Abstract
A city-wide cytomegalovirus serosurvey was conducted in Shanghai, China, and associated parameters were calculated by employing the catalytic model. The lowest seroprevalence was 60.37%, found in the >1- to 3-year age group. The value increased rapidly with age until 25 years, when a value of 97.03% was found, caused by the high force of infection (12.69) and by the reproductive rate (8.89).
Human cytomegalovirus (HCMV) is one of the eight human herpesviruses. Almost anyone of any age is susceptible to HCMV. The seropositivity of cytomegalovirus varies from 40% to 100%, with a pronounced disparity between developed and developing countries. Cytomegalovirus has a natural ability to enter latency after asymptomatic or symptomatic primary infection and undergoes periodic episodes of reactivation, especially in immunocompromised settings (17).
Primary HCMV infection in the mother during pregnancy has a high possibility of causing congenital infection (1, 11). However, pregnant women with HCMV reactivated from latency or reinfected by new strains can also transmit the virus to the fetus through the placenta, with the latter circumstance leading more easily to this effect (4, 11, 22).
In immunocompetent persons, adequate humoral and cellular immunity are required to restrain viral replication after primary infection and to maintain HCMV in a lifelong chronic state. Persistent cytomegalovirus infection could elicit the seemingly preferential expansion of HCMV-specific clones and lead to HCMV-related inflammation, which is harmful to adults, especially the elderly (2, 12, 15, 20, 21).
There were several surveys conducted in Shanghai, China, estimating HCMV seroprevalence rates several decades ago, in which the population was selected only from women of child-bearing age or those who were pregnant. Indeed, the overall seroprevalence rates of the entire population of Shanghai have not previously been investigated. Only when we survey and analyze the overall circumstances of HCMV infection in Shanghai can the public or government awareness be heightened and can a project be framed for prevention of HCMV-associated diseases, primary infection, or reinfection.
Ethics approval was obtained from the Ethics Committee of Ruijin Hospital. A total of 8,190 serum samples collected from June 2006 to May 2008 in Shanghai, China, were tested for HCMV seroprevalence. The serum samples provided for the HCMV seroprevalence survey were supplied by the Ruijin clinical laboratory after completion of clinically required testing. Specimens from organ transplants and from subjects who received a transfusion within the past 3 months or were known to be infected with human immunodeficiency virus were discarded. Serum samples were identified at Ruijin Hospital by the name of the subject, gender, age or date of birth, and date of collection to ensure that only one sample from any one subject was tested. HCMV-specific immunoglobulin G levels were tested by microparticle enzyme immunoassay technology (AxSYM). The presence of at least 15 antibody units per ml of sample is indicative of past or current infection with HCMV.
Due to Ruijin Hospital's reputation and size, samples collected from this hospital over a span of nearly 2 years are representative not only of the population of Shanghai, China, but also of the population of eastern China. Serum samples were collected from subjects between 0 and 106 years of age and were stratified into the following age groups: >1 to 3, >3 to 5, >5 to 10, >10 to 15, >15 to 20, >20 to 25, >25 to 30, >30 to 35, >35 to 40, and >40 years. Since the source of HCMV antibody in the ≤1 age group was not clear, this group was removed (3). Furthermore, as there were no significant differences in seroprevalence after the age of 40 years, the data after that age could be aggregated as >40 years. Prevalence was calculated separately for each age group and for Shanghai as a whole by weighting the prevalence estimates for each group by the age distribution of the 2004 Shanghai population. The median age of the oldest group (namely, >40 years) was assumed to be 55 years, because 70 years is generally considered to be the threshold age for the type I death rate (a death rate defined as type I indicates each person survives until the age specified, after which survival probability becomes zero [6]). Sample sizes were calculated to achieve a 95% confidence interval (CI) of approximately ±5% for each age group.
Three derived parameters, including the force of infection, the basic reproductive rate, and the average age of infection, were estimated by employing the catalytic model based on the underlying variable of age-specific seroprevalences (3, 6, 8, 14), which could noticeably reflect the severity of HCMV infection. The basic concepts of the above-mentioned three parameters were also discussed in the publication of Colugnati et al. (6).
