Abstract
The diversity of Lyme-borreliosis-inducing Borrelia species in Europe set high standards for the use of serodiagnostic test systems in terms of specificity and sensitivity. In the United States, the one-step C6 antibody test system based on the invariable domain IR6 of the VlsE molecule has been established as a successful diagnostic tool for testing canine samples. However, only a limited set of data are available regarding the antigenicity of the C6 peptides in an experimental murine model and sensitivity of the test regarding European Borrelia species. In order to investigate antibody reactions induced by these spirochetes, a total of 142 C3H/HeN mice were inoculated with Borrelia burgdorferi sensu stricto N40, B. garinii PBi, two isolates of B. afzelii, B. spielmanii A14S, B. valaisiana Rio6, B. valaisiana VS116, or B. lusitaniae. Infection of the mice was documented utilizing tissue culture and PCR. The IR6 sequences of B. burgdorferi sensu stricto B31, B. garinii IP90, and two B. afzelii ACAI strains have been used to synthesize and test additional C6 peptides. Compared to the well-established two-tiered test system, the results indicate that single C6 peptides derived from B. burgdorferi sensu stricto and B. garinii can be used in an enzyme-linked immunosorbent assay-based technique to detect murine antibodies induced by either agent. Little is known about the prevalence or pathogenicity of the B. afzelii strains in mammalian hosts, but our experimental data indicate differences in the C6 peptide test sensitivity for the detection of antibodies induced by different strains or isolates of B. afzelii.
Three members of the Borrelia burgdorferi sensu lato complex—B. burgdorferi sensu stricto, B. garinii, and B. afzelii (3)—are known to induce clinical symptoms associated with Lyme borreliosis (2, 3) in humans (43). Infections with B. burgdorferi sensu lato species are well documented in animals, especially infections in mice with B. burgdorferi sensu stricto, B. afzelii, and B. garinii have been proven (16, 31, 32) and in dogs B. burgdorferi sensu stricto has been demonstrated to cause infection and clinical disease (1). In Europe, B. lusitaniae (6, 21) and DNA from B. valaisiana, which can also be found in Asia (9, 39), have been detected in human patients with suspected Lyme borreliosis. B. spielmanii (36, 51) has been isolated from human patients. Regarding clinical symptoms, B. garinii and B. afzelii are the most prevalent pathogenic species in Europe and Asia, followed by B. burgdorferi sensu stricto, while in the United States, B. burgdorferi sensu stricto is the only species found to cause Lyme borreliosis in humans (42). The heterogeneity of Borrelia species in Europe led to the need of a species-dependent optimization of two-tiered serodiagnostic test systems (53) to determine specificity and sensitivity.
The variable surface protein VlsE (for variable major protein-like sequence, expressed) of B. burgdorferi sensu stricto is a highly specific serodiagnostic tool. The expression site vlsE undergoes a gene conversion mechanism (7, 58, 59) and, consequently, the VlsE protein itself is modified during infection in the mammalian host (58). The VlsE protein and especially its invariable region 6 (IR6) of B. garinii and B. burgdorferi sensu stricto were found to be highly immunogenic and specific for infection. Antibodies directed against these VlsE regions can be used in order to detect infections with B. burgdorferi sensu stricto strains in monkeys, mice, and humans (20, 27, 28). Based on these findings, a 26-mer synthetic peptide analogue of IR6, referred to as the C6 peptide, has been successfully established as a serodiagnostic tool for human and canine Lyme borreliosis in the United States and in Europe (5, 22-24, 26, 34, 35, 48). The diversity of potential pathogenic Borrelia species in Europe and the unexplained state of infectivity or pathogenicity of some species raised the question of whether a single IR6-derived peptide can detect antibodies to all of these species. In a previous study, IR6 derived from B. garinii VlsE could successfully detect murine antibodies against B. burgdorferi sensu stricto, B. garinii, and B. afzelii, and antibodies from U.S. patients with different clinical manifestations of Lyme borreliosis (25). Investigations have been conducted, in which human sera collected during various stages of clinical Lyme borreliosis in Europe and North America have been tested with IR6/C6 peptides derived from B. burgdorferi sensu stricto, B. garinii, and variants of B. afzelii. Several data indicate that IR6 sequences derived from B. afzelii are useful for the detection of C6 antibodies in human sera from the United States and that B. burgdorferi sensu stricto-derived C6 peptides can detect antibodies in sera from European patients. The B. garinii-derived C6 peptide did not detect antibodies against B. afzelii or B. burgdorferi sensu stricto, as well as the just-mentioned B. afzelii- and B. burgdorferi sensu stricto-derived C6 peptide variants (41). Regarding European patients’ sera, it was proposed that the use of a C6 peptide mixture might be more beneficial with regard of genospecies dependency and that early infections may not be detected by all C6 test preparations (12, 49). Previously reported data showed differences in the amino acid sequences between IR6 within the B. burgdorferi sensu lato complex (13), which might result in different reactivities of patient sera. These different observations taken together lead to the need of further investigations regarding the use of C6 peptides, especially for European applications. The antibody levels against different C6 peptides should be measured separately in individuals who were known to be infected with only one of the pathogenic Borrelia species to exclude the influences of cross-reactive antibodies induced by different species. To our knowledge, no experimental studies exist using C6 peptide sequences based on B. burgdorferi sensu stricto, B. garinii, and B. afzelii to detect immune responses at unique time points postinfection against different Borrelia genospecies, which also include B. spielmanii. This is important because B. spielmanii may be pathogenic, and serodiagnosis of specific antibodies against B. spielmanii might be recommended in the future. For this reason, we compared murine immune responses against B. spielmanii with Borrelia lysate antigen serology and C6 peptides. In our study, mice were inoculated with B. burgdorferi sensu stricto, B. garinii, B. afzelii, B. spielmanii, B. valaisiana, and B. lusitaniae. The potential infectivity of spirochetes was characterized with plasmid lp25 and lp28-1 PCR.
Murine sera were collected and tested with whole-cell lysate-based two-tiered test method and enzyme-linked immunosorbent assay (ELISA) containing C6 peptides originated from B. burgdorferi sensu stricto, B. garinii, or B. afzelii. Two-tiered testing was carried out to compare sensitivities with C6 peptide test system. In addition, to confirm results obtained by serodiagnosis, tissue culture and PCR were carried out to demonstrate the successful infection of the mice. We focused on the experimental assay and not on the induction of clinical symptoms by Borrelia species and strains or their correlation with C6 antibodies. For this reason, our results obtained for the sensitivity of C6 peptides in the murine model do not allow the unrestricted transfer of the conclusions to infections occurring in other host species such as humans and dogs. However, they should offer a comprehensive contribution to the question of whether serological assays using a single C6 peptide are sufficiently sensitive and cross-reactive to detect species-specific C6 antibodies induced by defined European Borrelia species or strains.
MATERIALS AND METHODS
Borrelia.
Six species from the B. burgdorferi sensu lato group were used for the experiments. B. burgdorferi sensu stricto N40 (passage 3) is an isolate from a skin punch biopsy of an experimentally infected dog (1). B. garinii strain PBi (passage 16) and B. afzelii PKo (passage 32), representing European serodiagnostic reference strains (55, 56), have been originally isolated from humans. A second, uncharacterized B. afzelii isolate was named as B. afzelii Slovakia in this publication was an isolate from an ixodid tick. This isolate was shown to be infective in C3H mice (17). Two strains of B. valaisiana, strain VS116 (passage 46) from our institute stock and a tick isolate strain Rio6 (passage unknown) from Spain (10) were used for inoculation. B. lusitaniae (passage 14) from Portugal was an isolate originated from human skin (6). B. spielmanii strain A14S (passage 10) was originally isolated in The Netherlands.
Cultivation of spirochetes.
To optimize bacterial growth in liquid media, BSK-H Medium (Sigma, Taufkirchen, Germany) and Barbour-Stoenner-Kelly (BSK-II) medium were prepared with different concentrations of heat-inactivated rabbit serum or gelatin for each Borrelia species and strain. All strains except B. afzelii PKo were grown at 33°C in BSK-II medium supplemented with 7.0 to 8.0% of rabbit serum and 8.5% bovine gelatin. B. afzelii PKo was grown in modified Kelly medium (MKP) (38). This protocol was kindly provided by Bettina Wilske, Max von Pettenkofer Institut, Munich, Germany.
Mice.
Female C3H/HeN (C3H) mice were bred and purchased by Harlan Winkelmann GmbH, Borchen, Germany. During the time of experiment, mice were kept under specific-pathogen-free conditions in individually ventilated cages at the animal facility of the Max Planck Institute for Evolutionary Anthropology (Leipzig, Germany). The animal experiment was carried out in accordance with the guidelines approved by the Animal Care and Usage Committee of the Regierungspräsidium, Leipzig, Germany.
Intradermal inoculation of B. burgdorferi sensu lato into mice.
In total, 142 mice were inoculated with B. burgdorferi sensu stricto N40, B. garinii PBi, B. afzelii PKo, B. afzelii Slovakia, B. valaisiana VS116, B. valaisiana Rio6, B. spielmanii A14S, or B. lusitaniae (Table 1) . Spirochetes were grown to late exponential phase until injection. The dose of spirochetes per mouse was between 6.0 × 105 and 1.0 × 106 in 100 μl of BSK-II or MKP medium. This volume was divided in two adjoining injections which were placed intradermally into shaven back of each mouse. Three C3H mice served as negative controls for tissue PCR.
TABLE 1.
Overview of B. burgdorferi sensu lato inoculation experimentsa
| Spirochete | Growth medium | Expt I |
Expt II |
Expt III |
Total no. of mice (n = 142 [7]) | |||
|---|---|---|---|---|---|---|---|---|
| Dose (CFU)/mouse | No. of mice (n = 48 [0]) | Dose (CFU)/mouse | No. of mice (n = 36 [2]) | Dose (CFU)/mouse | No. of mice (n = 58 [5]) | |||
| B. burgdorferi sensu stricto N40 | BSK-II | 106 | 6 | 106 | 10 | 106 | 5 | 21 |
| B. garinii PBi | BSK-II | 106 | 6 | 106 | 10 | 6.0 × 105 | 5 | 21 |
| B. afzelii PKo | MKP | 106 | 6 | - | 0 | 106 | 17 | 23 |
| B. afzelii Slovakia | BSK-II | 106 | 6 | - | 0 | 7.6 × 105 | 15 | 21 |
| B. valaisiana VS116 | BSK-II | 106 | 6 | - | 0 | - | 0 | 6 |
| B. valaisiana Rio6 | BSK-II | - | 0 | 106 | 8 | - | 0 | 8 |
| B. spielmanii A14S | BSK-II | 106 | 12 | - | 0 | 106 | 6 | 18 |
| B. lusitaniae | BSK-II | 106 | 6 | 106 | 8 | 106 | 10 | 24 |
For experiments I and III, the collection of murine serum samples occurred on days 28, 56, and 63 postinoculation, and mice were sacrificed on day 63. For experiment II, the collection of murine serum samples occurred on days 28, 56, and 84 postinoculation, and mice were sacrificed on day 90. n, Total number of mice (values in brackets indicate the number of negative-control mice). -, No inoculation with this spirochete.
