Abstract
Viral antigens complexed to heat shock proteins (HSPs) can enhance antiviral immunity. The present study evaluated the immunogenicity of a novel human immunodeficiency virus type 1B′ (HIV-1B′)-specific, human leukocyte antigen A2 (HLA-A2)-restricted peptide (FLQSRPEPTA, Gag448-457) and the cellular immune adjuvant effect of HSP gp96 using the HLA-A2 transgenic mouse model. It was found that gp96 could augment cytotoxic-T-lymphocyte responses specific for the 10-mer peptide of HIV-1B′. This study also evaluated the humoral immune adjuvant effect of HSP gp96 and its N-terminal fragment (N336) and found that immunization of BALB/c mice with a mixture of gp96 or its N-terminal fragment and HIV-1 p24 antigen or with an p24-N336 fusion protein resulted in a significant increase in anti-HIV p24 antibody titer. These results demonstrate the possibility of using gp96 and its N fragment as adjuvants to augment cellular and humoral immune responses against HIV-1 infection.
The heat shock protein (HSP) gp96 is a 96-kDa glycoprotein of the endoplasmic reticulum that is believed to be involved in antigen processing as an intermediate carrier of peptides. The mechanism of immunogenicity of gp96-peptide complexes involves the interaction of the gp96 with macrophages or dendritic cells through the receptor CD91 (5), followed by re-presentation of the gp96-chaperoned peptides by the major histocompatibility complex class I (MHC class I) and MHC class II molecules of the macrophage or dendritic cells (6, 11).
gp96-tumor antigen complexes induce antigen-specific cytotoxic-T-lymphocyte (CTL) responses against tumor cells (10, 16, 24), suggesting that these agents may be useful in vaccine development. Currently, human vaccine trials aimed at inducing tumor-specific immunity are in progress (4, 20, 21).
HSPs bound to viral antigens can enhance antiviral immunity, including NK activity, antibody-dependent cellular cytotoxicity, and CTL activities (2, 9, 23). The immunization of mice with HIV-1 p24 covalently linked to mycobacterial Hsp70 has been shown to elicit cellular and humoral responses to HIV-1 infection (28). Covalent linking is not necessary for the induction of immunity, as an HSP gp96 exogenously added to HIV-1 Gag-p24 peptide which contains multiple MHC class I- and II-restricted epitopes induced effective cellular immunity against HIV-1 infection (25). In addition, the significantly higher expression of the HSP receptor CD91 on monocytes of HIV-1-infected long-term nonprogressors suggests that HIV-1 antigen uptake and cross-presentation mediated by CD91 may contribute to host anti-HIV-1 defenses and play a role in protection against HIV-1 infection (15, 27).
Previously, we identified a new 10-mer HLA-A2-restricted peptide (FLQSRPEPTA, Gag448-457) of HIV-1B′ (13). In this study, in order to analyze the immunogenicity of the newly identified HIV-specific peptide and determine whether gp96 can enhance the peptide-specific CTL response, gp96 was isolated from human liver tissue. HLA-A2/kb transgenic mice were vaccinated with either peptide alone or in combination with gp96. Murine CTL responses were then analyzed, and the adjuvant effects of gp96 were determined.
It has been reported that the N-terminal fragments of gp96 are able to bind peptides and have binding affinity similar to that of the full-length gp96 (3, 7, 12), which may augment hepatitis B-specific peptide presentation in vitro and induce antigen-specific CTLs in mice (17, 29); however, whether the terminal fragment can induce HIV-specific immune responses as effectively as full-length gp96 remains to be determined. In this study, in order to evaluate the humoral immune adjuvant effect of HSP gp96 and its N-terminal fragment, BALB/c mice were coimmunized with mixtures of HIV-p24 antigen and gp96 or its N-terminal fragment (N336, amino acids 22 to 336) or with the HIV-1 fusion protein p24-N336, the resulting anti-p24 antibody titers were analyzed, and the humoral immune adjuvant effects of gp96 and its N-terminal fragment (N336) were determined.
MATERIALS AND METHODS
Peptides.
The 10-mer peptide FLQSRPEPTA (HIV-1B′ Gag448-457) and the non-A2-restricted control peptide QVPLRPMTFK (HIV-1B′ nef73-82) were synthesized at GL Biochem Ltd. (Shanghai, China). The purity (>98%) of the peptides was confirmed by high-performance liquid chromatography and mass spectrometry.
Purification of proteins.
HSP gp96 was purified from healthy human liver as described previously (22), which entails affinity chromatography over ConA-Sepharose (Sigma-Aldrich, St. Louis, MO), followed by ion-exchange chromatography over a MonoQ column (Amersham Pharmacia, Piscataway, NJ).
The portion of the gp96 gene corresponding to the N-terminal fragment (N336, amino acids 22 to 336) was cloned into a BamHI/XhoI-digested pGEX-6p-1 vector and expressed in Escherichia coli. The glutathione S-transferase fusion protein was cleaved with rhinovirus 3C protease, and the resultant protein mixture was applied to a glutathione Sepharose 4B column (GE, Piscataway, NJ), followed by treatment with Detoxi-Gel endotoxin-removing gel and columns (Pierce, Rockford, IL).