Since only the cross-sectional survey was conducted, we assumed that the force of infection would be age dependent, with the absence of time dependence at the beginning (equation 1), a circumstance likewise observed in the Colugnati study (6):
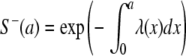 |
(1) |
where a is age, S−(a) is the age distribution for the seronegative samples, and λ is the force of infection, which is permitted to be a complicated function. After the integral transform method and the log-linear approach were employed, the primary equation was changed as follows:
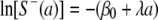 |
(2) |
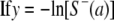 |
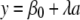 |
(3) |
Force of infection was assumed to be constant, and equation 3 was plotted as a scatter plot according to our data. Regressing on the overall population, it was found that there were no extreme deviations from linearity. Moreover, in combination with statistical analyses of linear regression, the model showed significance (P ≤ 0.05), indicating that the slope could be assumed to be constant. Therefore, the slope of the linear regression model (equation 3) was the estimate of the force of infection. Also, the estimates of the reproductive rate and the average age of infection were calculated using the equations seen in reference 6 under the age-independent assumption.
The seroprevalence comparisons were performed by the chi-square test or Fisher's exact test with SAS 6.12 TS level 0020 software. The force of infection was calculated by linear regression analysis also using SAS 6.12. P levels of 0.05 or less were considered statistically significant.
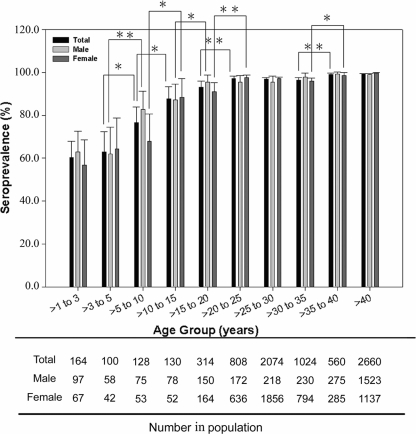
The weighted seroprevalence of the tested population of Shanghai, China, was 95.40% (95% CI, 94.94 to 95.85%), with an overall association identified between seroprevalence and increasing age. However, there were rapid increases seen before the 25-year end point, progressing then to a relatively stable stage. The lowest seroprevalence was 60.37%, seen in the >1- to 3-year group, indicating that the first few years of life is a crucial period for cytomegalovirus infection and vaccination. Seropositivity continued to rise rapidly with age up to 97.03% in >20- to 25-year-olds. Statistically significant increases were obvious among the continuous four groups from >3 to 25 years of age (Fig. 1).
FIG. 1.
HCMV seroprevalence by age group in Shanghai, China. T bars represent 95% CI. Black columns represent HCMV seroprevalence for an entire population. Light gray columns apply to males, and dark gray columns to females. Asterisks represent the statistical difference between group comparisons. One asterisk indicates significant difference (P ≤ 0.05 and P > 0.01), while two asterisks indicate extraordinary difference (P ≤ 0.01). Data below indicate the population number in each corresponding age group.
When male seroprevalence was weighted based upon the age distribution of females, there was little overall difference between males and females (95.55% and 95.84%, respectively; P = 0.511). However, there was a large discrepancy between genders in the group aged >5 to 10 years, with the male seroprevalence rate appearing to be much higher than that of the females (Fig. 1). Thus, the HCMV infectious surge in females was always seen to occur later than that in the male population before 25 years of age. This phenomenon could be explained by later stages being characterized by increased social activity among females. In general, there were no statistically significant differences between males and females in most age groups (Fig. 1).
The overall force of infection in >1-year-olds in Shanghai, China, was 8.04 per 100 persons per year (P = 0.0001 for the linear regression model). However, it was much higher among >1- to 25-year-olds (12.69; P = 0.0001), which led to a higher reproductive rate (8.89) before the 25-year end point. The overall basic reproductive rate was 5.65, indicating that, on average, an infected person transmits HCMV to nearly six susceptible people. The average age of HCMV infection was 7.87 years, based upon the force of infection before 25 years of age (Table 1).
TABLE 1.
