Abstract
Maintenance of the effective local concentration of antimicrobials on the tooth surface is critical for the management of cariogenic bacteria in the oral cavity. We report on the design of a simple tooth-binding micellar drug delivery platform that would effectively bind to tooth surfaces. To achieve tooth-binding ability, the chain termini of biocompatible Pluronic copolymers were modified with a biomineral-binding moiety (i.e., alendronate). The micelles formulated with this polymer were shown to be able to swiftly (<1 min) bind to hydroxyapatite (HA; a model tooth surface) and gradually release the encapsulated model antimicrobial (farnesol). These tooth-binding micelles were negatively charged and had an average effective hydrodynamic diameter of less than 100 nm. In vitro biofilm inhibition studies demonstrated that the farnesol-containing tooth-binding micelles were able to provide significantly stronger inhibition of Streptococcus mutans UA159 biofilm formation on HA discs than the untreated blank control micelles (P < 0.0001). Upon further optimization, this delivery platform could provide an effective tool for caries prevention and treatment.
Dental caries is defined as the localized destruction of susceptible dental hard tissues by acidic by-products from the bacterial fermentation of dietary carbohydrates (25). Overpopulation of the oral cavity by acid-producing bacteria is one of the three main pathological factors highlighted in the cariogenic process (6). Therefore, an important strategy that may be used to control or even eradicate dental caries is to target the bacterial aspect of the disease (5). Successful therapy against cariogenic bacteria largely depends on two major factors at the hard-tissue level: the specificity of the chemotherapeutic agent and the maintenance of its effective local concentration. Given that most of the available antimicrobial compounds do not have specificity for hard tissue (19), it is critical to develop mechanisms that retain these antimicrobials at the tooth surface, thereby maintaining their effective local concentration and improving their antimicrobial efficacy (4, 16).
Various delivery systems have been developed to maintain drug concentrations in the oral cavity. These include bioadhesive tablets (1, 7, 18), bioadhesive patches/films (22, 26), and bioadhesive gels and semisolids (10, 24, 29). However, these formulations provide the highest drug concentration at the mucosal epithelia instead of the tooth surface. Furthermore, local irritation at the site of adhesion and the uncomfortable sensation of a foreign object often lead to poor patient compliance (20, 27). To achieve a direct and long-lasting interaction of antimicrobials with the tooth surface, varnish formulations have also been developed (17, 28) and are generally applied by dental health practitioners during routine office visits. However, the long-term benefit of these periodic treatments is limited due to the episodic nature of dental caries.
We report here on the design of a novel tooth-binding micellar delivery platform that would effectively maintain the drug concentration on the tooth surface. By covalently attaching a prototype biomineral-binding moiety (alendronate [ALN]) to the chain termini of biocompatible Pluronic copolymers (11), these micelles are predicted to bind to hydroxyapatite (HA; the model tooth surface used in these studies) immediately upon exposure. The immobilized micelles should be able to act as a drug reservoir and gradually release the encapsulated antimicrobial. Farnesol (3,7,11- trimethyl-2,6,10-dodecatrien-1-ol), a recently identified anticaries natural product found in propolis with a MIC of 125 μM (28 μg/ml) for Streptococcus mutans UA159 (12-14), was chosen as the model drug for encapsulation in micelles. In the study presented here, it was found that the micelle-farnesol formulation is capable of providing the nearly complete inhibition of biofilm formation by the cariogenic bacterium S. mutans. It is anticipated that the tooth-binding micelles have the potential to be formulated into mouth rinses that may have the merits of simple application, cultural acceptance, and improved patient compliance.
MATERIALS AND METHODS
Chemicals.
Pentynoic acid 2,5-dioxo-pyrrolidin-1-yl ester (compound 1) and 1-hydroxy-4-pent-4-ynamidobutane-1,1-diyldiphosphonic acid (compound 2) were synthesized as described previously (15). ALN was purchased from Ultratech India Ltd. (New Mumbai, India). Farnesol was obtained from TCI America (Portland, OR). HA particles (DNA-grade, Bio-Gel HTP gel) were purchased from Bio-Rad (Hercules, CA). HA discs (13 mm in diameter by 1 to 1.5 mm thick) were purchased from Clarkson Chromatography Products, Inc. (South Williamsport, PA). LH-20 resin was purchased from GE Healthcare (Piscataway, NJ). Pluronic copolymers (P85 and P123) were generously provided by BASF Corporation (Florham Park, NJ). All other reagents and solvents were purchased from either Fisher Scientific (Pittsburgh, PA) or Acros Organics (Morris Plains, NJ), unless specified otherwise.
