Abstract
Ceftaroline is a broad-spectrum injectable cephalosporin exhibiting bactericidal activity against a variety of bacteria, including methicillin-resistant Staphylococcus aureus (MRSA). Using a two-compartment in vitro pharmacokinetic/pharmacodynamic (PK/PD) model, we evaluated the activity of ceftaroline at 600 mg every 8 h (q8h) and q12h in comparison with that of vancomycin at 1,000 mg q12h over a 72-h time period against six clinical MRSA isolates, including two heterogeneous vancomycin-intermediate S. aureus (hVISA) isolates. The MIC and minimum bactericidal concentration ranged between 0.125 to 2 and 0.5 to 2 μg/ml for ceftaroline and vancomycin, respectively. In the PK/PD model, ceftaroline was superior to vancomycin against all isolates (P < 0.05), except one to which it was equivalent. No difference in activity was observed between both q8 and q12h dosing regimens of ceftaroline. Bacterial regrowth was observed after 32 h for two isolates treated with ceftaroline. This regrowth was uncorrelated to resistance, instability of the drug, or tolerance. However, subpopulations with higher MICs to ceftaroline were found by population analysis for these two isolates. Finally, and in contrast to ceftaroline, MIC elevations up to 8 to 12 μg/ml were observed with vancomycin for the hVISA isolates. In conclusion, in addition to a lower potential to select resistant mutants, ceftaroline demonstrated activity equal to or greater than vancomycin against MRSA isolates. Although further in vitro and in vivo investigations are warranted, ceftaroline appears to be a promising alternative for the treatment of MRSA infections.
Bacteria exhibit an amazing ability to adapt to changes in their environment, and perhaps nowhere is this more visible than in the development of resistance to antibacterial agents. Antibiotic use and misuse have largely contributed to the emergence of multidrug-resistant (MDR) pathogens in the health care setting, leading to a critical need for the development of new antibacterial agents (23). Among the various MDR gram-positive pathogens, methicillin-resistant Staphylococcus aureus (MRSA) strains, particularly those that are heteroresistant to vancomycin, heterogeneous vancomycin-intermediate S. aureus (hVISA), represent a serious clinical concern because they have been associated with poor treatment outcomes, excess health care costs, and prolonged hospital stay (10, 11, 15). In MRSA, resistance to β-lactam antibiotics results from the production of a low-affinity penicillin-binding protein (PBP) called PBP2′ or PBP2a, that is not inhibited by most β-lactams owing to inefficient formation of a covalent enzyme-inhibitor complex (25). New compounds with higher affinity for PBP2a offer the potential to overcome this resistance and represent a novel option for treatment of infections caused by MRSA, including isolates with reduced susceptibility to glycopeptides (20, 21).
Ceftaroline is an investigational parenteral broad-spectrum cephalosporin that exhibits bactericidal activity against gram-positive and -negative pathogens, including MRSA, hVISA, and MDR Streptococcus pneumoniae (5, 18, 28). Administered as a water-soluble semisynthetic N-phosphono-type prodrug (ceftaroline fosamil), ceftaroline is currently in phase III development for treatment of complicated skin and skin structure infections (cSSSI) and community-acquired pneumonia (3). Several in vivo models have been used to compare the activity of ceftaroline versus those of vancomycin, linezolid, arbekacin, and teicoplanin against MRSA isolates (8, 9). In thigh muscle infection and endocarditis models, ceftaroline demonstrated a highly bactericidal effect and was significantly more active than linezolid against MRSA and hVISA isolates. Additionally, statistically significant differences between ceftaroline and vancomycin therapy were also observed against hVISA isolates, but not against MRSA, despite the fact that both agents achieved a bactericidal effect (9). Several factors, such as MIC values and the inoculum size, may affect the killing activity of β-lactam antimicrobials (24, 27). Until now, no in vitro studies of ceftaroline have been carried out using conditions that take into consideration the pharmacokinetic and pharmacodynamic (PK/PD) parameters of this agent. Thus, as the primary objective of this study, we evaluated the in vitro activity of ceftaroline in comparison with that of vancomycin against clinical MRSA and hVISA isolates using an experimental PK/PD model simulating human PK (16, 22, 26).
