Abstract
Previously it has been shown that tipranavir-ritonavir (TPV/r) does not affect efavirenz (EFV) plasma concentrations. This study investigates the effect of steady-state EFV on steady-state TPV/r pharmacokinetics. This was a single-center, open-label, multiple-dose study of healthy adult female and male volunteers. TPV/r 500/200 mg twice a day (BID) was given with food for 24 days. After dosing with TPV/r for 10 days, EFV 600 mg once a day was added to the regimen. Intensive pharmacokinetic (PK) sampling was done on days 10 and 24. Validated bioanalytical high-pressure liquid chromatography-tandem mass spectrometry methods were used to determine plasma tipranavir (TPV), ritonavir (RTV), and EFV concentrations. Thirty-four subjects were entered into the study, and 16 subjects completed it. The geometric mean ratios (90% confidence intervals) for TPV and RTV area under the curves, Cmaxs, and Cmins comparing TPV/r alone and in combination with EFV were 0.97 (0.87 to 1.09), 0.92 (0.81 to 1.03), and 1.19 (0.93 to 1.54) for TPV and 1.03 (0.78 to 1.38), 0.92 (0.65 to 1.30), and 1.04 (0.72 to 1.48) for RTV. Frequently observed adverse events were diarrhea, headache, dizziness, abnormal dreams, and rash. EFV had no effect on the steady-state PK of TPV or RTV, with the exception of a 19% increase in the TPV Cmin, which is not clinically relevant. TPV/r can be safely coadministered with EFV and without the need for a dose adjustment.
Tipranavir (TPV; Aptivus) is an approved protease inhibitor (PI) with potent activity against multiple-PI-resistant human immunodeficiency virus type 1 (HIV-1). The RESIST clinical studies showed better virological and immunological responses over 48 weeks with patients with extensive treatment experience for TPV-ritonavir (RTV) (TPV/r) with an optimized background regimen then with optimized boosted PI therapy (6). TPV is metabolized by cytochrome P450 3A4 (CYP3A4), and to achieve effective plasma TPV concentrations and a twice-daily (BID) dosing regimen with treatment-experienced patients, coadministration of TPV 500 mg with 200 mg of RTV (TPV/r) is essential (10). The TPV/r combination has both inducing and inhibiting effects on several CYP enzymes and has been shown to be a net inducer of CYP3A4 (M. Vourvahis, J. Dumond, K. Patterson, N. Rezk, N. White, S. Jennings, H. Tien, J. Sabo, T. MacGregor, and A. Kashuba, Effects of tipranavir/ritonavir [TPV/r] on the activity of hepatic and intestinal cytochrome P450 3A4/5 and P-glycoprotein [P-gp]: implications for drug interactions, presented at the 14th Conference on Retroviruses and Opportunistic Infections, Los Angeles, CA, 2007). As a result, drug interactions are to be expected when combining TPV/r with other medications (17). The combination of TPV/r with efavirenz (EFV) is an option in the treatment of HIV-infected individuals and should be assessed for possible drug interactions. Previously it has been shown that TPV/r does not affect EFV concentrations when administered together to healthy volunteers (13) and to HIV-1-infected patients (F. D. Goebel, J. P. Sabo, T. R. MacGregor, D. L. Mayers, and S. McCallister, Pharmacokinetic drug interaction screen of three doses of tipranavir/ritonavir [TPV/r] in HIV-infected patients on stable highly active antiretroviral therapy [HAART], presented at HIV DART Conference, Naples, FL, 2002). The objective of the current study was to determine the effects of steady-state EFV 600 mg once a day (QD) on the steady-state pharmacokinetics of TPV 500 mg and RTV 200 mg, both administered BID. The primary pharmacokinetic (PK) endpoints were area under the concentration-time curve (AUC), maximum concentration of drug in plasma (Cmax), and minimum concentration of drug in plasma (Cmin) for TPV and RTV. The secondary endpoints included other PK parameters as well as AEs (adverse events) and clinical laboratory test results.
MATERIALS AND METHODS
Study design.
This study was approved by the Ottawa Health Research Ethics Board and was performed at the Clinical Investigation Unit at the Ottawa Hospital. The study was carried out in compliance with the protocol, and the principles were laid down in the Declaration of Helsinki (October 1996), in accordance with the ICH Harmonised Tripartite Guideline for Good Clinical Practice and relevant Boehringer Ingelheim standard operating procedures.
