Abstract
Adhesion molecules are known to play major roles in the initiation and stabilization of cell-to-cell contacts during the immunological response. Human immunodeficiency virus type 1 (HIV-1) exploits those interactions to facilitate infection and propagation processes. The primary objective of the present study was to investigate the ability of antagonists specific for lymphocyte function-associated antigen 1 (LFA-1) to diminish HIV-1 infection and transmission. We demonstrate here that LFA-1 antagonists can significantly reduce HIV-1 replication in primary human cells and virus propagation by affecting cell-to-cell interactions. Moreover, the inhibition of LFA-1-mediated adhesion events also potentiates the antiviral efficacy of the peptide fusion inhibitor T-20. Altogether, our data suggest that LFA-1 antagonists represent promising antiviral agents. Antiadhesion therapy could be considered a complementary strategy targeting cellular functions essential for HIV-1 spreading and against which the combined therapy currently used displays a limited efficacy.
Actual strategies to treat human immunodeficiency virus type 1 (HIV-1)-infected individuals make use of highly active antiretroviral therapy. It has been demonstrated that it reduces viral load, improves survival time, and decreases AIDS-associated mortality. Unfortunately, highly active antiretroviral therapy presents numerous severe drawbacks including the emergence of resistant strains, cross-resistance to other drugs within the same class, transmission of drug-resistant strains, extensive and adverse side effects for patients under treatment, and considerable costs. Therefore, the development of alternative therapeutic approaches aimed at novel targets is urgently needed.
The present work focuses on the membrane glycoprotein lymphocyte function-associated antigen 1 (LFA-1), an integrin playing crucial roles in leukocyte trafficking, inflammation, and the orchestration of the immune response. Interactions between LFA-1 and its counterreceptors, called intercellular adhesion molecules (ICAMs), initiate the immune response by strengthening the adhesion between antigen-presenting cells (APCs), such as dendritic cells (DCs), macrophages, or B cells, and CD4+ T lymphocytes through the formation of the immunological synapse and by participating in leukocyte migration toward inflamed tissues or secondary lymphoid organs. LFA-1 is a heterodimeric receptor constituted of CD11a (α chain) and CD18 (β chain). LFA-1-mediated adhesion is regulated by conformational changes (affinity), lateral diffusion, and the spatial organization (avidity) of this integrin within the plasma membrane (19, 36, 54, 55, 61).
Cell-to-cell transmission is the most rapid and potent mechanism by which HIV-1 can infect CD4+ T cells (12). Indeed, the virus takes advantage of the normal communication between immune cells for its own propagation. A virological synapse (VS) is formed by the recruitment of multiple HIV-1 receptors and coreceptors, virus-encoded gp120 and gp41, adhesion molecules, and cytoskeleton elements at the interface of HIV-1 donor and target cells, thus favoring the directed budding and fusion of newly synthesized virions. The VS can be created between infected and uninfected CD4+ T cells (34, 44) as well as between DCs or other carrier cells exposed to viral particles and target T cells (1, 2, 7, 16, 59). VS formation relies on LFA-1-mediated adhesion via ICAMs, particularly ICAM-1 and ICAM-3 (4, 30, 31, 33, 35, 51). It has been demonstrated that interactions of LFA-1 and ICAM-1 can modulate HIV-1 transfer from immature DCs (iDCs) to CD4+ T cells (51). Moreover, the importance of interactions between LFA-1 and ICAM-1 in HIV-1 transmission has been confirmed using T cells from leukocyte adhesion deficiency type 1 patients (30). Furthermore, interactions between LFA-1 and ICAMs play relevant roles in cell-free HIV-1 infection. Virions are efficiently released by infected cells in the external environment, and even though free viruses have a short life span (25), a number of them can bind and productively infect target cells. The efficiency of this mode of infection is strongly increased by the incorporation of certain host molecules into the viral envelope. Indeed, it was previously shown that the insertion of host-derived ICAM-1 within HIV-1 particles significantly increases the infection process of primary human CD4+ T cells expressing cell surface LFA-1 molecules (21-23, 56).
Immune hyperactivation is an important feature of HIV-1 pathogenesis during the chronic phase of infection. Chronically infected individuals share multiple immune abnormalities, including a rapid turnover of CD4+ T cells, T-cell depletion, polyclonal B-cell activation, destruction of the architecture of some secondary lymphoid tissues, and immunodeficiency. A number of these clinical manifestations share similarities with the ones observed for allergic and autoimmune diseases. Interestingly, some of those conditions are currently being treated with agents that can block interactions between LFA-1 and ICAM-1 (13, 14, 29, 32).
Given the involvement of LFA-1 in different important physiological processes, the pharmaceutical industry has devoted great efforts to the development of potent antagonists over the past decade, with the aim of treating cancer as well as multiple inflammatory and autoimmune diseases. Consequently, various classes of LFA-1 inhibitors have been tested, which has allowed the highlighting of the complex mechanism of action of this integrin in various processes such as rolling, migration, and firm adhesion. Among those compounds, statins such as lovastatin and other molecules like XVA143 have been demonstrated to antagonize LFA-1-mediated cellular adhesion in different manners. For instance, lovastatin restrains the association between LFA-1 and ICAM-1 and impairs LFA-1-mediated immune functions such as homotypic adhesion, rolling, and transmigration in addition to inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase (49). Likewise, in the presence of XVA143, ICAM-1 interactions are not strong enough to allow firm attachment (52, 53), but an increase of the rolling of the immune cells on the endothelium is still observed (49).
It was previously reported that lovastatin inhibits the infection of CD4+ T cells by cell-free ICAM-1-bearing HIV-1 (26, 28). Moreover, lovastatin was also found to reduce viral load and increase CD4+ T-cell counts in chronically HIV-1-infected patients (15). Therefore, in an attempt to provide additional information on the efficacy of LFA-1 antagonists in limiting virus infection and propagation, we tested the capacity of XVA143 and lovastatin to modulate HIV-1 infection in monocultures enriched in activated CD4+ T cells and in cocultures containing iDCs and autologous CD4+ T cells. In addition, to investigate whether LFA-1 antagonists could help antiretroviral compounds prevent virus infection, experiments using T-20 in combination with LFA-1 antagonists were also performed.
MATERIALS AND METHODS
Antibodies and reagents.
The hybridoma cell lines FB202 and FB203, which produce anti-CD11a (clone TS1/22) and anti-CD18 (clone TS1/18), respectively, were kindly provided by P. Naccache (Université Laval, Québec, Canada). The blocking anti-LFA-1 monoclonal antibody MEM25 was purchased from Exbio Praha (Vestec, Czech Republic). XVA143 and its less active analogue LAA were kindly donated by P. Gillespie (Hoffmann-La Roche Inc., Nutley, NJ), while BIRT377 and its less active analogue, BIRT378, were provided by T. Kelly (Boehringer Ingelheim Pharmaceuticals Inc., Ridgefield, CT). Lovastatin and its inactive LFA-1 analogue pravastatin were purchased from Calbiochem (San Diego, CA). Endotoxin-free dimethyl sulfoxide (DMSO) was obtained from Sigma-Aldrich (Oakville, Ontario, Canada). The fusion inhibitor T-20 was provided by the AIDS Repository Reagent Program (Germantown, MD). The Cell Trace carboxyl fluorescent succinimidyl ester (CFSE) cell proliferation kit was purchased from Invitrogen (Burlington, Ontario, Canada).
Cells.
