Abstract
More than 20% of adults are persistently colonized with Staphylococcus aureus. When hospitalized, these carriers have increased risks of infection with their own strains. However, a recent study demonstrated a lower incidence of bacteremia-related death among carriers than among noncarriers, raising the question whether the adaptive immune system plays a protective role. In fact, S. aureus carriers mount a highly specific neutralizing antibody response against superantigens of their colonizing strains. We now used 2-dimensional immunoblotting to investigate the profiles of antibodies from healthy individuals against S. aureus extracellular proteins. Moreover, we tested whether symptom-free experimental colonization of these individuals with an S. aureus strain of low virulence, 8325-4, is sufficient to induce an antibody response. Sera obtained before and 4 weeks after colonization were screened for immunoglobulin G (IgG) antibody binding to extracellular staphylococcal proteins. At baseline, most volunteers harbored IgG directed against conserved virulence factors, including alpha-hemolysin (Hla), beta-hemolysin (Hlb), phospholipase C (Plc), staphylococcal serine protease (SspA), and cysteine protease (SspB). However, the variability of spot patterns and intensities was striking and could be important in case of infection. Experimental nasal colonization with S. aureus 8325-4 did not elicit new antibodies or boost the humoral response. Thus, the high antibody prevalence in humans is likely not induced by short-term nasal colonization, and presumably minor infections are required to trigger anti-S. aureus antibody responses.
Staphylococcus aureus is one of the most common causes of nosocomial infection, and the species is becoming increasingly resistant to antibiotics (2). Apart from being a major human pathogen, S. aureus is also a frequent colonizer of human skin and mucosa (34). The bacteria find their primary ecological niche in the human nose but are also able to colonize the throat, the intestines, and the perineal region, sometimes exclusively (1, 17). Approximately 20% of the adult population carry S. aureus in the nose persistently, and another 30% carry it intermittently, frequently only for a few days, whereas 50% are noncarriers (NC) (29, 30, 34). Nasal carriers stand an increased risk of developing severe S. aureus infections caused by their autologous strains, especially upon hospitalization or immune suppression (32, 35). This underlines the fact that host and environmental factors play a decisive role in determining the outcome of S. aureus host interactions.
In a recent large prospective study, carriers acquired S. aureus bacteremia more frequently than NC but, surprisingly, had a better survival rate than NC (35). This observation raises the question whether the adaptive immune system establishes immunity to the colonizing S. aureus strain, which could be of advantage in autologous infections. In support of this hypothesis, our group recently showed that S. aureus carriers raise a strong and strain-specific antibody response against the superantigen cocktail produced by their colonizing strain (12). However, S. aureus produces a broad repertoire of virulence factors, and the antibody response against superantigens is likely only the tip of an iceberg (8). In fact, anti-S. aureus antibodies against staphylococcal toxins, immune evasion molecules, and adhesins have been detected in healthy individuals as well as in patients (6, 7, 11, 31).
Virulence factor expression is strictly regulated in S. aureus. While adhesins are expressed by bacterial cells in logarithmic growth, the majority of known virulence factors, including most superantigens but also cytolytic toxins, proteases, lipases, and several immune evasion molecules, are secreted in the post-exponential-growth phase (23, 38). In contrast to intracellular and cell wall-associated proteins, secreted virulence factors can act systemically while bacteria remain localized. Consequently, these factors are the most likely stimuli of the adaptive immune system during epithelial colonization with S. aureus (28).
To date, a comprehensive investigation of anti-S. aureus antibody profiles from healthy individuals and their variability is still lacking. Moreover, it remains unknown which conditions (e.g., nasal colonization, minor or major infections) are required to trigger an antibody response against S. aureus. Therefore, we experimentally colonized the nares of 16 healthy human volunteers with S. aureus (36) and compared the anti-S. aureus antibody profiles before and 28 days after colonization. Our aims were to analyze the variability of the anti-S. aureus antibody profiles and to test whether experimental nasal colonization elicits or boosts an antibody response.
MATERIALS AND METHODS
S. aureus strains.
The superantigen-negative S. aureus strain 8325-4 was used for experimental nasal colonization of human volunteers as described previously (36). The genome sequence of the original 8325 strain is available at http://www.genome.ou.edu (NCBI database, accession no. NC_007795). Strain 8325-4 differs from strain 8325 by the absence of three prophages (20). DU5997 is a clfB-deficient mutant of 8325-4, and 8325-4 Δspa is a protein A-deficient mutant of 8325-4 (21, 36).
Study design and human experimental colonization.
Wertheim et al. conducted a human experimental colonization study of 16 healthy volunteers (36). Among them were six persistent carriers (PC), two intermittent carriers (IC), and eight NC (Table 1). In short, all enrolled volunteers underwent a decolonization treatment with mupirocin 5 weeks before experimental colonization. Volunteers were inoculated with a mixture of S. aureus strains 8325-4 and DU5997 (ΔclfB) in one nostril and with either 8325-4 or DU5997 in the other nostril (1 × 107 CFU of each strain). Follow-up cultures were performed on days 1, 2, 3, 4, 8, 15, 22, 28, and 29 after inoculation.
TABLE 1.
Characteristics of volunteers
| Volunteer no. | Carriage status | Day from which culture was negative |
|---|---|---|
| 12 | PC | 4 |
| 141 | IC | Remained a carrier |
| 165 | NC | 4 |
| 211 | NC | 4 |
| 216 | PC | 8 |
| 600 | NC | 29 |
| 601 | PC | Remained a carrier |
| 602 | IC | Remained a carrier |
| 603 | PC | 29 |
| 604 | NC | 3 |
| 605 | NC | 3 |
| 606 | PC | 3 |
| 607 | PC | 22 |
| 608 | NC | 15 |
| 609 | NC | 15 |
| 610 | NC | 28 |
Sera.
