Abstract
The aim of our study was to evaluate the outcome of acute prosthetic joint infections (PJIs) due to gram-negative bacilli (GNB) treated without implant removal. Patients with an acute PJI due to GNB diagnosed from 2000 to 2007 were prospectively registered. Demographics, comorbidity, type of implant, microbiology data, surgical treatment, antimicrobial therapy, and outcome were recorded. Classification and regression tree analysis, the Kaplan-Meier survival method, and the Cox regression model were applied. Forty-seven patients were included. The mean age was 70.7 years, and there were 15 hip prostheses and 32 knee prostheses. The median number of days from the time of arthroplasty was 20. The most frequent pathogens were members of the Enterobacteriaceae family in 41 cases and Pseudomonas spp. in 20 cases. Among the Enterobacteriaceae, 14 were resistant to ciprofloxacin, while all Pseudomonas aeruginosa isolates were susceptible to ciprofloxacin. The median durations of intravenous and oral antibiotic treatment were 14 and 64 days, respectively. A total of 35 (74.5%) patients were in remission after a median follow-up of 463 days (interquartile range, 344 to 704) days. By use of the Kaplan-Meier survival curve, a C-reactive protein (CRP) concentration of ≤15 mg/dl (P = 0.03) and receipt of a fluoroquinolone, when all GNB isolated were susceptible (P = 0.0009), were associated with a better outcome. By use of a Cox regression model, a CRP concentration of ≤15 mg/dl (odds ratio [OR], 3.57; 95% confidence interval [CI], 1.05 to 12.5; P = 0.043) and receipt of a fluoroquinolone (OR, 9.09; 95% CI, 1.96 to 50; P = 0.005) were independently associated with better outcomes. Open debridement without removal of the implant had a success rate of 74.5%, and the factors associated with good prognosis were a CRP concentration at the time of diagnosis ≤15 mg/dl and treatment with a fluoroquinolone.
Acute postoperative prosthetic joint infection (PJI) is an uncommon but severe complication after joint arthroplasty. The infection rate is 1 to 3%, and the most frequently isolated microorganisms are gram-positive cocci, including Staphylococcus aureus, coagulase-negative staphylococci, and Streptococcus spp. (12). However, it is of note that gram-negative bacilli (GNB) are isolated in 10% of cases of PJIs, and these infections are frequently polymicrobial (9).
The rate of success achieved by treatment with open debridement without implant removal and a prolonged course of antibiotics for staphylococcal acute PJIs is higher than 75% (2, 13, 17). However, there has been little experience with the use of the same surgical and antibiotic treatment for infections due to GNB (3, 8). In addition, a major concern associated with PJIs due to GNB is the emergence of strains resistant to many antibiotics and the lack of alternative treatments (15).
The aims of the present study were to review our experience with the treatment of acute PJIs due to GNB by the use of open debridement and retention of the implant, followed by antibiotic treatment, and to analyze those factors associated with the outcome.
MATERIALS AND METHODS
From January 2000 to December 2007, all patients with a PJI (hip hemiarthroplasty, total hip and knee arthroplasty) were prospectively registered in a database and the cases were retrospectively reviewed. All patients were treated in the bone and joint infection unit of the same hospital, which includes orthopedic surgeons and infectious disease specialists. Relevant information about each patient's demographics, comorbidity, type of implant (hip or knee prosthesis), clinical manifestations, leukocyte count, C-reactive protein (CRP) concentration at the time of admission for infection, surgical treatment, the microorganism isolated, antimicrobial therapy, and outcome were recorded. In the present study, only those cases with an acute, mono- or polymicrobial PJI due to GNB were included.
In the present study, an acute PJI due to GNB was defined by the presence of local inflammation of acute onset (symptom duration of <15 days), macroscopic evidence of extension of the infection through the capsule during open debridement, and isolation of GNB from deep samples. Infections were classified as follows: (i) postoperative infections were those diagnosed within the 90 days after joint arthroplasty, and (ii) hematogenous infections those that represented hematogenous seeding of the joint from another primary site with no previous dysfunction of the prosthesis.
