Abstract
Baylisascaris procyonis and Toxocara species are two important causes of larva migrans in humans. Larva migrans caused by Toxocara spp. is well known and is diagnosed serologically by enzyme immunoassay. Over a dozen cases of larva migrans and associated eosinophilic encephalitis caused by B. procyonis have also been reported, and at least a dozen additional cases are known. An enzyme-linked immunosorbent assay (ELISA) using the excretory-secretory (ES) antigen of B. procyonis larvae is currently being used in our laboratory as an aid in the diagnosis of this infection in humans. Clinically affected individuals show very high reactivity (measured as the optical density) on this ELISA; however, a one-way cross-reactivity with Toxocara spp. has been observed. As an approach to differentiate these two infections based on serology, we performed Western blots, wherein the B. procyonis ES antigen was reacted with serum samples from individuals known to be positive for either Toxocara spp. or B. procyonis larva migrans. Western blot results showed that B. procyonis antigens of between 30 and 45 kDa were specifically identified only by the sera from individuals with Baylisascaris larva migrans, thus allowing for differentiation between the two infections. This included human patient serum samples submitted for serologic testing, as well as sera from rabbits experimentally infected with B. procyonis. When used in conjunction with the ELISA, Western blotting could be an efficient tool for diagnosis of this infection in humans.
Larva migrans (LM) is a condition in which larvae of helminth parasites migrate and persist in different body organs and tissues, causing marked, usually eosinophilic inflammatory reactions (1, 18). Baylisascaris procyonis, the raccoon roundworm, and Toxocara spp., the canine and feline roundworms, are well-known causes of larva migrans in humans (9, 16, 20). Based on the organs infected, both Baylisascaris and Toxocara spp. can cause clinical visceral larva migrans, ocular larva migrans, and/or neural larva migrans. Both parasites can also produce mild clinical infection with nonspecific symptoms. Unlike Toxocara spp., Baylisascaris larvae molt and grow as they aggressively migrate, causing extensive mechanical damage and inflammation during infection (9, 16). Therefore, B. procyonis larvae have an increased capacity to damage the central nervous system, leading to eosinophilic meningoencephalitis and clinical neural larva migrans.
Larva migrans caused by Toxocara species is routinely diagnosed serologically by enzyme immunoassay (EIA), which utilizes Toxocara canis larval excretory-secretory (ES) antigens (8, 15). Serum samples from patients suspected to have Toxocara larva migrans are submitted to the Centers for Disease Control and Prevention (CDC) or other major laboratories or assessed using commercial kits (e.g., from Bordier Affinity Products, Cressier, Switzerland) for the detection of antibodies to Toxocara ES antigens. Toxocara infection is surprisingly common worldwide, including in the United States, where a recent national survey by the CDC showed a 14% seroprevalence of Toxocara in the population (29). Most cases of Toxocara larva migrans in humans involve visceral larva migrans, ocular larva migrans, or covert infection (20). Although B. procyonis also causes these conditions, it is being increasingly recognized as a cause of eosinophilic meningoencephalitis. Over a dozen cases of larva migrans with associated eosinophilic encephalitis caused by B. procyonis have been reported, with at least a dozen additional unpublished cases also known (our unpublished data). These cases have a wide spectrum of clinical disease varying from fatal or irreparable neurological damage (11, 23) to milder infection with improvement or even apparent recovery (6, 13, 25).
Similar to the EIA used for diagnosis of Toxocara larva migrans, considerable progress has been made in the serological diagnosis of Baylisascaris. Early on, in addition to exposure history, clinical cases of Baylisascaris neural larva migrans were diagnosed based on symptomatology, eosinophilic meningoencephalitis, and/or biopsy or autopsy findings. This was supported by the strong seroreactivity of patient serum and cerebrospinal fluid to Baylisascaris antigens in indirect immunofluorescence assays performed on frozen larval sections (14, 21). In cases where brain biopsy was performed and B. procyonis was seen, confirmation of the infection was also based on identification of the larvae by morphometry (5, 27). Currently, a larval ES antigen (BPES) enzyme-linked immunosorbent assay (ELISA) for serological testing in human patients is being performed in our laboratory at Purdue University and has shown great utility in assisting this diagnosis (6, 7, 27). However, it was found that the BPES ELISA has a one-way cross-reactivity with Toxocara spp. (Toxocara patients react positively on BPES ELISA, but Baylisascaris patients do not react on T. canis larval ES EIA). In light of this and earlier experiments (3, 4) showing some degree of cross-reactivity among the ascaridoid nematodes, in the present studies, we compared ELISA and Western blotting using BPES antigen for the serodiagnosis of Baylisascaris larva migrans. We examined the reactivity of serum samples from human patients with larva migrans and from rabbits experimentally infected with embryonated eggs of either Baylisascaris or Toxocara. Based on these studies, the utility of Western blot analysis in the diagnosis of Baylisascaris larva migrans is presented.
