Abstract
Summary: This papers aims at familiarizing psychiatric and nonpsychiatric readers with delusional infestation (DI), also known as delusional parasitosis. It is characterized by the fixed belief of being infested with pathogens against all medical evidence. DI is no single disorder but can occur as a delusional disorder of the somatic type (primary DI) or secondary to numerous other conditions. A set of minimal diagnostic criteria and a classification are provided. Patients with DI pose a truly interdisciplinary problem to the medical system. They avoid psychiatrists and consult dermatologists, microbiologists, or general practitioners but often lose faith in professional medicine. Epidemiology and history suggest that the imaginary pathogens change constantly, while the delusional theme “infestation” is stable and ubiquitous. Patients with self-diagnosed “Morgellons disease” can be seen as a variation of this delusional theme. For clinicians, clinical pathways for efficient diagnostics and etiology-specific treatment are provided. Specialized outpatient clinics in dermatology with a liaison psychiatrist are theoretically best placed to provide care. The most intricate problem is to engage patients in psychiatric therapy. In primary DI, antipsychotics are the treatment of choice, according to limited but sufficient evidence. Pimozide is no longer the treatment of choice for reasons of drug safety. Future research should focus on pathophysiology and the neural basis of DI, as well as on conclusive clinical trials, which are widely lacking. Innovative approaches will be needed, since otherwise patients are unlikely to adhere to any study protocol.
INTRODUCTION
The aim of this work is to sum up the current knowledge on delusional infestation (DI) or—using the narrower but more commonly used term—delusional parasitosis. (This review is based on 508 publications on the topic [publications known to us as of December 2008].) DI is characterized by patients' fixed belief that their skin and body (and rarely also their close personal environment) is infested by small, vivid (or less frequently inanimate) pathogens, although there is no medical or microbiological evidence for this. Most, but not all, patients blame the pathogens for causing various tactile sensations and itching (22, 78, 95, 136, 237). As a logical consequence of their belief, these psychiatric patients usually consult general practitioners, dermatologists, and microbiologists but are reluctant to see psychiatrists. This poses an intricate problem for the medical system in terms of adequate patient management and treatment settings. This article aims at preparing and familiarizing treating physicians from any specialty for this particularly demanding patient group. By using insets for excursions and additional information, we hope to serve the different interests and needs of the usual audience of Clinical Microbiology Reviews (microbiologists, infectious disease specialists, and public health personnel), as well as psychiatric and dermatologic readerships. The present work intends to foster cooperation of all medical specialties engaged in the field of DI.
A number of review articles on DI can be recommended as valuable further reading (for clinical aspects, see references 46, 78, 123, 192, 299, and 344; for pathophysiology, see references 30, 67, and 142; for management of patients, see references 117, 280, and351; for antipsychotic treatment, see references 178 and 322; and for comprehensive reviews, see references 22, 74, 95, 108, 136, 175, 300, and 346).
DELUSIONAL INFESTATION
The Clinical Picture
DI is very characteristic, and cases are often remembered by the treating practitioner (95, 108, 123, 136, 175). Despite its apparent uniformity and against common belief, DI is not a single psychiatric disorder (32, 43, 44, 46, 234, 299, 323). DI has two main forms, relating to the absence or presence of any other underlying cause (physical, toxic, or psychiatric). The primary form is an isolated, monosymptomatic delusional disorder sui generis (“pure form”) (322, 323). In this most interesting and important form of DI, psychopathology is limited to the delusions and abnormal tactile sensations related to the delusional theme. It is stunning to see that patients are otherwise entirely mentally healthy and argue rationally if they discuss issues other than infestation. In all secondary forms, another defined disorder or intoxication causes the symptoms of DI (30, 108, 299). In these cases, the symptoms of DI add to the symptoms of the disorder underlying. In this section, we also describe symptoms that are not included in the relevant diagnostic criteria in the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) (345a), or the Diagnostic and Statistical Manual of Psychiatric Disorders (DSM-IV-TR) (13a) and discern core and optional features of DI.
Core symptoms.
The chief complaints of patients with DI are (i) the rigid belief against all medical evidence that they are infested and (ii) abnormal sensations “as if” infectious agents evoke them (e.g., using description such as “crawling,” “biting,” “leaving marks,” and “building nests”). Thus, two complementary symptoms define DI. There has been a long debate in the psychiatric literature regarding which one of the two is the leading aspect. These two models can be described either as a delusional disorder versus the concept of a “chronic tactile hallucinosis” (32) or as a “cognitive approach” versus a “sensoralist approach” (30). Because disturbed reasoning and judgment are present in all cases of DI, while tactile symptoms were reported in only 82% (27 of 32 patients) of cases in one study (353), the picture is better characterized by the disturbed thought. The controversy in psychiatric academia on whether the rigid beliefs are overvalued ideas or real delusions is superfluous because it is widely accepted that the conviction of being infested can vary in intensity (spectrum) (77, 78, 300). In the most characteristic patients, physicians will find a fixed conviction. A true delusion has also been an inclusion criterion in influential studies (234). However, less severe cases in patients with a shakable belief prevail in number (300).
Nomenclature and minimal criteria.
Many names have denoted the clinical picture over the decades. In 1938, Ekbom used the German name “Dermatozoenwahn” (from the ancient Greek “derma [δɛ́ρμα]” = skin, “zóon [ζ ον]” = living being/animal, and German “Wahn” = delusion). Elsewhere, the name is cumbersome, so in many countries the term “Ekbom's syndrome” became widespread. This eponym, however, is ambiguous and not recommended, because it is also used to refer to restless legs syndrome. All names ending with “-phobia” that have been proposed over the years are also misleading, because there is no evidence of an anxiety disorder (such as entomo- [261, 338], acaro- [241, 319], or parasitophobia [259, 263]). Similarly, names highlighting the abnormal sensations and hallucinations, such as “organic hallucinosis,” used in ICD-10 (F06.0), are imprecise (cognitive symptoms are more important; often it is unclear whether illusions or hallucinations are present [see Inset 8]). The name “delusion of parasitosis” was introduced in 1946 (344), and it became the most common name in the literature. An alternative was “delusional parasitosis.” However, in recent years, patients have reported the presence of specific parasites less commonly, and the name thus has the disadvantage of covering only one sort of pathogens. We therefore recommend the use of the broader term “delusional infestation” in the future. This name highlights the core psychopathological feature (a thought disorder) and covers all kinds of (even “newly emerging”) imaginary pathogens by referring to the delusional theme “infestation” and not to a single species. Because of the presence of only one or two defining symptoms and often well-circumscribed etiological origins, the term “syndrome” does not appear to be justified (requiring at least three cardinal symptoms and unknown etiology). In Insets 1 to 3, we propose a set of minimal criteria for DI and illustrate the spectrum of clinical manifestations of DI in a collection of more or less typical patients.
ον]” = living being/animal, and German “Wahn” = delusion). Elsewhere, the name is cumbersome, so in many countries the term “Ekbom's syndrome” became widespread. This eponym, however, is ambiguous and not recommended, because it is also used to refer to restless legs syndrome. All names ending with “-phobia” that have been proposed over the years are also misleading, because there is no evidence of an anxiety disorder (such as entomo- [261, 338], acaro- [241, 319], or parasitophobia [259, 263]). Similarly, names highlighting the abnormal sensations and hallucinations, such as “organic hallucinosis,” used in ICD-10 (F06.0), are imprecise (cognitive symptoms are more important; often it is unclear whether illusions or hallucinations are present [see Inset 8]). The name “delusion of parasitosis” was introduced in 1946 (344), and it became the most common name in the literature. An alternative was “delusional parasitosis.” However, in recent years, patients have reported the presence of specific parasites less commonly, and the name thus has the disadvantage of covering only one sort of pathogens. We therefore recommend the use of the broader term “delusional infestation” in the future. This name highlights the core psychopathological feature (a thought disorder) and covers all kinds of (even “newly emerging”) imaginary pathogens by referring to the delusional theme “infestation” and not to a single species. Because of the presence of only one or two defining symptoms and often well-circumscribed etiological origins, the term “syndrome” does not appear to be justified (requiring at least three cardinal symptoms and unknown etiology). In Insets 1 to 3, we propose a set of minimal criteria for DI and illustrate the spectrum of clinical manifestations of DI in a collection of more or less typical patients.
Inset 1. Set of minimal criteria for DI.
Conviction of being infested by pathogens (small, vivid, inanimate [rare], often “new to science”) without any medical or microbiological evidence for this, ranging from overvalued ideas to a fixed, unshakable belief.
Abnormal sensations in the skin explained by the first criterion (usually meeting criteria of qualitatively abnormal sensations [level 2 cenesthesias according to G. Huber {140}], i.e., without delusions of control or so-called passivity phenomena according to the work of Kurt Schneider, except in cases secondary to schizophrenia).
Additional symptoms: additional facultative psychotic and nonpsychotic symptoms, e.g., visual illusions or hallucinations, may be present.
Location: (on, in, or under the) skin, but all parts of the body may be infested.
Duration: typically months or years (chronic), ranging from minutes (if secondary to toxic psychosis or delirium) to years.
Inset 2. Classic patients. As a clinical rule of thumb, there are some types of classic patients with DI. The most prominent is a middle-aged to elderly woman with few social contacts, no psychiatric history, and normal cognitive and social function (primary DI) (e.g., see reference 101). A second characteristic profile is an old, multimorbid patient with dementia and possible vision or hearing impairment living in a nursing home who gradually develops symptoms of DI next to paranoid symptoms such as ideas of being prosecuted and robbed (dementia-associated psychosis [DI secondary to dementia]). A third profile is an elderly patient with vascular encephalopathy and cortical atrophy (with or without dementia) who develops symptoms of DI secondary to a brain disorder (96, 97, 141). The fourth typical patient is a young male patient with sudden and transient symptoms of DI secondary to regular use of THC (delta-9-tetrahydrocannabinol), amphetamines, or cocaine (substance-induced DI).
Inset 3. Atypical manifestations. An example of an unusual presentation is this previously unpublished case of a 29-year-old woman who believed that her house was infested with rats (the atypical features are the comparatively large size of the alleged pathogens, i.e., rats, and the infestation of the close personal environment rather than the body). The patient misinterpreted bits of sand as rat droppings and started to have auditory hallucinations about vermin running around in her house. The environmental health department was never able to find any evidence of vermin. The delusions were triggered by a real sighting of a rat on one occasion. She started to spend most of her time outside the house, sealed off all food, lived in only one room of the house with her two young children, put trays with rat poison all around the house, and kept the lights on all night to be able to see the rats. This caused significant sleep disturbance in her and her children. A diagnosis of delusional disorder (atypical DI) was made in the absence of any other illness or substance misuse. After 6 weeks of nonpharmacological supportive intervention from the community mental health team, she accepted antipsychotic medication. She was started on amisulpride at 100 mg twice daily, which led to complete remission after 4 weeks. One short episode of 3 weeks in which she was noncompliant led to a reemergence of symptoms within 5 days. These subsided when amisulpride was restarted. She has been well now for 12 months on continuous medication.
The imaginary pathogens.
Patients make various presumptions about the nature of the infesting species. Some simply report vermin, insects, parasites, or “small animals” (32, 96, 177, 194, 342). Other patients have more specific assumptions, e.g., (itch) mites/scabies (32, 38, 111, 123, 136, 319, 344), (pubic) lice (32, 136, 289, 344), worms (32, 136), bugs (200), fleas (194), flies, ticks (38), and spiders (136). Microscopic pathogens such as bacteria (289, 342), viruses, and the like are reported much more rarely.
Alleged infestations by some kind of inanimate material, such as hair (96, 136), spots or dots (136), pigments, sand (123), threads, fibers, and the like, were rarities until the emergence of the “Morgellons phenomenon” in 2002. In a retrospective study of 385 published cases, patients reported the following pathogens, in decreasing order: insects, 84%; worms, 14%; bacteria, 2%; and fungi, 1% (323). In a later study with a sample of 35 patients, the same author found higher ratios for the “noninsect” groups (insects, 63%; bacteria, 20%; worms, 11%; and fungi, 6%) (323). This indicates that the imaginary pathogens vary and may undergo developments.
An interesting and indicative feature is the presumed size of the pathogens. They are often described as “almost too small to see” or tiny (123, 200, 289), such that it is difficult for the patient to catch one. Pathogens larger than centimeters are uncharacteristic for primary DI and rather indicate a toxic psychosis, delirium, or schizophrenia. In rare cases, the pathogenic agent cannot be described further (344), usually in those cases secondary to dementia, delirium, or intoxications.
The color of the pathogens is often black (32, 136, 194, 342), gray, or white (250), but all colors occur. Sometimes they are supposed to be skin-colored (38) or even make color changes, e.g., from red to green (198, 338).
The most frequent imaginary source of infestation is a transmission from other humans (50%), while plants, the garden, or some part of the patient's housing (33%), as well as animals and pets (17%), are less frequently blamed (239). A real infection of pets can be a trigger for DI in pet owners.
The most frequent localizations of infestation are the skin of the hands, arms, feet, lower legs, scalp, the upper back and breast region, and the genitals (78, 95, 97, 101). Body orifices such as the nose, ears, mouth, anus, and the whole gastrointestinal tract are often affected (78, 91, 110, 122, 130, 195, 245, 262), mainly in the elderly (234). DI of the eyes has been described in single cases (187, 287, 296, 327). An affection of the whole body is rare (323). The putative infestation is usually skin related. Most patients report the pathogens to be “on” the skin (43%), while vermin “in” or “under” the skin are less common (both 20%) (323). In cases of primary DI, the infestation was rather “in” or “under” the skin, while it was “in the body, blood or muscle” in organic brain syndromes and “on” the skin in cases secondary to schizophrenia (n = 20) (203). However, these relations are uncertain in view of the small study samples.
In many cases, patients believe that family members, friends, and other contacts, as well as personal belongings, their habitation, the garden, and pets, are also infested (38, 76, 96). The majority of patients (71 to 91%) experience an infestation of the body before that of the environment (239). If the patient believes that his or her partner, child, pet, or others are infested (and not him- or herself), then it is a DI by proxy (32, 77, 246, 353). The first report of this was a healthy dog who was cleaned excessively by the owner based on his delusional belief (194). Patients may present their healthy pets to veterinarians or their healthy children to pediatricians. If the patient and his or her partner, friend, or others develop a delusional belief that the patient, their environment, or both of them are infested, it is a shared delusional disorder (see below).
Lay research and theory.
Patients start to examine themselves, particularly their skin. The search gradually becomes more elaborate, and often instruments such as magnifying glasses and tweezers are used to examine the skin further (38, 95). These ritual-like “studies” can take hours each day (and may be confused with obsessive-compulsive disorder [OCD]). Patients often put down notes, pseudoscientific essays, and drawings with lay theory. In recent years, digital photos of alleged pathogens and skin lesions have become popular. Patients usually try to catch the pathogens as proof or for further investigation (136, 346). Further details on the imaginary “proof of infestation” are given in Inset 4.
Based on their own observations, patients can deliver impressive descriptions of the vermin, in particular in chronic primary DI with highly systemized delusions (234, 323). Patients describe where the pathogens reside in their body and how they spread, eat their skin, breed, or display other most fallacious behaviors. As patients do not experience tactile sensations continuously, they explain this with transient inactivity or resting cycles.
Doctor hopping.
Patients also consult numerous family doctors, dermatologists, entomologists, microbiologists, and tropical disease specialists (78, 234). The first doctor is usually (71%) the family doctor (with public health department doctors consulted in 9% of cases and dermatologists consulted in 6% of cases) (323). Some patients also seek help from pest control officers, federal institutions, and traditional healers. This is a direct consequence of their delusional belief. It is not surprising that patients do not seek psychiatric help and refuse psychiatric referral, as they do not believe that they have a psychiatric illness (192, 198, 237, 239, 286). In a recent study from Argentina, psychiatric referral was possible for only 1 of 12 patients (8%) (258).
Many patients ask the physician for a better or definitive diagnostic work-up, no matter how many dermatological and microbiological tests were unremarkable (similar to patients with hypochondriasis). The physician should not expect that patients be “relieved” by a negative finding. Instead, “nonfindings” will be interpreted as “incompetence” of the doctor, or patients will find another explanation to maintain their beliefs (e.g., “the sample was taken at the wrong site or the wrong time; a bad microscope, wrong medium, or wrong analytical method was used; a mix-up of samples, names, or reports occurred;” and others).
Most patients request more therapy rather than more diagnostics (similar to patients with somatoform disorders). They “already know” what they have and often ask to be prescribed the most aggressive antibiotic, virucide, pesticide, or other medication to get rid of the pathogens (95). It would be unethical and unhelpful to meet these irrational requests with the intention that the patient might learn from the lack of effect and gain insight into the nature of his or her symptoms. This approach will not work, because the lack of effect is reinterpreted by delusional elaboration (e.g., “it was the wrong or too weak an anti-infective”). Hence, prescription of any anti-infective without clear indication is no option and reinforces the delusional belief (95). A direct proposal of psychopharmacological treatment without accompanying measures irritates the patient. As a result, patients consult many doctors, and later specialists or academic institutions, but remain unsatisfied and eventually lose faith in professional medicine (234, 300).
Inset 4. The specimen sign. When patients are able to catch some of the pathogens, they are taken to the physician as proof of infestation. These specimens are usually presented in a small bin, vessel, bag, piece of paper, or plastic foil to protect it. It is telling that these allegedly dangerous pathogens are handled without disgust or anxiety of becoming infested. Instead, they are collected and stored like trophies (96). The proofs usually consist of dull and uncritical material, such as dander, crusts, scabs from healing skin lesions, hair, threads and other particles from clothes, fibers, dirt, or sand (114, 135, 136, 234, 346, 353). They can also consist of legs from flies or spiders (353) or breadcrumbs (115); this includes self-diagnosed “Morgellons” (17). More and more patients present digital photos or movies.
This clinical sign is characteristic and was first described by Perrin in 1896 (259). Frequencies of between 4% in India (35) and 92% in Argentina (258) have been reported (including frequencies of 14% [78], 26% [323], 29% [99], 35% [234], and 48% [353] [data refer to the rate of specimen presentation in a single consultation]). Clinical experience suggests that the majority of patients take or send specimens to physicians or microbiologists in the course of their illness, at least in primary DI and chronic forms. Still, the sign is neither obligatory nor sufficient to diagnose DI (95). Although the real nature of the specimen can be identified readily, the material should be examined.
This peculiar behavior was named the “matchbox sign” in an anonymous editorial (30 July 1983) and in a letter by W. R. Lee in The Lancet (169, 173). However, we propose the name “specimen sign,” because it seems more appropriate to point to the “pathogen” than the receptacle. The term is also comprehensive and covers all kinds of delivered material, including digital photos.
From a psychopathological point of view, to misconceive a real object to be a pathogen is an illusion (234), while a hallucination requires a perception without an external stimulus. Notably, the size of the material is usually minuscule, which certainly contributes to illusions and false interpretations. A study found that patients who send specimens to parasitologists are significantly older than those who do not (239). Impaired vision might play a crucial role in this subgroup of patients. An example of a classic matchbox sign from one of our patients is presented in Fig. 1 (101), and other examples can be found in recent publications (12, 101, 115, 327) and on the Internet.
FIG. 1.
Specimen sign. Patients provide all kinds of proof of infestation, in this case a matchbox filled with skin particles and crusts on a piece of white cotton.
Cleaning, self-therapy, and injuries.
Patients with DI show a distinct cleanliness with regard to their environment and their bodies. About 80% of patients also start intensive, repetitive, often dangerous self-cleansing to get rid of the pathogens (35, 78). Apart from frequent changing and washing of clothes, bed linen, and the like, this can be exaggerated to up to several disinfections of the whole house and the body each day or to changing underwear after some hours, even at night. Countless over-the-counter disinfectants, crèmes, soaps, and chemicals are used, often excessively in quantity and frequency. Some patients apply alcohol (32), rubbing alcohol (96), H2O2 (114, 192, 231), petroleum (32), gasoline, or kerosene (38). Mechanical force and instruments are also used to kill or catch the pathogens (rubbing or scratching with fingernails, nail files, tweezers, etc.) (101), and some patients work on their own bodies with dramatic physical strategies, including electric current, fire (78), ice packs, fluids (washing, bathing, and soaking for hours), and radiation (solarium) (95). In cases of infestation of the gut or body orifices, laxatives, enemas, ingestion, or lavages with vinegar or chili and manipulation with instruments occur and often result in injuries. Severe self-mutilations, e.g., of the eyes (296, 327), can occur, particularly in cases secondary to schizophrenia. Epilating the head and body hair, including trichotillomania, has been reported in many cases where patients associated their hair with the infestation (32, 34, 35, 96, 120, 136, 300, 319, 327). Relatives are sometimes urged to take part in these regimens, particularly in cases of shared delusional disorder. In DI by proxy, dependents can be subjected to cleaning excesses, causing concern about their health and safety. Child protection procedures must sometimes be considered in such cases. The self-therapies can be “self-invented” or recommended by other patients, e.g., via the Internet. The fewer patients feel that professional medicine can help, the more they use these measures. Other duties and personal contacts may be neglected (78). Patients perceive these measures as helpful to various degrees, but in the end, none of them is satisfactory. The cleaning strategies often change several times in the futile search for an effective measure.
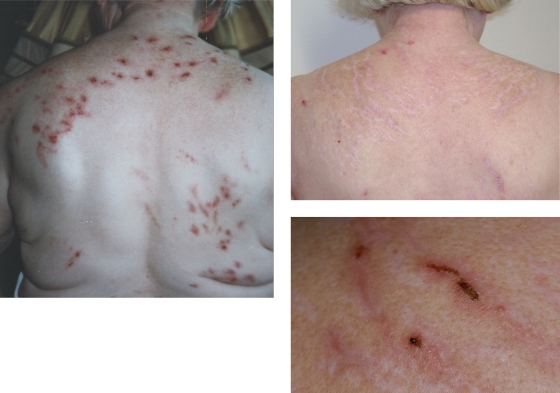
The most common complications of self-therapy are various skin lesions (e.g., erosions, scratch excoriations, ulcers, hemorrhagic crusts, hyper- and depigmentation, lichenification, bacterial superinfections, scars with nodes and plaques, chronic irritant contact dermatitis, lichen simplex chronicus, lichen amyloidosus, or prurigo nodularis). They have been reported with frequencies of 17% (299), 39% (353), 50% (250) and 63% (47). Severe lesions that require acute measures were found in 26% of the patients (9 of 35 patients) in one study (323). The presence of real skin problems appears to make it more believable that the patient has a real infestation of the skin. There is a real cause for itching and skin sensations. The different ages of the lesions and their distribution, however, are indicative of the nature of the manipulation. Lesions are usually limited to body parts within easy reach (300, 346). They are often more severe on the side opposite to the dominant hand (1, 101, 192). This is illustrated in Fig. 2, together with examples of typical acute and postacute skin alterations. The skin lesions are a crucial factor in pathogenesis and maintenance of the delusional disorder. The vicious circle of itching and scratching which is known from other skin disorders is also present in DI. It has an additional step, i.e., the delusional interpretation of the sensations, with the circle including itching, interpreted as an infestation (additional step), scratching/manipulations, skin damage (now partly real), itching, and so on. For adequate therapy, interventions at all steps of the circle are required, including local skin therapy to reduce itch, the prevention of further manipulations, and sufficient antipsychotic treatment of the fixed belief.
FIG. 2.
Skin lesions. Self-inflicted scratch excoriations in a patient with primary DI before (left) and after (upper right) antipsychotic treatment. Note that the skin lesions are limited to parts of the back that a right-handed elderly female can reach. At higher magnification, typical skin alterations in different stages of healing and scars of different ages can be seen (lower right).
Other dangerous consequences.
The more the patients try in vain to free themselves from the infestation, the more angry and desperate they may become. Hazardous actions such as burning one's “infested” furniture, other goods, and clothes (107) or even escaping from home (114, 198) have been described in the literature. Some patients rather develop depressive symptoms. Such secondary depression often develops within the course of the delusional disorder. Suicidal ideation (76, 280), attempted suicide (38, 123, 136), and committed suicide have been reported in single cases (224). Conversely, angry reactions occur. They range from becoming upset and hostile (if a psychiatric problem or referral is suggested), denying the possibility of a psychiatric problem and blaming the physicians for their incompetence, to aggressive assaults. Such actions are rarities (224, 231, 299). There is a single report of an attempted murder of a family doctor (48). A study of 20 patients, however, found a “rather low” overall aggressiveness (137). A lot of activity is characteristic of all monodelusional disorders and is found in primary DI as well (32). This contributes to high overall expenses that result from doctor hopping, buying disinfectants and medicines, destroying parts of the housekeeping, etc. Desperate patients also become susceptible to “profit-oriented providers of medical services.” Other psychopathological symptoms and details on Internet use in patients with DI are described in Insets 5 to 8.
Inset 5. The Internet and DI. Specialized Internet websites and, in particular, topic-related forums have become an important communication platform for almost any disease. They can be very helpful, but in the case of DI, absurd beliefs about unknown pathogens and uncritical recommendations of hazardous self-cleansing strategies are spread and shared (95, 337). Most patients with DI have sought help on websites these days. Hence, we encourage physicians to visit such websites and forums in order to know what information patients spread and read (e.g., www.morgellons.eu and www.curezone.com [with forum “Parasites: USP: Unidentified Skin Parasites,” last visited December 2008]). The supporters and many users of these websites believe that they suffer from an unknown infectious skin disease. They use the Internet to establish patient organizations and to organize and fund lay or professional research on unidentified infectious agents. For some years, the National Unidentified Skin Parasites Association (NUSPA), with the website www.skinparasites.com, was the main platform. The NUSPA negated the presence of a psychiatric problem in affected persons and ridiculed professional medicine for not being able to identify the pathogens.
Inset 6. Shared psychotic disorders. Another frequent feature in DI is the development of a shared psychotic disorder (SPD). The delusional beliefs of a first person—the inducer—are adopted by one or more persons, usually close relatives or dependent people (72, 78, 111, 192). This rare phenomenon was reported to occur in 14.4% of cases of DI (65 of 449 cases), according to the largest case collection to date (322). Our recent case selection found an SPD in 11% of the cases (99), but reported frequencies range from 8% to 49% (44, 72, 192, 216, 238, 250, 299, 353). The first report of an SPD in DI was published in 1923 (111). To date, there are many reports of a folie à deux or a folie à trois, i.e., delusional belief shared by two or three people, respectively, in DI (6, 60, 97, 111, 112, 115, 155, 165, 192, 194, 231, 250, 299, 300, 308, 322, 342). There is one report of a folie à cinq (288) and three reports of a folie à famille (53, 64, 213), i.e., the participation of five family members or all of them.
Inset 7. Other psychotic symptoms. Other rare psychotic symptoms in patients with DI are visual, auditory, or olfactory hallucinations, e.g., seeing, hearing (342), or smelling (41) the vermin, usually close to the person. Tactile and olfactory hallucinations related to the delusional theme are allowed in DSM-IV-TR delusional disorder, while visual hallucinations are more indicative of dementia or brain disorders (149).There are also single case reports where DI occurred in combination with other delusions, e.g., delusions of body smell (in a patient with schizophrenia) (318), delusions of jealousy (in a patient with stroke) (37), delusions of pregnancy (in a patient with posttraumatic epilepsy) (316), Capgras delusion (the delusional belief that a close person is replaced by a “Doppelgänger” or identical-looking impostor [observed in a patient with schizophrenia]) (210), or paranoia (persecutory delusions) (135, 231, 237, 300). Delusions of control are not seen in DI, except for cases secondary to schizophrenia.
Inset 8. Psychopathology. As for the abnormal tactile sensations seen in most patients with DI, there has been a controversy over whether they are properly described as illusions (78, 86, 87) or hallucinations (32, 194). The crucial point is the presence (in illusions) or absence (in hallucinations) of a “true” and sufficient explanation for the tactile symptoms. The problem for the proper psychopathological terminology is that in the tactile domain—in contrast to visual or auditory stimuli—the reality testing is more complicated for the physician (234). This makes the distinction between illusions and hallucinations almost impossible (123). (It is impossible with regard to stimuli within the body, i.e., proprio- or enteroception. It would be possible for usual tactile stimuli on the skin, i.e., exteroreception, but the presence of true skin lesions in DI and the microscopic nature of the alleged pathogens render it at least very difficult to prove or disprove a true cause for tactile phenomena.) Therefore, Musalek suggested using the descriptive term “(skin) sensations” (234).
As for the delusional intensity of the fixed belief, some authors have stressed that it represents an overvalued idea (i.e., still shakable) (107, 136, 300), and others have found the beliefs to represent a full-blown delusion with a fixed conviction, implicitly (319) or explicitly (78). A psychopathological study showed that the delusion was monothematic, that its elements were connected logically, and that in the vast majority of cases (32 of 34 cases) tactile symptoms did not precede the delusion (234). This favored the cognitive approach. But Marneros et al. pointed out that “(e)ven careful analysis cannot always answer the question, whether the main symptoms (…) have to be classified as delusions, hallucinations or misidentifications” (203). The controversy is mainly academic, however, because all of these symptoms are classed as being within the psychotic spectrum and warrant antipsychotic treatment.
History, Names, and Basic Concepts
First description.
The Parisian dermatologist Georges Thibierge is usually credited with the first detailed medical description of the clinical picture, in 1894 (319), although there are some indications of even older descriptions (Inset 9). Thibierge called the affected persons “les acarophobes.” (Les acarophobes, acarophobia, and entomophobia come from the ancient Greek “akari ['ακαρi]” = mite, ancient Greek “phobos [Φ'oβος]” = fear, and ancient Greek “entomos ['ɛντομος]” = incised/cut in [insects].) They had the false conviction that they had scabies (“la crainte non justifiée de la gale”). The author described the following two subgroups: those who really had scabies before (and were cured) and those who never had it. Thibierge also stated that a similar picture occurs in cocainism, as he learned from Saury and Seglas at a congress on mental health in Rouen (France) in 1890.
Only 2 years later, the dermatologist Perrin from Marseille (France) presented three comprehensive case histories, using the name “des névropathies parasitophobiques” (259). He stressed that patients had the morbid conviction of having a parasitic infestation (“la crainte morbide d'une affection parasitaire”). The term “phobia” is obviously false today, but the French name “phobie” did not refer to a neurotic symptom in those days (31, 226). While being ridiculed by some (344), it can clearly be seen from the above that Thibierge and Perrin correctly described wrong beliefs, not phobic disorders. Further details on the history of DI research are presented in Inset 10.
Inset 9. Earlier descriptions? In his book Brief Lives (1669-1696), the English writer John Aubrey (1626-1697) described a prisoner of the Tower of London, James Harrison, who suffered from a similar picture, as described by Lyell (192). Trabert (321, 323) indicated that Robert Willan of London might have discussed cases of DI in his 1799 book. Reviewing the German transcript of the book (343), it appears more likely that the author simply described “pruritus senilis.” The same applies to the German entomologic book of Johann Heinrich Jördens and the chapter on “the flea of the skin itching of the aged (Pulex pruriginis senilis),” which only reproduces the work of Willan.
Inset 10. The history of research on DI. The names used for DI over time and milestones in research are presented in Table 1.
TABLE 1.