A set of inclusion and exclusion criteria was used to select healthy volunteers for this study. Female and male subjects between 18 and 60 years old had to be in good health, with a body mass index between 18 and 29.9 kg/m2. Subjects had to be nonsmokers and able to adhere to the protocol, as well as not using any medications, herbs, or foods that could interfere with the study medications. Subjects were to be excluded if there were signs of disease (whether or not needing medical treatment), participation in other trials, blood donations, known hypersensitivities to the study drugs, and use of certain medications, including pharmacological contraceptives. For female subjects, pregnancy, breastfeeding, and not using a barrier method of birth control were exclusion criteria. All subjects had to sign an informed consent form before any study-related procedure could take place.
This was an open-label study of healthy male and female volunteers to investigate the effect of steady-state EFV on the pharmacokinetics of steady-state TPV and RTV. The subjects received 500 mg of TPV and 200 mg of RTV BID at 8:00 a.m. and 8:00 p.m. from day 1 to day 23 and received one last dose at 8:00 a.m. on day 24. On day 10, 600-mg EFV tablets were added to the treatment regimen as a single evening dose administered at 10:00 p.m. until day 23 of the study. This dosing time just before bedtime was chosen to minimize the central nervous system side effects commonly associated with EFV use. The treatment period ended on day 24, with the last dose of TPV/r administered at 8:00 a.m., and the trial ended after a follow-up period on day 38. The study was not designed as a crossover study (TPV/r alone to TPV/r plus EFV versus TPV/r plus EFV to TPV/r alone) because of the long half-life of EFV, which could influence the study results in such a design or would require a long washout.
Serial blood sampling for TPV and RTV was done just before (nominal time zero hours) and at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, and 12 h after intake of medication on study days 10 and 24. AE, safety, and concomitant medication data were collected throughout the study.
All three drugs in this study (TPV, RTV, and EFV) were to be consumed orally with 240 ml of water. TPV/r was administered with a light meal (357 kcal; 8.2 g fat and 10.6 g protein) at 8:00 a.m. and 8:00 p.m. during this study, while EFV was administered without food QD at 10:00 p.m. During the period that the subjects were self-dosing at home, the investigator provided a guideline for light meals that the subjects consumed with their medications.
Bioanalysis. (i) TPV and RTV.
Plasma samples were analyzed for TPV and RTV with a validated liquid chromatography-tandem mass spectrometry method at BASi Analytics, West Lafayette, IN. TPV, RTV, and the internal standard were extracted from human K3EDTA plasma by a two-step liquid-liquid extraction method that used an ethyl acetate-hexane mixture followed by a hexane wash. The analytes were separated and detected by a liquid chromatography-tandem mass spectrometry system that used a 2.0- by 30-mm Synergi Polar-RP column with a formic acid-acetic acid-acetonitrile mobile phase. The high-calibration curve ranged from 1,000 ng/ml to 20,000 ng/ml. For TPV, the intraday accuracy and precision were −13.8% to 7.6% and 1.9% to 9.6%, respectively. The interday accuracy and precision were −7.6% to 4.0% and 4.8% to 7.2%, respectively. For RTV, these numbers were −14.4% to 2.1%, 1.0% to 6.8%, −5.4% to 0.3%, and 5.6% to 8.7%, respectively.
For the low-calibration curve from 25.0 ng/ml to 2,000 ng/ml, the TPV intraday accuracy and precision were −10.3% to 7.9% and 1.6% to 7.7%, respectively. The interday accuracy and precision were −5.2% to −1.6% and 5.1% to 7.0%, respectively. For RTV, these numbers were −9.4% to 6.0%, 1.6% to 6.9%, −8.3% to 2.4%, and 5.4% to 6.9%, respectively.
(ii) EFV.
For the determination of EFV concentrations in plasma, a method similar to that for TPV and RTV was developed at BASi Analytics, West Lafayette, IN. EFV and the internal standard nevirapine were extracted from human K3EDTA plasma by a liquid-liquid extraction method using methyl t-butyl ether. The calibration curve ranged from 0.200 μg/ml to 10.0 μg/ml. The intraday accuracy and precision were −11.0% to 4.0% and 0.7% to 3.4%, respectively. The interday accuracy and precision were −4.2% to 0.0% and 2.9% to 4.8%, respectively.