Methods.
1H nuclear magnetic resonance (NMR) spectra were recorded on a Varian Inova Unity 500 NMR spectrometer. UV-visible spectra were measured on a Shimadzu UV-1601PC UV-visible spectrophotometer. Electrophoretic mobility measurements were performed with a ZetaPlus analyzer (Brookhaven Instrument Co.). The zeta potential of the micelles was calculated from the electrophoretic mobility values by using the Smoluchowski equation. The effective hydrodynamic diameter (Deff) of the micelles was measured by photon correlation spectroscopy (DLS) with a ZetaPlus analyzer equipped with a multiangle sizing option (BI-MAS). An Agilent 1100 high-pressure liquid chromatography (HPLC) system with a quaternary pump (with degasser), an autosampler, a fluorescence detector, and a diode array-based UV detector was used for drug release analysis.
Synthesis of p-toluenesulfonyl-terminated P123 (Tos-P123; compound 3).
P123 (10.5 g, 2 mmol) was dried by azeotropic evaporation with toluene (three times with 50 ml each time) and dissolved under argon in anhydrous dichloromethane (20 ml) together with 4-dimethylaminopyridine (0.122 g, 1 mmol) and triethylamine (2.02 g, 20 mmol). The reaction mixture was cooled to 0°C, and p-toluenesulfonyl chloride (Tos; 3.81 g, 20 mmol) was added. After overnight reaction at room temperature, the mixture was washed with hydrochloric acid (0.1 M, two times with 10 ml each time), water (two times with 10 ml each time), and saturated NaCl solution (brine, two times with 10 ml each time) and was then dried over anhydrous magnesium sulfide. After removal of the solvent under reduced pressure, the crude product was further purified by a LH-20 column. The yield of polymer was 60% (yield = [weight of the purified product/theoretical weight of the product] × 100). The ratio of tosylation was determined to be 100% by 1H NMR. 1H NMR (dimethyl sulfoxide [DMSO]-d6) δ (ppm) 7.79 (d, J = 8.29 Hz), 7.48 (d, J = 8.29 Hz), 4.11 (t, J = 4.88 Hz), 3.65 to 3.43 (m), 1.04 (d, J = 4.39 Hz).
Synthesis of azide-terminated P123 (Azido-P123; compound 4).
Tos-P123 (1.64 g, 0.27 mmol) was dissolved in dimethylformamide (20 ml). Sodium azide (0.176 g, 2.7 mmol) was then added. The reaction proceeded with stirring at 100°C for 1 day. After filtration and solvent removal, the crude product was dissolved in dichloromethane, washed with water (two times with 10 ml each time) and brine (two times with 10 ml each time), and then dried over anhydrous magnesium sulfide. After removal of the solvent under vacuum, the product was obtained. The yield of polymer was 96.2%. Complete azidation was supported by the disappearance of the Tos signals in the 1H NMR spectrum. 1H NMR (DMSO-d6) δ (ppm) 3.61 (t, J = 4.88 Hz), 3.56 to 3.43 (m), 1.04 (d, J = 4.39 Hz).
Synthesis of ALN-P123 conjugate (compound 5).
Azido-P123 (2.9 g, 0.5 mmol) and 1-hydroxy-4-pent-4-ynamidobutane-1,1-diyldiphosphonic acid (0.395 g, 1 mmol) were dissolved in an ethanol-H2O solution (1/1, 15 ml). Sodium ascorbate (0.198 g, 1 mmol) and copper sulfide pentahydrate (25 mg, 0.1 mmol) were then added separately under argon. The reaction mixture was allowed to stir for 3 days at room temperature. After removal of the solvent, the product was acidified and purified with a column with LH-20 resin and with methanol as the eluent. The yield of the polymeric product was 70%. The conversion ratio from azide to ALN at the P123 chain termini was estimated by 1H NMR to be 90%. See Fig. 1 for the entire synthetic route. 1H NMR (D2O) δ (ppm) 7.81 (s), 3.93 (t, J = 4.90 Hz), 3.80 to 3.39 (m), 3.14 (t, J = 6.83 Hz), 1.86 (m), 1.75 (m), 1.12 (d, J = 7.81 Hz).