For debridement, preexisting incisions were always used, necrotic tissue was excised, and the joint was washed out with 6 liters of sterile water. The components of the prosthesis were left in situ after it was confirmed at the time of surgery that there were no signs of loosening. In knee arthroplasties, the polyethylene component was removed and replaced with a new component, while in total hip arthroplasties, the polyethylene as well as the femoral head were replaced, when possible. The patients were taken back to the operating room to repeat the irrigation only when systemic or local signs of infection persisted after debridement. When this was performed within the week after the first debridement, it was not considered a failure. At least three deep samples of synovial fluid or periprosthetic tissue were submitted to the microbiology laboratory. Synovial fluid was aspirated, and 50% was inoculated into aerobic blood culture flasks and 50% was inoculated into anaerobic blood culture flasks (Bactec 9240 system; BD Diagnostic Systems). The volume inoculated in each flask was approximately 1 to 3 ml. Solid samples from periprosthetic tissue were taken and placed in sterile containers. Finally, swab cultures were obtained by passing a sterile swab over the intracapsular area, bone, or fluid and immediately placed in transport medium (Amies transport medium). Each sample for culture was transported to, processed, and analyzed in the Microbiology Laboratory. Blood culture flasks containing the aspirated synovial fluid were incubated in the Bactec 9240 system for up to 5 days (5). Positive cultures were Gram stained, and the microorganisms were identified by conventional microbiological methods. The periprosthetic tissue and swabs were cultured in thioglycolate broth, blood agar under aerobic conditions, and Schaedler agar under anaerobic conditions. All samples were incubated for up to 5 days. Positive cultures were regrown in the appropriate medium. All microorganisms isolated were identified by standard biochemical procedures. In addition, blood cultures were performed for patients with fever at the time of admission for infection. An antibiogram for all isolates was performed by the microdilution method.
After open debridement, a broad-spectrum intravenous antimicrobial regimen that included vancomycin at 1 g/12 h (monitored to attain a trough concentration of 15 mg/liter) plus ceftazidime at 2 g/8 h was started and was maintained until definitive microbiological results were obtained. The intravenous antibiotics used to treat infections caused by GNB susceptible to expanded-spectrum cephalosporins were ceftriaxone at 1 g/24 h or cefotaxime at 2 g/6 h, those used to treat infections caused by extended-spectrum β-lactamase (ESBL)-producing members of the family Enterobacteriaceae were imipenem at 1 g/8 h or meropenem at 1 g/8 h, and those used to treat infections caused by Pseudomonas aeruginosa were ceftazidime at 2 g/6 h plus ciprofloxacin at 400 mg/12 to 8 h. The oral antibiotics used were ciprofloxacin at 750 mg/12 h or levofloxacin at 500 mg/24 h for the treatment of infections caused by fluoroquinolone-susceptible gram-negative organisms and ceftibuten at 400 mg/24 h, co-trimoxazole at 800 mg/12 h, or amoxicillin (amoxicilline)-clavulanate at 875/125 mg/8 h for the treatment of infections caused by fluoroquinolone-resistant gram-negative organisms. For the treatment of infections caused by staphylococci and streptococci, patients received levofloxacin, co-trimoxazole, or linezolid at 600 mg/12 h, according to the findings of the antibiogram, in association with rifampin (rifampicin) at 600 mg/24 h. For the treatment of infections caused by enterococci, patients received amoxicillin at 1 g/8 h or linezolid, according to the findings of the antibiogram. The dosages were adjusted according to the patient's renal function. The duration of intravenous and oral antibiotic treatment was not standardized and was decided according to the clinical manifestations and the CRP concentrations of each case.
After the patients were discharged, they were followed up monthly while they were receiving treatment. Once the treatment was finished, they were followed up every 3 months, at which time the clinical response and any secondary effects of the antimicrobial therapy were recorded. The outcome was evaluated according to the following definitions: (i) remission was considered to have occurred when the patient showed no symptoms of infection, the prosthesis was retained, and the CRP concentration was less than 1 mg/dl; and (ii) failure was considered to have occurred when inflammatory signs and a high CRP concentration remained during the treatment or reappeared after the patient completed treatment (relapse or reinfection, depending on the microorganism isolated).