MATERIALS AND METHODS
B. procyonis larval ES antigen preparation.
Collection, preservation, and embryonation of B. procyonis eggs was performed as described by Kazacos et al. (19). In vitro culture of hatched larvae was performed as described by Boyce et al. (4) with slight modifications. Second-stage (L2) larvae were hatched aseptically from in vitro-embryonated B. procyonis eggs and placed into in vitro cultures, and samples of the culture medium was collected and replaced at weekly intervals. This culture medium containing the ES antigens of the parasite was dialyzed against 0.1 M ammonium bicarbonate solution, and the ES antigens were concentrated by lyophilization. Completely lyophilized antigen was then resuspended in 0.1 M ammonium bicarbonate. The protein concentration of the ES antigen was estimated according to the manufacturer's protocols using a BCA protein assay kit (Pierce/Thermo Fisher Scientific, Asheville, NC). Aliquots of the ES antigen were prepared and stored at −20°C until use.
Serum samples for ELISA and Western blotting. (i) Positive and negative control sera.
Anti-B. procyonis serum used as a positive control was obtained from the Division of Parasitic Diseases, CDC, Atlanta, GA. The serum was from a baboon that developed severe neural larva migrans after experimental infection with B. procyonis embryonated eggs. Serum from a healthy adult human with no history of exposure to raccoons or any clinical signs was used as negative control.
(ii) Human patient serum samples.
For Toxocara sera, a total of 30 human serum samples already tested for the presence of antibodies to Toxocara ES antigens during years 2003 to 2005 were obtained from the Division of Parasitic Diseases, CDC. These serum samples were from individuals of different age groups and of either sex (13 females, 16 males, and 1 unknown). Fifteen of these sera had Toxocara EIA results of <1:32 and were considered negative. The other 15 sera had Toxocara EIA results of >1:256, which are considered by the CDC as high positives. Another set of serum samples (Txc 1 through 7), obtained previously from CDC and also positive for Toxocara (titers unknown), were also used in the Western blot assay. All sera were divided into aliquots and stored at −20°C until use.
For Baylisascaris sera, human patient serum samples submitted to the Parasitology Laboratory, Department of Comparative Pathobiology, Purdue University, for BPES ELISA testing (from year 1986 to 2008) were considered as Baylisascaris larva migrans positive samples based on three criteria. (i) The serum sample was collected from a clinically affected individual, some of whom were confirmed through biopsy or autopsy. (ii) The serum tested positive on B. procyonis ES antigen ELISA. (iii) The serum tested negative on Toxocara EIA at the CDC. Twenty serum samples that met these three criteria were included in the present study. These 20 samples included sera from 13 published cases (including one cited in reference 7) of Baylisascaris larva migrans, and an additional seven patients who met the above criteria.
(iii) Rabbit serum samples.
Serum samples from seven rabbits (New Zealand White) experimentally infected with 10,000 embryonated eggs of T. canis and one rabbit infected with 10,000 embryonated eggs of B. procyonis, from a previous study conducted in our laboratory (4), were also used in the Western blot assays with BPES. Serum samples were collected from these rabbits preinfection and at 14 days (B. procyonis) and 56 days (T. canis) postinfection. Sera from three uninfected rabbits were used as negative controls.
ELISA.
