 μg/ml or 50 μg/ml, respectively. For the larval stage, pairwise log-rank tests indicate four groups (α = 1%): (i) praziquantel; (ii) PA1103, PA1259, and PA1120; (iii) PA1103, PA1252, and PA1308; and (iv) the control. For the adult stage, pairwise log-rank tests indicate five groups (α = 1%): (i) praziquantel, PA1110, PA1259, and PA1120; (ii) PA1252; (iii) PA1103; (iv) PA1308; and (v) control.
FIG. 3.
Survival of cultured S. mansoni larval-stage (A) or adult-stage (B) parasites treated with PA1259, artemether, artesunate, or praziquantel. Cultured worms were treated with compounds at indicated concentrations and times to produce 100% parasite death. For the larval stage, pairwise log-rank tests indicate four groups (α = 1%): (i) PA1259 (100 μg/ml and 300 μg/ml); (ii) PA1259 (5 and 10 μg/ml), praziquantel (5 and 10 μg/ml), and artemether (300 μg/ml); (iii) artesunate (300 μg/ml); and (iv) the control. For the adult stage, pairwise log-rank tests indicate four groups (α = 1%): (i) PA1259 (100 μg/ml and 300 μg/ml), (ii) praziquantel (5 and 10 μg/ml), (iii) artemether (300 μg/ml), and (iv) artesunate (300 μg/ml), PA1259 (5 and 10 μg/ml), and the control.
In conclusion, these results indicate that trioxaquines are efficient compounds against S. mansoni in vitro. One of these trioxaquines, PA1259, is as effective as praziquantel at killing worms in vitro. Experiments to evaluate these trioxaquines in vivo with mice infected by Schistosoma mansoni are in progress.
Acknowledgments
This work was supported by Palumed, the UMR 5244, and a grant from the Agence Nationale pour la Recherche (ANR grant no. ANR-08-MIEN-026-02).
We thank Sonia Kitoune and Christine Salle (both from Palumed) and Bernard Dejean (UMR 5244) for technical assistance.
Footnotes
Published ahead of print on 13 July 2009.
REFERENCES
- 1.Alonso, D., J. Muñoz, J. Gascón, M. E. Valls, and M. Corachan. 2006. Short report: failure of standard treatment with praziquantel in two returned travelers with Schistosoma haematobium infection. Am. J. Trop. Med. Hyg. 74:342-344. [PubMed] [Google Scholar]
- 2.Benoit-Vical, F., J. Lelièvre, A. Berry, C. Deymier, O. Dechy-Cabaret, J. Cazelles, C. Loup, A. Robert, J.-F. Magnaval, and B. Meunier. 2007. Trioxaquines are new antimalarial agents active on all erythrocytic forms, including gametocytes. Antimicrob. Agents Chemother. 51:1463-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boissier, J., and H. Mone. 2000. Experimental observations on the sex ratio of adult Schistosoma mansoni, with comments on the natural male bias. Parasitology 121:379-383. [DOI] [PubMed] [Google Scholar]
- 4.Colley, D. G., P. T. LoVerde, and L. Savioli. 2001. Medical helminthology in the 21st Century. Science 293:1437-1438. [DOI] [PubMed] [Google Scholar]
- 5.Coslédan, F., L. Fraisse, A. Pellet, F. Guillou, B. Mordmüller, P. G. Kremsner, A. Moreno, D. Mazier, J. P. Maffrand, and B. Meunier. 2008. Selection of a trioxaquine as a drug-candidate. Proc. Natl. Acad. Sci. USA 105:17579-17584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doenhoff, M. J., J. R. Kusel, G. C. Coles, and D. Cioli. 2002. Resistance of Schistosoma mansoni to praziquantel: is there a problem? Trans. R. Soc. Trop. Med. Hyg. 96:465-469. [DOI] [PubMed] [Google Scholar]
- 7.Gryseels, B., K. Polman, J. Clerinx, and L. Kestens. 2006. Human schistosomiasis. Lancet 368:1106-1118. [DOI] [PubMed] [Google Scholar]
- 8.Keiser, J., J. Chollet, S.-H. Xiao, J.-Y. Mei, P.-Y. Jiao, J. Utzinger, and M. Tanner. 2009. Mefloquine—an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl. Trop. Dis. 3:e350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laurent, S. A.-L., J. Boissier, F. Coslédan, H. Gornitzka, A. Robert, and B. Meunier. 2008. Synthesis of “Trioxaquantel” derivatives as potential new antischistosomal drugs. Eur. J. Org. Chem. 2008:895-913. [Google Scholar]
- 10.McManus, D. P., and A. Loukas. 2008. Current status of vaccines for schistosomiasis. Clin. Microbiol. Rev. 21:225-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meunier, B. 2008. Hybrid molecules with a dual mode of action: dream or reality? Acc. Chem. Res. 41:69-77. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira, M. F., S. W. Kycia, A. Gomez, A. J. Kosar, D. S. Bohle, E. Hempelmann, D. Menezes, M. A. Vannier-Santos, P. L. Oliveira, and S. T. Ferreira. 2005. Structural and morphological characterization of hemozoin produced by schistosoma mansoni and rhodnius prolixus. FEBS Lett. 579:6010-6016. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro-dos-Santos, G., S. Verjovski-Almeida, and L. C. C. Leite. 2006. Schistosomiasis—a century searching for chemotherapeutic drugs. Parasitol. Res. 99:505-521. [DOI] [PubMed] [Google Scholar]
- 14.Robert, A., F. Benoit-Vical, C. Claparols, and B. Meunier. 2005. The antimalarial drug artemisinin alkylates heme in infected mice. Proc. Natl. Acad. Sci. USA 102:13676-13680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sayed, A. A., A. Simeonov, C. J. Thomas, J. Inglese, C. P. Austin, and D. L. Williams. 2008. Identification of oxadiazoles as new drug leads for the control of schistosomiasis. Nat. Med. 14:407-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao, S., J. Chollet, J. Utzinger, H. Matile, J. Mei, and M. Tanner. 2001. Artemether administered together with haemin damages schistosomes in vitro. Trans R. Soc. Trop. Med. Hyg. 95:67-71. [DOI] [PubMed] [Google Scholar]
- 17.Xiao, S., M. Tanner, E. K. N′Goran, J. Utzinger, J. Chollet, R. Bergquist, C. Minggang, and Z. Jiang. 2002. Recent investigations of artemether, a novel agent for the prevention of Schistosomiasis japonica, mansoni and haematobia. Acta Trop. 82:175-181. [DOI] [PubMed] [Google Scholar]
- 18.Xiao, S.-H., Y.-L. Wu, M. Tanner, W.-M. Wu, J. Utzinger, J.-Y. Mei, B. Scorneaux, J. Chollet, and Z. Zhai. 2003. Schistosoma japonicum: in vitro effects of artemether combined with haemin depend on cultivation media and appraisal of artemether products appearing in the media. Parasitol. Res. 89:459-466. [DOI] [PubMed] [Google Scholar]
- 19.Xiao, S.-H., J. Keiser, J. Chollet, J. Utzinger, Y. Dong, Y. Endriss, J. L. Vennerstrom, and M. Tanner. 2007. In vitro and in vivo activities of synthetic trioxolanes against major human schistosome species. Antimicrob. Agents Chemother. 51:1440-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]