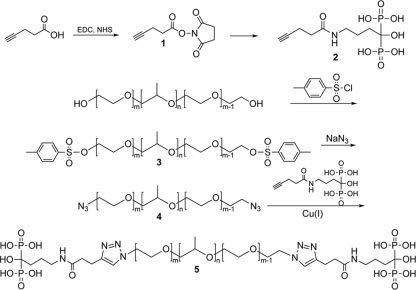
FIG. 1.
Synthesis of ALN-P123.
Preparation and characterization of tooth-binding micelle.
Different amounts of farnesol were added to aqueous Pluronic solution. The mixture was subjected to vortex mixing for 30 s and placed at 37°C overnight with gentle agitation to allow micelle formation. The resulting micelle solutions were filtered (filter pore size, 0.45 μm) and then subjected to measurement of the zeta potential and Deff by DLS. See Table 1 for the details for all micelle formulations.
TABLE 1.
Compositions and characterization of different micelle preparations
| Preparation | Pluronic P85 (%)a | ALN-P123 (%)a | Farnesol (%)a | Particle size (nm) | Polydispersity index | Zeta potential (mV) |
|---|---|---|---|---|---|---|
| 1 | 1.6 | 0.4 | 0 | 99.5 ± 5.8 | 0.29 | −27.0 |
| 2 | 1.6 | 0.4 | 0.4 | 45.1 ± 0.7 | 0.30 | −27.06 |
| 3 | 1.6 | 0.4 | 0.7 | 53.0 ± 0.6 | 0.26 | −26.1 |
| 4 | 1.6 | 0.4 | 1.0 | 68.2 ± 1.5 | 0.26 | −26.0 |
| 5 | 2.0 | 0 | 1.0 | 100.5 ± 3.1 | 0.29 | −0.37 |
| 6 | 1.95 | 0.05 | 0.4 | 95.4 ± 2.8 | 0.30 | −2.1 |
| 7 | 1.9 | 0.1 | 0.4 | 95.5 ± 1.6 | 0.31 | −8.53 |
| 8 | 1.8 | 0.2 | 0.4 | 83.7 ± 2.6 | 0.28 | −10.25 |
All percentages are presented in weight/volume.
Binding kinetics of tooth-binding micelle on HA particles.
Micelle solutions (preparations 2, 6, 7, and 8 in Table 1) were mixed with HA particles (20 mg/tube) in Eppendorf centrifuge tubes. The tubes were placed on a Labquake rotator (Thermo Fisher Scientific Inc.) to allow binding at room temperature. At each predetermined time point, three tubes were taken out and centrifuged (12,000 rpm, 0.5 min) and 100 μl of the supernatant was collected. The collected samples were then diluted 100 times and analyzed by HPLC under the following conditions: an Agilent C18 reverse-phase column (4.6 by 250 mm; particle size, 5 μm) with a mobile phase of acetonitrile-water (80:20, vol/vol) at a flow rate of 1 ml/min was used. The UV detection was set at 210 nm. The amount of farnesol that bound to HA particles via the micellar formulation was calculated by subtracting the amount of farnesol left in the supernatant from the initial amount of farnesol added.
In vitro release of farnesol from tooth-binding micelle immobilized on HA particles.
Micelle solutions (preparations 2, 3, and 4 in Table 1) were mixed with HA particles (100 mg) for 30 min to allow binding of the micelles to HA. The mixture was then centrifuged and the HA particles were washed with water three times to remove unbound micelles. The total amount of farnesol loaded on HA particles was calculated by subtracting the amount of unbound farnesol from the amount of farnesol added. The micelle-loaded HA particles were then resuspended in 1 ml of releasing medium (0.1 M phosphate-buffered saline, pH 7.4) and placed on a Labquake rotator to allow drug release at 37°C. At predetermined time intervals, samples were centrifuged; and all the supernatant was removed, replaced with 1 ml of fresh medium, and then resuspended. The collected supernatant (0.5 ml) was mixed with acetonitrile (0.5 ml), filtered (filter pore size, 0.2 μm), and analyzed by HPLC.