The DNA fragment representing the HIV-1 p24 was cloned into NdeI/XhoI-digested PET30α vector and expressed in E. coli. The soluble His-tagged protein was purified using nickel-nitrilotriacetic acid (Ni-NTA) chromatography (Invitrogen, Carlsbad, CA), followed by treatment with Detoxi-Gel endotoxin-removing gel and columns (Pierce).
The plasmid HIV-1p24-N336 was created by subcloning the N336 gene into the expression vector PET30α at the BamHI and XhoI sites and the HIV-1 p24 gene into the same expression vector at the NdeI and BamHI sites. The HIV-1 p24-N336 plasmid was expressed in E. coli. The inclusion body was purified and then refolded using Ni-NTA chromatography (Invitrogen), followed by treatment with Detoxi-Gel endotoxin-removing gel and columns (Pierce).
Protein concentrations were determined with a bicinchoninic acid protein concentration test kit (Pierce), and proteins were diluted with phosphate-buffered saline (PBS). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analyses were used to confirm the identity of the proteins.
Immunization of mice.
Female HLA-A2/kb mice (6 to 8 weeks old) were immunized subcutaneously (s.c.) at multiple sites with peptide either alone (50 μg per mouse) or together with gp96 (10 to 50 μg per mouse). The injection volume was adjusted to 200 μl. Spleens were removed 7 days after the last immunization, and the splenocytes were suspended in RPMI 1640 medium. The splenocytes were stimulated with peptides for 7 days. Cells were cultured in RPMI 1640 medium containing 10% fetal calf serum (FCS), 2-mercaptoethanol, l-glutamine, and antibiotics.
To analyze humoral immune responses to HIV-1 p24, female BALB/c mice were immunized intraperitoneally every 3 weeks with HIV-1 p24 (4 μg per mouse) with or without either gp96 (6 μg per mouse) or N336 (6 μg per mouse) protein or immunized with HIV-1 p24-N336 (10 μg per mouse). The injection volume was adjusted to 200 μl. Blood samples were harvested 21 days after the last immunization.
ELISpot assays.
Enzyme-linked immunospot (ELISpot) assays were performed according to the manufacturer's instructions. Briefly, 96-well polyvinylidene difluoride plates were preincubated with the coating antibody at 4°C overnight and blocked with PBS buffer containing 1% bovine serum albumin (BSA) for 1 h at 37°C. Freshly isolated splenocytes (3 ×105 cells/well pulsed with 10 μg/ml HIV-1 B′ peptide) were added to the well and incubated for 30 h at 37°C. The spots per well were counted using an automated ELISpot plate reader (Bioreader 3000 PRO).
Tetramer staining.
Spleens were harvested 7 days after the third immunization and dissociated into single-cell suspensions. A total of 3 ×106 cells per sample were washed with PBS and incubated in 50 μl of PBS containing phycoerythrin (PE)-conjugated HLA-A2Kb tetramer (5 μg/ml for fresh spleen cells) loaded with the Gag peptide (FLQSRPEPTA) for 20 min at 4°C and stained for 20 min (4°C) with fluorescein isothiocyanate (FITC)-conjugated anti-CD8 and PE-Cy5-conjugated anti-CD3. Cells were washed three times with PBS buffer and fixed by adding 500 μl of 2% polyformaldehyde. Samples were analyzed by fluorescence-activated cell sorting using CellQuest software (BD Biosciences). Lymphocytes were gated on CD8+, CD3+, and tetramer-positive cells. More than 105 events were acquired for each sample.
Nonradioactive cytotoxicity assay.
The cytolytic activity of the induced CTLs was determined by a nonradioactive cytotoxicity assay. Target cells (T2 cell line) were labeled with peptide (100 μg/ml) in RPMI 1640 with 10% FCS for 1 h at 37°C and then washed twice with plain RPMI 1640. Splenocytes were added as CTL effectors to the target cells (1 × 104) at various effector-to-target cell ratios in 96-well round-bottom plates in a final volume of 100 μl RPMI 1640 with 10% FCS. The cell mixture was then centrifuged to promote cell contact and incubated at 37°C for 4 h. Supernatant (50 μl/well) was harvested manually. Lactate dehydrogenase (LDH) released into the supernatant was measured with a 30-min coupled enzymatic assay, and visible wavelength absorbance data were obtained at 492 nm. The percent of specific release was calculated from the mean of duplicate cultures according to the following formula: percent specific release = [(experimental release − effector spontaneous release − target spontaneous release) × 100]/(target maximum − target spontaneous).
ELISA.
Serum antibody titers from immunized BALB/c mice with HIV-1 p24 were measured by a direct enzyme-linked immunosorbent assay (ELISA). Ninety-six-well plates coated overnight with 100 μl/well of 3 μg/ml recombinant HIV-1 p24 in PBS were blocked for 2 h with PBS containing 1% BSA. Sera were then added in serial dilutions and incubated for 2 h. The plates were washed three times with PBS containing 0.05% Tween 20 and incubated for 1 h with a 1/2,000 dilution of a peroxidase-conjugated rabbit anti-mouse secondary antibody (SBS, Auburn Hills, MI). The plates were washed three times, developed with PNPP (Sigma-Aldrich), and analyzed at 405 nm with a Multiscan MK3 ELISA plate reader.