DI: a Babel of names and milestones in research
| Yr of publication | Author(s) (country)a | Name used for DI | Milestones and new concepts |
|---|---|---|---|
| 1894 | Thibierge (F) | Les acarophobes | First clear description |
| 1896 | Perrin (F) | Des névrodermies parasitophobiques | Matchbox sign (first) |
| 1920 | Gamper (D) | Psychosen des Rückbildungsalter | Organic origin (hypothesized thalamic dysfunction) |
| 1921 | Pierce (USA) | Entomophobia | |
| Myerson (USA) | Acarophobia | ||
| 1923 | Giacardy (F) | Un cas d'acarophobie familiale | SPD (first) |
| 1925 | Grøn (FIN) | Les dermatophobies | Psychogenic origin |
| 1928 | MacNamara (USA) | “Cutaneous … hallucinations” | Hallucinations plus secondary delusions; affection of pets (first) |
| 1929 | Schwarz (D) | Circumscripte Hypochondrie | Occurs as a depressive symptom |
| 1930 | Mallet and Male (F) | Délire cénesthesique | |
| 1934 | Smith (USA) | Hallucinations of insect infestation | |
| 1935 | Wilhelmi (D) | Ungezieferwahn | |
| 1938 | Ekbom (S) | Der präsenile Dermatozoenwahn | Distinct disorder (first), organic/involutional, |
| Hase (D) | Pseudoparasitismus | illusion plus secondary delusions | |
| 1944 | Davis | Insect hallucination | |
| 1946 | Wilson and Miller (USA) | Delusion of parasitosis | Four different etiologies |
| 1949 | Harbauer (D) | Dermatozoenwahn (Ekbom) | Presenile or in depression, response to ECT (first), illusions or hallucinations occur |
| 1954 | Bers and Conrad (D) | Die chronische taktile Halluzinose | Multiple etiologies, mostly organic, with emphasis on hallucinations |
| Böttcher (D) | Das Syndrom des wahnhaften Ungezieferbefalls | First to gather cases from PCOsb emphasizes syndromal nature | |
| 1960 | Döhring (D) | Ungezieferwahn | Sample of 77 cases reported by a microbiologist |
| 1962 | Liebaldt and Klages (D) | Isolierte taktile Dermatozoenhalluzinose | Only postmortem histology (thalamocortical dysconnection) |
| 1965 | Tullett (UK) | Delusions of parasitosis | Separate entity, “monosymptomatic hypochondriasis” |
| 1966 | Schrut and Waldron (USA) | Delusory parasitosis (entomo-, acaro-, or dermatophobia) | Psychoanalytical model (unconscious sexual guilt) |
| 1970 | Hopkinson (CAN) | Delusions of infestation | First response to antidepressant in major depression |
| 1975 | Ganner and Lorenzi (D) | Der Dermatozoenwahn | “Independent” and “as a concomitant phenomenon” |
| Riding and Munro (UK) | Monosymptomatic hypochondriacal psychosis | First response to pimozide (case 3) | |
| 1978 | Annika Skott (S) | Delusions of infestation; Dermatozoenwahn (Ekbom's syndrome) | First real study, “primary delusion,” organic (>50% of cases) |
| 1979 | Frithz (S) | Delusions of infestation | First study with depot antipsychotics |
| 1982-1986 | Ungvari and Vladar (H), Hamann and Avnstorp (S) | Dermatozoenwahn, delusion(s) of infestation | Open and placebo-controlled studies with pimozide |
| 1983 | Lyell (UK) | Delusions of parasitosis | First and largest survey; DI starts from senile pruritus |
| 1985 | Berrios (UK) | Delusional parasitosis | Four pathogenetic mechanisms, a variety of etiologies |
| 1986 | Bourgeois (F) | Syndrome d'Ekbom et délires d'infestation cutanée | Large survey of French dermatologists |
| 1987 | Renvoize et al. (UK) | The syndrome of delusional infestation | Emphasis on syndromal nature |
| 1989 | Musalek (A) | Dermatozoenwahn | First interdisciplinary outpatient clinic; only SPECT study |
| 1991-1995 | Trabert (D) | Dermatozoenwahn | Only epidemiological study (D), meta-analysis of 1,223 cases |
| 1994 | Srinivasan et al. (IND) | Delusional parasitosis | Open study questioning superiority of pimozide |
| 1995 | Gallucci and Beard (US) | Delusions of parasitosis | Response to atypical antipsychotic risperidone (first) (n = 1) |
| 2007 | Lepping et al. (UK) | Delusional parasitosis | Systematic review of antipsychotics in DI (first) |
| 2008 | Freudenmann and Lepping (D) | Delusional parasitosis | Meta-analysis of cases treated with atypical antipsychotics (first) |
| Huber et al. (I) | Delusional parasitosis | Structural MRI study (first) |
F, France; D, Germany; USA, United States; FIN, Finland; S, Sweden; UK, United Kingdom; CAN, Canada; H, Hungary; A, Austria; IND, India; I, Italy.
PCO, pest control officer.
(i) Early 20th century. Magnan and Saury first described tactile symptoms in people with regular use of cocaine, now referred to as “cocaine bugs” (signe de Magnan) (196). The seminal works of Thibierge and Perrin, in 1894 and 1896, respectively, are described in the text. Other early insights into possible etiologies of DI came from Giacardy, who first described that the delusional belief can be obtained by another person as an induced psychosis (shared delusional belief in a couple) (111). MacNamara believed that the “hallucinations” are the main feature of the syndrome (194). Hanns Schwarz was the first to consider DI as a form of hypochondriasis within the spectrum of affective disorders (which is too narrow) and first noted premorbid anankastic personality traits (289, 290). Another etiology was stressed by the seminal work of Swedish psychiatrist Karl-Axel Ekbom, with the German title “Der präsenile Dermatozoenwahn” (78; English translation by Yorston [79]). A new disease model based on seven cases (all postmenopausal females) and a collection of all published cases was proposed that conceptualized a presenile involutional/organic brain syndrome. It would result from presenile pruritus, with “real perceptions” (tactile illusions) which were interpreted in a delusional way.
(ii) The 1940s. Some years later, Wilson and Miller reviewed all available cases and added six of their own (344). They described four different etiologies (toxins, schizophrenia, old-age depression, and paranoia/delusional disorder) but also noted that 8 of the 51 patients (15.9%) had cerebral atherosclerotic or senile disorders. The overall outcome was considered poor, with 82% of cases being “unchanged.” Hubert Harbauer, a medical assistant to Kurt Schneider in Heidelberg (Germany), was the first to use electroconvulsive therapy (ECT) successfully in two patients with psychotic depression (123). Accordingly, he disapproved of Ekbom's concept of an involutional disorder.
(iii) The 1950s. The following decade saw the first publications with larger samples, which are unknown in English literature. In an M.D. thesis, Böttcher was the first to gather cases from pest controllers (44). He stressed hypochondriacal features in the picture (43, 44). The entomologist Döhring reported 77 cases (72). Bers and Conrad confirmed positive but transient effects of ECT in two depression-related cases (32) and emphasized the syndromal aspect of the picture, which was characterized as a chronic tactile hallucinosis with an organic basis (similar to a hallucinosis in alcoholics or those with syphilis or other brain disorders). This concept is mainly used in ICD-10 (F06.0 organic hallucinosis). Gerd Huber, of Bonn (Germany), the most influential scholar of Kurt Schneider, reckoned DI as belonging to the cenesthetic subtype of paranoid schizophrenia described by him (138-140).
(iv) The 1970s. The first reports on positive effects of psychopharmacotherapy were published in the 1970s. Hopkinson reported on tricyclic antidepressants in patients with DI secondary to depression (136). In 1975, the high-potency antipsychotic pimozide was reported to be helpful in two cases (271, 272), stimulating further research. One of the authors, Alistair Munro, subsumed DI to a group of isolated psychotic disorders called “monosymptomatic hypochondriacal psychosis.” In 1978, Annika Skott performed a groundbreaking first study, including electroencephalograms and family genetics, which showed that organic brain disorders are present in more than half of cases and that the clinical course depends on the type of DI (299).
(v) The 1980s. Surveys among British and French dermatologists further established DI with its clinical features and provided the largest samples to date (46, 192, 268). The value of pimozide was further studied in the first open and small controlled trials (121, 331, 334, 335) (see below). Another study achieved excellent results with traditional depot antipsychotics (105). In order to secure sufficient psychiatric care, the first two specialized interdisciplinary outpatient clinics were founded in the 1980s, by Musalek (Austria) and Trabert (Germany). The projects yielded some of the best research on DI (some parts available only in German) (234, 235, 237, 239, 240, 321-323, 325, 326).
(vi) The 1990s. The alleged superiority of pimozide over other antipsychotics in the treatment of DI was questioned by a study from India, which showed a response to standard antipsychotics (304). In 1995, based on a collection of 1,223 published cases, Trabert found a significantly improved prognosis and outcome with the introduction of antipsychotics (322). In the same year, the first case report on positive effects of an atypical antipsychotic (risperidone) was published (106).
(vii) Since 2000. In recent years, our group has published guidelines for the clinical management of patients with DI (95, 175), a systematic review on the effects of antipsychotics in primary DI (178), a case-based meta-analysis on the effects of atypical antipsychotics in primary and secondary DI (99), and papers on the pathogenesis of DI (141, 142).
Although DI has been known for much longer than 100 years, DI research on the brain pathophysiology of the different forms of DI and clinical trials are inconclusive.
Epidemiology
Is DI a rare disorder?
DI is usually considered a rare disorder, particularly by psychiatrists. However, most psychiatrists underestimate its frequency. Recently, dermatologists and microbiologists have questioned that it is infrequent (20, 74, 312). There is a large unknown figure, and Internet forums give a vague idea of the true frequency. The mean number of cases of DI per institution or hospital and year has been found to range from 0.6 (dermatology [Singapore] [19]) to 20 (entomology [United States] [286] and psychiatry [Hungary] [334]) (20). Certainly there are other psychiatric and dermatological disorders that occur less frequently than DI (20, 312). Well over 1,400 definite cases have been published in the literature alone (Table 2) (there were already 1,223 cases in Trabert's case collection from 1995).
TABLE 2.
Epidemiology of DI: classic and large studies (n > 10)
| Author (yr) of study | Countrya | n | Sex ratio (female to male) | Mean or median age (or range) (yr) | Mean or median duration of illness (yr) | Presence of specimen sign or SPDb | Infestation |
|---|---|---|---|---|---|---|---|
| Perrin (1896) | F | 3 | 2:1 | 48 | 0.9 | SS | Unusual flea, lice, parasites |
| Schwarz (1929) | D | 5 | 5:0 | 54 | 1.5 | Lice, small animals and bacteria, insects | |
| Wilhelmi (1935) | D | 5 | 3:2 | 50 | 4 | SPD | Insects (new to science), bacteria |
| Ekbom (1938) | S | 7 | 7:0 | 58 | 1 | SS, SPD | Little insects/animals, mites, pubic lice, worms |
| Wilson and Miller (1946) | USA | 6 | 2:1 | 51 | 7 | SPD | Lice or insects (new to science) |
| Böttcher (1954) | D | 19 | 3.8:1 | 38-68 | SPD | Vermin | |
| Döhring (1960) | D | 77 | 3.5:1 | 50-70 | SS, SPD | Vermin | |
| Skott (1978) | S | 57 | 2.8:1 | 64 | 4 | SS, SPD | Insects, flies, ticks, etc. |
| Lyell (1983 [192]) | UK | 282 (survey) | 2:1 | 19-90 | SS, SPD | Parasites (survey) | |
| Reilly and Batchelor (1986) | UK | 53 (survey) | 1.5:1 | 20-92 | >1 (61%) | Parasites (survey) | |
| Bourgeois et al. (1986) | F | 150 (survey) | 5.7:1 | 68 | >2 (50%) | ||
| Marneros et al. (1988) | D | 28 and 20 | 4:1 | 49-78 | “In skin/body” (organic), “on skin” (schizophrenia) | ||
| Musalek et al. (1989) | A | 107 | 4.6:1 | >2 (29%) | SS | Parasites | |
| Ohtaki (1991) | J | 94 | 1.8:1 | 46/53 (males/females) | SPD | Mites | |
| Trabert (1991) | D | 115 (survey) | 3.1:1 | 60 | 3 (maximum, 35) | SS | |
| Trabert (1993) | D | 35 | 3.4:1 | 59 | 3.25 | SS, SPD | Insects, bacteria, worms, fungi |
| Trabert (1995) | D | 1,223 (review) | 2.4:1 | 57 | 3 | SPD | Review |
| Huber (1997) | D | 36 | 2.3:1 | 25-84 | SS, SPD | ||
| Zomer et al. (1998) | NL | 33 | 1.5:1 | 57 | 1.3 | SS, SPD | Parasites |
| Bhatia et al. (2000) | IND | 52 | 1.7:1 | >0.5 | Insects | ||
| Freudenmann and Lepping (2008) | D | 63 (review) | 1.3:1 | 66 (17-90) | 1.4 (median) | SS, SPD | Review |
| Summary | >1,400 | 1.3-5.7:1 | 17-92 | ∼3 (days to 35 yr) |
F, France; D, Germany; USA, United States; S, Sweden; UK, United Kingdom; A, Austria; IND, India; NL, The Netherlands; J, Japan.
SS, specimen sign; SPD, shared psychotic disorder.
Prevalence and incidence.
There is a relative dearth of reliable data on the epidemiology of DI for most countries. The only exceptions are two specific epidemiological studies that attempted to establish prevalence and incidence rates in Germany (based on careful estimations). Trabert conducted two surveys, one in all neurological, psychiatric, dermatologic, and geriatric hospitals, as well as public health departments, for the index year 1988 (1,015 institutions; 341 responded and reported; n = 115 cases) (325) and another, complementary survey of private practices (323). A prevalence of 5.58 cases per 1 million inhabitants was calculated based on cases reported to hospitals and public health services (323), while a much higher prevalence was estimated based on the survey of private practices (83.23 per million) (323). This indicated that patients with DI prefer outpatient contacts, and the figure may reflect doctor hopping. The estimated respective annual incidence rates were 2.37 and 17 per 1 million inhabitants per year, respectively. These figures are in keeping with available data for delusional disorders in general, which include DI. For example, Manschreck reported a prevalence of delusional disorders of 24 to 30 and an incidence of 0.7 to 3.0 per 100,000 inhabitants (201). Further epidemiological data from different specialties are presented in Inset 11.
Inset 11. Other epidemiological data from different specialties. (i) Entomologists. The entomologists Schrut and Waldron reported more than 100 consultations by patients with presumptive DI in only 5 years (192, 286), giving a vague idea of the real frequency of DI in the population. The second largest case collection reported by entomologists encompassed 77 patients who desired examinations of themselves and their proofs of infestation (some of them up to six times) in 10 years (1949-1958) (72). At the University Department of Parasitology in Vienna (Austria), 73 cases were seen in 30 years (1958-1987) (239).
(ii) Dermatology. About 90% of patients with DI seek help from dermatologists (346). While many psychiatrists do not see a single patient in their career, a large survey of British dermatologists showed that they all had seen at least one patient (192) (216 dermatologists reported 282 cases). Similarly, 134 French dermatologists reported 150 cases, and 13 had seen more than 1 case (47). In Poland, 85% of the dermatologists (n = 118) had seen at least one case, 7% had seen even more than 10, and almost 20% were reported to currently treat one patient (312). Thus, patients with DI are not rare in dermatological practice. The rate of DI in dermatological hospital admissions, however, is lower than that for psychiatry and has been reported as about 1 in 3,000 patients (0.3 per thousand) (299). Critically, almost every fifth dermatologist (19.5%) tried to explain the disorder to the patients, and 16.6% of patients received placebos (312). Many unspecific anxiolytics and sedatives were prescribed (47, 312). Trabert found in the survey study that the outcome was significantly better with psychiatric therapy than without (55.5% response or remission versus 16.6%; chi square = 4.33; df = 1; P < 0.04) (325).
(iii) Psychiatry. The frequency of DI in psychiatric settings is known only for admissions to hospital, as data on outpatients are lacking. In two psychiatric university hospitals in Germany, the rate of DI cases among all admissions was assessed retrospectively as 2.5 per thousand (15 among 6,000 admissions in 47 years) in Münster (214), while a much lower rate of 0.67 per thousand was found in Bonn (28 among 40,029 admissions in 30 years) (203). This is further evidence that patients with DI evade contact with psychiatrists.
A stable and ubiquitous picture.
In view of the lack of formal epidemiological studies, we extracted the main epidemiological parameters from other studies (Table 2). These parameters show that DI is found worldwide and is surprisingly stable over time. All main characteristics, such as mean age, sex ratio, course and duration of illness, and the presence of clinical characteristics such as the specimen sign and SPDs, can be traced back to the late 19th century (22, 95, 319). Only the imaginary pathogens change. Case reports on DI are available from all continents except Africa (and the Antarctic). While most classic reports came from Europe and North America, more recently an increasing number of cases from South America (249, 258), the Caribbean (80), the Middle East (110, 298), Asia, the Pacific Region, and Australia (19, 88, 187, 244) have been published.
Course and duration of illness.
The clinical course of DI is quite variable and depends mainly on the type of DI. It can be episodic, periodic, or chronic (299). The most characteristic primary form of DI has an insidious onset and a chronic course, usually for years. Chronic courses also occur in elderly patients with DI secondary to medical illnesses. In intoxication, the onset of DI is acute and symptoms last only for hours, days, or weeks. An episodic course with free intervals is usually found in cases secondary to recurrent major depression. Accordingly, durations of illness between days and 35 years have been reported, with an average of 3.13 years across the different forms of DI (325), as summarized in Table 2. This confirms the overall chronic course that is typical for delusional disorders.
Sex ratio.
The majority of studies found that more women than men suffer from DI (Table 2). The preponderance of females ranges from 1.33:1 (99) to 5.7:1 (47). In large samples, the ratio is usually about 2.5:1 (198, 215, 237, 268, 299, 325, 353). It was calculated to be 2.36:1 in the largest meta-analysis, with 1,196 cases (322). However, the female excess exists only for persons over 45 years of age (192, 250, 268, 322, 325) and in primary, not secondary, forms of DI (n = 63) (99). In young patients between 30 and 39 years, there can be more males (presumably due to DI secondary to intoxications) (325).
Age at onset.
DI is usually a disorder of middle-aged and elderly patients (237, 299, 300, 322, 325, 353). The mean age at clinical presentation was 57.02 (± 14.6) years according to Trabert's meta-analysis (322). It was 60 years in his own epidemiological study (n = 115) (325). The lowest known age at onset is 17 years, and the highest is 92 years. Men and schizophrenic patients (325) as well as patients with a learning disability (299) were significantly younger than the average patient. Table 2 summarizes findings from other studies.
Social background and functioning.
Patients with DI, in particular those with primary DI, do not have any particular socioeconomic status, low education, or childhood problems (234). Compared to a control group, they also did not differ in marital status and level of education and even had a higher job status (203). This reflects the circumscript nature of the delusional disorder in primary DI, which does not affect overall functioning. Medical students and physicians with DI have been described twice in the literature (73, 192). Many studies, however, have independently established that social isolation is common. At least 47% of patients are isolated or live alone (268). Other studies found rates of 69.8% of being unmarried, divorced, or widowed (239) and 77% of being isolated (markedly or moderate isolation) (323). It was unclear for a long time whether isolation was a causal factor for symptom development or a consequence of the disorder itself. Eventually, two studies found isolation to be a preexisting risk factor (239, 323). This obviously has therapeutic implications.
Classification and Types of DI
The nosology of DI.
The nosology and correct classification of DI have been controversial since the first description of the syndrome (Inset 10). Ekbom conceptualized the syndrome as a “presenile” involutional (progressive) or organic syndrome (32, 78, 203, 299, 315), while other authors saw DI within the spectrum of affective disorders (43, 44, 289), schizophrenia (138, 139), or delusional disorders/paranoia (299). The debate is futile, because “the” DI does not exist. It is an unspecific picture with many different, but only a few typical, etiologies and associations with other illnesses (30, 32, 95, 108, 178, 235, 236, 299). The presence of different etiologies and subforms of DI was first put forward clearly by Wilson and Miller (344). Berrios further described the respective mechanisms (30). Accordingly, DI is no diagnosis sensu stricto, and it has no category of its own in the current text revision of DSM-IV-TR or in ICD-10. However, in the chapter “Organic Hallucinosis” (ICD-10 F06.0), the name “Dermatozoenwahn” (German for “delusional infestation”) is listed as “corresponding” (95, 178).
Forms of DI.
Ganner and Lorenzi were the first to strictly distinguish primary and secondary forms of DI and used the terms “independent” and “as a concomitant phenomenon” to denote this difference (108). The decisive point is whether the syndrome is considered “secondary” to another medical condition or drug effect or not, in which case it is primary. Within secondary DI, there are a few typical subgroups depending on etiology, i.e., DI can be secondary to other psychiatric illnesses (e.g., secondary to schizophrenia or major depression), toxic psychoses (e.g., cocaine abuse), medication-induced psychoses, organic brain diseases (e.g., delirium, dementia, tumors, or vascular encephalopathy), and general medical conditions with pruritus (e.g., renal or hepatic failure, cancer, systemic rheumatic illnesses, or type 2 diabetes). Over the years, a myriad of associations have been described in the literature, many only in case reports (74, 95, 178, 192, 300, 353). Table 3 summarizes and groups these conditions as far as possible. The respective diagnostic ICD-10 and DSM-IV-TR codes have been included in the table to increase practical use. This distinction of primary and secondary forms of DI has become the most common classification (76, 94, 95, 178). It has been used and proven in clinical practice and in research. With this classification, it is easy to assign proper diagnoses to individual clinical cases and to determine their prognosis (299). The appropriate therapy for a patient with DI can be employed only when the type of DI is clear (95, 175, 235). Research and publications are often flawed because the type of DI has not been determined, impeding sound conclusions and scientific progress. We have therefore suggested minimum criteria for the reporting of cases, including information on sex and age of the patient as well as type of DI (primary or secondary), medication used (with dosages), length of follow-up, and outcome based on a three-point scale (no effect, partial effect, or full remission) (178).
TABLE 3.
DI: classification, diagnostic codes, and associationsa
| Classification | Diagnosis (code) |
Reference(s) | |
|---|---|---|---|
| ICD-10 | DSM-IV-TR | ||
| Primary DI | |||
| No underlying disorder or condition | Persistent delusional disorder (F22.0) | Delusional disorder, somatic type (297.1) | 30, 101, 108, 299, 322, 325 |
| Shared DI | Induced psychotic disorder (F24) | Shared psychotic disorder (297.3) | 6, 57, 60, 84, 103, 111, 112, 115, 155, 165, 192, 208, 213, 216, 231, 238, 299, 300, 322, 326, 344 |
| Secondary DI (secondary to another condition) | |||
| Psychiatric (“functional”) as concomitant symptom | |||
| Schizophrenia, usually paranoid | [Schizophrenia] (F20.x) | [Schizophrenia] (295.xx) | 6, 19, 47, 61, 116, 136, 192, 198, 299, 317, 318, 322, 337, 344, 352 |
| Affective disorders, mainly major depressive disorder with psychotic symptoms | [Mood disorder] (F3x.x) | [Mood disorder] (296.xx) | 6, 16, 35, 47, 85, 107, 110, 116, 123, 136, 172, 183, 192, 212, 237, 245, 289, 290, 295, 299, 300, 322, 344 |
| Oligophrenia/mental retardation | [Mental retardation] (F7x) | [Mental retardation] (317, 318) | 46, 212, 213, 259, 295, 299 |
| Anxiety disorders | 252, 300 | ||
| Borderline personality disorder | 237 | ||
| Substance- or medication-related (“toxic”) psychosis due to: | |||
| Psychotropic drugs | Acute intoxication (intoxication psychosis; F1x.05) or psychotic disorder, predominantly delusional (F1x.51) or predominantly hallucinatory (F1x.52) | Substance-induced psychotic disorder, with hallucinations (292.11), with delusions (292.12) | |
| Cocaine | 51, 76, 83, 221, 297, 330 | ||
| Crack cocaine | 337 | ||
| (Meth)amphetamine | 65, 81, 156, 205, 299, 323 | ||
| Amphetamine plus THC | 234 | ||
| Pemoline (dopamine-releasing agent) | 164 | ||
| l-DOPA | 311 | ||
| Methylphenidate | 118, 303 | ||
| THC | 76, 227 | ||
| Alcohol | 32, 47, 136, 183, 227, 237, 295, 299 | ||
| Phenelzine (unselective, irreversible monoamine oxidase inhibitor) | 7, 186 | ||
| Polysubstance use | 47, 76, 237 | ||
| Medications | Organic hallucinosis/delusional disorder (F06.0/F06.2) | Substance-induced psychotic disorder, with hallucinations (292.11), with delusions (292.12) | |
| Corticosteroids | 136, 299 | ||
| Antibiotics (clarithromycin, ciprofloxacin) | 306, 329 | ||
| Alpha b2 interferon plus ribavirin | 273 | ||
| Topiramate | 88 | ||
| Bromide intoxication | 182 | ||
| Based on other brain pathologies (“macroscopic”) or general medical conditions | Organic hallucinosis/delusional disorder (F06.0/F06.2) or dementia/delirium (see below) or persistant delusional disorder (F22.0), when DI is not considered to be a physiological consequence of the somatic disorder | Psychotic disorder due to … (indicate the general medical condition), with delusions (293.81) or with hallucinations (293.82), or dementia/dilirium (see below) or delusional disorder, somatic type (297.1), when DI is not considered to be a physiological consequence of the somatic disorder | |
| Brain disorders | |||
| Neurodegenerative disorders | |||
| Dementia, Alzheimer type | [Dementia] (F00-F03) | [Dementia] (294) | 35, 46, 47, 55, 108, 149, 198, 237, 251, 270, 299, 313, 323 |
| Cortical atrophy | 141 | ||
| Parkinson's disease and multiple system atrophy | 167, 311 | ||
| Huntington's disease | 226 | ||
| Multiple sclerosis | 192 | ||
| Brain stem and thalamus atrophy | 131 | ||
| Torticollis spasmodicus | 11 | ||
| Neurovascular disorders | |||
| Vascular/subcortical arteriosclerotic encephalopathy/dementia | 30, 68, 96, 97, 135, 138, 141, 183, 198, 299, 315, 333 | ||
| Stroke | 2, 37, 68, 123, 192, 242, 244, 278, 294, 308, 336 | ||
| Intracerebral hemorrhage | 89 | ||
| Subarachnoid hemorrhage | 68 | ||
| Brain tumors | 30, 138, 183, 217, 219, 283, 315, 333 | ||
| Pituitary tumor | 30, 183, 217, 315 | ||
| Craniopharyngioma | 333 | ||
| Infections | |||
| Meningitis | 268, 299 | ||
| Encephalitis | 299 | ||
| Neurosyphilis | 32, 78, 136, 299 | ||
| HIV encephalopathy | 106, 350 | ||
| Traumatic brain injury, including in thalamus | 14, 268, 299 | ||
| Posttraumatic epilepsy | 316 | ||
| Normal pressure hydrocephalus | 172 | ||
| Delirium, acute confusional states | [Delirium] (F05) | [Delirium] (293.0, 292.81, 780.09) | 47, 251 |
| Central anticholinergic delirium (doxepine, cetirizine) | 266 | ||
| General medical conditions (with pruritus or paresthesia) | |||
| Infections | |||
| Tuberculosis | 344 | ||
| Leprosy | 19, 33, 36 | ||
| Gonorrhea | 32 | ||
| Endocrine conditions | |||
| Diabetes mellitus | 19, 52, 192, 198, 268, 299, 345 | ||
| Hypothyroidism | 132 | ||
| Panhypopituitarism | 207 | ||
| Hypoparathyroidism | 324 | ||
| Postpartum | 170 | ||
| Oncologic/hematologic conditions | |||
| Solid tumors, lymphoma | 19, 30 | ||
| Leukemia | 30, 299 | ||
| Anemia | 192, 268 | ||
| Vitamin deficiency | |||
| B1 | 9 | ||
| Niacin (B3) (pellagra) | 192, 264 | ||
| Folic acid (B9) | 9, 192, 207, 299 | ||
| B12 | 192, 212, 263 | ||
| Rheumatic conditions | |||
| Systemic lupus erythematosus | 133 | ||
| M. Behçet | 67, 268 | ||
| Renal failure | 192, 268, 300 | ||
| Cholestasis with hyperbilirubinemia | 192, 247, 299 | ||
| Sensory deficits | |||
| Blindness | 295, 299 | ||
| Deafness | 295, 299 | ||
| Cardiovascular conditions | |||
| Congestive heart failure | 19, 78, 96, 135, 198 | ||
| Absolute arrhythmia | 198 | ||
| Arterial hypertension | 19, 78, 135, 198, 268 | ||
| Other | |||
| Hypernatremia | 89 | ||
Diagnoses appearing in square brackets need to be further specified according to the respective diagnostic system.
(i) Primary DI.
The name “primary” DI was first introduced by Skott in 1978 (299). Today, primary DI meets the criteria of “delusional disorder, somatic type,” according to DSM-IV-TR criteria (Inset 12). This disorder cannot be explained by any other condition and has been described as monosymptomatic, circumscript, or isolated. The diagnosis is valid for disorders characterized by nonbizarre delusions of at least 1-month's duration and unremarkable cognitive and social functioning with normal behavior (unless connected to the delusional theme). Odd or bizarre beliefs and behaviors must not be present. The belief of being infested and consecutive measures to free oneself of the possible pathogens, as seen in DI, are not “odd” or “bizarre” in this context (e.g., compared to a schizophrenic person who believes him- or herself to be influenced by electric fields and radio antennas that he or she can feel and hear interfering with the synapses of the brain). There are no signs of lasting disorganization of behavior (as is the case in schizophrenia). Hallucinations do not dominate the picture but can be present if they are related to the delusional theme, e.g., tactile sensations of “parasites crawling” while believing oneself to be infested by such vermin. First-rank symptoms for the diagnosis of schizophrenia according to the criteria of Kurt Schneider must be excluded. The possibility of depressive mood swings within the course of the delusional disorder is mentioned in the diagnostic criteria, but depression must be secondary to the delusion and have a shorter duration.
Inset 12. DSM-IV-TR diagnostic criteria for delusional disorder (code 297.1).
Nonbizarre delusions (i.e., involving situations that occur in real life, such as being followed, poisoned, infected, loved at a distance, or deceived by spouse or lover, or having a disease) of at least 1 month's duration.
Criterion A for schizophrenia has never been met. Note that tactile and olfactory hallucinations may be present in delusional disorder if they are related to the delusional theme.
Apart from the impact of the delusion(s) or its ramifications, functioning is not markedly impaired and behavior is not obviously odd or bizarre.
If mood episodes have occurred concurrently with delusions, their total duration has been brief relative to the duration of the delusional periods.
The disturbance is not due to the direct physiological effects of a substance (e.g., a drug of abuse or a medication) or a general medical condition.
Specify type (the following types are assigned based on the predominant delusional theme):
Erotomanic type: delusions that another person, usually of higher status, is in love with the individual.
Grandiose type: delusions of inflated worth, power, knowledge, identity, or special relationship to a deity or famous person.
Jealous type: delusions that the individual's sexual partner is unfaithful.
Persecutory type: delusions that the person (or someone to whom the person is close) is being treated malevolently in some way.
Somatic type: delusions that the person has some physical defect or general medical condition.
Mixed type: delusions characteristic of more than one of the above types, but no one theme predominates.
Unspecified type.
In ICD-10, primary DI belongs to the category “persistent delusional disorder” (code ICD-10 F22). There are minor differences in the criteria that cannot be discussed here. The most obvious one is that the research diagnostic criteria of ICD-10 require at least 3 months of symptom duration (only 1 month according to DSM-IV-TR).
(ii) Secondary DI.
All other forms of DI are secondary to another disorder, illness, or cause, which determines the subform of secondary DI and the diagnosis (Table 3).
In view of the different forms of DI, one may wonder which is the most frequent and characteristic. Many authors have considered primary DI to be prevailing (35, 137, 268, 322). For example, Bhatia et al. assumed a rate of 88% for primary DI (35). In contrast, Wilson and Miller found primary DI in only about 8% of cases (344). Most other authors believe that cases of DI secondary to macroscopic brain disorders (organic) are more typical than primary DI (32, 78, 203, 299, 315). Four studies suggest that primary DI is more frequent than organic DI but that all forms of secondary DI taken together dominate. In Trabert's analysis of 449 cases, 40.3% were primary DI and 21.8% were organic psychoses (induced psychotic disorder, 14.4%; schizophrenia, 10.6%; affective disorders, 9.1%; and neurosis, 3.5%) (322). In his own sample of 35 patients, almost half had a diagnosis of primary DI (49%), followed by schizophrenia (26%), organic psychosis (14%), depression, and dementia (both 6%) (323); toxic cases were included in the study. In our recent case collection, 44% of cases were primary DI (28 of 63 cases), while 35% were organic DI or dementia (19 plus 3 [respectively] of 63 cases) (99); all secondary forms together accounted for 56% of cases. In a cranial magnetic resonance imaging (MRI) study by Huber et al., 56% (five of nine patients) of patients had a macroscopic brain disorder, and only one single patient was diagnosed with primary DI (141). Taking these results together, it becomes clear that by using current diagnostic criteria, most cases can be assigned properly to one of the different forms of DI but that DI is not a single nosological entity and there is no prevailing form.
Pathogenesis
The biopsychosocial model and, more recently, the neurodevelopmental model have been established to explain the etiology of schizophrenia (291, 340). For other psychotic or delusional disorders, including DI, however, there is a distinct lack of a generally accepted pathogenetic model (further details are given in Inset 13). These nonschizophrenic psychotic disorders have hardly been studied by biological psychiatry and cognitive neuroscience.