(A portion of this work was presented at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy-46th Annual Meeting of the Infectious Diseases Society of America, Washington, DC, 25 to 28 October 2008 [29].)
MATERIALS AND METHODS
Bacterial strains and media.
Six clinical isolates of MRSA, including five organisms selected from the Anti-Infective Research Laboratory (Detroit, MI) collection, were investigated in the in vitro PK/PD model. All isolates were recovered from patients from different health care settings in Detroit between 1996 and 2007. On a routine basis in our laboratory, each isolate was screened for its heterogeneous susceptibility profile to vancomycin using the Macro Etest and modified population analysis methods, as previously described (31). MRSA 494 (1), R3804, R4039, and R5200 were found fully susceptible to vancomycin, whereas R1629 was characterized as hVISA (12). The well-described hVISA isolate Mu3 was also included in the study and was generously provided by K. Hiramatsu, Juntendo University, Japan (7).
Antimicrobials and media.
Ceftaroline (lot CI 170-07) was provided by Forest Laboratories, Inc. (New York, NY), and vancomycin was commercially purchased from Sigma-Aldrich Co. (Saint Louis, MO). Stock solutions were freshly prepared each week according to the manufacturers and were kept frozen at −4°C.
Mueller-Hinton broth (Difco, Detroit, MI), supplemented with 25 μg/ml calcium and 12.5 μg/ml magnesium (SMHB), was utilized for susceptibility testing and in vitro PK/PD models. Tryptic soy agar (TSA; Difco, Detroit, MI) plates were used for growth of isolates and for determination of colony counts in time-kill experiments. Brain heart infusion (BHI) agar (Difco, Detroit, MI) was used for population analysis profile (PAP) experiments and to investigate emergence of resistance.
Susceptibility testing.
The MICs of each agent were determined in duplicate by broth microdilution in SMHB according to Clinical and Laboratory Standards Institute (CLSI) guidelines (2). Five-microliter samples from visually clear wells were plated onto TSA plates and incubated at 35°C for 24 h for the determination of the minimum bactericidal concentration (MBC).
Ceftaroline and vancomycin population analysis.
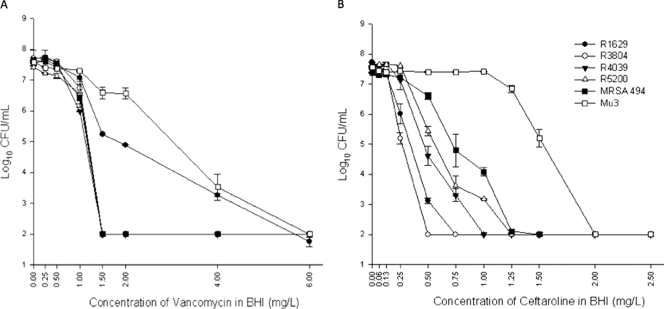
A bacterial suspension of ∼107 CFU/ml (similar to the initial inoculum used in the in vitro PK/PD model) was plated with an automatic spiral plating device (WASP; DW Scientific, West Yorkshire, United Kingdom) onto freshly prepared BHI agar plates containing 0.06 to 2.5 μg/ml of ceftaroline or 0.25 to 6 μg/ml of vancomycin. After 48 h of incubation at 35°C, colony counts were determined using an automated colony counter (Synoptics, Ltd., Frederick, MD). The lower limit of detection for colony counts was 2 log10 CFU/ml. PAP was performed in duplicate to ensure reproducibility, and curves were constructed by plotting mean colony counts (log10 CFU/ml) versus concentration.
In vitro PK/PD model.