Statistical analysis.
Continuous variables were expressed as the means and standard deviations or the medians and interquartile ranges (IQRs). The continuous variables were age, the time from the arthroplasty to the diagnosis of infection (the age of the implant), leukocyte count, and the duration of intravenous and oral antibiotic treatment. The categorical variables were sex, comorbidity (having or not having one or more of the following entities: diabetes mellitus, liver cirrhosis, chronic renal failure, rheumatoid arthritis, or chronic obstructive pulmonary disease), the type of infection (postsurgical or hematogenous), the type of prosthesis (hip or knee), positive blood cultures, the need for a second debridement, polymicrobial infection, infection due to Pseudomonas spp., and antibiotic treatment. The continuous variables were compared by using the Mann-Whitney U test, and the comparison of proportions was done by using the χ2 test or the Fisher exact test, when necessary.
The continuous and categorical variables were analyzed by the use of classification and regression tree analysis (CART) to identify the predictors of the outcomes and useful breakpoints for the continuous variables. The Kaplan-Meier survival method was used to estimate the cumulative probability of treatment failure from the time of open debridement to the time of the last visit. The log rank test was applied to evaluate the influence of each variable. A Cox regression model was applied to identify the independent variables associated with failure after open debridement. Only those variables with a significantly higher cumulative probability of failure by the Kaplan-Meier curve method were included in the Cox regression model. Statistical significance was defined as a two-tailed P value of <0.05. The analysis was done with the SPSS program (version 12.0; SPSS, Inc., Chicago, IL) and CART software (version 6.0; Salford Systems, San Diego, CA).
RESULTS
During the study period, a total of 47 patients met the criteria for inclusion in the study. The main characteristics of the patients according to the outcome are summarized in Table 1. The mean age was 70.7 years (standard deviation, 11.3 years); there were 15 hip prostheses and 32 knee prostheses. The median number of days from the time of arthroplasty to the time of diagnosis of the infection was 20 (IQR, 16 to 28 days). There were 44 acute postsurgical deep infections, and for these the median number of days from the time of arthroplasty was 19 (IQR, 15.25 to 25.50 days). There were also three hematogenous infections; and for these three cases the median numbers of days from the time of arthroplasty were 168, 673, and 682 days, respectively. The GNB isolated and their main traits of resistance are listed in Table 2. The most common isolates were members of the Enterobacteriaceae family in 41 cases (Escherichia coli in 20 cases) and Pseudomonas aeruginosa in 19 cases. There were 28 polymicrobial infections (59.5%): 10 were due to more than one GNB and 18 were due to mixed infections that included GNB and gram-positive cocci (Table 3). Two different microorganisms were isolated from 21 of 28 polymicrobial infections; in 7 cases, two different GNB were isolated, and in 14 cases a GNB and a gram-positive organism were isolated. In the rest of the polymicrobial infections (seven cases), three different microorganisms were identified: in three cases, there were three different GNB; in three cases, there were two different GNB and a gram-positive organism; and in one case, there were one GNB and two different gram-positive organisms. Among the Enterobacteriaceae, 14 were resistant to ciprofloxacin, 8 were ESBL producers, and 1 was a chromosomal beta-lactamase (AmpC) producer. All P. aeruginosa isolates were susceptible to ciprofloxacin. Thirteen patients had infections due to a fluoroquinolone-resistant GNB, including Bacteroides fragilis, and these infections were associated with poor outcomes (Table 1).
TABLE 1.