Serum samples were obtained from all 16 volunteers directly before experimental inoculation and 28 days afterwards. Sera were aliquoted and stored at −80°C.
Growth conditions of bacteria and preparation of protein extracts.
In this study, S. aureus strain NCTC8325-4 and its isogenic spa mutant DU5875 (8325-4 Δspa) were used. For the preparation of protein extracts, S. aureus 8325-4 and its isogenic spa mutant were inoculated into tryptic soy broth to an optical density at 540 nm (OD540) of 0.05 and were cultivated in 1.5 liters of tryptic soy broth at 37°C and 110 rpm until the bacterial culture entered the stationary phase at an OD540 of 8 to 10.
For preparation of the extracellular protein fraction, S. aureus cells were removed by centrifugation (9,164 × g for 10 min at 4°C), and extracellular proteins from three 1.5-liter culture supernatants were precipitated by addition of trichloroacetic acid to 10% (wt/vol) and centrifugation at 9,164 × g for 1 h at 4°C. Protein-containing pellets were then washed six times with 70% ethanol. After the final washing step, 100% ethanol was added and then removed by centrifugation (5 min, 16,060 × g, 4°C), and the protein pellet was air dried and finally resolved in rehydration buffer containing 8 M urea, 2 M thiourea, and 2% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate}. The protein concentration was determined according to Bradford (3) with a commercially available kit (Pierce, Thermo Scientific, Bonn, Germany). Sample aliquots were stored at −80°C.
Two-dimensional (2D) polyacrylamide gel electrophoresis (PAGE) with 2D minigels.
Isoelectric focusing was done with 7-cm Immobiline dry strips (GE Healthcare, Munich, Germany) with the pH ranges 4 to 7 and 6 to 11. For separations in the pI range from 4 to 7, strips were rehydrated overnight in 150 μl rehydration solution (8 M urea, 2 M thiourea, 33 mM CHAPS, 28 mM dithiothreitol [DTT], 2% Pharmalyte 3-10 carrier ampholytes, and a trace of bromophenol blue) containing 30 μg of protein extract. Strips were placed in a Multiphor II horizontal electrophoresis system (GE Healthcare), and proteins were separated at 20°C at a maximum of 2 mA and 5 W per strip by applying the following voltage profile: 200 V constant for 1 min, a gradient from 200 V to 3,500 V for 1.5 h, and 3,500 V constant for 1.5 h (total, 8,000 V·h).
For separations in the pI range from 6 to 11, strips were rehydrated overnight in 150 μl rehydration solution (8 M urea, 2 M thiourea, 10% [vol/vol] isopropanol, 5% [vol/vol] glycerol, 162 mM DTT, 1% [vol/vol] Pharmalyte 3-10, 1% [vol/vol] Pharmalyte 8.5-10.5, and a trace of bromophenol blue) without proteins, and 30 μg of crude protein extract was loaded with the aid of sample cups at the anode. The isoelectric focusing was done in a Multiphor II device at 20°C, 2 mA, and 5 W with the following voltage profile: 200 V constant for 1 min, a gradient from 200 V to 3,500 V for 1.5 h, and 3,500 V constant for 1 h 5 min (total, 6,500 V·h). After isoelectric focusing, the strips were either frozen for storage or immediately equilibrated with solution A (20% [vol/vol] glycerol, 0.375 M Tris-HCl [pH 8.8], 6 M urea, 4% [wt/vol] sodium dodecyl sulfate [SDS], and 65 mM DTT) and solution B (20% [vol/vol] glycerol, 0.375 M Tris-HCl [pH 8.8], 6 M urea, 4% [wt/vol] SDS, 135 mM iodoacetamide, and a trace of bromophenol blue) for 15 min each. Separation according to apparent molecular weight by SDS-PAGE in the second dimension was performed for 12 samples in parallel on 12.5% PAGE gels using a vertical Mini Protean II Multi cell (Bio-Rad, Munich, Germany) at a constant power of 10 mA per gel for 30 min and then at 15 mA for 90 min.
2D Western blot analysis.
The pH range 6 to 11 was probed with extracellular proteins of the S. aureus strain 8325-4. 2D immunoblotting (2D-IB) for the pH range 4 to 7 was performed with extracellular proteins from 8325-4 Δspa in order to avoid a high background due to immunoglobulin G (IgG) binding by protein A. The two strains produced very similar patterns of extracellular proteins, except for the difference in protein A (data not shown).
Thirty micrograms of extracellular proteins was separated on 12.5% 2D minigels as described above and was transferred to a polyvinylidene difluoride membrane for Western blotting using a semidry blotting device (Milliblot graphic electroblotter II; Millipore, Billerica, MA) according to the manufacturer's instructions. The quality of transfer was controlled by reversible staining with Pelikan Ink solution (10). After destaining, membranes were blocked with a solution of 5% nonfat dry milk in TBS-T buffer (Tris-buffered saline-Tween, comprising 20 mM Tris-HCl, 137 mM NaCl, and 0.1% [vol/vol] Tween 20 [pH 7.6]) for 1 h at room temperature. After five washes with TBS-T buffer, the membranes were incubated with the human sera overnight at 4°C (diluted 1:10,000 in 5% [wt/vol] bovine serum albumin in TBS-T). Bound human IgG was detected by incubation with peroxidase-conjugated goat anti-human IgG(H+L), diluted 1:50,000 in blocking buffer for 1 h at room temperature. After a wash, the membranes were incubated with the SuperSignal West Femto maximum-sensitivity substrate (Pierce) for 5 min, before signals were detected with a Lumi-Imager (Roche, Mannheim, Germany). With a 1-min recording time, both weak and strong signals were in the linear range.
