Abstract
A population pharmacokinetics analysis was performed after intravenous ganciclovir and oral valganciclovir in solid organ transplant patients with cytomegalovirus. Patients received ganciclovir at 5 mg/kg of body weight (5 days) and then 900 mg of valganciclovir (16 days), both twice daily with dose adjustment for renal function. A total of 382 serum concentrations from days 5 and 15 were analyzed with NONMEM VI. Renal function given by creatinine clearance (CLCR) was the most influential covariate in CL. The final pharmacokinetic parameters were as follows: ganciclovir clearance (CL) was 7.49·(CLCR/57) liter/h (57 was the mean population value of CLCR); the central and peripheral distribution volumes were 31.9 liters and 32.0 liters, respectively; intercompartmental clearance was 10.2 liter/h; the first-order absorption rate constant was 0.895 h−1; bioavailability was 0.825; and lag time was 0.382 h. The CLCR was the best predictor of CL, making dose adjustment by this covariate important to achieve the most efficacious ganciclovir exposure.
Cytomegalovirus (CMV), the most common opportunistic pathogen affecting solid organ transplant (SOT) patients (24), is associated with significant morbidity and mortality (25). Intravenous (i.v.) ganciclovir has been the gold standard for both prevention and treatment of CMV disease in SOT patients (20). The risk of neutropenia, the need for catheter access, and the costs and inconvenience of patient hospitalization after i.v. ganciclovir use have led to the development of oral formulations. Oral ganciclovir is effective in preventing CMV infection and disease, but its low bioavailability (F) limits the degree of viral suppression and may lead to the emergence of viral resistance (6, 18). The development of valganciclovir, an orally available ester prodrug of ganciclovir, has overcome the low F of ganciclovir (7). Following oral intake, the majority of valganciclovir is rapidly converted to ganciclovir by intestinal and hepatic esterases. Ganciclovir is entirely cleared by renal excretion (7).
The efficacy and safety of oral valganciclovir (900 mg) for the treatment of CMV disease in SOT patients have been shown to be comparable to the efficacy and safety of i.v. ganciclovir (5 mg/kg of body weight) when both drugs were given twice daily for 21 days and followed by 900 mg of valganciclovir daily for 28 days (1, 2).
The pharmacokinetics of ganciclovir after i.v. ganciclovir and/or oral valganciclovir administration have been studied in different patient populations (4, 8, 11, 28, 29). Some authors (21) have demonstrated equivalence between different single doses of oral ganciclovir and valganciclovir and i.v. ganciclovir using noncompartmental pharmacokinetic analysis. However, this approach cannot provide a clinically applicable model for dose adjustment. Such a model would be very useful to achieve the required ganciclovir exposure to ensure efficacy and avoid hematological adverse events and/or CMV resistance. It could be the prerequisite step for characterization of the relationship between ganciclovir exposure and viral eradication. In a previous study of SOT recipients with donor-positive/recipient-negative CMV serostatus (27), viremia was suppressed during prophylaxis with ganciclovir exposure values of 40 to 50 μg·hr/ml and there was only a weak tendency to increased neutropenia and leukopenia with higher ganciclovir exposure. Therefore, dose adjustment could be based on this exposure value. Yuen et al. (29) have described the population pharmacokinetics of ganciclovir in kidney transplant and human immunodeficiency virus (HIV)-positive patients. Wiltshire et al. (26) also described the population pharmacokinetics of oral ganciclovir compared with those of valganciclovir in SOT patients. However, population studies dealing with pharmacokinetics of ganciclovir after i.v. ganciclovir followed by oral valganciclovir in SOT patients infected by CMV where drug disposition and F could be estimated are lacking.
The aims of this study were (i) to establish the population pharmacokinetics of ganciclovir after i.v. ganciclovir followed by oral valganciclovir in SOT patients infected by CMV; (ii) to evaluate the effects of several physiopathological factors on the disposition kinetics of ganciclovir and to find potential predictive factors for dosage individualization; and (iii) to evaluate the effectiveness of the manufacturer's recommended adjusted dose in preventing under- or overexposure in SOT patients.
MATERIALS AND METHODS
Study design.
A prospective clinical trial performed according to good clinical practice and in accordance with the Declaration of Helsinki was carried out at the Hospital Universitari de Bellvitge, Barcelona, Spain, between March 2004 and February 2006. All patients provided written informed consent (clinicaltrials.gov identifier NCT00730769).
Patients with established CMV infection undergoing allogeneic SOT (kidney, liver, or heart) were eligible for inclusion if they were ≥18 years old and presented with positive CMV antigenemia (pp65) defined as ≥20 positive cells/105 peripheral blood mononuclear cells. Patients with severe CMV tissue-invasive disease, absolute neutrophil counts of <500/mm3, platelet counts of <25,000/mm3, hemoglobin at <80 g/liter, or estimated glomerular filtration rates of <10 ml/min (according to the Cockroft-Gault formula [9]) were excluded. Demographic characteristics such as age, gender, weight, transplant type, clinical laboratory measurements (creatinine [CR] and CR clearance [CLCR] estimated according to the Cockcroft-Gault formula [5]), and concomitant immunosuppressive medication were recorded.
Ganciclovir/valganciclovir administration.
Patients received i.v. ganciclovir (Cymevene; F. Hoffmann-La Roche Ltd., Basel, Switzerland) at 5 mg/kg over 1 h twice daily for 5 days followed by 900 mg of oral valganciclovir (Valcyte; F. Hoffmann-La Roche Ltd., Basel, Switzerland) twice daily for 16 days (21 days of treatment). For patients with impaired renal function, i.v. and oral doses were adjusted by CLCR (Cockcroft-Gault formula [5]) in accordance with the manufacturer's recommendations (Table 1).
TABLE 1.
Dosing adjustments based on glomerular filtration rate in the solid organ transplant populationa
| Drug | CLCR (ml/min) | Dose (mg/kg)/interval (h) |
|---|---|---|
| Ganciclovir | >50 | 5/12 |
| 25-50 | 2.5/12 | |
| <25 | 2.5/24 | |
| Valganciclovir | ≥60 | 900/12 |
| 40-59 | 450/12 | |
| 25-39 | 450/24 | |
| 10-24 | 450/48 |
The glomerular filtration rate was calculated using the Cockcroft-Gault formula.