Statistics.
Statistical analysis and graphical presentation were done using SigmaPlot 11.5 (SPSS Inc., Chicago, IL) and Microsoft Excel. Results are given with standard deviations or as medians with ranges.
RESULTS
Preparation of proteins.
In this study we purified HSP gp96 to homogeneity from healthy human liver. The protein was purified on a MonoQ column that eluted between 300 and 1,000 mM NaCl.
The immune effects of gp96 may depend on its ability to bind peptides (12, 24). It was reported that gp96 might contain a peptide-binding element within its N terminus (3, 7, 12); however, whether the peptide-binding domains can generate immune effects is not known. To address this issue, the N-terminal fragment of gp96, N336 (amino acids 22 to 336) was cloned into the pGEX-6p1 expression vector and expressed in bacteria as described in Materials and Methods.
The p24-N336 fusion protein and HIV-1 p24 proteins were found to be expressed at very high levels in E. coli. The p24-N336 fusion protein was purified as an inclusion body, and the subsequently refolded protein was further purified by using Ni-NTA columns. His-tagged HIV-1 p24 was isolated by using Ni-NTA columns.
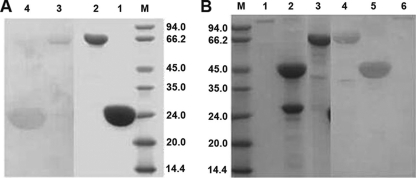
The purified proteins were determined by Coomassie blue staining to be at least 90% pure (Fig. 1), and their identities were confirmed by Western blotting with either anti-gp96 or anti-HIV-1 p24 monoclonal antibodies. HIV-1 p24 (Fig. 1A, lane 4) and HIV-1 p24-N336 (lane 3) were recognized by anti-HIV-1 p24 antibody. HSP gp96 (Fig. 1B, lane 6), N336 (lane 5), and HIV-1 p24-N336 (lane 4) were recognized by anti-gp96 monoclonal antibody. There were two bands of N336 protein (Fig. 1B, lane 2); one is not recognized by anti-gp96 antibody (Fig. 1B, lane 5) and is about 26 kDa; the other is recognized by anti-gp96 antibody and is about 45 kDa. The 26-kDa band may be due to the degradation of the N336 protein.
FIG. 1.
SDS-PAGE and Western blot analysis of gp96, N336, P24, and p24-N336. (A) Purified p24 (lane 1) and p24-N336 (lane 2) were analyzed on a 12% SDS-PAGE gel and were immunoblotted with an anti-p24 monoclonal antibody, respectively (lanes 4 and 3). (B) Purified gp96 (lane 1), N336 (lane 2), and p24-N336 (lane 3) were analyzed on a 12% SDS-PAGE gel and immunoblotted with an anti-gp96 monoclonal antibody (lanes 6, 5, and 4).
Glycoprotein 96-stimulated HIV-1 Gag448-457 CTL response in HLA-A2/kb transgenic mice.
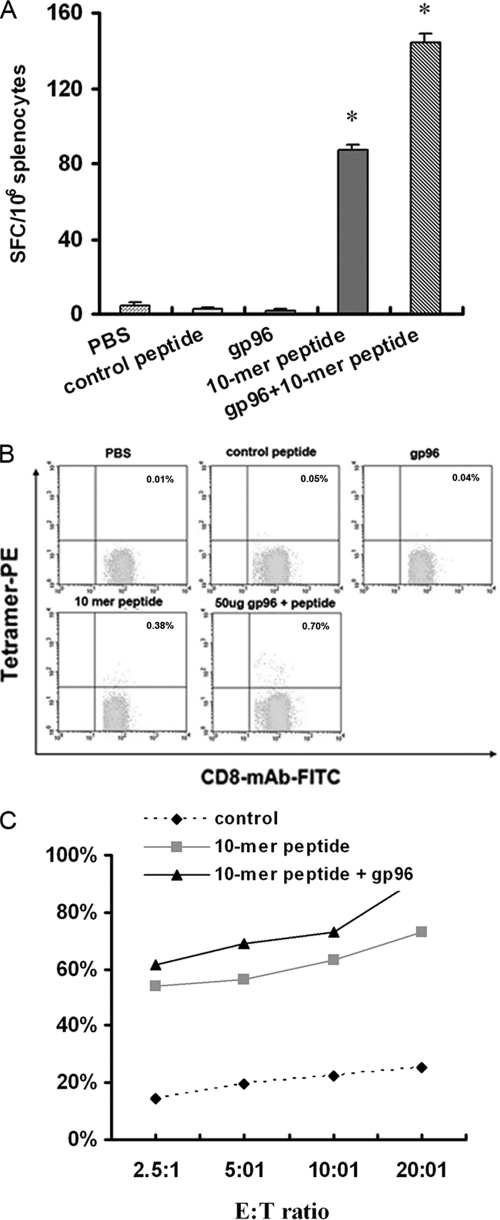
To determine whether the HIV-1 Gag448-457 peptide can elicit a CTL response and whether gp96 could enhance the peptide-specific CTL response in vivo, HLA-A2/kb transgenic mice were immunized s.c. with 50 μg of peptide either alone or together with 50 μg of gp96. CTL responses were monitored 3 weeks thereafter. Freshly isolated splenocytes from the immunized mice were assayed in an ex vivo ELISpot assay. As shown in Fig. 2A, the mean frequency response for the 10-mer peptide was 87 spot-forming cells (SFC)/106 splenocytes in the group of mice immunized s.c. with the peptide only, whereas it was 144 SFC/106 splenocytes in the group of mice coimmunized s.c. with 50 μg of gp96. The number of SFCs increased 66% for the HIV-1 Gag448-457 peptide with the use of gp96. No specific CTL responses were observed in mice immunized with PBS, control peptide, or gp96 only.