Serum samples before and 28 days after colonization from one volunteer were always analyzed in the same experiment. Each serum was tested in two to four technical replicates in independent experiments. To monitor reproducibility, a pool of all 32 sera was applied in parallel throughout the experiments. Spot intensity was quantified with good reproducibility: the level of variation of total spot intensity of technical replicates was around 30% (data not shown).
Spot detection, quantitation, and identification.
The 2D-IB images were analyzed with the Delta-2D software package, version 3.4 (Decodon GmbH, Greifswald, Germany). All IB images were matched to a master IB, which was developed with a pool of all 32 sera. A fused image was obtained using the union fuse option. Spots on the fused gel image were automatically detected and manually validated by comparing the original gel images with the fused gel image. Subsequently, the spot map and the corresponding labels of the fused gel image were transferred to all gel images included in the project, thus ensuring uniform analysis throughout the study. Spot intensities were calculated based on the areas and pixel intensities of spots. The intensity values (spot volumes) were corrected based on the local background. Spot intensities are given as the median ± interquartile range of background-corrected raw values of technical replicates. Because sera differed strongly in signal intensity, we did not perform normalization based on the total signal intensity but analyzed the raw volume data instead.
Highly basic proteins (pH > 9.8) were blotted to polyvinylidene difluoride membranes with different efficiencies. Therefore, the 29 most basic protein spots (pI > 9.8) were excluded from data analysis, including spots corresponding to autolysin (Atl), autolysin precursor (Aaa), immunodominant antigen B (IsaB), and iron-regulated surface determinant protein A (IsdA).
To reveal the proteins behind the IB spots, a reference map on which protein spots were identified was matched with the master IB containing the spot map and corresponding spot labels in Delta-2D. By these means, protein names could be assigned to 44% (52/117) and 36% (43/119) of the IB spots for pH 6 to 11 and pH 4 to 7, respectively (see Fig. S2 in the supplemental material).
Statistics.
Statistical analyses were performed with GeneSpring, version 3.7.1. Graphs were created with GraphPad Prism, version 5. Medians are given with their interquartile ranges. For each spot, the median spot intensity of technical replicates was calculated from the replicates. “Strong” IB signals were defined as the top 10% of all spots from all 16 volunteers (cutoff, 5.6 Gy).
Nonparametric correlations (Mann-Whitney U test, GeneSpring) were estimated for comparison of median spot intensities of S. aureus carriers and NC on the single-spot level. P-values (two-tailed) below 0.05 were considered statistically significant.
To reveal colonization-induced changes in the IB spot intensities, we calculated the median ratios (post- versus precolonization) of two to four technical replicates. We applied stringent selection criteria and defined the change in IB spot intensity as significant only if (i) the median intensity ratio between post- and precolonization sera exceeded 5, (ii) the intensity ratio exceeded 2.5 in at least two technical replicates, and (iii) the difference between the spot intensities of post- and precolonization sera was more than two times the standard deviation of the spot intensity of precolonization sera.
RESULTS
Our investigations were performed in the context of an experimental colonization study that compared the adherence and colonization properties of S. aureus 8325-4 and its isogenic clumping factor B mutant (36). 8325-4 is a laboratory strain with low virulence and without superantigen genes; it was chosen for safety reasons. As expected, all volunteers remained completely free from clinical symptoms (36). Sera obtained from these volunteers before and 4 weeks after the colonization were used to investigate the anti-S. aureus IgG profiles in healthy adults.
High variability of baseline IgG binding to S. aureus antigens.
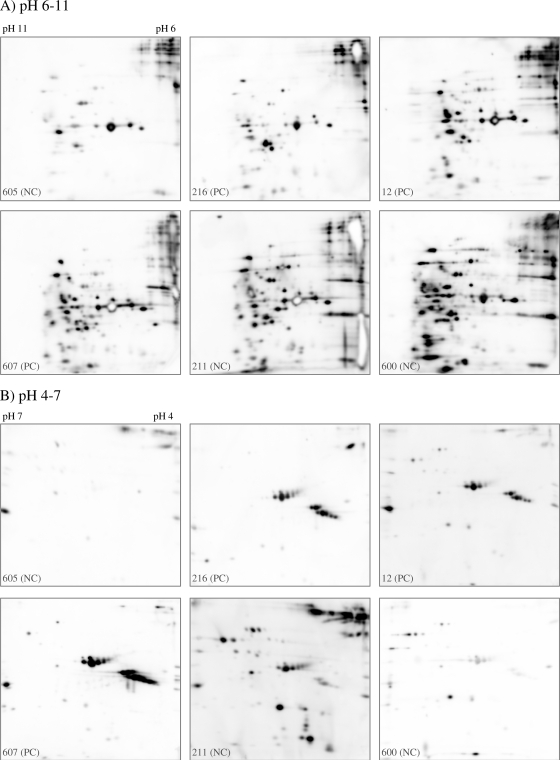
Because S. aureus releases many virulence factors, including hemolysins, proteases, and superantigens, into the extracellular space, especially during post-exponential growth (37), we focused our investigation on serum IgG binding to these antigens. We used 2D-IB to obtain a comprehensive picture of the anti-S. aureus antibody profiles against the post-exponential-phase extracellular proteome of S. aureus strain 8325-4. Before experimental colonization, we found marked interindividual variation in baseline IgG binding when we probed 2D blots with the precolonization sera from the 16 volunteers. Six representative cases are depicted in Fig. 1, which shows serum IgG binding to bacterial proteins resolved in the pH ranges 6 to 11 (A) and 4 to 7 (B) (all results are given in Fig. S1 in the supplemental material). The strong heterogeneity in IB patterns comprised total IgG binding, spot patterns, and spot intensity.