Blood sampling and drug analysis.
Blood samples were collected before and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, and 12 h after dosing on treatment days 5 (after i.v. ganciclovir) and 15 (after oral valganciclovir). Further sampling to 24 h was performed in one patient with CLCR values of 10 ml/min (day 5) and 16 ml/min (day 15). Time deviations were taken into account in the pharmacokinetic analysis. Blood samples were centrifuged, and serum samples collected and stored at −20°C until analysis. Ganciclovir concentrations were measured by high-performance liquid chromatography with UV detection, according to a previously validated method (22). Linearity was shown over a range of 0.5 to 30 μg/ml, with an intra- and interassay precision of <7.0% and an accuracy of <5%. The lower limit of quantification was 0.5 μg/ml.
Pharmacokinetic data analysis.
Pharmacokinetic data were analyzed simultaneously using NONMEM (nonlinear mixed-effects modeling) software, version VI (3). As NONMEM estimated the pharmacokinetic parameters of ganciclovir, valganciclovir doses were converted to their equivalent ganciclovir content by multiplying the valganciclovir dose by 0.720 (corresponding to the ratio between the molecular weights of ganciclovir and valganciclovir).
The modeling process consisted of the following three steps: (i) development of the base pharmacokinetic model, incorporating interoccasion variability; (ii) covariate selection; and (iii) model validation. The first-order conditional estimation method with interaction was used throughout the entire model-building process.
Base population pharmacokinetics model.
Models of one and two open compartments with first-order absorption were evaluated, as well as models with and without lag time. The standard lag time models and the transit compartment models (23) were tested to describe the absorption profile. Interpatient and interoccasion (16) variability were assumed to have a log-normal distribution. Additive, proportional, and combined (additive plus proportional) models were compared to assess the residual error (RE) in drug concentrations. The individual contribution to the RE was accounted for by including an interpatient variability term in the RE model when first-order conditional estimation with interaction was applied (14). This term was implemented as an exponential model in the RE model (17). The adequacy of the models developed was evaluated by (i) changes in the minimum objective function value [−2log (likelihood); MOFV] obtained in NONMEM VI; (ii) the precision of the parameter estimates; and (iii) goodness-of-fit plots. For nested models, the ratio of the MOFV is asymptotically chi-square distributed, with degrees of freedom equal to the difference in the number of model parameters. A significance level of 0.05% denoted a significant improvement of fit (drop in the MOFV by >7.879) for a one-parameter difference. Goodness-of-fit was evaluated through plots of typical population model-predicted or individual Bayesian predicted concentrations versus observed concentrations or plots of conditional weighted residuals versus time (12). The Xpose program (13), version 4.0, was used to guide the model-building process.
Covariate model.
The effects of all physiologically reasonable covariates, summarized in Table 2, on model parameters were investigated by plotting empirical Bayes's estimates of pharmacokinetic parameters against covariates, using the generalized additive models procedure (13), and then testing all of them with NONMEM.
TABLE 2.
Demographic and clinical characteristics of the population studieda
| Patient characteristicb | All | Type of transplant |
||
|---|---|---|---|---|
| Kidney | Liver | Heart | ||
| No. of patients | 20 | 10 | 5 | 5 |
| Weight (kg) | 66.2 (12.9) | 65.9 (12.9) | 69.6 (10.8) | 65.8 (5.5) |
| Age (yr) | 55.7 (11.8) | 54.6 (10.3) | 53.2 (8.7) | 54.4 (17.3) |
| CLCR (ml/min) | 57.0 (25.3) | 39.9 (22.9) | 75.1 (18.0) | 73.2 (5.5) |
| Gender (male/female) | 10/10 | 4/6 | 2/3 | 4/1 |
| Concomitant immunosuppressive medication | ||||
| MMF (yes/no) | 16/4 | 9/1 | 4/1 | 5/0 |
| CsA (yes/no) | 11/9 | 3/7 | 4/1 | 4/1 |
| TAC (yes/no) | 8/12 | 6/4 | 1/4 | 1/4 |
| SRL (yes/no) | 1/19 | 1/9 | 0/5 | 0/5 |
Data are given as mean (standard deviation) values or counts.
CsA, cyclosporine; MMF, mycophenolate mofetil; SRL, sirolimus; TAC, tacrolimus. CLCR was estimated according to the Cockcroft-Gault formula.
Covariates were first tested univariately in the model and then by the cumulative forward inclusion/backward elimination procedures. Significance levels of 5% (reduction in the MOFV of >3.841 points) and 1% (increase in the MOFV of >6.635 points) were employed during the forward addition and backward elimination steps. A decrease of at least 10% in interpatient variability associated with a specific pharmacokinetic parameter was considered clinically relevant for the inclusion of that specific covariate. All evaluated relationships between pharmacokinetic parameters and continuous covariates were centered around the mean. The covariates were introduced into the base model, in terms of linear relationships. Because the primary goal of the population pharmacokinetic analysis was to estimate individual patient ganciclovir pharmacokinetic parameters, the extent of Bayesian shrinkage was evaluated for each parameter in the final model using the following formula (15): shrinkage = 1 − (SDηparameter/Ωparameter). In this formula, SDηparameter is the standard deviation of the individual estimates of η (interpatient variability random effect) for each parameter and Ωparameter is the estimate of the standard deviation of the estimated population variance. Large values of shrinkage would be associated with generally poor individual estimates of that parameter.
Model validation.
The bootstrap method with replacement was used to assess the stability of the final model and to construct confidence intervals (CIs) of pharmacokinetic parameters (10), using PsN-Toolkit version 2.2.6 (19). Two hundred data sets were reconstructed by resampling from the original data. The final model was fitted to the replicate data sets, and parameter estimates for each of the replicate data sets were obtained. The mean values of the parameters obtained were compared with those estimated from the original data. The lower and upper limits of the 95% CI for each parameter accounted for its corresponding variability.
Model-based simulations.