Time-kill model experiments were performed over a 72-h period with an initial inoculum of ∼107 CFU/ml, using a hollow fiber model (cartridge C2011; Fibercell System, Inc., Frederick, MD) simulating human PK, as previously described (4). Fresh SMHB medium was continuously supplied and removed from a reservoir chamber via a peristaltic pump (Masterflex; Cole-Parmer Instrument Co., Chicago, IL) set to simulate the half-lives of the antibiotics (average of 2 h for ceftaroline and 6 h for vancomycin). The dosing regimens were 600 mg of ceftaroline every 8 h (q8h) and 12 h (q12h) (free maximum concentration of drug in serum [fCmax] targeted, 15.2 μg/ml; protein binding, 20%) (22), and 1,000 mg of vancomycin every 12 h (targeted fCmax, 18 μg/ml; protein binding, 50%) (16). The pump rates were set at 2.3 and 0.7 ml/min for ceftaroline and vancomycin, respectively. After inoculation of the bacteria into the extracapillary space of the cartridge, antibiotic solution was infused in the reservoir chamber via a dosing port. All experiments were performed at 37°C in duplicate to ensure reproducibility.
PD analysis.
Culture samples (1 ml) were removed from each model at 0, 1, 2, 4, 8, 24, 28, 32, 48, 56, and 72 h. Appropriate dilutions were plated using an automatic spiral plating device to enumerate CFU/ml and avoid antibiotic carryover. Plates were then incubated at 35°C for 24 h, and bacterial counts were determined by an automated colony counter. Time-kill curves were constructed by plotting mean colony counts (log10 CFU/ml) versus time, and bactericidal activity was defined as a ≥3-log10-CFU/ml (99.9%) reduction from the starting inoculum.
PK analysis.
To assess the concentration of the antibiotic, samples (∼1 ml) were removed from an injection port located between the reservoir chamber and the cartridge at 0.5, 1, 2, 4, 8, 24, 28, 32, 48, 56, and 72 h. Samples were stored at −4°C until analysis. Vancomycin concentrations were determined by fluorescence polarization immunoassay (Abbott Diagnostics TDx). The limit of detection for this assay was 2 μg/ml (r2 = 0.99; between-day and intraday coefficient of variation for low, medium, and high standards, ≤2.5%). Ceftaroline concentrations were determined by microbioassay using Bacillus subtilis ATCC 6633 as the indicator organism. Sterile blank 0.25-in. disks (BD, Sparks, MD) were spotted with 10 μl of samples or standards prepared in SMHB. Concentrations of 5, 10, and 20 μg/ml were used as standards. Each standard and sample was tested in duplicate by placing disks on antibiotic assay medium 2 agar plates swabbed with a 0.5 McFarland suspension of the test organism. Zone sizes were measured after 18 to 24 h of incubation at 35°C. This assay was linear over the range tested (r2 = 0.95; between-day and intraday coefficient of variation for low, medium, and high standards, ≤12.8%). Ceftaroline half-life, peak, and trough values were calculated from concentration-versus-time plots. Elimination half-lives, free area under the concentration-time curve between two consecutive dosings at steady state (fAUC), fCmax, and free minimum (trough) concentration of drug in serum (fCmin) were determined by trapezoidal methods utilizing PK Analyst software (Micromath, Salt Lake City, UT).
Emergence of resistance.
To investigate the development of resistance, 100-μl samples from the 24-, 48-, and 72-h time points were plated directly onto TSA plates containing threefold the MIC of ceftaroline or vancomycin. Plates were examined after 48 h of incubation at 35°C. Any growth observed on the antibiotic-containing plates after 48 h of incubation was considered as emergence of resistance, and MIC testing was performed to determine the level of resistance.
Statistical analysis.
Differences in the bacterial densities observed at 24, 48, and 72 h with each dosing regimen were analyzed by t test or analysis of variance with Tukey's post hoc test. All statistical analyses were performed using SPSS statistical software (release 17.0; SPSS, Inc., Chicago, IL). A P value of <0.05 was considered significant.