B. procyonis ES antigen ELISA was performed by using Immulon-2HB flat-bottom microtiter plates (Thermo Scientific, Asheville, NC). The wells were coated with 0.1 μg of ES antigen per well, followed by incubation at 37°C for 90 min with shaking. Three percent fish gelatin (Sigma-Aldrich, St. Louis, MO) prepared in Tris-buffered saline (TBS)-0.05% Tween 20 (TBST) was used as the blocking agent. Primary antibody (human patient sera) at a 1:200 dilution and secondary antibody (alkaline phosphatase-conjugated goat anti-human immunoglobulin G [IgG(H+L)]; Bethyl Laboratories, Inc., Montgomery, TX) at a 1:5,000 dilution were prepared in TBST. Incubations with blocking agent and primary and secondary antibodies were performed for 1 h at room temperature with shaking. All washing steps were performed with TBST except the wash before the addition of the substrate, where TBS was used. In each step, washing was done three times, with each wash lasting ∼5 min. p-Nitrophenyl phosphate (Sigma-Aldrich) was used as the substrate, and the plates were incubated for 30 min. The optical density (OD) of the antigen-antibody reaction in the microtiter plate was read in a THERMOmax absorbance microplate reader (Molecular Devices, Sunnyvale, CA) at 405 nm. All serum samples in the ELISA were run in triplicate, and the results were averaged.
SDS-PAGE and Western blotting.
Portions (5 μg) of BPES antigen per lane were resolved by sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis (SDS-12% PAGE). The gels were stained with either Coomassie brilliant blue R-250 (Bio-Rad Laboratories, Richmond, CA) or silver stained (22). For Western blotting, the resolved proteins from the gel were transferred onto a 0.45-μm-pore-size nitrocellulose membrane (Bio-Rad) as described by Towbin et al. (28). The nitrocellulose membrane was cut into individual lanes prior to reaction with the serum samples. Blocking was performed with 5% nonfat dry milk prepared in TBS. Primary antibody (human patient serum samples) and secondary antibody [peroxidase-conjugated Affinipure goat anti-human IgG(H+L)] dilutions were prepared in TBS-0.01% Tween 20. Primary antibody was used at a dilution of 1:500 or 1:1,000, and secondary antibody was used at a dilution of 1:10,000. Supersignal West Pico chemiluminescent substrate (Pierce/Thermo Scientific) was used as the substrate, and the chemiluminescence detection and documentation was performed by using a Kodak Digital Science Image Station 440CF (Eastman Kodak Company Scientific Imaging Systems, Rochester, NY). For Western blot assays with serum samples from experimentally infected rabbits, a similar protocol was used except that the primary antibody (rabbit serum) was used at a 1:500 dilution, and the secondary antibody [peroxidase-conjugated Affinipure goat anti-rabbit IgG(H+L)] was used at a 1:10,000 dilution.
RESULTS
SDS-PAGE analysis of B. procyonis ES antigen.
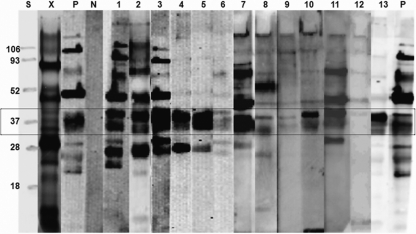
The ES antigen from B. procyonis larval culture was a heterogeneous mixture of proteins ranging in molecular size from approximately 10 to 200 kDa. All individual 2-week cultures had the same composition. Most of the proteins could be seen upon staining with Coomassie brilliant blue R-250, although better visualization of the protein bands, especially the 35- and 43-kDa proteins, occurred with silver staining (Fig. 1).
FIG. 1.
SDS-PAGE profile of B. procyonis larval ES antigen and Western blot profiles showing the components recognized by sera from patients with Baylisascaris larva migrans. Antigenic components of between 30 and 45 kDa are recognized by sera from patients with different spectra of clinical larva migrans (box). Lane X, SDS-PAGE profile; lane S, protein standard; lane P, serum from B. procyonis-infected baboon as positive control; lane N, human negative control serum; lanes 1 to 13, Baylisascaris larva migrans-positive human patient sera. Five-microgram portions of antigen per lane were used. All sera were used at a 1:1,000 dilution.