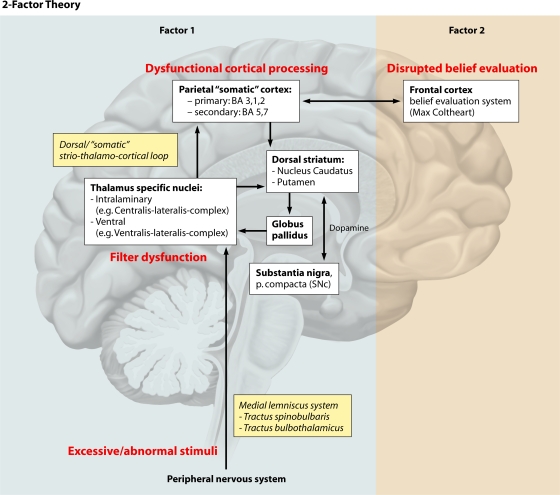
Inset 13. Neural correlates and models of psychotic symptoms. Some basic insight into the origin of key psychopathological features of these disorders has emerged, namely, for delusions and hallucinations (104). First, for the development of delusions, the “two-factor cognitive model” has been proposed by Bell et al. (26). The first factor consists of abnormal sensory perceptions which prompt the contents of the different delusions, while the second factor is a dysfunctional “belief evaluation system” located in the prefrontal cortex, which is thought to be common to all delusional disorders and “prevents the person from rejecting the belief in the light of strong evidence against it (…).” The model has been applied successfully to delusional disorders, mainly Capgras and Cotard syndrome (“two-factor theory of monothematic delusions”) (58, 59), but was not supported by all studies (25). Second, regarding the origin of hallucinations, neuroimaging studies indicate an overactivity in primary or particular secondary cortices of the respective sensory systems (10, 71). This overactivity might result from a disinhibition caused by reduced cognitive control and reduced inhibitory input from the prefrontal cortex and from other top-down processes (8, 10). Hallucination formation has also been linked to dysfunctional processing in subcortical regions and the cerebellum (10) and to a reduced filter function in the thalamus, e.g., in alcohol hallucinosis (302). Lepping and Swinton proposed a disturbance of the filtering function of primary sensory stimuli in the parietal lobes in patients with borderline personality disorder who experience psychotic symptoms (179).
Pathophysiology of DI.
For all forms of DI, an integrated pathophysiological model is absent, and there is a dearth of pathophysiological research (74, 95, 136, 141, 142, 300, 346). The interplay and sequence of abnormal sensations in the development of a delusional conviction are unclear. We only know that pathological tactile perceptions do not always precede the delusion (234). For example, in primary DI, the delusional belief arises as “delusions proper” (K. Jaspers [144, 145]), which are not based on other psychopathologies. Conversely, in secondary DI, the underlying disorder may be associated with tactile sensations or itching which is misinterpreted in a delusional way (95). Accordingly, looking at the different forms of DI, primary DI is understood the least, while in the various secondary forms, associations with disorders and triggers such as toxins hint to a possible pathophysiology and related neurotransmitter systems (Table 3). Despite limitations to current knowledge, pathogenesis is likely to be multifactorial. Possible factors, such as genetics, organic factors, premorbid traits, acute triggers, and social vulnerability, are known from other psychotic disorders, and we discuss these item by item.
(i) Genetics and heredity.
The first study on family history showed that patients with DI have significantly more affected relatives (about two times more) than do controls (45 versus only 23 affected persons among 200 relatives; P < 0.01) (299). But this only indicated a higher general level of vulnerability for psychiatric disorders and no DI-specific association (only a single other case of DI was reported among relatives; 3 suicides, 22 psychiatric outpatients, and 19 inpatients came from only 20 of the 45 families) (299). Another study reported a positive psychiatric family history of DI in 15% of the cases; this rate did not differ from that for schizophrenia (16%), but both were higher than those for control families (203). Other studies found a positive family history in 11% (323) or 32% (20% were first-degree relatives) (234) of cases. The numbers of cases were too small and the diagnoses of the affected relatives were too heterogeneous for any further conclusions.
As for specific susceptibility genes encoding functionally relevant proteins in the brain, studies are limited to samples of the superordinate diagnoses or syndromes. Serretti et al. showed that long alleles in exon 3 of the dopamine receptor D4 (DRD4) gene were associated with delusional severity in psychotic disorders in general (n = 2,011) and, more specifically, in delusional disorders (n = 104) (292). This association was confirmed in an independent Mexican sample (5). In contrast, an association with polymorphisms in the gene encoding the presynaptic dopamine transporter was absent in delusional disorder (260).
(ii) Neural basis and relevant brain structures.
Evidence for brain structures affected in DI from neuroimaging and lesion studies is limited to case reports, with the exception of a recent structural MRI study with a small sample of nine patients with DI from our group (141).
(a) Structural and functional neuroimaging. Using the historical method of pneumencephalography, eight cases have been investigated by different authors (23, 29, 54, 198). Cortical atrophy was present in six of eight cases, while subcortical atrophy with dilatation of the third ventricle was observed in six cases and subcortical atrophy with dilatation of the lateral ventricles was observed in four cases. All patients exhibited at least one abnormality (67).
The only structural cranial MRI study, by Huber et al., revealed macroscopic brain disorder in five of nine cases, which accordingly were classified as secondary organic DI (141). This subgroup of DI cases showed mainly vascular lesions of the basal ganglia with a predominant bilateral affection of the striatum, in particular the putamen, and subcortical white matter lesions in the centrum semiovale. Severe cortical atrophy was present in three of the cases, while cortical lesions were absent except for one patient with left-sided fronto-temporal lesions. A left-sided thalamic lesion was found in one case. The finding of striatal lesions pointed to a tentative role of a disturbed “somatic loop” (striato-thalamo-cortical loop) and may be an indication for dopaminergic dysfunction, as far as such conclusions can be drawn from this small exploratory study. A marked affection of the putamen was also reported in a case of DI secondary to multiple-system atrophy with nigrostriatal degeneration (hyperintensity in the FLAIR sequence) (167).
Functional neuroimaging to study the neural substrate and the pathophysiology of DI is lacking completely, with the exception of a single case report. Narumoto and coworkers compared pre- and posttreatment regional cerebral blood flow derived from single-photon emission computed tomography (SPECT) in a patient with DI secondary to a right temporo-parietal stroke (244). Under effective treatment with risperidone, they found a marked improvement of regional cerebral blood flow, including that in the bilateral frontal and left temporo-parietal cortices, the right parietal operculum, and the basal ganglia, bilaterally. Similarly, for a patient with delusional disorder, somatic type, Hayashi et al. reported hypoperfusion of the left parietal and temporal regions in a second case, which responded to paroxetine (130). No functional MRI studies are available at present.
(b) Brain lesions. Another limited source of information about the neural basis of DI results from patients in whom symptoms developed secondary to localized brain lesions (ischemic lesions, tumors, etc.). Ischemic brain lesions in these patients were seen predominantly in subcortical regions and in temporo-parietal regions after stroke (2, 37, 68, 90, 97, 195, 242, 244, 278, 314). Other case reports linked the origin of symptoms with a brain tumor in the hypophyseal region (30, 183, 217, 219, 315) or a craniopharyngioma (333). A potential pathogenetic role of the thalamus in DI was first hypothesized in 1920 (107). Amler reported a tactile hallucinosis with traumatic thalamic lesions (14). The first and only cerebral histopathology of a patient with DI secondary to a large tumor in the hypophyseal region led to a hypothesis of disturbed thalamocortical connectivity. The tumor had also invaded the hypothalamus and ventromedial and dorsolateral parts of the thalamus and led to a secondary atrophy of the prefrontal and parietal cortex (“due to retrograde thalamocortical degeneration”), involving somatosensory cortices (183). As mentioned above, generalized cortical and subcortical atrophy and striatal lesions were reported for DI (67, 141). In summary, limited data from imaging and lesion studies cautiously suggest a network of striatum/putamen, thalamus, and fronto-temporo-parietal cortices to be involved in the formation of DI.
(c) Oxidative cell stress. Ng et al. asserted in a recent systematic review that “oxidative stress has been implicated in the pathogenesis of diverse disease states, and may be a common pathogenic mechanism underlying many major psychiatric disorders, as the brain has comparatively greater vulnerability to oxidative damage.” They concluded that multidimensional data support the role of oxidative stress in diverse psychiatric disorders (248).This has led to suggestions that antipsychotic medication may be neuroprotective, a claim that could not be supported in a recent review (174). There are no studies to date linking DI with cell stress.
(iii) Role of dopaminergic neurotransmission.
Several lines of evidence suggest that dopaminergic neurotransmission plays the most important role in the etiology of DI, as with other disorders in the spectrum of psychotic and delusional disorders. This is because DI can be secondary to drugs such as cocaine, amphetamine, pemoline, or methylphenidate, which increase synaptic dopamine levels by blocking presynaptic dopamine reuptake at the dopamine transporter (118, 141, 142, 164, 303). Furthermore, antidopaminergic substances, such as antipsychotics, are helpful in the majority of patients with DI (99, 178). Among antipsychotics, the effects on DI appear to correlate with their potency to block postsynaptic D2 receptors, e.g., haloperidol and pimozide among traditional substances (in contrast to low-potency agents such as levomepromazine) and risperidone, olanzapine, and amisulpride among atypical antipsychotics (in contrast to, e.g., quetiapine or ziprasidone) (99, 176). Neuroimaging studies have shown that the nigrostriatal dopaminergic system was damaged in at least a subgroup of patients (lesions in the putamen and the caudate nucleus) (141). Striatal dysfunction and an altered dopaminergic neurotransmission in DI were also indicated by cases secondary to Parkinson's and Huntington's disease (226) and by multiple-system atrophy (167), pointing again to dysfunctional dopaminergic pathways.
Importantly, there is a convergence of findings on the relevant brain structures and on neurotransmitter systems. Both highlight the role of the nigrostriatal and mesolimbocortical dopaminergic systems.
(iv) Premorbid features.
Apart from these neurobiological aspects, some other premorbid factors have consistently been reported for DI. Obsessive-compulsive (anancastic) personality traits with a distinct sense of cleanliness were found in about 50% of patients with DI, and it was shown that they preceded the DI, pointing to a vulnerability for the development of DI associated with this personality type (135, 136, 234, 268, 289). Another common predisposing factor is social isolation (see “Epidemiology”). True skin diseases or infections in the patient, contact persons, or pets were found in some cases, e.g., previous scabies (136, 319) or previous syphilis (78). Other predisposing factors are sensory deficits, such as impaired vision or hearing (44, 183). In Skott's sample, about two-thirds of patients had impaired vision and one-third had impaired hearing (299). In the larger and more recent French sample of Bourgeois and Nguyen-Lan, 31% (47 of 150 patients) of patients had reduced sight (46). Vision impairment definitely adds to mistaking small particles for some kind of vermin (illusions) and the development of the specimen sign.
DIAGNOSIS
Diagnosing Delusion
Delusions and DI.
A patient with DI is easy to recognize (117). It is usually a bigger challenge to exclude the multitude of possible associated illnesses and to evaluate their causative role. But there is also a more fundamental problem. All known pathogens can be ruled out readily, making the diagnosis very likely, but it is impossible to exclude 100% that an unknown or new infectious species is present. The history of medicine is full of examples where diseases eventually turned out to be caused by an infectious agent. In particular, the history of infectious diseases is full of falsities (e.g., malaria is not “bad air”). Even since the 1980s, new infectious diseases or even species have been found (e.g., Helicobacter pylori in gastritis, human papillomaviruses in cervical cancer, retroviruses in acquired immunodeficiency, and prions). To put it the other way round, how can you be sure that a patient has a delusion, not a great new insight and the perseverance needed? Is it not possible that an infectious agent new to science is present in this patient with DI? Beliefs are rather easy to evaluate when they refer to your specialty, but physicians—psychiatrists in particular—are regularly required to judge opinions that tap into areas beyond their expertise (physics, astronomy, etc.). This is the problem of diagnosing delusions. In order to know how to proceed in such situations, it is helpful to know what delusions are and what we can learn from the German psychiatrist Karl Jaspers (1883-1969). This is summed up in Inset 14.
Inset 14. Defining delusions. The definition of “delusions” is not easy, and countless proposals exist. A delusion is usually defined as a “fixed false belief out of keeping with the patient's cultural background” (152), but recently in clinical psychiatry the aspect of being “false” is considered less important than the aspect of being “fixed.” Most definitions go back to the famous three criteria of delusions defined by Karl Jaspers in his groundbreaking book General Psychopathology (German Allgemeine Psychopathologie, first published in 1913, 9th edition published in 1973 [144, 145]). According to Jaspers, delusions can be recognized by (i) an “extraordinary conviction” and an “incomparable, subjective certainty” (“subjective Gewissheit”), (ii) which cannot be influenced by experience or logical conclusions (“imperviousness”), although (iii) “their content is impossible” (“Unmöglichkeit des Inhalts”). Jaspers also stressed the “incorrigibility of delusion” (“Unkorrigierbarkeit”) and added that falsities of healthy people are also often unshakable, but that—in this case—the majority of the people around them share the beliefs. Regarding the origin of delusions, Jaspers separates “delusions proper” (“echte Wahnideen”), which are autochthonous and not secondary to another pathology, from “delusion-like ideas” (“wahnhafte Ideen”), which result from pathological interpretations of real objects (e.g., “delusional perceptions” [Kurt Schneider]), deluded memories of the past (delusional memory, or “Wahnerinnerung” [K. Jaspers]), or other psychopathologic symptoms. Delusional conviction is not established 100% at the beginning, when patients still doubt their own beliefs (similar to when delusions start to respond to antipsychotic medication). Jaspers most clearly described that in delusion formation patients “look for some kind of connection” and try to find explanations. Real perceptions, facts, and their own past are misinterpreted by the same thought process, which is “trying to link them harmoniously” in order to erase all doubts (“zu widerspruchslosem Zusammenhang gebracht”). Jaspers called this process (delusional) “elaboration” (“Wahnarbeit”; a dysfunctional “belief evaluation system” in current terminology). Particularly in chronic, lucid psychotic disorders, such as monothematic delusional disorders, this work can absorb all intellectual capacities of an individual. The result is a “delusional system” (“Wahnsystem”).
Using Jaspers's criteria properly.
It may appear easy to diagnose any delusion with Jaspers's criteria. The intuition would be to prove the patient wrong and to use Jaspers's criterion of the impossibility of the content to identify a delusion. However, nobody can be an expert in every discipline, which makes this criterion difficult. Jaspers himself indicated that supernatural metaphysical beliefs presented by patients “cannot be adjudged true or untrue, correct or false,” and that “it is difficult enough to be decisive” even where “empirical reality is concerned” (145). Therefore, psychiatrists identify delusions not primarily by judging the reality or falsity of the content of the belief, although this might seem the most obvious. They (i) use the criterion that patients maintain their belief despite all evidence to the contrary (second criterion) and (ii) look at the patients' explanations and proofs, which can often easily be falsified, in contrast to the content of the delusion itself. Accordingly, the best practice in diagnosing delusions is to look at the form of reasoning, not the contents, because the third Jasperian criterion of delusions can be a pitfall. For example, a patient with schizophrenia may believe him- or herself to be persecuted by the Mafia (possible; difficult, if not impossible, to prove the contrary); asked to give a substantiation for this belief, the patient refers to strikingly many black Italian cars in front of his or her house and too many mentions of organized crime on TV (obviously insufficient explanation or proof).
We can use this psychopathological knowledge in DI. When a patient claims to be infested by an infectious species new to science, it is therefore recommended to listen to the line of reasoning and to examine the proofs of infestation instead of trying to challenge the belief that a hitherto unknown species causes the symptoms (impossible to falsify). A true infestation has to be ruled out, as far as possible. The patient has the right to be examined with regard to known infectious agents and other disorders that mimic or cause DI without any prejudice, but only to a reasonable extent. With this approach, it will be possible to handle almost any patient with due care. A diagnosis of DI can best be made when microbiologists, dermatologists, and psychiatrists cooperate. The former prove the impossibility of the patients' belief as far as pathogens are known, while the latter ascertains the presence of a delusion, if this was not otherwise possible.
Likelihood of overlooking an infestation.
Still, well-informed patients with DI will argue that new infectious agents are discovered continuously and that patients have falsely received a diagnosis of DI and eventually turned out to be truly infested. We are aware of 13 cases of this kind in the medical literature (n = 1 [328], n = 6 [192], n = 2 [351], with 1 of them reported twice [77], n = 1 [119], n = 1 [73], and n = 2 [280]). In terms of figures, based on at least 1,400 published cases of DI (conservative estimate of prevalence), the risk of overlooking an infestation would be less than 1%. In Lyell's survey of dermatologists, the risk was 2.1% (6 of 282 patients) (192). A recent publication reported a much higher rate of 22% (two of nine patients), which was undoubtedly due to the particularly small sample size (280).
Delusional themes versus contents.
The syndrome of DI is very stable over time, with the exception of the exact alleged pathogens. To explain this, classical psychopathology is helpful. In all delusional and psychotic disorders, the overarching delusional themes are stable over time, but the specific delusional contents within these themes are variable and influenced by culture and zeitgeist. For example, within the theme “persecution” (usually seen in paranoid schizophrenia or paranoia), patients have blamed many different persecutors, which obviously depend on real threats of the respective era, e.g., communists, Nazis, agents (KGB, Gestapo, Mossad, MI-6, or Central Intelligence Agency), the Mafia, religious fanatics or a sect, etc. Other delusional themes, such as “grandiosity” or, in our case, “infestation,” have time-specific manifestations. In the 19th and early 20th centuries, patients believed themselves to be infested by scabies, typhus, or the pest (Table 2). This changed to parasites or insects and then, increasingly, to bacteria, fungi, viruses, or unknown species. In recent years, there are new alleged pathogens, such as “stealth-adapted virus” and “alternative cellular energy pigments” (206). Even more recently, the alleged pathogens can be threads, hairs, and fibers, a phenomenon which afflicted persons call “Morgellons.” The theme “infestation” is influenced by modern literature and films (outbreaks of new pathogens from secret labs, etc.).
The Morgellons Phenomenon
With good knowledge of all key features of DI, the psychopathology of delusions and delusional themes, the epidemiology and the history of DI, we have the armamentarium to examine the so-called “Morgellons disease” (background information is given in Inset 15). In the following, we prefer the term “Morgellons.”
Inset 15. Morgellons. Since 2002, an increasing number of patients have contacted physicians and complained about “having Morgellons disease,” a disorder or name not known to medical science. Usually, patients had read or heard about the “disease” on the Internet. The first patients described were from the United States (mainly California). They claimed to be infested by fibers and threads infesting their skin, causing skin problems and unspecific neuropsychiatric symptoms. They provided proofs of that infestation, often digital photos. Dermatological and microbiological tests were unremarkable, but patients questioned the negative results. Morgellons sufferers usually believed themselves to be infested by inanimate material, but some also saw associations with a fungus (new to science), genetically modified food, “nanotechnology gone awry, an immune disorder, an infectious agent, mass psychogenic hysteria, the effects of airline contrails (…), illegal immigrants, Lyme disease, and others” (222). Not all people with self-diagnosed Morgellons presented such “proliferating theories” about the nature of the infestation (222), which resemble conspiracy theories. On the Internet, affected people have started to use the diminutive “Morgie(s)” for Morgellons. Over the last few years, in times of “cyberchondria,” Morgellons has become a real mass phenomenon. It has been called a “socially transmitted disease over the internet” (256). The obviously extensive Internet use of Morgellons sufferers explains its fast spread. So far, there is no sign of a real (infectious) epidemic. Meanwhile, “Morgellons” has arrived in Europe (274).
It is easy to retrace the history of Morgellons. The key role involves an organization called the Morgellons Research Foundation (MRF). The MRF runs a comprehensive website called www.morgellons.org, which receives a lot of traffic and serves as the main platform and resource for the fast-growing community of people interested in this “disease.” The material on the website shows how self-diagnosed Morgellons sufferers think and act. We recommend visiting this website to get one's own impression. Other websites and file-sharing platforms have also become popular for spreading and exchanging lay material and digital proofs of infestation. The MRF website provides the following background of the organization: “The Morgellons Research Foundation (MRF) is a 501I3 nonprofit organization established in 2002 in honor of a two-year-old child with an unknown illness, which his mother labeled ‘Morgellons disease’” (www.morgellons.org; accessed 8 December 2008). According to the website, the child has suffered from “numerous symptoms outside known illness categories.” One physical sign became the “defining” characteristic of the illness, namely, small-diameter “filaments” protruding from lesions near the child's mouth and other body areas that were both sensitive and painful. In searching for others who might have encountered the same phenomenon, the MRF was created. Its initial function was to convey details of the phenomenon to others and simultaneously to provide a central registration site to foster broader communication (www.morgellons.org; accessed 18 October 2008). The mother is biologist Mary M. Leitao. She is the founder and current “executive director” of the MRF. In 2006, Leitao and two officials of the MRF published the first paper on Morgellons, in a dermatological journal (281). The two other authors are Ginger Savely, a registered nurse (part of the “medical advisory board” of the MRF), and a physician, William T. Harvey (“chairman” of the MRF). In this paper, the authors postulate that Morgellons “(…) is characterized by fiber-like strands extruding from the skin in conjunction with various dermatological and neuropsychiatric symptoms” and that it “(…) resembles and may be confused with delusional parasitosis,” and they suggest an “(…) association with Lyme disease” with an “apparent response to antibacterial therapy.” Although there are no data to support these conclusions, the authors indicate it “may be linked to an undefined infectious process” (281).
Online interviews with Leitao, which can easily be found on the Internet (66, 124, 223), further explain how she coined the previously unknown name “Morgellons.” She reported that, in 2001, soon after the unexpected death of her husband, she examined her 2-year-old-son and discovered red, blue, black, and white “bundles of fibers,” which several doctors failed to identify. In search of an explanation, she found a letter by a Sir Thomas Brown (1690) reproduced by C. E. Kellett from 1935 and called “Sir Thomas Browne and the disease called the Morgellons” (1, 153, 233). Brown described symptoms in children in the Languedoc area in the South of France (“…that Endemial Distemper of little Children in Languedock, called the Morgellons, wherein they critically break out with harsh Hairs on their Backs, which takes off the Unquiet Symptoms of the Disease, and delivers them from Coughs and Convulsions”). After choosing the name Morgellons for the symptoms in her youngest son, Leitao founded the MRF (1, 337). She said that she later started to believe that her other two teenage children suffered from the same disease (223).
As of December 2008, there are 14 publications on Morgellons listed in PubMed. None of them provides any proof of a true infestation. Papers promoting the existence of Morgellons can easily be traced back to the MRF by checking the authors' addresses and affiliations (49, 128, 281). Next to Leitao, the most important protagonist of the Morgellons story is William T. Harvey, the present “chairman” of the MRF. Apart from his recent papers on Morgellons, it is interesting to see that all of his earlier publications—before the name Morgellons was coined in 2002—deal with neuropsychiatric symptoms in himself which he considered to be “emerging illnesses” or “an unrecognized borreliosis pandemic” in connection with Lyme disease and Candida tropica (127, 129). Previously, Harvey had also founded an organization called the International Lyme and Associated Diseases Society (www.ilads.org) (129) and described his self-treatment with ultrahigh doses of antibiotics to free himself from bacteria. Together with another physician who treated Morgellons patients with “modest courses of antibiotics” (49), he expanded his theories and recently suggested an association of Lyme disease and autism (50). In 2007, Harvey published an angry and vague reply to Caroline Koblenzer, who had reported on having treated Morgellons patients effectively with the antipsychotic pimozide as if they had DI (128, 157, 158). The author stated that “all patients with Morgellons carry elevated laboratory proinflammatory markers, elevated insulin levels, and verifiable serologic evidence of 3 bacterial pathogens” (without naming them) but emphasized that “(m)ost importantly they will improve, and most recover on antibiotics directed at the above pathogens.” He fails to provide any specific data supporting this claim but calls for “evidence-based medicine” for Morgellons (128). Paquette criticized that some members of the MRF board offer extended therapy with antibiotics and fungicides without any clear medical indication (256), capitalizing on the true despair of patients with so-called Morgellons (66, 124).
With the help of their website, many interviews, and a lot of media coverage, Leitao and the MRF have managed to raise money and support from more than “13,000 registered families,” according to the hit counter on the MRF website. The MRF's most astonishing achievement, next to the publications in medical journals, was that they were able to persuade the U.S. Centers for Disease Control and Prevention (CDC) to conduct a large study in Morgellons sufferers in late April 2006. This decision of the CDC was also covered in Nature Medicine with the title “Mysterious ‘Morgellons disease’ prompts US investigation” (204). The CDC had turned down several previous applications by the MRF since 2004. But on 5 April 2006, California Senator Dianne Feinstein, stimulated by mail from the Morgellons community, directed a letter to the CDC and asked them to take action (accessible via the MRF website [http://www.morgellons.org/cdc_timeline.htm]). Further U.S. senators sent letters to the CDC in December 2006 and encouraged the CDC to accelerate their work. On 28 March 2007, the CDC removed “The Delusional Parasitosis Page” from its website. Instead, on 12 June 2007, a site on “unexplained dermopathy (aka Morgellons)” was launched. Since then, the CDC's definition of the picture is as follows: “Persons who suffer from this unexplained skin condition report a range of cutaneous (skin) symptoms including crawling, biting and stinging sensations; granules, threads, fibers, or black speck-like materials on or beneath the skin; and/or skin lesions (e.g., rashes or sores). In addition to skin manifestations, some sufferers also report fatigue, mental confusion, short term memory loss, joint pain, and changes in vision” (http://www.cdc.gov/unexplaineddermopathy/general_info.html; accessed 18 October 2008).
On 1 August 2007, the CDC set up a study protocol with three phases, as follows (www.cdc.gov; visited 16 July 2008). “To learn more about this condition, CDC is conducting an epidemiologic investigation. CDC has awarded a contract to Kaiser Permanente's Northern California Division of Research to assist CDC in the investigation of this condition. The study is being designed and led by CDC. The primary goals of the investigation are to help us learn more about who may be affected by this condition, the symptoms they experience, and to generate hypotheses about factors that may contribute to it. The investigation will involve: determining the clinical and epidemiologic features of this condition; assessing the histopathology of skin biopsies from affected patients; characterizing foreign material such as fibers or threads obtained from persons with the condition; and estimating rates of illness among the study population.”
Phase I was for identifying and recruiting eligible participants and has been completed. In phase II, starting in May 2008 and still ongoing, the participants are examined clinically, with skin biopsies, blood tests, and a state mental examination. Treatment will not be provided. Of course, the results of the CDC investigation are highly desired, but the CDC has not published any results so far. Instead, the MRF presented a summary of phase I (16 June 2008) on their website. It does not provide any significant data but gives a lengthy account of organisms isolated from a single person's cold and hot water tank (which had not been used for years). The largest part of the summary consists of new theories about possible pathogens which shall cause Morgellons [“There are six particle types that are consistently recovered from the skin surface of those suffering from Morgellons disease, 1) ribbon-like fibers, 2) rounded fibers, 3) capsule-like particles, 4) black flakes/grains, 5) worm-like particles, and 6) stellate-shaped (“starfish-shaped”) particles. The fibers are often pigmented…”].
We tried to obtain usable information on the CDC study directly from the CDC's lead investigator, Michele L. Pearson, in late 2008. Our request was answered as follows: “Our investigation is ongoing. So, we don't have any results to share at this time” (M. L. Pearson, personal communication). In interviews, Pearson has been cited as saying that Morgellons is “a complex condition” and “multifactorial,” that there is “nothing systematic,” and that “people are suffering. (…) Some have incurred high medical costs for alternative therapies” (222). Elsewhere, she was quoted as follows: “We don't know if it's infectious. We don't have any good evidence that it's communicable” (66).
Outside the MRF, almost all other authorities doubt that Morgellons is a new infectious disease. The National Institutes of Health disapproved a request by the MRF to investigate Morgellons by the NIH Undiagnosed Diseases Program (27 August 2008, as reported on the MRF website). The Robert-Koch-Institute in Berlin, Germany, does not list Morgellons among “Infectious Diseases A to Z” and the list of “new or more frequently occurring infectious diseases” (www.rki.de; last visited 29 December 2008). A standard textbook on parasitology summarizes the following in the section “Pseudoparasites and Artifacts”: “…many expert parasitologists, medical entomologists and other microbiologists have in fact carefully examined fibers and other materials expressed or extracted from such patients and found that biological organisms are not present. Although an apparent association of the condition with the presence of Lyme disease has been reported (Savely et al., 2006, Am J Clin Dermatol, 7:1-6), further research will be needed to help resolve the validity of Morgellons disease. Until then, whether Morgellons disease is another name for delusional parasitosis or a real disease entity with a biologic or physiologic basis will remain up in the air” (17). There is first low-level evidence from case reports and expert opinion that patients with self-diagnosed Morgellons respond to antipsychotic medication in cases of sufficient adherence (158).
Critical appraisal as of December 2008.
It is apparent that Morgellons and its sufferers exhibit all typical clinical signs of DI (mainly primary DI, in view of a lack of mental decline outside the delusional theme). The origin of Morgellons, in the particular mother-child constellation, has similarities with DI by proxy (70, 124). The desperate search for an explanation and a name for the symptoms that led to the name “Morgellons” resembles phenomena such as delusional elaboration. Looking at the early history of Morgellons, the high level of activity seen in patients with self-diagnosed Morgellons, important members of the MRF, and its supporters, their reactions to criticism, and the persistent ignoring of all evidence against an infestation are very evocative of the behavioral and thought patterns observed in patients with delusional disorders. It is compelling to believe that Morgellons is a new manifestation and variation of the delusional theme “infestation” (1), but in the present situation with the ongoing CDC study readers may draw their own conclusions. At present, Morgellons appears to be one of the first “Internet-transmitted diseases.” At any rate, the self-described symptoms of Morgellons patients meet our minimal criteria of DI detailed above and are “very similar, if not identical, to those of delusions of parasitosis” (1). Therefore, the practical conclusion for the medical community must be to treat Morgellons as DI as long as there is no better explanation. In view of many patients complaining about an infestation with species other than parasites (including Morgellons) and the syndromal aspect of the picture, we prefer the broader term “delusion infestation” instead of delusional parasitosis. It fits into the picture that the former main website of patients with DI, www.skinparasites.com, has disappeared because of the shift to Morgellons. From a psychiatric point of view, we expect further variations and manifestations of DI, including new names and pathogens. It is important for dermatologists, microbiologists, and generalists to know the phenomenon of name shifts and variations within a delusional theme. Morgellons will not be the last variation to emerge. When talking to patients with DI or self-diagnosed Morgellons, the use of the name “Morgellons” cannot be recommended as a “rapport-enhancing” term, as recently proposed by Murase et al. (233), because this would contribute to establishing a lay term unknown to medical science. We suggest using the name “unexplained dermopathy,” as introduced by the CDC, because it is neutral and correct. It might aid physicians in keeping patients' cooperation without being forced to be imprecise.
Diagnostic Procedure
Two-stage clinical pathway.
Most authors recommend a graded pathway with two major steps for optimized clinical management of patients with DI (76, 234, 280, 351). It has to be stressed, however, that all of these clinical pathways for DI have not been evaluated in terms of sensitivity, specificity, or cost-efficiency and represent expert opinion only. Nonetheless, all syndromes with multiple etiologies and many differential diagnoses require thorough diagnostic examination (step 1) in order to facilitate an etiology-specific treatment (step 2). The first diagnostic step is summarized in Fig. 3 (based on references 95 and 175). It aims at facilitating the choice of efficient measures in investigating the large number of associated disorders.
FIG. 3.
Diagnostic overview (step 1).
Diagnostic work-up and diagnosis.