FIG. 2.
Detection of HLA-A2 transgenic murine CTLs by using ELISpot, tetramer, and nonradioactive cytotoxicity assays. (A) HLA-A2 transgenic mice were immunized three times with 50 μg of HIV-1 Gag448-457 peptide alone or mixtures of 50 μg of peptides and 50 μg of gp96 in PBS. Seven days after the last inoculation, splenocytes were isolated from the immunized mice and were tested for 40 h by ELISpot assay. Bars represent the means, with standard deviations indicated. *, P < 0.05. (B) Splenocytes were stained with HLA-A2/kb-β2-microglobulin-10-mer peptide or HLA-A2/kb-β2-microglobulin-control peptide tetramer together with anti-CD8-FITC and anti-CD3-PE-Cy5 antibodies. The analyzed splenocytes were gated on CD3+, CD8+, and tetramer-positive cells. Numbers are percentages of tetramer-positive cells within CD8+ CD3+ T lymphocytes. (C) Splenocytes from the same mice were in vitro restimulated with 10 μM of the peptides. Five days later, a nonradioactive cytotoxicity assay was performed on T2 target cells pulsed with the same peptide.
The HIV-1 Gag448-457 peptide was then used to generate tetramer complexes, which stained peptide-specific CTLs from the stimulated mouse splenocytes, and the proportion of peptide-specific CD8+ T cells was thus determined. As shown in Fig. 2B, in the group of mice immunized s.c. with the Gag peptide only, about 0.38% of the CD8+ T cells were peptide specific, while in the group of mice coimmunized s.c. with 50 μg of gp96, the frequency of the HIV-1 Gag448-457-specific CD8+ T cells increased to about 0.70%. No specific staining was observed with the splenocytes isolated from mice immunized with PBS, control peptide, or gp96 only.
In addition, the cytolytic potential of the HIV-1 Gag448-457-specific CTLs was confirmed by cytotoxicity assay. Splenocytes from these immunized mice were restimulated in vitro with the corresponding peptides. A nonradioactive cytotoxicity assay was performed on the peptide-pulsed T2 cell line at 7 days poststimulation. Splenocytes from mice immunized with the control peptide served as controls. As shown in Fig. 2C, the HIV-1 Gag448-457-specific CD8+ T cells were HLA A2 dependent, as CTL-mediated cytotoxicity of the A2 peptide was much higher than that of the HLA A2-independent control peptide. CTL-mediated cytolysis of target cells was 73.1% and 92.4%, respectively, in mice immunized with HIV-1 Gag448-457 peptide alone and together with gp96 when effector-to-target cell ratio was 20:1. The results from the three independent CTL assays showing that the HIV-specific peptide was immunogenic and produced a functional CTL response. The high cytotoxic activity in the nonradioactive cytotoxicity assay might be due to too much LDH activity, or the target and effector cells may have released too much LDH.
From the results of ELISpot, tetramer, and CTL assays, it is concluded that the new peptide (FLQSRPEPTA, Gag448-457) can induce an HIV-1B′-specific HLA-A2-restricted CTL response in HLA-A2 transgenic mice, and human gp96 can serve as an adjuvant to increase its immunogenicity.
Correlation between CTL response and gp96 concentrations.
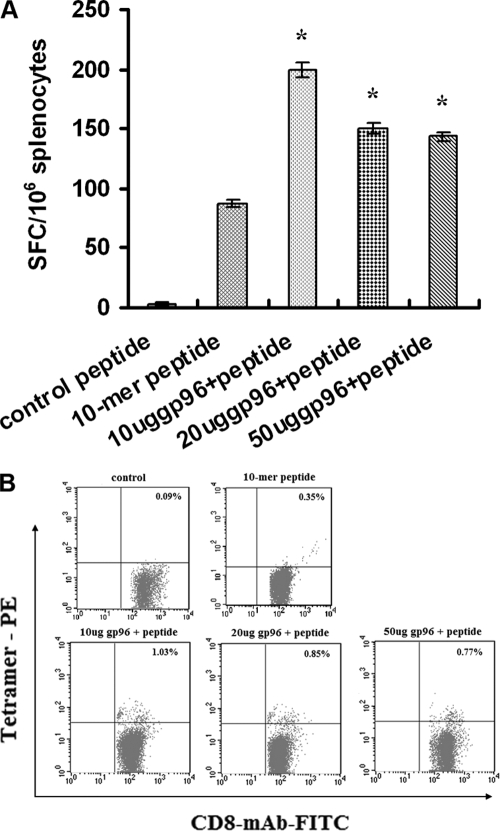
HIV-1 Gag448-457 peptide-specific CTL responses in splenocytes from mice immunized with different amounts of gp96 (0, 10, 20, and 50 μg) and a constant amount (50 μg) of the peptide were compared.