B. procyonis larval ES antigen-based ELISA of human patient serum samples.
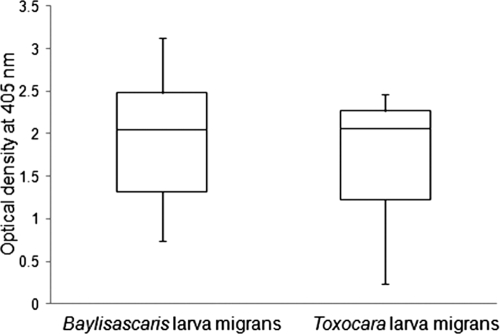
ES antigen prepared from B. procyonis larvae was used in this ELISA. The putative cutoff for this assay is an OD at 405 nm of 0.250, with negatives considered <0.150 and suspect reactor/indeterminates of 0.150 to 0.250. Serum samples from human patients with Baylisascaris larva migrans reacted strongly to BPES antigen and produced high OD values ranging from 0.744 to 3.132 (Fig. 2). None of the Baylisascaris larva migrans-positive serum samples had an OD of <0.500; all except one were negative on Toxocara ELISA at the CDC. Marked cross-reactivity was observed when Toxocara larva migrans serum samples (T. canis larval ES EIA titer of >1:256) were tested in the BPES ELISA. Of the 15 positive serum samples obtained from the CDC, 11 showed a high OD ranging between 1.997 and 2.459 and 4 of the sera had low values (OD < 0.500) (Fig. 2). The human patient serum samples from the CDC with a T. canis larval ES EIA titer of <1:32 (i.e., 1:2 and 1:8) all had very low ODs on the BPES ELISA (data not shown).
FIG. 2.
Reactivity of human serum samples positive for either Baylisascaris or Toxocara larva migrans on Baylisascaris procyonis larval ES antigen ELISA. Sera from patients with Toxocara larva migrans showed high ODs similar to those of patients with Baylisascaris larva migrans. The range box plots depict the median, upper quartile, lower quartile, maximum, and minimum OD values of patients with Baylisascaris larva migrans or Toxocara larva migrans.
B. procyonis larval ES antigen-based Western blot assay with human patient serum samples.
Anti-B. procyonis serum from the baboon reacted with most of the proteins in the BPES antigen. The negative control serum did not react to any BPES antigen on the Western blot (Fig. 1). Although all of the 20 Baylisascaris larva migrans-positive serum samples identified the BPES antigens, a difference in reactivity with individual serum samples was observed. This difference was not considered significant since all of the samples identified most components of the BPES antigen, differing quantitatively. The most significant observation was the consistent recognition of BPES proteins between 30 and 45 kDa by all of the Baylisascaris larva migrans-positive serum samples (Fig. 1).
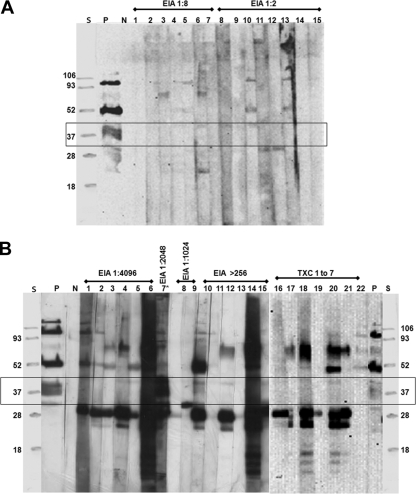
Toxocara larva migrans-negative serum samples (EIA titers of 1:2 and 1:8) showed no or very mild reactivity with the BPES antigens (Fig. 3A). On the other hand, Toxocara larva migrans-positive serum samples (EIA titer of >1:256) showed a strong reactivity to different components of the BPES antigen, but there was no reactivity seen with the 30- to 45-kDa proteins except for one sample that reacted strongly with this group (from a patient with suspected dual infection) (Fig. 3B). The other set of Toxocara serum samples (Txc 1 through 7) also showed no reactivity to the 30- to 45-kDa components (Fig. 3B).
FIG. 3.