RESULTS
MIC and MBC results for the tested clinical isolates are summarized in Table 1. Both drugs displayed very similar MIC ranges, and MBCs were found to be equal to or 1 dilution higher than the respective MICs. No changes in ceftaroline MICs and MBCs were observed throughout all the experiments. In contrast, vancomycin exposure in the PK/PD model resulted in an elevation in MIC against both hVISA R1629 and Mu3. MICs increased to 8 μg/ml for both isolates at 24 h and up to 12 μg/ml for Mu3 at 48 h.
TABLE 1.
In vitro susceptibility of tested MRSA determined by the microdilution method or PAP and analysis of observed PK parameters
| Drug and isolate | MIC (μg/ml)b | MBC (μg/ml) | Half-life (h) | fCmax (μg/ml)a | fCmin (μg/ml)a | T99.9% (h)a | fAUC/MIC (h−1)a | % fTb,c |
|
|---|---|---|---|---|---|---|---|---|---|
| >MIC | >2× MIC | ||||||||
| Ceftaroline | 2.1 ± 0.2 | 17.4 ± 0.7 | 1.3 ± 0.3 | ||||||
| R1629 | 0.125 (0.75) | 0.25 | 12 | 100 (100)/100 (67) | 100 (75)/100 (50) | ||||
| R3804 | 0.25 (0.5) | 0.5 | 26.8 | 100 (100)/100 (67) | 100 (94)/83 (62.5) | ||||
| R4039 | 0.5 (1) | 1 | 6.5 | 100 (94)/83 (62.5) | 100 (69)/62.5 (46) | ||||
| R494 | 0.5 (1.2) | 0.5 | 28.3-35 | 100 (85)/83 (57) | 94 (62.5)/62.5 (42) | ||||
| R5200 | 1 (1) | 1 | 6.8 | 94/62.5 | 69/46 | ||||
| Mu3 | 2 (2) | 2 | 5.05-44 | 69/46 | 47/31 | ||||
| Vancomycin | 6.8 ± 1.6 | 18.0 ± 0.2 | 5.2 ± 1.2 | ||||||
| R1629 | 1 (6) | 2 | 57.9 | ||||||
| R3804 | 0.5 | 1 | 231.8 | ||||||
| R4039 | 1 | 1 | 115.9 | ||||||
| R494 | 0.5 | 1 | 231.8 | ||||||
| R5200 | 0.5 | 0.5 | 231.8 | ||||||
| Mu3 | 2 (6) | 2 | 57.9 | ||||||
fCmax, free peak concentration of drug in serum; fCmin, free trough concentration of drug in serum; fAUC, free area under the concentration-time curve; T99.9%, time to achieve bactericidal activity with the q12h regimen.
Parentheses indicate values computed with the minimal concentration required to inhibit growth of the subpopulation determined by PAP at an initial inoculum of 107 CFU/ml.
%fT > MIC, percentage of time the q8h/q12h dosing regimen is above the MIC.
Observed PK parameters were within 15% of the targeted values, similar to those observed in human therapy (16, 22). Maximum concentrations of drug (fCmax) were 17.4 ± 0.7 and 18.0 ± 0.2 μg/ml, and half-lives were 2.1 ± 0.2 and 6.8 ± 1.6 h for ceftaroline and vancomycin, respectively. The main PK parameters for β-lactams and glycopeptides (time of the dosing regimen above the MIC [fT > MIC] and fAUC/MIC ratio) were analyzed and are reported in Table 1. For ceftaroline, fT > MIC and fT > 2× MIC were achieved for 42% to 100% of the dosing interval for all isolates, except the Mu3 isolate, for which values were strongly reduced. For vancomycin, the fAUC/MIC ratio was greater than 125 h−1 for MRSA 494 and R3804 (231.8 ± 43.3 h−1), but <125 h−1 for the two hVISA isolates (57.9 ± 10.8 h−1 for R1629 and Mu3) and the MRSA isolate R4039 (115.9 ± 21.7 h−1).