Pharmacokinetics.
Noncompartmental methods were used for PK analysis (WinNonlin version 5.01, Pharsight Corporation, Mountain View, CA). The highest observed plasma concentration was defined as the Cmax, with the corresponding sampling time as tmax. The elimination rate constant (λz) was determined by least-squares linear regression analysis (log C versus t) of the last data points (n ≥ 3). The t1/2 was calculated by the equation t1/2 = ln2/λz. The AUC was estimated using the linear-log trapezoidal rule (linear up/log down) for 0 to 12 h postdose. The concentration at 12 h postdose was defined as the Cmin. The Cl/F, where F represents the oral bioavailability, was calculated as dose/AUC, and the volume of distribution (V) was calculated as (Cl/F)/λz.
Safety.
Safety data, including biochemistry, vital signs, physical exams, and AEs were collected throughout the study. All data were captured in an electronic online case report form. All subjects treated with at least one dose of the study drugs were included in the safety analysis.
Statistics.
Statistical analysis was done with SAS (SAS Institute Inc., Cary, NC; release 8.02). For TPV and RTV, geometric means and coefficients of variation were calculated for all PK parameters. The ratio of the geometric means (GMR) of the TPV AUC, Cmax, and Cmin for TPV/r plus EFV to those for TPV/r alone was used to assess if there was a drug-drug interaction (18). The null hypothesis tested was that these ratios were either below the lower boundary of relevance (0.80) or above the upper boundary of relevance (1.25). The alternative hypothesis was that these ratios were contained within the relevance boundaries. The null hypothesis was rejected in favor of the hypothesis of the absence of a relevant interaction if the 90% confidence interval (CI) for the GMR was completely contained in the acceptance region of 0.80 to 1.25.
Sample size.
A sample size of 24 completed subjects was planned in order to provide at least 80% power to conclude no drug-drug interaction between TPV and EFV. This sample size was based on variability estimates from previous TPV PK studies, including a study that examined the effect of TPV coadministration on EFV pharmacokinetics. Due to discontinuations of subjects in the study, data from 16 subjects were included in the PK analyses. The planned analyses were carried out despite the knowledge that, because of a smaller sample size than planned, there would likely be higher variability than planned in our estimates of the GMR. This could have resulted in our primary objective of staying completely within boundaries of 80 to 125% being less likely to be attained.
RESULTS
Study population.
The study enrolled 34 subjects (18 males and 16 females; 33 Caucasians and 1 black), and their median (minimum to maximum) age, height, weight, and body mass index were 37 (21 to 58) years, 167.5 (156 to 189) cm, 76.3 (55 to 103.5) kg, and 26.1 (19.9 to 30.2) kg/m2, respectively. A total of 18 subjects were discontinued (5 for AEs [4 for liver enzyme elevations and 1 for viral pleurisy], 1 for noncompliance to the protocol, 1 for withdrawal of consent, and 11 because the study was prematurely discontinued). Boehringer Ingelheim closed this trial prior to completion after discussion with Health Canada regarding a safety finding of intracranial hemorrhage in a small number of HIV-infected patients receiving TPV/r in long-term follow-up of the clinical development program (2, 8). PK data were available for 16 subjects. Safety data for this study were based on all 34 subjects that received study medication.
Pharmacokinetics.