(A) Western blot profiles of B. procyonis larval ES antigenic components recognized by human serum samples negative (EIA titers of 1:2 and 1:8) for Toxocara spp. Toxocara-negative sera show no or very mild reactivity with some of the antigenic components of B. procyonis larval ES antigens and none with the 30- to 45-kDa antigens (Box). Lane S, protein standard; lane P, serum from B. procyonis-infected baboon as a positive control; lane N, human negative control serum; lanes 1 to 15, Toxocara larva migrans-negative human patient sera. Five-microgram portions of antigen per lane were used. All sera were used at a 1:1,000 dilution. (B) Western blot profiles of B. procyonis larval ES antigenic components recognized by human serum samples specifically positive (EIA titer of >1:256) for Toxocara spp. Toxocara-specific sera of high or unknown (Txc 1 to 7) EIA titers recognize different antigenic components of B. procyonis larval ES antigens, but not the 30- to 45-kDa antigenic components (except for lane 7*) (box). Lane S, protein standard; lane P, serum from B. procyonis-infected baboon as a positive control; lane N, human negative control serum; lanes 1 to 22, Toxocara larva migrans-positive human patient sera. Five-microgram portions of antigen per lane were used. All sera were used at a 1:1,000 dilution. (Suspected dual infection with Toxocara spp. and B. procyonis is indicated by an asterisk.)
B. procyonis larval ES antigen-based Western blot assays with experimentally infected rabbit serum samples.
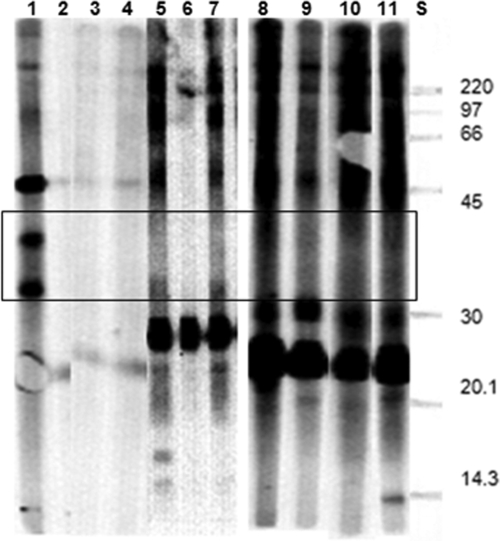
The serum from the rabbit experimentally infected with B. procyonis showed strong reactivity to all of the components of the BPES antigen, including at 30 to 45 kDa, whereas the uninfected rabbits (negative controls) did not show any reactivity to the BPES antigen (Fig. 4). The serum from T. canis-infected rabbits showed strong reactivity to high- and low-molecular-mass proteins in the BPES antigen but did not show any reactivity to the 30- to 45-kDa components of the BPES antigen (Fig. 4).
FIG. 4.
Western blot profiles of B. procyonis larval ES components recognized by serum from rabbits experimentally infected with either B. procyonis or T. canis infective eggs. Lane S, protein standard; lane 1, serum from rabbit infected with embryonated B. procyonis eggs; lanes 2, 3, and 4, uninfected control rabbit serum; lanes 5 to 11, serum from rabbits infected with embryonated T. canis eggs. Only the rabbit in lane 1 recognizes the 30- to 45-kDa B. procyonis ES antigens (box). Five-microgram portions of antigen per lane were used.
DISCUSSION
Western blots have been used to study cross-reactivity with heterologous antigens in different parasitic infections (2, 24), and it has been observed that while there are major proteins that cross-react, there are some which do not, thus providing a way to differentiate between infections. Boyce et al. (3) demonstrated by using Western blot assays that there is widespread cross-reactivity among ascarid antisera raised in mice. However, certain components of larval BPES were recognized only by B. procyonis antiserum. In a different study, these authors (4) showed that anti-T. canis rabbit serum cross-reacted with several components of BPES antigen, except those of 39 and 43 kDa. In the present study, we have shown the immediate utility of a BPES antigen-based Western blot assay in separating Baylisascaris larva migrans from Toxocara larva migrans. Our study revealed information that could ultimately be useful for the development of a specific serological test based on defined native or recombinant antigens of B. procyonis.
During the present study, the specific recognition of 30- to 45-kDa BPES components by sera from patients with Baylisascaris larva migrans was observed. A previous study by Boyce et al. (2) showed that 33- to 45-kDa components of BPES were specifically recognized by serum from a child who died of Baylisascaris larva migrans, as confirmed at autopsy (10). Cunningham et al. (7) showed strong reactivity of a patient′s serum to B. procyonis larval ES antigens on a Western blot, including those in the 30- to 45-kDa range. In the present study, when serum from this patient was used in the BPES Western blot assay, although the reactivity was no longer as strong, a similar pattern involving recognition of the 30- to 45-kDa proteins was observed (Fig. 1, lane 6). Cited in the same study (7) is a 21-year-old male from Oregon who was seropositive for B. procyonis infection and also had characteristic lesions on brain biopsy. The serum from this patient also identified the 30- to 45-kDa components of BPES antigen (data not shown), confirming the infection. Toxocara larva migrans-positive serum samples, on the other hand, did not react with the 30- to 45-kDa BPES antigenic components (Fig. 3B), indicating that these antigens were specifically recognized only by serum from individuals with Baylisascaris larva migrans. Parasite-specific human patient serum is often difficult to obtain, and therefore this observation of a different pattern of reactivity with Baylisascaris and Toxocara patient sera was further strengthened by confirming the reactivity pattern with parasite-specific sera from experimentally infected rabbits (4) (Fig. 4).