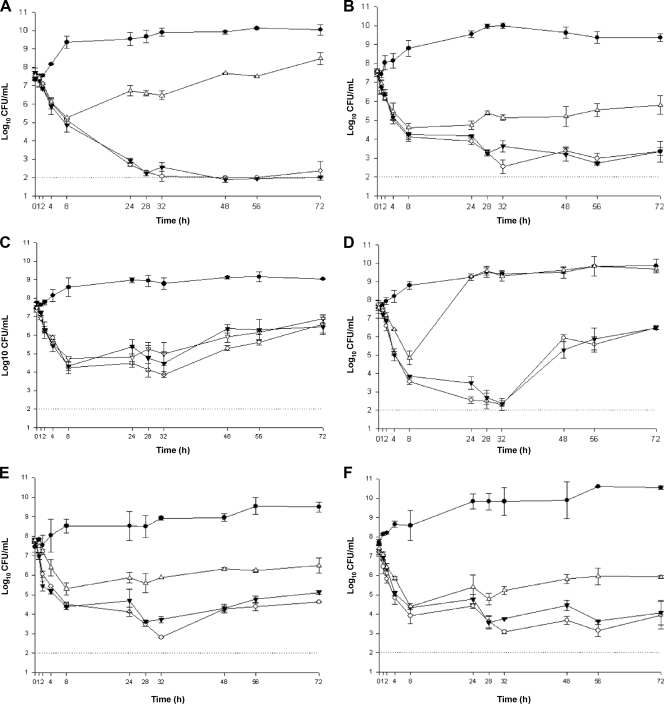
Quantitative changes in log10 CFU/ml over the 72-h time period are graphically displayed in Fig. 1. Comparison of the bacterial densities at 24, 48, and 72 h of drug exposure did not demonstrate any significant difference between the q8h and q12h dosing regimens against any of the tested isolates. Bactericidal activity (i.e., ≥3-log kill) was achieved with ceftaroline at 6.5, 6.8, 26.8, and 12 h for the MRSA isolates R4039, R5200, R3804, and hVISA R1629, respectively. For MRSA 494, bactericidal activity was temporarily achieved between 25.5 and 37.5 h and 28.3 and 35 h with the q8h and q12h dosing regimens, respectively. For Mu3, bactericidal activity was demonstrated with ceftaroline between 5.05 and 44 h with both dosing intervals. Bacterial regrowth observed secondary to ceftaroline exposure against Mu3 and MRSA 494 was not associated with tolerance, instability of the drug, or emergence of resistance (data not shown). In an attempt to explain the results, we investigated the presence of resistant subpopulations in the initial samples. Using PAP performed at an inoculum size similar to that used in the in vitro PK/PD models, two of the six tested isolates (MRSA 494 and Mu3) demonstrated the presence of subpopulations with higher MICs (≥1 μg/ml) to ceftaroline (Fig. 2). The standard vancomycin regimen (1,000 mg q12h) did not lead to a bactericidal effect against any of the six tested isolates. Ceftaroline dosed at 600 mg q8h or q12h demonstrated a better killing effect than a standard dose of vancomycin against the two hVISA (R1629 and Mu3) and three MRSA (R3804, R4039, and R5200) isolates (P < 0.05). Against one isolate, MRSA 494, no significant difference in killing between the two agents was observed (Fig. 1).
FIG. 1.
In vitro killing activity of ceftaroline at 600 mg q8h (open circle) and q12h (filled inverted triangles) compared with that of vancomycin at 1,000 mg q12h (open triangles) in the two-compartment in vitro PK/PD model against hVISA R1629 (A), MRSA R3804 (B), MRSA 494 (C), hVISA Mu3 (D), MRSA R4039 (E), and MRSA R5200 (F). Growth control curves (filled circles) demonstrate viability of the organisms without antibiotic treatment over the 72-h period, and in each panel, the dotted line represents the limit of detection (2 log10 CFU/ml).