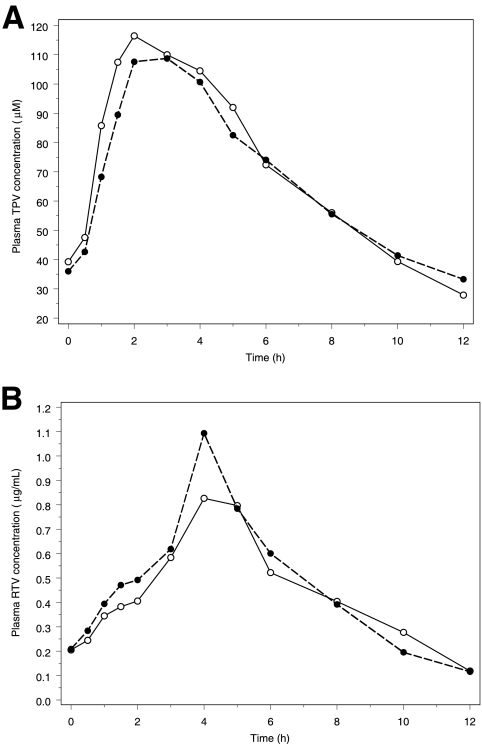
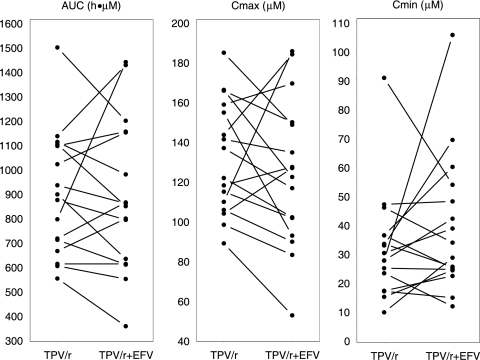
The PK results for TPV, RTV, and EFV were based on the data from the 16 subjects that completed the study. For TPV, the GMR and 90% CI for the AUC, Cmax, and Cmin comparing TPV/r alone and in combination with EFV were 0.97 and 0.87 to 1.09, 0.92 and 0.81 to 1.03, and 1.19 and 0.93 to 1.54, respectively. For RTV, the GMR and 90% CI for the AUC, Cmax, and Cmin were 1.03 and 0.78 to 1.38, 0.92 and 0.65 to 1.30, and 1.04 and 0.72 to 1.48, respectively. The details of the PK results for TPV and RTV can be found in Table 1. In Fig. 1, the plasma drug concentration-time profiles for TPV and RTV based on the geometric mean concentrations from the 16 subjects at each time point are presented. Figure 2 visualizes the individual effect of EFV on the TPV AUC, Cmax, and Cmin for the 16 subjects. This figure shows an increase of TPV pharmacokinetics with some subjects, whereas a decrease was observed with others, after the addition of EFV.
TABLE 1.
Summary of the steady-state pharmacokinetics for TPV and RTVa
| PK parameter | TPV/r | TPV/r + EFV | GMR (90% CI) |
|---|---|---|---|
| TPV | |||
| AUC (h·μM) | 858.9 (28.9) | 834.2 (39.2) | 0.97 (0.87-1.09) |
| Cmax (μM) | 128.7 (22.0) | 118.0 (34.0) | 0.92 (0.81-1.03) |
| Cmin (μM) | 27.9 (57.1) | 33.3 (63.1) | 1.19 (0.93-1.54) |
| Tmax (h) | 2.0 (1.5-2.0) | 3.0 (1.5-4.0) | |
| t1/2 (h) | 4.0 (32.1) | 4.7 (32.4) | |
| Vz/F (liter) | 5.54 (31.4) | 6.72 (50.7) | |
| CL/F (liter/h) | 0.97 (28.9) | 0.99 (39.2) | |
| RTV | |||
| AUC (h·μg/ml) | 6.48 (50.8) | 6.70 (71.4) | 1.03 (0.78-1.38) |
| Cmax (μg/ml) | 1.52 (65.6) | 1.40 (89.1) | 0.92 (0.65-1.30) |
| Cmin (μg/ml) | 0.112 (101.5) | 0.116 (87.0) | 1.04 (0.72-1.48) |
| Tmax (h) | 4.0 (1-10.1) | 4.0 (1.5-12.0) | |
| t1/2 (h)b | 1.8 (23.6) | 2.0 (55.7) | |
| Vz/F (liter)b | 76.7 (54.2) | 78.3 (137.9) | |
| CL/F (L/h) | 30.9 (50.8) | 29.9 (71.4) |
Values are geometric means, with the geometric CV (%) given parenthetically, except for the Tmax value, which is a median, with the minimum to maximum values given parenthetically.
n = 15; for all others, n = 16.
FIG. 1.
Steady-state geometric mean plasma TPV (A) and RTV (B) concentration-time profile for subjects receiving TPV/r 500/200 mg alone BID and TPV/r 500/200 mg BID plus EFV 600 mg QD. Filled bullets and dashed line, TPV/r plus EFV; open bullets and solid line, TPV/r alone.