The simulated values of the area under the concentration-time curve (AUC) following (i) the manufacturer's dosing strategy taking into account a body weight of 66.2 kg (mean population value) and (ii) the i.v. dosing estimated as the target AUC (AUCtarget; 45 μg·hr/ml) multiplied by the population CL (CLpop; predicted by the final population model) or (iii) the oral dosing estimated as AUCtarget multiplied by the ratio between CLpop and population bioavailability (Fpop; also predicted by the final population model) for 10 different cutoffs of estimated CLCR values (from 10 to 100 ml/min) were compared.
The simulated AUCs were calculated as dose/individual CL (for i.v. ganciclovir) or as individual F·dose/individual CL (for oral valganciclovir). AUCtarget was estimated from the mean value of the AUCtarget range proposed by Wiltshire et al. (27) in SOT patients. The percentages of patients achieving AUCtarget were estimated in all cases. These simulations evaluated the competence of recommended CLCR-adjusted doses regarding their ability to provide target exposure values following i.v. ganciclovir and oral valganciclovir treatment.
RESULTS
Patients.
Twenty-one Caucasian patients with established CMV infections undergoing allogeneic SOT (kidney, liver, or heart) were eligible for inclusion in this study. One patient (liver transplantation) was excluded from the population pharmacokinetics analysis because this patient did not complete the treatment due to pancytopenia.
Baseline demographic and clinical data for all patients, separated by SOT type, are summarized in Table 2. Dose adjustment according to renal function followed the manufacturer's recommendations (Table 1) with the exception of four patients: one with a CLCR of 27 ml/min who received i.v. ganciclovir at 5 mg/kg twice per day; one patient with a CLCR of 55 ml/min who received 900 mg of oral valganciclovir twice per day; and two patients with CLCR values of 38 and 25 ml/min, respectively, who received 450 mg of oral valganciclovir twice per day.
Ganciclovir serum levels.
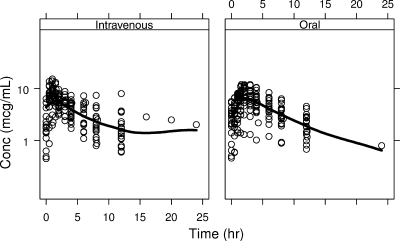
The time profiles of ganciclovir serum levels following i.v. ganciclovir and oral valganciclovir administration are displayed in Fig. 1. The smooth line shows the general trend of the data, and the sampling points adequately define the overall pharmacokinetics profiles of ganciclovir after both i.v. and oral administration. The final data set used for the pharmacokinetics analysis consisted of 382 ganciclovir concentrations, 190 from i.v. ganciclovir and 192 from oral valganciclovir. Only six samples were below the limit of quantification.
FIG. 1.
Ganciclovir serum concentrations versus time postdose following i.v. administration of ganciclovir (left) and oral administration of valganciclovir (right) in SOT patients. Circles represent observed ganciclovir concentrations. Solid lines represent the smooth line indicating the general data trend.
Population pharmacokinetics model. (i) Base population pharmacokinetics model.
The pharmacokinetic disposition of ganciclovir was best described by a two-compartment open linear model with first-order absorption process and lag time, parameterized in terms of total ganciclovir CL, central compartment distribution volume (V1), intercompartmental clearance, peripheral compartment distribution volume (V2), first-order absorption rate constant (ka), F, and lag time. The ka represents both absorption and hydrolysis of valganciclovir in the gut wall and liver, before reaching the systemic circulation. The transit compartment model for modeling the absorption/metabolism process did not result in further improvement of the model. The pharmacokinetic parameters estimated from the base model are listed in Table 3. The logit transformation was applied to F to restrain its estimate values between 0 and 1. Interpatient variability could be included in CL, V1, ka, and F. Consideration of interoccasion variability in disposition pharmacokinetic parameters did not significantly reduce MOFV (P > 0.05). RE was described by a combined error model where the proportional part estimated in terms of variance was 0.144 or 37.95% (expressed as coefficient of variation [CV%]) and the additive part in terms of a standard deviation was 0.475 μg/ml. The inclusion of interpatient variability in the RE model resulted in a decrease in MOFV of 43.43 points and was estimated to be 0.099 (expressed as variance) or 31.46% (expressed as CV%). In addition to resulting in an improvement in individual fits, this led to a marked decrease in the proportional residual variability of 24.21%.
TABLE 3.
Ganciclovir population pharmacokinetic parameter estimates for the base and final models and bootstrap results for the final modela
| Parameter | Unit of measure | Base model parameter estimate (relative SE) | Final model parameter estimate (relative SE) | Mean (95% CI) bootstrap resultsb |
|---|---|---|---|---|
| Pharmacokinetic parameters | ||||
| CL | Liter/h | 6.34 (15.93) | 7.49 (7.76)·(CLCR/57)c | 7.48 (6.27-8.71) |
| V1 | Liter | 32.0 (13.28) | 31.90 (9.81) | 32.76 (21.93-41.87) |
| CLD | Liter/h | 9.65 (18.13) | 10.20 (11.86) | 9.80 (6.47-13.93) |
| V2 | Liter | 31.1 (19.23) | 32.0 (17.78) | 32.93 (11.57-52.42) |
| ka | h−1 | 0.971 (18.43) | 0.895 (10.77) | 0.91 (0.546-1.244) |
| F | 0.760 (6.99) | 0.825 (7.07) | 0.81 (0.698-0.952) | |
| Lag time | h | 0.395 (3.67) | 0.382 (4.69) | 0.38 (0.34-0.42) |
| Interpatient variability ind: | ||||
| CL | 0.466 (26.39) | 0.107 (40.56) | 0.106 (0.027-0.187) | |
| V1 | 0.239 (61.09) | 0.227 (63.00) | 0.208 (−0.045-0.499) | |
| ka | 0.478 (39.96) | 0.464 (36.85) | 0.446 (0.068-0.860) | |
| F | 0.026 (48.58) | 0.049 (63.90) | 0.060 (−0.014-0.352) | |
| Residual variability | ||||
| σ1 | μg/ml | 0.475 (24.84) | 0.465 (15.74) | 0.453 (0.235-0.695) |
| σ22 | 0.144 (24.58) | 0.143 (19.30) | 0.145 (0.069-0.217) | |
| ω2RE | 0.099 (59.84) | 0.116 (54.48) | 0.134 (−0.048-0.28) |
CLD, intercompartmental clearance between central and peripheral compartments; σ1, standard deviation of additive component of residual variability; σ22, variance of proportional component of residual variability; ω2RE, interpatient variability in residual error.