The pathway starts from a patient with symptoms similar to those of DI, passes a stage of suspected DI, and gradually approaches a confirmed diagnosis. The first measure is to rule out a true infestation and a number of common differential diagnoses and associated disorders by basic, cost-effective measures. This includes some basic laboratory tests (inflammation markers) and a dermatological skin examination with magnification and mineral oil skin scrapings, possibly biopsies where indicated. This is the most important step because a real infestation must be excluded (346). Bacterial skin superinfections may be caused by self-therapy and may elevate inflammation markers. The examination of the patient and the specimen, if available, must be thorough, even if the physician does not suspect an infection. Careful and understanding history taking (e.g., asking about any possible tropical vacations) and questions about the subjective burden of disease are essential for establishing at least some trust. After the exclusion of real skin infections, suspected DI must be separated from “similar conditions” from the dermatopsychiatric spectrum, e.g., formication, acne excoriée, etc. When these disorders are excluded, DI is confirmed (minimal criteria are met), but this still is no diagnosis in the narrow sense. Only now are more cost-intensive special diagnostics useful to allow for the determination of the form of DI. Additional history taking and further investigations are usually needed at this stage to rule out general medical conditions with pruritus and psychiatric symptoms (endocrine, renal, hepatic, malignant, rheumatoid, nutritional, and neurological conditions and pregnancy). Some laboratory tests are mandatory (complete blood cell count, erythrocyte sedimentation rate, C-reactive protein, serum creatinine and electrolytes, liver function, thyroid stimulating hormone, fasting glucose, urine analysis [obligatory for illicit drug use testing], and pregnancy test). Other parameters should be considered in specific clinical constellations (borrelia, treponema, hepatitis, and human immunodeficiency virus [HIV] serologies, vasculitis screening, allergy testing, B vitamins and folate, and carbohydrate-deficient transferrin) (95, 175). Urine testing for illicit drugs is obligatory, in particular testing for cocaine, amphetamines, and cannabinoids. A chest X-ray and an electrocardiogram (ECG) can be necessary. A cranial MRI is also needed to rule out brain disorders and to establish a psychiatric diagnosis. With the results of the above measures, a diagnosis according to DSM-IV-TR or ICD-10 criteria can be made.
Urgency.
In most cases, symptom onset is insidious and the disorder is present for months, so standard priority in diagnostics and therapy is sufficient. Cases that require immediate or emergency care and admission to hospital are rare, e.g., acute symptom onset with obvious somatic disease, delirium, substance withdrawal or acute intoxications, or rare cases of psychiatric risk, such as neglect, risk of suicide or aggression, or dangerous and excessive self-cleaning of oneself or affected others, such as children.
Special diagnostic issues.
It is helpful to rate the degree of conviction of being infested before attempting psychiatric referral in vain. For a psychiatric referral to be successful, the patient usually needs to be willing to accept the possibility of an alternative (i.e., psychiatric) explanation of his or her symptoms. If psychiatric referral is impossible, the treating physician should at least review the case with a psychiatrist by phone to use psychiatric expertise for the differential diagnosis and psychopharmacology.
For each patient with DI, the possibility of an SPD in family members or other close contacts (e.g., asking if there are close persons with similar problems) has to be considered and assessed. Otherwise, it might be unrecognized that another person is the inducer who needs antipsychotic treatment (see below).
In addition, each patient with signs of manifest depression (low mood, loss of interest and enjoyment, reduced energy and motivation, disturbed appetite and sleep, and difficulty concentrating) should be questioned about suicidal ideation, intent, and plans. A risk assessment has to be performed regarding the patient and significant others, especially children. Sometimes, patients hold a grudge against former physicians. In case of frank signs of hostility, it is inevitable to perform a full risk assessment and to consider the need for compulsory committal to hospital. A psychiatrist, the community mental health team, or local authorities such as the police must be informed in the rare cases of imminent danger.
Diagnostic problems.
A formal problem in the diagnostic process can be to assign one of the DSM-IV-TR or ICD-10 diagnoses to the single patient suffering from DI, although the different diagnostic criteria per se are clear. The first typical situation is the question of whether a general medical condition causes brain dysfunction or not (e.g., moderate chronic renal failure). If the former is assumed, then the diagnosis would be “psychotic disorder due to a medical condition”; in the latter case, it remains a delusional disorder, somatic type. The diagnostic uncertainty is reflected by high rates of patients who did not get any formal diagnosis, including 16% in psychiatry, 54% in public health departments, and even 80% in dermatology (325).
Differential Diagnosis
The differential diagnosis of DI is broad and can be grouped and approached as follows.
Organic or substance-induced DI.
Important differential diagnoses are organic or substance-induced cases of diseases and disorders that cause secondary DI (general medical conditions, brain disorders, prescribed medication, and illicit substances). They are listed in Table 3 and do not need further explanation.
Formication.
The symptom of “feeling ants crawling on the skin” (from Latin “formica” = ant) is very frequent and unspecific and does not justify a diagnosis of DI. The absence of a fixed belief of being infested would indicate that DI is not present. Further diagnostics, mainly neurological, can be needed if additional neurological deficits are identified.
Major psychiatric disorders.
All major psychiatric disorders with body-related delusions, hallucinations, and self-inflicted skin lesions are important differential diagnoses and can be a cause of secondary DI.
(i) Schizophrenia.
Delusions and behaviors in schizophrenia are often odd or bizarre, in contrast to delusions in delusional disorders such as primary DI. In schizophrenia, persecution is a more typical delusional theme than infestation. Schneiderian first-rank symptoms, such as delusions of control (passivity phenomena), are almost pathognomonic. Hallucinations are rather auditory and, much rarer, tactile. In contrast to delusional disorders, blunted or flat affect, negative symptoms, formal thought disorder, and a “deteriorating course of illness” are observed. The manifestation is usually earlier than in delusional disorders (in the late 20s). DSM-IV-TR diagnostic criteria must be met. DI can also occur secondary to schizophrenia (e.g., the patient believes a nest of a genetically manipulated virus has been implanted in the brain via an invisible needle by a malign force, and he or she can hear the viruses comment on his or her actions and believes they can also control his or her thoughts).
(ii) Psychotic depression.
Patients with a psychotic major depression usually present with delusions of guilt and nihilistic or hypochondriacal ideas, while psychotic symptoms from the spectrum of DI are rare complications (e.g., within a depression getting worse a patient feels empty, dead, and as if he or she is being eaten by worms which creep in the skin and in the mouth, and he or she believes this is justified punishment for sinful deeds). However, two clinical constellations in the context of DI and depressive symptoms are important. A major depression with psychotic symptoms causing secondary DI (“primary depression”) must be discerned from the inverse constellation, a “secondary depression” that develops in the course of DI. The two variants can best be separated by comparing the durations of DI and depression: the initial disorder determines the diagnosis, main therapy, and prognosis.
(iii) Other delusional disorders, somatic type.
Other delusional disorders of the somatic type are very similar disorders with a delusional belief related to the patient's body. In contrast to being infested, patients may believe that they have a sexually transmitted disease or that the body has a defective shape or emits a foul odor (olfactory reference syndrome) (275) or dangerous breath (delusional halitosis) (22).
(iv) Dementia.
Patients with dementia suffer from global and progressive memory and cognitive disturbances that affect activities of daily living. Various psychotic symptoms, including delusions, illusions, and hallucinations, can occur as a dementia-related psychosis or within recurring delirium. Feelings of being persecuted or robbed and optic illusions are common. In some cases, these psychotic symptoms include DI and abnormal tactile sensations (DI secondary to various forms of dementia).
(v) Toxic psychosis.
Psychotropic drugs, in particular cocaine (“cocaine bugs” [196]), amphetamines, and, less frequently, cannabinoids, alcohol dependence (with peripheral neuropathy), and alcohol withdrawal with delirium can cause DI-like symptoms (secondary DI).
Similar psychiatric disorders.
Other psychiatric disorders have similarities with DI and should be considered as part of the differential diagnosis.
(i) OCD.
Patients with the form of OCD that focuses on cleanliness have intrusive and often also obsessive thoughts about contamination and/or infestation and usually perform ritualized, time-demanding cleaning rituals of their own bodies and accommodation, resulting in rashes and skin lesions. Because of three mutual symptoms (preoccupation with infections, intensive cleaning, and skin lesions), OCD is sometimes mistaken for DI. OCD patients try to ignore or suppress thoughts about infections and recognize them as unreasonable, in contrast to patients with DI. Delusions or hallucinations are absent. OCD is not observed as a complication or comorbidity of DI.
(ii) Trichotillomania.
Trichotillomania is an isolated impulse control disorder or occurs as an unspecific symptom, e.g., in mental retardation. In contrast to patients with DI, patients with trichotillomania remove their hair for no specific reason and do not believe themselves to be infested or associate their hair with pathogens.
(iii) Hypochondriasis.
Patients with hypochondriasis are worried about having a severe or fatal, sometimes infectious, illness. They consult numerous doctors in order to get more and better diagnostic investigations. Despite this partial overlap with symptoms of DI, it differs because of the absence of overvalued ideas or delusions.
(iv) Burning mouth disorder (“orodynia”/“glossodynia”).
Patients with this little understood and comparatively “new” disorder suffer from sensations, usually “burning,” in the oral mucosa or the tongue without detectable changes. They are usually middle-aged to elderly females, similar to the case for DI. In contrast to DI, patients do not believe themselves to be infested and have no other delusional convictions. No treatment is well established.
Psychodermatoses.
Dermatologists are consulted by two other patient groups with psychiatric disorders, which also differ from DI in the lack of psychotic symptoms. Both disorders result in skin picking.
(i) Dermatitis artifacta.
Dermatitis artifacta represents a form of factitious disorder. It can also occur by proxy.
(ii) Psychogenic excoriation.
Patients with psychogenic excoriation scratch and pick their skin in stereotyped rituals (“skin picking disorder”; also called dermatotillomania). A special form is selective skin picking on the face, which is mostly seen in young females (acne excoriée des jeunes filles). The skin manipulations and lesions can be similar to those seen in DI, but they are not based on an imaginary infestation of the skin. The picture occurs as an impulse control disorder, within the spectrum of OCDs or based on an imagined defect in appearance (body dysmorphic disorder/dysmorphophobia). It is treated with serotonergic antidepressants and cognitive behavioral therapy.
Other causes of pruritus.
In a broader sense, all conditions with chronic itch or pruritus (sine materia) belong to the differential diagnosis of DI.
(i) Senile pruritus.
Senile pruritus is frequent in the elderly (with no psychotic symptoms).
(ii) Medication.
Many of the most frequently prescribed substances can cause pruritus, paresthesia, or adverse dermatological effects (134; http://www.uni-duesseldorf.de/AWMF/ll/013-048.htm [last accessed 21 November 2008]) (Inset 16). This is important when new-onset DI correlates with the introduction of any of these substances.
Inset 16. Drugs with frequent pruritogenic or dermal adverse effects (erythema, itch, and rashes). Hydroxyethyl starch (up to 40%), gold, cytostatic drugs (bleomycin, vincristin, and tacrolimus), clonidine, corticosteroids, estrogens, ACE inhibitors and ATII antagonists, calcium antagonists, alpha1-antagonists, beta blockers, diuretics, antibiotics (macrolides, quinolones, cotrimoxazole, penicillin, and cephalosporins), antidiabetics (metformin, glipizide), statins, digitalis, omeprazole, nonsteroidal anti-inflammatory drugs, opioids (sufentanil up to 25%), selective serotonin reuptake inhibitors, tricyclic antidepressants, lamotrigine, and conventional antipsychotics can all have pruritogenic or dermal adverse effects. Of particular interest are codeine preparations. Cocodamol (codeine-paracetamol at 8 and 500 mg, respectively) preparations are regularly among the three most prescribed drugs in the United Kingdom. Because codeine is metabolized to morphine, it has opioid side effects, including pruritus.
(iii) Diseases or physiological states with the highest rates of itch/pruritus as a symptom.
The diseases with the highest rates of pruritus as a symptom include atopic dermatitis (100%), psoriasis (84%), primary biliary cirrhosis (80 to 100%), varicella-zoster virus infections, including zoster and postherpetic neuralgia (58 or 30%), anorexia (58%), polycythemia vera (48%), renal failure (22 to 66%), Hodgkin's disease (25 to 35%), pregnancy (18%), hyperthyroidism (4 to 7.5%), diabetes mellitus (3%), and solid malignant tumors (http://www.uni-duesseldorf.de/AWMF/ll/013-048.htm; last accessed 21 November 2008).
TREATMENT AND OUTCOME
Approaching Patients with DI
According to the first specific paper on the management of patients with DI, by Gould and Gragg, “(t)here is probably no disorder in dermatology that is so difficult to treat and for which the published advice is so inappropriate and impossible to follow” as DI (117). Basic communication is difficult (a “single false word” insinuating a psychiatric disorder too early will enrage patients).
The stepwise diagnostic approach presented above appears easy to follow. However, the clinical reality in patients with DI is often different, necessitating an idealized flow chart. For the physician, it is easy to be sure of the right diagnosis when true infections have been ruled out and Jasperian criteria of a delusion are met, but how is it possible to present the “negative results” to the patient? You may keep him in care by promising yet another skin swab, but sooner or later the patient wants to know the diagnosis and whether you think he has “something psychiatric” or not. “Overdiagnosing” (too many diagnostic investigations just to keep the patient) is not advisable in the long run, but neither is “underdiagnosing” (too early diagnosis of DI without proper tests). A critical point is reviewing the diagnostic findings from samples and skin swabs. If no pathogens are found (again), the patient will not be reassured but will question the quality of the measure (examples of delusional elaboration) and reinterpret the findings against all evidence to maintain the belief. Things are even more complicated if the report of microbiological skin swabs mentions any bacteria (whether they are part of the physiological flora or not) or real superinfections (due to the manipulations), because in those cases the patient will probably see it as proof of infestation.
It is an even bigger challenge to secure a psychiatric evaluation/referral and to engage the patient in a trial of psychopharmacological treatment.
Possible strategies.
To better prepare the treating physician, some thoughtful approaches have been proposed, which are highly recommended readings (77, 95, 117, 163, 175, 191-193, 234, 280, 300, 351, 353). They tried to overcome previous inappropriate suggestions from the 1940s, which suggested convincing the patient that the disorder is psychological and trying immediate psychiatric referral while telling the patient that a dermatologist definitely cannot help (344). This caused most patients never to return but not to engage in meaningful treatment either. Gould and Gragg were the first who tried to keep patients in dermatology and suggested guidelines “preferable to no treatment or to neglect” which “may be used by most physicians” (117). These guidelines were explained based on only two cases, but they were a starting point for refined suggestions over the years (280, 300, 351). The effectiveness of these approaches has hardly been studied systematically. The only evidence available is a report of dermatologists who were able to engage three of four patients in pimozide treatment by using such strategies (n = 33) (353). There is also a short note by Trabert on his experience with 35 patients where a similar approach was used and “accepted by almost all patients,” without giving any numbers (323). Thus, the suggestions have a low level of evidence. This also applies to our summary of the most important recommendations for clinical management (Inset 17).
Inset 17. Do's and don'ts.
Take time; take a careful history, including trips to tropical resorts.
Perform the diagnostic investigations needed (even if you are sure that the patient has no infection).
Examine all specimens carefully.
Acknowledge the patient's suffering. Show empathy and offer to help to reduce distress!
Paraphrase the symptoms (“you are itching,” “the sensations,” “the crawling,” etc.) instead of reinforcing or questioning them.
Indicate that you are familiar with the problem and that you were able to help other patients not instantly, but after a while.
Answer that you did not find any pathogens so far, but you are sure that the patient really suffers from his perceptions.
Indicate that this may be due to overactivity in the nervous system and to normal neuron-adaptive processes in the brain.
Try to introduce antipsychotics as the only substances helpful against these processes, as suggested by current research.
Use the names “unexplained dermopathy” or “Ekbom's syndrome” if the patients asks for the diagnosis.
Introduce antipsychotics as helpful against the patient's distress and itching (antihistaminic component of many antipsychotics).
Do not try to convince the patient or question the patient's beliefs.
Do not attempt immediate psychiatric referral or try to establish psychopharmacological therapy too soon.
Do not use words like “delusion(al),” “psychotic,” “psychological,” “psychiatric,” etc. (too early).
Do not use phrases like “calm down” (“be happy it's not infectious,” “it is only psychogenic,” etc.); this will upset the patient.
Do not simply prescribe an antipsychotic because different approaches are needed according to the type of DI.
Do not prescribe antibiotics or any other anti-infective without a real infection (further reinforcing the delusion).
Do not overlook frank aggression against other health care professionals.
Do not forget to ask patients with despair and signs of manifest depression about suicidal ideation and to evaluate any risk to others.
We would like to propose some additional techniques for the crucial points in the communication with the patient, as follows.
(i) When it comes to explaining the diagnosis and microbiological (non)findings, it may be indicated that there is the possibility of infestations that cannot be identified at present but that everything possible will be done to test for known species. The physician might indicate that there is also a second possibility, of an “oversensitivity and overactivity in the peripheral and central nervous systems,” which can be the cause for tickling, crawling, and itching. If the patient indicates disbelief, it can be helpful to explain this with analogies to other disorders (pain) and to healthy people, in whom such phenomena can be present transiently. It can be explained that all chronic symptoms, such as chronic itch/paresthesia or chronic pain, cause adaptive and neuroplastic reactions in the responsible neuronal networks and sensory brain areas. Alternatively, if you want to evade pathology, the analogy that all repeated stimuli or actions are represented in the brain by neuroplasticity (e.g., in violin players or when you learn to swim, learn to ride a bike, learn a language, etc.) may be used. When asked for a diagnosis, the term “unexplained dermopathy” from the CDC study or “Ekbom's syndrome” can be used. The recently proposed name “pseudoparasitic dysesthesia,” however, appears unhelpful (339).
(ii) In case it was possible to win the patient's trust, e.g., by means of thorough examination, proper diagnostics, and a good explanation of the symptoms, the physician might introduce the idea that a trial of “neuroleptics” seems promising and reasonable and is usually helpful after a few weeks (the older term might be better than “antipsychotics” here). This can be explained by the physician's personal experience and results from research (referring to references 99 and 322). If the patient insists that what he or she considers to be a causal therapy is needed, it might be indicated to say that for many diseases (e.g., some forms of cancer) and some infections (e.g., many viral infections), no effective countermeasures are available. However, “… all that can be done is to treat the symptoms, but often patients are happy and satisfied with the relief of symptoms” (193). Obviously, the patient has to be informed that in his or her case, the substance chosen is not indicated for schizophrenia, but antipsychotics generally are used for many indications other than schizophrenia. In desperate cases, even a bargain might be indicated (further examination and diagnostic investigations for accepting a treatment trial) (351).
The group around James Slaughter was the first to propose a stepwise approach for dermatologists, with straightforward recommendations for the first and later visits (77, 300, 351). A similar guideline for interdisciplinary dermatology-psychiatry clinics was recently published (280), but its suitability for daily use was illustrated with a single case only. The order of measures is similar to our suggestions (Fig. 3 and 4; Inset 17) (95, 175). At the first, usually unscheduled consultation, dermatology and differential diagnosis are the focus (examination of skin and samples, laboratory tests). The physician should express interest in the patient's beliefs and promise a thorough evaluation of the case. Skin lesions are treated topically, and oral antihistamines can be considered against the itch. Unfortunately, we did not find a superior efficacy of particularly antihistaminergic antipsychotics (99, 178), which means that antihistamines are often required on top of antipsychotics. It is the aim of the first visit to rule out true infections and any other organic or toxic cause, as well as the effect of prescribed medication. Later visits start with highlighting the patient's suffering while pointing out that something can be done. A shared disease model is needed at some point to ensure compliance (see above). For self-diagnosed Morgellons, it has been recommended to introduce the model of nerve endings in the skin forming a “nerve fiber network” which has become “hypersensitive,” sending abnormal signals to the brain (skillfully alluding to the alleged “fibers”) (280). Antipsychotics are introduced to the patients as necessary to treat such sensory alterations (as a “filter”). For dermatological services, follow-up visits every 2 weeks for 2 months have been proposed (351). For joint dermatology-psychiatry clinics, the psychiatrist is introduced only after that period (for evaluation of mental status, comorbidities, and substance abuse and the choice of adequate medication) (280). Further appointments are made with the dermatologist. When delusions start to respond to antipsychotic treatment, referral to a psychiatrist is more likely to be possible than in untreated patients (351). In other words, it will more commonly be the dermatologist or general practitioner who has to initiate antipsychotic treatment rather than the psychiatrist.
FIG. 4.
Therapy overview (step 2).
Advice to microbiologists and public health departments.
Microbiologists, parasitologists, infectious disease specialists, and public health departments are involved in the field of DI in a different way from the specialties mentioned above. They usually do not deal with the patients directly and do not struggle with establishing effective treatment. Their dilemma is the question of how to deal with the plentitude of specimens, letters, e-mails, and digital photos and videos sent to them. Obviously, it is impossible to analyze or comment on all of the material without a formal request and with no cost-covering compensation. However, not answering these requests will do a disservice to the patients (increasing their despair, the likelihood of dangerous self-therapy, and their delusional belief). Instead, prearranged standard letters are desirable. They should be short and factual and avoid criticism or therapeutic recommendations. Reports of diagnostic findings to another physician should leave as little room for misinterpretation as possible, because patients will insist on reading them.
Etiology-Specific Multimodal Therapy
Etiology-specific therapy.
The multietiological nature of DI implies that therapy needs to be customized for the various forms of DI. The therapeutic approach to DI (as summarized in Fig. 3) depends primarily on etiology (95, 99, 175, 178, 234, 323). Although antipsychotics certainly have a pivotal role in the psychopharmacotherapy of DI, it would be an oversimplification to believe that they are the only therapy and that they are always of the same importance. The main indication for antipsychotics is the presence of recurring or persistent psychotic symptoms (overvalued ideas or delusions, hallucinations), while simple formication is no indication. A major depression with concomitant DI symptoms may respond to mere antidepressive therapy (shown for electroconvulsive therapy or imipramine) without antipsychotic treatment (32, 123, 136). The first formal study investigating the different therapy approaches with a mixed sample of 34 patients showed that all 12 patients with a depressive disorder responded to antidepressants (75% remission and 25% response; amitriptyline, doxepine, and maprotiline were used), while only 31% of the patients in the organic/dementia subgroup profited from treatment (23% remission, 8% response, and 69% no effect; nootropics plus antipsychotics were used). For the whole sample in this open trial, a moderate outcome was found, with 50% full remission, 15% partial remission, and 35% no response (235). Please compare these rates to the effects of antipsychotic therapy in other forms of DI, summarized in Table 4.
TABLE 4.
Acute treatment for DIa
| Study | No. of primary DI cases/total no. of cases | Design | Treatment | Outcome (%) |
|||
|---|---|---|---|---|---|---|---|
| ++ | + | 0 | Other | ||||
| Wilson and Miller (1946) | 4/51 | Retrospective | No antipsychotics | 10 | 8 | 82 | |
| Frithz (1979) | 15/15 | Open | Fluphenazine dec. i.m. (n = 10) or cisflupenthixol dec. i.m. (n = 5) | 73b | 20b | 7b | |
| Hamann and Avnstorp (1982) | 5/11 | Double-blind | Pimozide | 91 | 9 | ||
| Crossover | Placebo | 9 | 89 | ||||
| Munro (1982) | 9/25 | Open | Pimozide | 33c | 56c | 11c | |
| Lyell (1983 [193]) | ?/282 | Survey of dermatologists in United Kingdom | Pimozide (n = 66) | 67 | 24 | 9 | |
| Ungvari (1983, 1984) | 19/26 | Open | Pimozide (n = 22) | 64e | 36 | ||
| Haloperidol (n = 4) | 75 | 25 | |||||
| Ungvari and Vladar (1984, 1986) | 10/10 | On-off-on | Pimozide and placebo | 100d | 90% relapse | ||
| Musalek et al. (1989) | ?/34 | Open (specialized clinic) | Overall (n = 34) | 50 | 15 | 35 | |
| TCAs for depression (n = 12) | 75 | 25 | |||||
| Nootropics plus APs for organic/dementia-associated DI (n = 13) | 23 | 8 | 69 | ||||
| Paholpak (1990) | 9/10 | Open | Haloperidol | 100 | |||
| Trabert (1993) | 17/35 | Open (specialized clinic) | Pimozide (n = 15), butyrophenones (n = 15), fluspirilene i.m. (n = 2), additional ADs in seven cases | 27b,f | 54b | 18b | |
| Srinivasan et al. (1994) | 19/19 | Open (randomized) | Trifluoperazine (n = 6), chlorpromazine (n = 3), haloperidol (n = 2) | 58b | 32b | 11b | |
| Bilateral ECT (n = 8) | 75 | 13 | 13 | ||||
| Trabert (1995) | ?/1223 | Meta-analysis of 193 articles | No antipsychotics (n = 56) | 34 | 29 | 38 | |
| Typical APs (n = 206) | 52 | 32 | 17 | ||||
| Zomer et al. (1998) | ?/33 | Retrospective | Pimozide (n = 24) | 33 | 28 | 39 | |
| Other (n = 9) | 7 | 13 | 80 | ||||
| Bhatia et al. (2000) | 32/52 | Open | Pimozide (n = 46) | 52 | 35 | 13 | |
| Freudenmann and Lepping (2008) | 28/63 | Meta-analysis of 434 papers | Atypical APs (n = 63) | 37 | 38 | 5 | 21 |
Abbreviations: ECT, electroconvulsive therapy; TCAs, tricyclic antidepressants; AD, antidepressants; APs, antipsychotics; i.m., intramuscularly; dec., decanoate; ++, full remission; +, response; 0, no effect.
Cannot be assigned further to the single substances.
Primary DI only.
In both “on” phases.
One patient also received fluspirilene intramuscularly.
Data available on antipsychotics for only 32 patients and on outcome for only 33 patients.
In cases of primary DI and those secondary to schizophrenia, the main intervention is antipsychotic medication (usually given orally; intramuscular depot formulations are used less frequently). The inducer in shared psychotic disorders is also treated in this way, while the receiver often remits when separated from the inducer or once the inducer starts to recover. Cases secondary to depression are treated as any other major depressive episode with psychotic symptoms, with antidepressants plus antipsychotics. For details on the state-of-the-art therapy of schizophrenia, major depression, dementia-associated psychosis, and delirium, we must refer to the current guidelines. For cases secondary to chronic drug abuse, the therapeutic mainstay is, of course, abstinence and symptomatic treatment with an antipsychotic for a limited period (no generally accepted guidelines are available).
Multimodal therapy.
The second principle in therapy of DI is multimodality (74, 95, 96, 175, 280, 323, 351). Multimodal treatment is needed to address all symptoms and “pathogenetic” factors according to our current pathophysiological understanding. It targets peripheral sensation and itching, skin lesions and superinfections, risk factors (such as isolation or sensory deficits), treatment of any underlying illness, and a specific psychopharmacotherapy according to the type of DI. Accordingly, this complex approach requires close collaboration across medical specialties (dermatologist, microbiologist, general practitioner, and psychiatrist).
Within this approach, basic measures and specific psychiatric therapy can be differentiated as follows. (i) Basic measures include dermatological local treatment of skin lesions (in some cases, transient light dressings render continued manipulations more difficult) and the consideration of nonsedating antihistamines against pruritus (topical and oral, e.g., loratadine or levocetirizine). In the presence of an underlying general medical condition, sufficient treatment must be established, if needed with the help of the patient's general practitioner or other specialists. Moreover, maintaining factors of DI should be reduced as far as possible, if present (social isolation, vision and hearing impairment), and possible triggers such as pruritogenic medication should be stopped whenever possible. (ii) In contrast, specific psychiatric treatment is aimed at the dissolution of false beliefs and abnormal sensations. Almost all known forms of treatment have been used in DI, including psychopharmacotherapy (various classes and combinations), psychotherapy, ECT, and psychosurgery, because the illness was long considered treatment refractory. However, they have hardly been studied systematically (see below).
Special Therapeutic Issues
Ethical considerations.
As we have seen, it is a big challenge to initiate psychiatric medication in patients with DI. This is reinforced by the need for shared decision-making and full informed consent. It particularly hinders the recruitment of patients for the research of delusional disorders because of the need to indicate the diagnosis on information leaflets. We believe that the recommendations made above about how to approach the patient allow for effective treatment without misinforming the patient. The recommendations form a stepwise approach to full consent in very particular and difficult circumstances. We accept that this is ethically more problematic in research, although we suggest that pragmatic solutions be developed to enable research to occur with these patients.
How often is psychopharmacotherapy possible?
Strategies to increase the likelihood of engaging patients in psychopharmacotherapy instead of the anti-infective that they desire have already been delineated. If the patient's confidence has not been shaken by constant questioning of their beliefs, early psychiatric referral, or premature introduction of psychotropic medication, some studies suggest that 75% of patients can be motivated to take antipsychotic medication for a trial period (n = 33 [353] and n = 12 [258]). If patients have become wary, however, it will be literally impossible to convince any patient, even for a skilled psychiatrist. About 60% of patients accepted psychiatric medication in a survey of British dermatologists (n = 53) (268). An excellent rate of 89% of patients (eight of nine patients) accepted antipsychotic treatment in a psychiatric outpatient setting for an elderly patient sample (mean, 75 years) (141). Despite these promising reports, the majority of patients will reject psychiatric medication if no particular measures are taken.
Treatment goals.
Another important premise of treatment of DI is to define a reasonable therapeutic goal. At first sight, this might be the complete resolution of the core symptoms (delusions and abnormal sensations) and full retrospective insight (insight into the fact that he or she never had any real infestation but had a psychiatric disorder). However, as we realize from the treatment of schizophrenia and delusional disorders, chronic systematized delusions are often out of therapeutic reach. In one study, only 9% of patients with mixed DI (n = 35) gained full insight under sufficient psychiatric therapy (323). Therefore, realistic goals for therapy are needed, particularly in primary DI. These are (with a decreasing likelihood of being reached) (i) a reduction of distress (caused by the imaginary infestation) and tactile sensations/itching, i.e., improved quality of life; (ii) a reduction of skin lesions due to less self-cleansing (also saving time and money); and (iii) a reduction of the delusional conviction of being infested, at least to a degree which enables psychiatric treatment and normal social contacts. A full remission of symptoms and full retrospective insight are a rarity in primary DI but can more commonly be achieved in secondary DI (99, 234, 235).
Legal considerations and off-label use.
For nonpsychiatric audiences, it might be surprising that in view of the frank presence of a psychotic disorder, the massive subjective distress, and a reasonable outcome whenever psychiatric treatment is established, patients are not forced to accept psychiatric treatment. In most countries, however, involuntary committal to a psychiatric hospital or enforced treatment in the community is not possible unless danger to the patient's life or a third party's well-being is imminent. This treatment dilemma persists in primary DI but also in other forms of monodelusional disorders, in contrast to psychotic disorders such as schizophrenia or mania, where the willingness to use compulsory admissions appears higher. It remains to be seen to what extent community treatment orders will be used for monodelusional disorders, as some federal states in the United States and Australia, as well as England and Wales, have recently introduced provisions for compulsive community treatment under certain conditions. In other parts of the European Union, such community treatment orders are rarely used even where they are legally allowed.
Off-label use is frequently applied in psychiatric pharmacotherapy because for many disorders or clinical situations no approved measure exists, so both clinicians and patients are left without clear choices (146). No substance is specifically approved for treating DI or the overarching categories “delusional disorder, somatic type,” or “delusional disorder.” Only some typical antipsychotics, with their often-broader approval, might cover DI, e.g., haloperidol and fluphenazine (28, 305). In contrast, most atypical antipsychotics have explicit approval only for “acute therapy” and/or “maintenance treatment of schizophrenia” in most countries. Only risperidone has been approved by the U.S. Food and Drug Administration (FDA) for treating “other psychotic disorders” (305). Off-label use and package inserts that mention “schizophrenia” further complicate the situation when clinicians try to engage patients with DI to try an antipsychotic.
The best treatment setting.
Another important therapeutic issue is the question of the best treatment setting. More specifically, it is the question of how far conventional services are able to offer the structures needed to provide the care needed for this truly interdisciplinary patient group or in which organizational structures the necessary cooperation between dermatology, microbiology, and psychiatry can be implemented. The answer obviously depends on the health system of the country in question, but some general rules are useful to remember.
A management in psychiatry is possible for only a minority of patients (5 of 66 cases [190]). These cases had a better outcome than those treated in dermatology (325) or other specialties (99). This was explained by better antipsychotic therapy but might rather be due to selection bias (studying only those with more insight or who were less severely ill). However, a subgroup of patients can be treated successfully in psychiatric outpatient clinics, as recently shown by our group (seven of nine patients [78%] reached full remission) (101, 141). Psychiatric inpatient treatment for DI remains a rarity (96, 97). It is mainly restricted to patients with dementia-associated psychosis treated in old-age psychiatry departments.