FIG. 2.
PAP for the six tested isolates against vancomycin (A) and ceftaroline (B) using an inoculum size of 107 to 108 CFU/ml.
DISCUSSION
Six isolates with different susceptibility profiles to vancomycin and ceftaroline were exposed to both agents over 72 h using a two-compartment in vitro PK/PD model mimicking human PK. No difference was observed between the q8h and q12h regimens of ceftaroline, despite a relatively short half-life (between 2 and 2.5 h) and a time-dependent activity. Our results appeared therefore favorable to a q12h interval, which is in agreement with clinical trials (22). Also consistent with previous in vitro and in vivo investigations, ceftaroline appeared superior to vancomycin against three MRSA and two hVISA strains (P < 0.05), achieving sustained bactericidal activity against the majority of these isolates (9, 18, 28). In contrast to vancomycin, for which both hVISA isolates developed intermediate resistance with significant increases in MIC, none of the tested isolates developed resistance to ceftaroline. This result represents an important property of ceftaroline, since antimicrobial resistance represents a current and serious concern (9, 21). Against one MRSA isolate (MRSA 494), ceftaroline was bactericidal (unlike vancomycin), but this activity was transient. Although unclear at present, the presence of subpopulations with higher MICs might explain these results. Target attainment studies performed with cephalosporins reported that bacteriostatic and bactericidal effects are achieved for staphylococci when free drug concentrations exceed the MIC for 30% or 50% of the dosing interval, respectively (14). In this study, % fT > MIC correlating with bactericidal effect was achieved for all isolates, except MRSA 494 and Mu3, for which % fT > MICs were lowest when considering the subpopulation with the highest MIC. This partial explanation to the bacterial regrowth is also emphasized by the fact that we did not observe this effect against isolates with homogeneous populations. The use of the standard inoculum of 107 CFU/ml may be, however, a limitation of this study, since it may not be optimal to investigate potential selection of resistant populations. Further experiments using higher inocula are warranted to evaluate the impact of the subpopulations on the in vitro activity of ceftaroline against MRSA.
Treatment of infections caused by MRSA, and especially those exhibiting heterogeneous resistance to vancomycin, represents one of the most important challenges for clinicians (13). At this time, daptomycin and linezolid are among the last available options to treat hVISA and VISA infections, but despite their restricted use, resistance has already emerged (6, 17, 30). Progress toward development of new anti-MRSA agents includes a new class of cephalosporins that exhibit high affinity for PBP2a, the determinant of β-lactam resistance in this pathogen (19). Ceftaroline has recently demonstrated encouraging results in phase III trials for the treatment of cSSSI (3, 19). In the present in vitro study, ceftaroline demonstrated higher activity than a standard regimen of vancomycin against five out of six clinical MRSA and hVISA isolates. In addition to its low potential for selecting resistant mutants, ceftaroline represents therefore a promising option for treatment of serious infections caused by MDR S. aureus strains, including those with reduced susceptibility to glycopeptides.
Acknowledgments
This study was funded by a research grant from Forest Laboratories. Scientific Therapeutics Information, Inc. (Springfield, NJ), provided editorial assistance on this manuscript. Funding for editorial assistance was provided by Forest Laboratories, Inc.
Footnotes
Published ahead of print on 8 September 2009.
REFERENCES
- 1.Allen, G. P., G. W. Kaatz, and M. J. Rybak. 2004. In vitro activities of mutant prevention concentration-targeted concentrations of fluoroquinolones against Staphylococcus aureus in a pharmacodynamic model. Int. J. Antimicrob. Agents 24:150-160. [DOI] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2008. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 8th ed. CLSI, Wayne, PA.