HCMV force of infection, basic reproductive rates, and average ages of infection in Shanghai, China
| Shanghai population characteristic | Force of infectiona (95% CI) | Basic reproductive rate (95% CI) | Average age (yr) of infectionb (95% CI) |
|---|---|---|---|
| Entire Shanghai population | 8.04 (6.36-9.72) | 5.65 (4.50-6.81) | |
| Aged >1 to 25 yr | 12.69 (11.49-13.90) | 8.89 (8.05-9.73) | 7.87 (7.19-8.68) |
| Total males | 7.69 (5.59-9.78) | 5.41 (3.99-6.86) | |
| Males, >1 to 20 yr | 13.40 (10.20-16.59) | 9.38 (7.15-11.61) | 7.46 (6.03-9.75) |
| Total females | 9.16 (7.59-10.72) | 6.42 (5.34-7.51) | |
| Females, >1 to 25 yr | 13.30 (10.23-16.36) | 9.31 (7.17-11.45) | 7.52 (6.11-9.72) |
Number of individuals infected by HCMV per 100 susceptible individuals per year. If the relevant confidence intervals did not overlap, the parameters based on the different groups were considered to have statistical difference.
Since the average age of infection was less than 25 years based on the overall force of infection, it should be calculated more accurately according to the force of infection among >1- to 25-year-olds.
When force of infection was stratified by gender overall, females (9.16; P = 0.0001 for the linear regression model) scored a bit higher than males (7.69; P = 0.0001) (Table 1). Females maintained a high force of infection (13.30) from >1 to 25 years of age, while males showed a similar force of infection (13.40) from >1 to only 20 years.
In comparison with the other five countries listed in Table 2, HCMV force of infection in Shanghai, China, ranks at the top, as does the basic reproductive rate. Also, the average age of infection in Shanghai is the youngest observed. HCMV force of infection in Shanghai is 5.14-fold higher than that in the United States (1988 to 1994), 4.35-fold higher than that in Australia (2002), and so on. The high force of infection is directly associated with the high basic reproductive rate (Table 2). While in the other five countries, it is delayed almost to child-bearing age (in São Paulo, we focus mainly on the second stage of HCMV infection [3]), the average age of infection in Shanghai occurs nearly at the end of childhood, even though the data are calculated almost based on the second stage of the infectious surge due to the deficiency of the data in the first year.
TABLE 2.
Comparison of HCMV force of infection, basic reproductive rates, and average ages of infection in different countries
| Location (yr studied)a | Population | Force of infectionb (95% CI) | Basic reproductive rate (95% CI) | Average age (yr) of infection (95% CI) | Reference |
|---|---|---|---|---|---|
| Shanghai, China (2006-2008) | Entire Shanghai population | 8.04 (6.36-9.72) | 5.65 (4.50-6.81) | 7.87 (7.19-8.68) | |
| Australia (2002) | Entire Australia population | 1.89 (1.53-2.26) | 1.80 (1.63-1.99) | 27.50 (26.14-28.87) | 19 |
| São Paulo, Brazil (1990-1991) | Entire São Paulo population | Age dependentc | 5.02 mo, 19.84 yr | 3 | |
| ARM, Spain (1993) | Entire ARM population | 4.98 (2.91-7.05) | 3.60 (2.34-4.97) | 17.87 (13.68-23.87) | 7 |
| ARM, Spain (1999) | Entire ARM population | 3.37 (2.63-4.11) | 2.61 (2.19-3.05) | 22.37 (20.15-24.82) | 7 |
| London, UK (1975-1982) | Pregnant women | 3.10 | 2.40 | 32.00 | 10 |
| London, UK (1983-1985) | Pregnant women | 3.50 | 2.70 | 29.00 | 10 |
| United States (1988-1994) | Entire U.S. population | 1.60 (1.30-1.90) | 1.70 (1.50-1.80) | 28.60 (27.30-29.40) | 6 |
ARM, Autonomous Region of Madrid; UK, United Kingdom.
No. of individuals infected by HCMV per 100 susceptible individuals per year.
The age-dependent force of infection has its highest value early in childhood and then drops quickly to nearly 5 years of age. Afterward, it increases slowly (from 5 to 15 years old) and then decreases again at a minimum speed during the life span (3).
An important limitation of this study is the limited population of >1- to 15-year-olds, for which the grouping may not be sufficiently powered, and the associated parameters therefore may not be calculated accurately. However, the basic conclusion can still be made based upon the captured information, and the general overall trend is still apparent. Moreover, the Farrington equation is flexible (6, 8).