In the case of larva migrans in humans, simultaneous or independent exposure to both B. procyonis and T. canis is quite possible. Recently, a 17-year-old boy from Oregon with acute meningoencephalitis due to B. procyonis neural larva migrans was reported to be seropositive for both Baylisascaris and Toxocara (6). In addition to evidence of severe neurological disease (more common with Baylisascaris), the reactivity of this patient's serum to 30- to 45-kDa BPES proteins on a Western blot indicated definitively a Baylisascaris infection. However, exact exposure to both parasites could not be determined. We believe that dual infection is also a likely explanation for why the serum from one CDC Toxocara-positive patient also showed strong reactivity to the 30- to 45-kDa proteins of the BPES antigen (Fig. 3B, lane 7). Since raccoons frequently occur in urban and suburban areas in close proximity to humans and their pets (16, 26), exposure to infective Baylisascaris eggs in the same environment as that of Toxocara eggs is likely a common occurrence. Most cases of infection with either or both parasites would be low level, resulting in covert infection with mild or nonspecific signs. Given this possibility and considering the one way cross-reactivity mentioned earlier, it might not be surprising to see background levels of antibodies to Toxocara spp. in some patients with Baylisascaris larva migrans. The fact that dogs can also act as definitive hosts for B. procyonis, developing patent infections and shedding B. procyonis eggs along with those of T. canis, could also lead to simultaneous exposure of humans to both parasites (12, 16, 17).
All of the Baylisascaris cases discussed in the present study had a high reactivity on the BPES ELISA and showed the 30- to 45-kDa antigen reactivity pattern. However, we are not sure whether the reactivity pattern described here would necessarily be seen with serum from patients with mild infection or disease. For example, serum from a 4-year-old Baylisascaris patient from Louisiana (25) reacted moderately on the BPES ELISA but showed very weak to no reactivity in a Western blot (Fig. 1, lane 12). We believe that detectable levels of antibodies to 30- to 45-kDa BPES proteins might not have been formed yet during the course of infection in this patient. Also, it is not known at this point how factors such as low infection or early diagnosis and treatment would affect the outcome of the Western blot analysis described here.
Although the emphasis of the present study has been on the serologic differentiation between Baylisascaris and Toxocara larva migrans, an important consideration in any serodiagnostic testing is knowledge of potential cross-reactivity. Current information about cross-reactivity of Baylisascaris with other parasites, including other nematodes, is very limited. Fortunately, many of the parasitic diseases can be excluded from the differential diagnosis based on epidemiology, particularly geographic distribution, and/or clinical symptoms. For example, the occurrence of other nematode parasites commonly associated with eosinophilic meningitis, such as Angiostrongylus cantonensis and Gnathostoma spinigerum (27), is restricted to certain geographical regions, primarily Asia, the Pacific Islands, and Caribbean. With a good travel and exposure history, they and other infections can be ruled out in many cases, leaving B. procyonis and Toxocara spp. as the primary focus for differential diagnosis. In North America and Europe, where both raccoons and dogs occur in many of the same areas, B. procyonis should be very high on the differential list for eosinophilic meningitis, particularly in children (11, 23). In fact, many infectious disease specialists in these areas are now aware of this parasite and provide information on it when potential cases arise (K. Kazacos, unpublished data). In clinical cases of Baylisascaris larva migrans, an initial screening with ELISA, followed by Western blot analysis using BPES antigen should prove very useful for specific diagnosis (Table 1). This would include differentiation of Baylisascaris from Toxocara spp. larva migrans when the etiology of eosinophilic meningitis is suspected to be due to one of these parasites.
TABLE 1.