In dermatology, better care of the skin lesions and easier differential diagnosis are possible, but dermatologists do not always feel prepared to initiate antipsychotic treatment (74). Some studies suggested that psychopharmacological therapy might be less effective administered by dermatologists than elsewhere (325), while others reported excellent outcomes (105, 121). The inferiority of mere dermatological to psychopharmacological and combined therapy was suggested by a retrospective survey (11 versus 56 versus 60% response) (268). However, a meta-analysis of DI cases treated with atypical antipsychotics found that outcomes in psychiatry and dermatology did not differ (99). Quite on the contrary, the outcome from combined dermatological-psychiatric treatment was worse (61% partial or full remission) than that for psychiatry (87% partial or full remission), but this result was limited by the retrospective design and a possible publication bias. In view of these heterogeneous findings and the lack of direct comparison of the different treatment settings in a prospective study, we do not have conclusive evidence for the superiority of any setting.
Specialized clinics.
At any rate, close cooperation between specialties is needed for seamless diagnostic investigations and optimized treatment (74). At present, many patients fall through the net of a rigid medical system and end up in dangerous self-therapy. One possibility is the establishment of low-threshold, specialized joint clinics in dermatology with an attending psychiatrist (77, 234, 235, 239, 280, 300, 323, 351). This treatment setting was first introduced in 1986 by Michael Musalek in Vienna, Austria, to attract patients, meet their particular needs, and avoid the problematic interface between dermatology and psychiatry. A few years later (1989 to 1991), Wolfgang Trabert used a very similar approach in Homburg, Germany (323). One group reported that about two of three patients return in this specialized setting (77), while apparently some patients cannot be kept in professional medicine. Trabert reported that 80% of the patients came for more than one visit, and 46% came more than six times (323). While 57% came back for a maximum of 3 months, 23% were seen for more than a year (11% for more than 2 years) (323). Of these, the majority attended the service for less than 3 months (57%) (323), but this might be enough for significant treatment effects (99). Trabert did not actively inform patients that he is a psychiatrist. Most patients eventually asked him and he introduced himself as a psychiatrist. At this point, only a single patient with a primary DI immediately refused further contacts, while the vast majority accepted the approach, some even encouraged him, and all of them were glad that they were treated in dermatology (323). The clinics of Musalek and Trabert closed some years ago with the end of their research. Unfortunately, this approach is limited to a few similar services and initiatives today (for example, James Slaughter's group at the University of Missouri [77, 300, 351], John Koo's group at UCLA [161-163], R. E. Accordino at the New York Presbyterian Hospital's Psychodermatology Clinic [1], Uwe Gieler in Giessen, Germany [112], and Ruth E. Taylor in London, United Kingdom). Outcome research from such clinics would be welcome to evaluate this treatment setting.
Psychiatric Therapy Other than Antipsychotics
Before we turn to psychopharmacotherapy, other treatment approaches in DI need to be reviewed, as follows.
Antidepressants are frequently used for treatment of DI. In cases secondary to major depression, they represent the standard treatment that follows current guidelines. They can also be necessary in cases of depressive symptoms within primary DI or an antipsychotic-induced depression. As in other forms of depressive disorders, selective serotonin reuptake inhibitors are usually first-line agents. Among these, escitalopram and sertraline are associated with the lowest risk of hepatic interactions with concurrent antipsychotic therapy and the highest acceptability and were recently shown in a meta-analysis of 12 new antidepressants to be the most efficacious together with mirtazapine and venlafaxine (56).
Going back to the early stages of psychiatric pharmacotherapy in the 1920s and 1930s, there are anecdotal reports of patients with DI treated with opium (32, 78, 289, 307) phenobarbital (78), or placebo (40, 93). Placebo therapy cannot be recommended at all (346).
In 1991, the use of opioid antagonists against associated itching was suggested (42), but their use did not become accepted and was not replicated or studied systematically for any form of DI.
Some authors have used psychotherapy, with or without confrontational denial (40, 320). It is helpful in 10% of cases, at best (346). Only Slaughter and his group emphasize a role for psychotherapy, particularly suggestion, in cases with a “shakable conviction” (300, 351). In their sample of 20 cases, 9 were treated with suggestion (45%), with a reported full remission (“cured”) in all cases (351). The time to remission ranged from 1 to 12 months.
Conversely, prefrontal leucotomy (neurosurgery of the cerebral white matter) has been described for a single case of DI (257). The intervention was effective and, furthermore, led to a remission in other people involved in an SPD.
Other groups have tried to influence the peripheral tactile symptoms by means of transcutaneous electric stimulation (347). No other reports on this strategy have been published.
The most interesting nonpharmacological approach is ECT. We were able to identify a total of 20 published cases treated with ECT since 1949, mainly for DI secondary to severe depression (3, 24, 32, 108, 123, 136, 304). The best evidence is provided by a study from India in which six of eight patients (75%) treated with ECT remitted and only one patient did not respond (304). The response rate was superior to that for antipsychotic treatment, but the study was limited by its small sample size (n = 19). In conclusion, ECT can be a treatment option, in particular in cases of DI secondary to medication-resistant depression and elderly patients.
In general, evidence is very limited for all of these nonstandard therapies for DI.
Antipsychotics
Antipsychotics are by far the most important substance group in the treatment of DI, particularly in primary DI. In this review, an approximately chronological and substance-specific approach is used. The evidence for the effectiveness of typical antipsychotics and atypical antipsychotics in the acute treatment of DI is summarized in Table 4. Twelve studies investigating effects of acute antipsychotic treatment in DI were selected, in addition to two case-based meta-analyses, from the eras of typical antipsychotics (322) and atypical antipsychotics (99). They can be compared to the “baseline” outcome determined in the time before the introduction of antipsychotics, based on data provided by Wilson and Miller (344) and Trabert (322). In view of the heterogeneity of outcome measures and criteria used, we tried to assign the outcomes reported in the single papers to a simple three-point scale (full remission, partial remission, or no response), as far as possible. Many studies failed to consider the presence of different etiologic forms of DI. We tried to establish from the papers how many of the patients in the samples had primary or secondary DI because it does not make sense to conduct research on therapy and outcome for DI without a strict separation of the different etiological forms.
Evidence-based medicine.
Looking at the selected studies, it appears that the results are heterogeneous. There is a complete lack of randomized controlled clinical trials on the use of antipsychotics in DI with adequate sample sizes. For primary DI, a systematic review from our group did not retrieve any published or unpublished controlled trials (178). This dearth of data exists for secondary DI, too. Evidence is limited to small open and retrospective trials, surveys, and case studies for all antipsychotic substances and equals a level of evidence between IIa and IV according to the criteria of the Agency for Health Care Policy and Research (AHCPR) (4). In this situation, a Cochrane review or other meta-analyses of trials are not possible. Accordingly, using today's standards in clinical medicine, there is no conclusive evidence of superiority of any antipsychotic over placebo but sufficient evidence to justify antipsychotic treatment in certain types of DI (Fig. 3).
Looking at the rates of nonresponse across studies, it appears that only between 0 and 39% of cases treated with antipsychotics do not respond at all (with the exception of a study of Musalek et al. and a subgroup of organic and dementia-related cases treated with nootropics/cognitive enhancers and antipsychotics). This overall favorable outcome, however, needs cautious interpretation for methodological reasons (lack of controlled trials, small samples, and publication and selection biases).
Course of response, dosage, and maintenance therapy.
Other important but hardly studied aspects in antipsychotic treatment of DI are the questions of the onset and the time of maximum effect, the dosage, and the need for maintenance therapy after an initial response to treatment (99). The available data on these important practical aspects are summarized in Inset 18.
Inset 18. Practical aspects of psychopharmacotherapy in DI. A first response of symptoms in DI has been observed after between 1 (227) and 3 or 4 weeks (322, 351). In cases treated with atypical antipsychotics, the median onset of effects already occurred after 1.5 weeks (99).
The maximum effect of treatment was observed after 2 weeks in most cases, and in some cases after 6 to 8 weeks, using pimozide (228). When using atypical antipsychotics, the maximum response was seen after 6 weeks (median), and it occurred significantly earlier in secondary (3 weeks; range, 0.5 to 8 weeks) than in primary DI (10 weeks; range, 3 to 16 weeks) (99). These time aspects merit further study because very few data are available.
Despite the chronic course of DI, relatively small doses of antipsychotics were sufficient to improve symptoms compared to those for schizophrenia, acute psychotic disorders, or mania, even in young patients (98, 99, 158, 159, 175, 211, 280). Dose-defining studies for the treatment of DI as well as other forms of delusional disorders are lacking (99). When treating elderly patients with DI, low age-adapted doses of antipsychotics are mandatory.
Another important question for the clinician is that of the optimal duration of acute therapy and the need for maintenance therapy after a response. In the study by Zomer et al., pimozide was tapered off in 18 patients 6 weeks after clinical improvement (type of DI not known) (353). Only five remained in full remission without medication. In another study, discontinuation of pimozide after successful acute therapy was followed up for 19 to 48 months in 14 patients (type of DI unknown) (188). Half of them remained in remission, while a reintroduction of pimozide in the other half was not always helpful. In primary DI, after the end of depot antipsychotic injections, 6 of 10 cases relapsed within 3 months, and only 2 remained completely asymptomatic (105). In mixed DI samples, relapse occurred within months after stopping treatment (121, 331). In a few cases (two of nine), antipsychotics were tapered off successfully (141). Other authors noted a recurrence of symptoms in cases of nonadherence to medication (41, 304).
To conclude, further research on these aspects of antipsychotic therapy in DI could provide valuable information for the planning of treatment, scheduling of appointments, and evaluation of treatment. The duration of treatment should be at least 3 weeks for secondary DI and 10 weeks for primary DI (time of maximum effect). Therapy switches in cases of an unsatisfactory response should be made only with those periods in mind. Zomer et al. recommended continuing the antipsychotic for another 6 weeks after symptom dissolution and then tapering it off (353). In many cases, however, a low-dose maintenance therapy appears reasonable (previous severe DI, a good response, few adverse effects, and sufficient therapy monitoring).
Typical Antipsychotics
Trabert's meta-analysis.
An influential publication from 1995 by Trabert established that significant improvements in the outcome of DI were achieved with the introduction of typical antipsychotics (322). In this vast work, 1,223 published cases were identified from 193 articles. Sufficient data on outcomes were available for only 56 cases from the time before 1960 and for 206 cases after 1960. With the introduction of antipsychotics, the rate of full remission increased significantly, from 33.9% to 51.9%, as did the rate of response (little or marked), from 28.5% to 31.5% (chi square = 13.7; df = 3; P = 0.003). Vice versa, the rate of nonresponse decreased from 37.5% to 16.5%. Outcome data were available for more than the cases described above, i.e., for a total of 301 cases (treated and untreated); among those, full remission was achieved in 51.4%, marked and little improvement in 16.9% and 11.6%, respectively, and no change in 19.9%. The author concluded that the overall clinical course of DI was better than previously thought and that prognosis improved with antipsychotics. Publication bias was inevitable in this study due to its design. A tabulation of diagnoses was presented for 449 of the patients, but the exact diagnoses for the 206 cases in the “antipsychotic era” cannot be extrapolated.
Pimozide.
Looking at individual substances from the group of typical antipsychotics, Lyell eloquently remarked that the introduction of pimozide divided the psychiatric therapy of DI into two eras, “B.P.” and “A.P.” (before and after pimozide) (192). Before, the disorder was considered almost untreatable (in particular for cases not secondary to depression). The outcome in the prepsychopharmacological era was unfavorable, as reported by Trabert (see above), and it was even worse in other studies, with 10% “cured,” 8% “ameliorated,” and 82% “unchanged” (344). In placebo arms of a study from 1982, about 10 of 11 patients (91%) did not respond (121). With the introduction of pimozide, in contrast, combined rates of partial (response) and full remissions of more than 90% were reached (Table 4). This made pimozide the “treatment of choice” for DI in the mid-1980s, according to most authors (for today's perspective, see below). Pimozide is characterized in detail in Inset 19.
Inset 19. Pimozide. Pimozide was launched in 1963 and approved for treating Gilles de la Tourette syndrome in the United States and for schizophrenia and chronic psychosis in Europe but not the United States (here it is licensed only as a second-line substance for Tourette's). Pimozide was never approved for the treatment of delusional disorders or DI in particular. In 1975, first case reports indicated positive effects in “monosymptomatic hypochondriacal psychosis” (271, 272). Further case reports (18, 171, 229, 232, 269), surveys (192, 269), and open (331, 332) and two so-called “controlled” studies (121, 334, 335) showed good efficacy. Thus, supported by continuous appraisal of the substance by Alistair Munro (227, 229, 230), pimozide became the “substance of choice” for DI since the late 1980s, according to scientific publications and numerous textbooks of psychiatry and dermatology (163, 353).
(i) Chemistry. The substance is a diphenylbutylpiperidine derivate (like fluspirilene). The molecule is not tricyclic and has similarities to high-potency butyrophenones such as haloperidol.
(ii) Pharmacodynamics. Its mechanism of action is dominated by its very high potency to block dopamine D2 receptors. It has the highest antipsychotic potency together with benperidol, which is only rarely used these days for the treatment of deviant antisocial sexual behavior, and fluspirilene. Pimozide also blocks D1, D3, and D4 receptors, as well as serotonin 5-HT2A receptors, mediating antipsychotic effects. Its antagonistic properties at opiate receptors are remarkable (45, 62) and have been associated with its possible antipruritogenic effects and as an explanation for its particular effects in DI (147, 189). Its low affinity at histamine H1 receptors explains why pimozide causes little to no sedation (265). Anticholinergic effects are also infrequent. Astonishingly, in mice pimozide has antibacterial effects against the neurotropic intracellular bacterium Listeria monocytogenes (185). The importance of this very recent unreplicated finding for humans is not clear at present. At any rate, the fact that all potent antipsychotics are effective in delusional disorders and DI does not support any speculations about a true infection being cured by antibiotic effects (which are bound to arise).
(iii) Pharmacokinetics and interactions. The substance has special pharmacokinetic properties as well, in particular a long elimination half-life of about 55 h (among commonly used antipsychotics, only aripiprazole and sertindole act longer). It was the first antipsychotic to allow a once-daily administration. It is only available for oral administration (its brand name “Orap” stands for “oral antipsychotic”). Its hepatic breakdown is catalyzed by CYP 3A4 and, to a lesser extent, 1A2 (69). Thus, potent inhibitors of CYP 3A4 can evoke significantly elevated plasma levels and increase the toxicity of pimozide, e.g., a prolongation of the QTc interval (e.g., by azole antifungals, macrolide antibiotics, nefazodone, sertraline, protease inhibitors such as ritonavir, and grapefruit juice). Blockers of CYP 1A2, such as fluvoxamine, might also potentiate pimozide toxicity. Carbamazepine, a potent inducer of CYP 3A4 and 1A2, can cause low pimozide serum concentrations. Coadministration of any other proarrhythmogenic substance is contraindicated (especially amiodarone, chinolones, cisapride, tricyclic antidepressants, and other antipsychotics, such as thioridazine, sertindole, or ziprasidone). Pimozide itself inhibits CYP 2D6 and therefore might interfere with CYP 2D6 substrates, in particular in poor metabolizers (69).
(iv) Adverse effects. The profile of untoward effects is that of a very potent typical antipsychotic and results mainly from its strong D2 antagonism. Apart from hyperprolactinemia, depression (by partly blocking the reward system in the ventral striatum), and metabolic complications, there are two major risks. (i) Even low doses of pimozide frequently cause extrapyramidal side effects. The risk of inducing tremor and the need for the use of antiparkinsonian medication were higher than those for other typical antipsychotics (265). Critically, the risk of malignant neuroleptic syndrome and irreversible tardive dyskinesia is usually considered one of the highest of all antipsychotics. (ii) Pimozide causes a dose-dependent prolongation of the QTc interval (75). This is due to the blocking of the myocardial hERG potassium rectifier channel and interferes with proper cardiac repolarization (150, 151, 160). A prolongation of the QTc interval has been associated with an increased risk of torsades de points tachycardia and sudden death (113, 267). However, the best calculation formula for QTc, its limits, and the strengths of the association remain controversial (109). In any case, pimozide is certainly among the most risky antipsychotics in this respect (after thioridazine and droperidol) (113, 267). Thus, an ECG before treatment initiation and periodic ECG monitoring at follow-up are mandatory under pimozide, but highly recommended for all antipsychotics.
The excellent reputation of pimozide in the treatment of DI resulted mainly from the following two placebo-controlled studies.
(i) Hamann and Avnstorp, two Danish dermatologists, conducted a double-blind crossover trial with a 4-week washout phase comparing the effects of pimozide and placebo in 11 patients with DI (121). The sample was mixed (five patients had primary DI, and six had secondary DI). Therapy with pimozide was started with 2 mg daily, followed by an open dosage phase (1 to 5 mg daily), with 6 weeks in total. Pimozide was significantly more effective than placebo against itching and delusions and showed a trend to affect the perceived “presence of vermin” as well as excoriations in a positive way. Ten of 11 patients reached partial remission (no full remissions), while only 1 of 9 patients responded to placebo. The onset of effects against delusions was observed after 6 weeks. Eight of the patients (73%) experienced adverse effects, such as drowsiness, extrapyramidal symptoms, or depression. After the end of the trial, an open phase was added. Two of the remaining four patients stopped pimozide without relapse (5 months).
(ii) Ungvari and Vladar, two Hungarian psychiatrists, performed the second placebo-controlled trial, with an on-off-on design and a well-defined sample of 10 patients with primary DI (published twice [334, 335]). In the first “on” phase (3 weeks), individual daily doses of 2 to 8 mg pimozide were administered (initial dose, 1 mg). The “off” phase consisted of placebo therapy for 3 weeks. The second “on” phase was like the first (but lasted only 2 weeks). All 10 patients responded in both pimozide phases, whereas under placebo 9 of 10 patients had a symptom increase. No patient reached full remission (with full retrospective insight). The authors deliberately did not choose a crossover design because they expected to lose patients in the “placebo first” group.
However, a thorough examination of these two “standard” studies shows their limitations, including (i) small samples (both); (ii) a mix of diagnoses, i.e., primary and secondary forms of DI (Hamann and Avnstorp study); (iii) a lack of randomized allocation to treatment arms (both); and (iv) an otherwise problematic design (in the Ungvari and Vladar study, with its on-off-on design, order effects are likely and a true “double-blind method” unlikely).
The other open or retrospective studies (Table 4) and case reports published after these two studies did not improve evidence on the use of pimozide in DI (39, 63, 92, 122, 125, 161, 172, 220, 270). Still, the second, open study by Ungvari is noteworthy (published twice [331, 332]). It had a larger but mixed sample of 26 cases (19 cases of primary DI and 7 cases secondary to schizophrenia, brain tumors, mental retardation, or alcoholism). All patients but one were treated with pimozide (1 mg daily, acute therapy with 2 to 5 mg, and maintenance with 1 to 2 mg daily, in one case with haloperidol). They showed either full (12 of 18 patients) or partial (6 of 18 patients) remission, although no patient reached full retrospective insight. This shows that the heterogeneity of the distribution of full and partial remissions seen in Table 4 largely depends on divergent definitions of response and outcome criteria, despite all our efforts to reassess the studies. In this study, 10 of 26 patients had adverse effects such as extrapyramidal symptoms (n = 7) or sedation (n = 5), but a substance change was not necessary. Looking at primary DI only, an analysis of published cases found that pimozide showed a response in 94% of cases (50 of 53 cases), with full remission in 45% of cases (24 of 53) (178). Pimozide therefore has the best level of evidence of any antipsychotic in the treatment of DI (178). According to AHCPR criteria, the level of evidence is IIa (at least one well-designed, controlled trial without randomization) (4). There is converging low-level evidence from experimental trials, open and retrospective studies, and case series that pimozide is effective in both primary and secondary DI. However, Cochrane reviews failed to find controlled trials with pimozide for DI and for any delusional disorder (in contrast to 35 eligible trials for schizophrenia) (265, 309).
Other typical antipsychotics.
Typical antipsychotics other than pimozide have hardly been studied for DI. The most important study is an open trial with 19 cases of primary DI, which were randomly assigned to one of three typical antipsychotics or ECT (304). Ratings were blind. Trifluoperazine (n = 6; dose, 10 to 15 mg/day), chlorpromazine (n = 3; dose, 150 to 300 mg/day), and haloperidol (n = 2; dose, 10 mg/day) were studied for 4 to 8 weeks. With these typical antipsychotics, 90% of the patients responded to therapy (58% with full remission). It was concluded that the findings would question the superiority of pimozide (this would require larger samples and a head-to-head comparison with randomized allocation). At any rate, the study indicated that a good outcome is not a substance-specific effect of pimozide but probably a class effect relating to antidopaminergic medication. This is supported further by the second open study, which used a typical antipsychotic other than pimozide (255). Low doses of haloperidol (1 to 5 mg/day) in a sample of 10 patients (9 with primary DI) led to a response in all cases, but no full remission.
Individual case reports with typical antipsychotics do not allow further statistics, but they show that a variety of antipsychotic substances have been used for DI over the last years, including chlorpromazine (304), haloperidol (7, 15, 46, 172, 255, 293, 304, 351), fluphenazine (23), trifluoperazine (304), sulpiride (46, 314), perazine (143), and thioridazine (19, 172, 251).
Traditional depot antipsychotics.
Only a single open study investigating traditional depot antipsychotics in DI exists (105). Fifteen patients with primary DI (14 female) were given either fluphenazine at 7.5 to 25 mg intramuscularly every 3 weeks or cisflupenthixol at 6 to 20 mg intramuscularly every 3 weeks for 3 to 12 months by the Swedish dermatologist Anders Frithz. These doses are relatively low compared to those used in schizophrenia, but 93% of patients (14 of 15 patients) responded to therapy, and 73% even fully remitted (11 of 15 patients). This outcome is even more astonishing as many of the patients had not responded to oral antipsychotics before (although noncompliance was not formally ruled out by assessing drug serum levels). Within 3 months after stopping this regimen, 6 of 10 patients had relapsed, while only 2 remained symptom-free. The excellent overall therapy response was never replicated. The study is limited by its small sample size and the lack of a nonactive control.
The group around James Slaughter underlines the necessity to use the often-transient insight for the application of depot antipsychotics. Their personal experience with haloperidol decanoate given intramuscularly was good, but this was based on only 2 of 20 cases (351). Recently, the group reported the use of haloperidol decanoate at 100 mg given intramuscularly, or 50 mg for older adults, in cases of an unshakable belief and little adherence to medication (77), but they did not provide further details on the sample.
Fluspirilene for intramuscular injection, a diphenylbutylpiperidine like pimozide, was reported to be helpful in five cases, among these some secondary to mental retardation or drug abuse (n = 2 [323], n = 1 [331], n = 1 [301], and n = 1 [154]).
In conclusion, limited data suggest that depot antipsychotics might be a treatment option in cases with transient or persistent insight into the illness. Conclusive evidence for superiority of this approach is lacking not only for DI but also for all delusional disorders. In schizophrenia, however, depot antipsychotics have proven to have advantages in terms of therapy adherence and long-term outcome (285).
Atypical Antipsychotics
As for “atypical” antipsychotics, the data basis for treatment for both primary and secondary DI is even more limited. As shown in Table 4, prospective trials, and even open trials, are completely lacking. The first report of DI responding to an atypical antipsychotic was published in 1995 (106). In a 72-year-old male with a 5-year history of primary DI which failed to respond to haloperidol decanoate, symptoms started to abate after only 1 week of oral risperidone therapy. Meanwhile, there are well over 30 published cases with risperidone for all forms of DI (3, 53, 57, 68, 102, 103, 141, 155, 168, 172, 197, 212, 213, 225, 243, 244, 249, 254, 258, 278, 282, 341). Since the mid-1990s, further atypical antipsychotics have been licensed for the treatment of schizophrenia, and all of these substances have since been used, with various effects, in cases of primary or secondary DI, including olanzapine (41, 89, 96, 101, 141, 167, 172, 199, 211, 212, 249, 280), sertindole (348), quetiapine (19, 37, 76, 155, 218, 249, 341), amisulpride (99, 175-177, 212, 253), ziprasidone (99), aripiprazole (141, 165, 166, 276), and paliperidone (97).
Case-based meta-analysis.
In view of these anecdotal reports, a meta-analysis of outcome and treatment response to atypical antipsychotics in all known cases of DI was performed by our group (99). The study was designed to complement Trabert's meta-analysis of the cases treated with conventional antipsychotics from 1995 and to sum up the knowledge on the use of atypical antipsychotics in DI. Sixty-three cases (59 published cases up to June 2007, with 4 of our own cases) were available from more than 434 publications on DI. Of these, 44% had a diagnosis of primary DI. We used the criteria set out by our group earlier to make sure that the reported cases satisfied minimum inclusion criteria (178). In all cases, the type of DI and diagnosis had been reassessed by two independent raters. The sample was typical in terms of all major clinical features (age, gender, duration of illness, and presence of SPDs). The reported clinical outcome was reassessed and assigned to standardized outcome codes in order to homogenize the data as far as possible. It was determined if a clinical change could really be ascribed to the effect of single substances or not, i.e., in cases of multiple medication changes prior to a response. We also sought to gather data on the time course of response (onset and time of maximum effect), the dosage needed (compared with schizophrenia), and the relation of outcome and duration of sufficient treatment. It was found that 69% of the patients responded or remitted with the use of the first atypical antipsychotic, and 75% responded after therapy switches (final outcome). The rate of full remissions, however, was only 37% (Table 4). First effects were noted after 1.5 weeks, on average, and the maximum effect was observed after 6 weeks (later in primary than in secondary DI [10 versus 3 weeks]; P < 0.004). If a treatment of more than 8 weeks could be established, all cases responded at least partially. As for the different forms of DI, there was a nonsignificant trend that secondary cases were more likely to respond than primary ones (78% versus 59%). Unexpectedly, only four of nine cases of DI secondary to major depression responded to antidepressants and antipsychotics (44%). Risperidone and olanzapine were the most widely used atypical antipsychotics. Risperidone was helpful in 24 of 35 situations (69% partial or full remission), and olanzapine was helpful in 13 of 18 situations (72%). Risperidone was particularly effective and achieved significantly more full than partial remissions in secondary than in primary DI cases. For the other atypical antipsychotics, data were too scarce to perform quantitative analyses. The effective dosages chosen by the various clinicians were rather low compared to those used for schizophrenia (more data are needed for the newer atypical antipsychotics). In sum, this study provides low-level evidence that atypical antipsychotics are effective for all forms of DI and suggests that not only typical but also atypical antipsychotics are helpful in the majority of cases, if sufficient treatment is established. Despite all efforts made to increase the informative value of the case collection, publication and selection biases were inevitable in this retrospective study. The sample size and data on some atypical antipsychotics were limited (e.g., amisulpride, ziprasidone, sertindole, and aripiprazole). Updates with more cases are desirable.
Depot atypical antipsychotics.
The first atypical antipsychotic available as a depot for intramuscular injection is risperidone microspheres. Data on its use in DI are limited to two cases, which both fully remitted (212). In view of the available data for oral risperidone and the experience with conventional depot antipsychotics, a risperidone depot is theoretically a promising substance. Its formulation and slow release, with an onset of action after 3 weeks, and its short duration of action (14 days), however, are possible disadvantages.
Very recently, olanzapine pamoate was licensed for maintenance therapy in patients with schizophrenia who have responded to olanzapine in acute therapy. Its launch was impaired by the so-called postinjection delirium sedation syndrome. This syndrome can occur in any intravasal or subcutaneous injection. It is mediated by an excessive dissolution of olanzapine from the pamoate salt (no anaphylactic reaction). Injection intervals are every 2 or 4 weeks, depending on the dose. No data on its (off-label) use for any delusional disorder are available.
Antipsychotic Therapy of DI
When it comes to the question of the antipsychotic substance of choice for DI, sufficient evidence for clear recommendations is lacking. As indicated, a Cochrane review would abstain from any suggestion in view of the absence of randomized controlled trials. The methodological quality of most of the available studies is low (uncontrolled or retrospective design, small sample size, and/or mix of primary and secondary DI cases). However, we try to make careful recommendations based on the low-level evidence summarized in Table 4 in order to meet clinicians' needs as far as possible. The substance of choice has to be both effective and safe.
In Inset 20, we compare the use of typical and atypical antipsychotics in DI in terms of efficacy measures.
Inset 20. Comparing typical and atypical antipsychotics for treatment of DI. Looking at the rate of full remissions, only 37% of cases became asymptomatic when treated with atypical antipsychotics (99), compared to 52% when typical antipsychotics were used (322). In primary DI alone, the rates were 25% with atypical antipsychotics (99) and 51% with substances from the typical arsenal (based on 92 published cases) (178). The highest remission rate, 73%, was seen with typical antipsychotic depots (105), followed by an open trial using pimozide (64%) (331, 332). However, these rates have not been replicated. It follows that the criterion of remission would favor typical over atypical antipsychotics, as far as conclusions are possible from these comparisons.
The rates of response (full and partial remissions), however, were similar with atypical antipsychotics (75%) (99) and typical antipsychotics (84%) (322). These rates are also very favorable and clearly better than those in “prepharmacological times” (18% [344] to 63% [322]). An optimum response rate of 100% was described for pimozide by the same author in two studies (331, 332, 334, 335) and for haloperidol with a small sample (255), but these results remain unreplicated. Conversely, other studies with pimozide found response rates inferior to those with other typical and atypical antipsychotics (61% [353] and 67% [192]). This exemplifies the heterogeneity of the data. In the overall response, adding partial remission to full remission rates, atypical and typical antipsychotics appear almost equally helpful. Pimozide does not seem to be superior, but cautious interpretation is needed.
The lowest rate of nonresponse or cases left unchanged was seen with atypical antipsychotics (only 5%). It was 17% with typical antipsychotics (322) and much higher before the introduction of psychopharmacotherapy (38% or 82%). This criterion would favor atypical antipsychotics, but a positive publication bias is particularly likely given the relative novelty of published data on atypical antipsychotics.
Hence, divergent conclusions regarding the superiority of typical or atypical antipsychotics in DI can be drawn using different efficacy parameters (Inset 20). This results from the heterogeneity of study designs, samples, and outcome measures (Table 4), despite all our efforts to homogenize data (by reassessing diagnoses and outcomes and using the same three outcome codes). We needs to explain, however, why outcomes with atypical antipsychotics might appear worse in view of the lower remission and response rates in our previous study (99). The reason is that other studies have not reported dropouts, patients lost to follow-up, and those who turned down a proposed trial with antipsychotics, which accounted for one-fifth of the whole sample (21%) in our study. Therefore, it is rather surprising that atypical antipsychotics were still noninferior or superior in other outcome parameters (e.g., the lowest rate of nonresponding cases).
However, in the present situation of limited efficacy data, one could argue that drug safety might be an equally important criterion in choosing the most appropriate antipsychotic, as well as patient characteristics (age) and comorbidities. Randomized clinical trials with head-to-head comparisons and a clear selection of primary or secondary forms of DI are highly desired and need to investigate the superiority of any substance in terms of efficacy and/or tolerability, yet they are difficult to conduct (100). Innovative designs and ways to engage patients in such a trial and to meet criteria of full informed consent will be needed.
Final remarks on pimozide.
Pimozide is no longer a first-line antipsychotic for any indication (schizophrenia or tic disorder) for reasons of drug safety, according to the FDA and other authorities (28, 305). This reassessment largely results from a high risk of a QTc prolongation and hepatic drug-drug interactions (mainly via CYP 3A4). Accordingly, pimozide is not the substance of choice in any form of DI either, in view of the available evidence, safety issues, and treatment alternatives (99, 175). We do not concur with recent recommendations to further use the substance as a first-line agent in DI (21, 158, 349). It is only possibly more effective (IIa level of evidence), but it certainly has higher risks than many other typical and atypical antipsychotics. Its potential benefits do not override its risks. It must be admitted that these risks are less pronounced if low doses are used (dose-dependent QTc prolongation) and that the treating physician must be familiar with any other antipsychotics and their specific risk profiles as well. In view of their higher average age, safety issues make any substance problematic for patients with DI. The necessity to discuss potential risks with a patient group that can hardly be engaged in taking such medication reinforces the problem. However, pimozide might still be considered for DI under some premises, e.g., (i) in case of a failure of at least two other antipsychotics, (ii) in younger patients, (iii) without additional medication (no risk of interactions), (iv) in those with no history of cardiac disease, (v) in low doses (reducing the risk of extrapyramidal symptoms, hyperprolactinemia, and depression), and (vi) provided that pre- and posttreatment QTc interval monitoring and lab controls are guaranteed.