293T cells were purchased from the American Type Culture Collection (Manassas, VA), whereas TZM-bl cells were obtained from the AIDS Repository Reagent Program. Peripheral blood lymphocytes (PBLs) from healthy donors were obtained by first isolating peripheral blood mononuclear cells (PBMCs) by Ficoll-Hypaque gradient centrifugation. Next, CD14-expressing cells (i.e., monocytes) were eliminated by adherence, and the remaining cells, which are a mix of CD4+ and CD8+ T cells and B lymphocytes, were called PBLs. CD4+ T cells were purified from freshly isolated PBMCs by immunomagnetic negative selection (Stem Cell Technologies Inc., Vancouver, Canada). Both purified CD4+ T cells and PBLs were cultured for 2 days in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (Wisent, St-Bruno, Québec, Canada) in the presence of phytohemagglutinin (PHA) (1 μg/ml) and recombinant human interleukin-2 (IL-2) (30 U/ml) before virus infection or initiation of coculture with iDCs. To obtain iDCs, monocytes (CD14+) were purified from freshly isolated PBMCs by immunomagnetic positive selection (Stem Cell Technologies Inc.). Purified CD14+ cells were cultured for 7 days in RPMI 1640 medium supplemented with 10% fetal bovine serum, granulocyte-macrophage colony-stimulating factor (1,000 U/ml), and IL-4 (200 U/ml) to obtain iDCs as previously described (27).
Plasmids and virus production.
pNL4-3 (X4) is a full-length infectious molecular clone of HIV-1 (provided by the AIDS Repository Reagent Program). pNL4-3Balenv is a plasmid where the env gene from the NL4-3 strain was replaced by that of the R5-tropic Bal strain (supplied by R. J. Pomerantz, Thomas Jefferson University, Philadelphia, PA) (18). pCD1.8 is a eukaryotic expression vector containing the entire human ICAM-1 (a generous gift from T. Springer, the Center for Blood Research, Boston, MA). Viruses differing by only the absence (called NL4-3) or the presence (called NL4-3/ICAM-1) of host-derived ICAM-1 proteins on their surface were produced by the calcium phosphate coprecipitation method using 293T cells as described previously (22). It should be noted that 293T cells do not constitutively express cell surface ICAM-1 (data not shown). Briefly, virus preparations devoid of host ICAM-1 were obtained upon the transfection of 293T cells with pNL4-3 alone, whereas ICAM-1-bearing virions were produced following the cotransfection of 293T cells with pNL4-3 and pCD1.8. The efficient incorporation of host-derived ICAM-1 within emerging HIV-1 particles was confirmed through the use of a previously described virus capture test (10, 41). In some experiments, laboratory and clinical isolates of HIV-1 (i.e., 92HT599/X4 tropic and 93HT054/R5 tropic, supplied by the AIDS Repository Reagent Program) were generated upon acute infection of PHA-stimulated PBLs. Briefly, viral stocks of NL4-3, NL4-3Balenv, 92HT599, or 93HT054 (10 ng per 105 cells) were mixed with PHA-IL-2-activated PBLs (20 × 106 PBLs) in a final volume of 4 ml and incubated at 37°C for 4 h. Cells were resuspended at 2 × 106 cells per ml and incubated at 37°C for 7 days. Supernatants were filtered, and the aliquots were kept frozen at −80°C until use. Virus preparations were normalized for virion content by using an in-house enzymatic assay specific for the major p24gag viral protein as previously described (8).
Virus infection studies.
PHA-activated PBLs (105 cells) were plated into 96-well flat-bottom tissue culture plates (in triplicates) and either left untreated or treated with the tested compounds at different concentrations for 30 min at 37°C. Cells were then infected with various viral preparations (i.e., NL4-3 and NL4-3/ICAM-1 produced in 293T cells as well as 92HT599 and 93HT054 expanded in PHA-stimulated PBLs) (2 ng of p24gag/105 cells) in a final volume of 200 μl. Cells were washed 24 h later, and cell-free supernatants were harvested at 4 and 7 days postinfection. In some experiments, LFA-1 antagonists were added 24 h postinfection. When indicated, the fusion inhibitor T-20 was added at various concentrations, in addition to the LFA-1 antagonists. Virus replication was estimated by measuring the p24 content by enzyme-linked immunosorbent assay or by incubating cell-free supernatants (75 μl) with TZM-bl (104 cells), which is an LFA-1-negative indicator cell line that enables the sensitive and quantitative analysis of both X4- and R5-tropic HIV-1 isolates since this cell line expresses high surface levels of CD4, CXCR4, and CCR5 (45). This technique, in contrast to the measurement of extracellular p24 content, permits assessments of the amount of infectious virus particles contained in the supernatants from infected cells. TZM-bl cells were lysed 72 h postinfection, and luciferase activity (expressed in relative light units) was monitored as described previously (22).
Homotypic adhesion.
To assess the effect of LFA-1 antagonists on CD4+ T-cell aggregate formation and disaggregation, cells were first activated with the mitogenic agent PHA (1 μg/ml) for 48 h to allow the activation of LFA-1 molecules and homotypic cell-to-cell adhesion. Cells were then washed, resuspended in culture medium, and transferred into 96-well plates (105 cells in 200 μl per well). Thereafter, LFA-1 antagonists or DMSO was added at the indicated concentrations, and cells were incubated at 37°C under a 5% CO2 atmosphere for 24 h in the presence of IL-2. The cells were examined for homotypic adhesion using a light microscope connected to a camera. Representative pictures of each well were taken at a ×20 magnification. The quantification of aggregation was performed with CellProfiler image analysis software.
Proliferation assay.
iDCs (2 × 104 cells per well) were either left untreated or treated with lipopolysaccharide (LPS) (100 ng/ml) for 24 h at 37°C and then washed with 1× phosphate-buffered saline. Resting and PHA-activated CD4+ T cells were labeled with CFSE (2 μM) according to instructions provided by the manufacturer (Invitrogen) and kept at 37°C for 24 h. Next, CD4+ T cells were washed, left untreated, or treated with LFA-1 antagonists at different concentrations or with the solvent DMSO for 30 min at 37°C and then added to DCs (i.e., iDCs or LPS-matured DCs) at a 10:1 ratio (i.e., at a final concentration of 2 × 105 CD4+ T cells per well). Under some conditions, the bacterial superantigen SEE from Staphylococcus aureus (100 pg/ml) was added in the coculture to mimic antigenic presentation. After 5 days of coculture, cells were fixed in paraformaldehyde (2%) and analyzed by flow cytometry. The proliferation analysis was performed with FCS Express software (De Novo Software, Los Angeles, CA).
Virus transfer experiments.
iDCs (5 × 104 cells) were seeded into 96-well flat-bottom tissue culture plates and pulsed for 1 h at 37°C with NL4-3Balenv (2 ng of p24gag). Next, cells were washed three times with phosphate-buffered saline and cocultured with CD4+ T cells (1 × 105 cells). Primary human CD4+ T cells were either left untreated or treated for 15 min at 37°C with XVA143 (5 μM), LAA (5 μM), lovastatin (10 μM), pravastatin (10 μM), or the appropriate concentration of DMSO (diluent) before incubation with iDCs. Cocultured cells were washed 24 h later, and cell-free supernatants were harvested at 3 days following the initiation of the coculture. Virus production was estimated by measuring the p24 content in cell-free supernatants by enzyme-linked immunosorbent assay.
Analysis of efficacy of the drug combinations tested.
The interaction between two different compounds was defined by the fractional inhibitory concentration (FIC) index (FICI) (43). The FICI is the sum of the FIC of each of the drugs and is expressed mathematically as follows: FICI = FICA + FICB = (IC50 drug A in combination/IC50 drug A alone) + (IC50 drug B in combination/IC50 drug B alone). The 50% inhibitory concentrations (IC50s) of the drug alone and in combination were calculated by nonlinear regression analysis using Prism software. Synergy is present when the effect of the drug combination exceeds the additive effect of the individual compounds (i.e., FICI < 0.8), partial synergy or an additive effect is seen when the effect of the drug combination is equal to that of the sum of the individual components (i.e., 0.8 < FICI < 1.25), and an antagonistic effect is observed when the effect of the drug combination is reduced compared to that of the most effective compound (i.e., FICI > 1.25).