FIG. 2.
Effect of steady-state EFV on the steady-state TPV AUC, Cmax, and Cmin. Note that the symbols and lines represent individual subjects.
For EFV, the mean ± standard deviation for plasma concentration on day 24 at 22 h was 1.54 ± 0.63 mg/liter, with a minimum to maximum of 0.50 to 2.73 mg/liter. All 16 subjects had detectable concentrations of EFV over the TPV and RTV sampling interval (9.8 to 22 h after the last dose of EFV).
Safety.
All 34 subjects that received study medications were included in the safety evaluation. No deaths or serious AEs occurred during this study. No significant changes in vital signs or physical findings were observed during the study. Table 2 summarizes the safety data for this study. Five subjects were discontinued because of AEs (4 of them because liver enzymes were elevated and 1 because of a viral pleurisy). Of the subjects who received study medications, 30/34 (88.2%) reported gastrointestinal AEs, with diarrhea being the most frequent (27/34; 79.4%), followed by abdominal pain (13/34; 38.2%), nausea (12/34; 35.3%), dyspepsia (4/34; 11.8%), and flatulence (3/34; 8.8%). Nervous system disorders were reported by 25/34 (73.5%) subjects, with headache being the most frequent (15/34; 44.1%), followed by dizziness (14/34; 41.2%), paraesthesia (6/34; 17.6%), and hypoesthesia (4/34; 11.8%). Psychiatric disorders were reported by 9/34 (26.5%), with abnormal dreams (5/34; 14.7%) being the most common. Skin and subcutaneous tissue disorders were reported by 6/34 (17.6%), with rash (4/34; 11.8%) being the most common, followed by pruritus (2/34; 5.9%). General disorders were reported by 18/34 (52.9%), with fatigue being the most frequent (11/34; 32.4%), followed by feeling drunk (9/34; 26.5%).
TABLE 2.
AEs and laboratory abnormalities
| System organ class or preferred term | TPV/r | TPV/r + EFV | Total |
|---|---|---|---|
| No. of subjects (%) | 34 (100.0) | 31 (100.0) | 34 (100.0) |
| Total no. with adverse events (%) | 29 (85.3) | 25 (80.6) | 32 (94.1) |
| Gastrointestinal disorders | 28 (82.4) | 18 (58.1) | 30 (88.2) |
| Nervous system disorders | 11 (32.4) | 19 (61.3) | 25 (73.5) |
| Psychiatric disorders | 3 (8.8) | 8 (25.8) | 9 (26.5) |
| Skin and subcutaneous tissue disorders | 2 (5.9) | 5 (16.1) | 6 (17.6) |
| General disorders | 7 (20.6) | 12 (38.7) | 18 (52.9) |
| No. with AST increase (%) | 5 (14.7) | 5 (16.1) | |
| No. with AST grade 3 or 4 (%) | 1 (2.9) | 2 (6.5) | |
| No. with ALT increase (%) | 12 (35.3) | 13 (41.9) | |
| No. with ALT grade 3 or 4 (%) | 3 (8.8) | 6 (19.4) | |
| Total no. with cholesterol increase (%) | 0 | 6 (19.4) | |
| Total no. with cholesterol grade 3 or 4 (%) | 0 | 3 (9.7) |
DISCUSSION
In this healthy-volunteer drug interaction study, the effect of steady-state EFV on the pharmacokinetics of steady-state TPV and RTV was investigated. The number of evaluable subjects was lower than the 24 subjects planned by sample size calculation and resulted in less statistical power. Nevertheless, the narrow CIs for the GMRs of TPV AUC, 0.87 to 1.09 (GMR of 0.97), and Cmax, 0.81 to 1.03 (GMR of 0.92), suggest a nonsignificant decrease after the coadministration of EFV. TPV Cmin increased by 19% after coadministration of EFV, with a GMR and CI of 1.19 and 0.93 to 1.54, respectively. RTV exposure was unaffected, as shown by the GMRs; however, a larger variability between subjects resulted in wider CIs for the AUC, Cmax, and Cmin of 0.78 to 1.38 (GMR of 1.03), 0.65 to 1.30 (0.92), and 0.72 to 1.48 (1.04).