As shown in Fig. 3A, a dose-dependent adjuvant effect was observed for gp96 in the ELISpot assay, with the peak activity at 10 μg, but gp96 alone elicited no CTL response. Larger amounts (50 μg) of gp96 were detrimental to the adjuvant effect, presumably due to the fact that excess gp96 might be immunosuppressive and decrease the ability of mice to generate immune T-cell responses, which was also consistent with previous reports (17).
FIG. 3.
CTL responses to the HIV-1 Gag448-457 peptide in HLA-A2 transgenic mice in the presence of different amounts of gp96 as an adjuvant. Four groups of mice were immunized with 50 μg of the 10-mer peptide and different amounts (0, 10, 20, and 50 μg) of gp96. Splenocytes were obtained 7 days after the last immunization. (A) ELISpot assay. Splenocytes at 106 cells per well were tested in the presence of 10 μM of the peptide. Bars represent the means from three independent experiments. *, P < 0.05. (B) Tetramer staining. Splenocytes isolated from the immunized mice were stained with HLA-A2/kb-β2-microglobulin-10-mer peptide tetramer along with anti-CD8-FITC and anti-CD3-PE-Cy5 antibodies. The analyzed splenocytes were gated on CD3+, CD8+, and tetramer-positive cells. Numbers are percentages of tetramer-positive cells within the CD8+ and CD3+ T lymphocytes.
Splenocytes from the immunized mice were further analyzed in a tetramer staining assay (Fig. 3B). Consistent with the ELISpot assay, the highest percentage of peptide-specific CD8+ T cells (1.03%) was observed when 10 μg of gp96 was used. Taken collectively, these results demonstrate that the HSP gp96 can serve as a potential adjuvant to enhance HIV-1 Gag448-457 peptide-specific CTL responses in mice.
Enhancement of humoral immune responses to HIV-1 p24 by gp96 and its N-terminal fragment.
It has been reported that without a suitable adjuvant, a small synthetic peptide can elicit only a weak CTL response. In this study, we evaluated the humoral adjuvant effect of gp96 and its N-terminal fragment using Gag-p24 instead of the small 10-mer peptide. It is possible that gp96 acts as an adjuvant, which may stimulate the humoral immune response to an antigen mixed with gp96 (18). Mice were injected with 4 μg of p24 mixed with 6 μg of gp96 or N336, and the anti-HIV-1 p24 antibody titer was measured. The titer of anti-p24 antibody was 105.0 in mice injected with a mixture of gp96 and p24 and 105.2 in mice injected with a mixture of N336 and HIV-1 p24, while it was much lower (104.6) in mice immunized with p24 only (Fig. 4).
FIG. 4.
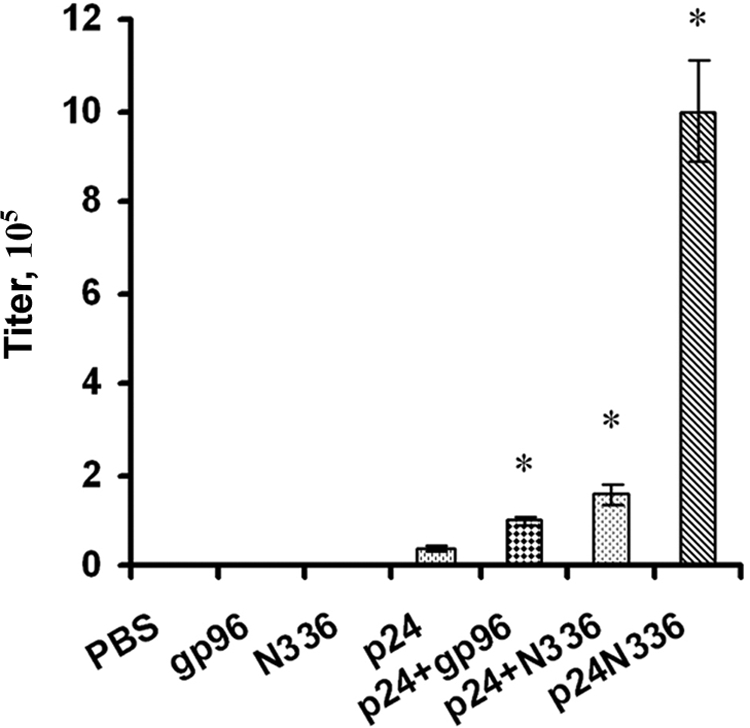
Induced humoral immune response in BALB/c mice by gp96. Groups of BALB/c mice (eight per group) were immunized with: PBS only (group 1), 6 μg gp96 only (group 2), 6 μg N336 only (group 3), 4 μg HIV-1 p24 only (group 4), 4 μg HIV-1 p24 plus 6 μg gp96 (group 5), 4 μg HIV-1 p24 plus 6 μg N336 (group 6), or 10 μg p24-N336 (group 7). At day 0, mice were immunized with the first vaccination; at days 21 and d 42, mice were reimmunized with the same material; at day 63, sera were collected and stored at −20°C, and titers at different dilutions were tested at the same time. *, P < 0.05.