Statistical analysis.
Analysis was performed by one-way analysis of variance or matched-pair t test. P values of less than 0.05 were considered to be statistically significant.
RESULTS
Lovastatin reduces infection with both HIV-1 particles lacking host-derived ICAM-1 and ICAM-1-bearing virions.
In order to corroborate the capability of the LFA-1 antagonist lovastatin to control HIV-1 replication, X4-tropic NL4-3 virus stocks either lacking (i.e., NL4-3) or bearing (i.e., NL4-3/ICAM-1) host-derived ICAM-1 were produced upon transient transfection in 293T cells and used to infect primary human cells (i.e., PBLs). Target cells were initially pretreated with the drug diluent DMSO (as a control), lovastatin, or pravastatin before virus infection. Statins such as lovastatin and pravastatin act as inhibitors of HMG-CoA reductase. However, some statins like lovastatin can also interfere with interactions between LFA-1 and ICAM-1, a process leading to a modulation of T-cell activation. The LFA-1 inactive analogue pravastatin, which does not affect LFA-1-mediated adhesion, was used to estimate the fraction of inhibition that is due to the effect on HMG-CoA reductase. Results depicted in Fig. 1A indicate that virus replication is inhibited in a dose-dependent manner by lovastatin but is almost unaffected by pravastatin. Interestingly, comparable IC50s were obtained in the presence of lovastatin when the two tested virus preparations were used (i.e., NL4-3 and NL4-3/ICAM-1). The colorimetric MTS cell proliferation assay revealed that the observed reduction in the level of virus production is not due to cell toxicity since cellular viability remains the same in the presence of all tested concentrations of lovastatin (data not shown). To provide physiological significance to our results and considering that clinical isolates of HIV-1 are less susceptible than laboratory strains to some antiretroviral drugs that target, for example, early steps in virus replication, similar studies were performed with two field isolates of HIV-1 displaying distinct tropisms (i.e., 92HT599/X4 and 93HT054/R5). It should be noted that both field isolates of HIV-1 were produced in natural cellular reservoirs (i.e., mitogen-stimulated PBLs) to more closely parallel natural conditions. Interestingly, previous studies revealed that HIV-1 particles expanded in such primary human cells are known to incorporate various host-derived constituents including ICAM-1 (5, 8, 9, 11, 57). Again, lovastatin leads to a dose-dependent inhibition of 92HT599 and 93HT054 replication (Fig. 1B), which provides additional credence to our findings. It also seems that lovastatin is affecting primarily cell-to-cell virus transmission since virus production is similarly affected when treatment is taking place either 30 min before or 24 h following virus infection (Fig. 1C).
FIG. 1.
Virus replication is inhibited by lovastatin. (A and B) PHA-activated PBLs were either left untreated or treated with the listed concentrations of lovastatin, pravastatin, or the appropriate dilutions of DMSO for 30 min. Cells were next infected with NL4-3 either lacking or bearing host-derived ICAM-1 (A) and the two clinical viral strains 92HT599 and 93HT054 (B). Cells were washed extensively after 24 h to eliminate uninternalized virions, and cell-free supernatants were harvested at 3 days (A) or 4 days (B) postinfection. Virus production was assessed by measuring the p24 content. The data depicted in the left and middle graphs of A and B represent the means ± standard errors of the means (SEM) from quadruplicate samples of a single experiment and are representative of four separate experiments. Results illustrated in the right graph of A depict the effect of lovastatin and represent the IC50s of these four independent experiments, with the horizontal bars representing the means (P value determined by paired t test; n = 4). (C) PHA-activated PBLs were either left untreated or treated with the listed concentrations of lovastatin or the appropriate dilutions of DMSO. Samples were treated with lovastatin at either 30 min before infection or 24 h postinfection with 92HT599 and 93HT054. Cell-free supernatants were harvested at 4 days postinfection, and virus replication was assessed by estimating the p24 content. Results are expressed as the IC50s of eight or three independent experiments, as indicated, with the horizontal bars representing the means (P value determined by unpaired t test).
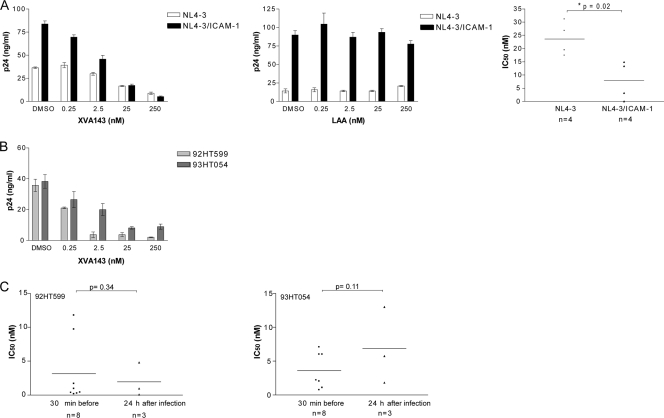
The LFA-1 inhibitor XVA143 is more efficient at inhibiting infection with ICAM-1-bearing virions.
Since lovastatin exerts multiple physiological effects, notably on cholesterol metabolism through an inhibition of the HMG-CoA reductase, we repeated the above-described experiments with a more specific LFA-1 antagonist. XVA143 has been demonstrated to be a powerful agent that blocks LFA-1-mediated firm adhesion without affecting HMG-CoA reductase (49). Accordingly, we investigated the ability of XVA143 to limit HIV-1 infection. Data from our experiments indicated that the XVA143 compound can also inhibit the replication of both NL4-3 and NL4-3/ICAM-1 virus stocks in PBLs (Fig. 2A). However, a statistically significant difference between the IC50s of the two virus preparations tested was seen in the presence of XVA143 (P = 0.02). Indeed, the replication of ICAM-1-bearing virus is more sensitive to XVA143 (i.e., IC50 = 8 nM) than isogenic virus that lacks host-derived ICAM-1 (i.e., IC50 = 24 nM). The less active analogue of XVA143, LAA, was used as a control, and virus replication was unaffected by similar concentrations of this compound. The LFA-1 antagonist XVA143 is also very efficient at controlling infection with clinical isolates 92HT599 and 93HT054 (mean IC50s from seven donors of 4.5 nM and 3 nM, respectively) (Fig. 2B). In agreement with what was seen with lovastatin, the LFA-1 antagonist XVA143 also exerts its effect mostly on cell-to-cell virus transfer because the IC50s were not significantly affected when the compound was added 24 h following HIV-1 infection (Fig. 2C). The process of infection with virions lacking the adhesion molecule ICAM-1 is also affected by the two LFA-1 antagonists lovastatin and XVA143. Antiviral activity was seen in cells pretreated for 30 min before the addition of viruses or cells treated at 24 h postinfection (data not shown). These findings further support the idea that LFA-1 antagonists affect primarily cell-to-cell virus propagation events. The XVA143-mediated inhibition of HIV-1 replication is not due to cell toxicity, since cellular viability as monitored by the MTS test remained unchanged in the presence of all tested concentrations of XVA143 (data not shown).
FIG. 2.