In vitro inhibition of S. mutans biofilm growth on HA discs.
S. mutans UA159 (21) was used in this study. S. mutans frozen stock cultures were maintained in 25% (vol/vol) glycerol at −80°C. For each experiment, S. mutans was streaked from a frozen stock onto Todd-Hewitt-yeast extract (THYE agar; Todd-Hewitt broth containing 0.3% [wt/vol] yeast extract) agar (1.5%, wt/vol). After 48 h of growth at 37°C under a 5% CO2 atmosphere, a single colony of bacteria was inoculated into 3 ml of THYE broth (2) and allowed to grow statically overnight at 37°C with 5% CO2. On the next day, the overnight culture was diluted to a density of 2 × 104 CFU/ml in chemically defined medium containing 0.25% (wt/vol) glucose and sucrose (CDM), prepared as described previously (2).
Autoclaved HA discs were incubated with different micelle solutions (preparations 2, 3, 4, and 5 in Table 1), farnesol solution (1% [wt/vol] in ethanol), or CDM in a 24-well plate for 1 h. The discs were then removed from the wells and dip washed with CDM three times to remove unbound micelles. For the farnesol-ethanol solution group, the discs were dip washed three times with ethanol and then washed with CDM. The HA discs were then transferred to wells containing 1 ml of the diluted S. mutans culture and cultured statically for 48 h to allow biofilm growth at 37°C with 5% CO2. The CDM was changed at 24 h.
After 48 h of growth, the HA discs were dip washed three times in THYE medium to remove loosely attached bacteria and then placed in 1 ml of THYE medium. The surfaces of the HA discs were gently scraped with a sterile spatula to harvest the adherent cells, and the number of viable cells in each sample was quantified by the track dilution method (9) to achieve countable CFU. All plates were incubated for 48 h at 37°C with 5% CO2 prior to counting of the colonies. These experiments were repeated three times.
Statistical analysis.
The Kruskal-Wallis test, a nonparametric alternative to one-way analysis of variance that does not assume that data follow a normal distribution, was used for overall comparison of the mean ranks of the numbers of CFU under different types of treatments. To evaluate specific differences between experimental groups, Tukey's nonparametric multiple-comparison method was used; P values of <0.05 were considered significant in both tests.
RESULTS
Preparation and characterization of the tooth-binding micelle.
The multistep synthesis of ALN-P123 is critical for the success of tooth-binding micelles. Each reaction step was accomplished with a reasonable yield of polymer of at least 60%. For modification of the P123 chain termini, both the tosylation and the azidation steps were completed with 100% conversion. For the last step of conjugation to ALN with click chemistry, the conversion is 90%, or an average of 1.8 ALN molecules per P123 chain (Fig. 1). DLS analyses (Table 1) suggest that both empty micelles and farnesol-loaded nonbinding micelles had the largest particle size (∼100 nm). Farnesol-loaded tooth-binding micelles had a relatively smaller size, which increased as the farnesol load was raised. Over the range of loads tested, however, the Deff of farnesol-loaded tooth-binding micelles was <100 nm.
Binding kinetics of tooth-binding micelle to HA particles.
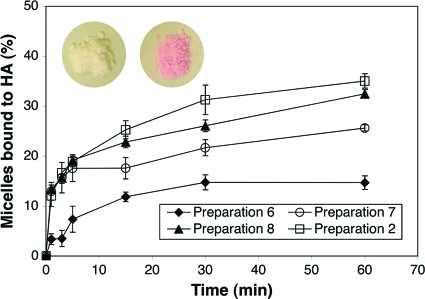
As shown in Fig. 2, the amount of micelle bound to HA particles was increased by raising the ALN-P123 content. However, the HA-binding kinetics did not change significantly after the proportion of ALN-P123 was increased to above 0.1% (wt/vol) (preparation 7). Preparations 7, 8, and 2 (containing 0.1% [wt/vol], 0.2% [wt/vol], and 0.4% [wt/vol] ALN-P123, respectively) bound to the HA particles quickly (<1 min) and reached a binding plateau within 5 min.
FIG. 2.