The approved combination of TPV with RTV (500/200 mg BID) has been shown to be a net inducer of CYP3A4 (M. Vourvahis, J. Dumond, K. Patterson, N. Rezk, N. White, S. Jennings, H. Tien, J. Sabo, T. MacGregor, and A. Kashuba, Effects of tipranavir/ritonavir [TPV/r] on the activity of hepatic and intestinal cytochrome P450 3A4/5 and P-glycoprotein [P-gp]: implications for drug interactions, presented at the 14th Conference on Retroviruses and Opportunistic Infections, Los Angeles, CA, 2007). EFV is also an inducer of CYP3A4 (11) and was expected to decrease the PK parameters of TPV and RTV in the studied combination. Previously it has been shown that EFV results in lower exposure of other RTV-boosted PIs, such as indinavir (1), lopinavir (4, 7, 15), atazanavir (5), fosamprenavir (19), and more recently darunavir (14). In a healthy-volunteer study combining RTV-boosted nelfinavir with EFV, increases in the nelfinavir AUC and Cmax of 30% and 29% were noted as well as a 48% increase in the Cmin as a result of the combination with EFV (9). The absence of additional induction of the metabolism of TPV by EFV is in contrast with these previous results, but in line with the results from the nelfinavir study. However, limits to induction may influence interaction with very potent inducers, such as TPV.
Notwithstanding the findings described above, the combination of three drugs influencing metabolism and transport results in multiple bidirectional interactions, of which the net effect is unpredictable, and increases interpatient variability. This might explain the interindividual differences in the directions of the interaction between EFV and TPV/r, as shown in Fig. 2.
The EFV plasma concentrations measured with this healthy-volunteer population after the final dose of EFV confirm adequate EFV exposure. The average steady-state EFV concentration measured 22 h after the last dose of EFV was 1.54 ± 0.63 mg/liter (minimum to maximum of 0.50 to 2.73 mg/liter) and is in line with data from the EFV product monograph (3). The current study was not designed to assess the effect of TPV/r on the pharmacokinetics of EFV, as this has been investigated in previous studies, and it has been shown that TPV/r does not affect EFV concentrations when administered together to healthy volunteers (13) and to HIV-1-infected patients (F. D. Goebel, J. P. Sabo, T. R. MacGregor, D. L. Mayers, and S. McCallister, Pharmacokinetic drug interaction screen of three doses of tipranavir/ritonavir [TPV/r] in HIV-infected patients on stable highly active antiretroviral therapy [HAART], presented at HIV DART Conference, Naples, FL, 2002).
The most frequently observed AEs were gastrointestinal disorders (88%), nervous system disorders (73%), psychiatric disorders (26%), and skin disorders (18%). Nervous system and psychiatric disorders, consistent with the effects of EFV, occurred more frequently in the second treatment phase of the study after EFV was added to TPV/r. Consistent with other TPV trials, an increase in liver enzymes (ALT and AST) and in total cholesterol levels was observed; however, all laboratory abnormalities returned to baseline after the study drugs were discontinued. In general, both treatment combinations, TPV/r and TPV/r plus EFV, were tolerated, with the majority of AEs being mild in intensity. The observed 19% increase in the TPV Cmin is unlikely to result in limiting toxicity, as this has been documented in the literature. A subanalysis from the RESIST studies has shown that the 45.6% increase in the median Cmin of TPV as a result of coadministration with enfuvirtide did not result in more hepatotoxicity (12). In addition it was shown that TPV Cmins of >120 μM were associated with a higher incidence of grade 3 and 4 elevations in transaminases, compared to lower concentrations (16). In the present study all subjects had TPV Cmins of <120 μM. Finally, the tolerability levels of TPV/r at different doses with EFV were comparable in studies with both HIV-infected patients (F. D. Goebel, J. P. Sabo, T. R. MacGregor, D. L. Mayers, and S. McCallister, Pharmacokinetic drug interaction screen of three doses of tipranavir/ritonavir [TPV/r] in HIV-infected patients on stable highly active antiretroviral therapy [HAART], presented at HIV DART Conference, Naples, FL, 2002) and healthy volunteers (13). Due to the design of the current study, the safety effect of adding TPV/r to existing treatment with EFV could not be studied; however, the previous studies in which TPV/r was added to EFV did not reveal additional EFV-related toxicity (13; F. D. Goebel, J. P. Sabo, T. R. MacGregor, D. L. Mayers, and S. McCallister, Pharmacokinetic drug interaction screen of three doses of tipranavir/ritonavir [TPV/r] in HIV-infected patients on stable highly active antiretroviral therapy [HAART], presented at HIV DART Conference, Naples, FL, 2002).