Derived from 200 successful bootstrap sample runs.
CLCR, creatinine clearance, estimated with the Cockroft-Gault formula (ml/min); 57 = mean population CLCR value (ml/min).
Estimates of interpatient variability are expressed as variance values (ω2).
(ii) Covariate analysis.
When covariates were tested univariately, the inclusion of CLCR reduced the MOFV by −33.478 (P < 0.001) and contributed to a decrease of interpatient variability in ganciclovir CL from 68.19% to 32.71%, with CLCR explaining the 52.03% interpatient variability in CL. The inclusion of gender and the coadministration of tacrolimus decreased the MOFV by 9.196 (P < 0.01) and 6.354 points (P < 0.05), respectively. The inclusion of body weight in CL was not statistically significant and explained only 4.74% of the interpatient variability in this parameter. According to these results, CLCR was the most influential covariate on CL. Cumulative inclusion of gender in the CLCR covariate model failed to cause a further significant drop in the MOFV (−1.289, P > 0.05). After incorporation of tacrolimus administration or body weight in the CLCR covariate model, an increase in the MOFV was observed in both cases. During the backward elimination, exclusion of any of these covariates, one at a time, from the CLCR model did not result in a significant increase in the MOFV. Therefore, a model which regarded CLCR as the predictor variable of ganciclovir CL was the most suitable, from both a statistical and clinical viewpoint. In addition, CLCR explained the majority of interpatient variability in CL, providing good estimates and significance for all pharmacokinetic parameters.
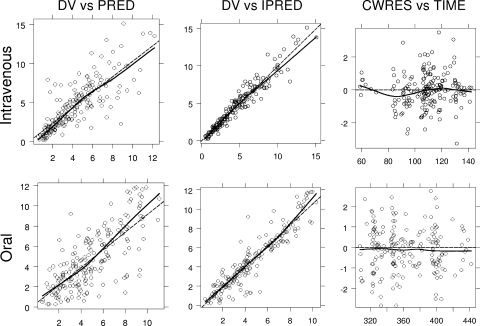
Figure 2 shows the goodness-of-fit plots, demonstrating that the measured and model-predicted concentrations were in good general agreement for both methods of administration (i.v. and oral). Neither treatment showed a specific trend for conditional weighted residuals versus time.
FIG. 2.
Goodness-of-fit plots for the population pharmacokinetics model. Upper panels show results for i.v. administration of ganciclovir and lower panels show results for oral administration of valganciclovir. Ganciclovir concentrations are given in μg/ml and time in hours from the start of the treatment. DV, observed concentrations; PRED, population predictions; IPRED, individual predictions; CWRES, conditional weighted residuals; dashed line, identity line; solid line, smooth line indicating the general trend of the data.
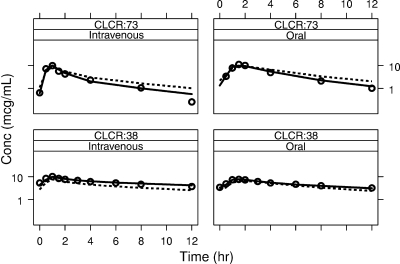
Figure 3 shows superimposed values of the observed, individually predicted, and population-predicted ganciclovir serum concentrations observed in two patients of the population studied, showing CLCR values of 73 ml/min (median) and 38 ml/min (low), respectively, after both i.v. ganciclovir and oral valganciclovir administration. These plots support the fact that the final model satisfactorily fits the data.
FIG. 3.
Superimposed values of the observed (DV, open circles), individually predicted (IPRED, solid line), and population-predicted (PRED, dashed lines) ganciclovir serum concentrations versus time postdose in two patients belonging to the population studied, showing CLCR values of 73 and 38 ml/min, respectively, after both i.v. ganciclovir (left) and oral valganciclovir (right) administration.
The parameter estimates corresponding to the final model are also listed in Table 3. Interpatient variability values for CL were decreased in the final model (interpatient variability of 0.107, expressed as variance, or 32.71%, expressed as CV%) compared with the values for the base model (interpatient variability of 0.466, expressed as variance, or 68.26%, expressed as CV%). Residual variability and interpatient variability in RE were, in both cases, comparable and estimated with similar precision with respect to the base model (Table 3). Estimates of the shrinkage for all the pharmacokinetic parameters were less than 17%, suggesting that in all the cases, the individual estimates were reasonably robust.
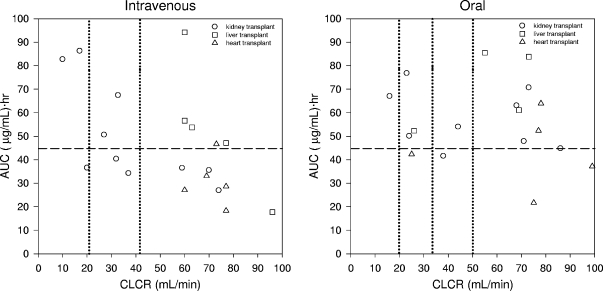
Figure 4 shows the relationship between ganciclovir exposure predicted by the developed model versus CLCR after i.v. ganciclovir and oral valganciclovir administration in SOT patients. These results show a tendency to higher ganciclovir systemic exposure after valganciclovir compared to that after i.v. ganciclovir specifically for patients showing CLCR values of 60 ml/min and higher.
FIG. 4.
Relationship between ganciclovir exposure (AUC) and estimated CLCR in SOT patients after administration of i.v. ganciclovir (left) and oral valganciclovir (right) according to the manufacturer's recommended dosage. Vertical lines show dosage adjustments based on CLCR. Horizontal lines show the AUCtarget value that was considered efficacious.
Model validation.