XVA143 reduces HIV-1 production in primary human cells. (A and B) PHA-activated PBLs were either left untreated or treated with the listed concentrations of XVA143, its less active analogue LAA, or the appropriate dilutions of DMSO for 30 min. Cells were next infected with NL4-3 either lacking or bearing host-derived ICAM-1 (A) and the two clinical viral strains 92HT599 and 93HT054 (B). Cells were washed extensively after 24 h to eliminate uninternalized virions, and cell-free supernatants were harvested at 3 days (A) or 4 days (B) postinfection. Virus production was assessed by measuring the p24 content. The data depicted in left and middle graphs of A and B represent the means ± SEM from quadruplicate samples of a single experiment and are representative of four separate experiments. Results illustrated in the right graph of A depict the effect of XVA143 and represent the IC50s of these four independent experiments, with the horizontal bars representing the means (*, P < 0.05 by paired t test; n = 4). (C) PHA-activated PBLs were either left untreated or treated with the listed concentrations of XVA143 or the appropriate dilutions of DMSO. Samples were treated with the listed LFA-1 antagonists at either 30 min before infection or 24 h postinfection with 92HT599 and 93HT054. Cell-free supernatants were harvested at 4 days postinfection, and virus replication assessed by estimating the p24 content. Results are expressed as the IC50s of eight or three independent experiments, as indicated, with the horizontal bars representing the means (P value determined by unpaired t test).
LFA-1 antagonists affect cell cluster formation in response to mitogenic stimulation as well as DC-induced T-cell proliferation.
We next tried to shed light on the putative mechanism(s) by which the LFA-1 antagonists studied can exert their antiviral action. Given that the VS is considered an efficient process for viral dissemination, and LFA-1-mediated adhesion plays a crucial role in this cell-to-cell communication, we addressed the possibility that LFA-1 antagonists prevent this phenomenon. To this end, CD4+ T cells were activated with the mitogenic agent PHA, which is known to induce LFA-1-mediated homotypic cell-to-cell adhesion. Cells were then treated with different concentrations of XVA143 or lovastatin to evaluate the ability of the LFA-1 antagonists to alter aggregate formation and trigger the disaggregation of cell clusters. We observed that XVA143 disrupts preformed cell clusters and also prevents the formation of new ones (Fig. 3A). This observation was made when doses of XVA143 ranging from 5 to 5,000 nM were used (Fig. 3B). Homotypic cell-to-cell adhesion was less efficiently reduced by lovastatin. As expected, controls consisting of samples treated with DMSO and the less active analogues LAA and pravastatin have no effect on the formation of cell clusters in PHA-activated PBLs.
FIG. 3.
XVA143 strongly reduces PHA-mediated aggregation of CD4+ T cells. (A) PHA-activated human CD4+ T cells (105 cells) were either left untreated or treated with XVA143 (5 μM), LAA (5 μM), lovastatin (10 μM), pravastatin (10 μM), or the appropriate dilution of DMSO and incubated for 24 h at 37°C to allow further aggregation or disaggregation. Representative pictures of each well were taken at a ×20 magnification. (B) Quantitative measurements of the cellular aggregates were achieved with CellProfiler image analysis software. The areas of the clumps (aggregates) were used to make the histograms. The measurement units for the clump areas are displayed in pixels. Ctrl, control. (C) CFSE-labeled resting or PHA-activated CD4+ T cells were pretreated or not with LFA-1 antagonists or the solvent DMSO for 30 min and then cocultured with iDCs or LPS-matured DCs in the absence or presence of SEE (100 pg/ml). Proliferation was stopped 5 days later with paraformaldehyde by use of a fixation procedure. Cells were analyzed using a flow cytometer with a 488-nm excitation-and-emission filter appropriate for fluorescein. Proliferation analysis was performed with FCS Express software. Results are expressed as percentages of divided cells.
Considering that LFA-1 antagonists affect cell-to-cell adhesion and since T-cell activation following immunological synapse formation relies heavily on interactions between LFA-1 and ICAM-1, we next investigated whether XVA143 and lovastatin can impair DC-mediated T-cell proliferation. This is based on the idea that DCs are known to be very potent at generating the proliferation of resting T cells. Quiescent and PHA-activated CD4+ T cells were labeled with the stably integrated fluorescent probe CFSE to estimate cell division. Next, such cells were either left untreated or treated with LFA-1 antagonists and then cocultured with iDCs or LPS-treated DCs in absence or presence of LPS or SEE. In our experiments, LPS was used to trigger the maturation of DCs and to promote the activation of resting CD4+ T cells, whereas the superantigen SEE will cause massive polyclonal T-cell proliferation when presented by APCs such as DCs. As expected, a significant DC-mediated proliferation of resting CD4+ T cells was obtained with LPS and SEE when used individually, and cellular proliferation could not be achieved in already-activated CD4+ T cells (i.e., PHA treated) except when the two agents were used in combination (Fig. 3C). Normal T-cell functions were affected by LFA-1 antagonists since the percentages of resting CD4+ T cells in division are notably reduced in the presence of XVA143 and to a much lesser extent by lovastatin. These data suggest that the contact between the two cell types (i.e., DCs and CD4+ T cells), which is required for T-cell priming, is affected by the LFA-1 antagonists.
DC-mediated HIV-1 transfer is strongly reduced by lovastatin and XVA143.
We also investigated whether the two tested LFA-1 antagonists could affect HIV-1 transmission from iDCs to autologous CD4+ T cells. For this purpose, iDCs were initially pulsed with HIV-1 and extensively washed to eliminate unadsorbed viruses. Next, autologous PHA-activated CD4+ T cells, which were either left untreated or treated with the different compounds, were then cocultured with virus-loaded iDCs to allow virus transfer. We tested concentrations of LFA-1 antagonists that can potently prevent infection with cell-free virions and also impair cell-to-cell interactions (i.e., 5 μM for XVA143 and 10 μM for lovastatin). Results illustrated in Fig. 4 indicate that virus transmission is markedly affected by both lovastatin (98% ± 0.9% inhibition) and XVA143 (88% ± 3% inhibition). Virus transfer was also modulated by the less active analogue LAA (i.e., 35% ± 14%) but to a lower extent than with XVA143. Pravastatin, which has no impact on LFA-1-mediated adhesion, also affects viral transfer (50% ± 5%), therefore suggesting an involvement of HMG-CoA reductase in this phenomenon.
FIG. 4.
HIV-1 transmission from iDCs to CD4+ T cells is significantly diminished by LFA-1 antagonists. iDCs (5 × 104 cells) were initially seeded into 96-well flat-bottom tissue culture plates and pulsed with NL4-3Balenv for 1 h at 37°C (2 ng of p24gag). Next, cells were washed to remove excess virus and cocultured with autologous CD4+ T cells (1 × 105 cells) that had previously been either left untreated or treated with XVA143 (5 μM), LAA (5 μM), lovastatin (10 μM), pravastatin (10 μM), or the appropriate dilutions of DMSO for 15 min at 37°C. Cocultured cells were washed 24 h later, and cell-free supernatants were harvested 3 days following the initiation of the coculture. Virus replication was estimated by measuring the p24 content. The data shown are the means ± SEM for quadruplicate samples and are representative of five separate experiments. Percentages of inhibition compared to DMSO-treated cells are provided above each bar (*, P < 0.05; **, P < 0.01 [one-way analysis of variance Dunnet posttest]).
Positive drug interactions between LFA-1 antagonists and T-20.
The entry inhibitor T-20 (enfuvirtide; Fuzeon) is currently administered in combination therapy to treat individuals infected with multidrug-resistant viral strains. The potency of HIV-1 entry inhibitors in inhibiting cell-to-cell HIV-1 transmission is still poorly known. However, it was recently reported that virions carrying host-derived ICAM-1 are two- to fivefold less sensitive to T-20 than viruses lacking this cell adhesion molecule (6). The next set of investigations was thus aimed at defining whether the antiviral activity of T-20 could be potentiated by an LFA-1 antagonist. In contrast to more traditional approaches for measuring drug combination efficacies, which rely on established cell lines, we performed our work with primary human cells to more closely parallel natural conditions. As expected, we obtained a certain donor-to-donor variation, and it is thus more complicated to select compound concentrations for the pairing. For this study, we first selected the mean concentration from different donors and viral preparations resulting in a 50% inhibition of infection to select the dose response. Next, we included two lower and two upper concentrations in order to have five different concentrations. We tried various combinations in order to obtain a good nonlinear regression curve and calculated the IC50s of the drugs when used alone and in combination. Results presented in Fig. 5 represent the best conditions found.