In order to prove that the HIV-1 p24 antigen covalently linked to N336 can also mediate a humoral immune response, we examined the ability of the HIV-1 p24-N336 fusion protein to elicit anti-HIV-1 p24 humoral immune responses. BALB/c mice were inoculated intraperitoneally with 10 μg p24-N336 fusion protein in PBS. Serum samples were obtained 3 weeks after the third immunization, and anti-p24 immunoglobulin G antibody titers were determined by ELISA. Mice immunized with the p24-N336 fusion protein had an anti-p24 antibody titer of 106.0, which was higher than that of mice immunized with p24 alone (104.6). In addition, mice immunized with the HIV-1 p24-N336 fusion protein had an anti-HIV-1 p24 titer 10 times greater than mice immunized with a mixture of HIV-1 p24 and gp96 or N336 (Fig. 4). The significant difference in anti-HIV-1 p24 antibody induced by the coadministration of p24 and HSP gp96 (or N336) and the p24-N336 fusion protein might be due to the fact that the adjuvant and p24 do not necessarily come into contact with the same antigen-presenting cell during coadministration.
These results demonstrate that HSP gp96 and its N-terminal fragment (N336) can serve as potential adjuvants to enhance humoral immune response to HIV-1 in mice and that the N336 covalently linked to HIV-1 p24 antigen can further augment the humoral immune response to HIV-1 in mice.
DISCUSSION
HSPs are involved in eliciting a specific cellular immune response, which has been suggested to be dependent on their ability to chaperone a large variety of peptides. gp96, as one of the most abundant intracellular HSPs, possesses multiple functions. Among these functions, its ability to bridge the innate and adaptive immune systems has attracted extensive interest (14, 19, 26). An optimized peptide epitope specific for MHC class I molecules can potentially boost the antigen-specific cytotoxic-T-cell response. However, without suitable adjuvant, small synthetic peptides can elicit only a weak CTL response (1). Thus, the viral antigens need to be combined with efficient adjuvant with the potential for use in humans. Different investigations have shown that gp96 could act as a potential adjuvant for eliciting a peptide-specific CTL response in mice (11, 17, 25).
In this study, we demonstrated that the novel 10-mer HIV-specific HLA-A2-restricted peptide was indeed immunogenic in HLA-A2/kb transgenic mice and produced peptide-specific CTLs (Fig. 2). In addition, we also demonstrated the adjuvant effects of gp96 for eliciting a peptide-specific CTL response. The data presented in this report are in agreement with the results of several earlier studies that showed the presentation of HIV-1-specific peptides to MHC class I molecules by HSPs (11, 25). A dose-dependent adjuvant effect was observed for gp96, and larger amounts (50 μg) of gp96 were detrimental to the adjuvant effect, presumably because an excess amount of gp96 can be immunosuppressive, which is consistent with previous reports (17).
Most studies so far on the immune effect of gp96 focused mainly on its enhancement of CTLs; recently, several studies clearly demonstrated the potential of using gp96 or its N-terminal fragment as an adjuvant to augment the humoral immunity to hepatitis B virus (18) and human papillomavirus type 16 infection (8). However, whether normal tissue-derived gp96 could influence humoral immune response to HIV-1 p24 has not been fully addressed. In this study, BALB/c mice were coimmunized with gp96 or its N-terminal fragment and the HIV-p24 protein, and the presence of anti-p24 antibody was 105.0 and 105.2 in mice injected with a mixture of gp96 or its N-terminal fragment and p24, respectively, which was higher than the 104.6 antibody titer of mice immunized with p24 alone. The data demonstrated that gp96 or its N-terminal fragment can influence antibody production. The reason that gp96 enhances humoral response might be (i) stimulation of antigen-presentating cells to increase the efficiency of processing exogenous antigen and presenting peptides to MHC class II molecules, (ii) activation of bystander CD4+ T cells, or (iii) a Th2-specific costimulatory function.
Previous studies in virus and parasite model systems have focused on the use of codelivery of viral protein or DNA antigen with gp96 or its N-terminal fragment without covalent linkage (8, 18). In this study, we established the N-terminal fragment of gp96 fusion protein (HIV-1 p24-N336). Our results demonstrated that HIV-1 p24, when genetically fused to the N terminus of gp96, could induce 25 times more anti-p24 antibody than p24 alone and at least 10 times more anti-p24 antibody than the mixture of HIV-1 p24 plus either gp96 or N336. The significant difference in anti-HIV-1 p24 antibody induced by the codelivery of p24 with HSP gp96 (or N336) and that induced by the p24-N336 fusion protein may be due to the fact that the adjuvant and p24 do not necessarily come into contact with the same antigen-presenting cell during coadministration. Eliciting neutralizing antibodies to HIV is now believed to be key for the development of vaccines against HIV infection. Our results indicate that codelivery of HIV-1 p24 with gp96 or its N-terminal fragment with or without covalent linkage could increase the production of antibody to HIV-1 p24 in mice.
Ideal therapeutic vaccines for infectious diseases should elicit not only the cellular immune response but also the humoral immune response. This study likely provides an additional option for the potential of using gp96 as a cellular and humoral adjuvant to induce immune responses to HIV-1 infection. However, more research on codelivery of gp96 or its N-terminal or C-terminal fragments with different viral proteins or epitopes of HIV are required in order to provide more potent vaccine candidate in future studies.