Sera.
Blood samples from all mice were obtained at days 28 and 56 after borrelia inoculation by retrobulbary bleeding under anesthesia using hematocrit glass capillaries (200 μl of blood) or by intracardial bleeding at day of sacrifice using a syringe. Serum was prepared in serum separators (BD Microtainer; Becton Dickinson) by a centrifugation step at 5,000 × g for 5 min at room temperature. Sera were stored frozen at −80°C. For the calculation of ELISA cutoffs, 27 stored sera from uninfected C3H/HeN mice served as negative controls.
Tissue samples for cultivation and PCR.
Mice were sacrificed at day 63 or 90 after borrelia inoculation. Tissues from the heart, skin from the right ear, right tarsal joint, bladder, and skin from inoculation area of the back were collected under sterile conditions. Tissue samples were washed in 70% of ethanol and in phosphate-buffered saline (PBS). Whole bladder and joint and parts of the ear, heart, and skin of back were squashed in 200 μl of BSK-II or MKP. These tissue mixtures were each individually transferred into 6 ml of BSK-II or MKP. Tissue cultures were kept at 33°C for 8 weeks and observed weekly for the presence of viable spirochetes. Except in experiment I, parts of the heart and skin from the ear and back were frozen at −80°C for later PCR. In the case of borrelia-positive tissue cultures, spirochetes were stored at −80°C in 100-μl aliquots containing added glycerol.
KELA using Borrelia lysate as antigen.
A computerized kinetic ELISA (KELA) was performed as described previously (50). Sonicated whole cell-lysate of B. burgdorferi sensu stricto N40 served as the antigen. Murine sera were diluted 1:100 in PBS with 0.05% of Tween 20 and 2% milk powder (PBSTM). Borrelia-specific antibodies were detected with horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG; R&D Systems, Minneapolis, MN) in dilutions of 1:500 and 1:1,000 in PBSTM.
Western blotting.
Western blot analysis of selected mice sera was performed with a test kit using B. afzelii lysate antigen and recombinant VlsE (B. afzelii + VlsE EcoBlot IgG Western Blot; Genzyme Virotech GmbH, Rüsselsheim, Germany). The test procedure was carried out according to the instructions supplied with the kit. Alkaline phosphatase conjugated affinity purified goat anti-mouse IgG (Rockland, Inc., Gilbertsville, PA) at a dilution of 1:4,000 was used as the secondary antibody. The substrate reaction was stopped after 10 min. The interpretation of test results was performed according to included kit-specific protein band templates and based on published criteria (14, 15).
C6 peptide-specific ELISA commercial test system.
The Lyme Quantitative C6-antibody test kit (Lyme Quant C6 Test; IDEXX Laboratories, Inc., Westbrook, ME) was used as a prescreening test for C6-specific antibodies (24, 34). This test kit was originally developed for canine serum samples to measure the levels of C6-specific antibody in the sera of positive dogs. Kit instructions were used but adapted with the following modifications: murine samples sera were diluted 1:100, and antibodies were detected with horseradish peroxidase-conjugated goat anti-mouse IgG (R&D Systems) using dilutions of 1:1,000 and 1:500 in PBSTM. The optical densities (OD) of the samples were measured spectrophotometrically at 650 nm and compared to sera from uninfected mice and sera from mice infected with B. burgdorferi sensu stricto N40.
ELISAs with Borrelia species-specific C6 peptides serving as the antigen.
Four synthetic C6 peptides derived from the IR6 region of B. burgdorferi sensu stricto, B. garinii, or B. afzelii were synthesized with an N-terminal cysteine that was used for conjugation. The origin and amino acid sequences of the four synthetic C6 peptides are shown in Table 2. Microtiter plates were coated with 100 μl of each C6 peptide/well at a concentration of 0.5 μg/ml in a 0.05 M sodium carbonate coating buffer. After an incubation step at room temperature, the peptide solution was aspirated, and the plates were washed two times with PBS-Tween wash buffer. Plates were blocked by using 200 μl of detergent containing sucrose in 0.1 M Tris buffer/well for 2 h at room temperature. The blocking buffer was aspirated, and the plates were dried overnight at room temperature. Plates were stored in Mylar bags with desiccants at 4°C until use. ELISA was carried out and evaluated according to the protocol described previously for the commercial C6 test system.
TABLE 2.
B. burgdorferi sensu lato C6 peptide sequences used for ELISA
| Species origin and recombination cassette | Peptide | Amino acid sequence (26-mer)a | Accession no. |
|---|---|---|---|
| B. burgdorferi B31 Vls15 | B. burgdorferi C6 | CMKKDDQIAAAIALRGMAKDGKFAVK | AAC45189.1 |
| B. garinii IP90 Vls7 | B. garinii C6 | CMKKDDQIAAAMVLRGMAKDGQFALK | AAN87831.1 |
| B. afzelii ACAI Vls1 | B. afzelii C6-1 | CMKKSDKIAAAIVLRGVAKSGKFAVA | AAN87809.1 |
| B. afzelii ACAI Vls3 | B. afzelii C6-2 | CMKKRNDKIVAAIVLRGVAKDGKFAAA | AAN87811.1 |
Including the N-terminal cysteine (C) conjugated.
Statistical evaluation of ELISA data.
For each plate, mean background values (wells incubated without serum but with anti-mouse IgG) were subtracted from mean samples values for each sample. For each infection group (mice infected with the same Borrelia species), the means (μ) and standard deviations (SD) (σ) of the obtained ELISA values were calculated. Cutoffs for each antigen tested in an ELISA were calculated by adding five SD to the mean values obtained by measuring 27 samples from uninfected mice. Samples were considered positive if the measured OD was equal or greater than the cutoff value.
Infectivity of Borrelia strains used for inoculation.
To determine the infectivity of the Borrelia species used for inoculation, a PCR was performed that detected sequences of the linear plasmids lp25 and lp28-1. Lack of the lp25 plasmid results in a complete loss of infectivity and lack of the lp28-1 plasmid has been reported to result in diminished virulence (18, 19). Borrelia species and strains that were originally used for inoculation and those that could be isolated from murine tissues postinoculation were grown in BSK-II or MKP as previously described. Cultures were centrifuged at 10,000 × g for 15 min at 15°C. The supernatant was discharged, and the pellet was suspended in 200 μl of PBS. Centrifugation was repeated at 4°C. Supernatant was discarded again, and the pellet was suspended in 180 μl of PBS. Spirochetes and water used as a negative control were applied to a Qiagen Blood & Tissue kit (Qiagen GmbH, Hilden, Germany). DNA was eluted in 100 μl of distilled water. The yield of DNA and the purity were measured with a spectrophotometer (Eppendorf, Hamburg, Germany) at wavelengths λ = 230, 260, 280, and 320 nm. In the case of weak spirochetal growth, 100-μl portions of spirochete cultures were centrifuged and suspended in PBS and then boiled at 94°C for 5 min. A total of 105 spirochetes per μl were directly used for PCR. The primer sequences for plasmid lp25 (618 bp) and plasmid lp28-1 (291 bp) were previously described (16, 17). As DNA template, 5 μl of DNA were used. PCRs (50 μl) contained 4.0 mM MgCl2, 1.0× PCR buffer, 1.25 U of Taq polymerase (AmpliTaq Gold DNA Polymerase with Gold Buffer and MgCl2; Applied Biosystems Deutschland GmbH, Darmstadt, Germany), 0.2 mM concentrations of deoxynucleoside triphosphates (Fermentas GmbH, St. Leon-Rot, Germany), 1.0 μM concentrations of each primer (synthesized by Metabion International AG, Martinsried, Germany), and water. The PCR protocol was structured as follows: 94°C for 3 min; 40 cycles at 94°C for 1 min, 45°C for 1 min, and 72°C for 2 min; followed by a final extension step at 72°C for 6 min. A 1.6% agarose gel separation with ethidium bromide staining was used to resolve and visualize the PCR products.
Preparation and extraction of murine and borrelia DNA for positive control standard.
Two mice, which were kept under specific-pathogen-free conditions as mentioned above, were sacrificed. Livers, spleens, and hearts were removed under sterile conditions. Tissue portions of 0.5 g were subjected to a phenol-chloroform-isoamyl alcohol extraction protocol as described previously (52). After extraction, DNA pellets were dried under vacuum and then dissolved in 200 μl of distilled water. B. burgdorferi sensu stricto N40, B. garinii PBi, B. afzelii PKo, and B. spielmanii A14S were grown as described previously. DNA extraction of 107 spirochetes per ml of medium was carried out as previously described. DNA was eluted in 55 μl of distilled water. Then, 55 μl of eluted DNA from 107 borrelia was used for the preparation of dilutions containing DNA from 107 to 10−2 borrelia in distilled water. Portions (200 μl) of DNA dilution from uninfected mice tissues were combined with 50 μl of each spirochete DNA dilution in a final DNA concentration of 240 ng/μl.
Extraction of DNA from mice tissues samples inoculated with Borrelia spp.
Skin tissue samples from the ear or back of mice inoculated with B. burgdorferi sensu stricto, B. garinii PBi, B. afzelii PKo, B. afzelii Slovakia, or B. spielmanii were used for DNA extraction. Additional tissue samples from three uninfected C3H mice served as negative controls. DNA extraction was carried out using a Qiagen Blood & Tissue kit.
DNA quantification.