FIG. 1.
High variability in serum IgG binding to S. aureus extracellular proteins in the pH ranges 6 to 11 (A) and 4 to 7 (B). IB for pH 4 to 7 was performed with extracellular proteins from 8325-4 Δspa in order to avoid background due to IgG binding to protein A. S. aureus extracellular proteins from cells in the stationary-growth phase were resolved by 2D-PAGE and were probed with precolonization sera from 16 volunteers. Data for six representative individuals are shown.
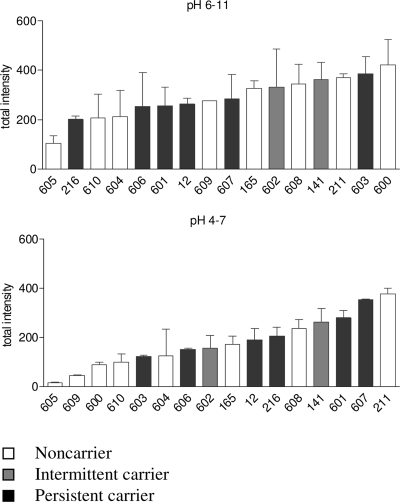
The total IgG binding, i.e., the sum of spot intensities, differed between individuals up to a factor of 20 in the pH range from 4 to 7 and a factor of 3.6 in the pH range from 6 to 11, where more immune-reactive proteins were present (Fig. 2). S. aureus carriers and NC did not differ significantly in total IgG binding, but the numbers are small. Moreover, total IgG binding did not correlate with persistence of strain 8325-4 in the nose following experimental colonization (data not shown).
FIG. 2.
Variability of total IgG binding to S. aureus 8325-4 extracellular proteins. The total spot intensity of all spots analyzed was taken as a measure for global serum IgG binding. Human serum IgG reacted more frequently with basic proteins (top) than with neutral or acidic proteins (bottom). The total spot intensity differed by a factor as high as 20 for IBs with the pH range 4 to 7. Each value was based on two to four technical replicates. Means and standard deviations are shown.
Individuals differed strongly in their spot patterns and spot intensities (Fig. 1). For example, serum 605 reacted only with a few staphylococcal proteins, especially in the pH range from 4 to 7, while serum 211 contained high titers of IgG against a range of staphylococcal exoproteins, including the immune dominant staphylococcal protein A (IsaA).
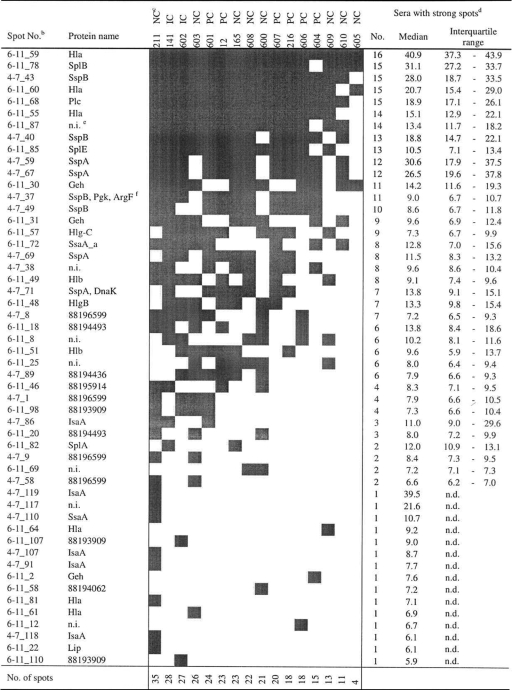
As a measure for immune dominance, we identified the 10% of spots that were strongest on all 2D-IBs (cutoff, 5.6 arbitrary units [Au]). In Table 2 we provide an overview of their distribution. The spots (rows) were ranked by their frequencies among the 16 volunteers. Similarly, volunteers were ranked by their numbers of strong spots (columns). At the top of Table 2 are proteins with strong IgG binding in many individuals. These spots represent the pattern of immune dominance or the core immune proteome of S. aureus 8325-4. Most immune-reactive proteins could be identified by matching the IBs with the protein reference maps of strain 8325-4 (for annotated reference blots, see Fig. S2 in the supplemental material). Among the 10% strongest spots that were present in at least 75% (12/16) of all individuals, we found the virulence factors alpha-hemolysin (Hla), cysteine protease (SspB), phospholipase C (Plc), serine protease-like proteins B and E (SplB, SplE), and staphylococcal serine protease (V8 protease; SspA). These virulence factors are highly conserved in the species S. aureus.
TABLE 2.
S. aureus extracellular proteins with strong IgG bindinga
As a measure for immune dominance, we identified the 10% of spots that were strongest (gray squares) on all 2D-IBs (cutoff, 5.6 Au). This table combines the results for the pH ranges of 6 to 11 and 4 to 7. An annotated reference blot with the spot numbers is provided in Fig. S2 in the supplemental material.
IB spots were ranked by their frequencies among the 16 volunteers.
The carrier status is given for each of the 16 volunteers. Volunteers were ranked by the number of strong spots for each.
The number of sera in which the respective IB spot was defined as strong is given, along with the median spot volume (arbitrary units) and interquartile range. n.d., not determined.
n.i., not identified.
A strong 2D-IB spot covered two (or more) closely located protein spots.