Acknowledgments
We thank Songdong Meng for thoughtful comments for improving the manuscript. We thank Yuxia Zhang, who kindly provided the N-terminal fragment of the gp96 plasmid. We thank Weihua Zhang for her help during the preparation of the manuscript.
This work was supported by a grant from the Major State Basic Research Development Program of China (973 Program) (no. 2006CB504202 and 2006CB504208).
Footnotes
Published ahead of print on 23 September 2009.
REFERENCES
- 1.Audibert, F. 2003. Adjuvants for vaccines, a quest. Int. Immunopharmacol. 3:1187-1193. [DOI] [PubMed] [Google Scholar]
- 2.Babaahmady, K., W. Oehlmann, M. Singh, and T. Lehner. 2007. Inhibition of human immunodeficiency virus type 1 infection of human CD4+ T cells by microbial HSP70 and the peptide epitope 407-426. J. Virol. 81:3354-3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker-LePain, J. C., M. Sarzotti, T. A. Fields, C. Y. Li, and C. V. Nicchitta. 2002. GRP94 (gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression. J. Exp. Med. 196:1447-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belli, F., A. Testori, L. Rivoltini, M. Maio, G. Andreola, M. R. Sertoli, G. Gallino, A. Piris, A. Cattelan, I. Lazzari, M. Carrabba, G. Scita, C. Santantonio, L. Pilla, G. Tragni, C. Lombardo, F. Arienti, A. Marchianò, P. Queirolo, F. Bertolini, A. Cova, E. Lamaj, L. Ascani, R. Camerini, M. Corsi, N. Cascinelli, J. J. Lewis, P. Srivastava, and G. Parmiani. 2002. Vaccination of metastatic melanoma patients with autologous tumor-derived heat shock protein gp96-peptide complexes: clinical and immunologic findings. J. Clin. Oncol. 20:4169-4180. [DOI] [PubMed] [Google Scholar]
- 5.Binder, R. J., and P. K. Srivastava. 2004. Essential role of CD91 in re-presentation of gp96-chaperoned peptides. Proc. Natl. Acad. Sci. USA 101:6128-6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binder, R. J., and P. K. Srivastava. 2005. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat. Immunol. 6:593-599. [DOI] [PubMed] [Google Scholar]
- 7.Biswas, C., U. Sriram, B. Ciric, O. Ostrovsky, S. Gallucci, and Y. Argon. 2006. The N-terminal fragment of GRP94 is sufficient for peptide presentation via professional antigen presenting cells. Int. Immunol. 18:1147-1157. [DOI] [PubMed] [Google Scholar]
- 8.Bolhassani, A., F. Zahedifard, M. Taghikhani, and S. Rafati. 2008. Enhanced immunogenicity of HPV16E7 accompanied by Gp96 as an adjuvant in two vaccination strategies. Vaccine 26:3362-3370. [DOI] [PubMed] [Google Scholar]
- 9.Brenner, B. G., and Z. Wainberg. 2001. Heat shock proteins: novel therapeutic tools for HIV-infection? Expert Opin. Biol. Ther. 1:67-77. [DOI] [PubMed] [Google Scholar]
- 10.Chandawarkar, R. Y., M. S. Wagh, and P. K. Srivastava. 1999. The dual nature of specific immunological activity of tumor-derived gp96 preparations. J. Exp. Med. 189:1437-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doody, A. D., J. T. Kovalchin, M. A. Mihalyo, A. T. Hagymasi, C. G. Drake, and A. J. Adler. 2004. Glycoprotein 96 can chaperone both MHC class I- and class II-restricted epitopes for in vivo presentation, but selectively primes CD8+ T cell effector function. J. Immunol. 172:6087-6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gidalevitz, T., C. Biswas, H. Ding, D. Schneidman-Duhovny, H. J. Wolfson, F. Stevens, S. Radford, and Y. Argon. 2004. Identification of the N-terminal peptide binding site of glucose-regulated protein 94. J. Biol. Chem. 279:16543-16552. [DOI] [PubMed] [Google Scholar]
- 13.Gong, X., X. Gui, Y. Zhang, and P. Tien. 2006. Screening for CD8 cytotoxic T lymphocytes specific for Gag of human immunodeficiency virus type 1 subtype B′ Henan isolate from China and identification of novel epitopes restricted by the HLA-A2 and HLA-A11 alleles. J. Gen. Virol. 87:151-158. [DOI] [PubMed] [Google Scholar]
- 14.Hilf, N., H. Singh-Jasuja, P. Schwarzmaier, C. Gouttefangeas, H. G. Rammensee, and H. Schild. 2002. Human platelets express heat shock protein receptors and regulate dendritic cell maturation. Blood 99:3676-3682. [DOI] [PubMed] [Google Scholar]