To detect the ospA gene from B. burgdorferi sensu stricto N40 primer, probe sequences and an amplification protocol were used as previously described (44). Tests showed that the probe specific for B. burgdorferi sensu stricto ospA could detect B. garinii and B. afzelii ospA but not the corresponding B. spielmanii ospA sequence. The following primer and probe sequences were synthesized by Metabion: for B. garinii PBi ospA, BgPBi-ospA-16F (5′-AAATGTTAGCAGCCTTGATGAAA-3′) and BgPBi-ospA-119R (5′-GACTGTAATTACCATCTTTGTCTTT-3′); for B. afzelii PKo ospA, BaPKo-ospA-15F (5′-AAAATGTTAGCAGCCTTGATGAA-3′) and BaPKo-ospA-119R (5′-GACTGTACTTACCGTCTTTGTCTT-3′); and for B. spielmanii ospA, Bsp-ospA-F (5′-AATGTTAGCGGCCTTGACGAGAA-3′), Bsp-ospA-R (5′-AGGCTGTATTTACCGTCTTTGTCCT-3′), and Bsp-ospA-P (FAM-5′-AACAGCACTTCAGTAGATGTACCTGG-3′-TAMRA).
A volume of 2.5 μl of DNA per well in triplicate was used with 25 μl of Mastermix for testing. Species-specific DNA from borrelia served as the positive control in each specific quantitative PCR (qPCR) assay. Measurements were carried out with an iCycler iQ Multi-Color real-time PCR detection system (Bio-Rad Laboratories GmbH, Munich, Germany). Optimal results regarding slope, efficacy, and sensitivity could be achieved with a total DNA concentration of 120 ng/μl (300 ng/well). For every sample, threshold cycle mean values and SD were calculated and correlated to the values of the positive-standard dilution series to define the number of spirochetes per 300 ng of extracted murine DNA.
Image processing.
Images taken from Western blots, and PCR gels were processed (size, contrast, brightness, and labeling) using CorelDRAW 9 computer software.
RESULTS
Re-cultivation of spirochetes.
To determine whether the inoculated Borrelia species and strains were able to disseminate through the host's tissue, samples of murine tissues were cultivated in liquid media for eight weeks. The results are shown in Table 3. From 100% of the murine tissues and mice inoculated with B. burgdorferi sensu stricto N40 spirochetes could be reisolated, while 14 of 20 mice (70.0%) inoculated with B. afzelii PKo and 11 of 20 mice (55.0%) inoculated with B. afzelii Slovakia were culture positive. The majority of these cultures were found to be positive for heart tissue and bladder tissue samples. No tissues from mice inoculated with B. garinii PBi, B. spielmanii, B. lusitaniae, and B. valaisiana Rio6 were positive. Tissues from mice inoculated with B. valaisiana VS116 were not assayed.
TABLE 3.
Recultivation of spirochetes from murine tissues
| Borrelia inoculated | No. of mice tested/no. of mice inoculateda | No. of tissue samples positive for spirochetes/no. of cultivated tissue samples |
Total mice with Borrelia-positive tissue samples |
|||||
|---|---|---|---|---|---|---|---|---|
| Heart | Bladder | Joint | Ear | Back | No. of mice/total no. of mice examined | Rate (%) | ||
| B. burgdorferi sensu stricto N40 | 12/20 | 12/12 | 12/12 | 12/12 | 12/12 | 6/6 | 12/12 | 100.0 |
| B. garinii PBi | 18/21 | 0/18 | 0/18 | 0/18 | 0/18 | 0/15 | 0/18 | 0.0 |
| B. afzelii PKo | 20/23 | 9/20 | 6/20 | 1/20 | 5/20 | 0/17 | 14/20 | 70.0 |
| B. afzelii Slovakia | 20/21 | 6/20 | 7/20 | 4/20 | 0/20 | 2/14 | 11/20 | 55.0 |
| B. valaisiana VS116 | 0/6 | -b | - | - | - | - | - | - |
| B. valaisiana Rio6 | 8/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0/8 | 0.0 |
| B. spielmanii A14S | 18/18 | 0/18 | 0/18 | 0/18 | 0/18 | 0/12 | 0/18 | 0.0 |
| B. lusitaniae | 24/24 | 0/24 | 0/24 | 0/24 | 0/24 | 0/18 | 0/24 | 0.0 |
That is, the number of mice tested in tissue culture/the number of mice inoculated with Borrelia.
-, not tested.
IgG antibody response to B. burgdorferi sensu stricto lysate antigen KELA.
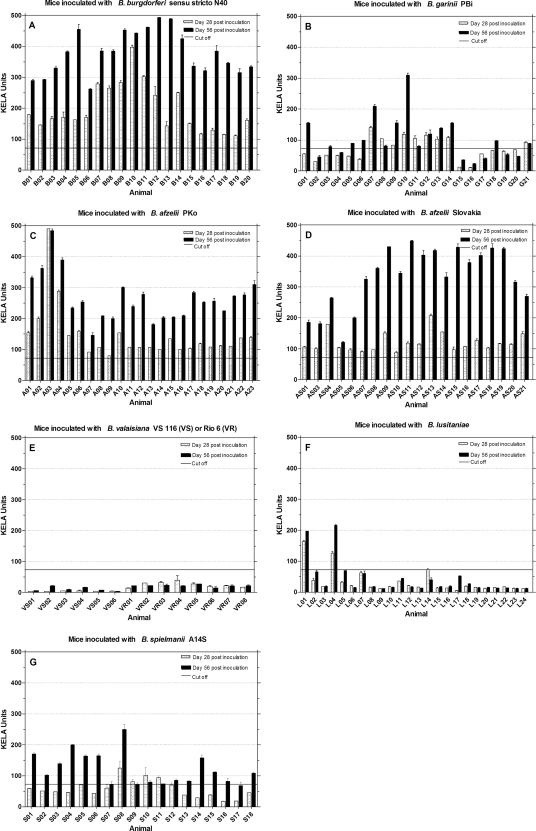
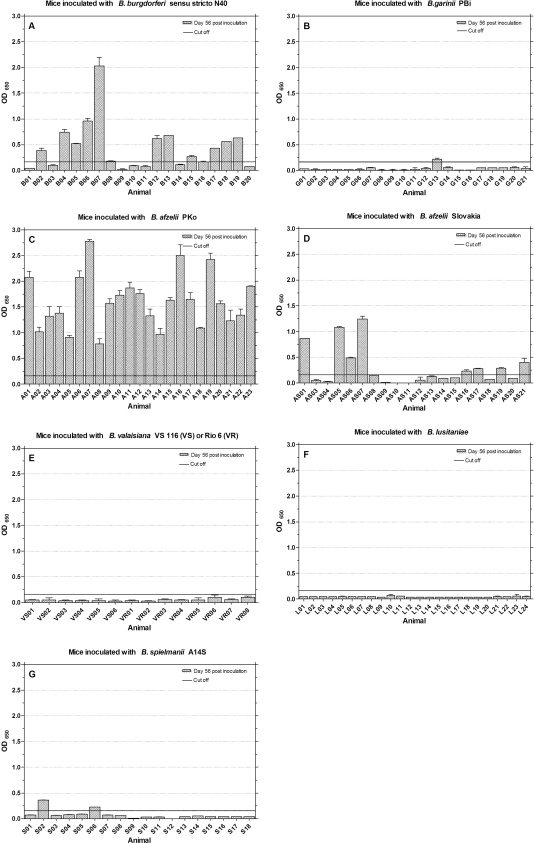
As a first serological screening test for immunological responses to borrelia, murine sera were tested at days 28 and 56 postinoculation for rising antibody reactions with a KELA based on whole-cell lysate antigen (Fig. 1). All sera from mice inoculated with B. burgdorferi sensu stricto N40 (Fig. 1A), B. afzelii PKo (Fig. 1C), and B. afzelii Slovakia (Fig. 1D) showed strong and rising total antibody levels during the first 56 days postinoculation. The group of mice which had been inoculated with B. garinii PBi reacted heterogeneously (Fig. 1B). Seven murine sera (G02, G04, G15 to G17, G19, and G20) remained antibody negative, while three mice sera (G01, G07, and G10) showed high and eleven mice sera (G03, G05, G06, G08, G09, G11 to G14, G18, and G21) showed moderate antibody levels. These variations were also found in mice inoculated with B. spielmanii A14S (Fig. 1G). Six sera (S07, S09 to S12, and S17) showed low antibody levels, while twelve sera showed a clear rising of IgG levels over time. Of 24 sera from mice inoculated with B. lusitaniae, two (L01 and L04) responded positively (Fig. 1F). The rest of the serum samples did not exceed the cutoff line, including four sera (L02, L05, L07, and L17) that showed a weak increase in antibodies over time. None of the mice which had been inoculated with B. valaisiana VS116 or Rio6 showed a detectable antibody reaction (Fig. 1E).
FIG. 1.
IgG antibody response to B. burgdorferi sensu stricto lysate antigen. Mice were inoculated with different Borrelia species (A to G), and sera were tested at days 28 and 56 postinoculation for the presence of a rising overall Borrelia IgG response. The cutoff (73.03) was set at mean KELA units value of sera from 27 uninfected mice plus five times the SD of the mean.
Confirmation of previous KELA results with Western blots based on B. afzelii lysate antigen.