In vitro binding kinetic study of the tooth-binding micelle formulations (listed in Table 1) to the HA surface. All data are means ± standard deviations (n = 3). (Color inset) HA particles treated with nonbinding micelles (left) and tooth-binding micelles (right). Both micelles were labeled with rhodamine B (a pink dye).
In vitro release of tooth-binding micelles.
The in vitro release profile of farnesol from the tooth-binding micelles that bound to HA particles was evaluated over a 48-h period. About 10 to 20% of the farnesol was released into the medium by 48 h. Sustained drug release profiles were observed (Fig. 3), regardless of the amount of farnesol loaded in the micelle formulation.
FIG. 3.
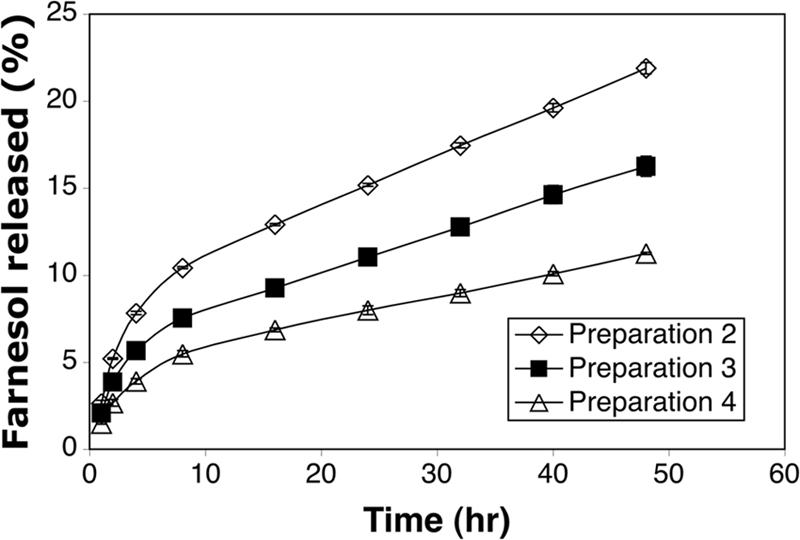
In vitro release of farnesol from tooth-binding micelle formulations (listed in Table 1) bound to HA particles. All data are expressed as means ± standard deviations (n = 3).
Inhibition of S. mutans biofilm growth on HA discs.
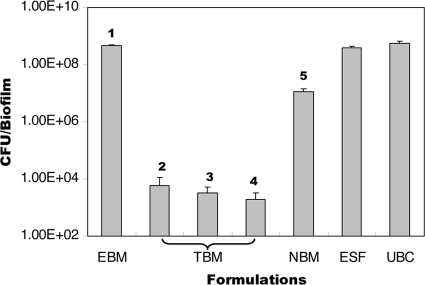
As shown in Fig. 4, all farnesol-containing tooth-binding micelle groups (containing low, medium, and high doses of farnesol) showed significantly (P < 0.0002) higher levels of biofilm inhibition than the untreated blank control, empty tooth-binding micelles, farnesol-containing nonbinding micelles (containing the same amount of farnesol as the high-dose tooth-binding micelles), and an ethanol solution of farnesol (containing the same amount of farnesol as the high-dose tooth-binding micelles). No significant differences were observed between the three groups treated with the farnesol-containing tooth-binding micelles (low, medium, and high doses), as each displayed an approximately 4-log decrease in the numbers of CFU per biofilm recovered from the HA discs. A rather weak but significant (P < 0.006) inhibition of the biofilm was also observed when the HA discs were treated with nonbinding farnesol-containing micelles relative to the effects observed with the untreated blank control, the empty tooth-binding micelle, and the ethanol solution of farnesol groups. This may have been due to a nonspecific interaction of the micelle with the HA disc and the subsequent release of residual farnesol.
FIG. 4.
Average number of CFU of S. mutans recovered per HA disc after 48 h of incubation. HA discs were pretreated with the following formulations: tooth-binding micelle (TBM; preparations 2, 3, and 4, corresponding to low, medium, and high doses of farnesol, respectively); empty tooth-binding micelle (EBM); nonbinding micelle (NBM; containing the same amount of farnesol as the high-dose tooth-binding micelle); ethanol solution of farnesol (ESF; containing same amount of farnesol as the high-dose tooth-binding micelle); and an untreated blank control (UBC). The boldface numbers refer to the preparation numbers listed in Table 1. P < 0.0001 (Kruskal-Wallis test). All data are expressed as means ± standard deviations (n = 3).