Differential diagnosis of B. procyonis and Toxocara larva migrans using ES antigen-based serological assays
| Cause of larva migrans | Reactivity of serum on Toxocara ES antigen-based EIA | Reactivity on B. procyonis ES antigen-based ELISA | B. procyonis ES antigen-based Western blotsa |
|---|---|---|---|
| B. procyonis | − | + | + |
| Toxocara spp. | + | +b | − |
30- to 45-kDa antigen recognition.
Due to cross-reactivity.
Acknowledgments
We thank Mark Eberhard of the Division of Parasitic Diseases, CDC, Atlanta, GA, for providing serum from the experimentally infected baboon; Marianna Wilson at the CDC for ongoing dialogue on Toxocara serology and providing samples of Toxocara-positive human serum; and Jennifer Robichaud for assistance in the development of the BPES ELISA.
Footnotes
Published ahead of print on 9 September 2009.
REFERENCES
- 1.Beaver, P. C. 1969. The nature of visceral larva migrans. J. Parasitol. 55:3-12. [PubMed] [Google Scholar]
- 2.Boyce, W. M., D. J. Asai, J. K. Wilder, and K. R. Kazacos. 1989. Physicochemical characterization and monoclonal and polyclonal antibody recognition of Baylisascaris procyonis larval excretory-secretory antigens. J. Parasitol. 75:540-548. [PubMed] [Google Scholar]
- 3.Boyce, W. M., B. A. Branstetter, and K. R. Kazacos. 1988. Comparative analysis of larval excretory-secretory antigens of Baylisascaris procyonis, Toxocara canis, and Ascaris suum by Western blotting and enzyme immunoassay. Int. J. Parasitol. 18:109-113. [DOI] [PubMed] [Google Scholar]
- 4.Boyce, W. M., B. A. Branstetter, and K. R. Kazacos. 1988. In vitro culture of Baylisascaris procyonis and initial analysis of larval excretory-secretory antigens. Proc. Helminthol. Soc. Wash. 55:15-18. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2002. Raccoon roundworm encephalitis-Chicago, Illinois, and Los Angeles, California, 2000. MMWR Morb. Mortal. Weekly Rep. 50:1153-1155. [PubMed] [Google Scholar]
- 6.Chun, C. S., K. R. Kazacos, C. Glaser, D. Bardo, S. Dangoudoubiyam, and R. Nash. Global neurological deficits with Baylisascaris encephalitis in a previously healthy teenager. Pediatr. Infect. Dis. J., in press. [DOI] [PubMed]
- 7.Cunningham, C. K., K. R. Kazacos, J. A. Lucas, J. B. McAuley, E. J. Wozniak, and L. B. Weiner. 1994. Diagnosis and management of Baylisascaris procyonis infection in an infant with non-fatal meningoencephalitis. Clin. Infect. Dis. 18:868-872. [DOI] [PubMed] [Google Scholar]
- 8.De Savigny, D. H., A. Voller, and A. W. Woodruff. 1979. Toxocariasis: serological diagnosis by enzyme immunoassay. J. Clin. Pathol. 32:284-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Despommier, D. 2003. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin. Microbiol. Rev. 16:265-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox, A. S., K. R. Kazacos, N. S. Gould, P. T. Heydemann, C. Thomas, and K. M. Boyer. 1985. Fatal eosinophilic meningoencephalitis and visceral larva migrans caused by the raccoon ascarid Baylisascaris procyonis. N. Engl. J. Med. 312:1619-1623. [DOI] [PubMed] [Google Scholar]
- 11.Gavin, P. J., K. R. Kazacos, and S. T. Shulman. 2005. Baylisascariasis. Clin. Microbiol. Rev. 18:703-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greve, J. H., and S. E. O'Brien. 1989. Adult Baylisascaris infections in two dogs. Comp. Anim. Pract. 19:41-43. [Google Scholar]
- 13.Hajek, J., Y. Yau, P. Kertes, T. Soman, S. Laughlin, R. Kannani, K. R. Kazacos, S. Dangoudoubiyam, and M. A. Opavsky. Raccoon roundworm meningoencephalitis, Ontario, Canada. Can. J. Infect. Dis., in press. [DOI] [PMC free article] [PubMed]
- 14.Huff, D. S., R. C. Neafie, M. J. Binder, G. A. De Leon, L. W. Brown, and K. R. Kazacos. 1984. Case 4. The first fatal Baylisascaris infection in humans: an infant with eosinophilic meningoencephalitis. Pediatr. Pathol. 2:345-352. [DOI] [PubMed] [Google Scholar]
- 15.Jacquier, P., B. Gottstein, Y. Stingelin, and J. Eckert. 1991. Immunodiagnosis of toxocarosis in humans: evaluation of a new enzyme-linked immunosorbent assay kit. J. Clin. Microbiol. 29:1831-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kazacos, K. R. 2001. Baylisascaris procyonis and related species, p. 301-341. In W. M. Samuel, M. J. Pybus, and A. A. Kocan (ed.), Parasitic diseases of wild mammals, 2nd ed. Iowa State University Press, Ames.