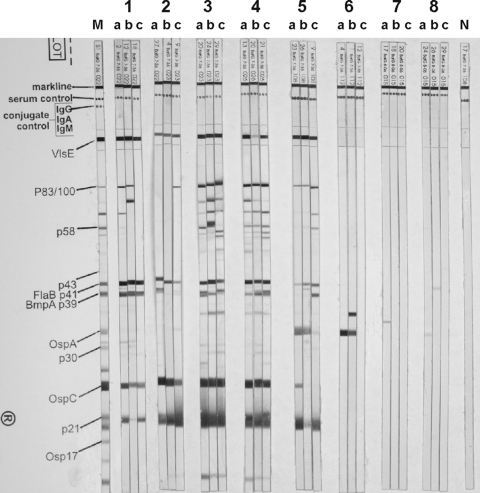
Following the two-tiered testing method for serodiagnosis, immune responses detected with ELISA were confirmed by using a lysate antigen-based Western blot with sera obtained day 56 postinoculation. When samples that had exceeded the KELA cutoff line were tested, mice were considered overall positive for a specific contact with borrelial antigen if protein bands for recombinant VlsE and two of the following bands were detectable: p83/100, p58, p43, p39, p30, p23 (OspC), p21, and p17. The number of sera that reacted against the different protein bands and the percentages are shown in Table 4. A representative selection of murine serum samples is shown in the Western blots in Fig. 2. The majority of mice (Table 4) inoculated with B. burgdorferi sensu stricto N40 (represented by number 1 and the sera B02, B12, and B19 on stripes a, b, and c) showed serum reactions against the VlsE protein, p41, p39, OspC, and p21. All sera were considered seropositive. A total of 100% of mice that were inoculated with B. afzelii PKo and 85.0% of B. afzelii Slovakia-inoculated mice showed antibody reactions against recombinant VlsE (Table 4). All sera responded against p83/p100, p41, OspC, and p21, as shown with serum samples A08, A12, and A17 for B. afzelii PKo (Fig. 2, number 3, stripes a to c) and AS07, AS14, and AS15 for B. afzelii Slovakia (Fig. 2, number 4, stripes a to c). In summary, all mice inoculated with B. afzelii were considered seropositive. Seventeen mice inoculated with B. garinii PBi showed no or weak antibody responses on Western blots (data not shown). Despite the fact that 18 sera reacted against OspC (Table 4), 17 were considered seronegative because of missing reactions against VlsE and combinations of infection-specific proteins. Three mice reacted moderately seropositive with recombinant VlsE (Fig. 2, number 2, G07, G13, and G18 on stripes a to c). In addition, these three sera produced antibody reactions against OspC and p21 and were considered seropositive. Mice inoculated with B. spielmanii A14S are represented by sera S09, S22, and S25 (Fig. 2, number 5, stripes a to c). As shown in Table 4, 94.4% of sera reacted positively against VlsE. A total of 77.8% showed antibody responses to p83/p100, and 72.2% showed antibody responses to p21. Eight sera responded to OspA (44.4%) and 16 to p41 (88.9%). The 17 VlsE-positive sera were considered seropositive. None of the mice inoculated with B. lusitaniae showed antibodies to VlsE or other proteins specific for Borrelia infections. As represented by the sera L04, L01, and L12 (Fig. 2, number 6, stripes a to c), 29.2% of the mice inoculated with B. lusitaniae (Table 4) reacted against OspA. None of the mice were considered seropositive, which is also true for mice inoculated with B. valaisiana VS116 and B. valaisiana Rio6.
TABLE 4.
Western blot immune responses of murine sera inoculated with different Borrelia species against lysate antigen proteins
| Protein | No. of sera with specific present protein bands and expressed as % of tested sera |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
B. burgdorferi sensu stricto N40 |
B. garinii PBi |
B. afzelii PKo |
B. afzelii Slovakia |
B. spielmanii A14S |
B. lusitaniae |
B. valaisiana VS116 |
B. valaisiana Rio6 |
|||||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| VlsE | 20 | 100.0 | 3 | 15.0 | 23 | 100.0 | 17 | 85.0 | 17 | 94.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| p83/p100 | 15 | 75.0 | 1 | 5.0 | 23 | 100.0 | 20 | 100.0 | 14 | 77.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| p58 | 7 | 35.0 | 1 | 5.0 | 18 | 78.3 | 11 | 55.0 | 3 | 16.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| p43 | 0 | 0.0 | 0 | 0.0 | 1 | 4.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| p41 | 19 | 95.0 | 13 | 65.0 | 23 | 100.0 | 20 | 100.0 | 16 | 88.9 | 1 | 4.2 | 0 | 0.0 | 0 | 0.0 |
| p39 | 14 | 70.0 | 4 | 20.0 | 23 | 100.0 | 17 | 85.0 | 12 | 66.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| OspA | 1 | 5.0 | 4 | 20.0 | 0 | 0.0 | 4 | 20.0 | 8 | 44.4 | 7 | 29.2 | 0 | 0.0 | 0 | 0.0 |
| p30 | 6 | 30.0 | 0 | 0.0 | 2 | 8.7 | 3 | 15.0 | 3 | 16.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| OspC | 16 | 80.0 | 18 | 90.0 | 23 | 100.0 | 20 | 100.0 | 3 | 16.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| p21 | 14 | 70.0 | 13 | 65.0 | 23 | 100.0 | 20 | 100.0 | 13 | 72.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| p17 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total sera | 20 | 100.0 | 20 | 100.0 | 23 | 100.0 | 20 | 100.0 | 18 | 100.0 | 24 | 100.0 | 6 | 100.0 | 8 | 100.0 |
FIG. 2.
Antibody response to recombinant VlsE and Borrelia lysate antigen on day 56 postinoculation in a Western blot. M, positive control marker for protein bands; N, negative murine serum. Number 1, stripes a to c: mice inoculated with B. burgdorferi sensu stricto N40 (B02, B12, and B19). Number 2, stripes a to c: mice inoculated with B. garinii PBi (G07, G13, and G18). Number 3, stripes a to c: mice inoculated with B. afzelii PKo (A08, A12, and A17). Number 4, stripes a to c: mice inoculated with B. afzelii Slovakia (AS07, AS14, and AS15). Number 5, stripes a to c: mice inoculated with B. spielmanii A14S (S09, S22, and S25). Number 6, stripes a to c: mice inoculated with B. lusitaniae (L04, L01, and L12). Number 7, stripes a to c: mice inoculated with B. valaisiana VS116 (VS02, VS03, and VS05). Number 8, stripes a to c: mice inoculated with B. valaisiana Rio6 (VR03, VR05, and VR08).
C6 peptide-specific quantitative ELISA results obtained with a commercial test system.
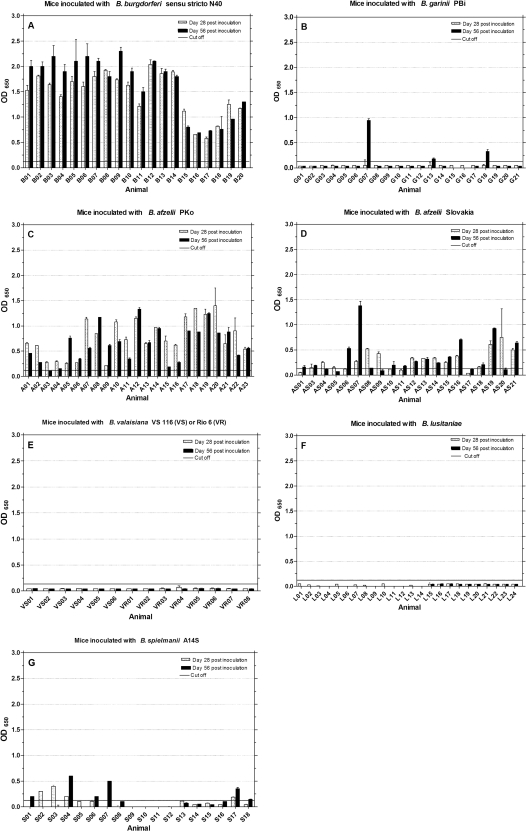
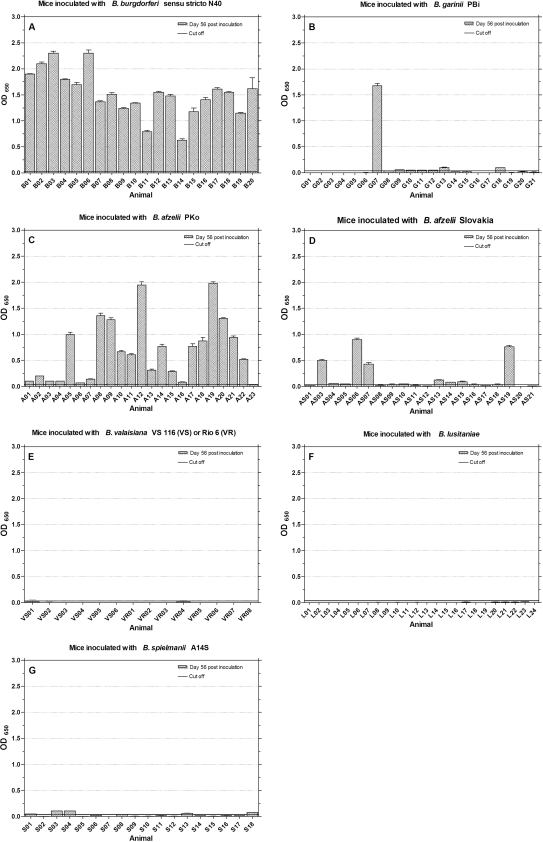
All murine sera were tested with the Lyme Quant C6 Test to document the relative level of C6-specific antibodies over time. The data are shown in Fig. 3. Mice inoculated with B. burgdorferi sensu stricto N40 developed strong and mostly rising antibody responses over time from days 28 to 56 postinoculation (μ = 1.7; σ = 0.56 at day 56) (Fig. 3A). In comparison, sera from mice inoculated with B. afzelii PKo (Fig. 3C) produced weaker C6 signals (μ = 0.65; σ = 0.36 at day 56). Thirteen mice (A01 to A04, A07, A10, A11, A15 to A18, A20, and A22) showed a decline in antibody levels from days 28 to 56. Of the mice inoculated with B. afzelii Slovakia, seven (AS04, AS05, AS08, AS09, AS12, AS14, and AS20) (Fig. 3D) showed a decline in C6 antibody levels and five (AS06, AS07, AS16, AS19, and AS21) had higher antibody reactions (OD > 0.5; μ = 0.35; σ = 0.33) at day 56. Only three mice inoculated with B. garinii PBi showed rising antibody reactions (G07, G13, and G18) with OD values of μ = 0.49 and σ = 0.40 at day 56. Six mice inoculated with B. spielmanii A14S (Fig. 3G) developed rising antibody levels over time (S01, S04, S06, S07, S17, and S18). Two sera (S02 and S03) showed a decrease in C6 antibodies from days 28 to 56 (in total μ = 0.13, σ = 0.18 at day 56). Mice inoculated with B. valaisiana or B. lusitaniae strains, with the results depicted in Fig. 3E (μ = 0.04, σ = 0.0) or Fig. 3F (μ = 0.02 and σ = 0.02), respectively, had no detectable C6 antibody response.
FIG. 3.
IgG antibody response to Lyme Quant C6 Test ELISA. Mice were inoculated with different Borrelia species (A to G), and sera were tested at days 28 and 56 postinoculation for the presence of a rising anti-C6 peptide IgG response. The cutoff (OD = 0.13) was set at mean OD value of sera from 27 uninfected mice plus five times the SD of the mean.