Table 3 lists results corresponding to the means and 95% CIs of parameter bootstrap estimates. The parameter values for the final model generated from the bootstrap analysis were similar to those of the model developed using the 20 patients in this study. The percentage of difference between the final model and mean bootstrap sample data was lower than 8.5% for all the structural pharmacokinetic and random-effect parameters, with the exception of interpatient variability in RE, where the difference was 15.64%. All final model estimates lie within the 95% CI of the bootstrap values. These results indicate that the estimates for the fixed and random effects in the final model are accurate and that the model is stable.
Model-based simulations.
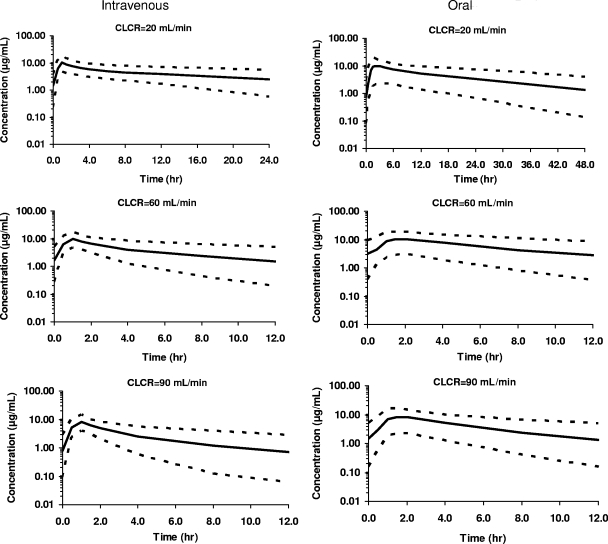
Figure 5 shows the simulated ganciclovir serum levels and 95% CIs for three estimated CLCR values (low, 20 ml/min; median, 60 ml/min; and high, 90 ml/min) following the administration of i.v. ganciclovir and oral valganciclovir according to the adjusted manufacturer's recommended dose (Table 1). A tendency toward lower model-predicted concentrations with higher CLCR values, more noticeable after i.v. administration than after oral administration, is shown.
FIG. 5.
Simulated ganciclovir serum concentrations versus time. Mean and 95% CIs obtained from 1,000 simulations of ganciclovir serum concentration-time profiles after i.v. administration of ganciclovir (left) and oral administration of valganciclovir (right) to patients showing CLCR values of 20, 60, and 90 ml/min and 66.2 kg of body weight and treated according to the manufacturer's recommendations. Solid lines show 50th-percentile mean predictions, and dotted lines show 2.5th and 97.5th percentiles.
Table 4 summarizes the average predicted AUC and 95% CI values using (i) the manufacturer's recommended dosing strategy, taking into account a body weight of 66.2 kg (mean population value), and (ii) the dose adjustment strategy based on AUCtarget·CLpop (for i.v. administration) or AUCtarget·(CLpop/Fpop) (for oral administration) for 10 different cutoffs of estimated CLCR values (from 10 to 100 ml/min). The percentages of patients achieving AUCtarget after each strategy/administration route are also shown. The dose adjustment strategy (based on AUCtarget in the simulations) resulted in a decreased range of AUCs with respect to those resulting from the manufacturer's recommended strategy for both i.v. and oral administration.
TABLE 4.
Mean predicted AUC values from different simulated dosing strategies for patients, showing various estimated CLCR values and percentages of patients achieving AUCtargeta
| CLCR (ml/min) | Value predicted using: |
|||||||
|---|---|---|---|---|---|---|---|---|
| Initial dosing according to manufacturer's instructions |
Dose adjustment according tob: |
|||||||
| AUCtarget·CLpop |
AUCtarget·(CLpop/Fpop) |
|||||||
| IV |
Oral |
IV |
Oral |
|||||
| AUC (95% CI) | % of patients achieving target | AUC (95% CI) | % of patients achieving target | AUC (95% CI) | % of patients achieving target | AUC (95% CI) | % of patients achieving target | |
| 10 | 128.74 (67.88-247.90) | 100.0 | 190.35 (71.31-393.63) | 98.8 | 44.70 (23.72-86.93) | 49.5 | 41.15 (14.31-85.07) | 41.3 |
| 20 | 62.12 (34.85-123.22) | 83.6 | 93.46 (30.94-196.96) | 92.4 | 44.46 (23.93-84.19) | 48.9 | 40.46 (15.53-90.96) | 40.0 |
| 30 | 41.30 (22.67-79.41) | 38.9 | 61.81 (19.87-134.64) | 75.4 | 45.17 (24.70-83.72) | 50.7 | 42.25 (14.62-85.51) | 45.0 |
| 40 | 30.88 (17.03-60.83) | 13.8 | 46.46 (14.27-99.48) | 52.0 | 44.35 (24.06-84.90) | 48.3 | 40.19 (13.55-87.60) | 40.3 |
| 50 | 25.36 (13.49-47.05) | 3.5 | 38.23 (12.63-78.10) | 33.2 | 44.78 (23.92-86.83) | 49.1 | 40.22 (13.83-82.89) | 40.0 |
| 60 | 41.29 (22.13-81.13) | 41.0 | 62.15 (20.39-133.25) | 77.2 | 45.43 (24.41-87.61) | 51.6 | 41.92 (14.37-91.07) | 43.0 |
| 70 | 35.67 (18.19-71.31) | 22.3 | 54.41 (16.90-117.60) | 65.5 | 45.35 (23.18-85.84) | 50.7 | 42.52 (13.85-91.53) | 44.4 |
| 80 | 32.70 (17.22-61.83) | 14.4 | 48.36 (15.19-105.25) | 56.7 | 45.79 (25.42-82.89) | 51.8 | 42.20 (13.45-83.20) | 44.4 |
| 90 | 28.14 (15.47-53.09) | 7.3 | 42.92 (13.50-89.43) | 45.0 | 44.62 (24.15-85.52) | 49.1 | 40.92 (13.77-86.92) | 42.1 |
| 100 | 26.55 (13.81-51.58) | 3.9 | 38.13 (9.43-79.44) | 35.1 | 44.62 (24.46-85.50) | 49.1 | 41.79 (14.33-86.84) | 43.7 |
Mean predicted AUC values, expressed as μg·hr/ml, are from different simulated dosing strategies in patients showing estimated CLCR values from 10 to 100 ml/min and body weight of 66.2 kg (mean population value). See Table 1 for dosing adjustments.