- 3.Corey, G. R., M. Wilcox, G. H. Talbot, T. Baculik, and D. Thye. 2008. CANVAS-1: randomized, double-blinded, phase 3 study (P903-06) of the efficacy and safety of ceftaroline vs. vancomycin plus aztreonam in complicated skin and skin structure infections (cSSSI), abstr. L-1515a, p. 321. Final Program Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 4.Drusano, G. L., W. Liu, D. L. Brown, L. B. Rice, and A. Louie. 2009. Impact of short-course quinolone therapy on susceptible and resistant populations of Staphylococcus aureus. J. Infect. Dis. 199:219-226. [DOI] [PubMed] [Google Scholar]
- 5.Ge, Y., D. Biek, G. H. Talbot, and D. F. Sahm. 2008. In vitro profiling of ceftaroline against a collection of recent bacterial clinical isolates from across the United States. Antimicrob. Agents Chemother. 52:3398-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hayden, M. K., K. Rezai, R. A. Hayes, K. Lolans, J. P. Quinn, and R. A. Weinstein. 2005. Development of daptomycin resistance in vivo in methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 43:5285-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 8.Iizawa, Y., J. Nagai, T. Ishikawa, S. Hashiguchi, M. Nakao, A. Miyake, and K. Okonogi. 2004. In vitro antimicrobial activity of T-91825, a novel anti-MRSA cephalosporin, and in vivo anti-MRSA activity of its prodrug, TAK-599. J. Infect. Chemother. 10:146-156. [DOI] [PubMed] [Google Scholar]
- 9.Jacqueline, C., J. Caillon, V. Le Mabecque, A. F. Miègeville, A. Hamel, D. Bugnon, J. Y. Ge, and G. Potel. 2007. In vivo efficacy of ceftaroline (PPI-0903), a new broad-spectrum cephalosporin, compared with linezolid and vancomycin against methicillin-resistant and vancomycin-intermediate Staphylococcus aureus in a rabbit endocarditis model. Antimicrob. Agents Chemother. 51:3397-3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones, R. N. 2008. Key considerations in the treatment of complicated staphylococcal infections. Clin. Microbiol. Infect. 14(Suppl. 2):3-9. [DOI] [PubMed] [Google Scholar]
- 11.Kluytmans, J., and M. Struelens. 2009. Meticillin resistant Staphylococcus aureus in the hospital. BMJ 338:b364. [DOI] [PubMed] [Google Scholar]
- 12.Leonard, S. N., C. Vidaillac, and M. J. Rybak. 2009. Activity of telavancin against Staphylococcus aureus strains with various vancomycin susceptibilities in an in vitro pharmacokinetic/pharmacodynamic model with simulated endocardial vegetations. Antimicrob. Agents Chemother. 53:2928-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levine, D. P. 2008. Vancomycin: understanding its past and preserving its future. South. Med. J. 101:284-291. [DOI] [PubMed] [Google Scholar]
- 14.Lodise, T. P., Jr., R. Pypstra, J. B. Kahn, B. P. Murthy, H. C. Kimko, K. Bush, G. J. Noel, and G. L. Drusano. 2007. Probability of target attainment for ceftobiprole as derived from a population pharmacokinetic analysis of 150 subjects. Antimicrob. Agents Chemother. 51:2378-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maor, Y., M. Hagin, N. Belausov, N. Keller, D. Ben-David, and G. Rahav. 2009. Clinical features of heteroresistant vancomycin-intermediate Staphylococcus aureus bacteremia versus those of methicillin-resistant S. aureus bacteremia. J. Infect. Dis. 199:619-624. [DOI] [PubMed] [Google Scholar]
- 16.Matzke, G. R., G. G. Zhanel, and D. R. Guay. 1986. Clinical pharmacokinetics of vancomycin. Clin. Pharmacokinet. 11:257-282. [DOI] [PubMed] [Google Scholar]