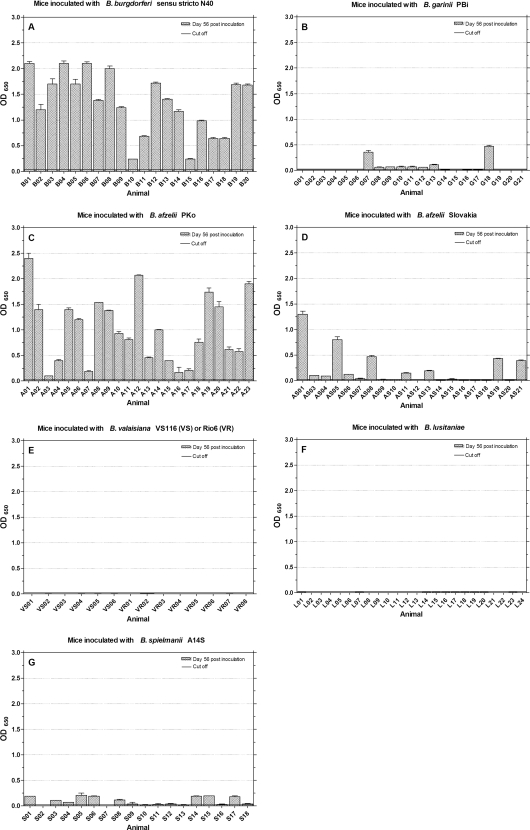
Antibody responses against Borrelia species-specific C6 peptides at day 56 postinoculation.
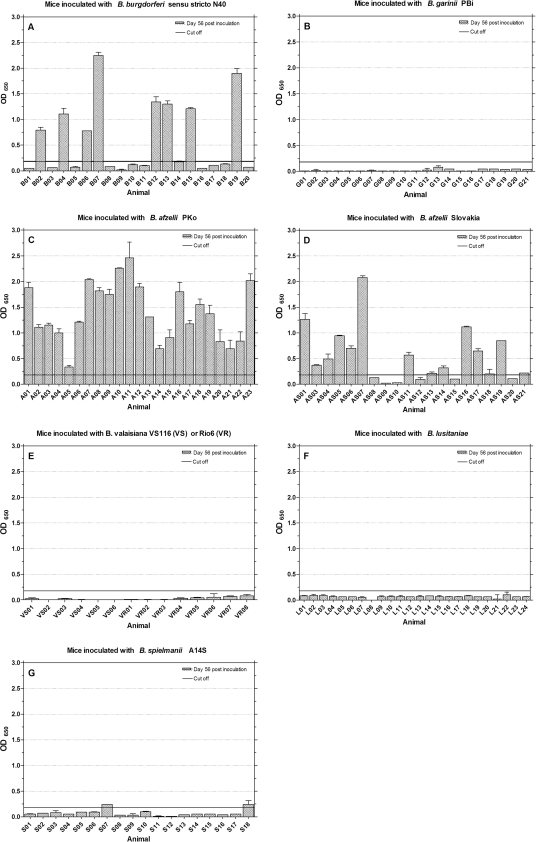
Figure 4 to 7 show IgG antibody reactions of murine sera against B. burgdorferi sensu stricto C6 (Fig. 4), B. garinii C6 (Fig. 5), and B. afzelii C6 peptides with sequence variable 1 (B. afzelii C6-1) (Fig. 6) and variable 2 (B. afzelii C6-2) (Fig. 7). Considerable overall antibody levels were found in mice inoculated with B. burgdorferi sensu stricto N40 (Fig. 4 to 7A). The highest overall responses were detected to B. garinii C6 with (μ = 1.50; σ = 0.43) in Fig. 5A, followed by B. burgdorferi C6 (μ = 1.33; σ = 0.17) in Fig. 4A. Lower and individually different immune responses were detected using B. afzelii C6-1 (μ = 0.59; σ = 0.70) and B. afzelii C6-2 (μ = 0.43; σ = 0.46). Twelve (Fig. 6A) and seven sera (Fig. 7A), respectively, did not exceed the cutoff, while specific sera showed higher antibody levels (for example, B02, B07, B12, or B19 in Fig. 6A and B04 to B07 and B17 to B19 in Fig. 7A). Mice inoculated with B. afzelii PKo (Fig. 4 to Fig. 7C) built peak antibody responses against B. afzelii C6-1 (μ = 1.40; σ = 0.55) and B. afzelii C6-2 (μ = 1.60; σ = 0.57). Some sera reacted more strongly against B. afzelii C6-1 (A08); others reacted more strongly against B. afzelii C6-2 (A05). Responses to B. burgdorferi C6 (μ = 1.00; σ = 0.65; Fig. 4C) varied. The lowest overall antibody responses were detected against B. garinii C6 peptide (μ = 0.71; σ = 0.60). Here, eight mice developed reactions of <0.25 OD (Fig. 5C).
FIG. 4.
IgG antibody response to B. burgdorferi sensu stricto C6 peptide antigen. Mice were inoculated with different Borrelia species (A to G), and sera were tested at day 56 postinoculation. The cutoff (0.02) was set at the mean OD value of sera from 27 uninfected mice plus five times the SD of the mean.
FIG. 7.
IgG antibody response to B. afzelii C6-2 peptide antigen. Mice were inoculated with different Borrelia species (A to G), and sera were tested at day 56 postinoculation. The cutoff (= 0.16) was set at mean OD value of sera from 27 uninfected mice plus five times the SD of the mean.
FIG. 5.
IgG antibody response to B. garinii C6 peptide antigen. Mice were inoculated with different Borrelia species (A to G), and sera were tested at day 56 postinoculation. The cutoff (0.04) was set at mean the OD value of sera from 27 uninfected mice plus five times the SD of the mean.
FIG. 6.
IgG antibody response to B. afzelii C6-1 peptide antigen. Mice were inoculated with different Borrelia species (A to G), and sera were tested at day 56 postinoculation. The cutoff (0.18) was set at the mean OD value of sera from 27 uninfected mice plus five times the SD of the mean.
Mice inoculated with B. afzelii Slovakia (Fig. 4 to Fig. 7D) responded most strongly to B. afzelii C6-1 (μ = 0.50; σ = 0.52), as shown in Fig. 6D, followed by peptide B. afzelii C6-2 (μ = 0.27; σ = 0.36), as shown in Fig. 7D. The highest levels were reached by the sera AS01, AS05 to AS07, AS16, AS17, AS19, and AS21. Twelve sera did not reach cutoff line (Fig. 7D). Four sera reached moderate antibody levels against B. garinii C6 in Fig. 5D (AS03, AS06, AS07, and AS19), while the overall titer was μ = 0.16 with σ = 0.20. Three mice inoculated with B. garinii PBi (G07, G13, and G18 in Fig. 5B) had detectable antibody levels against B. garinii C6 (μ = 0.10; σ = 0.36). Mouse G07 reacted the most strongly with an OD of 1.68. This distribution of antibody reactions can also be seen in Fig. 4B for B. burgdorferi C6, but the overall titers are clearly lower (μ = 0.06; σ = 0.12). Only one mouse (G13) exceeded the cutoff if B. afzelii C6-2 (μ = 0.04; σ = 0.05) antibodies were detected (Fig. 7B). No marked antibody responses could be detected against B. afzelii C6-1 (μ = 0.02; σ = 0.04), as shown in Fig. 6B. In mice inoculated with B. spielmanii (Fig. 4 to Fig. 7G), only weak overall responses to the different C6 peptides were detected. The highest levels were reached using B. burgdorferi C6 (Fig. 4G) with μ = 0.09 and σ = 0.07. Nine sera showed moderate titers with OD values of <0.25 (S01, S03 to S06, S08, S14, S15, and S17). Five sera (S01, S03, S04, S13, and S18) showed weak responses to B. garinii C6 (Fig. 5G) and overall ODs with μ = 0.03 and σ = 0.03. Two sera (S02 and S06) exceeded the cutoff with responses against B. afzelii C6-2 (Fig. 7G), while S07 and S18 showed weak reactions to B. afzelii C6-1 (Fig. 6G). No immune responses against B. burgdorferi C6, B. garinii C6, or B. afzelii C6-1 and B. afzelii C6-2 peptides were detected in sera from B. valaisiana- or B. lusitaniae-inoculated mice (Fig. 4 to Fig. 7E and F.)
Sensitivity of different C6 peptides compared to two-tiered testing method.
Table 5 demonstrates the sensitivity of different C6 peptides compared to the two-tiered testing method whose results were set as the standard. Calculations show that sensitivities of 100.0% were reached by homologous systems with C6 peptides and C6 antibodies originated from the same Borrelia species such as B. burgdorferi sensu stricto and B. garinii. In the case of C6 antibody detection against B. afzelii C6-1, B. afzelii C6-2, and B. burgdorferi C6, 100.0% sensitivity was obtained for B. afzelii PKo antibodies; however, clearly lower sensitivities of 70.6% (B. afzelii C6-1) and 47.1% (B. afzelii C6-2) were obtained when the sera of mice inoculated with B. afzelii Slovakia were tested. According to this, B. burgdorferi C6 (58.8%) and B. garinii C6 (64.7%) detected B. afzelii Slovakia antibodies with less sensitivity than B. afzelii PKo (100.0 and 95.7%, respectively). C6 antibodies against B. garinii PBi were not detected with B. afzelii C6-1, while 33.3% of the same sera reacted with B. afzelii C6-2. The highest sensitivity for B. spielmanii A14S C6 antibodies was achieved using B. burgdorferi C6 with 52.9%; it was less with B. garinii C6 (23.5%) and weakest with the two B. afzelii peptide preparations (11.8% each).
TABLE 5.
Sensitivity of different C6 peptides used as ELISA antigens compared to two-tiered testinga
| Borrelia strain | C6 peptide sensitivity (%)b |
|||
|---|---|---|---|---|
| B. burgdorferi C6 | B. garinii C6 | B. afzelii C6-1 | B. afzelii C6-2 | |
| B. burgdorferi sensu stricto N40 | 100.0 | 100.0 | 40.0 | 65.0 |
| B. garinii PBi | 100.0 | 100.0 | 0.0 | 33.3 |
| B. afzelii PKo | 100.0 | 95.7 | 100.0 | 100.0 |
| B. afzelii Slovakia | 58.8 | 64.7 | 70.6 | 47.1 |
| B. spielmanii A14S | 52.9 | 23.5 | 11.8 | 11.8 |
True positive was defined as KELA positive and Western blot positive in two-tiered testing.
C6 peptides from B. burgdorferi sensu stricto, B. garinii, and B. afzelii.
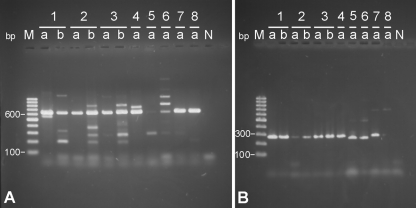
Plasmid content of Borrelia used for inoculation.