CLpop and Fpop values were estimated from the final model.
The percentages of patients with AUCs over the target after the manufacturer's recommended strategy were in general higher after oral administration than after i.v. administration for patients with CLCR values of 20 ml/min and up. The highest percentages of patients with AUCs over the target were observed at CLCR values of <30 ml/min after both i.v. and oral administration.
DISCUSSION
Considerable variability exists regarding the pharmacokinetics of ganciclovir following i.v. ganciclovir and oral valganciclovir administration in SOT patients. This study was undertaken to develop a clinically applicable population pharmacokinetics model to quantify inter- and intrapatient variability and to analyze the effects of physiopathological factors on the pharmacokinetics of ganciclovir, with the aim of explaining the observed variability.
Therapeutic drug monitoring of ganciclovir after valganciclovir administration is not employed in the treatment of SOT patients infected with CMV. A previous study demonstrated viremia suppression during prophylaxis with ganciclovir exposure (AUC) of 40 to 50 μg·ml/hr (27), suggesting that dose adjustment to a target exposure value would justify the development of a clinically applicable population pharmacokinetics model. To date, no pharmacokinetics/pharmacodynamics relationship or target exposure (AUC) values have been established for the treatment of CMV disease in infected SOT patients. The current study represents a first step toward developing optimal ganciclovir dosing schedules for the treatment of CMV disease on the basis of a pharmacokinetics/pharmacodynamics relationship.
The pharmacokinetic disposition of ganciclovir was best described by a two-compartment open linear model with first-order absorption process and elimination from the central compartment. This corresponds with previous studies of i.v. ganciclovir (29) and oral valganciclovir in SOT patients (26). The population pharmacokinetics model obtained in this work yielded basic pharmacokinetic parameter estimates that were in agreement with previous results (29).
In the current study, CL of ganciclovir was influenced by renal function through the estimated CLCR according to the Cockcroft-Gault formula (5), a result that is expected with a drug that is excreted unchanged by the kidney (20) and which has been reported previously (29). Explicitly, the CL of ganciclovir was estimated to increase with estimated CLCR. This supports a CLCR-tiered dosing regimen for ganciclovir/valganciclovir to ensure similar exposure across the renal function range in transplant patients.
CLCR was shown to be the most influential covariate; the estimated CLCR explained most of the interpatient variability in CL (52.03%). Other covariates tested, statistically justifiable for the model, including gender or coadministration of tacrolimus, were correlated with CLCR. Of note, gender influence may be confounded because 6 of 10 females were kidney transplant recipients having CLCR values of <38 ml/min, whereas only 4 of 10 males were kidney transplant recipients and three of the four had CLCR values of >58 ml/min. Similarly, the influence of tacrolimus coadministration could be attributed to the fact that six out of eight patients (75.0%) taking tacrolimus were kidney transplant recipients and three of these five were treated with tacrolimus because of an acute rejection episode, so they had compromised renal function, evidenced by their estimated CLCR values (lower than 35 ml/min). The results of the present study indicated that no pharmacokinetic parameters were statistically related to body weight. The influence of body weight on CL was not statistically significant and explained only 4% of interpatient variability in CL. Therefore, in transplant patients, variability due to renal function appeared to be more relevant than that due to differences in body weight. In fact, the therapeutic impact of this may be irrelevant since estimation of CLCR is adjusted by body weight, and hence, this factor is implicit in dose adjustment using CLCR calculations.
In the present study, the fact that body weight did not statistically influence CL was inconsistent with the results of a previous population pharmacokinetics study (29) in which i.v. ganciclovir (1.2 to 5.0 mg/kg) was given to HIV-positive patients and kidney transplant patients and which demonstrated that even when CL was corrected by body weight, CLCR, and disease process, the interpatient variability associated with ganciclovir CL was 47.5% (CV%). By comparison, the present study demonstrated an interpatient variability in ganciclovir CL of only 32.71% (CV%).
On the other hand, according to that study (29), the ganciclovir CL estimated for a 66.2-kg (mean body weight of the population of the present study) kidney transplant patient with a CLCR value of 40 ml/min was shown to be 6.43 liter/h [estimated from CL = 0.382 + 0.168·body weight·(CLCR/100)·(1 − T)· (1 − CMV), where T = 0/0.76 for nontransplant/transplant patients and CMV = 0/0.41 for HIV-positive patients shedding CMV into urine/HIV-positive patients with CMV retinitis], whereas a value of 5.26 liter/h [estimated from CL = 7.49·(CLCR/57)] was given by the model developed in the present study. These discrepancies could be attributed to the fact that only 5 of the 53 patients included in the previous study were kidney transplant patients, and hence, it is possible that CL values could not be accurately estimated in the kidney transplant subpopulation.
The results of the present study predicted that typical CL would range from 1.31 liter/h (for a typical patient with CLCR of 10 ml/min) to 13.14 liter/h (for a typical patient with CLCR of 100 ml/min) [both values were estimated from CL = 7.49·(CLCR/57)]. The therapeutic implications of this are that patients with CLCR values of 100 ml/min would require considerably larger doses (around 10 times) of ganciclovir than those with CLCR values of 10 ml/min.
Regarding the other pharmacokinetic parameters estimated by the final model reported herein, the V1 and V2 values were consistent with those estimated from results previously reported by Yuen et al. (29) (V1 = 0.391·body weight and V2 = 0.511·body weight; i.e., for a patient of 66.2 kg, V1 was 31.90 liter herein versus 25.88 liter in the study of Yuen et al. and V2 was 32.0 liter versus 33.83 liter, respectively), as was intercompartmental clearance (10.20 liter/h versus 13.4 liter/h, respectively).
Both Yuen et al. (29) after administration of i.v. ganciclovir and Wiltshire et al. (26) after administration of oral ganciclovir and oral valganciclovir to SOT patients reported that body weight was statistically significant in V1 and V2; however, no significant relationship between distribution volumes and body weight could be demonstrated in the present study.