FIG. 5.
LFA-1 antagonists potentiate the antiretroviral activity of T-20. PHA-activated PBLs were either left untreated or treated with the listed concentrations of T-20, XVA143, and lovastatin used either alone or in combination. Cells were next infected with 92HT599 or 93HT054, which were produced in primary human cells. Cell-free supernatants were harvested at 6 days postinfection, and virus production was assessed using TZM-bl indicator cells. Results are expressed as a percentage of inhibition of infection compared to DMSO-treated control cells. The data shown represent the means ± SEM for quadruplicate samples and are representative of three separate experiments. The asterisks indicate statistically significant differences (*, P < 0.05; **, P < 0.01; ***, P < 0.001 [paired t test with raw data]) between the following treatment groups: T-20 plus lovastatin versus T-20 and lovastatin, and T-20 plus XVA143 versus T-20 and XVA143.
Briefly, cells were pretreated with different concentrations of T-20 and LFA-1 antagonists, used either alone or in combination, and then infected with the two clinical variants 92HT599 and 93HT054. Finally, virus production was monitored by inoculating TZM-bl indicator cells with culture supernatants from such treated PBLs. Data shown in Fig. 5 demonstrate that the antiviral activity of T-20 is enhanced when used in combination with either lovastatin or XVA143. For example, the IC50s of T-20 were equal to 1.9 and 8.4 ng/ml when infection was carried out with 92HT599 and 93HT054, respectively, compared to 0.4 and 1.3 ng/ml in the presence of XVA143 and 0.14 and 1.1 ng/ml with lovastatin. To determine the precise nature of these interactions (i.e., additive or synergistic), we estimated the FICI, which is the ratio of the IC50 of the combination of drugs to that of the drug of interest alone. Overall, the FICIs of three independent experiments indicate that XVA143 synergizes with T-20, whereas lovastatin demonstrates partial synergistic/additive effects (Table 1).
TABLE 1.
FICIs of IC50s of LFA-1 antagonists and T-20
| Compound | Donor | FICIa |
|
|---|---|---|---|
| 92HT599 | 93HT054 | ||
| XVA143 | 1 | 0.07 | 0.18 |
| 2 | 0.13 | NA | |
| 3 | NA | 0.57 | |
| Lovastatin | 1 | 0.67 | 1.20 |
| 2 | 0.08 | 1.19 | |
| 3 | 0.52 | 0.11 | |
An FICI of <0.8 indicates synergy; 0.8 < FICI < 1.25 indicates a partial synergy/additive effect; an FICI of >1.25 indicates antagonism. NA, not applicable (the inhibitory activity of XVA143 alone was too high for any effect to be seen when used in combination with T-20).
DISCUSSION
In the present study, we demonstrate that the impairment of LFA-1-ICAM-1 interactions with LFA-1 antagonists can reduce infection with cell-free HIV-1 particles and cell-to-cell virus transmission. In addition, we report that the combination of LFA-1 antagonists and the fusion inhibitor T-20 produces synergic or additive/synergic activity. A major strength of this study is that the antiviral efficacy of LFA-1 antagonists was not assessed in artificial cell systems (i.e., established cell lines) but in a more relevant experimental model. Indeed, all experiments were performed under conditions as close as possible to in vivo conditions, namely, in primary human target cells and using viral strains expanded in PBLs. However, this model system comes with a major caveat, that is, the intrinsic variability between the tested donor samples.
Our data indicate that XVA143 is more effective than lovastatin at reducing infection with cell-free viruses. This observation is not surprising since XVA143 was specifically designed to be an inhibitor of LFA-ICAM-1 interactions through a disturbance of firm adhesion between these two molecules (38, 50). Under our experimental conditions, the superior antiviral activity of this LFA-1 antagonist is most likely associated with its greater ability to inhibit LFA-1-mediated cell-to-cell aggregation. It is well known that homotypic adhesion between CD4+ T cells is due mostly to LFA-1 and ICAM-1 interactions (20, 47). Several stimuli can promote LFA-1 adhesiveness including the lectin PHA, which cross-links different cell surface molecules including the T-cell receptor and triggers a signaling cascade mimicking antigen presentation. These signals conduct in the generation of second messengers that increase the avidity of LFA-1 and therefore adhesiveness to ICAMs (20, 40, 60). Our findings revealed that XVA143 and lovastatin, in a much less effective manner, promote the disaggregation of PHA-induced cell-to-cell adhesion. These results are perfectly in line with data from a recent study showing that the LFA-1 inhibitors XVA143 and LFA878 (the latter is a lovastatin-derived compound) prevent the homotypic adhesion of NK cells and promote the disaggregation of existing NK cell clusters (65). It is also well documented that the stabilization of the contact between APCs and T cells during antigenic presentation is mediated mostly by interactions between LFA-1 and ICAM-1. As expected, we show here that LFA-1 antagonists also alter the DC-mediated proliferation of resting CD4+ T cells in the presence of LPS and SEE. These results are consistent with other previously reported results showing that statin-derived compounds and XVA143 impair T-cell and NK cell activation as well as proliferation through an inhibition of LFA-1-mediated adhesion (64, 65).
Considering that cell-to-cell transmission is the most efficient way used by HIV-1 for its propagation and since the stabilization of the cell-to-cell adhesion process is mediated mostly by interactions between LFA-1 and ICAM-1 (3, 34, 35, 48), we have further investigated the potential inhibitory effect of LFA-1 antagonists on the formation of VSs between CD4+ T cells. Based on our results, we propose that XVA143 is affecting later steps in the virus life cycle, such as the creation of the VS and virus-mediated syncytium formation. Both the VS and syncytia are thought to play an important role in HIV-1 spreading between live cells, because the dissemination of this retrovirus is estimated to be at least 2 to 3 orders of magnitude more efficient when cells can physically interact with each other (12, 17). Interestingly, interactions between LFA-1 and ICAM-1 are also needed to facilitate HIV-1-induced syncytium formation (21, 33, 63). However, our results are in conflict with data from a recent study demonstrating that although interactions between LFA-1 and ICAM-1 strongly facilitate cell-to-cell contacts that promote HIV-1 transmission, viral transfer between virus-infected effector T cells and quiescent CD4+ T cells can occur even in the absence of LFA-1-ICAM-1 interactions (46). The conclusions made by those authors were based mainly on results obtained by using MOLT T cells as HIV-1 effector cells and primary resting CD4+ T cells as acceptor cells in the presence of anti-LFA-1 or anti-ICAM-1 antibody. While that study is complete and well described, those authors did not provide any functional assay proving that the anti-LFA-1 and anti-ICAM-1 antibodies used in their work impaired LFA-1-mediated cell-to-cell adhesion and/or blocked the adhesion of their cells onto ICAM-1-coated plates. Moreover, cells were not pretreated with their tested antibodies before initiating the coculture step with acceptor cells. Thus, there is a possibility that interactions between LFA-1 and ICAM-1 are taking place despite the presence of the studied antibodies. In our study, when T cells were pretreated with the XVA143 before or 24 h after the addition of the viruses (i.e., virions lacking or bearing host-derived ICAM-1), the level of HIV-1 replication was strongly reduced, therefore confirming that LFA-1-mediated cell-to-cell contacts play an important role in HIV-1 spreading.