DISCUSSION
The purpose of this study was to develop a simple yet efficient drug delivery system that would maintain a long-lasting effective concentration of drug on the tooth surface. Such an approach, in combination with potent antimicrobials, may effectively prevent biofilm formation on the tooth surface by cariogenic bacteria such as S. mutans and the subsequent development of dental caries. The important and novel element of this delivery system is the introduction of a tooth-binding moiety that would anchor the delivery system of choice onto the tooth surface.
ALN, a bisphosphonate, was chosen as a prototype binding moiety because of its high affinity for HA crystals (the main component of tooth enamel) and the fact that it has been used clinically for the treatment of osteoporosis for many years (23). However, a potential risk of ALN usage in an oral rinse formulation would be the systemic ingestion of the drug. Concerns have been raised about the long-term impact of bisphosphonates on bone resorptive activity and its rare association with osteonecrosis of the jaw (3). These risks would likely be minimal with a mouth rinse formulation that used the micellar delivery system described here due to (i) the chemical conjugation of ALN to the chain termini of Pluronic and (ii) the expectoration of the vast majority of the formulation, which would result in a very low systemic dose. Biodegradable biomineral-binding moieties (e.g., acidic peptides) have been used extensively in the development of bone-targeting delivery systems (30), and they could be used as alternative tooth-binding moieties if long-term toxicity is found to be a concern with ALN-functionalized Pluronic.
Farnesol is a hydrophobic compound with a water solubility of merely 1.2 mM (8). In previous investigations, organic solvents (ethanol and DMSO) were required to assist with the dissolution of farnesol in water (12-14). Using the Pluronic block copolymers, which can readily self-assemble into micelles in aqueous solutions with a core (hydrophobic)-shell (hydrophilic) structure, farnesol can be encapsulated into the hydrophobic core of the Pluronic micelle [poly(propylene oxide) segment], which acts as the hosting reservoir that dissolves and readily disperses the hydrophobic farnesol in water. Therefore, the benefits of this formulation may also include the prevention of irritation caused by organic solvents and potentially improved patient compliance.
The binding kinetics of the tooth-binding micelles, as demonstrated in Fig. 2, indicates that even with a relatively low content of the binding moiety, these micelles exhibit swift kinetics of binding to HA particles. Additional studies will be performed in the future to clarify the impacts of other in vivo factors (e.g., the salivary pellicle) on the binding kinetics of these micelles. The small size (∼100 nm) of the micelles developed in this study may also facilitate their ability to access locations on and between teeth that normal toothbrushing misses, and the micelles are unlikely to cause the uncomfortable sensation of a foreign object in patients. As shown in Fig. 3, the newly designed delivery system has the potential to provide a sustained drug-releasing profile and maintain a long-lasting effective drug concentration on the tooth surface. It is important to keep in mind, however, that the release of drug in the oral cavity may become faster due to the presence of physiological factors, such as saliva flow.
One of the biggest challenges in dental antimicrobial therapy lies in the fact that most antimicrobials have a low level of retention, if any, in the oral cavity (especially the tooth surface). As a consequence, the level of cariogenic bacteria can readily be reestablished between each antimicrobial exposure. As demonstrated in Fig. 4, neither the ethanol solution of farnesol nor the farnesol-loaded nonbinding micelle could very effectively prevent S. mutans biofilm formation due to the lack of farnesol retention on the HA disc surface. On the other hand, because of their ability to bind to the HA surface, tooth-binding micelles at all dosing levels demonstrated nearly complete biofilm inhibition, even after extensive washing. Although only one strain of S. mutans was tested in this proof-of-principle study, future testing of the inhibitory effect of these tooth-binding micelles will extend to clinical strains of S. mutans and other cariogenic bacteria.
In summary, a tooth-binding micelle delivery platform for the prevention and treatment of dental caries has been designed and prepared in this study. It was found to bind to the HA surface swiftly and release the encapsulated antimicrobial (farnesol) in a sustained manner. HA discs treated with this formulation could effectively inhibit in vitro biofilm formation by cariogenic S. mutans strains. Further optimization and exploration are needed to translate this novel delivery platform into clinical application for the prevention and treatment dental caries.