However, there was also much interindividual variation in spot intensities as well as in spot patterns. IgG binding to the immune-dominant antigens differed between individuals by factors of 2.3 (6-11_68; Plc) to 18.9 (4-7_59; SspA). The spot with the highest intensity in every individual represents Hla (6-11_59), which was also the most abundant spot in the protein 2D-PAGE analysis. In this single case, spot quantification was out of the linear range. Finally, the lower part of Table 2 shows that many immune-reactive spots were present in only a few individuals. Twenty-four of the 52 strongest spots appeared in fewer than half of the volunteers, underlining the impressive interindividual diversity in the adaptive immune response against S. aureus in the healthy population.
Differences in baseline IgG binding between carriers and NC.
The broad interindividual variability in baseline IgG binding raised the question whether antibody binding differed between S. aureus carriers and NC. In fact, five spots were stronger in carriers (P = 0.05 by the Mann-Whitney U test), but none were stronger in NC (Table 3). These spots represent IgG binding to the conserved virulence factors SspA, SspB, and IsaA, which belong to the core immune proteome, as well as to two unidentified proteins. SspA, SspB, and IsaA are represented on 2D gels by several isoforms, all of which show the same tendency, i.e., stronger serum IgG binding in carriers than in NC.
TABLE 3.
Differences in baseline IgG binding between carriers and NC
| Spot label | Proteina | Median level (in arbitrary units) of IgG binding (interquartile range) for: |
Pb | |
|---|---|---|---|---|
| Carriers (PC + IC) | NC | |||
| 4-7_101 | NI | 0.7 (0.4-1.3) | 0.3 (0.2-0.4) | 0.027 |
| 4-7_119 | IsaA_6, IsaA_7, IsaA_8 | 1.4 (1.1-1.9) | 0.5 (0.4-0.7) | 0.027 |
| 4-7_49 | SspB_1 | 8.6 (6.2-12.9) | 4.3 (2.6-6.6) | 0.036 |
| 4-7_69 | SspA_1, SspA_6 | 9.6 (5.6-12.6) | 2.7 (0.8-5.5) | 0.046 |
| 4-7_70 | NI | 0.1 (0.1-0.2) | 0.1 (0.1-0.1) | 0.046 |
Only proteins that differed significantly in IgG binding between carriers and NC are listed. NI, not identified.
By the Mann-Whitney U test.
Impact of experimental colonization on the serum IgG response.
To test whether the observed highly variable anti-S. aureus antibody profiles are induced by bacterial colonization, we compared the sera obtained before and 4 weeks after colonization with S. aureus 8325-4.
In contrast with the pronounced interindividual differences in baseline antibody patterns, experimental colonization induced only moderate changes (see Fig. S1 in the supplemental material). In total, 19 spots gained intensity by a factor of at least 5 (Table 4). These moderate increases in IgG binding were observed predominantly for weak spots (exceptions are 4-7_8 and 4-7_77), which also hampered spot identification. We observed no commonality in spots with increased IgG binding, i.e., the observed serum IgG responses to experimental colonization were very individual. In line with these findings, we observed no major differences in the serum IgA response, which was analyzed in four representative probands (see Fig. S2 in the supplemental material). In conclusion, human experimental colonization with S. aureus 8325-4 did not elicit a strong humoral immune response.
TABLE 4.
Spots with more than fivefold increases after experimental colonization
| Spot labela | Protein | Volunteer no. | Carriage status | Median fold increase in spot intensity (minimum-maximum)b | Spot intensityc |
|---|---|---|---|---|---|
| 4-7_34 | NId | 165 | NC | 11.5 (4.2-18.7) | 0.1 |
| 4-7_31 | NI | 165 | NC | 7.7 (4.7-10.7) | 0.1 |
| 4-7_5 | 88196599_1 | 165 | NC | 6.49 (3.4-9.3) | 0.1 |
| 6-11_71 | NI | 165 | NC | 5.9 (2.1-11.0) | 0.3 |
| 6-11_15 | NI | 604 | NC | 8.4 (7.2-9.6) | 0.1 |
| 6-11_42 | NI | 604 | NC | 7.4 (3.2-11.6) | 0.3 |
| 4-7_8 | 88196599_2 | 608 | NC | 12.0 (8.1-15.8) | 4.0 |
| 4-7_9 | 88196599_10 | 608 | NC | 8.6 (3.3-13.8) | 2.3 |
| 4-7_61 | NI | 608 | NC | 6.6 (2.6-10.5) | 0.1 |
| 6-11_21 | NI | 601 | PC | 7.1 (3.7-11.6) | 0.9 |
| 6-11_113 | NI | 601 | PC | 2.7 (2.6-17.9) | 0.3 |
| 4-7_77 | NI | 603 | PC | 5.6 (5.6-5.7) | 2.6 |
| 4-7_115 | SsaA_b_1 | 606 | PC | 24.8 (19.4-30.2) | 0.3 |
| 4-7_3 | 88196599_8 | 606 | PC | 9.7 (2.5-16.9) | 0.7 |
| 4-7_23 | PdhD_1, PdhD_2, PdhD_3 | 606 | PC | 7.4 (5.0-9.9) | 0.1 |
| 4-7_5 | 88196599_1 | 606 | PC | 5.9 (4.3-7.4) | 0.2 |
| 4-7_88 | NI | 606 | PC | 5.3 (4.8-5.7) | 0.1 |
| 6-11_6 | Pbp3 | 607 | PC | 18.6 (13.6-23.6) | 0.2 |
| 6-11_7 | Geh_13 | 607 | PC | 15.4 (14.9-16.0) | 0.2 |
An annotated reference blot with the spot numbers is provided in Fig. S2 in the supplemental material.
The ratio of the spot intensity in the postcolonization serum to that in the precolonization serum was determined for each volunteer and for each technical replicate separately. A spot increase was defined as (i) a ≥5-fold increase in the mean ratio, (ii) a ≥2.5-fold increase in at least two technical replicates, and (iii) a difference in spot intensity between the postcolonization and precolonization sera exceeding twofold the standard deviation of the technical replicates. The median, minimum, and maximum of the ratios from two to four technical replicates are given.