- 17.Mendes, R. E., L. M. Deshpande, M. Castanheira, J. DiPersio, M. A. Saubolle, and R. N. Jones. 2008. First report of cfr-mediated resistance to linezolid in human staphylococcal clinical isolates recovered in the United States. Antimicrob. Agents Chemother. 52:2244-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mushtaq, S., M. Warner, Y. Ge, K. Kaniga, and D. M. Livermore. 2007. In vitro activity of ceftaroline (PPI-0903M, T-91825) against bacteria with defined resistance mechanisms and phenotypes. J. Antimicrob. Chemother. 60:300-311. [DOI] [PubMed] [Google Scholar]
- 19.Page, M. G. 2006. Anti-MRSA beta-lactams in development. Curr. Opin. Pharmacol. 6:480-485. [DOI] [PubMed] [Google Scholar]
- 20.Parish, D., and N. Scheinfeld. 2008. Ceftaroline fosamil, a cephalosporin derivative for the potential treatment of MRSA infection. Curr. Opin. Investig. Drugs 9:201-209. [PubMed] [Google Scholar]
- 21.Ratnaraja, N. V., and P. M. Hawkey. 2008. Current challenges in treating MRSA: what are the options? Expert Rev. Anti Infect. Ther. 6:601-618. [DOI] [PubMed] [Google Scholar]
- 22.Riccobene, T., E. Fang, and D. Thye. 2008. A single- and multiple-dose study to determine the safety, tolerability, and pharmacokinetics (PK) of ceftaroline (CPT) administered by intramuscular (IM) injection to healthy subjects, abstr. A-1888, p. 34. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 23.Rybak, M. J. 2004. Resistance to antimicrobial agents: an update. Pharmacotherapy 24:203S-215S. [DOI] [PubMed] [Google Scholar]
- 24.Sadaba, B., J. R. Azanza, M. A. Campanero, and E. Garcia-Quetglas. 2004. Relationship between pharmacokinetics and pharmacodynamics of beta-lactams and outcome. Clin. Microbiol. Infect. 10:990-998. [DOI] [PubMed] [Google Scholar]
- 25.Stapleton, P. D., and P. W. Taylor. 2002. Methicillin resistance in Staphylococcus aureus: mechanisms and modulation. Sci. Prog. 85:57-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talbot, G. H., D. Thye, A. Das, and Y. Ge. 2007. Phase 2 study of ceftaroline versus standard therapy in treatment of complicated skin and skin structure infections. Antimicrob. Agents Chemother. 51:3612-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turnidge, J. D. 1998. The pharmacodynamics of beta-lactams. Clin. Infect. Dis. 27:10-22. [DOI] [PubMed] [Google Scholar]
- 28.Vidaillac, C., S. N. Leonard, and M. J. Rybak. 2008. In vitro activity and aminoglycoside synergy of ceftaroline against clinical isolates of hospital-acquired (HA) methicillin-resistant Staphylococcus aureus (MRSA), abstr. C1-3719, p. 134. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 29.Vidaillac, C., S. N. Leonard, and M. J. Rybak. 2008. In vitro activity of ceftaroline (CPT) vs. vancomycin (VM) against MRSA and hVISA strains in a pharmacokinetic/pharmacodynamic (PK/PD) model, abstr. A-979, p. 21. Abstr. 48th Annu. Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC.
- 30.Wilson, P., J. A. Andrews, R. Charlesworth, R. Walesby, M. Singer, D. J. Farrell, and M. Robbins. 2003. Linezolid resistance in clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 51:186-188. [DOI] [PubMed] [Google Scholar]
- 31.Wootton, M., R. A. Howe, R. Hillman, T. R. Walsh, P. M. Bennett, and A. P. MacGowan. 2001. A modified population analysis profile (PAP) method to detect hetero-resistance to vancomycin in Staphylococcus aureus in a UK hospital. J. Antimicrob. Chemother. 47:399-403. [DOI] [PubMed] [Google Scholar]