Acknowledgments
This work was supported in part by NIH grants AR053325 (to D.W.) and AI038901 (to K.W.B.).
We also acknowledge James Booth for the helpful discussion at the beginning of the project.
Footnotes
Published ahead of print on 24 August 2009.
REFERENCES
- 1.Ali, J., R. Khar, A. Ahuja, and R. Kalra. 2002. Buccoadhesive erodible disk for treatment of oro-dental infections: design and characterisation. Int. J. Pharm. 238:93-103. [DOI] [PubMed] [Google Scholar]
- 2.Biswas, I., L. Drake, and S. Biswas. 2007. Regulation of gbpC expression in Streptococcus mutans. J. Bacteriol. 189:6521-6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards, B. J., J. W. Hellstein, P. L. Jacobsen, S. Kaltman, A. Mariotti, and C. A. Migliorati. 2008. Updated recommendations for managing the care of patients receiving oral bisphosphonate therapy: an advisory statement from the American Dental Association Council on Scientific Affairs. J. Am. Dent. Assoc. 139:1674-1677. [DOI] [PubMed] [Google Scholar]
- 4.Featherstone, J. D. 2006. Delivery challenges for fluoride, chlorhexidine and xylitol. BMC Oral Health 6(Suppl. 1):S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Featherstone, J. D. 2000. The science and practice of caries prevention. J. Am. Dent. Assoc. 131:887-899. [DOI] [PubMed] [Google Scholar]
- 6.Featherstone, J. D., S. M. Adair, M. H. Anderson, R. J. Berkowitz, W. F. Bird, J. J. Crall, P. K. Den Besten, K. J. Donly, P. Glassman, P. Milgrom, J. R. Roth, R. Snow, and R. E. Stewart. 2003. Caries management by risk assessment: consensus statement, April 2002. J. Calif. Dent. Assoc. 31:257-269. [PubMed] [Google Scholar]
- 7.Giunchedi, P., C. Juliano, E. Gavini, M. Cossu, and M. Sorrenti. 2002. Formulation and in vivo evaluation of chlorhexidine buccal tablets prepared using drug-loaded chitosan microspheres. Eur. J. Pharm. Biopharm. 53:233-239. [DOI] [PubMed] [Google Scholar]
- 8.Hornby, J. M., E. C. Jensen, A. D. Lisec, J. J. Tasto, B. Jahnke, R. Shoemaker, P. Dussault, and K. W. Nickerson. 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl. Environ. Microbiol. 67:2982-2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jett, B. D., K. L. Hatter, M. M. Huycke, and M. S. Gilmore. 1997. Simplified agar plate method for quantifying viable bacteria. BioTechniques 23:648-650. [DOI] [PubMed] [Google Scholar]
- 10.Jones, D. S., C. R. Irwin, A. D. Woolfson, J. Djokic, and V. Adams. 1999. Physicochemical characterization and preliminary in vivo efficacy of bioadhesive, semisolid formulations containing flurbiprofen for the treatment of gingivitis. J. Pharm. Sci. 88:592-598. [DOI] [PubMed] [Google Scholar]
- 11.Kabanov, A. V., P. Lemieux, S. Vinogradov, and V. Alakhov. 2002. Pluronic block copolymers: novel functional molecules for gene therapy. Adv. Drug Deliv. Rev. 54:223-233. [DOI] [PubMed] [Google Scholar]
- 12.Koo, H., S. K. Pearson, K. Scott-Anne, J. Abranches, J. A. Cury, P. L. Rosalen, Y. K. Park, R. E. Marquis, and W. H. Bowen. 2002. Effects of apigenin and tt-farnesol on glucosyltransferase activity, biofilm viability and caries development in rats. Oral Microbiol. Immunol. 17:337-343. [DOI] [PubMed] [Google Scholar]