Median spot intensity (in arbitrary units) of technical replicates of IBs developed with postcolonization sera.
NI, not identified.
DISCUSSION
When we probed extracellular S. aureus proteins with serum samples from 16 volunteers, we observed a broad spectrum of anti-S. aureus antibodies in every individual. There was pronounced interindividual variability in total IgG binding, spot patterns, and spot intensities. Compared to this strong IgG response, experimental nasal colonization with S. aureus 8325-4, a laboratory strain of low virulence, induced only minor changes in the IgG binding patterns. Similar findings were obtained for IgA.
The study was focused on extracellular proteins, because virulence factors are enriched in this subproteome, and because of our finding of S. aureus strain-specific antibody responses to superantigens (12). The fluorescence-based 2D-IB method chosen for investigation is relatively simple, is highly reproducible, and allows signal quantification. In contrast to (multiplexed) enzyme-linked immunosorbent assay and genomic expression approaches, it permits the simultaneous analysis of the proteome of an individual S. aureus strain and the serum antibody response against it. However, there are limitations: Conformational epitopes may be denatured; nonprotein antigens are not resolved; and the assay measures antibody binding but not function. Further studies are needed to assess the antibody response against cell wall-associated antigens and to correlate the observed antibody binding with neutralizing or opsonizing properties. Moreover, due to our study design, we may have missed exclusive throat and intestinal carriers (1, 17).
The high prevalence of a broad spectrum of antistaphylococcal antibodies in healthy adults can be explained by the ubiquity of S. aureus. Carriers are exposed to the same S. aureus strain for a long time and will experience multiple minor invasive episodes from it. Even though NC by definition are not constantly colonized on nasal epithelia, they will transiently contact different S. aureus strains, and most individuals experience minor staphylococcal skin infections. To what degree cross-reactive antibodies elicited by antigens from other bacterial species shaped the observed IgG binding patterns will need to be addressed in the future.
The core immune proteome of S. aureus 8325-4, defined as strong spots that were present in at least 75% of the individuals, consists of the well-known virulence factors Hla, SspB, Plc, SplB, SplE, and SspA. These virulence factors are conserved within the species S. aureus, and they appear to be immune dominant. IgG binding to IsaA, SspA, SspB, and two unidentified proteins was stronger in carriers than in NC. This confirms and extends our earlier observation that S. aureus carriers raise a strong antibody response against the superantigen cocktail produced by their colonizing strain (12). Similarily, Verkaik et al. observed higher IgG titers against TSST-1 and staphylococcal enterotoxin A in persistent carriers than in NC (31). We observed no spots that were more prominent in NC than in carriers.
The most striking finding with our unbiased approach, however, was the high degree of variability in IgG binding to S. aureus exoproteins in healthy adults. This may have been underrated in studies with selected antigens or serum pools. The data clearly show that our previous observation of a highly heterogeneous spectrum of superantigen-neutralizing antibodies in healthy adults was just the tip of the iceberg (11, 12; also unpublished data). Similarly, a broad range of anti-S. aureus antibody titers was reported for some toxins and surface-associated antigens (6, 31).
The high variability in antibody profiles likely reflects the individual history of encounters with S. aureus as well as the genetic diversity of the species. The latter is due to mobile genetic elements, which carry many virulence and resistance genes. Two strains can differ in as much as 20% of their genomes (15). Thus, S. aureus strains are expected to leave different imprints on the adaptive immune system. It is also possible that some antibody specificities were cross-reactive, i.e., induced by non-S. aureus antigens.
The broad and very personal antibody repertoires against staphylococcal virulence factors will likely put patients at different starting positions in case of S. aureus invasion. Evidence for a protective role of anti-S. aureus antibodies is still limited. Toxic shock syndrome patients, who commonly lack anti-TSST-1-antibodies, can be effectively treated by therapeutic application of immunoglobulins (26, 27). Moreover, antibodies targeted at surface proteins or toxins improve the outcome in animal models of S. aureus infections (13, 16, 22, 25, 33). However, for most antibody specificities observed in this study, the possible biological significance has not yet been investigated.
Until now, information about the behavior of the microorganism in the different phases of its encounters with the host has been scarce and difficult to obtain (9). The adaptive immune system may be considered a sensitive reporter system for in vivo-expressed bacterial antigens, and the complex IgG binding patterns demonstrate that our analysis was focused on a highly relevant subproteome.
How are these immune responses induced? Do proteins released by S. aureus confront the immune system during colonization of intact epithelia, or are minor infections necessary to trigger the observed antibody patterns? Are some of them cross-reactive with highly conserved antigens from other bacteria? Experimental colonization induced only minor changes in the levels of IgG binding to a number of weak spots, which were not conserved between individuals. Moreover, serum IgA responses were not affected by experimental colonization, either (see Fig. S3 in the supplemental material). As in earlier colonization studies with wild-type strains (18, 29, 36), the duration of carriage was variable, and only 10 out of 16 volunteers were colonized for more than 8 days. This resembles the behavior of S. aureus in IC and NC (29, 30), but not in persistent colonization, which frequently lasts for months or years. Our findings suggest that short-term colonization with a strain of low virulence does not trigger or boost strong IgG or IgA responses. The antibody patterns observed in carriers as well as in NC could, therefore, be due either to long-term, high-density colonization or, as we consider more likely, to (minor) S. aureus infections, as has been reported for selected antigens (4, 5, 14). This could imply that serum antibodies are directed predominantly against infection-related antigens and are less involved in the control of colonization.