Suggested antipsychotics.
It is quite easy to decide against pimozide as a first-line substance for DI. However, at present, evidence-based recommendations in favor of any typical or atypical antipsychotic for primary or secondary DI are not possible. With the intent to come to any cautious recommendation, an option is to use information from antipsychotic therapy of schizophrenia (typical versus atypical antipsychotics [180], head-to-head comparisons of different atypical antipsychotics [181]) and to combine it with low-level evidence for DI (Table 4). However, it is controversial to what extent more recent independently funded studies of schizophrenia (e.g., Clinical Antipsychotic Trials of Intervention Effectiveness [CATIE] [184], Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study [CUtLASS 1] [148]) can be extrapolated to other psychotic disorders. Accordingly, all subsequent recommendations can only be preliminary.
The first-line agent when establishing an antipsychotic treatment for all forms of DI should be an atypical antipsychotic, such as olanzapine, risperidone, or amisulpride, or one of the following three well-known and comparatively safe typical antipsychotics: haloperidol, sulpiride, or perphenazine. The fact that quetiapine has been relatively disappointing regarding its efficacy in DI, despite its strong antihistaminic component, suggests that the antipsychotic efficacy of the chosen substance is likely to be more important than its antihistaminic properties (99). In dementia-related DI, antipsychotics need to be used cautiously because of a slightly increased risk of cardiovascular events. Clinicians need to balance this risk carefully with any potential improvements in the patient's quality of life (see below).
When using typical antipsychotics, the lowest effective dose should be used to minimize typical adverse reactions of potent D2-receptor blockers (e.g., extrapyramidal side effects such as dystonias or akathisia, hyperprolactinemia, and resulting galactorrhea). Conventional depots can also be considered in selected cases (e.g., haloperidol decanoate), while pimozide is only a third-line treatment. Patients must be informed about potential irreversible tardive dyskinesia.
As for atypical antipsychotics, at present, the best but inconclusive evidence for DI is available for risperidone and olanzapine (99). Determining if atypical antipsychotics are effective for DI as a group, or more specifically, whether there are differences between the individual substances requires further data. Using high-level evidence from schizophrenia research for choosing between atypical antipsychotics, it has been found that clozapine, amisulpride, olanzapine, and risperidone are more effective than typical antipsychotics (in this order; small to medium effect sizes) (180). Direct head-to-head comparisons favored olanzapine (and risperidone) among the atypical antipsychotics included in the study (181). The recommendation to use atypical antipsychotics as the antipsychotic treatment of choice is in line with all current guidelines for the treatment of schizophrenia. It is commonly accepted that they have fewer extrapyramidal side effects than conventional substances. Recently, it was shown that this finding holds when atypical antipsychotics are compared with low doses of haloperidol (180), but to a much lesser extent compared with perphenazine (184). Conversely, atypical antipsychotics (particularly olanzapine and clozapine) are associated with more weight gain and consecutive metabolic disorders than are typical antipsychotics, with the exception of ziprasidone and aripiprazole (180). This emphasizes that atypical antipsychotics are by no means a homogeneous group in terms of mechanism of action, pharmacokinetics, efficacy, and profile of adverse effects, despite their shared umbrella name. When it comes to choosing a particular substance for treatment initiation, comparatively small differences in efficacy will be less important than large differences in risk profile (180, 181). Therefore, the choice of single atypical antipsychotics for DI treatment must be made individually based on concomitant illnesses and the risk profile of the substance rather than merely on efficacy considerations alone. Substances with a longer elimination half-life, allowing for single daily doses, may also have theoretical advantages (e.g., olanzapine, paliperidone, aripiprazole, sertindole, and long-acting quetiapine).
Therapy monitoring.
Any antipsychotic treatment with a typical or atypical antipsychotic must be monitored with adequate controls of clinical status (psychiatry, neurology), laboratory investigations (white blood cell count, liver and renal functions, serum electrolytes), ECG (QTc interval), and metabolism indices (body weight, glucose and lipid metabolism). With typical antipsychotics, the clinician should pay particular attention to extrapyramidal side effects, and with atypical antipsychotics (particularly olanzapine), metabolic complications of excessive weight gain must be monitored, according to large consensus conferences (13).
Dementia-related psychosis.
DI often develops secondary to dementia. Dementia-related psychosis (also referred to as “behavioral and psychological symptoms in dementia” [BPSD]) can present with various psychotic symptoms (most frequently paranoid ideas) and mild to severe agitation (146, 279). For many years, BPSD was treated with high- and low-potency antipsychotics (off-label). But in April 2005, the FDA requested drug manufacturers to add black box warnings to prescription information and informed physicians that atypical antipsychotics are associated with an increased mortality due to cerebrovascular events in dementia-related psychosis (odds ratio, 1.6 to 1.7 versus placebo). In June 2008, this warning was expanded to typical substances, indicating a class effect. Time-to-discontinuation studies (CATIE-AD) showing problems in tolerability further questioned the use of atypical antipsychotics in this patient group (284), although positive effects were seen in some symptom domains, such as anger, aggression, and paranoid ideas. However, this did not reduce care needs or improve the quality of life (310). Psychotropic medication should be used for BPSD only if nonpharmacological measures are ineffective (279). Psychosocial treatments are also hardly evaluated, leaving heath care providers without clear and safe options for managing these patients (146). In cases of DI secondary to dementia, issues of quality of life and side effects from antipsychotic medication have to be balanced with an increase in risk of cardiovascular events. A blanket dismissal of antipsychotics as a possible treatment option, however, is not justified.
DISCUSSION
Main Findings
The present paper is the most comprehensive review of all major aspects and all forms of DI and is based on more than 500 publications. Our review showed that the clinical picture itself is well established, with many details and facultative features described in large surveys, numerous case series, and previous reviews (47, 74, 95, 178, 192, 234, 299, 300, 322, 323).
Definitions.
Countless more or less appropriate names and various diagnostic criteria exist for this clinical picture, however. In order to reduce confusion, we have proposed a set of minimal criteria and suggested using the name “delusional infestation.” This underlines the overarching delusional theme “infestation” and incorporates variations of the delusional theme over time, including newly emerging imaginary pathogens such as the so-called “Morgellons.” Names such as delusional parasitosis or “Dermatozoenwahn” falsely suggest a single alleged pathogen and have a narrower remit. Of the two major symptoms, delusions and abnormal sensations, the former was shown to be more important, so it does not seem justified to list the disease exclusively in the ICD-10 category “organic hallucinosis.” Despite the one single name “delusional infestation,” there is not one single form of DI (236); instead, there is a need to differentiate between primary and several secondary forms. In order to improve terminology, we also propose to use the name “specimen sign” instead of “matchbox sign” when patients present proof of infestation.
Epidemiology and psychopathology.
It appears that DI is a stable and ubiquitous picture and that it is more frequent than most physicians think, although reliable epidemiological data are scarce (323, 325). The number of undetected cases is likely to be high. Microbiologists, infectious disease specialists, dermatologists, and generalists have the most realistic estimation of the real number of affected persons. This is also reflected in the numerous websites created since the 1990s. Our summary of the epidemiology and history of DI as well as an excursus on the psychopathology of delusions showed that the axiom of changing delusional contents within stable delusional themes is also present in DI.
Morgellons.
In view of the origins of Morgellons, the subsequent development of the movement, its precise correspondence with the minimal criteria of DI, and the presence of all other typical clinical features, it seems justified to consider “Morgellons” to be a new, and not the last, variation of the delusional theme “infestation.” So far, there is no dermatological or entomological evidence for a true infestation with a new species of “fibers,” “filaments,” or “threads” (1, 17, 157, 158, 222). Official and usable results of the CDC study on the search for the unknown pathogen are not available. DI certainly exists, as shown by our historical and epidemiological accounts and uncontradicted psychiatric knowledge over more than 100 years (321, 322). However, it is not a psychiatric but a microbiological task to prove or disprove the existence of Morgellons. From a psychiatric point of view, Morgellons sufferers show all signs of DI, and as long there is no better explanation, Morgellons can be seen as a form of DI. A further characterization as primary DI or one of the secondary forms, however, is needed for each individual sufferer to determine the most appropriate psychiatric and other treatment.
Clinical aspects.
The presented work also provides a complete review of all known associations and underlying disorders, diseases, and toxic agents in patients with DI (Table 3). To enhance clinical usability, all DSM-IV-TR and ICD-10 chapter F diagnostic codes were included. For the same reason, a detailed, graded pathway for the diagnosis work-up, differential diagnosis, and determination of the form of DI was presented. We hope that physicians no longer consider DI a single diagnostic entity that can be treated with the same approach (235) but apply the appropriate treatment regimen using the suggested etiology-specific pathway.
Need for research on pathogenesis and therapy.
The assessment of the published literature showed a notable paucity of original research, which clearly contrasts with a large number of case series and reviews. The number of studies on the pathophysiology of DI is limited to studies by Skott (299), Musalek (234, 240), and Huber et al. (141). Although the complex process of pathogenesis in DI is little understood, we try to explain symptom formation from a cognitive and psychopathological point of view, provide a hypothetical model on the neuronal correlates of DI, and try to apply the two-factor theory of delusional disorders to DI for the first time. Details can be found in Inset 21.
Inset 21. A pathogenetic model (hypothesis). (i) Symptom formation. In primary DI, the belief of being infested with something arises unexpectedly at some point in time, without a previous abnormal sensation or a transient physiological tactile sensation. It is falsely ascribed to the presence of an infestation. It is likely that the formation of this false belief is based on a biopsychosocial vulnerability to develop a psychiatric illness. Similar to other major mental illnesses, acute stressors or life events, or someone itching or talking about an infectious disease, may trigger an episode of DI. The feeling of bugs crawling underneath or on the skin gradually becomes a conviction, and the highly unlikely possibility that an infestation is the cause is favored because of “errors in probabilistic reasoning” (76). Cognitive biases such as selective attention and an attention shift to skin sensations contribute to both formation and maintenance of such a wrong belief. In such stressful situations, there is a tendency to blame others, and sources of danger are attributed externally (209), similar to the case in paranoia (persecutory type of delusional disorders). In the case of DI, an “infestation by something” is made responsible for causing the itch. The exact “choice of the imaginary pathogen” within the delusional theme of infestation is modulated culturally and may have biographic explanations (e.g., a certain event that the individual perceives as “causal for the infestation,” such as a visit by a person with a dog). In the end, the cognitive “belief evaluation system” (M. Coltheart [58, 59]) fails and does not reject the hypothesis that an infestation is present. This delusional nucleus is gradually enriched to systemized delusions by expanding the theory behind it and seeking reasons why it is true (delusional elaboration), as well as reinterpreting past experiences according to the delusional logic (delusional memory), according to Karl Jaspers. Sensory input and current surroundings may also be mistaken according to the belief (illusion; e.g., dandruff may be mistaken for head lice). These cognitive processes eliminate all doubts and link the beliefs. Patients will “look for some kind of connection” (K. Jaspers) and desperately seek until they find a subjectively sound explanation for how and when they became infested, although specialists have ruled out true infections several times (stealth viruses, strange fibers, Morgellons, or whatever). Criticism or alternative views are no longer allowed by the dysfunctional belief evaluation system. Instead, judgment is used to seal off and immunize the systematized delusion.
(ii) Model of the neuronal basis of DI. This idealized process of DI formation, findings from imaging and lesion studies pointing to certain brain structures, and the associations with the dopaminergic system can be integrated into a hypothetical model of the neuronal basis of DI. Since this model results from low-level evidence (case reports), it cannot be more than a starting point. However, a model allows for testing and falsification in future studies, for example, using neuroimaging techniques. It does not include developmental or social aspects. It is also open to the temporal lobe pathology of the described pathological processes and their connections. It can be assumed that the neuronal structures mentioned are not linked by chance, but rather influence each other reciprocally in the course of a more and more chronic delusional disorder.
As measured by today's standards, defined by evidence-based medicine, high-level evidence for psychopharmacotherapy in DI and the effects of different treatment settings (psychiatry versus dermatology versus joint clinics) is absent. In order to meet clinicians' needs, we gave some careful recommendations on psychiatric pharmacotherapy based on the existing low-level evidence. In addition, we saw theoretical advantages of low-threshold specialized outpatient clinics in dermatology with a collaborating psychiatrist in order to meet the particular needs of patients with DI. A specialist team of clinicians may be best equipped to care for patients suffering from DI and to keep their faith in medicine. No evaluated or manualized guidelines are available, but some do's and don'ts have proven helpful (95, 117, 175, 280, 300). These suggestions and pathways should be tested in formal studies, though.
There are several possible explanations for the lack of research in DI. While dermatologists have DI patients but do not study psychotic disorders, psychiatrists hardly come into contact with these patients in order to recruit a sufficient sample. In addition, pharmacological companies, perhaps for economic reasons, neglect clinical studies on “psychotic disorders other than schizophrenia.” The whole group of delusional disorders, including DI, represent “blind spots” or “forgotten disorders” in research. For example, Manschreck and Khan found only 134 cases of delusional disorder described with sufficient data to inform their review on the treatment of delusional disorders (202). Further explanations lie in the patient group itself and current legal restraints for research. Full informed consent for clinical trials and sufficient adherence to study protocols are difficult, and often impossible, to obtain in this patient sample (227, 334). No deluded patient with DI will sign a patient agreement form if it is required for him or her to be fully informed about the administration of an antipsychotic for a delusional disorder in which the patient does not believe. Accordingly, well-meant measures to secure patient safety eventually turn against the patients because they impair the development and evaluation of more effective treatments (99, 277).
Perspectives and Future Research
The most important and promising research areas in the field of DI are the better characterization of its pathophysiology and prospective clinical trials. Studies in both areas should determine the types of DI and differentiate between primary and secondary forms of DI, using our classification presented in Table 3 (e.g., see references 99, 141, and 178). From a psychiatric point of view, primary DI is the most valuable form for future research, because it might help to improve our understanding of the etiology and optimized therapy of delusional disorders of the somatic type.
Pathophysiology.
Apart from the results of the CDC study, modern neuroimaging techniques would be most appropriate to further study the neuronal basis of DI and the interplay between abnormal, mostly tactile sensations and the delusion. Structural MRI studies with larger samples and functional MRI studies comparing primary DI and controls under tactile stimulation or pharmacological itch-induction appear to be promising approaches in order to identify the relevant brain structures and to confirm or falsify the model presented in Fig. 5 (82, 126). Functional imaging studies would be helpful to explore alterations in the striatal dopaminergic neurotransmitter system (e.g., using 11C-raclopride-positron emission tomography) and the role of the presynaptic dopamine transporter in DI (e.g., using 123I-FP-CIT-SPECT). Genetic research on the spectrum of delusional and psychotic disorders gives rise to the hope that susceptibility genes different from those found in schizophrenia can be identified.
FIG. 5.
Pathogenetic model (hypothesis). This illustration integrates the limited evidence available on pathophysiology and neural correlates of DI in a hypothetical network model.
Therapy.
Multidisciplinary efforts from clinicians and researchers as well as pharmaceutical companies are inevitable to provide more evidence for the effects and tolerability of antipsychotics in delusional disorder according to DSM-IV-TR criteria, including delusional disorder of the somatic type and primary DI. The most important study would be to prove the effectiveness of typical and atypical antipsychotics against placebo in trials with well-characterized samples of adequate size, not to mention independent studies that compare different antipsychotics and allow the evaluation of the time course of clinical response (onset of effects, time of maximum effects). Until then, we encourage colleagues to report further case reports and series, using our case reporting criteria (178), for subsequent pooling of data. To minimize publication and selection biases and to obtain realistic numbers, all cases where treatment was intended should be reported (99). For the treating physician, the situation is complicated further by the limited choice of antipsychotics approved for psychotic disorders other than schizophrenia.
Except for progress in psychopharmacotherapy for DI, treatment and outcome of DI may be improved by the creation of more specialized joint clinics for DI according to Musalek's model (234), although evidence for the superiority of this model is mixed. These institutions are best placed in dermatology clinics, i.e., the most “attractive” place with the lowest threshold, and aim at evading patient attrition at the interface of dermatology and psychiatry. They also secure sufficient dermatologic and psychiatric care. In building up such outpatient departments, a well-planned “disappearance” of psychiatry is needed in advertisements, letterheads, websites, and initial patient contact. The name “Integrative Dermatology” (University of Rochester) is well chosen in order to avoid the name “psych…” (280). Instead of referring to “delusional parasitosis,” the clinical picture could be called “unexplained dermopathy” in publications for the layperson. To attract patients, multimodal diagnostic and therapeutic measures could be offered. Treatment is initiated by dermatologists under the auspices of the psychiatrist, who stands in the background until partial insight has been achieved (e.g., by antipsychotics). Unfortunately, such clinics have not been evaluated systematically by controlled prospective trials with a head-to-head comparison to other settings, treatment as usual, or a waiting list. At any rate, joint clinics should be best equipped to handle the less severely deluded patients and are most promising in severe cases (99, 175, 258, 280). Since a definite trial on the best treatment setting will be an almost impossible task, it appears justified to encourage colleagues to establish local joint services with dermatologists, entomologists, and psychiatrists anyway. Even with all of these efforts made, it is difficult to say how many patients with DI will be reached.
On the search for new nonpharmacological interventions in psychotic disorders, a pilot study indicated that it is possible to disrupt “magical thinking” with single-pulse transcranial magnetic stimulation over the lateral temporal lobe in 12 healthy subjects (27). The effect was shown in comparison with a control group stimulation over the vertex and could not be explained by simple slowing. In analogy to other cortical hyperexcitability syndromes (e.g., tinnitus), it might also be interesting to see whether it is possible to interfere with the disturbed belief evaluation system with single-pulse or repetitive transcranial magnetic stimulation.
This review intended to provide a common basis for an improved cooperation of dermatologists, microbiologists, and psychiatrists in the field of DI both in clinical practice and in research. Improvements in medical care can be achieved if medical specialties work together in order to meet the needs of this truly interdisciplinary patient group. We hope that our work contributes to reducing patients' distress caused by the imaginary pathogens, whatever their current name might be.
Biography
Roland Freudenmann graduated from the University of Ulm and received his medical doctorate in 2001 after studying medicine in Ulm, Germany, and Montpellier, France. For his residency training, he joined the Department of Psychiatry and Psychotherapy with Manfred Spitzer, where he has held a position as Senior Physician and Assistant Professor since 2007. He became board certified in psychiatry and psychotherapy in 2007 and in addiction medicine in 2008. Dr. Freudenmann is a member of the German Society for Psychiatry (DGPPN), its section on Emergency Psychiatry, the Arbeitsgemeinschaft Psychosomatische Medizin (APD), and the European Society for Dermatology and Psychiatry (ESDaP). He is on the advisory editorial board of The Open Addiction Journal. Since meeting his first patient with delusional infestation almost 10 years ago, his research in the field has led to several publications, many of them coauthored with Dr. Lepping. Dr. Freudenmann has also published papers on psychopharmacotherapy, addiction, the history of psychiatry, and neuroimaging.
Peter Lepping studied medicine in Münster, Germany. He moved to the United Kingdom in 1995, where he received his postgraduate education as a psychiatrist. He is a member of the Royal College of Psychiatrists. He worked in and around Liverpool, and since 2004 he has been a consultant psychiatrist in Wrexham, North Wales. He is also an Honorary Senior Lecturer with the University of Wales and an Associate Medical Director for Ethics, Capacity, and Consent with the North Wales NHS Trust. He has been interested in delusional infestations for some years and has collaborated with Dr. Freudenmann on most of his research into this disorder. He also publishes in the areas of violence, ethics, mental health assessment tools, and systematic reviews. He is a member of various European research networks and has won two research prizes, in 2002 and 2004.
REFERENCES
- 1.Accordino, R. E., D. Engler, I. H. Ginsburg, and J. Koo. 2008. Morgellons disease? Dermatol. Ther. 21:8-12. [DOI] [PubMed] [Google Scholar]
- 2.Adunsky, A. 1997. Early post-stroke parasitic delusions. Age Ageing 26:238-239. [DOI] [PubMed] [Google Scholar]
- 3.Aftab, A., and K. M. Ranjha. 2005. Ekboms disease (delusional disorder, somatic type): a case report. J. Pak. Assoc. Dermatol. 15:96-98. [Google Scholar]
- 4.Agency for Health Care Policy and Research. 1992. AHCPR publication 92-0032. Agency for Health Care Policy and Research, Rockville, MD.
- 5.Aguirre, A. J., R. Apiquian, A. Fresan, and C. Cruz-Fuentes. 2007. Association analysis of exon III and exon I polymorphisms of the dopamine D4 receptor locus in Mexican psychotic patients. Psychiatry Res. 153:209-215. [DOI] [PubMed] [Google Scholar]
- 6.Ait-Ameur, A., P. Bern, M. P. Firoloni, and P. Menecier. 2000. Delusional parasitosis or Ekbom's syndrome. Rev. Med. Interne 21:182-186. (In French.) [DOI] [PubMed] [Google Scholar]
- 7.Aizenberg, D., B. Schwartz, and Z. Zemishlany. 1991. Delusional parasitosis associated with phenelzine. Br. J. Psychiatry 159:716-717. [DOI] [PubMed] [Google Scholar]
- 8.Aleman, A., K. B. Bocker, R. Hijman, E. H. de Haan, and R. S. Kahn. 2003. Cognitive basis of hallucinations in schizophrenia: role of top-down information processing. Schizophr. Res. 64:175-185. [DOI] [PubMed] [Google Scholar]
- 9.Aleshire, I. 1954. Delusions of parasitosis. Report of a successful care with antipellagrous treatment. JAMA 155:15-27. [DOI] [PubMed] [Google Scholar]
- 10.Allen, P., F. Laroi, P. K. McGuire, and A. Aleman. 2008. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci. Biobehav. Rev. 32:175-191. [DOI] [PubMed] [Google Scholar]
- 11.Amancio, E. J., C. M. Peluso, A. C. G. Santos, C. C. P. Magalhaes, M. F. C. Pires, A. P. P. Dias, and F. A. A. Debs. 2002. Ekbom's syndrome and spasmodic torticollis: case report. Arq. Neuropsiquiatr. 60:155-158. (In Portuguese.) [DOI] [PubMed] [Google Scholar]
- 12.Amato Neto, V., J. G. Amato, V. S. Amato, and C. S. Ferreira. 2007. Ekbom syndrome (delusory parasitosis): ponderations on two cases. Rev. Inst. Med. Trop. Sao Paulo 49:395-396. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. 2004. Consensus development conference on antipsychotic drugs and obesity and diabetes. J. Clin. Psychiatry 65:267-272. [DOI] [PubMed] [Google Scholar]
- 13a.American Psychiatric Association. 2000. Diagnostic and statistical manual of psychiatric disorders: DSM-IV-TR. American Psychiatric Association, Washington, DC.
- 14.Amler, G. 1959. Tactile hallucinosis in traumatic lesions of the thalamus. Fortschr. Neurol. Psychiatr. 27:595-601. (In German.) [PubMed] [Google Scholar]
- 15.Andrews, E., J. Bellard, and W. G. Walter-Ryan. 1986. Monosymptomatic hypochondriacal psychosis manifesting as delusions of infestation: case studies of treatment with haloperidol. J. Clin. Psychiatry 47:188-190. [PubMed] [Google Scholar]
- 16.Angelini, C., and L. Leppo. 1966. Deliroide dermatozoico in corso di una fase di psicosi maniaco-depressiva. Riv. Psichiatr. 1:73-76. [Google Scholar]
- 17.Ash, L. R., and T. C. Orihel. 2007. Atlas of human parasitology, 5th ed. American Society for Clinical Pathology Press, Chicago, IL.
- 18.Avnstorp, C., K. Hamann, and P. W. Jepsen. 1980. Delusions of parasite infestation treated with pimozide (Orap). Ugeskr. Laeger 142:2191-2192. (In Danish.) [PubMed] [Google Scholar]
- 19.Aw, D. C., J. Y. Thong, and H. L. Chan. 2004. Delusional parasitosis: case series of 8 patients and review of the literature. Ann. Acad. Med. Singapore 33:89-94. [PubMed] [Google Scholar]
- 20.Bak, R., P. Tumu, C. Hui, D. Kay, J. Burnett, and D. Peng. 2008. A review of delusions of parasitosis, part 1: presentation and diagnosis. Cutis 82:123-130. [PubMed] [Google Scholar]
- 21.Bak, R., P. Tumu, C. Hui, D. Kay, and D. Peng. 2008. A review of delusions of parasitosis, part 2: treatment options. Cutis 82:257-264. [PubMed] [Google Scholar]
- 22.Baker, P. B., B. L. Cook, and G. Winokur. 1995. Delusional infestation. The interface of delusions and hallucinations. Psychiatr. Clin. N. Am. 18:345-361. [PubMed] [Google Scholar]
- 23.Bauer, A., and A. Mosler. 1970. Die Behandlung des Dermatozoenwahns. Arzneimittelforschung 20:884-886. [PubMed] [Google Scholar]
- 24.Baumer, L. 1951. Die Behandlung des Juckreizes insbesondere beim “Dermatozoenwahn” mit Elektroschock. Hautarzt 2:131-132. [Google Scholar]
- 25.Bell, V., P. W. Halligan, and H. D. Ellis. 2008. Are anomalous perceptual experiences necessary for delusions? J. Nerv. Ment. Dis. 196:3-8. [DOI] [PubMed] [Google Scholar]
- 26.Bell, V., P. W. Halligan, and H. D. Ellis. 2006. Explaining delusions: a cognitive perspective. Trends Cogn. Sci. 10:219-226. [DOI] [PubMed] [Google Scholar]
- 27.Bell, V., V. Reddy, P. Halligan, G. Kirov, and H. Ellis. 2007. Relative suppression of magical thinking: a transcranial magnetic stimulation study. Cortex 43:551-557. [DOI] [PubMed] [Google Scholar]
- 28.Benkert, O., and H. Hippius. 2009. Kompendium der Psychiatrischen Pharmakotherapie, 7th ed. Springer, Heidelberg, Germany.
- 29.Bergmann, B. 1957. Zur Genese der taktilen Halluzinose bzw. des Dermatozoenwahns. Nervenarzt 28:22-27. [PubMed] [Google Scholar]
- 30.Berrios, G. E. 1985. Delusional parasitosis and physical disease. Compr. Psychiatry 26:395-403. [DOI] [PubMed] [Google Scholar]
- 31.Berrios, G. E. 1982. Tactile hallucinations: conceptual and historical aspects. J. Neurol. Neurosurg. Psychiatry 45:285-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bers, N., and K. Conrad. 1954. Die chronische taktile Halluzinose. Fortschr. Neurol. Psychiatr. 22:254-270. [Google Scholar]
- 33.Bhatia, M. S., R. Chandra, and A. Kamra. 2002. Delusional parasitosis in leprosy. Indian J. Lepr. 74:159-160. [PubMed] [Google Scholar]
- 34.Bhatia, M. S., R. K. Gautam, S. Shome, and G. K. Bedi. 1994. Delusional parasitosis with trichotillomania. J. Indian Med. Assoc. 92:389. [PubMed] [Google Scholar]
- 35.Bhatia, M. S., T. Jagawat, and S. Choudhary. 2000. Delusional parasitosis: a clinical profile. Int. J. Psychiatry Med. 30:83-91. [DOI] [PubMed] [Google Scholar]
- 36.Bhatia, M. S., S. Shome, and R. K. Gautam. 1996. Delusional parasitosis in leprosy. Lepr. Rev. 67:230. (Letter.) [PubMed] [Google Scholar]
- 37.Blasco-Fontecilla, H., M. D. Bragado Jimenez, L. M. Garcia Santos, and J. M. Barjau Romero. 2005. Delusional disorder with delusions of parasitosis and jealousy after stroke: treatment with quetiapine and sertraline. J. Clin. Psychopharmacol. 25:615-617. [DOI] [PubMed] [Google Scholar]
- 38.Bleumel, C. S. 1938. The troubled mind. Williams & Wilkins Company, Baltimore, MD.
- 39.Bond, W. S. 1989. Delusions of parasitosis: a case report and management guidelines. DICP 23:304-306. [DOI] [PubMed] [Google Scholar]
- 40.Borel, J., and H. Ey. 1932. Obsession hallucinatoire zoopathique guérie par psychothérapie. Ann. Med. Psychol. 90:181-185. [Google Scholar]
- 41.Bosmans, A., and P. Verbanck. 2008. Successful treatment of delusional disorder of the somatic type or “delusional parasitosis” with olanzapine. Pharmacopsychiatry 41:121-122. [DOI] [PubMed] [Google Scholar]
- 42.Botschev, C., and N. Muller. 1991. Opiate receptor antagonists for delusions of parasitosis. Biol. Psychiatry 30:530-531. (Letter.) [DOI] [PubMed] [Google Scholar]
- 43.Böttcher, W. 1952. “Circumscripte Hypochondrie” (Das Syndrom des wahnhaften Ungezieferbefalls). Psychiatr. Neurol. Med. Psychol. 4:95. [Google Scholar]
- 44.Böttcher, W. 1954. Das Syndrom des wahnhaften Ungezieferbefalles. Dissertation. Humboldt Universität, Berlin, Germany.
- 45.Boublik, J. H., and J. W. Funder. 1984. Interaction of dopamine receptor ligands with subtypes of the opiate receptor. Eur. J. Pharmacol. 107:11-16. [DOI] [PubMed] [Google Scholar]
- 46.Bourgeois, M., and A. Nguyen-Lan. 1986. Ekbom's syndrome and delusion of skin infestation. 1. Review of the literature. Ann. Med. Psychol. (Paris) 144:321-340. (In French.) [PubMed] [Google Scholar]
- 47.Bourgeois, M., P. Rager, F. Peyre, A. Nguyen-Lan, and J. J. Etchepare. 1986. Fréquence et aspects du syndrome d'Ekbom enquête auprès des dermatologues francais (a propos de 150 cas). Ann. Med. Psychol. (Paris) 144:659-668. [PubMed] [Google Scholar]
- 48.Bourgeois, M. L., P. Duhamel, and H. Verdoux. 1992. Delusional parasitosis: folie a deux and attempted murder of a family doctor. Br. J. Psychiatry 161:709-711. [DOI] [PubMed] [Google Scholar]
- 49.Bransfield, R. C. 2007. Morgellons disease. Psychiatr. News 42:24. [Google Scholar]
- 50.Bransfield, R. C., J. S. Wulfman, W. T. Harvey, and A. I. Usman. 2008. The association between tick-borne infections, Lyme borreliosis and autism spectrum disorders. Med. Hypotheses 70:967-974. [DOI] [PubMed] [Google Scholar]
- 51.Brewer, J. D., A. Meves, J. M. Bostwick, K. L. Hamacher, and M. R. Pittelkow. 2008. Cocaine abuse: dermatologic manifestations and therapeutic approaches. J. Am. Acad. Dermatol. 59:483-487. [DOI] [PubMed] [Google Scholar]
- 52.Busch, G. 1960. Syndrom des wahnhaften Ungezieferbefalles bei einem Diabetiker. Z. Gesamt. Inn. Med. 15:411-417. [PubMed] [Google Scholar]
- 53.Caimi, M., G. P. Dona, and G. Colombo. 16 June 2008, accession date. Ekbom's syndrome. G. Ital. Psicopatol. 9. http://www.gipsicopatol.it/italiano/rivista/2003/vol9-1/caimi.htm. (In Italian.)