- 13.Koo, H., P. L. Rosalen, J. A. Cury, Y. K. Park, and W. H. Bowen. 2002. Effects of compounds found in propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob. Agents Chemother. 46:1302-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koo, H., B. Schobel, K. Scott-Anne, G. Watson, W. H. Bowen, J. A. Cury, P. L. Rosalen, and Y. K. Park. 2005. Apigenin and tt-farnesol with fluoride effects on S. mutans biofilms and dental caries. J. Dent. Res. 84:1016-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu, X. M., H. T. Lee, R. A. Reinhardt, L. A. Marky, and D. Wang. 2007. Novel biomineral-binding cyclodextrins for controlled drug delivery in the oral cavity. J. Control. Release 122:54-62. [DOI] [PubMed] [Google Scholar]
- 16.Liu, X. M., R. A. Reinhardt, and D. Wang. 2006. Drug delivery strategies for common orofacial diseases. J. Drug Target. 14:583-597. [DOI] [PubMed] [Google Scholar]
- 17.Matthijs, S., and P. A. Adriaens. 2002. Chlorhexidine varnishes: a review. J. Clin. Periodontol. 29:1-8. [DOI] [PubMed] [Google Scholar]
- 18.Minghetti, P., B. Pacchetti, L. Montanari, C. Ronchi, and F. Berlati. 1997. Buccoadhesive tablets for the slow delivery of cetylpyridinium chloride: design and in vitro/in vivo analysis. Boll. Chim. Farm. 136:543-548. [PubMed] [Google Scholar]
- 19.Moshrefi, A. 2002. Chlorhexidine. J. West. Soc. Periodontol. Periodontal. Abstr. 50:5-9. [PubMed] [Google Scholar]
- 20.Mulhbacher, J., P. Ispas-Szabo, M. Ouellet, S. Alex, and M. A. Mateescu. 2006. Mucoadhesive properties of cross-linked high amylose starch derivatives. Int. J. Biol. Macromol. 40:9-14. [DOI] [PubMed] [Google Scholar]
- 21.Murchison, H. H., J. F. Barrett, G. A. Cardineau, and R. Curtiss III. 1986. Transformation of Streptococcus mutans with chromosomal and shuttle plasmid (pYA629) DNAs. Infect. Immun. 54:273-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nafee, N. A., M. A. Boraie, F. A. Ismail, and L. M. Mortada. 2003. Design and characterization of mucoadhesive buccal patches containing cetylpyridinium chloride. Acta Pharm. 53:199-212. [PubMed] [Google Scholar]
- 23.Russell, R. G. 2007. Bisphosphonates: mode of action and pharmacology. Pediatrics 119(Suppl. 2):S150-S162. [DOI] [PubMed] [Google Scholar]
- 24.Schiff, T. 2007. Anticalculus effect of a cetylpyridinium chloride/zinc gluconate mucoadhesive gel: results of a randomized, double-blind, controlled clinical trial. J. Clin. Dent. 18:79-81. [PubMed] [Google Scholar]
- 25.Selwitz, R. H., A. I. Ismail, and N. B. Pitts. 2007. Dental caries. Lancet 369:51-59. [DOI] [PubMed] [Google Scholar]
- 26.Senel, S., G. Ikinci, S. Kas, A. Yousefi-Rad, M. F. Sargon, and A. A. Hincal. 2000. Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery. Int. J. Pharm. 193:197-203. [DOI] [PubMed] [Google Scholar]
- 27.Sudhakar, Y., K. Kuotsu, and A. K. Bandyopadhyay. 2006. Buccal bioadhesive drug delivery—a promising option for orally less efficient drugs. J. Control. Release 114:15-40. [DOI] [PubMed] [Google Scholar]
- 28.Thneibat, A., M. Fontana, M. A. Cochran, C. Gonzalez-Cabezas, B. K. Moore, B. A. Matis, and M. R. Lund. 2008. Anticariogenic and antibacterial properties of a copper varnish using an in vitro microbial caries model. Oper. Dent. 33:142-148. [DOI] [PubMed] [Google Scholar]
- 29.Vinholis, A. H., L. C. Figueiredo, E. Marcantonio, Jr., R. A. Marcantonio, S. L. Salvador, and G. Goissis. 2001. Subgingival utilization of a 1% chlorhexidine collagen gel for the treatment of periodontal pockets. A clinical and microbiological study. Braz. Dent. J. 12:209-213. [PubMed] [Google Scholar]
- 30.Wang, D., S. C. Miller, P. Kopečková, and J. Kopeček. 2005. Bone-targeting macromolecular therapeutics. Adv. Drug Deliv. Rev. 57:1049-1076. [DOI] [PubMed] [Google Scholar]