Responses of a different magnitude were obtained with the other LFA-1 antagonist tested, i.e., lovastatin. Indeed, lovastatin displays a lower capacity to control acute HIV-1 infection of CD4+ T cells. Similar results were obtained with another LFA-1 inhibitor (i.e., BIRT377), which blocks LFA-1 in its inactive form as lovastatin (data not shown). This is perhaps related to some unique features of this class of LFA-1 inhibitors, which exert a smaller effect on LFA-1 molecules when they are partially activated. Surprisingly, lovastatin displays a higher efficiency than XVA143 in DC-mediated virus transfer. Given that lovastatin can inhibit both LFA-1-mediated adhesion and HMG-CoA reductase activity while XVA143 can abrogate interactions between LFA-1 and ICAM-1 without affecting HMG-CoA reductase, it can be proposed that the superior activity of lovastatin with respect to DC-mediated HIV-1 transfer is most likely due to the inhibitory effect of lovastatin on HMG-CoA reductase. This hypothesis is supported by our findings that pravastatin, which affects only HMG-CoA reductase, can reduce HIV-1 transfer from iDCs to CD4+ T cells by 50%, while it has no impact on cell-free HIV-1 infection as well as on cell-to-cell transmission between CD4+ T cells (data not shown). The inhibition of HMG-CoA reductase has been shown to affect the mevalonate pathway, leading to the impairment of cholesterol synthesis and other isoprenoid intermediates, which are added during the posttranslational modification of various signaling proteins, like G- and Ras-like proteins, involved in cytoskeleton remodeling and endocytosis (37, 39, 62). Therefore, it can be postulated that a disruption of the mevalonate pathway by lovastatin or pravastatin perturbs the signaling cascade involved in iDC-mediated HIV-1 transmission that is independent of LFA-1-mediated cell adhesion.
Our in vitro evaluation of the anti-HIV-1 interactions between LFA-1 antagonists and T-20 revealed a synergy taking place with XVA143 and an additive/synergistic effect with lovastatin. Similar observations were made with another entry inhibitor, the CXCR4 inhibitor AMD-3100 (data not shown). It remains unclear whether the antiretroviral drugs targeting binding and fusion events can keep their full inhibitory effect when viruses are transmitted at the interface between infected and uninfected cells. Interestingly, it was recently reported that the fusion inhibitor T-20 is much less effective against cell-to-cell HIV-1 transfer than against infection with cell-free virus (58). Consequently, by limiting cell-to-cell contact, particularly in the context of a hyperactivation of the immune system, antiadhesion compounds could cooperate with the current antiretroviral drugs to reduce HIV-1 infection and dissemination. Furthermore, this drug combination should reduce the emergence of drug-resistant viral strains and improve the treatment of infected individuals. The potential use of LFA-1 antagonists in addition to the current combination of antiretroviral therapy is supported by our findings that the concentration of T-20 necessary to reduce virus production by 50% is approximately four times lower in the presence of LFA-1 antagonists.
Altogether, the data presented in this study lead us to propose that treatment with LFA-1 antagonists might help to control virus replication both by reducing infection with cell-free virions and by limiting the cell-to-cell transmission of HIV-1. Virus production might also be affected by a more indirect process based on the idea that LFA-1 antagonists could reduce the well-described immune hyperactivation state seen in chronically HIV-1-infected individuals. Indeed, a chronic activation of the immune system leads to a rapid turnover of CD4+ T cells, T-cell depletion, polyclonal B-cell activation, destruction of the architecture of some secondary lymphoid tissues, and immunodeficiency. A number of these clinical manifestations share similarities with the ones observed for allergic and autoimmune diseases. Interestingly, some of those conditions are currently being treated with agents that block interactions between LFA-1 and ICAM-1 (13, 14, 29, 32). Interestingly, we report here that LFA-1 antagonists significantly reduce the DC-mediated proliferation of resting CD4+ T cells. This feature is expected to further decrease virus production in HIV-1-infected patients by diminishing the persistent immune activation state seen in infected individuals. LFA-1 antagonists can also be useful in the context of an HIV-1 infection to decrease the migration of virus carrier cells like DCs and macrophages to lymphoid organs, reduce the nonspecific generation of effector CD4+ T cells through the stimulation of APCs, diminish the recruitment of permissive CD4+ T cells in infected tissues, and abrogate the nonspecific killing of immune and nonimmune cells by cytotoxic T lymphocytes, all phenomena thought to be responsible for the destruction of cells and tissues in HIV-1-infected patients. Obviously, this treatment should not be given during the acute infection phase to avoid interfering with the establishment of an effective antiviral response, which requires the formation of the immunological synapse between DCs and T cells. In other respects, LFA-1 antagonists could be considered for microbicide cocktails to prevent HIV-1 transmission and primary infection. These drug combinations are usually designed to inhibit the first steps in the viral life cycle. The use of antiadhesion compounds might prevent the binding of virus-carrying and/or virus-infected immune cells from semen to mucosal epithelial cells and DCs. Moreover, by reducing the migration of virus-loaded immune cells from mucosal tissues to lymph nodes, antiadhesive molecules should limit HIV-1 dissemination.
It is well established that HIV-1 infection upregulates the expression of both ICAM-1 and LFA-1 in lymphoid tissues and PBLs (24, 42). Moreover, systemic immune activation is detected in HIV-1-infected persons, and LFA-1-mediated adhesion plays a dominant role in HIV-1 infection and dissemination processes. Consequently, the use of LFA-1 antagonists represents an avenue worth exploring for therapeutic intervention.
Acknowledgments
We thank Sylvie Méthot for editorial assistance.
This study was made possible through operating grants to M.J.T. from the Canadian Institutes of Health Research (CIHR) HIV/AIDS Research Program (HOP-14438 and MOP-79542). C.G. and S.T. are the recipients of a fellowship award and a doctoral award, respectively, from the CIHR HIV/AIDS Research Program. M.J.T. holds the Canada Research Chair in Human Immuno-Retrovirology (tier 1 level).
Footnotes
Published ahead of print on 31 August 2009.