The ka and lag time parameters estimated in the current study differed from those reported by Wiltshire et al. (26) (0.895 versus 0.36 h−1 for ka and 0.382 h versus 0.661 h for lag time, respectively). These differences could be attributed to the sampling design used in each study. In the present study, the absorption process was characterized by sampling at 0.5, 1, 1.5, 2, and 3 h postadministration, while in the Wiltshire study (26), only one sample covered the absorption phase (1 to 3 h). The absolute F after oral administration of valganciclovir was 0.825 in the present study. To our knowledge, this is the first report of this parameter in an SOT population infected by CMV.
One of the purposes of this study was to use the model developed to evaluate the adequacy of the manufacturer's recommended CLCR-adjusted doses to prevent under- or overexposure to ganciclovir. On the basis of previous work (27), viremia can be suppressed during prophylaxis with exposures of 40 to 50 μg·hr/ml; hence, in the current study, a target exposure of 45 μg·hr/ml was considered adequate for that purpose.
Comparison of exposures achieved after the manufacturer's recommended initial doses, taking into account a body weight of 66.2 kg (mean population value in this study), to those after dose adjustment by AUCtarget using the model developed (Table 4) showed that orally administered doses resulted in adequate exposures in all cases, with the exception of patients with CLCR values under 30 ml/min, in which case overexposure occurred. Actually, mean AUC values of 94.46 μg·hr/ml (CLCR = 20 ml/min) and 190.35 μg·hr/ml (CLCR = 10 ml/min) were found with the 92.4% and 98.8%, respectively, of patients showing exposure values higher than 45 μg·hr/ml. After i.v. administration, a tendency to underexposure for patients with CLCR values over 80 ml/min (mean AUC values were 28.14 μg·hr/ml for patients with a CLCR of 90 ml/min and 26.55 μg·hr/ml for those with a CLCR of 100 ml/min) and overexposure for patients with CLCR values under 30 ml/min was observed (mean AUC values were 62.12 μg·hr/ml for patients with a CLCR of 20 ml/min and 128.74 μg·hr/ml for those with a CLCR of 10 ml/min). Patients with CLCR values of 50 ml/min were specifically underdosed after i.v. administration (mean AUC values were 25.36 μg·hr/ml, and only 3.5% of patients achieved an exposure higher than 45 μg·hr/ml). These results are also graphically displayed in Fig. 4 and are in agreement with the percentages of patients achieving exposure values over 45 μg·hr/ml for each CLCR cutoff value (Table 4).
Dose adjustment by the model developed in this study provided mean AUC values closer to the target of 45 μg·hr/ml in all cases. Moreover, the percentages of patients achieving exposures higher than 45 μg·hr/ml ranged from 40 to 50%, which is more in line with the percentages that should be expected.
The results presented here also indicate that our model could provide initial dose optimization based on CLCR values and the target exposure (AUC) required to achieve efficacy, as well as further therapeutic drug monitoring, specifically for those patients in whom changes in renal function can occur throughout the treatment period.
In conclusion, adaptive dosing following the start of the treatment was feasible in the population studied, since they received repeated doses of ganciclovir/valganciclovir. An approach allowing modification of the ganciclovir/valganciclovir dose to achieve a target plasma exposure from the start of the treatment is likely to improve outcome in SOT patients infected by CMV. Studies with a larger number of patients would be necessary to confirm the optimum dosage regimen after both i.v. and oral administration. Hence, the results from this study might assist the optimization of initial dosing regimens aimed at achieving AUCtarget from the start of the treatment.
Acknowledgments
We thank the Biochemistry Department (Hospital Vall d'Hebron) for their help in determining ganciclovir serum concentrations and the nurses of the Nephrology Department of Hospital de Bellvitge, Barcelona, for their technical support in ganciclovir/valganciclovir administration and blood sampling. We also thank Anne-Marie Stephani of Wolters Kluwer Health for editorial assistance with the preparation of the manuscript.
Support for this study was partly provided by Instituto Carlos III (Spain), plan 2005-2008.
The authors have no financial or personal conflicts of interest related to the manuscript.
The registration number at clinicaltrials.gov is NCT00730769.
Footnotes
Published ahead of print on 8 September 2009.
REFERENCES
- 1.Asberg, A., A. Humar, H. Rollag, A. G. Jardine, H. Mouas, M. D. Pescovitz, D. Sgarabotto, M. Tuncer, I. L. Noronha, and A. Hartmann. 2007. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am. J. Transplant. 7:2106-2113. [DOI] [PubMed] [Google Scholar]
- 2.Avery, R. K. 2007. Valganciclovir versus IV ganciclovir for therapy of cytomegalovirus viremia: has victory been achieved? Am. J. Transplant. 7:2062-2063. [DOI] [PubMed] [Google Scholar]
- 3.Beal, S. L., and L. B. Sheiner (ed.). 1992. NONMEM VI user's guide. Globomax LLC, Hanover, MD.