- 17.Kazacos, K. R. 2006. Unusual fecal parasite in a dog. NAVC Clinician's Brief. 4:37-39. [Google Scholar]
- 18.Kazacos, K. R. 1997. Visceral, ocular, and neural larva migrans, p. 1459-1473. In D. H. Connor, F. W. Chandler, D. A. Schwartz, H. J. Manz, and E. E. Lack (ed.), Pathology of infectious diseases, vol. 2. Appleton and Lange, Stamford, CT. [Google Scholar]
- 19.Kazacos, K. R., W. L. Wirtz, P. P. Burger, and C. S. Christmas. 1981. Raccoon ascarid larvae as a cause of fatal central nervous system disease in subhuman primates. J. Am. Vet. Med. Assoc. 179:1089-1094. [PubMed] [Google Scholar]
- 20.Magnaval, J. F., L. T. Glickman, P. Dorchies, and B. Morassin. 2001. Highlights of human toxocariasis. Korean J. Parasitol. 39:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moertel, C. L., K. R. Kazacos, J. H. Butterfield, H. Kita, J. Watterson, and G. J. Gleich. 2001. Eosinophil-associated inflammation and elaboration of eosinophil-derived proteins in 2 children with raccoon roundworm (Baylisascaris procyonis) encephalitis. Pediatrics 108:e93. http://www.pediatrics.org/cgi/content/full/108/105/e193. [DOI] [PubMed] [Google Scholar]
- 22.Morrissey, J. H. 1981. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal. Biochem. 117:307-310. [DOI] [PubMed] [Google Scholar]
- 23.Murray, W. J., and K. R. Kazacos. 2004. Raccoon roundworm encephalitis. Clin. Infect. Dis. 39:1484-1492. [DOI] [PubMed] [Google Scholar]
- 24.Nunes, C. M., R. N. Tundisi, J. F. Garcia, M. B. Heinemann, S. Ogassawara, and L. J. Richtzenhain. 1997. Cross-reactions between Toxocara canis and Ascaris suum in the diagnosis of visceral larva migrans by Western blotting technique. Rev. Inst. Med. Trop. Sao Paulo 39:253-256. [DOI] [PubMed] [Google Scholar]
- 25.Pai, P. J., B. G. Blackburn, K. R. Kazacos, R. P. Warrier, and R. E. Begue. 2007. Full recovery from Baylisascaris procyonis eosinophilic meningitis. Emerg. Infect. Dis. 13:928-930. [DOI] [PubMed] [Google Scholar]
- 26.Roussere, G. P., W. J. Murray, C. B. Raudenbush, M. J. Kutilek, D. J. Levee, and K. R. Kazacos. 2003. Raccoon roundworm eggs near homes and risk for larva migrans disease, California communities. Emerg. Infect. Dis. 9:1516-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowley, H. A., R. M. Uht, K. R. Kazacos, J. Sakanari, W. V. Wheaton, A. J. Barkovich, and A. W. Bollen. 2000. Radiologic-pathologic findings in raccoon roundworm (Baylisascaris procyonis) encephalitis. Am. J. Neuroradiol. 21:415-420. [PMC free article] [PubMed] [Google Scholar]
- 28.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Won, K. Y., D. Kruszon-Moran, P. M. Schantz, and J. L. Jones. 2008. National seroprevalence and risk factors for zoonotic Toxocara spp. infection. Am. J. Trop. Med. Hyg. 79:552-557. [PubMed] [Google Scholar]