Figure 8 shows stained electrophoresis gels with PCR products from lp25 (Fig. 8A, 618 bp) and lp28-1 (Fig. 8B, 291 bp). All Borrelia strains and species with the exception of B. valaisiana VS116 (number 5a) and B. valaisiana Rio6 (number 6a) contained lp25. DNA from lp28-1 (Fig. 8B) was found in all Borrelia species and strains except B. lusitaniae (number 8a), B. valaisiana VS116 (number 5a), and B. valaisiana Rio6 (number 6a). In the case of these B. valaisiana strains, DNA products with lower sizes (∼280 bp) were found.
FIG. 8.
Detection of linear plasmids lp25 (A) and lp28-1 (B) in B. burgdorferi sensu lato (numbers 1 to 8). Number 1, B. burgdorferi sensu stricto N40; number 2, B. afzelii PKo; number 3, B. afzelii Slovakia; number 4, B. garinii PBi; number 5, B. valaisiana VS116; number 6, B. valaisiana Rio6; number 7, B. spielmanii A14S; number 8, B. lusitaniae. a, Used for inoculation; b, reisolated from murine tissue. M, marker for DNA band size in base pair length. N, negative control (water).
Quantitative detection of spirochetes in selected murine tissues.
Table 6 shows the results from qPCR analyses of DNA from the tissues of selected mice. Tissues from the ears or backs of five mice inoculated with B. burgdorferi sensu stricto N40 were tested for the presence of the ospA gene. Except for two mice (B08 and B12), specific borrelia DNA was found in all of the animals. The spirochete content per 300 ng of murine DNA was between 146,000 and 20.9 organisms. Reisolation from these mice with culture could be confirmed by qPCR. No DNA from B. garinii ospA was detected in tissues from four tested mice inoculated with B. garinii PBi, and recultivation of the spirochetes also failed. In all four tested ears from mice that were inoculated with B. afzelii PKo, DNA from B. afzelii ospA was detected with a spirochete content ranging from 19 to 222 organisms per 300 ng of murine DNA. The culture results were confirmed for three of the four mice. DNA from B. afzelii ospA was detected in the ears of two of three mice inoculated with B. afzelii Slovakia with contents ranging from 16.8 and 30.2 organisms per 300 ng of murine DNA. Three mice inoculated with B. spielmanii A14S were tested with DNA from ears and backs. ospA was present in all three samples obtained from the backs.
TABLE 6.
Quantitative detection of species-specific ospA DNA in selected murine tissues and comparison with reisolation results from skin tissue
| Borrelia strain and mouse no. | Skin type | Test resulta |
|
|---|---|---|---|
| qPCR | Culture | ||
| B. burgdorferi sensu stricto N40 | |||
| B07 | Ear | 146,000 | + |
| Back | 4,940 | + | |
| B08 | Ear | 0.0 | + |
| B12 | Ear | 0.0 | + |
| Back | 199 | + | |
| B11 | Ear | 568 | + |
| B15 | Ear | 20.9 | + |
| B20 | Ear | 531 | + |
| B. garinii PBi | |||
| G07 | Ear | 0.0 | - |
| Back | 0.0 | - | |
| G09 | Ear | 0.0 | - |
| Back | 0.0 | - | |
| G13 | Ear | 0.0 | - |
| Back | 0.0 | - | |
| G18 | Ear | 0.0 | - |
| Back | 0.0 | - | |
| B. afzelii PKo | |||
| A08 | Ear | 34.3 | - |
| A13 | Ear | 208 | + |
| A15 | Ear | 222 | + |
| A17 | Ear | 19 | + |
| B. afzelii Slovakia | |||
| AS07 | Ear | 30.2 | + |
| AS14 | Ear | 16.8 | -b |
| AS15 | Ear | 0.0 | -c |
| B. spielmanii A14S | |||
| S13 | Ear | 0.0 | - |
| Back | 13.8 | - | |
| S14 | Ear | 0.0 | - |
| Back | 13.6 | - | |
| S17 | Ear | 0.0 | - |
| Back | 32.59 | - | |
qPCR results are indicated as the number of spirochetes per 300 ng of extracted murine DNA. Culture results are indicated as positive (+) or negative (-).
Due to contamination of the culture medium, Borrelia could be reisolated from the joint.
Due to contamination of the culture medium, Borrelia could be reisolated from the bladder.
DISCUSSION
The focus of this investigation was to verify the applicability of single C6 peptides originated from defined Borrelia species for antibody testing in terms of cross-reactivity and sensitivity to species-specific C6 antibodies in the mouse. In this context, the sensitivity of these C6 peptide-based test systems was compared to the two-tiered test method using lysate antigen-based ELISA and Western blotting. Because of the experimental design of the present study, specificity as defined by an epidemiological approach was not determined. The data we obtained show clearly, that in a mouse model, antibodies against B. burgdorferi C6 or B. garinii C6 can be detected with sensitivities of 100% for each of the two corresponding Borrelia species compared to two-tiered approach (Table 5). This confirms similar results from previous studies (25, 26, 29), which used a single C6 peptide derived from B. burgdorferi or B. garinii and concluded that IR6 is antigenetically conserved among strains of B. garinii and B. burgdorferi sensu stricto. We observed that the antibody levels against B. burgdorferi sensu stricto and B. garinii were generally higher when B. garinii C6 was used as detection antigen; however, individual antibody levels varied clearly among the experimentally infected mice. For example, in mice B10 and B15 a less intense reaction to B. burgdorferi C6 than to B. garinii C6 (Fig. 4 and 5A) was observed. When we focused on the detection of C6 antibodies against the B. afzelii PKo strain and the B. afzelii Slovakia isolate, it became apparent that immune responses against C6 peptides were clearly varied. To assure genospecies identity, B. afzelii PKo and B. afzelii Slovakia were characterized by amplification and sequencing based on a 600-nucleotide fragment of their gene encoding the 16S rRNA as described previously (37). The results confirmed that both are B. afzelii (data not shown).
The overall C6 antibody levels were noticeably lower in mice inoculated with B. afzelii Slovakia compared to mice inoculated with B. afzelii PKo. Furthermore, compared to B. afzelii PKo-inoculated mice, more B. afzelii Slovakia inoculated-animals were nonreactive against the different C6 peptides (Fig. 4 to 7D). In contrast, antibody detection with Borrelia lysate antigens (KELA and Western blot) did not show broad differences between the B. afzelii isolate and the strain PKo. However, a smaller fraction of B. afzelii Slovakia-inoculated animals was responsive to recombinant VlsE in Western blot analyses (85.0%; 17 of 20 mice; Table 4), which might be a sign for a less immune effective VlsE presentation of this borrelia isolate, which probably results in lower C6 responses of sera obtained from these mice. In addition, antibody populations in the B. afzelii PKo-infected mice might bind more efficiently to C6 peptides based on B. afzelii ACAI than antibodies of B. afzelii Slovakia-infected mice. We assume that this observation may be dependent on the B. afzelii strain or isolate we used for this experiment and that there appear to be general differences in the sensitivity to detect specific antibodies against B. afzelii genospecies members. The choice of C6 peptides for European applications might be reviewed focusing on this aspect. For example, in another study, there was little difference in sensitivity using a C6 ELISA to detect antibodies against diverse ospC genotypes or strains of B. burgdorferi sensu stricto in U.S. patients (57).
In our study, the measured C6 responses against B. burgdorferi sensu stricto N40, B. garinii PBi, and B. afzelii PKo support the experimental data, which show that B. burgdorferi sensu stricto N40 successfully induces infection and clinical symptoms in dogs (45, 46) and, furthermore, B. garinii PBi and B. afzelii PKo in mice (4, 8). In addition, B. afzelii PKo and B. garinii PBi have been isolated from humans and detected in patients (11, 54) and are therefore recommended for human serodiagnosis (14).
No broad data are available regarding the prevalence of these defined Borrelia strains in naturally infected hosts such as humans and dogs or the clinical impact of the isolate B. afzelii Slovakia. For this isolate, the clinical relevance cannot be estimated. Further studies should focus especially on the impact of defined single-species or single-strain borrelia infections on the detection of C6 antibodies, including their potential inducing clinical signs in different susceptible hosts.
Standardized experimental conditions in our mouse model allowed a careful characterization of the C6 peptide antibody detection capabilities in animals with defined monoinfections. The results of the present study show that the use of C6 peptides derived from B. garinii and B. burgdorferi works best for the detection of immune responses against B. burgdorferi, B. garinii, and B. afzelii PKo. Looking at the quantities of B. afzelii antibodies detected with B. afzelii C6-1 or B. afzelii C6-2, it can be concluded that both peptides are sufficiently reactive to detect B. afzelii PKo (100.0% each). B. afzelii C6-1 is more sensitive for the detection of antibodies against B. afzelii Slovakia than B. afzelii C6-2.
A quantitative C6 ELISA test, which was originally developed to monitor changes in C6 antibody levels in dogs, was used to demonstrate changes in C6 antibody levels over time (day 28 to day 56). This test kit was adequate in terms of sensitivity for the detection of C6 antibodies against B. burgdorferi sensu stricto N40, B. garinii PBi (100.0%), B. afzelii PKo (95.7%), and B. afzelii Slovakia (88.2%) compared to two-tiered testing (data not shown). Antibodies against B. spielmanii were detected with a sensitivity of 35.3%. Unexpectedly, detectable C6 antibody levels to B. afzelii declined from days 28 to 56, which could be observed in sera from mice inoculated with B. afzelii PKo and in sera from mice inoculated with B. afzelii Slovakia (Fig. 3C and D). This decline may be part of individual variations in mice over time and a reflection of the specific characteristics of the C6 immune responses induced by B. afzelii. Another explanation may be that some strains of B. afzelii are less C6 immunoreactive in mice, resulting in a corresponding decline of the C6 antibody level. However, viable spirochetes could be isolated from B. afzelii-inoculated mice via tissue culture, which confirms the ongoing process of infection.