- 54.Campanella, G. 1969. Study on the delusion of parasitosis (Ekbom's syndrome). Acta Neurol. (Napoli) 24:903-927. (In Italian.) [PubMed] [Google Scholar]
- 55.Carvalho, C. N., O. Bastos, and O. Gouveia. 2001. Ekbom's syndrome as prodromal manifestation of dementia. Rev. Psiquiatr. Clin. 28:129-133. (In Portuguese.) [Google Scholar]
- 56.Cipriani, A., T. A. Furukawa, G. Salanti, J. R. Geddes, J. P. Higgins, R. Churchill, N. Watanabe, A. Nakagawa, I. M. Omori, H. McGuire, M. Tansella, and C. Barbui. 2009. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet 373:746-758. [DOI] [PubMed] [Google Scholar]
- 57.Colombo, G., M. Caimi, and G. P. Dona. 2004. Shared Ekbom's syndrome. A case study. Eur. Psychiatry 19:115-116. [DOI] [PubMed] [Google Scholar]
- 58.Coltheart, M. 2007. Cognitive neuropsychiatry and delusional belief. Q. J. Exp. Psychol. (Colchester) 60:1041-1062. [DOI] [PubMed] [Google Scholar]
- 59.Coltheart, M., R. Langdon, and R. McKay. 2007. Schizophrenia and monothematic delusions. Schizophr. Bull. 33:642-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cordeiro, Q., Jr., and C. E. Corbett. 2003. Delusional parasitic infestation and folie a deux: case report. Arq. Neuropsiquiatr. 61:872-875. [DOI] [PubMed] [Google Scholar]
- 61.Cornbleet, T., and M. Brown. 1948. Dermatological manifestations in psychiatric disorders. JAMA 136:152-157. [DOI] [PubMed] [Google Scholar]
- 62.Creese, I., A. P. Feinberg, and S. H. Snyder. 1976. Butyrophenone influences on the opiate receptor. Eur. J. Pharmacol. 36:231-235. [DOI] [PubMed] [Google Scholar]
- 63.Damiani, J. T., F. P. Flowers, and D. K. Pierce. 1990. Pimozide in delusions of parasitosis. J. Am. Acad. Dermatol. 22:312-313. [DOI] [PubMed] [Google Scholar]
- 64.Daniel, E., and T. N. Srinivasan. 2004. Folie a famille: delusional parasitosis affecting all the members of a family. Indian J. Dermatol. Venereol. Leprol. 70:296-297. [PubMed] [Google Scholar]
- 65.Daube, H. 1949. Pervitinpsychosen. Nervenarzt 15:20-25. [Google Scholar]
- 66.Davis, J. L. 2008. Rare skin problem baffles experts. CDC launches investigation of Morgellons disease. WebMD Health News. http://www.medicinenet.com/script/main/art.asp?articlekey=86523.
- 67.de Leon, J., R. E. Antelo, and G. Simpson. 1992. Delusion of parasitosis or chronic tactile hallucinosis: hypothesis about their brain physiopathology. Compr. Psychiatry 33:25-33. [DOI] [PubMed] [Google Scholar]
- 68.De Leon, O. A., K. M. Furmaga, A. L. Canterbury, and L. G. Bailey. 1997. Risperidone in the treatment of delusions of infestation. Int. J. Psychiatry Med. 27:403-409. [DOI] [PubMed] [Google Scholar]
- 69.Desta, Z., T. Kerbusch, N. Soukhova, E. Richard, J. W. Ko, and D. A. Flockhart. 1998. Identification and characterization of human cytochrome P450 isoforms interacting with pimozide. J. Pharmacol. Exp. Ther. 285:428-437. [PubMed] [Google Scholar]
- 70.DeVita-Raeburn, E. 2007. The Morgellons mystery. Psychol. Today. http://www.psychologytoday.com/articles/200702/the-morgellons-mystery.
- 71.Dierks, T., D. E. Linden, M. Jandl, E. Formisano, R. Goebel, H. Lanfermann, and W. Singer. 1999. Activation of Heschl's gyrus during auditory hallucinations. Neuron 22:615-621. [DOI] [PubMed] [Google Scholar]
- 72.Döhring, E. 1960. Zur Häufigkeit des Syndroms “Wahnhafter Ungezieferbefall.” Münch. Med. Wochenschr. 102:2158-2160. [PubMed] [Google Scholar]
- 73.Donabedian, H. 2007. Delusions of parasitosis. Clin. Infect. Dis. 45:e131-e134.
- 74.Driscoll, M. S., M. J. Rothe, J. M. Grant-Kels, and M. S. Hale. 1993. Delusional parasitosis: a dermatologic, psychiatric, and pharmacologic approach. J. Am. Acad. Dermatol. 29:1023-1033. [DOI] [PubMed] [Google Scholar]
- 75.Drolet, B., G. Rousseau, P. Daleau, R. Cardinal, C. Simard, and J. Turgeon. 2001. Pimozide (Orap) prolongs cardiac repolarization by blocking the rapid component of the delayed rectifier potassium current in native cardiac myocytes. J. Cardiovasc. Pharmacol. Ther. 6:255-260. [DOI] [PubMed] [Google Scholar]
- 76.Dunn, J., M. B. Murphy, and K. M. Fox. 2007. Diffuse pruritic lesions in a 37-year-old man after sleeping in an abandoned building. Am. J. Psychiatry 164:1166-1172. [DOI] [PubMed] [Google Scholar]
- 77.Edison, K. E., J. R. Slaughter, and R. D. Hall. 2007. Psychogenic parasitosis: a therapeutic challenge. Mol. Med. 104:132-137. [PubMed] [Google Scholar]
- 78.Ekbom, K. A. 1938. Der praesenile Dermatozoenwahn. Acta Psychiatr. Neurol. Scand. 13:227-259. [Google Scholar]
- 79.Ekbom, K. A., G. Yorston, M. Miesch, S. Pleasance, and S. Rubbert. 2003. The pre-senile delusion of infestation. Hist. Psychiatry 14:229-256. [DOI] [PubMed] [Google Scholar]
- 80.Elena, M., R. Barreras, B. Mantecon Fernandez, V. Capo de Paz, J. M. Almeida Lorente, D. Gonzalez Rubio, and R. Diaz Fernandez. 2005. Delusional parasitosis: apropos of a case. Rev. Cubana Med. Trop. 57:233-236. [PubMed] [Google Scholar]
- 81.Ellingwood, E. H. 1967. Amphetamine psychosis: description of the individuals and process. J. Nerv. Ment. Dis. 44:273-283. [Google Scholar]
- 82.Ellis, H. D. 2007. Delusions: a suitable case for imaging? Int. J. Psychophysiol. 63:146-151. [DOI] [PubMed] [Google Scholar]
- 83.Elpern, D. J. 1988. Cocaine abuse and delusions of parasitosis. Cutis 42:273-274. [PubMed] [Google Scholar]
- 84.Evans, P., and H. Merskey. 1972. Shared beliefs of dermal parasitosis: folie partagee. Br. J. Med. Psychol. 45:19-26. [DOI] [PubMed] [Google Scholar]
- 85.Even, C., J. L. Goeb, and R. Dardennes. 1997. Bipolar affective disorder and Ekbom syndrome: apropos of a case. Encephale 23:397-399. [PubMed] [Google Scholar]
- 86.Fleck, U. 1955. Bemerkungen zur chronischen taktilen Halluzinose. Arch. Psychiatr. Nervenkr. 193:261-276. [DOI] [PubMed] [Google Scholar]
- 87.Fleck, U. 1957. Ergänzung der Bemerkungen zur chronischen taktilen Halluzinose. Nervenarzt 28:231-233. [PubMed] [Google Scholar]
- 88.Fleury, V., J. Wayte, and M. Kiley. 2008. Topiramate-induced delusional parasitosis. J. Clin. Neurosci. 15:597-599. [DOI] [PubMed] [Google Scholar]
- 89.Floris, G., A. Cannas, M. Melis, P. Solla, and M. G. Marrosu. 2008. Pathological gambling, delusional parasitosis and adipsia as a post-haemorrhagic syndrome: a case report. Neurocase 14:385-389. [DOI] [PubMed] [Google Scholar]
- 90.Flynn, F. G., J. L. Cummings, J. Scheibel, and W. Wirshing. 1989. Monosymptomatic delusions of parasitosis associated with ischemic cerebrovascular disease. J. Geriatr. Psychiatry Neurol. 2:134-139. [DOI] [PubMed] [Google Scholar]
- 91.Ford, E. B., D. P. Calfee, and R. D. Pearson. 2001. Delusions of intestinal parasitosis. South. Med. J. 94:545-547. [PubMed] [Google Scholar]
- 92.Frances, A., and A. Munro. 1989. Treating a woman who believes she has bugs under her skin. Hosp. Community Psychiatry 40:1113-1114. [DOI] [PubMed] [Google Scholar]
- 93.Frankel, E. B. 1983. Treatment of delusions of parasitosis. J. Am. Acad. Dermatol. 9:772-773. (Letter.) [DOI] [PubMed] [Google Scholar]
- 94.Freinhar, J. P. 1984. Delusions of parasitosis. Psychosomatics 25:47-49, 53. [DOI] [PubMed] [Google Scholar]
- 95.Freudenmann, R. W. 2002. Delusions of parasitosis: an up-to-date review). Fortschr. Neurol. Psychiatr. 70:531-541. (In German.) [DOI] [PubMed] [Google Scholar]
- 96.Freudenmann, R. W. 2003. A case of delusional parasitosis in severe heart failure. Olanzapine within the framework of a multimodal therapy. Nervenarzt 74:591-595. (In German.) [DOI] [PubMed] [Google Scholar]
- 97.Freudenmann, R. W., P. Kühnlein, P. Lepping, and C. Schönfeldt-Lecuona. 2009. Secondary delusional parasitosis treated with paliperidone. Clin. Exp. Dermatol. 34:375-377. [DOI] [PubMed] [Google Scholar]
- 98.Freudenmann, R. W., and P. Lepping. 2006. Dopaminergic antipsychotics in delusional parasitosis. Prog. Neurol. Psychiatry 10:20. [Google Scholar]
- 99.Freudenmann, R. W., and P. Lepping. 2008. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J. Clin. Psychopharmacol. 28:500-508. [DOI] [PubMed] [Google Scholar]
- 100.Freudenmann, R. W., and C. Schönfeldt-Lecuona. 2005. Delusional parasitosis: treatment with atypical antipsychotics. Ann. Acad. Med. Singapore 34:141-142. (Letter.) [PubMed] [Google Scholar]
- 101.Freudenmann, R. W., C. Schönfeldt-Lecuona, and P. Lepping. 2007. Primary delusional parasitosis treated with olanzapine. Int. Psychogeriatr. 19:1161-1168. [DOI] [PubMed] [Google Scholar]
- 102.Freyne, A., E. Kenny, and C. Cooney. 1999. Delusions of infestation—a case report of response to risperidone. Ir. Med. J. 92:435. [PubMed] [Google Scholar]
- 103.Friedmann, A. C., A. Ekeowa-Anderson, R. Taylor, and A. Bewley. 2006. Delusional parasitosis presenting as folie a trois: successful treatment with risperidone. Br. J. Dermatol. 155:841-842. [DOI] [PubMed] [Google Scholar]
- 104.Frith, C. 2005. The neural basis of hallucinations and delusions. C. R. Biol. 328:169-175. [DOI] [PubMed] [Google Scholar]
- 105.Frithz, A. 1979. Delusions of infestation: treatment by depot injections of neuroleptics. Clin. Exp. Dermatol. 4:485-488. [DOI] [PubMed] [Google Scholar]
- 106.Gallucci, G., and G. Beard. 1995. Risperidone and the treatment of delusions of parasitosis in an elderly patient. Psychosomatics 36:578-580. [DOI] [PubMed] [Google Scholar]
- 107.Gamper, E. 1920. Klinischer Beitrag zur Kenntnis der Psychosen im Rückbildungsalter und zur Frage des Wahnbildung aus überwertiger Idee. Jahrb. Psychiatr. Neurol. 40:111-169. [Google Scholar]
- 108.Ganner, H., and E. Lorenzi. 1975. Der Dermatozoenwahn. Psychiatr. Clin. 8:31-44. [PubMed] [Google Scholar]
- 109.Garson, A., Jr. 1993. How to measure the QT interval—what is normal? Am. J. Cardiol. 72:14B-16B. [DOI] [PubMed] [Google Scholar]
- 110.Ghaffari-Nejad, A., and K. Toofani. 2006. Delusion of oral parasitosis in a patient with major depressive disorder. Arch. Iran. Med. 9:76-77. [PubMed] [Google Scholar]
- 111.Giacardy, P. 1923. Un cas d'acarophobie familiale. J. Med. Bordeaux 53:479-480. [Google Scholar]
- 112.Gieler, U., and M. Knoll. 1990. Delusional parasitosis as ‘folie a trois.’ Dermatologica 181:122-125. [PubMed] [Google Scholar]
- 113.Glassman, A. H., and J. T. Bigger, Jr. 2001. Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. Am. J. Psychiatry 158:1774-1782. [DOI] [PubMed] [Google Scholar]
- 114.Goddard, J. 1995. Analysis of 11 cases of delusions of parasitosis reported to the Mississippi Department of Health. South. Med. J. 88:837-839. [DOI] [PubMed] [Google Scholar]
- 115.Gonul, M., A. Kilic, S. Soylu, U. Gul, C. Aydemir, and O. Kocak. 2008. Folie a deux, diagnosed by delusional parasitosis. Eur. J. Dermatol. 18:95-96. [DOI] [PubMed] [Google Scholar]
- 116.González Seijo, J. C., I. Lastra Martinez, and Y. M. Ramos Vicente. 1993. Parasitic delusions: literature review and report of new cases. Actas Luso Esp. Neurol. Psiquiatr. Cienc. Afines 21:56-62. [PubMed] [Google Scholar]
- 117.Gould, W. M., and T. M. Gragg. 1976. Delusions of parasitosis. An approach to the problem. Arch. Dermatol. 112:1745-1748. [DOI] [PubMed] [Google Scholar]
- 118.Gross-Tsur, V., A. Joseph, and R. S. Shalev. 2004. Hallucinations during methylphenidate therapy. Neurology 63:753-754. [DOI] [PubMed] [Google Scholar]
- 119.Guarneri, F., C. Guarneri, G. Mento, and A. Ioli. 2006. Pseudo-delusory syndrome caused by Limothrips cerealium. Int. J. Dermatol. 45:197-199. [DOI] [PubMed] [Google Scholar]
- 120.Halprin, K. M. 1966. The art of self-mutilation. II. Delusions of parasitosis. JAMA 198:1207. [PubMed] [Google Scholar]
- 121.Hamann, K., and C. Avnstorp. 1982. Delusions of infestation treated by pimozide: a double-blind crossover clinical study. Acta Dermatol. Venereol. 62:55-58. [PubMed] [Google Scholar]
- 122.Hanumantha, K., P. V. Pradhan, and B. Suvarna. 1994. Delusional parasitosis—study of 3 cases. J. Postgrad. Med. 40:222-224. [PubMed] [Google Scholar]
- 123.Harbauer, H. 1949. Das Syndrom des “Dermatozoenwahns” (Ekbom). Nervenarzt 20:254-258. [Google Scholar]
- 124.Harlan, C. 23 July 2006. Mom fights for answers on what's wrong with her son. Pittsburgh Post-Gazette, Pittsburgh, PA. http://www.post-gazette.com/pg/06204/707970-85.stm.
- 125.Harper, R., and G. Moss. 1992. Delusional infestation associated with post-herpetic neuralgia and EEG abnormalities. Br. J. Psychiatry 161:411-412. [DOI] [PubMed] [Google Scholar]
- 126.Hartwig, V., C. Cappelli, N. Vanello, E. Ricciardi, E. P. Scilingo, G. Giovannetti, M. F. Santarelli, V. Positano, P. Pietrini, L. Landini, and A. Bicchi. 2006. A compatible electrocutaneous display for functional magnetic resonance imaging application. Conf. Proc. IEEE Eng. Med. Biol. Soc. 1:1021-1024. [DOI] [PubMed] [Google Scholar]
- 127.Harvey, W. T. 1989. A flight surgeon's personal view of an emerging illness. Aviat. Space Environ. Med. 60:1199-1201. [PubMed] [Google Scholar]
- 128.Harvey, W. T. 2007. Morgellons disease. J. Am. Acad. Dermatol. 56:705-706. [DOI] [PubMed] [Google Scholar]
- 129.Harvey, W. T., and P. Salvato. 2003. ‘Lyme disease’: ancient engine of an unrecognized borreliosis pandemic? Med. Hypotheses 60:742-759. [DOI] [PubMed] [Google Scholar]
- 130.Hayashi, H., S. Oshino, J. Ishikawa, S. Kawakatsu, and K. Otani. 2004. Paroxetine treatment of delusional disorder, somatic type. Hum. Psychopharmacol. 19:351-352. [DOI] [PubMed] [Google Scholar]
- 131.Heim, M., and J. Morgner. 1980. Zur Problematik der chronischen taktilen Halluzinose. Psychiatr. Neurol. Med. Psychol 32:405-411. [PubMed] [Google Scholar]
- 132.Helmchen, H. 1961. Zur Analyse des sogenannten Dermatozoenwahnes. Beitrag Syndrom-Genese. Nervenarzt 32:509-513. [Google Scholar]
- 133.Hernandez-Albujar, S., G. Rubio, J. Gopar, G. Galeote, R. Rey, and A. Gil. 1996. Parasitic delirium in patient with multiorganic pathology: a complex situation. An. Med. Interna 13:549-551. [PubMed] [Google Scholar]
- 134.Hinkle, N. 2000. Delusory parasitosis. Am. Entomol. 46:17-25. [Google Scholar]
- 135.Hoffmann, S. O. 1973. Rückbildung eines sogenannten Dermatozoenwahns nach Schrittmacherimplantation. Zum Problem der symptomatischen Psychosen bei Herzkrankheiten. Nervenarzt 44:48-51. [PubMed] [Google Scholar]
- 136.Hopkinson, G. 1970. Delusions of infestation. Acta Psychiatr. Scand. 46:111-119. [DOI] [PubMed] [Google Scholar]
- 137.Hornstein, O. P., P. Hofmann, and P. Joraschky. 1989. Delusions of parasitic skin infestation in elderly dermatologic patients. Z. Hautkr. 64:981-982, 985-989. (In German.) [PubMed] [Google Scholar]
- 138.Huber, G. 1997. Delusional parasitosis. A till today not sufficiently known psychiatric syndrome. Psiquiatr. Biol. 5:105-115. [Google Scholar]
- 139.Huber, G. 1957. Die coenästhetische Schizophrenie. Fortschr. Neurol. Psychiatr. 25:491-520. [PubMed] [Google Scholar]
- 140.Huber, G. 1987. Psychiatrie, 4th ed. Schattauer, Stuttgart, Germany.
- 141.Huber, M., M. Karner, E. Kirchler, P. Lepping, and R. W. Freudenmann. 2008. Striatal lesions in delusional parasitosis revealed by magnetic resonance imaging. Prog. Neuropsychopharmacol. Biol. Psychiatry 32:1967-1971. [DOI] [PubMed] [Google Scholar]
- 142.Huber, M., E. Kirchler, M. Karner, and R. Pycha. 2006. Delusional parasitosis and the dopamine transporter. A new insight of etiology? Med. Hypotheses 68:1351-1358. [DOI] [PubMed] [Google Scholar]
- 143.Jakuszkowiak-Wojten, K., W. J. Cubala, and J. Szeliga-Lewinska. 2007. Delusional parasitosis: case report. Psychiatr. Pol. 41:571-578. [PubMed] [Google Scholar]
- 144.Jaspers, K. 1973. Allgemeine Psychopathologie, 9th ed. Springer, Heidelberg, Germany.
- 145.Jaspers, K. 1963. General psychopathology, 4th ed. Manchester University Press, Manchester, United Kingdom.
- 146.Jeste, D. V., D. Blazer, D. Casey, T. Meeks, C. Salzman, L. Schneider, P. Tariot, and K. Yaffe. 2008. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology 33:957-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Johnson, G. C., and R. F. Anton. 1983. Pimozide in delusions of parasitosis. J. Clin. Psychiatry 44:233. (Letter.) [PubMed] [Google Scholar]
- 148.Jones, P. B., T. R. Barnes, L. Davies, G. Dunn, H. Lloyd, K. P. Hayhurst, R. M. Murray, A. Markwick, and S. W. Lewis. 2006. Randomized controlled trial of the effect on quality of life of second- versus first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1). Arch. Gen. Psychiatry 63:1079-1087. [DOI] [PubMed] [Google Scholar]
- 149.Kanazawa, A., and T. Hata. 1992. Coexistence of the Ekbom syndrome and lilliputian hallucination. Psychopathology 25:209-211. [DOI] [PubMed] [Google Scholar]
- 150.Kang, J., X. L. Chen, and D. Rampe. 2001. The antipsychotic drugs sertindole and pimozide block erg3, a human brain K(+) channel. Biochem. Biophys. Res. Commun. 286:499-504. [DOI] [PubMed] [Google Scholar]
- 151.Kang, J., L. Wang, F. Cai, and D. Rampe. 2000. High affinity blockade of the HERG cardiac K(+) channel by the neuroleptic pimozide. Eur. J. Pharmacol. 392:137-140. [DOI] [PubMed] [Google Scholar]
- 152.Kaplan, H. I., and B. J. Sadock. 1998. Synopsis of psychiatry, 8th ed. Williams & Wilkins, Baltimore, MD.
- 153.Kellett, C. E. 1935. Sir Thomas Browne and the disease called the Morgellons. Ann. Med. Hist. 7:467-469. [PMC free article] [PubMed] [Google Scholar]
- 154.Kienbaum, S., C. Schaller, G. Herrmann, C. Fritsch, and T. Ruzicka. 1994. Delusions of parasitosis with severe skin artefacts. Therapy with fluspirilene (Imap®). Z. Hautkr. 69:711-713. [Google Scholar]
- 155.Kim, C., J. Kim, M. Lee, and M. Kang. 2003. Delusional parasitosis as ‘folie a deux.’ J. Korean Med. Sci. 18:462-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kirk, L. 1975. Ad notam. Amfetaminpsykose. Ugeskr. Laeger 137:644. [Google Scholar]
- 157.Koblenzer, C. S. 2006. The challenge of Morgellons disease. J. Am. Acad. Dermatol. 55:920-922. [DOI] [PubMed] [Google Scholar]
- 158.Koblenzer, C. S. 2006. Pimozide at least as safe and perhaps more effective than olanzapine for treatment of Morgellons disease. Arch. Dermatol. 142:1364. [DOI] [PubMed] [Google Scholar]
- 159.Koblenzer, C. S., P. J. Koblenzer, and F. A. Tausk. 2008. What is psychocutaneous medicine? Cutis 81:487. [PubMed] [Google Scholar]
- 160.Kongsamut, S., J. Kang, X. L. Chen, J. Roehr, and D. Rampe. 2002. A comparison of the receptor binding and HERG channel affinities for a series of antipsychotic drugs. Eur. J. Pharmacol. 450:37-41. [DOI] [PubMed] [Google Scholar]
- 161.Koo, J., and C. Gambla. 1996. Delusions of parasitosis and other forms of monosymptomatic hypochondriacal psychosis. General discussion and case illustrations. Dermatol. Clin. 14:429-438. [DOI] [PubMed] [Google Scholar]
- 162.Koo, J., and A. Lebwohl. 2001. Psychodermatology: the mind and skin connection. Am. Fam. Physician 64:1873-1878. [PubMed] [Google Scholar]
- 163.Koo, J., and C. S. Lee. 2001. Delusions of parasitosis. A dermatologist's guide to diagnosis and treatment. Am. J. Clin. Dermatol. 2:285-290. [DOI] [PubMed] [Google Scholar]
- 164.Krauseneck, T., and M. Soyka. 2005. Delusional parasitosis associated with pemoline. Psychopathology 38:103-104. [DOI] [PubMed] [Google Scholar]
- 165.Kumbier, E., and S. C. Herpertz. 2007. Induced delusion of parasitosis (folie à deux). A case report. Nervenheilkunde 26:1013-1017. (In German.) [Google Scholar]
- 166.Kumbier, E., and J. Höppner. 2008. The neuroleptic treatment of delusional parasitosis: first experiences with aripiprazole. Hautarzt 59:728-730, 732-733. (In German.) [DOI] [PubMed] [Google Scholar]
- 167.Kumbier, E., and M. Kornhuber. 2002. Delusional ectoparasitic infestation in multiple system atrophy. Nervenarzt 73:378-381. (In German.) [DOI] [PubMed] [Google Scholar]
- 168.Kuruppuarachchi, K. A., and S. S. Williams. 2003. Delusional parasitosis. Ceylon Med. J. 48:96-97. [DOI] [PubMed] [Google Scholar]
- 169.Lancet. 1983. The matchbox sign. Lancet ii:261. (Editorial.) [Google Scholar]
- 170.Lanczik, M., K. Hofberg, and I. F. Brockington. 1999. Delusion of infestation with post-partum onset: case report. Psychopathology 32:277-280. [DOI] [PubMed] [Google Scholar]
- 171.Langeland, T., and J. Austad. 1980. Delusions of parasitosis (parasitophobia). Tidsskr. Nor. Laegeforen. 100:1275-1276. (In Norwegian.) [PubMed] [Google Scholar]
- 172.Le, L., and P. N. Gonski. 2003. Delusional parasitosis mimicking cutaneous infestation in elderly patients. Med. J. Aust. 179:209-210. [DOI] [PubMed] [Google Scholar]
- 173.Lee, W. R. 1983. Matchbox sign. Lancet ii:457-458. (Letter.) [DOI] [PubMed] [Google Scholar]
- 174.Lepping, P., J. Delieu, R. Mellor, J. H. H. Williams, P. R. Hudson, and C. Hunter-Lavin. Antipsychotic medication and oxidative cell stress—a systematic review. J. Clin. Psychiatry, in press. [DOI] [PubMed]
- 175.Lepping, P., and R. W. Freudenmann. 2008. Delusional parasitosis: a new pathway for diagnosis and treatment. Clin. Exp. Dermatol. 33:113-117. [DOI] [PubMed] [Google Scholar]
- 176.Lepping, P., R. Gil-Candon, and R. W. Freudenmann. 2006. Amisulpride as first line treatment for delusional parasitosis. Prog. Neurol. Psychiatry 10:28. [Google Scholar]
- 177.Lepping, P., R. Gil-Candon, and R. W. Freudenmann. 2005. Delusional parasitosis treated with amisulpride. Prog. Neurol. Psychiatry 9:12-16. [Google Scholar]
- 178.Lepping, P., I. Russell, and R. W. Freudenmann. 2007. Antipsychotic treatment of delusional parasitosis: systematic review. Br. J. Psychiatry 191:198-205. [DOI] [PubMed] [Google Scholar]
- 179.Lepping, P., and M. Swinton. 2004. Borderline personality disorder associated with psychotic symptoms and parietal lobe abnormalities. Psychiatr. Prax. 31:96-99. (In German.) [DOI] [PubMed] [Google Scholar]
- 180.Leucht, S., C. Corves, D. Arbter, R. R. Engel, C. Li, and J. M. Davis. 2009. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 373:31-41. [DOI] [PubMed] [Google Scholar]
- 181.Leucht, S., K. Komossa, C. Rummel-Kluge, C. Corves, H. Hunger, F. Schmid, C. Asenjo Lobos, S. Schwarz, and J. M. Davis. 2009. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. Am. J. Psychiatry 166:152-163. [DOI] [PubMed] [Google Scholar]
- 182.Levin, M. 1967. Transitory schizophrenias produced by bromide intoxication. Am. J. Psychiatry 103:229-237. [DOI] [PubMed] [Google Scholar]
- 183.Liebaldt, G., and W. Klages. 1961. Morphologische Befunde bei einer “isolierten taktilen Dermatozoenhalluzinose.” Nervenarzt 32:157-171.13761976 [Google Scholar]
- 184.Lieberman, J. A., T. S. Stroup, J. P. McEvoy, M. S. Swartz, R. A. Rosenheck, D. O. Perkins, R. S. Keefe, S. M. Davis, C. E. Davis, B. D. Lebowitz, J. Severe, and J. K. Hsiao. 2005. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N. Engl. J. Med. 353:1209-1223. [DOI] [PubMed] [Google Scholar]
- 185.Lieberman, L. A., and D. E. Higgins. 2009. A small-molecule screen identifies the antipsychotic drug pimozide as an inhibitor of Listeria monocytogenes infection. Antimicrob. Agents Chemother. 53:756-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liebowitz, M. R., E. J. Nuetzel, A. E. Bowser, and D. F. Klein. 1978. Phenelzine and delusions of parasitosis: a case report. Am. J. Psychiatry 135:1565-1566. [DOI] [PubMed] [Google Scholar]
- 187.Lim, G. C., Y. F. Chen, L. Liu, S. C. Huang, K. K. Lin, and C. H. Hsiao. 2006. Camphor-related self-inflicted keratoconjunctivitis complicating delusions of parasitosis. Cornea 25:1254-1256. [DOI] [PubMed] [Google Scholar]
- 188.Lindskov, R., and O. Baadsgaard. 1985. Delusions of infestation treated with pimozide: a follow-up study. Acta Dermatol. Venereol. 65:267-270. [PubMed] [Google Scholar]
- 189.Lorenzo, C. R., and J. Koo. 2004. Pimozide in dermatologic practice: a comprehensive review. Am. J. Clin. Dermatol. 5:339-349. [DOI] [PubMed] [Google Scholar]
- 190.Lyell, A. 1983. Delusions of parasitosis. J. Am. Acad. Dermatol. 8:895-897. [DOI] [PubMed] [Google Scholar]
- 191.Lyell, A. 1983. Delusions of parasitosis. Semin. Dermatol. 2:189-195. [Google Scholar]
- 192.Lyell, A. 1983. Delusions of parasitosis (the Michelson Lecture). Br. J. Dermatol. 108:485-499. [DOI] [PubMed] [Google Scholar]
- 193.Lynch, P. J. 1993. Delusions of parasitosis. Semin. Dermatol. 12:39-45. [PubMed] [Google Scholar]
- 194.MacNamara, E. D. 1928. Cutaneous and visual hallucinations. Lancet i:807-808. [Google Scholar]
- 195.Maeda, K., Y. Yamamoto, M. Yasuda, and K. Ishii. 1998. Delusions of oral parasitosis. Prog. Neuropsychopharmacol. Biol. Psychiatry 22:243-248. [DOI] [PubMed] [Google Scholar]
- 196.Magnan, V., and M. Saury. 1889. Trois cas de cocainisme chronique. C. R. Seances Soc. Biol. 1889:60-63. [Google Scholar]
- 197.Mahgoub, N. A. 2008. Successful treatment of delusion of parasitosis with risperidone. J. Neuropsychiatr. Clin. Neurosci. 20:112-113. [DOI] [PubMed] [Google Scholar]
- 198.Maier, C. 1987. Zum Problem des Dermatozoenwahnsyndroms. Nervenarzt 58:107-115. [PubMed] [Google Scholar]
- 199.Makhija, M., and S. Bhalerao. 2004. Reconsidering pimozide for new-onset delusions of parasitosis. Can. J. Psychiatry 49:643-644. [DOI] [PubMed] [Google Scholar]
- 200.Mallet, R., and P. Male. 1930. Délire cénesthesique. Ann. Med. Psychol. 88:198-201. [Google Scholar]
- 201.Manschreck, T. C. 1996. Delusional disorder: the recognition and management of paranoia. J. Clin. Psychiatry 57(Suppl. 3):32-38. [PubMed] [Google Scholar]
- 202.Manschreck, T. C., and N. L. Khan. 2006. Recent advances in the treatment of delusional disorder. Can. J. Psychiatry 51:114-119. [DOI] [PubMed] [Google Scholar]
- 203.Marneros, A., A. Deister, and A. Rohde. 1988. Delusional parasitosis. A comparative study to late-onset schizophrenia and organic mental disorders due to cerebral arteriosclerosis. Psychopathology 21:267-274. [DOI] [PubMed] [Google Scholar]
- 204.Marris, E. 2006. Mysterious ‘Morgellons disease’ prompts US investigation. Nat. Med. 12:982. [DOI] [PubMed] [Google Scholar]
- 205.Marschall, M. A., R. F. Dolezal, M. Cohen, and S. F. Marschall. 1991. Chronic wounds and delusions of parasitosis in the drug abuser. Plast. Reconstr. Surg. 88:328-330. [DOI] [PubMed] [Google Scholar]
- 206.Martin, W. J. 2005. Alternative cellular energy pigments mistaken for parasitic skin infestations. Exp. Mol. Pathol. 78:212-214. [DOI] [PubMed] [Google Scholar]
- 207.May, W. W., and M. S. Terpenning. 1991. Delusional parasitosis in geriatric patients. Psychosomatics 32:88-94. [DOI] [PubMed] [Google Scholar]
- 208.McAndrews, J., R. Jung, and V. Derbes. 1956. Delusions of dermal parasitosis (acaraphobia) manifested by a folie à deux. J. La. State Med. Soc. 108:279-288. [PubMed] [Google Scholar]
- 209.McKay, R., R. Langdon, and M. Coltheart. 2005. Paranoia, persecutory delusions and attributional biases. Psychiatry Res. 136:233-245. [DOI] [PubMed] [Google Scholar]
- 210.McLaughlin, J. A., and A. Sims. 1984. Co-existence of the Capgras and Ekbom syndromes. Br. J. Psychiatry 145:439-441. [DOI] [PubMed] [Google Scholar]
- 211.Meehan, W. J., S. Badreshia, and C. L. Mackley. 2006. Successful treatment of delusions of parasitosis with olanzapine. Arch. Dermatol. 142:352-355. [DOI] [PubMed] [Google Scholar]
- 212.Mercan, S., I. K. Altunay, N. Taskintuna, O. Ogutcen, and S. Kayaoglu. 2007. Atypical antipsychotic drugs in the treatment of delusional parasitosis. Int. J. Psychiatry Med. 37:29-37. [DOI] [PubMed] [Google Scholar]
- 213.Mercan, S., Ö. Ögütcen, I. K. Altunay, and O. Karamustafalioglu. 2005. Folie à famille ve Delüzyonel Parazitoz: Olgu Sunumu ve Literatürün Gözden Gecirilmesi. Klin. Psikofarmakol. Bül. 15:71-78. [Google Scholar]
- 214.Mester, H. 1977. Das Syndrom des wahnhaften Ungezieferbefalles. Angew. Parasitol. 18:70-84. [PubMed] [Google Scholar]
- 215.Mester, H. 1981. Der Ungezieferwahn-ein Beitrag über die Ätiologie und den Aufbau dieser Halluzinose. Fortschr. Neurol. Psychiatr. 49:136-144. [DOI] [PubMed] [Google Scholar]
- 216.Mester, H. 1975. Induced“acarophobia.” Psychiatr. Clin. 8:339-348. [PubMed] [Google Scholar]
- 217.Meumann, E. 1932. Psychosen bei Hypophysentumoren. Arch. Psychiatr. Nervenkrankh. 96:609-622. [Google Scholar]
- 218.Milia, A., M. G. Mascia, G. Pilia, A. Paribello, D. Murgia, E. Cocco, and M. G. Marrosu. 2008. Efficacy and safety of quetiapine treatment for delusional parasitosis: experience in an elderly patient. Clin. Neuropharmacol. 31:310-312. [DOI] [PubMed] [Google Scholar]
- 219.Miller-Kreuser, E. 1962. Symptombild der chronischen taktilen Halluzinose bei Hypophysenveränderung. Med. Welt. 2:88-91.14474125 [Google Scholar]
- 220.Mitchell, C. 1989. Successful treatment of chronic delusional parasitosis. Br. J. Psychiatry 155:556-557. [DOI] [PubMed] [Google Scholar]
- 221.Mitchell, J., and A. D. Vierkant. 1991. Delusions and hallucinations of cocaine abusers and paranoid schizophrenics: a comparative study. J. Psychol. 125:301-310. [DOI] [PubMed] [Google Scholar]
- 222.Molyneux, J. 2008. AKA ‘Morgellons.’ Am. J. Nurs. 108:25-26. [DOI] [PubMed] [Google Scholar]
- 223.Monaghan, E. 19 May 2006. All in the head? Times Online, London, United Kingdom. http://www.timesonline.co.uk/tol/comment/article721795.ece.