Characteristics of patients according to outcome
| Characteristic | Value for patients with treatment: |
Pb | |
|---|---|---|---|
| Failure (n = 12)a | Remission (n = 35) | ||
| Median (IQR) age (yr) | 71 (64.2-79) | 75 (63-79) | 0.96 |
| No. (%) female | 7 (58.3) | 24 (68.3) | 0.37 |
| No. (%) of patients with a comorbidityc | 6 (50) | 15 (42.9) | 0.46 |
| No. (%) of patients with the following type of prosthesis: | |||
| Hip | 4 (33.5) | 11 (31.4) | |
| Knee | 8 (66.7) | 24 (68.6) | 0.58 |
| Median (IQR) age of prosthesis (days) | 19 (17.2-22.7) | 21 (13-31) | 0.71 |
| No. (%) of patients with the following type of infection: | |||
| Postsurgical | 12 | 32 (91.4) | |
| Hematogenous | 0 | 3 (8.6) | 0.40 |
| Median (IQR) leukocyte count (no. of cells/mm3) | 8,750 (4,925-11,122) | 8,100 (6,150-10,417) | 0.97 |
| Median (IQR) CRP concn (mg/dl) | 11.4 (1.4-21.4) | 4.2 (0.8-12.9) | 0.20 |
| No. (%) of patients with: | |||
| CRP concn of >15 mg/dl | 6 (50) | 6 (17.1) | 0.03 |
| Bacteremia | 0 | 4 (11.4) | 0.29 |
| Polymicrobial infection | 7 (58.3) | 21 (60) | 0.59 |
| Mixed infection with gram-positive organisms | 3 (25) | 15 (42.9) | 0.32 |
| Infection due to a Pseudomonas sp.d | 4 (33.5) | 16 (45.7) | 0.34 |
| Infection due to a fluoroquinolone-resistant strain | 8 (66.7) | 5 (14.3) | 0.001 |
| Infection due to an ESBL-producing member of Enterobacteriaceae family | 4 (33.3) | 4 (11.4) | 0.17 |
| Need for a second debridement | 3 (25) | 4 (11.4) | 0.24 |
| Median (IQR) duration of antibiotic therapy (oral and intravenous) (days) | 84.5 (35.5-167.2) | 82 (47-135) | 0.94 |
| Median (IQR) duration of oral antibiotic therapy (days)e | 95 (27.7-165.2) | 64 (36-101.5) | 0.46 |
| No. (%) of patients treated with fluoroquinolones | 7 (58.3) | 28 (80) | 0.13 |
| No. (%) of patients treated with fluoroquinolones when all isolated GNB were susceptible | 2 (16.7) | 26 (74.3) | 0.001 |
Including relapse and reinfection.
Mann-Whitney U test, χ2 test, or Fisher exact test, when necessary.
Diabetes mellitus, liver cirrhosis, chronic renal failure, rheumatoid arthritis, or chronic obstructive pulmonary disease.
P. aeruginosa in 19 cases and Pseudomonas stutzeri in 1 case.
The information is for those patients who received oral antibiotics (n = 41).
TABLE 2.
GNB isolated from deep periprosthetic samples from patients with either monomicrobial and polymicrobial infections
| GNB | No. (%) of patients |
|---|---|
| Totala | 63 |
| Enterobacteriaceae | 41 (65) |
| Escherichia coli | 20 (48.8) |
| Resistant to ciprofloxacin | 10 |
| ESBL producerb | 7 |
| AmpC-producerb | 1 |
| Proteus mirabilis | 8 (19.5) |
| Proteus mirabilis resistant to ciprofloxacin | 2 |
| Enterobacter cloacae | 7 (17) |
| Enterobacter cloacae resistant to ciprofloxacin | 0 |
| Klebsiella pneumoniae | 2 (4.9) |
| Klebsiella pneumoniae resistant to ciprofloxacin | |
| and ESBL producer | 1 |
| Other Enterobacteriaceaec | 4 (9.5) |
| Other Enterobacteriaceae resistant to ciprofloxacin | 1 |
| Nonfermenting GNB | 21 (33.3) |
| Pseudomonas aeruginosa | 19 (90.4) |
| Pseudomonas aeruginosa resistant to ciprofloxacin | 0 |
| Other nonfermentersd | 2 (9.6) |
| Other nonfermenters resistant to ciprofloxacin | 0 |
| Anaerobes | 1 (1.6) |
| Bacteroides fragilis | 1 |
The number of microorganisms isolated was higher than the number of patients due to polymicrobial infections.
Five ESBL producers and the AmpC producer were resistant to ciprofloxacin.
Citrobacter diversus, Serratia marcescens, Providencia rettgeri, and Morganella morgagnii.
Pseudomonas stutzeri and Acinetobacter baumannii.