The lowest seroprevalence of HCMV is 60.37%, found among subjects that were >1 to 3 years of age. If we assume a seroprevalence of zero at birth, then the force of infection of the earliest few years is very high, suggesting that the first 2 or 3 years of life form a crucial stage of HCMV infection and vaccination. Therefore in developing countries, the force of infection always exhibits at least two stages, from a steep to a smooth curve, a circumstance likewise observed in the Almeidaa study (3). They had used a modified Farrington method to estimate the force of infection, which was age dependent. It showed its highest value early in childhood and then fell quickly to nearly 5 years of age. Subsequently, it went up slowly and then decreased again. The first curve was assumed to be the first stage. In this article, it is mainly the second stage that was investigated.
Proceeding forward nearly 20 years, the seroprevalence is seen to increase to 97.03% under a high force of infection and reproductive rate. In contrast with the developed countries, where there exists a large proportion of susceptible women of child-bearing age, almost every woman of child-bearing age in Shanghai, China, is infected with cytomegalovirus. However, the incidence of congenital HCMV infection is not infrequent. According to nation-wide or province-wide surveys done in China, the positivity of HCMV-specific immunoglobulin M in newborns is 1.5 to 3.7% (5, 13, 16, 23), much higher than that in developed countries where the incidence of congenital HCMV infection has been reported to vary between 0.15 and 2.0% (9, 11, 17). Since preconception immunity against HCMV provides only partial protection from intrauterine transmission, a substantial proportion of congenital HCMV infections occur in the offspring of women with preconception immunity, possibly due to the acquisition of a new strain (4, 11, 22). Therefore, HCMV vaccination is deemed to be necessary and effective for preventing congenital HCMV infection or disease (18, 22).
A higher HCMV force of infection and basic reproductive rates in childhood and adolescence dictate an earlier age of HCMV infection, leading in turn to a greater number of years spent infected with HCMV on average by the time an individual reaches old age. This is a circumstance which carries increased detrimental consequences (12, 15, 20, 21) and has important implications for the necessity of early life vaccination.
At this time, a clinically licensed vaccine is still not available, but significant progress has been made. Scientists in the relevant fields continue to study approaches, including the use of polyepitopes which can induce both humoral and cellular immunity to prevent infection. Increased effective longevity may be gained from a combination of strategy and regular booster immunizations, building defenses against HCMV multistrains given the expectation of reinfection by different strains of HCMV in humans (18, 22).
Acknowledgments
This work was supported by the Science and Technology Commission of Shanghai Municipality for project 074119521. There is no potential conflict of interest for any of us.
We are exceptionally grateful to Xiao-Yi Lin and Zhi-Dong Gu for help with serologic testing and to Sheng Ji for help with data retrieval. We also acknowledge Ying Jin and Ling-Jie Li for critical reading of the manuscript.
Author contributions are as follows: Feng-Qin Fang, conception and design, collection of data, data analysis and interpretation, and manuscript writing; Qi-Shi Fan, Yi-Bing Peng, and Yue Zhang, conception and design and sample collection; Zhi-Jun Yang, data analysis and interpretation and manuscript revision; Li Zhang and Ke-Zi Mao, collection of data; Yu-Hua Ji, conception and design, financial support, data interpretation, and manuscript revision.
Footnotes
Published ahead of print on 23 September 2009.