REFERENCES
- 1.Abbate, I., F. Dianzani, F. Bianchi, G. Mosiello, F. Carletti, D. Fiumara, and M. R. Capobianchi. 1999. RANTES stimulates cell-mediated transmission of HIV-1 infection. J. Interferon Cytokine Res. 19:345-350. [DOI] [PubMed] [Google Scholar]
- 2.Arias, R. A., L. D. Munoz, and M. A. Munoz-Fernandez. 2003. Transmission of HIV-1 infection between trophoblast placental cells and T-cells take place via an LFA-1-mediated cell to cell contact. Virology 307:266-277. [DOI] [PubMed] [Google Scholar]
- 3.Arthos, J., C. Cicala, E. Martinelli, K. Macleod, D. Van Ryk, D. Wei, Z. Xiao, T. D. Veenstra, T. P. Conrad, R. A. Lempicki, S. McLaughlin, M. Pascuccio, R. Gopaul, J. McNally, C. C. Cruz, N. Censoplano, E. Chung, K. N. Reitano, S. Kottilil, D. J. Goode, and A. S. Fauci. 2008. HIV-1 envelope protein binds to and signals through integrin alpha4beta7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 9:301-309. [DOI] [PubMed] [Google Scholar]
- 4.Barbeau, B., J. F. Fortin, N. Genois, and M. J. Tremblay. 1998. Modulation of human immunodeficiency virus type 1-induced syncytium formation by the conformational state of LFA-1 determined by a new luciferase-based syncytium quantitative assay. J. Virol. 72:7125-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastiani, L., S. Laal, M. Kim, and S. Zolla-Pazner. 1997. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J. Virol. 71:3444-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beauséjour, Y., and M. J. Tremblay. 2004. Susceptibility of HIV type 1 to the fusion inhibitor T-20 is reduced on insertion of host intercellular adhesion molecule 1 in the virus membrane. J. Infect. Dis. 190:894-902. [DOI] [PubMed] [Google Scholar]
- 7.Bounou, S., J.-F. Giguère, R. Cantin, C. Gilbert, M. Imbeault, G. Martin, and M. J. Tremblay. 2004. The importance of virus-associated host ICAM-1 in human immunodeficiency virus type 1 dissemination depends on the cellular context. FASEB J. 18:1294-1296. [DOI] [PubMed] [Google Scholar]
- 8.Bounou, S., J. E. Leclerc, and M. J. Tremblay. 2002. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4+-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J. Virol. 76:1004-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantin, R., J. F. Fortin, and M. Tremblay. 1996. The amount of host HLA-DR proteins acquired by HIV-1 is virus strain- and cell type-specific. Virology 218:372-381. [DOI] [PubMed] [Google Scholar]
- 10.Cantin, R., G. Martin, and M. J. Tremblay. 2001. A novel virus capture assay reveals a differential acquisition of host HLA-DR by clinical isolates of human immunodeficiency virus type 1 expanded in primary human cells depending on the nature of producing cells and the donor source. J. Gen. Virol. 82:2979-2987. [DOI] [PubMed] [Google Scholar]
- 11.Capobianchi, M. R., S. Fais, C. Castilletti, M. Gentile, F. Ameglio, and F. Dianzani. 1994. A simple and reliable method to detect cell membrane proteins on infectious human immunodeficiency virus type 1 particles. J. Infect. Dis. 169:886-889. [DOI] [PubMed] [Google Scholar]
- 12.Carr, J. M., H. Hocking, P. Li, and C. J. Burrell. 1999. Rapid and efficient cell-to-cell transmission of human immunodeficiency virus infection from monocyte-derived macrophages to peripheral blood lymphocytes. Virology 265:319-329. [DOI] [PubMed] [Google Scholar]
- 13.Cather, J. C., J. C. Cather, and A. Menter. 2003. Modulating T cell responses for the treatment of psoriasis: a focus on efalizumab. Expert Opin. Biol. Ther. 3:361-370. [DOI] [PubMed] [Google Scholar]
- 14.Curley, G. P., H. Blum, and M. J. Humphries. 1999. Integrin antagonists. Cell. Mol. Life Sci. 56:427-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.del Real, G., S. Jimenez-Baranda, E. Mira, R. A. Lacalle, P. Lucas, C. Gomez-Mouton, M. Alegret, J. M. Pena, M. Rodriguez-Zapata, M. Alvarez-Mon, A. C. Martinez, and S. Manes. 2004. Statins inhibit HIV-1 infection by down-regulating Rho activity. J. Exp. Med. 200:541-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Milito, A. 2004. B lymphocyte dysfunctions in HIV infection. Curr. HIV Res. 2:11-21. [DOI] [PubMed] [Google Scholar]
- 17.Dimitrov, D. S., R. L. Willey, H. Sato, L. J. Chang, R. Blumenthal, and M. A. Martin. 1993. Quantitation of human immunodeficiency virus type 1 infection kinetics. J. Virol. 67:2182-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dornadula, G., H. Zhang, S. Shetty, and R. J. Pomerantz. 1999. HIV-1 virions produced from replicating peripheral blood lymphocytes are more infectious than those from nonproliferating macrophages due to higher levels of intravirion reverse transcripts: implications for pathogenesis and transmission. Virology 253:10-16. [DOI] [PubMed] [Google Scholar]
- 19.Dustin, M. L., T. G. Bivona, and M. R. Philips. 2004. Membranes as messengers in T cell adhesion signaling. Nat. Immunol. 5:363-372. [DOI] [PubMed] [Google Scholar]
- 20.Dustin, M. L., and T. A. Springer. 1989. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature 341:619-624. [DOI] [PubMed] [Google Scholar]
- 21.Fortin, J.-F., B. Barbeau, H. Hedman, E. Lundgren, and M. J. Tremblay. 1999. Role of the leukocyte function antigen-1 conformational state in the process of human immunodeficiency virus type 1-mediated syncytium formation and virus infection. Virology 257:228-238. [DOI] [PubMed] [Google Scholar]
- 22.Fortin, J.-F., R. Cantin, G. Lamontagne, and M. Tremblay. 1997. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 71:3588-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortin, J.-F., R. Cantin, and M. J. Tremblay. 1998. T cells expressing activated LFA-1 are more susceptible to infection with human immunodeficiency virus type 1 particles bearing host-encoded ICAM-1. J. Virol. 72:2105-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garrido, M., A. Mozos, A. Martinez, F. Garcia, A. Serafin, V. Morente, M. Caballero, C. Gil, E. Fumero, J. M. Miro, N. Climent, J. M. Gatell, and L. Alos. 2007. HIV-1 upregulates intercellular adhesion molecule-1 gene expression in lymphoid tissue of patients with chronic HIV-1 infection. J. Acquir. Immune Defic. Syndr. 46:268-274. [DOI] [PubMed] [Google Scholar]
- 25.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 26.Giguère, J.-F., and M. J. Tremblay. 2004. Statin compounds reduce human immunodeficiency virus type 1 replication by preventing the interaction between virion-associated host intercellular adhesion molecule 1 and its natural cell surface ligand LFA-1. J. Virol. 78:12062-12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gilbert, C., C. Barat, R. Cantin, and M. J. Tremblay. 2007. Involvement of Src and Syk tyrosine kinases in HIV-1 transfer from dendritic cells to CD4+ T lymphocytes. J. Immunol. 178:2862-2871. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert, C., M. Bergeron, S. Méthot, J.-F. Giguère, and M. J. Tremblay. 2005. Statins could be used to control replication of some viruses, including HIV-1. Viral Immunol. 18:474-489. [DOI] [PubMed] [Google Scholar]
- 29.Gottlieb, A., J. G. Krueger, R. Bright, M. Ling, M. Lebwohl, S. Kang, S. Feldman, M. Spellman, K. Wittkowski, H. D. Ochs, P. Jardieu, R. Bauer, M. White, R. Dedrick, and M. Garovoy. 2000. Effects of administration of a single dose of a humanized monoclonal antibody to CD11a on the immunobiology and clinical activity of psoriasis. J. Am. Acad. Dermatol. 42:428-435. [DOI] [PubMed] [Google Scholar]
- 30.Groot, F., T. W. Kuijpers, B. Berkhout, and E. C. de Jong. 2006. Dendritic cell-mediated HIV-1 transmission to T cells of LAD-1 patients is impaired due to the defect in LFA-1. Retrovirology 3:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groot, F., T. M. van Capel, M. L. Kapsenberg, B. Berkhout, and E. C. de Jong. 2006. Opposing roles of blood myeloid and plasmacytoid dendritic cells in HIV-1 infection of T cells: transmission facilitation versus replication inhibition. Blood 108:1957-1964. [DOI] [PubMed] [Google Scholar]