TABLE 3.
Other pathogens isolated from polymicrobial infections
| Gram-positive coccia | No. (%) of patients |
|---|---|
| Total | 19 |
| Coagulase-negative staphylococci | 8 (42.1) |
| Staphylococcus aureus | 3 (15.8) |
| Enterococcus faecalis | 6 (31.6) |
| Streptococcus viridans | 1 (5.2) |
| Streptococcus agalactiae | 1 (5.2) |
The number of patients with polymicrobial infections that included GNB and gram-positive cocci was 18 (see text), but the total number of gram-positive cocci was 19 because two different gram-positive cocci were isolated from one patient.
The median durations of intravenous and oral antibiotic treatment were 14 days (IQR, 8 to 24 days) and 64 days (IQR, 27.5 to 101.5 days), respectively. The intravenous antibiotic regimens were a beta-lactam (n = 24), a beta-lactam with activity against P. aeruginosa plus ciprofloxacin (n = 17), and ciprofloxacin alone (n = 6). Fluoroquinolones (ciprofloxacin or levofloxacin) were the definitive antibiotic treatment in 35 cases. Among these 35 patients, 28 had infections due to fluoroquinolone-susceptible GNB, while the other 7 had infections due to susceptible and resistant GNB, and they received other antibiotics (co-trimoxazole in 5 cases and amoxicillin-clavulanate in 2 cases), in addition to fluoroquinolones. Among the 12 patients who did not receive fluoroquinolones, all of them received an intravenous beta-lactam and 8 switched to oral therapy with co-trimoxazole in 6 cases and ceftibuten and amoxicillin-clavulanate in one case each. The success rate was high (26 of 28 [92.8%]) for those patients with infections due to a fluoroquinolone-susceptible GNB who received a fluoroquinolone (n = 28) (Table 1).
A total of 35 (74.5%) patients were in remission after a median follow-up of 463 days (IQR, 344 to 704 days; range, 219 to 1,090 days) after open debridement. There were 12 (24.5%) failures, 9 relapses, and 2 reinfections; and 1 case could not be classified because no microorganism was identified. Among the cases with relapses, in eight cases the same GNB responsible for the index infection was isolated: E. coli in six cases (four ESBL producers) and B. fragilis and Enterobacter cloacae in one case each. The other relapse was due to the gram-positive organism component (coagulase-negative staphylococci) responsible for the index infection. Reinfections were due to S. aureus and coagulase-negative staphylococci. Although P. aeruginosa was part of the flora identified in the index infection of 4 of 12 treatment failures, in no case was this microorganism the cause of a relapse.
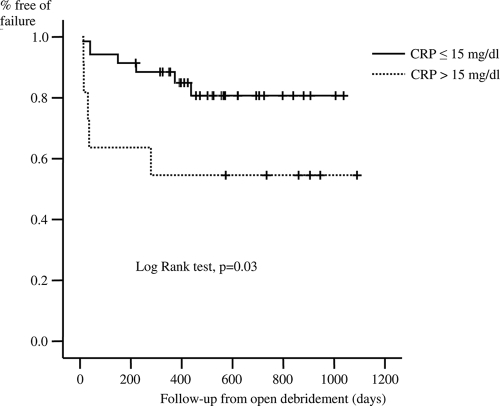
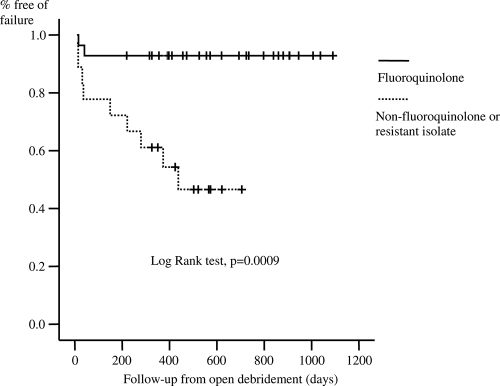
The CART model identified only the CRP concentration as a predictor of the outcome, and the breakpoint for the delineation of a risk of failure was 15 mg/dl. By using the Kaplan-Meier survival curve, a CRP concentration of ≤15 mg/dl (P = 0.03) and receipt of a fluoroquinolone, when all GNB isolated were susceptible to fluoroquinolones (P = 0.0009), were associated with better outcomes (Fig. 1 and 2). There were infections due to more than one GNB with different susceptibilities to fluoroquinolones; and treatment with fluoroquinolones indeed showed a trend toward a better outcome, but it was not significant (P = 0.13) (Table 1). In the Cox regression model, the inclusion of significant variables in univariate analysis (Table 1), a CRP concentration of ≤15 mg/dl (odds ratio, 3.57; 95% confidence interval, 1.05 to 12.5; P = 0.043), and receipt of a fluoroquinolone, when all the isolated GNB were susceptible to fluoroquinolones (odds ratio, 9.09; 95% confidence interval, 1.96 to 50; P = 0.005), were independently associated with better outcomes. Having an infection due to a fluoroquinolone-resistant strain was not an independent predictor of treatment failure.