- 15.Kebba, A., J. Stebbing, S. Rowland, R. Ingram, J. Agaba, S. Patterson, P. Kaleebu, N. Imami, and F. Gotch. 2005. Expression of the common heat-shock protein receptor CD91 is increased on monocytes of exposed yet HIV-1-seronegative subjects. J. Leukoc. Biol. 78:37-42. [DOI] [PubMed] [Google Scholar]
- 16.Kojima, T., K. Yamazaki, Y. Tamura, S. Ogura, K. Tani, J. Konishi, N. Shinagawa, I. Kinoshita, N. Hizawa, E. Yamaguchi, H. Dosaka-Akita, and M. Nishimura. 2003. Granulocyte-macrophage colony-stimulating factor gene-transduced tumor cells combined with tumor-derived gp96 inhibit tumor growth in mice. Hum. Gene Ther. 14:715-728. [DOI] [PubMed] [Google Scholar]
- 17.Li, H., M. Zhou, J. Han, X. Zhu, T. Dong, G. F. Gao, and P. Tien. 2005. Generation of murine CTL by a hepatitis B virus-specific peptide and evaluation of the adjuvant effect of heat shock protein glycoprotein 96 and its terminal fragments. J. Immunol. 174:195-204. [DOI] [PubMed] [Google Scholar]
- 18.Li, H. T., J. B. Yan, J. Li, M. H. Zhou, X. D. Zhu, Y. X. Zhang, and P. Tien. 2005. Enhancement of humoral immune responses to HBsAg by heat shock protein gp96 and its N-terminal fragment in mice. World J. Gastroenterol. 11:2858-2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, Z., J. Dai, H. Zheng, B. Liu, and M. Caudill. 2002. An integrated view of the roles and mechanisms of heat shock protein gp96-peptide complex in eliciting immune response. Front Biosci. 7:d731-d751. [DOI] [PubMed] [Google Scholar]
- 20.Li, Z., Y. Qiao, B. Liu, E. J. Laska, P. Chakravarthi, J. M. Kulko, R. D. Bona, M. Fang, U. Hegde, V. Moyo, S. H. Tannenbaum, A. Ménoret, J. Gaffney, L. Glynn, C. D. Runowicz, and P. K. Srivastava. 2005. Combination of imatinib mesylate with autologous leukocyte-derived heat shock protein and chronic myelogenous leukemia. Clin. Cancer Res. 11:4460-4468. [DOI] [PubMed] [Google Scholar]
- 21.Mazzaferro, V., J. Coppa, M. G. Carrabba, L. Rivoltini, M. Schiavo, E. Regalia, L. Mariani, T. Camerini, A. Marchianò, S. Andreola, R. Camerini, M. Corsi, J. J. Lewis, P. K. Srivastava, and G. Parmiani. 2003. Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin. Cancer Res. 9:3235-3245. [PubMed] [Google Scholar]
- 22.Meng, S. D., J. Song, Z. Rao, P. Tien, and G. F. Gao. 2002. Three-step purification of gp96 from human liver tumor tissues suitable for isolation of gp96-bound peptides. J. Immunol. Methods 264:29-35. [DOI] [PubMed] [Google Scholar]
- 23.Oglesbee, M. J., M. Pratt, and T. Carsillo. 2002. Role for heat shock proteins in the immune response to measles virus infection. Viral Immunol. 15:399-416. [DOI] [PubMed] [Google Scholar]
- 24.Rivoltini, L., C. Castelli, M. Carrabba, V. Mazzaferro, L. Pilla, V. Huber, J. Coppa, G. Gallino, C. Scheibenbogen, P. Squarcina, A. Cova, R. Camerini, J. J. Lewis, P. K. Srivastava, and G. Parmiani. 2003. Human tumor-derived heat shock protein 96 mediates in vitro activation and in vivo expansion of melanoma- and colon carcinoma-specific T cells. J. Immunol. 171:3467-3474. [DOI] [PubMed] [Google Scholar]
- 25.SenGupta, D., P. J. Norris, T. J. Suscovich, M. Hassan-Zahraee, H. F. Moffett, A. Trocha, R. Draenert, P. J. Goulder, R. J. Binder, D. L. Levey, B. D. Walker, P. K. Srivastava, and C. Brander. 2004. Heat shock protein-mediated cross-presentation of exogenous HIV antigen on HLA class I and class II. J. Immunol. 173:1987-1993. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava, P. 2002. Roles of heat-shock proteins in innate and adaptive immunity. Nat. Rev. Immunol. 2:185-194. [DOI] [PubMed] [Google Scholar]
- 27.Stebbing, J., B. Gazzard, L. Kim, S. Portsmouth, A. Wildfire, I. Teo, M. Nelson, M. Bower, F. Gotch, S. Shaunak, P. Srivastava, and S. Patterson. 2003. The heat-shock protein receptor CD91 is up-regulated in monocytes of HIV-1-infected “true” long-term nonprogressors. Blood 101:4000-4004. [DOI] [PubMed] [Google Scholar]
- 28.Suzue, K., and R. A. Young. 1996. Adjuvant-free hsp70 fusion protein system elicits humoral and cellular immune responses to HIV-1 p24. J. Immunol. 156:873-879. [PubMed] [Google Scholar]
- 29.Zhang, Y., Y. Zan, M. Shan, C. Liu, M. Shi, W. Li, Z. Zhang, N. Liu, F. Wang, W. Zhong, F. Liao, G. F. Gao, and P. Tien. 2006. Effects of heat shock protein gp96 on human dendritic cell maturation and CTL expansion. Biochem. Biophys. Res. Commun. 344:581-587. [DOI] [PubMed] [Google Scholar]