Regarding characteristics of inoculated species used in the murine model, B. spielmanii A14S was not reisolated from mice, which may be a result of the demands of this particular strain for in-vitro growth conditions (data not shown). lp25 and lp28-1 were present in the organisms used for inoculation and DNA from B. spielmanii ospA was detected in all mice that were tested. Interestingly, B. spielmanii-specific DNA was not detected in skin tissues from ear but was detected in skin from the back near the injection site. This may be a result of B. spielmanii-specific dissemination behavior and tissue presence in murine hosts. In summary, low C6 antibody levels were detected in mice inoculated with B. spielmanii A14S. The assay using the C6 peptide derived from B. burgdorferi sensu stricto showed the highest sensitivity (52.9%). The B. garinii C6, B. afzelii C6-1, and B. afzelii C6-2 peptides were clearly less capable of detecting specific antibodies. To our knowledge, this is the first investigation using C6 peptides to document B. spielmanii immune responses. No data are available for the vlsE gene sequences of B. spielmanii. Western blots show that antibodies against VlsE are produced as a result of the host's B. spielmanii-specific immune response. Phenomena such as antigenic variation including the invariable regions of VlsE, recombination, and segmental gene conversion occurrence similar to that described in B. burgdorferi sensu stricto B31 (58-60) might be expected. Specific IR6 sequences and C6 peptides derived from B. spielmanii VlsE should be investigated for future applications.
Serial in vitro passages of Borrelia can result in the loss of infectivity (33, 40, 47). This may have been one reason why only three mice inoculated with B. garinii PBi reacted clearly seropositive and none of the 21 mice was culture positive or PCR positive, although lp25 and lp28-1 were present in the inoculated spirochetes. B. lusitaniae contained lp25, but the reduced infection rate may have be due to the lack of lp28-1, as previously shown for B. burgdorferi sensu stricto B31 (19). This would explain the absence of the immune responses against VlsE and the C6 peptides. Regarding the results for B. valaisiana VS116 and Rio6, no positive results could be obtained for culture, serology, or plasmid PCR. For this reason, we concluded that these passages of strains most likely are not infective in mice.
Our data verify that the use of Borrelia lysate antigen as a two-tiered approach with ELISA, followed by Western blotting, is a sensitive and specific method for the detection of antibodies against B. burgdorferi sensu stricto, B. garinii, and B. afzelii. We can furthermore show that this test system detects antibodies directed against B. spielmanii and B. lusitaniae.
The addition of VlsE to the diagnostic test systems (30, 35) could specify the decision for the presence of acute Lyme borreliosis. With the use of recombinant VlsE in our study, the immune responses to successful infections with B. burgdorferi sensu lato was confirmed and was related to the lp28-1 contents of spirochetes used for infection. Despite the time-consuming method and difficult interpretation criteria, the two-tiered test-systems is still recommended primarily because of its increased sensitivity for European strains of B. burgdorferi sensu lato.
In summary, we conclude that the use of the present single-step C6 peptide test system to detect murine immune responses against B. burgdorferi sensu lato occurring in Europe is excellent in terms of sensitivity, when sera are tested only for antibodies against B. burgdorferi sensu stricto, B. garinii, and B. afzelii PKo. In our study we found that antibodies against the isolate B. afzelii Slovakia were less reactive with the C6 antigen compared to B. afzelii PKo. To understand the impact of this finding on the C6 test performance in Europe, it would be important to analyze B. afzelii Slovakia's prevalence and pathogenicity versus the other recognized strains of B. burgdorferi sensu lato. A combination of C6 antigens originally derived from different strains of B. burgdorferi, B. garinii, or B. afzelii may provide improved sensitivity. Further experimental investigations in natural hosts such as dogs should be performed to clarify the transferability of the results obtained from our experimental murine infection model.
Acknowledgments
We are very grateful to following scientists for the providing the Borrelia species and strains used in this study: Margarida Collares-Pereira (Institute of Hygiene and Tropical Medicine, Lisbon, Portugal) for B. lusitaniae; Christian Epe (Institute of Parasitology, University of Veterinary Medicine, Hannover, Germany) for B. spielmanii A14S; Raquel Escudero (Servicio de Bacteriología, Centro Nacional de Microbiología-Instituto de Salud Carlos III, Madrid, Spain) for B. valaisiana Rio6; Maria Kazimirova (Institute of Zoology, Slovak Academy of Sciences, Bratislava, Slovakia) for the B. afzelii isolate; and Bettina Wilske (Max von-Pettenkofer Institute, Munich, Germany) for B. garinii PBi and B. afzelii PKo. We thank Dania Richter (Institute of Pathology, Section Parasitology, Charité Berlin, Germany) for the sequencing and confirmation of Borrelia species identity. We are grateful to Viktor Dyachenko (Institute of Parasitology, College of Veterinary Medicine, University of Leipzig, Leipzig, Germany) for scientific and technical assistance.
Footnotes
Published ahead of print on 2 September 2009.
REFERENCES
- 1.Appel, M. J. G., S. Allen, R. H. Jacobson, T.-L. Lauderdale, Y.-F. Chang, S. J. Shin, J. W. Thomford, R. J. Todhunter, and B. A. Summers. 1993. Experimental Lyme disease in dogs produces arthritis and persistent infection. J. Infect. Dis. 167:651-664. [DOI] [PubMed] [Google Scholar]
- 2.Balmelli, T., and J. C. Piffaretti. 1995. Association between different clinical manifestations of Lyme disease and different species of Borrelia burgdorferi sensu lato. Res. Microbiol. 146:329-340. [DOI] [PubMed] [Google Scholar]
- 3.Baranton, G., D. Postic, G. Saint, I., P. Boerlin, J. C. Piffaretti, M. Assous, and P. A. Grimont. 1992. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int. J. Syst. Bacteriol. 42:378-383. [DOI] [PubMed] [Google Scholar]
- 4.Barthold, S. W. 1999. Specificity of infection-induced immunity among Borrelia burgdorferi sensu lato species. Infect. Immun. 67:36-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cinco, M., and R. Murgia. 2006. Evaluation of the C6 enzyme-linked immunoadsorbent assay for the serodiagnosis of Lyme borreliosis in northeastern Italy. New Microbiol. 29:139-141. [PubMed] [Google Scholar]
- 6.Collares-Pereira, M., S. Couceiro, I. Franca, K. Kurtenbach, S. M. Schafer, L. Vitorino, L. Goncalves, S. Baptista, M. L. Vieira, and C. Cunha. 2004. First isolation of Borrelia lusitaniae from a human patient. J. Clin. Microbiol. 42:1316-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coutte, L., D. J. Botkin, L. Gao, and S. J. Norris. 2009. Detailed analysis of sequence changes occurring during vlsE antigenic variation in the mouse model of Borrelia burgdorferi infection. PLoS Pathog. 5:e1000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Craig-Mylius, K. A., M. Lee, K. L. Jones, and L. J. Glickstein. 2009. Arthritogenicity of Borrelia burgdorferi and Borrelia garinii: comparison of infection in mice. Am. J. Trop. Med. Hyg. 80:252-258. [PubMed] [Google Scholar]
- 9.Diza, E., A. Papa, E. Vezyri, S. Tsounis, I. Milonas, and A. Antoniadis. 2004. Borrelia valaisiana in cerebrospinal fluid. Emerg. Infect. Dis. 10:1692-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escudero, R., M. Barral, A. Perez, M. M. Vitutia, A. L. Garcia-Perez, S. Jimenez, R. E. Sellek, and P. Anda. 2000. Molecular and pathogenic characterization of Borrelia burgdorferi sensu lato isolates from Spain. J. Clin. Microbiol. 38:4026-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fingerle, V., U. C. Schulte-Spechtel, E. Ruzic-Sabljic, S. Leonhard, H. Hofmann, K. Weber, K. Pfister, F. Strle, and B. Wilske. 2007. Epidemiological aspects and molecular characterization of Borrelia burgdorferi sensu lato from southern Germany with special respect to the new species Borrelia spielmanii sp. nov. Int. J. Med. Microbiol. 298:279-290. [DOI] [PubMed] [Google Scholar]
- 12.Gomes-Solecki, M. J., L. Meirelles, J. Glass, and R. J. Dattwyler. 2007. Epitope length, genospecies dependency, and serum panel effect in the IR6 enzyme-linked immunosorbent assay for detection of antibodies to Borrelia burgdorferi. Clin. Vaccine Immunol. 14:875-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottner, G., U. Schulte-Spechtel, and B. Wilske. 2004. Heterogeneity of the immunodominant surface protein VlsE among the three genospecies of Borrelia burgdorferi pathogenic for humans. Int. J. Med. Microbiol. 293(Suppl. 37):172-173. [DOI] [PubMed] [Google Scholar]
- 14.Hauser, U., G. Lehnert, R. Lobentanzer, and B. Wilske. 1997. Interpretation criteria for standardized Western blots for three European species of Borrelia burgdorferi sensu lato. J. Clin. Microbiol. 35:1433-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauser, U., G. Lehnert, and B. Wilske. 1999. Validity of interpretation criteria for standardized Western blots (immunoblots) for serodiagnosis of Lyme borreliosis based on sera collected throughout Europe. J. Clin. Microbiol. 37:2241-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hovius, J. W., X. Li, N. Ramamoorthi, A. P. van Dam, S. W. Barthold, P. T. van der, P. Speelman, and E. Fikrig. 2007. Coinfection with Borrelia burgdorferi sensu stricto and Borrelia garinii alters the course of murine Lyme borreliosis. FEMS Immunol. Med. Microbiol. 49:224-234. [DOI] [PubMed] [Google Scholar]
- 17.Kazimirova, M., M. Derdakova, E. Dvoroznakova, J. Koci, V. Taragelova, D. Selyemova, E. Eleckova, and M. Labuda. 2005. Experimental infection of C3H/N mice with Borrelia afzelii, abstr. 54. 10th International Conference on Lyme Borreliosis and Other Tick-borne Diseases, Vienna, Austria.