- 32.Harlan, J. M., and R. K. Winn. 2002. Leukocyte-endothelial interactions: clinical trials of anti-adhesion therapy. Crit. Care Med. 30:S214-S219. [DOI] [PubMed] [Google Scholar]
- 33.Hildreth, J. E., and R. J. Orentas. 1989. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science 244:1075-1078. [DOI] [PubMed] [Google Scholar]
- 34.Jolly, C., K. Kashefi, M. Hollinshead, and Q. J. Sattentau. 2004. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J. Exp. Med. 199:283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jolly, C., I. Mitar, and Q. J. Sattentau. 2007. Adhesion molecule interactions facilitate human immunodeficiency virus type 1-induced virological synapse formation between T cells. J. Virol. 81:13916-13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katagiri, K., A. Maeda, M. Shimonaka, and T. Kinashi. 2003. RAPL, a Rap1-binding molecule that mediates Rap1-induced adhesion through spatial regulation of LFA-1. Nat. Immunol. 4:741-748. [DOI] [PubMed] [Google Scholar]
- 37.Kato, T., H. Hashikabe, C. Iwata, K. Akimoto, and Y. Hattori. 2004. Statin blocks Rho/Rho-kinase signalling and disrupts the actin cytoskeleton: relationship to enhancement of LPS-mediated nitric oxide synthesis in vascular smooth muscle cells. Biochim. Biophys. Acta 1689:267-272. [DOI] [PubMed] [Google Scholar]
- 38.Kim, M., C. V. Carman, and T. A. Springer. 2003. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science 301:1720-1725. [DOI] [PubMed] [Google Scholar]
- 39.Liao, J. K., and U. Laufs. 2005. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 45:89-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lub, M., Y. van Kooyk, and C. G. Figdor. 1995. Ins and outs of LFA-1. Immunol. Today 16:479-483. [DOI] [PubMed] [Google Scholar]
- 41.Martin, G., and M. J. Tremblay. 2004. HLA-DR, ICAM-1, CD40, CD40L, and CD86 are incorporated to a similar degree into clinical human immunodeficiency virus type 1 variants expanded in natural reservoirs such as peripheral blood mononuclear cells and human lymphoid tissue cultured ex vivo. Clin. Immunol. 111:275-285. [DOI] [PubMed] [Google Scholar]
- 42.Ng, T. T., C. Guntermann, K. E. Nye, J. M. Parkin, J. Anderson, J. E. Norman, and W. J. Morrow. 1995. Adhesion co-receptor expression and intracellular signalling in HIV disease: implications for immunotherapy. AIDS 9:337-343. [PubMed] [Google Scholar]
- 43.Odds, F. C. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1. [DOI] [PubMed] [Google Scholar]
- 44.Piguet, V., and Q. Sattentau. 2004. Dangerous liaisons at the virological synapse. J. Clin. Investig. 114:605-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabat. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puigdomenech, I., M. Massanella, N. Izquierdo-Useros, R. Ruiz-Hernandez, M. Curriu, M. Bofill, J. Martinez-Picado, M. Juan, B. Clotet, and J. Blanco. 2008. HIV transfer between CD4 T cells does not require LFA-1 binding to ICAM-1 and is governed by the interaction of HIV envelope glycoprotein with CD4. Retrovirology 5:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothlein, R., and T. A. Springer. 1986. The requirement for lymphocyte function-associated antigen 1 in homotypic leukocyte adhesion stimulated by phorbol ester. J. Exp. Med. 163:1132-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rudnicka, D., J. Feldmann, F. Porrot, S. Wietgrefe, S. Guadagnini, M. C. Prevost, J. Estaquier, A. T. Haase, N. Sol-Foulon, and O. Schwartz. 2009. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J. Virol. 83:6234-6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salas, A., M. Shimaoka, A. N. Kogan, C. Harwood, U. H. von Andrian, and T. A. Springer. 2004. Rolling adhesion through an extended conformation of integrin alphaLbeta2 and relation to alpha I and beta I-like domain interaction. Immunity 20:393-406. [DOI] [PubMed] [Google Scholar]
- 50.Salas, A., M. Shimaoka, U. Phan, M. Kim, and T. A. Springer. 2006. Transition from rolling to firm adhesion can be mimicked by extension of integrin alphaLbeta2 in an intermediate affinity state. J. Biol. Chem. 281:10876-10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanders, R. W., E. C. de Jong, C. E. Baldwin, J. H. Schuitemaker, M. L. Kapsenberg, and B. Berkhout. 2002. Differential transmission of human immunodeficiency virus type 1 by distinct subsets of effector dendritic cells. J. Virol. 76:7812-7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shimaoka, M., A. Salas, W. Yang, G. Weitz-Schmidt, and T. A. Springer. 2003. Small molecule integrin antagonists that bind to the beta2 subunit I-like domain and activate signals in one direction and block them in the other. Immunity 19:391-402. [DOI] [PubMed] [Google Scholar]
- 53.Shimaoka, M., and T. A. Springer. 2004. Therapeutic antagonists and the conformational regulation of the beta2 integrins. Curr. Top. Med. Chem. 4:1485-1495. [DOI] [PubMed] [Google Scholar]
- 54.Shimaoka, M., T. Xiao, J. H. Liu, Y. Yang, Y. Dong, C. D. Jun, A. McCormack, R. Zhang, A. Joachimiak, J. Takagi, J. H. Wang, and T. A. Springer. 2003. Structures of the alpha L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell 112:99-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takagi, J., B. M. Petre, T. Walz, and T. A. Springer. 2002. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110:599-611. [DOI] [PubMed] [Google Scholar]
- 56.Tardif, M. R., and M. J. Tremblay. 2003. Presence of host ICAM-1 in human immunodeficiency virus type 1 virions increases productive infection of CD4+ T lymphocytes by favoring cytosolic delivery of viral material. J. Virol. 77:12299-12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tardif, M. R., and M. J. Tremblay. 2005. Regulation of LFA-1 activity through cytoskeleton remodeling and signaling components modulates the efficiency of HIV type-1 entry in activated CD4+ T lymphocytes. J. Immunol. 175:926-935. [DOI] [PubMed] [Google Scholar]
- 58.Terrazas-Aranda, K., Y. Van Herrewege, D. Hazuda, P. Lewi, R. Costi, R. Di Santo, A. Cara, and G. Vanham. 2008. Human immunodeficiency virus type 1 (HIV-1) integration: a potential target for microbicides to prevent cell-free or cell-associated HIV-1 infection. Antimicrob. Agents Chemother. 52:2544-2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turville, S. G., J. J. Santos, I. Frank, P. U. Cameron, J. Wilkinson, M. Miranda-Saksena, J. Dable, H. Stossel, N. Romani, M. Piatak, Jr., J. D. Lifson, M. Pope, and A. L. Cunningham. 2004. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 103:2170-2179. [DOI] [PubMed] [Google Scholar]
- 60.van Kooyk, Y., P. van de Wiel-van Kemenade, P. Weder, T. W. Kuijpers, and C. G. Figdor. 1989. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature 342:811-813. [DOI] [PubMed] [Google Scholar]
- 61.van Kooyk, Y., S. J. van Vliet, and C. G. Figdor. 1999. The actin cytoskeleton regulates LFA-1 ligand binding through avidity rather than affinity changes. J. Biol. Chem. 274:26869-26877. [DOI] [PubMed] [Google Scholar]
- 62.Verhulst, A., P. C. D'Haese, and M. E. De Broe. 2004. Inhibitors of HMG-CoA reductase reduce receptor-mediated endocytosis in human kidney proximal tubular cells. J. Am. Soc. Nephrol. 15:2249-2257. [DOI] [PubMed] [Google Scholar]
- 63.Vermot-Desroches, C., D. Rigal, S. Escaich, J. Bernaud, C. Pichoud, J. P. Lamelin, and C. Trepo. 1991. Functional epitope analysis of the human CD11a/CD18 molecule (LFA-1, lymphocyte function-associated antigen 1) involved in HIV-1-induced syncytium formation. Scand. J. Immunol. 34:461-470. [DOI] [PubMed] [Google Scholar]
- 64.Wang, Y., D. Li, R. Nurieva, J. Yang, M. Sen, R. Carreno, S. Lu, B. W. McIntyre, J. J. Molldrem, G. B. Legge, and Q. Ma. 2009. LFA-1 affinity regulation is necessary for the activation and proliferation of naive T cells. J. Biol. Chem. 284:12645-12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weitz-Schmidt, G., S. Chreng, and S. Riek. 2009. Allosteric LFA-1 inhibitors modulate natural killer cell function. Mol. Pharmacol. 75:355-362. [DOI] [PubMed] [Google Scholar]