FIG. 1.
Cumulative probability of survival (free of failure) according to the CRP concentration at the time of admission for infection.
FIG. 2.
Cumulative probability of survival (free of failure) according to receipt of a fluoroquinolone when isolates were susceptible or nonreceipt of a fluoroquinolone or receipt of a fluoroquinolone when at least one isolate was resistant to ciprofloxacin.
Among the patients with a CRP concentration of ≤15 mg/dl, those who received a fluoroquinolone when all the isolated strains were susceptible to ciprofloxacin had a remission rate of 95.4% (21 of 22 patients), while those who were infected with resistant strains or who did not receive a fluoroquinolone had a remission rate of 61.5% (8 of 13 patients). In addition, among the patients with a CRP concentration of >15 mg/dl, those who received a fluoroquinolone when all the isolated strains were susceptible to ciprofloxacin had a remission rate of 83.3% (five of six patients), while those who were infected with resistant strains or who did not receive a fluoroquinolone had a remission rate of only 16.7% (one of six patients) (Table 4).
TABLE 4.
Outcome according to CRP concentration and antimicrobial therapy received
| CRP concn and treatment or condition | No. (%) of patients with treatment: |
Pa | |
|---|---|---|---|
| Failure (n = 12) | Remission (n = 35) | ||
| ≤15 mg/dl | |||
| No fluoroquinolone or infection with a resistant strain (n = 13) | 5 (83.3) | 8 (27.6) | |
| Fluoroquinolone (n = 22) | 1 (16.7) | 21 (72.4) | 0.01 |
| >15 mg/dl | |||
| No fluoroquinolone or infection with a resistant strain (n = 11) | 5 (83.3) | 1 (16.7) | |
| Fluoroquinolone (n = 12) | 1 (16.7) | 5 (83.3) | 0.04 |
Fisher exact test.
DISCUSSION
The present study suggests that open debridement with retention of the implant followed by antibiotic treatment is a good option for the treatment of acute PJIs due to GNB. The success rate was 74.5%, which is similar to that reported by other authors who used this method for the treatment of acute PJIs due to staphylococci (2, 4, 7, 13). A CRP concentration of ≤15 mg/dl and treatment with a fluoroquinolone when all the isolated GNB were susceptible to fluoroquinolones were factors independently associated with a better outcome. In fact, patients who received a fluoroquinolone when all the isolated strains were susceptible to fluoroquinolones had a remission rate higher than 90% (26 of 28 patients). Previously published clinical experience with a similar therapeutic approach is scarce but supports the efficacy of fluoroquinolones. Brouqui et al. (3), using a combination of ceftazidime and ciprofloxacin, cured nine osteosynthetic devices and four of five PJIs due to P. aeruginosa. Recently, Legout et al. (8) reviewed their experience without removing the implant in 12 patients with an orthopedic device infection (internal fixation or joint prosthesis) due to a GNB. Antibiotic treatment consisted of intravenous cefepime for 4 weeks combined with oral ofloxacin or ciprofloxacin for 3 to 9 months, and the cure rate was 67% (8 of 12 patients). Fluoroquinolones are probably efficacious for the treatment of implant infections and osteomyelitis due to GNB for two reasons: (i) their diffusion to synovial fluid and bone (11) and (ii) their activity against biofilms. In an in vitro model of a Pseudomonas biofilm, Tanaka et al. (14) showed that the bactericidal action of beta-lactams against biofilm cells was affected by the low rate of cell growth inside the biofilm, while that of fluoroquinolones was considerably greater and independent of the growth rate.