- 4.Brown, F., L. Banken, K. Saywell, and I. Arum. 1999. Pharmacokinetics of valganciclovir and ganciclovir following multiple oral dosages of valganciclovir in HIV- and CMV-seropositive volunteers. Clin. Pharmacokinet. 37:167-176. [DOI] [PubMed] [Google Scholar]
- 5.Cockcroft, D. W., and M. H. Gault. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31-41. [DOI] [PubMed] [Google Scholar]
- 6.Crumpacker, C. S. 1996. Ganciclovir. N. Engl. J. Med. 335:721-729. [DOI] [PubMed] [Google Scholar]
- 7.Cvetkovic, R. S., and K. Wellington. 2005. Valganciclovir: a review of its use in the management of CMV infection and disease in immunocompromised patients. Drugs 65:859-878. [DOI] [PubMed] [Google Scholar]
- 8.Czock, D., C. Scholle, F. M. Rasche, D. Schaarschmidt, and F. Keller. 2002. Pharmacokinetics of valganciclovir and ganciclovir in renal impairment. Clin. Pharmacol. Ther. 72:142-150. [DOI] [PubMed] [Google Scholar]
- 9.Dubois, D., and E. Dubois. 1916. A formula to estimate the approximate surface area if height and weight are known. Arch. Intern. Med. 17:863-871. [Google Scholar]
- 10.Efron, B. 1979. Bootstrap methods: another look at the jacknife. Ann. Stat. 7:1-26. [Google Scholar]
- 11.Einsele, H., P. Reusser, M. Bornhauser, P. Kalhs, G. Ehninger, H. Hebart, Y. Chalandon, N. Kroger, B. Hertenstein, and F. Rohde. 2006. Oral valganciclovir leads to higher exposure to ganciclovir than intravenous ganciclovir in patients following allogeneic stem cell transplantation. Blood 107:3002-3008. [DOI] [PubMed] [Google Scholar]
- 12.Ette, E. I., and T. M. Ludden. 1995. Population pharmacokinetic modeling: the importance of informative graphics. Pharm. Res. 12:1845-1855. [DOI] [PubMed] [Google Scholar]
- 13.Jonsson, E. N., and M. O. Karlsson. 1999. Xpose: an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 58:51-64. [DOI] [PubMed] [Google Scholar]
- 14.Karlsson, M. O., E. N. Jonsson, C. G. Wiltse, and J. R. Wade. 1998. Assumption testing in population pharmacokinetic models: illustrated with an analysis of moxonidine data from congestive heart failure patients. J. Pharmacokinet. Biopharm. 26:207-246. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson, M. O., and R. M. Savic. 2007. Diagnosing and model diagnostics. Clin. Pharmacol. Ther. 82:17-20. [DOI] [PubMed] [Google Scholar]
- 16.Karlsson, M. O., and L. B. Sheiner. 1993. The importance of modeling interoccasion variability in population pharmacokinetic analyses. J. Pharmacokinet. Biopharm. 21:735-750. [DOI] [PubMed] [Google Scholar]
- 17.Kerbusch, T., P. A. Milligan, and M. O. Karlsson. 2004. Assessment of the relative in vivo potency of the hydroxylated metabolite of darifenacin in its ability to decrease salivary flow using pooled population pharmacokinetic-phamacodynamic data. Br. J. Clin. Pharmacol. 57:170-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limaye, A. P. 2002. Ganciclovir-resistant cytomegalovirus in organ transplant recipients. Clin. Infect. Dis. 35:866-872. [DOI] [PubMed] [Google Scholar]
- 19.Lindbom, L., P. Pihlgren, and N. Jonsson. 2005. PsN-Toolkit: a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput. Methods Programs Biomed. 79:241-257. [DOI] [PubMed] [Google Scholar]
- 20.McGavin, J. K., and K. L. Goa. 2001. Ganciclovir: an update of its use in the prevention of cytomegalovirus infection and disease in transplant recipients. Drugs 61:1153-1183. [DOI] [PubMed] [Google Scholar]
- 21.Pescovitz, M. D., J. Rabkin, R. M. Merion, C. V. Paya, J. Pirsch, R. B. Freeman, J. O'Grady, C. Robinson, Z. To, K. Wren, L. Banken, W. Buhles, and F. Brown. 2000. Valganciclovir results in improved oral absorption of ganciclovir in liver transplant recipients. Antimicrob. Agents Chemother. 44:2811-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pou, L., F. Campos, B. Almirante, et al. 1993. A rapid liquid chromatographic (HPLC) method for determination of ganciclovir in serum, p. 183-186. In M. M. Galteau, G. Siest, and J. Henny (ed.), Biology prospective. Comptes rendís du 8ieme Coloque de Pont-a-Mousson. John Libbey Eurotext, Paris, France.
- 23.Savic, R. M., D. M. Jonker, T. Kerbusch, and M. O. Karlsson. 2007. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J. Pharmacokinet. Pharmacodyn. 34:711-726. [DOI] [PubMed] [Google Scholar]
- 24.Tolkoff-Rubin, N. E., and R. H. Rubin. 1999. The impact of cytomegalovirus infection on graft function and patient outcome. Graft 2:S101-S103. [Google Scholar]
- 25.Vancíková, Z., and P. Dvorak. 2001. Cytomegalovirus infection in immunocompetent and immunocompromised individuals: a review. Curr. Drug Targets Immune Endocr. Metabol. Disord. 1:179-187. [PubMed] [Google Scholar]
- 26.Wiltshire, H., S. Hirankarn, C. Farrell, C. Paya, M. D. Pescovitz, A. Humar, E. Dominguez, K. Washburn, E. Blumberg, B. Alexander, R. Freeman, and N. Heaton. 2005. Pharmacokinetic profile of ganciclovir after its oral administration and from its prodrug, valganciclovir, in solid organ transplant recipients. Clin. Pharmacokinet. 44:495-507. [DOI] [PubMed] [Google Scholar]
- 27.Wiltshire, H., C. V. Paya, M. D. Pescovitz, A. Humar, E. Dominguez, K. Washburn, E. Blumberg, B. Alexander, R. Freeman, N. Heaton, and K. P. Zuideveld. 2005. Pharmacodynamics of oral ganciclovir and valganciclovir in solid organ transplant recipients. Transplantation 79:1477-1483. [DOI] [PubMed] [Google Scholar]
- 28.Winston, D. J., L. R. Baden, D. A. Gabriel, C. Emmanouilides, L. M. Shaw, W. R. Lange, and V. Ratanatharathorn. 2006. Pharmacokinetics of ganciclovir after oral valganciclovir versus intravenous ganciclovir in allogeneic stem cell transplant patients with graft-versus-host disease of the gastrointestinal tract. Biol. Blood Marrow Transplant. 12:635-640. [DOI] [PubMed] [Google Scholar]
- 29.Yuen, G. J., G. L. Drusano, C. Fletcher, E. Capparelli, J. D. Connor, J. P. Lalezari, L. Drew, S. Follansbee, D. Busch, M. Jacobson, et al. 1995. Population differences in ganciclovir clearance as determined by nonlinear mixed-effects modelling. Antimicrob. Agents Chemother. 39:2350-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]