- 18.Labandeira-Rey, M., J. Seshu, and J. T. Skare. 2003. The absence of linear plasmid 25 or 28-1 of Borrelia burgdorferi dramatically alters the kinetics of experimental infection via distinct mechanisms. Infect. Immun. 71:4608-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labandeira-Rey, M., and J. T. Skare. 2001. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69:446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lawrenz, M. B., J. M. Hardham, R. T. Owens, J. Nowakowski, A. C. Steere, G. P. Wormser, and S. J. Norris. 1999. Human antibody responses to VlsE antigenic variation protein of Borrelia burgdorferi. J. Clin. Microbiol. 37:3997-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le, F. A., D. Postic, K. Girardet, O. Peter, and G. Baranton. 1997. Characterization of Borrelia lusitaniae sp. nov. by 16S ribosomal DNA sequence analysis. Int. J. Syst. Bacteriol. 47:921-925. [DOI] [PubMed] [Google Scholar]
- 22.Levy, S., T. P. O'Connor, J. L. Hanscom, and P. Shields. 2002. Utility of an in-office C6 ELISA test kit for determination of infection status of dogs naturally exposed to Borrelia burgdorferi. Vet. Ther. 3:308-315. [PubMed] [Google Scholar]
- 23.Levy, S. A. 2002. Use of a C6 ELISA test to evaluate the efficacy of a whole-cell bacterin for the prevention of naturally transmitted canine Borrelia burgdorferi infection. Vet. Ther. 3:420-424. [PubMed] [Google Scholar]
- 24.Levy, S. A., T. P. O'Connor, J. L. Hanscom, P. Shields, L. Lorentzen, and A. A. Dimarco. 2008. Quantitative measurement of C6 antibody following antibiotic treatment of Borrelia burgdorferi antibody-positive nonclinical dogs. Clin. Vaccine Immunol. 15:115-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang, F. T., E. Aberer, M. Cinco, L. Gern, C. M. Hu, Y. N. Lobet, M. Ruscio, P. E. Voet, Jr., V. E. Weynants, and M. T. Philipp. 2000. Antigenic conservation of an immunodominant invariable region of the VlsE lipoprotein among European pathogenic genospecies of Borrelia burgdorferi SL. J. Infect. Dis. 182:1455-1462. [DOI] [PubMed] [Google Scholar]
- 26.Liang, F. T., R. H. Jacobson, R. K. Straubinger, A. Grooters, and M. T. Philipp. 2000. Characterization of a Borrelia burgdorferi VlsE invariable region useful in canine Lyme disease serodiagnosis by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 38:4160-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang, F. T., and M. T. Philipp. 1999. Analysis of antibody response to invariable regions of VlsE, the variable surface antigen of Borrelia burgdorferi. Infect. Immun. 67:6702-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang, F. T., and M. T. Philipp. 2000. Epitope mapping of the immunodominant invariable region of Borrelia burgdorferi VlsE in three host species. Infect. Immun. 68:2349-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang, F. T., A. C. Steere, A. R. Marques, B. J. Johnson, J. N. Miller, and M. T. Philipp. 1999. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi vlsE. J. Clin. Microbiol. 37:3990-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marangoni, A., A. Moroni, S. Accardo, and R. Cevenini. 2008. Borrelia burgdorferi VlsE antigen for the serological diagnosis of Lyme borreliosis. Eur. J. Clin. Microbiol. Infect. Dis. 27:349-354. [DOI] [PubMed] [Google Scholar]
- 31.Moody, K. D., and S. W. Barthold. 1995. Animal models of human disease: Lyme borreliosis in laboratory mice. Comp. Pathol. Bull. 1995:4-6. [Google Scholar]
- 32.Moody, K. D., G. A. Terwilliger, G. M. Hansen, and S. W. Barthold. 1994. Experimental Borrelia burgdorferi infection in Peromyscus leucopus. J. Wildl. Dis. 30:155-161. [DOI] [PubMed] [Google Scholar]
- 33.Norris, S. J., J. K. Howell, S. A. Garza, M. S. Ferdows, and A. G. Barbour. 1995. High- and low-infectivity phenotypes of clonal populations of in vitro-cultured Borrelia burgdorferi. Infect. Immun. 63:2206-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Connor, T. P., K. J. Esty, J. L. Hanscom, P. Shields, and M. T. Philipp. 2004. Dogs vaccinated with common Lyme disease vaccines do not respond to IR6, the conserved immunodominant region of the VlsE surface protein of Borrelia burgdorferi. Clin. Diagn. Lab. Immunol. 11:458-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peltomaa, M., G. McHugh, and A. C. Steere. 2004. The VlsE (IR6) peptide ELISA in the serodiagnosis of Lyme facial paralysis. Otol. Neurotol. 25:838-841. [DOI] [PubMed] [Google Scholar]
- 36.Richter, D., D. B. Schlee, R. Allgower, and F. R. Matuschka. 2004. Relationships of a novel Lyme disease spirochete, Borrelia spielmanii sp. nov., with its hosts in central Europe. Appl. Environ. Microbiol. 70:6414-6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richter, D., D. B. Schlee, and F. R. Matuschka. 2003. Relapsing fever-like spirochetes infecting European vector tick of Lyme disease agent. Emerg. Infect. Dis. 9:697-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ruzic-Sabljic, E., and F. Strle. 2004. Comparison of growth of Borrelia afzelii, B. garinii, and B. burgdorferi sensu stricto in MKP and BSK-II medium. Int. J. Med. Microbiol. 294:407-412. [DOI] [PubMed] [Google Scholar]
- 39.Saito, K., T. Ito, N. Asashima, M. Ohno, R. Nagai, H. Fujita, N. Koizumi, A. Takano, H. Watanabe, and H. Kawabata. 2007. Case report: Borrelia valaisiana infection in a Japanese man associated with traveling to foreign countries. Am. J. Trop. Med. Hyg. 77:1124-1127. [PubMed] [Google Scholar]
- 40.Schwan, T. G., W. Burgdorfer, and C. F. Garon. 1988. Changes in infectivity and plasmid profile of the Lyme disease spirochete, Borrelia burgdorferi, as a result of in vitro cultivation. Infect. Immun. 56:1831-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sillanpaa, H., P. Lahdenne, H. Sarvas, M. Arnez, A. Steere, M. Peltomaa, and I. Seppala. 2007. Immune responses to borrelial VlsE IR6 peptide variants. Int. J. Med. Microbiol. 297:45-52. [DOI] [PubMed] [Google Scholar]
- 42.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 43.Steere, A. C., R. T. Schoen, and E. Taylor. 1987. The clinical evolution of Lyme arthritis. Ann. Intern. Med. 107:725-731. [DOI] [PubMed] [Google Scholar]
- 44.Straubinger, R. K. 2000. PCR-Based quantification of Borrelia burgdorferi organisms in canine tissues over a 500-day postinfection period. J. Clin. Microbiol. 38:2191-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Straubinger, R. K., A. F. Straubinger, B. A. Summers, R. H. Jacobson, and H. N. Erb. 1998. Clinical manifestations, pathogenesis, and effect of antibiotic treatment on Lyme borreliosis in dogs. Wien. Klin. Wochenschr. 110:874-881. [PubMed] [Google Scholar]
- 46.Straubinger, R. K., B. A. Summers, Y. F. Chang, and M. J. Appel. 1997. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J. Clin. Microbiol. 35:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas, V., J. Anguita, S. Samanta, P. A. Rosa, P. Stewart, S. W. Barthold, and E. Fikrig. 2001. Dissociation of infectivity and pathogenicity in Borrelia burgdorferi. Infect. Immun. 69:3507-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tjernberg, I., T. Schon, J. Ernerudh, A. C. Wistedt, P. Forsberg, and I. Eliasson. 2008. C6-peptide serology as diagnostic tool in neuroborreliosis. APMIS 116:393-399. [DOI] [PubMed] [Google Scholar]
- 49.Tjernberg, I., H. Sillanpaa, I. Seppala, I. Eliasson, P. Forsberg, and P. Lahdenne. 2009. Antibody responses to Borrelia IR(6) peptide variants and the C6 peptide in Swedish patients with erythema migrans. Int. J. Med. Microbiol. 299:439-446. [DOI] [PubMed] [Google Scholar]
- 50.Toepfer, K. H., and R. K. Straubinger. 2007. Characterization of the humoral immune response in dogs after vaccination against the Lyme borreliosis agent A study with five commercial vaccines using two different vaccination schedules. Vaccine 25:314-326. [DOI] [PubMed] [Google Scholar]
- 51.Wang, G., A. P. van Dam, and J. Dankert. 1999. Phenotypic and genetic characterization of a novel Borrelia burgdorferi sensu lato isolate from a patient with Lyme borreliosis. J. Clin. Microbiol. 37:3025-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiley, J. 1993. Preparation of genomic DNA from mammalian tissue, ch. 10.1-10.1.3 and 10.2.1-10.2.3. In R. E. Coico (ed.), Current protocols in immunology, vol. 2. John Wiley & Sons, Inc., New York, NY. [Google Scholar]
- 53.Wilske, B. 2003. Diagnosis of Lyme borreliosis in Europe. Vector-Borne Zoonotic Dis. 3:215-227. [DOI] [PubMed] [Google Scholar]
- 54.Wilske, B., U. Busch, H. Eiffert, V. Fingerle, H. W. Pfister, D. Rossler, and V. Preac-Mursic. 1996. Diversity of OspA and OspC among cerebrospinal fluid isolates of Borrelia burgdorferi sensu lato from patients with neuroborreliosis in Germany. Med. Microbiol. Immunol. 184:195-201. [DOI] [PubMed] [Google Scholar]
- 55.Wilske, B., V. Fingerle, V. Preac-Mursic, S. Jauris-Heipke, A. Hofmann, H. Loy, H. W. Pfister, D. Rossler, and E. Soutschek. 1994. Immunoblot using recombinant antigens derived from different genospecies of Borrelia burgdorferi sensu lato. Med. Microbiol. Immunol. 183:43-59. [DOI] [PubMed] [Google Scholar]
- 56.Wilske, B., C. Habermann, V. Fingerle, B. Hillenbrand, S. Jauris-Heipke, G. Lehnert, I. Pradel, D. Rossler, and U. Schulte-Spechtel. 1999. An improved recombinant IgG immunoblot for serodiagnosis of Lyme borreliosis. Med. Microbiol. Immunol. 188:139-144. [DOI] [PubMed] [Google Scholar]
- 57.Wormser, G. P., D. Liveris, K. Hanincova, D. Brisson, S. Ludin, V. J. Stracuzzi, M. E. Embers, M. T. Philipp, A. Levin, M. Guero-Rosenfeld, and I. Schwartz. 2008. Effect of Borrelia burgdorferi genotype on the sensitivity of C6 and two-tier testing in North American patients with culture-confirmed Lyme disease. Clin. Infect. Dis. 47:910-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang, J. R., J. M. Hardham, A. G. Barbour, and S. J. Norris. 1997. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell 89:275-285. [DOI] [PubMed] [Google Scholar]
- 59.Zhang, J. R., and S. J. Norris. 1998. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversion. Infect. Immun. 66:3698-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang, J. R., and S. J. Norris. 1998. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect. Immun. 66:3689-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]