Unfortunately, 14 of 43 (32.5%) isolates of the Enterobacteriaceae family recovered in our study were resistant to ciprofloxacin. In addition, eight PJIs were due to ESBL producers of the Enterobacteriaceae, and to our knowledge, this is the first description of these pathogens as causes of PJIs. The cure rate for patients infected with these organisms was only 50%. The activity of tigecycline against biofilms caused by GNB (Acinetobacter baumannii and Klebsiella pneumoniae) in vitro was evaluated in a previous study (1), and it was lower than against biofilms caused by gram-positive organisms (6, 10); however, there was a considerable synergism between N-acetylcysteine and tigecycline. In the future, it will be necessary to evaluate new strategies for the treatment of implant-related infections due to GNB.
The duration of antimicrobial therapy for acute PJIs has not been well established. On the basis of expert opinion, a duration of 3 to 6 months is recommended (16). In our protocol, antibiotic therapy was maintained until the clinical resolution of infectious symptoms and the normalization of the CRP concentration were attained. We have obtained good results using this protocol, especially when a fluoroquinolone was administered.
More than 60% of the infections in our study were polymicrobial; and although it might seem to be a high rate, a recent article from Oxford (9) described 12 acute infections due to GNB, and 8 (66.6%) were polymicrobial. In fact, in that study 47% of acute PJIs were polymicrobial.
The suggested duration of follow-up for patients with PJIs is at least 2 years on the basis of experience with cases in which the major pathogens were gram-positive organisms. A drawback of our study was that only 40% of our patients reached the 2-year follow-up; however, data on the outcome and the natural history of PJIs due to GNB are scarce, and in fact, this is the largest reported series of patients treated with conservative surgery and antimicrobial therapy. However, the small sample size implies a limited statistical power, and the conclusions should be interpreted with caution. Another drawback of our study was that the antimicrobial therapy was not randomized but was administered according to the susceptibility pattern of the microorganisms isolated. The design of clinical trials for the evaluation of the best treatments for these infections is difficult, and for this reason, it is necessary to progress from observational cohort studies.
In conclusion, open debridement without removal of the implant in patients with acute PJIs due to GNB had a success rate of 74.5%. The factors associated with a good prognosis were a CRP concentration at the time of diagnosis of ≤15 mg/dl and treatment with a fluoroquinolone when all the strains isolated were susceptible to ciprofloxacin. In the future, it will be necessary to evaluate new therapeutic strategies for those infections due to fluoroquinolone-resistant strains.
Acknowledgments
This study was supported by the Red Española para la Investigación en Patología Infecciosa.
At the time of publication, none of the authors disclosed any potential conflicts of interest.
Footnotes
Published ahead of print on 17 August 2009.