In conclusion, the coadministration of multiple-dose EFV 600 mg QD with TPV/r 500/200 mg BID had no effect on the steady-state pharmacokinetics of TPV or RTV for healthy volunteer subjects. However, there was a notable exception of a 19% increase in the TPV Cmin, which is not a clinically relevant effect. Therefore, TPV/r can be safely coadministered with EFV and without the need for a dose adjustment.
Acknowledgments
We thank the volunteers that participated in this study. I Séguin, D. Côté, and D. Young are kindly acknowledged for their invaluable help with this study.
D.W.C. is supported by a Career Scientist Award from the Ontario HIV Treatment Network of the Ontario Ministry of Health. D.W.C. and C.J.L.L.P. have received grants and contracts for this and other research from BI through their institution and employer, the University of Ottawa at The Ottawa Hospital and the Ottawa Hospital Research Institute. C.J.L.L.P., J.P.S., L.B., and D.W.C. were all involved in conducting the study and writing the manuscript. This study was supported by Boehringer Ingelheim Canada Ltd.
Footnotes
Published ahead of print on 31 August 2009.
REFERENCES
- 1.Aarnoutse, R. E., K. J. Grintjes, D. S. Telgt, M. Stek, Jr., P. W. Hugen, P. Reiss, P. P. Koopmans, Y. A. Hekster, and D. M. Burger. 2002. The influence of efavirenz on the pharmacokinetics of a twice-daily combination of indinavir and low-dose ritonavir in healthy volunteers. Clin. Pharmacol. Ther. 71:57-67. [DOI] [PubMed] [Google Scholar]
- 2.AIDS Patient Care STDS. 2006. Intracranial hemorrhage with Aptivus. AIDS Patient Care STDS 20:889. [PubMed] [Google Scholar]
- 3.Bristol-Myers Squibb. 2007. Efavirenz product monograph, p. 39. Bristol-Myers Squibb Canada, Montreal, Quebec, Canada.
- 4.Dailly, E., C. Allavena, F. Raffi, and P. Jolliet. 2005. Pharmacokinetic evidence for the induction of lopinavir metabolism by efavirenz. Br. J. Clin. Pharmacol. 60:32-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dailly, E., O. Tribut, P. Tattevin, C. Arvieux, P. Perre, F. Raffi, and P. Jolliet. 2006. Influence of tenofovir, nevirapine and efavirenz on ritonavir-boosted atazanavir pharmacokinetics in HIV-infected patients. Eur. J. Clin. Pharmacol. 62:523-526. [DOI] [PubMed] [Google Scholar]
- 6.Hicks, C. B., P. Cahn, D. A. Cooper, S. L. Walmsley, C. Katlama, B. Clotet, A. Lazzarin, M. A. Johnson, D. Neubacher, D. Mayers, and H. Valdez. 2006. Durable efficacy of tipranavir-ritonavir in combination with an optimised background regimen of antiretroviral drugs for treatment-experienced HIV-1-infected patients at 48 weeks in the Randomized Evaluation of Strategic Intervention in multi-drug reSistant patients with Tipranavir (RESIST) studies: an analysis of combined data from two randomised open-label trials. Lancet 368:466-475. [DOI] [PubMed] [Google Scholar]
- 7.Hsu, A., J. Isaacson, S. Brun, B. Bernstein, W. Lam, R. Bertz, C. Foit, K. Rynkiewicz, B. Richards, M. King, R. Rode, D. J. Kempf, G. R. Granneman, and E. Sun. 2003. Pharmacokinetic-pharmacodynamic analysis of lopinavir-ritonavir in combination with efavirenz and two nucleoside reverse transcriptase inhibitors in extensively pretreated human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 47:350-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Justice, A. C., D. S. Zingmond, K. S. Gordon, S. L. Fultz, J. L. Goulet, J. T. King, Jr., D. M. Bravata, H. Valdez, M. Kraft, and K. M. Mattocks. 2008. Drug toxicity, HIV progression, or comorbidity of aging: does tipranavir use increase the risk of intracranial hemorrhage? Clin. Infect. Dis. 47:1226-1230. [DOI] [PubMed] [Google Scholar]