Our results have implications for vaccine development. Strategies are usually focused on conserved staphylococcal antigens (6, 24). Our data show that most healthy adults already possess strong antibody responses against many conserved S. aureus proteins. It remains to be shown how much vaccination could add to this. Moreover, our data illustrate the pronounced heterogeneity of clinical S. aureus isolates and host responses (15). The variable S. aureus virulence factors might be equally important as conserved bacterial antigens and should be considered in the design of S. aureus vaccines.
Supplementary Material
Acknowledgments
Work in the laboratories of Michael Hecker, B. M. Bröker, and U. Völker was supported by the Deutsche Forschungsgemeinschaft (SFB-TR34, GRK840). T. T. H. Nguyen received a grant from the Vietnamese Ministry of Education and Training (MOET).
The authors have declared no conflict of interest.
We thank Tim Foster (Dublin, Ireland) for providing S. aureus strain DU5875 (8325-4 Δspa).
Footnotes
Published ahead of print on 16 September 2009.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Acton, D. S., M. J. Plat-Sinnige, W. van Wamel, N. de Groot, and A. van Belkum. 2009. Intestinal carriage of Staphylococcus aureus: how does its frequency compare with that of nasal carriage and what is its clinical impact? Eur. J. Clin. Microbiol. Infect. Dis. 28:115-127. [DOI] [PubMed] [Google Scholar]
- 2.Boucher, H. W., and G. R. Corey. 2008. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 46:S344-S349. [DOI] [PubMed] [Google Scholar]
- 3.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 4.Burlak, C., C. H. Hammer, M. A. Robinson, A. R. Whitney, M. J. McGavin, B. N. Kreiswirth, and F. R. Deleo. 2007. Global analysis of community-associated methicillin-resistant Staphylococcus aureus exoproteins reveals molecules produced in vitro and during infection. Cell. Microbiol. 9:1172-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colque-Navarro, P., B. Soderquist, H. Holmberg, L. Blomqvist, P. Olcen, and R. Mollby. 1998. Antibody response in Staphylococcus aureus septicaemia—a prospective study. J. Med. Microbiol. 47:217-225. [DOI] [PubMed] [Google Scholar]
- 6.Dryla, A., S. Prustomersky, D. Gelbmann, M. Hanner, E. Bettinger, B. Kocsis, T. Kustos, T. Henics, A. Meinke, and E. Nagy. 2005. Comparison of antibody repertoires against Staphylococcus aureus in healthy individuals and in acutely infected patients. Clin. Diagn. Lab. Immunol. 12:387-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Etz, H., D. Minh, T. Henics, A. Dryla, B. Winkler, C. Triska, A. Boyd, J. Söllner, W. Schmidt, U. von Ahsen, M. Buschle, S. Gill, J. Kolonay, H. Khalak, C. Fraser, A. von Gabain, E. Nagy, and A. Meinke. 2002. Identification of in vivo expressed vaccine candidate antigens from Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 99:6573-6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster, T. J. 2004. The Staphylococcus aureus “superbug.” J. Clin. Investig. 114:1693-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goerke, C., and C. Wolz. 2004. Regulatory and genomic plasticity of Staphylococcus aureus during persistent colonization and infection. Int. J. Med. Microbiol. 294:195-202. [DOI] [PubMed] [Google Scholar]
- 10.Hancock, K., and V. C. Tsang. 1983. India ink staining of proteins on nitrocellulose paper. Anal. Biochem. 133:157-162. [DOI] [PubMed] [Google Scholar]
- 11.Holtfreter, S., K. Bauer, D. Thomas, C. Feig, V. Lorenz, K. Roschack, E. Friebe, K. Selleng, S. Lovenich, T. Greve, A. Greinacher, B. Panzig, S. Engelmann, G. Lina, and B. M. Bröker. 2004. egc-encoded superantigens from Staphylococcus aureus are neutralized by human sera much less efficiently than are classical staphylococcal enterotoxins or toxic shock syndrome toxin. Infect. Immun. 72:4061-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holtfreter, S., K. Roschack, P. Eichler, K. Eske, B. Holtfreter, C. Kohler, S. Engelmann, M. Hecker, A. Greinacher, and B. M. Bröker. 2006. Staphylococcus aureus carriers neutralize superantigens by antibodies specific for their colonizing strain: a potential explanation for their improved prognosis in severe sepsis. J. Infect. Dis. 193:1275-1278. [DOI] [PubMed] [Google Scholar]
- 13.Josefsson, E., O. Hartford, L. O'Brien, J. M. Patti, and T. Foster. 2001. Protection against experimental Staphylococcus aureus arthritis by vaccination with clumping factor A, a novel virulence determinant. J. Infect. Dis. 184:1572-1580. [DOI] [PubMed] [Google Scholar]
- 14.Kanclerski, K., B. Soderquist, M. Kjellgren, H. Holmberg, and R. Mollby. 1996. Serum antibody response to Staphylococcus aureus enterotoxins and TSST-1 in patients with septicaemia. J. Med. Microbiol. 44:171-177. [DOI] [PubMed] [Google Scholar]
- 15.Lindsay, J. A., and M. T. Holden. 2006. Understanding the rise of the superbug: investigation of the evolution and genomic variation of Staphylococcus aureus. Funct. Integr. Genomics 6:186-201. [DOI] [PubMed] [Google Scholar]
- 16.McKenney, D., K. L. Pouliot, Y. Wang, V. Murthy, M. Ulrich, G. Döring, J. C. Lee, D. A. Goldmann, and G. B. Pier. 1999. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science 284:1523-1527. [DOI] [PubMed] [Google Scholar]
- 17.Mertz, D., R. Frei, B. Jaussi, A. Tietz, C. Stebler, U. Fluckiger, and A. F. Widmer. 2007. Throat swabs are necessary to reliably detect carriers of Staphylococcus aureus. Clin. Infect. Dis. 45:475-477. [DOI] [PubMed] [Google Scholar]
- 18.Nouwen, J., H. Boelens, A. van Belkum, and H. Verbrugh. 2004. Human factor in Staphylococcus aureus nasal carriage. Infect. Immun. 72:6685-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reference deleted.