REFERENCES
- 1.Aslam, S., B. W. Trautner, V. Ramanathan, and R. O. Darouiche. 2007. Combination of tigecycline and N-acetylcysteine reduces biofilm-embedded bacteria on vascular catheters. Antimicrob. Agents Chemother. 51:1556-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barberan, J., L. Aguilar, G. Carroquino, M. J. Giménez, B. Sánchez, D. Martínez, and J. Prieto. 2006. Conservative treatment of staphylococcal prosthetic joint infections in elderly patients. Am. J. Med. 119:e7-e10. [DOI] [PubMed] [Google Scholar]
- 3.Brouqui, P., M. C. Rousseau, A. Stein, M. Drancourt, and D. Raoult. 1995. Treatment of Pseudomonas aeruginosa-infected orthopedic prostheses with ceftazidime-ciprofloxacin antibiotic combination. Antimicrob. Agents Chemother. 39:2423-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giulieri, S. G., P. Graber, P. E. Ochsner, and W. Zimmerli. 2004. Management of infection associated with total hip arthroplasty according to a treatment algorithm. Infection 32:222-228. [DOI] [PubMed] [Google Scholar]
- 5.Hughes, J. G., E. A. Vetter, R. Patel, C. D. Schleck, S. Harmsen, L. T. Turgeant, and F. R. Cockerill III. 2001. Culture with BACTEC Peds Plus/F bottle compared with conventional methods for detection of bacteria in synovial fluid. J. Clin. Microbiol. 39:4468-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labthavikul, P., P. J. Petersen, and P. A. Bradford. 2003. In vitro activity of tigecycline against Staphylococcus epidermidis growing in an adherent-cell biofilm model. Antimicrob. Agents Chemother. 47:3967-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laffer, R. R., P. Graber, P. E. Ochsner, and W. Zimmerli. 2006. Outcome of prosthetic knee-associated infection: evaluation of 40 consecutive episodes at a single centre. Clin. Microbiol. Infect. 12:433-439. [DOI] [PubMed] [Google Scholar]
- 8.Legout, L., E. Senneville, R. Stern, Y. Yazdanpanah, C. Savage, M. Roussel-Delvalez, B. Rosele, H. Migaud, and Y. Mouton. 2006. Treatment of bone and joint infections caused by gram-negative bacilli with a cefepime-fluoroquinolone combination. Clin. Microbiol. Infect. 12:1030-1033. [DOI] [PubMed] [Google Scholar]
- 9.Moran, E., S. Masters, A. R. Berendt, P. McLardy-Smith, I. Byren, and B. L. Atkins. 2007. Guiding empirical antibiotic therapy in orthopaedics: the microbiology of prosthetic joint infection managed by debridement, irrigation and prosthesis retention. J. Infect. 55:1-7. [DOI] [PubMed] [Google Scholar]
- 10.Raad, I., H. Hanna, Y. Jiang, T. Dvorak, R. Reitzel, G. Chaiban, R. Sherertz, and R. Hachem. 2007. Comparative activities of daptomycin, linezolid, and tigecycline against catheter-related methicillin-resistant Staphylococcus bacteremic isolates embedded in biofilm. Antimicrob. Agents Chemother. 51:1656-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rimmele, T., E. Boselli, D. Breilh, S. Djabarouti, J. C. Bel, R. Guyot, M. C. Saux, and B. Allaouchiche. 2004. Diffusion of levofloxacin into bone and synovial tissues. J. Antimicrob. Chemother. 53:533-535. [DOI] [PubMed] [Google Scholar]
- 12.Soriano, A., G. Bori, S. Garcia-Ramiro, J. C. Martinez-Pastor, T. Miana, C. Codina, F. Maculé, M. Basora, J. A. Martínez, J. Riba, S. Suso, and J. Mensa. 2008. Timing of antibiotic prophylaxis for primary total knee arthroplasty performed during ischemia. Clin. Infect. Dis. 46:1009-1014. [DOI] [PubMed] [Google Scholar]
- 13.Soriano, A., S. Garcia, G. Bori, M. Almela, X. Gallart, F. Macule, J. Sierra, J. A. Martínez, S. Suso, and J. Mensa. 2006. Treatment of acute post-surgical infection of joint arthroplasty. Clin. Microbiol. Infect. 12:930-933. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka, G., M. Shigeta, H. Komatsuzawa, M. Sugai, H. Suginaka, and T. Usui. 1999. Effect of the growth rate of Pseudomonas aeruginosa biofilms on the susceptibility to antimicrobial agents: beta-lactams and fluoroquinolones. Chemotherapy 45:28-36. [DOI] [PubMed] [Google Scholar]
- 15.Wenzel, R. P. 2004. The antibiotic pipeline—challenges, costs, and values. N. Engl. J. Med. 351:523-526. [DOI] [PubMed] [Google Scholar]
- 16.Zimmerli, W., A. Trampuz, and P. E. Ochsner. 2004. Prosthetic-joint infections. N. Engl. J. Med. 351:1645-1654. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerli, W., A. F. Widmer, M. Blatter, R. Frei, and P. E. Ochsner. 1998. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. JAMA 279:1537-1541. [DOI] [PubMed] [Google Scholar]