- 224.Monk, B. E., and Y. J. Rao. 1994. Delusions of parasitosis with fatal outcome. Clin. Exp. Dermatol. 19:341-342. [DOI] [PubMed] [Google Scholar]
- 225.Moretti, M., and G. Varga. 2000. Psychosomatic dermatologic symptoms. Orv. Hetil. 141:169-172. (In Hungarian.) [PubMed] [Google Scholar]
- 226.Morris, M. 1991. Delusional infestation. Br. J. Psychiatry 1991(Suppl.):83-87. [PubMed] [Google Scholar]
- 227.Munro, A. 1982. Delusional hypochondriasis. A description of monosymptomatic hypochondriacal psychosis (MHP). Clarke Institute of Psychiatry Monograph Series, Department of Psychiatry, University of Toronto, Toronto, Ontario, Canada.
- 228.Munro, A. 1983. Delusional parasitosis: a form of monosymptomatic hypochondriacal psychosis. Semin. Dermatol. 2:197-202. [Google Scholar]
- 229.Munro, A. 1978. Monosymptomatic hypochondriacal psychoses: a diagnostic entity which may respond to pimozide. Can. Psychiatr. Assoc. J. 23:497-500. [DOI] [PubMed] [Google Scholar]
- 230.Munro, A. 1980. Monosymptomatic hypochondriacal psychosis. Br. J. Hosp. Med. 24:34, 36-38. [PubMed] [Google Scholar]
- 231.Munro, A. 1988. Monosymptomatic hypochondriacal psychosis. Br. J. Psychiatry 1988(Suppl.):37-40. [PubMed] [Google Scholar]
- 232.Munro, A. 1978. Two cases of delusions of worm infestation. Am. J. Psychiatry 135:234-235. [DOI] [PubMed] [Google Scholar]
- 233.Murase, J. E., J. J. Wu, and J. Koo. 2006. Morgellons disease: a rapport-enhancing term for delusions of parasitosis. J. Am. Acad. Dermatol. 55:913-914. [DOI] [PubMed] [Google Scholar]
- 234.Musalek, M. 1991. Der Dermatozoenwahn. Thieme, Stuttgart, Germany.
- 235.Musalek, M., M. Bach, K. Gerstberger, O. M. Lesch, V. Passweg, J. Wancata, and H. Walter. 1989. Drug therapy of delusional parasitosis. The importance of differential diagnosis for psychopharmacologic treatment of patients with delusional parasitosis. Wien. Med. Wochenschr. 139:297-302. (In German.) [PubMed] [Google Scholar]
- 236.Musalek, M., M. Bach, V. Passweg, and S. Jaeger. 1990. The position of delusional parasitosis in psychiatric nosology and classification. Psychopathology 23:115-124. [DOI] [PubMed] [Google Scholar]
- 237.Musalek, M., J. Grunberger, O. M. Lesch, L. Linzmayer, H. Walter, and W. Gebhart. 1988. Psychopathologie der Dermatozoenwahnkranken. Nervenarzt 59:603-609. [PubMed] [Google Scholar]
- 238.Musalek, M., and E. Kutzer. 1990. The frequency of shared delusions in delusions of infestation. Eur. Arch. Psychiatry Neurol. Sci. 239:263-266. [DOI] [PubMed] [Google Scholar]
- 239.Musalek, M., and E. Kutzer. 1989. Psychiatric and parasitologic aspects of dermatozoon delusion. Wien. Klin. Wochenschr. 101:153-160. [PubMed] [Google Scholar]
- 240.Musalek, M., I. Podreka, H. Walter, E. Suess, V. Passweg, D. Nutzinger, R. Strobl, and O. M. Lesch. 1989. Regional brain function in hallucinations: a study of regional cerebral blood flow with 99m-Tc-HMPAO-SPECT in patients with auditory hallucinations, tactile hallucinations, and normal controls. Compr. Psychiatry 30:99-108. [DOI] [PubMed] [Google Scholar]
- 241.Myerson, A. 1921. Two cases of acarophobia. Boston Med. Surg. J. 1921:184. [Google Scholar]
- 242.Nagaratnam, N., and L. O'Neile. 2000. Delusional parasitosis following occipito-temporal cerebral infarction. Gen. Hosp. Psychiatry 22:129-132. (Letter.) [DOI] [PubMed] [Google Scholar]
- 243.Narayana Godwa, B. S., S. Heebbar, and M. T. Sathyanarayan. 2002. Delusional parasitosis responding to risperidone. Indian J. Psychiatry 44:382-383. [PMC free article] [PubMed] [Google Scholar]
- 244.Narumoto, J., H. Ueda, H. Tsuchida, T. Yamashita, Y. Kitabayashi, and K. Fukui. 2006. Regional cerebral blood flow changes in a patient with delusional parasitosis before and after successful treatment with risperidone: a case report. Prog. Neuropsychopharmacol. Biol. Psychiatry 30:737-740. [DOI] [PubMed] [Google Scholar]
- 245.Nejad, A. G., and K. Toofani. 2005. Delusion of oral parasitosis in a patient with major depressive disorder. Can. J. Psychiatry 50:301-302. [DOI] [PubMed] [Google Scholar]
- 246.Nel, M., J. P. Schoeman, and R. G. Lobetti. 2001. Delusions of parasitosis in clients presenting pets for veterinary care. J. S. Afr. Vet. Assoc. 72:167-169. [DOI] [PubMed] [Google Scholar]
- 247.Nevo, A., and A. Bleich. 1996. Pruritus due to obstructive jaundice, with delusions of parasitosis. Harefuah 130:93-94, 143. [PubMed] [Google Scholar]
- 248.Ng, F., M. Berk, O. Dean, and A. I. Bush. 2008. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int. J. Neuropsychopharmacol. 11:851-876. [DOI] [PubMed] [Google Scholar]
- 249.Nicolato, R., H. Correa, M. A. Romano-Silva, and A. L. Teixeira, Jr. 2006. Delusional parasitosis or Ekbom syndrome: a case series. Gen. Hosp. Psychiatry 28:85-87. [DOI] [PubMed] [Google Scholar]
- 250.Ohtaki, N. 1991. Delusions of parasitosis—report of 94 cases. Nippon Hifuka Gakkai Zasshi 101:439-446. [PubMed] [Google Scholar]
- 251.O'Keeffe, S. T. 1995. Delusions of infestation during delirium in a patient with restless legs syndrome. J. Geriatr. Psychiatry Neurol. 8:120-122. [DOI] [PubMed] [Google Scholar]
- 252.Oruc, L., and P. Bell. 1995. Multiple rape trauma followed by delusional parasitosis. A case report from the Bosnian war. Schizophr. Res. 16:173-174. (Letter.) [DOI] [PubMed] [Google Scholar]
- 253.Ozkan, A. T., and K. Y. Mumcuoglu. 2008. Entomophobia and delusional parasitosis. Turkiye Parazitol. Derg. 32:366-370. [PubMed] [Google Scholar]
- 254.Pacan, P., A. Reich, and J. C. Szepietowski. 2004. Delusional parasitosis successfully controlled with risperidone—two case reports. Dermatol. Psychosom. 5:193-195. [Google Scholar]
- 255.Paholpak, S. 1990. Delusion of parasitosis: a report of ten cases at Srinagarind Hospital. J. Med. Assoc. Thai. 73:111-114. [PubMed] [Google Scholar]
- 256.Paquette, M. 2007. Morgellons: disease or delusions? Perspect. Psychiatr. Care 43:67-68. [DOI] [PubMed] [Google Scholar]
- 257.Partridge, M. 1950. One operation cures three people. Effect of prefrontal leucotomy on a case of folie a deux et demie. Arch. Neurol. Psychiatr. 64:792-796. [PubMed] [Google Scholar]
- 258.Pavlovsky, F., V. Peskin, L. Di Noto, and J. C. Stagnaro. 2008. Delusion of parasitosis: report of twelve cases. Vertex 19:99-111. [PubMed] [Google Scholar]
- 259.Perrin, L. 1896. Des névrodermies parasitophobiques. Ann. Dermatol. Syphiligr. (Paris) 7:129-138. [Google Scholar]
- 260.Persico, A. M., and M. Catalano. 1998. Lack of association between dopamine transporter gene polymorphisms and delusional disorder. Am. J. Med. Genet. 81:163-165. [PubMed] [Google Scholar]
- 261.Pierce, D. W. 1921. Sanitary entomology. Richard D. Badger, Boston, MA.
- 262.Podoll, K., F. Bofinger, B. von der Stein, W. Stuhlmann, and C. Kretschmar. 1993. Delusions of intestinal parasitosis in a female patient with endogenous depression. Fortschr. Neurol. Psychiatr. 61:62-66. [DOI] [PubMed] [Google Scholar]
- 263.Pope, F. M. 1970. Parasitophobia as the presenting symptom of vitamin B12 deficiency. Practitioner 204:421-422. [PubMed] [Google Scholar]
- 264.Prakash, R., S. Gandotra, L. K. Singh, B. Das, and A. Lakra. 2008. Rapid resolution of delusional parasitosis in pellagra with niacin augmentation therapy. Gen. Hosp. Psychiatry 30:581-584. [DOI] [PubMed] [Google Scholar]
- 265.Rathbone, J., and T. McMonagle. 2007. Pimozide for schizophrenia or related psychoses. Cochrane Database Syst. Rev. 2007:CD001949. [DOI] [PubMed] [Google Scholar]
- 266.Reichenberg, J. S., M. Magid, and L. A. Drage. 2007. A cure for delusions of parasitosis. J. Eur. Acad. Dermatol. Venereol. 21:1423-1424. [DOI] [PubMed] [Google Scholar]
- 267.Reilly, J. G., S. A. Ayis, I. N. Ferrier, S. J. Jones, and S. H. Thomas. 2000. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet 355:1048-1052. [DOI] [PubMed] [Google Scholar]
- 268.Reilly, T., and D. Batchelor. 1986. The presentation and treatment of delusional parasitosis: a dermatological perspective. Int. Clin. Psychopharmacol. 1:340-353. [DOI] [PubMed] [Google Scholar]
- 269.Reilly, T. M., W. H. Jopling, and A. W. Beard. 1978. Successful treatment with pimozide of delusional parasitosis. Br. J. Dermatol. 98:457-459. [DOI] [PubMed] [Google Scholar]
- 270.Renvoize, E. B., J. Kent, and H. M. Klar. 1987. Delusional infestation and dementia: a case report. Br. J. Psychiatry 150:403-405. [DOI] [PubMed] [Google Scholar]
- 271.Riding, B. E., and A. Munro. 1975. Pimozide in monosymptomatic psychosis. Lancet i:400-401. (Letter.) [DOI] [PubMed] [Google Scholar]
- 272.Riding, J., and A. Munro. 1975. Pimozide in the treatment of monosymptomatic hypochondriacal psychosis. Acta Psychiatr. Scand. 52:23-30. [DOI] [PubMed] [Google Scholar]
- 273.Robaeys, G., J. De Bie, M. Van Ranst, and F. Buntinx. 2007. An extremely rare case of delusional parasitosis in a chronic hepatitis C patient during pegylated interferon alpha-2b and ribavirin treatment. World J. Gastroenterol. 13:2379-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Robertson, K. S. 12 October 2007, posting date. Old problem, new name? Br. J. Psychiatry 191:198-205. (eLetter.) http://bjp.rcpsych.org/cgi/eletters/191/3/198.17766758 [Google Scholar]
- 275.Robles, D. T., S. Romm, H. Combs, J. Olson, and P. Kirby. 2008. Delusional disorders in dermatology: a brief review. Dermatol. Online J. 14:2. [PubMed] [Google Scholar]
- 276.Rocha, F. L., and C. Hara. 2007. Aripiprazole in delusional parasitosis: case report. Prog. Neuropsychopharmacol. Biol. Psychiatry 31:784-786. [DOI] [PubMed] [Google Scholar]
- 277.Ryan, C. J., G. de Moore, and M. Patfield. 1995. Becoming none but tradesmen: lies, deception and psychotic patients. J. Med. Ethics 21:72-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Safer, D. L., B. Wenegrat, and W. T. Roth. 1997. Risperidone in the treatment of delusional parasitosis: a case report. J. Clin. Psychopharmacol. 17:131-132. (Letter.) [DOI] [PubMed] [Google Scholar]
- 279.Salzman, C., D. V. Jeste, R. E. Meyer, J. Cohen-Mansfield, J. Cummings, G. T. Grossberg, L. Jarvik, H. C. Kraemer, B. D. Lebowitz, K. Maslow, B. G. Pollock, M. Raskind, S. K. Schultz, P. Wang, J. M. Zito, and G. S. Zubenko. 2008. Elderly patients with dementia-related symptoms of severe agitation and aggression: consensus statement on treatment options, clinical trials methodology, and policy. J. Clin. Psychiatry 69:889-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Sandoz, A., M. LoPiccolo, D. Kusnir, and F. A. Tausk. 2008. A clinical paradigm of delusions of parasitosis. J. Am. Acad. Dermatol. 59:698-704. [DOI] [PubMed] [Google Scholar]
- 281.Savely, V. R., M. M. Leitao, and R. B. Stricker. 2006. The mystery of Morgellons disease: infection or delusion? Am. J. Clin. Dermatol. 7:1-5. [DOI] [PubMed] [Google Scholar]
- 282.Scheinfeld, N. 2003. Delusions of parasitiosis: a case with a review of its course and treatment. Skinmed 2:376-378. [DOI] [PubMed] [Google Scholar]
- 283.Schilder, P. 1927. Psychische Symptome bei Mittel-und Zwischenhirnerkrankungen. Wien. Klin. Wochenschr. 36:1147-1148. [Google Scholar]
- 284.Schneider, L. S., P. N. Tariot, K. S. Dagerman, S. M. Davis, J. K. Hsiao, M. S. Ismail, B. D. Lebowitz, C. G. Lyketsos, J. M. Ryan, T. S. Stroup, D. L. Sultzer, D. Weintraub, and J. A. Lieberman. 2006. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's disease. N. Engl. J. Med. 355:1525-1538. [DOI] [PubMed] [Google Scholar]
- 285.Schooler, N. R., J. Levine, J. B. Severe, B. Brauzer, A. DiMascio, G. L. Klerman, and V. B. Tuason. 1980. Prevention of relapse in schizophrenia. An evaluation of fluphenazine decanoate. Arch. Gen. Psychiatry 37:16-24. [DOI] [PubMed] [Google Scholar]
- 286.Schrut, A. H., and W. G. Waldron. 1963. Psychiatric and entomological aspects of delusory parasitosis. Entomophobia, acarophobia, dermatophobia. JAMA 186:429-430. [DOI] [PubMed] [Google Scholar]
- 287.Schuller, R. A., and L. Skorin. 2006. Floppy eyelid syndrome and conjunctivits in a patient with delusional parasitosis. Clin. Refract. Optom. 17:440-442. [Google Scholar]
- 288.Schwartz, E., E. Witztum, and K. Y. Mumcuoglu. 2001. Travel as a trigger for shared delusional parasitosis. J. Travel Med. 8:26-28. [DOI] [PubMed] [Google Scholar]
- 289.Schwarz, H. 1929. Circumscripte Hypochondrien. Monatsschr. Psychiatr. Neurol. 72:150-164. [Google Scholar]
- 290.Schwarz, H. 1959. Circumskripte Hypochondrie, Dermatozoenwahn oder taktile Halluzinose? Nervenarzt 30:203-211. [PubMed] [Google Scholar]
- 291.Sei, Y., R. Ren-Patterson, Z. Li, E. M. Tunbridge, M. F. Egan, B. S. Kolachana, and D. R. Weinberger. 2007. Neuregulin1-induced cell migration is impaired in schizophrenia: association with neuregulin1 and catechol-o-methyltransferase gene polymorphisms. Mol. Psychiatry 12:946-957. [DOI] [PubMed] [Google Scholar]
- 292.Serretti, A., R. Lilli, C. Lorenzi, E. Lattuada, and E. Smeraldi. 2001. DRD4 exon 3 variants associated with delusional symptomatology in major psychoses: a study on 2,011 affected subjects. Am. J. Med. Genet. 105:283-290. [DOI] [PubMed] [Google Scholar]
- 293.Shah, P. A. 1988. Delusions of parasitosis. South. Med. J. 81:939-940. [DOI] [PubMed] [Google Scholar]
- 294.Shelley, W. B., and E. D. Shelley. 1988. Delusions of parasitosis associated with coronary bypass surgery. Br. J. Dermatol. 118:309-310. (Letter.) [DOI] [PubMed] [Google Scholar]
- 295.Sheppard, N. P., S. O'Loughlin, and J. P. Malone. 1986. Psychogenic skin disease: a review of 35 cases. Br. J. Psychiatry 149:636-643. [DOI] [PubMed] [Google Scholar]
- 296.Sherman, M. D., G. N. Holland, D. S. Holsclaw, J. M. Weisz, O. H. Omar, and R. A. Sherman. 1998. Delusions of ocular parasitosis. Am. J. Ophthalmol. 125:852-856. [DOI] [PubMed] [Google Scholar]
- 297.Siegel, R. K. 1978. Cocaine hallucinations. Am. J. Psychiatry 135:309-314. [DOI] [PubMed] [Google Scholar]
- 298.Sirota, P., and Y. Melamed. 1994. Delusions of parasitosis. Harefuah 127:336-339. (In Hebrew.) [PubMed] [Google Scholar]
- 299.Skott, A. 1978. Delusions of infestation. Dermatozoenwahn-Ekbom's syndrome. Report from the Psychiatric Research Center Number 13. St. Jörgen Hospital, University of Goteborg, Goteborg, Sweden.
- 300.Slaughter, J. R., K. Zanol, H. Rezvani, and J. Flax. 1998. Psychogenic parasitosis. A case series and literature review. Psychosomatics 39:491-500. [DOI] [PubMed] [Google Scholar]
- 301.Sojka, E., and Z. Afeltowicz. 1978. Ekbom's syndrome in a patient with mitral valve disease and subacute endocarditis. Pol. Tyg. Lek. 33:691-692. [PubMed] [Google Scholar]
- 302.Soyka, M., W. Koch, and K. Tatsch. 2005. Thalamic hypofunction in alcohol hallucinosis: FDG PET findings. Psychiatry Res. 139:259-262. [DOI] [PubMed] [Google Scholar]
- 303.Spensley, J., and D. A. Rockwell. 1972. Psychosis during methylphenidate abuse. N. Engl. J. Med. 286:880-881. [DOI] [PubMed] [Google Scholar]
- 304.Srinivasan, T. N., T. R. Suresh, V. Jayaram, and M. P. Fernandez. 1994. Nature and treatment of delusional parasitosis: a different experience in India. Int. J. Dermatol. 33:851-855. [DOI] [PubMed] [Google Scholar]
- 305.Stahl, S. M. 2006. Essential psychopharmacology. The prescriber's guide. Cambridge University Press, Cambridge, United Kingdom.
- 306.Steinert, T., and H. Studemund. 2006. Acute delusional parasitosis under treatment with ciprofloxacin. Pharmacopsychiatry 39:159-160. [DOI] [PubMed] [Google Scholar]
- 307.Strandberg, J. 1925. Psyche und Hautkrankheiten, p. 258. In Psychogenese und Psychotherapie körperlicher Symptome. Oswald Schwarz, Vienna, Austria.
- 308.Sugahara, H., Y. Otani, and M. Sakamoto. 2000. Delusional parasitosis accompanied by word deafness due to cerebral infarction: folie a deux. Psychosomatics 41:447-448. [DOI] [PubMed] [Google Scholar]
- 309.Sultana, A., and T. McMonagle. 2000. Pimozide for schizophrenia or related psychoses. Cochrane Database Syst. Rev. 2000:CD001949. [DOI] [PubMed] [Google Scholar]
- 310.Sultzer, D. L., S. M. Davis, P. N. Tariot, K. S. Dagerman, B. D. Lebowitz, C. G. Lyketsos, R. A. Rosenheck, J. K. Hsiao, J. A. Lieberman, and L. S. Schneider. 2008. Clinical symptom responses to atypical antipsychotic medications in Alzheimer's disease: phase 1 outcomes from the CATIE-AD effectiveness trial. Am. J. Psychiatry 165:844-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Swick, B. L., and H. W. Walling. 2005. Drug-induced delusions of parasitosis during treatment of Parkinson's disease. J. Am. Acad. Dermatol. 53:1086-1087. [DOI] [PubMed] [Google Scholar]
- 312.Szepietowski, J. C., J. Salomon, E. Hrehorow, P. Pacan, A. Zalewska, and A. Sysa-Jedrzejowska. 2007. Delusional parasitosis in dermatological practice. J. Eur. Acad. Dermatol. Venereol. 21:462-465. [DOI] [PubMed] [Google Scholar]
- 313.Takahashi, P. Y., and L. Krajn. 1999. Case report: delusions of parasitosis as an unusual presenting feature of dementia. Ann. Long Term Care 7:153-155. [Google Scholar]
- 314.Takahashi, T., H. Ozawa, S. Inuzuka, Y. Harada, T. Hanihara, and N. Amano. 2003. Sulpiride for treatment of delusion of parasitosis. Psychiatry Clin. Neurosci. 57:552-553. [DOI] [PubMed] [Google Scholar]
- 315.Takeda, M., S. Tanino, K. Nishinuma, T. Matsubayashi, S. Yamashita, and T. Nishimura. 1985. A case of hypophyseal prolactinoma with treatable delusions of dermatozoiasis. Acta Psychiatr. Scand. 72:470-475. [DOI] [PubMed] [Google Scholar]
- 316.Tenyi, T., R. Herold, S. Fekete, A. Kovacs, and M. Trixler. 2001. Coexistence of delusions of pregnancy and infestation in a male. Psychopathology 34:215-216. [DOI] [PubMed] [Google Scholar]
- 317.Tenyi, T., and M. Trixler. 1990. The Ekbom symptom in schizophrenic psychoses. Orv. Hetil. 131:2575-2578. (In Hungarian.) [PubMed] [Google Scholar]
- 318.Tenyi, T., and M. Trixler. 1993. Coexistence of the delusions of infestation and body smell in schizophrenia: a case report. Psychopathology 26:292-293. [DOI] [PubMed] [Google Scholar]
- 319.Thibierge, G. 1894. Les acarophobes. Rev. Gen. Clin. Ther. 32:373-376. [Google Scholar]
- 320.Torch, E. M., and E. R. Bishop, Jr. 1981. Delusions and parasitosis: psychotherapeutic engagement. Am. J. Psychother. 35:101-106. [DOI] [PubMed] [Google Scholar]
- 321.Trabert, W. 1997. 100 years of delusional parasitosis. Med. Welt. 48:33-37. (In German.) [Google Scholar]
- 322.Trabert, W. 1995. 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology 28:238-246. [DOI] [PubMed] [Google Scholar]
- 323.Trabert, W. 1993. Delusional parasitosis. Studies on frequency, classification and prognosis. Dissertation. Universität des Saarlandes, Homburg/Saar, Germany. (In German.)
- 324.Trabert, W. 1985. Dermatozoon delusion in hypoparathyroidism—reflections on the genesis of delusions. Psychiatr. Neurol. Med. Psychol. (Leipzig) 37:648-655. (In German.) [PubMed] [Google Scholar]
- 325.Trabert, W. 1991. Epidemiology of delusional ectoparasitic infestation. Nervenarzt 62:165-169. (In German.) [PubMed] [Google Scholar]
- 326.Trabert, W. 1999. Shared psychotic disorder in delusional parasitosis. Psychopathology 32:30-34. [DOI] [PubMed] [Google Scholar]
- 327.Trager, M. J., T. N. Hwang, and T. J. McCulley. 2008. Delusions of parasitosis of the eyelids. Ophthal. Plast. Reconstr. Surg. 24:317-319. [DOI] [PubMed] [Google Scholar]
- 328.Traver, J. R. 1951. Unusual scalp dermatitis in humans caused by the mite Dermatophagoides. Entomol. Soc. Wash. 53:1-25. [Google Scholar]
- 329.Tse, K. C., F. K. Li, S. Tang, M. F. Lam, T. M. Chan, and K. N. Lai. 2001. Delusion of worm infestation associated with clarithromycin in a patient on peritoneal dialysis. Perit. Dial. Int. 21:415-416. [PubMed] [Google Scholar]
- 330.Tullett, G. L. 1965. Delusions of parasitosis. Br. J. Dermatol. 77:448-455. [DOI] [PubMed] [Google Scholar]
- 331.Ungvari, G. 1984. Treatment of a dermatozoon delusion with neuroleptics. Psychiatr. Prax. 11:116-119. (In German.) [PubMed] [Google Scholar]
- 332.Ungvari, G. 1983. Neuroleptic treatment of delusions of parasitic infestation. Ideggyogy Sz. 36:391-397. (In Hungarian.) [Google Scholar]
- 333.Ungvari, G., and B. Pethö. 1980. Zur Pathogenese des Dermatozoenwahns. Psychiatr. Neurol. Med. Psychol. 32:353-358. [PubMed] [Google Scholar]
- 334.Ungvari, G., and K. Vladar. 1986. Pimozide treatment for delusion of infestation. Act. Nerv. Super. (Prague) 28:103-107. [PubMed] [Google Scholar]
- 335.Ungvari, G., and K. Vladar. 1984. Pimozide therapy in dermatozoon delusion. Dermatol. Monatsschr. 170:443-447. (In German.) [PubMed] [Google Scholar]
- 336.Valbuena Briones, A., J. J. Avila Escribano, C. de Dios Perrino, and M. Castaneda Saiz de Jauregui. 1984. Dermatozoic (parasitic) delusion. Med. Clin. 83:249-252. [PubMed] [Google Scholar]
- 337.Vila-Rodriguez, F., and B. G. Macewan. 2008. Delusional parasitosis facilitated by web-based dissemination. Am. J. Psychiatry 165:1612. [DOI] [PubMed] [Google Scholar]
- 338.Waldron, W. G. 1962. The role of the entomologist in delusional parasitosis (entomophobia). Bull. Entomol. Soc. Am. 8:81-83. [Google Scholar]
- 339.Walling, H. W., and B. L. Swick. 2007. Psychocutaneous syndromes: a call for revised nomenclature. Clin. Exp. Dermatol. 32:317-319. [DOI] [PubMed] [Google Scholar]
- 340.Weinberger, D. R. 1987. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry 44:660-669. [DOI] [PubMed] [Google Scholar]
- 341.Wenning, M. T., L. E. Davy, G. Catalano, and M. C. Catalano. 2003. Atypical antipsychotics and the treatment of delusional parasitosis. Ann. Clin. Psychiatry 15:233-239. [DOI] [PubMed] [Google Scholar]
- 342.Wilhelmi, J. 1935. Ungezieferwahn. Med. Welt. 9:351-354. [Google Scholar]
- 343.Willan, R. 1799. Die Hautkrankheiten und ihre Behandlung. Systematisch beschrieben (Übersetzung aus dem Englischen von Friese F.G.). J. F. Korn, Breslau, Germany.
- 344.Wilson, J. W., and H. E. Miller. 1946. Delusions of parasitosis (acarophobia). Arch. Dermatol. 54:39-56. [DOI] [PubMed] [Google Scholar]
- 345.Winkler, L. 1957. Der wahnhafte Ungezieferbefall. Z. Haut Geschlechtskr. 22:47-52. [PubMed] [Google Scholar]
- 345a.World Health Organization. 2007. International statistical classification of diseases and related health problems, 10th revision. WHO, Geneva, Switzerland. http://apps.who.int/classifications/apps/icd/icd10online/.
- 346.Wykoff, R. F. 1987. Delusions of parasitosis: a review. Rev. Infect. Dis. 9:433-437. [DOI] [PubMed] [Google Scholar]
- 347.Yamashita, T. 1986. Treatment of delusions of parasitosis: efficacy of transcutaneous electrical stimulation. Seishin Shinkeigaku Zasshi 88:1-13. [PubMed] [Google Scholar]
- 348.Yorston, G. 1997. Treatment of delusional parasitosis with sertindole. Int. J. Geriatr. Psychiatry 12:1127-1128. (Letter.) [DOI] [PubMed] [Google Scholar]
- 349.Yosipovitch, G., and L. S. Samuel. 2008. Neuropathic and psychogenic itch. Dermatol. Ther. 21:32-41. [DOI] [PubMed] [Google Scholar]
- 350.Zamperetti, M., and G. F. Goldwurm. 1988. Delirio dermatozoico di Ekbom in un soggetto HIV positivo e cirrptico successivamente indotto nei familiari. Riv. Psichiatr 1988:135-141. [Google Scholar]
- 351.Zanol, K., J. Slaughter, and R. Hall. 1998. An approach to the treatment of psychogenic parasitosis. Int. J. Dermatol. 37:56-63. [DOI] [PubMed] [Google Scholar]
- 352.Ziese, P. 1967. Dermatozoenwahn bei konfabulatorischer Paraphrenie. Munch. Med. Wochenschr. 49:2584-2587. [PubMed] [Google Scholar]
- 353.Zomer, S. F., R. F. De Wit, J. E. Van Bronswijk, G. Nabarro, and W. A. Van Vloten. 1998. Delusions of parasitosis. A psychiatric disorder to be treated by dermatologists? An analysis of 33 patients. Br. J. Dermatol. 138:1030-1032. [DOI] [PubMed] [Google Scholar]