- 20.Novick, R. 1967. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology 33:155-166. [DOI] [PubMed] [Google Scholar]
- 21.Patel, A., P. Nowlan, E. Weavers, and T. Foster. 1987. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect. Immun. 55:3103-3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rennermalm, A., Y. H. Li, L. Bohaufs, C. Jarstrand, A. Brauner, F. R. Brennan, and J. I. Flock. 2001. Antibodies against a truncated Staphylococcus aureus fibronectin-binding protein protect against dissemination of infection in the rat. Vaccine 19:3376-3383. [DOI] [PubMed] [Google Scholar]
- 23.Rogasch, K., V. Rühmling, J. Pané-Farré, D. Höper, C. Weinberg, S. Fuchs, M. Schmudde, B. M. Bröker, C. Wolz, M. Hecker, and S. Engelmann. 2006. The influence of the two-component system SaeRS on global gene expression in two different Staphylococcus aureus strains. J. Bacteriol. 188:7742-7758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaffer, A. C., and J. C. Lee. 2008. Vaccination and passive immunisation against Staphylococcus aureus. Int. J. Antimicrob. Agents 32(Suppl. 1):S71-S78. [DOI] [PubMed] [Google Scholar]
- 25.Schaffer, A. C., R. M. Solinga, J. Cocchiaro, M. Portoles, K. B. Kiser, A. Risley, S. M. Randall, V. Valtulina, P. Speziale, E. Walsh, T. Foster, and J. C. Lee. 2006. Immunization with Staphylococcus aureus clumping factor B, a major determinant in nasal carriage, reduces nasal colonization in a murine model. Infect. Immun. 74:2145-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlievert, P. 2001. Use of intravenous immunoglobulin in the treatment of staphylococcal and streptococcal toxic shock syndromes and related illnesses. J. Allergy Clin. Immunol. 108:S107-S110. [DOI] [PubMed] [Google Scholar]
- 27.Stolz, S. J., J. P. Davis, J. M. Vergeront, B. A. Crass, P. J. Chesney, P. J. Wand, and M. S. Bergdoll. 1985. Development of serum antibody to toxic shock toxin among individuals with toxic shock syndrome in Wisconsin. J. Infect. Dis. 151:883-889. [DOI] [PubMed] [Google Scholar]
- 28.van Belkum, A. 2006. Staphylococcal colonization and infection: homeostasis versus disbalance of human (innate) immunity and bacterial virulence. Curr. Opin. Infect. Dis. 19:339-344. [DOI] [PubMed] [Google Scholar]
- 29.van Belkum, A., N. J. Verkaik, C. P. de Vogel, H. A. Boelens, J. Verveer, J. L. Nouwen, H. A. Verbrugh, and H. F. Wertheim. 2009. Reclassification of Staphylococcus aureus nasal carriage types. J. Infect. Dis. 199:1820-1826. [DOI] [PubMed] [Google Scholar]
- 30.VandenBergh, M. F. Q., E. P. F. Yzerman, A. van Belkum, H. A. M. Boelens, M. Sijmons, and H. A. Verbrugh. 1999. Follow-up of Staphylococcus aureus nasal carriage after 8 years: redefining the persistent carrier state. J. Clin. Microbiol. 37:3133-3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verkaik, N. J., C. P. de Vogel, H. A. Boelens, D. Grumann, T. Hoogenboezem, C. Vink, H. Hooijkaas, T. J. Foster, H. A. Verbrugh, A. van Belkum, and W. J. van Wamel. 2009. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. J. Infect. Dis. 199:625-632. [DOI] [PubMed] [Google Scholar]
- 32.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 344:11-16. [DOI] [PubMed] [Google Scholar]
- 33.Wardenburg, J. B., and O. Schneewind. 2008. Vaccine protection against Staphylococcus aureus pneumonia. J. Exp. Med. 205:287-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wertheim, H. F., D. C. Melles, M. C. Vos, W. van Leeuwen, A. van Belkum, H. A. Verbrugh, and J. L. Nouwen. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5:751-762. [DOI] [PubMed] [Google Scholar]
- 35.Wertheim, H. F., M. C. Vos, A. Ott, A. van Belkum, A. Voss, J. A. Kluytmans, P. H. van Keulen, C. M. Vandenbroucke-Grauls, M. H. Meester, and H. A. Verbrugh. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703-705. [DOI] [PubMed] [Google Scholar]
- 36.Wertheim, H. F., E. Walsh, R. Choudhurry, D. C. Melles, H. A. Boelens, H. Miajlovic, H. A. Verbrugh, T. Foster, and A. van Belkum. 2008. Key role for clumping factor B in Staphylococcus aureus nasal colonization of humans. PLoS Med. 5:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ziebandt, A. K., D. Becher, K. Ohlsen, J. Hacker, M. Hecker, and S. Engelmann. 2004. The influence of agr and σB in growth phase dependent regulation of virulence factors in Staphylococcus aureus. Proteomics 4:3034-3047. [DOI] [PubMed] [Google Scholar]
- 38.Ziebandt, A. K., H. Weber, J. Rudolph, R. Schmid, D. Hoper, S. Engelmann, and M. Hecker. 2001. Extracellular proteins of Staphylococcus aureus and the role of SarA and σB. Proteomics 1:480-493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.