REFERENCES
- 1.Adler, S. P., and B. Marshall. 2007. Cytomegalovirus infections. Pediatr. Rev. 28:92-100. [DOI] [PubMed] [Google Scholar]
- 2.Almanzar, G., S. Schwaiger, and B. Jenewein. 2005. Long-term cytomegalovirus infection leads to significant changes in the composition of the CD8+ T-cell repertoire, which may be the basis for an imbalance in the cytokine production profile in elderly persons. J. Virol. 79:3675-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almeidaa, L. N., R. S. Azevedoa, M. Amakub, and E. Massad. 2001. Cytomegalovirus seroepidemiology in an urban community of São Paulo, Brazil. Rev. Saude Publica 35:124-129. [DOI] [PubMed] [Google Scholar]
- 4.Boppana, S. B., L. B. Rivera, K. B. Fowler, M. Mach, and W. J. Britt. 2001. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N. Engl. J. Med. 344:1366-1371. [DOI] [PubMed] [Google Scholar]
- 5.Chen, C. Z., and J. Z. Bu. 2007. The investigation of inflection of TORCH in neonate. Matern. Child Health Care China 22:3099-3100. (In Chinese.) [Google Scholar]
- 6.Colugnati, F. A., S. A. Staras, S. C. Dollard, and M. J. Cannon. 2007. Incidence of cytomegalovirus infection among the general population and pregnant women in the United States. BMC Infect. Dis. 7:71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Ory, F., R. Ramírez, L. García Comas, P. León, M. J. Sagües, and J. C. Sanz. 2004. Is there a change in cytomegalovirus seroepidemiology in Spain? Eur. J. Epidemiol. 19:85-89. [DOI] [PubMed] [Google Scholar]
- 8.Farrington, C. P. 1990. Modelling forces of infection for measles, mumps and rubella. Stat. Med. 9:953-967. [DOI] [PubMed] [Google Scholar]
- 9.Gaytant, M. A., J. M. Galama, B. A. Semmekrot, W. J. Melchers, J. M. Sporken, H. P. Oosterbaan, P. A. van Dop, A. Huisman, H. M. Merkus, and E. A. Steegers. 2005. The incidence of congenital cytomegalovirus infections in The Netherlands. J. Med. Virol. 76:71-75. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths, P. D., A. McLean, and V. C. Emery. 2001. Encouraging prospects for immunisation against primary cytomegalovirus infection. Vaccine 19:1356-1362. [DOI] [PubMed] [Google Scholar]
- 11.Kenneson, A., and M. J. Cannon. 2007. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev. Med. Virol. 17:253-276. [DOI] [PubMed] [Google Scholar]
- 12.Koch, S., R. Solana, O. Dela Rosa, and G. Pawelec. 2006. Human cytomegalovirus infection and T cell immunosenescence: a mini review. Mech. Ageing Dev. 127:538-543. [DOI] [PubMed] [Google Scholar]
- 13.Li, K. H., H. Liu, J. Y. Liu, and Y. J. Li. 2003. Analysis of serological findings and clinical manifestations of TORCH infections in newborns. Chin. J. Exp. Clin. Virol. 17:283-284. (In Chinese.) [PubMed] [Google Scholar]
- 14.Li, W. C., D. T. Zhao, Q. B. Zhao, L. C. Wang, and H. Zhang. 2007. The choice and calculation of force of infection in regression of catalytic model. J. Math. Med. 20:135-136. (In Chinese.) [Google Scholar]
- 15.Pawelec, G., and C. Gouttefangeas. 2006. T-cell dysregulation caused by chronic antigenic stress: the role of CMV in immunosenescence? Aging Clin. Exp. Res. 18:171-173. [DOI] [PubMed] [Google Scholar]
- 16.Ping, Y. 2007. The status and treatment of CMV infection in newborns. Shanghai Med. Pharm. J. 28:224-226. (In Chinese.) [Google Scholar]
- 17.Rawlinson, W., and G. Scott. 2003. Cytomegalovirus: a common virus causing serious disease. Aust. Fam. Physician 32:789-793. [PubMed] [Google Scholar]
- 18.Schleiss, M. R., and T. C. Heineman. 2005. Progress toward an elusive goal: current status of cytomegalovirus vaccines. Expert Rev. Vaccin. 4:381-406. [DOI] [PubMed] [Google Scholar]
- 19.Seale, H., C. R. MacIntyre, H. F. Gidding, J. L. Backhouse, D. E. Dwyer, and L. Gilbert. 2006. National serosurvey of cytomegalovirus in Australia. Clin. Vaccine Immunol. 13:1181-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stassen, F. R., T. Vainas, and C. A. Bruggeman. 2008. Infection and atherosclerosis. An alternative view on an outdated hypothesis. Pharmacol. Rep. 60:85-92. [PubMed] [Google Scholar]
- 21.Streblow, D. N., J. Dumortier, A. V. Moses, S. L. Orloff, and J. A. Nelson. 2008. Mechanisms of cytomegalovirus-accelerated vascular disease: induction of paracrine factors that promote angiogenesis and wound healing. Curr. Top. Microbiol. Immunol. 325:397-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhong, J., and R. Khanna. 2007. Vaccine strategies against human cytomegalovirus infection. Expert Rev. Anti-Infect. Ther. 5:449-459. [DOI] [PubMed] [Google Scholar]
- 23.Zhou, P., L. Yao, and S. F. Wang. 2004. Research on the status of CMV infection in the pregnant and newborns. J. Harbin Med. Univ. 38:393-395. (In Chinese.) [Google Scholar]