- 9.la Porte, C. J. L., M. J. de Graaff-Teulen, E. P. Colbers, D. S. Voncken, S. M. Ibanez, P. P. Koopmans, Y. A. Hekster, and D. M. Burger. 2004. Effect of efavirenz treatment on the pharmacokinetics of nelfinavir boosted by ritonavir in healthy volunteers. Br. J. Clin. Pharmacol. 58:632-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacGregor, T. R., J. P. Sabo, S. H. Norris, P. Johnson, L. Galitz, and S. McCallister. 2004. Pharmacokinetic characterization of different dose combinations of coadministered tipranavir and ritonavir in healthy volunteers. HIV Clin. Trials 5:371-382. [DOI] [PubMed] [Google Scholar]
- 11.Mouly, S., K. S. Lown, D. Kornhauser, J. L. Joseph, W. D. Fiske, I. H. Benedek, and P. B. Watkins. 2002. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin. Pharmacol. Ther. 72:1-9. [DOI] [PubMed] [Google Scholar]
- 12.Raffi, F., M. Battegay, S. Rusconi, M. Opravil, G. Blick, R. T. Steigbigel, M. Kraft, D. Neubacher, and J. P. Sabo. 2007. Combined tipranavir and enfuvirtide use associated with higher plasma tipranavir concentrations but not with increased hepatotoxicity: sub-analysis from RESIST. AIDS 21:1977-1980. (Erratum, 22:i, 2008.) [DOI] [PubMed] [Google Scholar]
- 13.Roszko, P. J., K. Curry, B. Brazina, A. Cohen, E. L. Turkie, J. P. Sabo, T. R. MacGregor, and S. McCallister. 2003. Standard doses of efavirenz (EFV), zidovudine (ZDV), tenofovir (TDF), and didanosine (ddI) may be given with tipranavir/ritonavir (TPV/r). Antivir. Ther. 8(Suppl. 1):S428. [Google Scholar]
- 14.Sekar, V. J., M. De Pauw, K. Marien, M. Peeters, E. Lefebvre, and R. M. Hoetelmans. 2007. Pharmacokinetic interaction between TMC114/r and efavirenz in healthy volunteers. Antivir. Ther. 12:509-514. [PubMed] [Google Scholar]
- 15.Solas, C., I. Poizot-Martin, M. P. Drogoul, I. Ravaux, C. Dhiver, A. Lafeuillade, T. Allegre, M. Mokhtari, J. Moreau, G. Lepeu, N. Petit, A. Durand, and B. Lacarelle. 2004. Therapeutic drug monitoring of lopinavir/ritonavir given alone or with a non-nucleoside reverse transcriptase inhibitor. Br. J. Clin. Pharmacol. 57:436-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valdez, H., S. McCallister, V. Kohlbrenner, and D. Mayers. 2005. Tipranavir/ritonavir (TPV/r) 500 mg/200mg BID drives week 24 viral load (VL) below 400 copies/mL when combined with a second active drug (T-20) in highly protease inhibitor-experienced HIV patients, abstr. WeOa0205. Abstr. 3rd Int. AIDS Soc. Conf. HIV Pathog. Treat. International AIDS Society, Geneva, Switzerland.
- 17.Vourvahis, M., and A. D. Kashuba. 2007. Mechanisms of pharmacokinetic and pharmacodynamic drug interactions associated with ritonavir-enhanced tipranavir. Pharmacotherapy 27:888-909. [DOI] [PubMed] [Google Scholar]
- 18.Williams, R. L., M. L. Chen, and W. W. Hauck. 2002. Equivalence approaches. Clin. Pharmacol. Ther. 72:229-237. [DOI] [PubMed] [Google Scholar]
- 19.Wire, M. B., C. Ballow, S. L. Preston, C. W. Hendrix, P. J. Piliero, Y. Lou, and D. S. Stein. 2004. Pharmacokinetics and safety of GW433908 and ritonavir, with and without efavirenz, in healthy volunteers. AIDS 18:897-907. [DOI] [PubMed] [Google Scholar]