Abstract
Experimental leishmaniasis in BALB/c and C57BL/6 mice are the most investigated murine models that were used for the preclinical evaluation of Leishmania vaccine candidates. We have previously described two new inbred mouse strains named PWK and MAI issued from feral founders that also support the development of experimental leishmaniasis due to L. major. In this study, we sought to determine whether different mouse inbred strains generate concordant or discordant results when used to evaluate the potential of Leishmania proteins to protect against experimental leishmaniasis. To this end, two Leishmania proteins, namely, LACK (for Leishmania homolog of receptor for activated C kinase) and LmPDI (for L. major protein disulfide isomerase) were compared for their capacity to protect against experimental leishmaniasis in PWK, MAI, BALB/c, and C57BL/6 inbred mouse strains. Our data show that the capacity of Leishmania proteins to confer protection depends on the mouse strain used, stressing the important role played by the genetic background in shaping the immune response against the pathogen. These results may have important implications for the preclinical evaluation of candidate Leishmania vaccines: rather than using a single mouse strain, a panel of different inbred strains of various genetic backgrounds should be tested in parallel. The antigen that confers protection in the larger range of inbred strains may have better chances to be also protective in outbred human populations and should be selected for clinical trials.
The leishmaniasis are parasitic diseases due to a protozoan of the genus Leishmania that are endemic in 88 countries. Three hundred and fifty million people are exposed to the infection risk and 14 million people are known to be infected. Two million new cases, including 1.5 million of the cutaneous leishmaniasis, are estimated to appear annually (39). The leishmaniasis represent a worldwide major public health problem because of several therapeutic challenges such as drug toxicity, parasite resistance to current drugs, and the high cost of the new treatments. The problem is particularly serious since the disease affects the poorest classes of the developing countries. The cutaneous leishmaniasis are among the rare parasitic diseases that might be potentially vaccine preventable. However, even if theoretically feasible, there is still no human Leishmania vaccine available today (17). One serious obstacle facing such a goal is the lack of experimental animal models that tightly mimic the disease as it occurs in humans.
The experimental infection of inbred BALB/c and C57BL/6 mice by Leishmania major parasites has established the functionality of the Th1/Th2 dichotomy of CD4+ T helper cells and the contrasted pathogenic roles played in protection or disease promotion by the two Th subsets (33). Thus, C57BL/6 mice infected with L. major develop a Th1 response and efficiently control the disease within few weeks. In contrast, susceptible BALB/c mice mount a Th2 response and develop a severe, unremitting, and ultimately lethal disease (37). The susceptibility of BALB/c mice to L. major infection has been ascribed to the occurrence within the lymph nodes draining the inoculation site, of an early burst (at 16 h postinoculation) of interleukin-4 (IL-4) that polarizes the immune response toward the Th2 pathway (15, 24). The contrasted immunopathogenic mechanisms at work in BALB/c and C57BL/6 strains likely reflect differences in their genetic background. Since the majority of studies evaluating vaccine candidates have been conducted in the BALB/c model, it would be hazardous to extrapolate the conclusions drawn from these experiments to other inbred strains of different genetic backgrounds or to out bred animal models (i.e., primates): one given vaccine could be promising in one strain and still fail to protect in another strain (17). Thus, the criteria that would help to select at the preclinical stage a Leishmania antigen as a promising vaccine candidate worth entering the clinical trial stage are still not clearly defined.
We have recently identified two new inbred mouse strains derived from feral founders, named PWK and MAI, that are susceptible to L. major infection (1). MAI mice develop an infiltrated lesion at the site of parasite inoculation that enlarges over time in an unremitted way. In this strain, the primary infection does not induce protection against reinfection. Although the immune response to Leishmania antigens in MAI mice was characterized by a Th2 cytokine profile, IL-4 did not seem to play a dominant role in disease phenotype as in BALB/c mice. In PWK mice, the experimental disease induced by L. major infection is featured by a nodule that develops at the site of parasite inoculation. This nodule is larger and of a much longer duration (30 weeks to complete healing) than the one that develops in C57BL/6. PWK mice acquire a solid immunity after a primary infection and are completely refractory to a secondary challenge. They develop during infection a mixed Th1/Th2 cytokine pattern, with IL-10 playing a disease-promoting role.
The diverse disease patterns induced by L. major in PWK, MAI, C57BL/6, and BALB/c mice and the heterogeneity in the immunopathogenic mechanisms at work in each strain are likely shaped by the genetic background of the mice. This assumption led us to explore the effect of the genetic diversity of inbred mouse strains on the protection potentially conferred by Leishmania proteins against L major infection. Two Leishmania promising vaccine candidates were used, namely, the Leishmania homolog of receptor for activated C kinase (LACK) (31) and the L. major protein disulfide isomerase (LmPDI) (5).
MATERIALS AND METHODS
Mice.
Six- to eight-week-old males and females from inbred wild-mouse derived strains (MAI/Pas and PWK/Pas) bred by 20 to 50 brother-sister crosses were used in the present study. These strains were obtained from Institut Pasteur, Paris, France. Six- to eight-week-old Female BALB/cJ Rj and C57BL/6J Rj mice maintained in animal facilities at Institut Pasteur de Tunis (Tunis, Tunisia) were also used.
Parasite culture.
A highly virulent L. major isolate (zymodeme MON25; MHOM/TN/94/GLC94), obtained from a human ZCL lesions, was used in the present study (16). Parasites were cultivated on NNN medium at 26°C and then progressively adapted to RPMI 1640 medium (Sigma, St. Louis, MO) containing 2 mM l-glutamine, 100 U of penicillin/ml, 100 μg of streptomycin/ml, and 10% heat-inactivated fetal calf serum (Gibco, Grand Island, NY). The stationary phase was reached after 6 days of culture, and metacyclic parasites were then purified by using a Ficoll density gradient (Sigma) as previously described (36).
Expression and purification of the recombinant LmPDI and LACK proteins in Escherichia coli.
In the present study, we used the recombinant plasmids pET-LACK (23) and pET-LmPDI (5). E. coli BL21 cells harboring the recombinant plasmids were grown in LB medium, induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside; Amersham-Pharmacia) for 4 h, and then lysed. Recombinant LmPDI-His6 and whole LACK-His6 were synthesized. Insoluble proteins were solubilized in 6 M guanidine-HCl and then purified by affinity chromatography over Ni-NTA resin using an imidazole gradient elution according to the manufacturer's recommendations (GE Healthcare Biosciences, Uppsala, Sweden). The purity was demonstrated by using sodium dodecyl sulfate (SDS)-12% polyacrylamide gels and Coomassie blue staining. Recombinant proteins were also analyzed by Western blot experiments. Briefly, E. coli BL21 lysates expressing proteins or purified recombinant proteins were incubated in 1× SDS sample buffer, boiled for 10 min, separated on a 12% or 18% SDS-polyacrylamide gels, and electrotransferred to nitrocellulose membranes. Filters were blocked with phosphate-buffered saline (PBS)-0.1% Tween 20 (PBS-T) containing 5% nonfat dried milk at room temperature for 1 h and then incubated overnight at 4°C with the anti-histidine (anti-His) antibody (Amersham-Pharmacia). Filters were then washed three times with PBS-T, incubated with goat anti-mouse secondary antibody coupled to peroxidase (Amersham-Pharmacia) for 1 h at room temperature, washed three times with PBS-T, and revealed by using the diaminobenzidine tetrahydrochloride (Sigma).
Immunization of mice and parasite challenge.
Mice (five animals per group) were injected by the subcutaneous route in the left footpad with 25 μg of purified recombinant proteins produced in E. coli mixed with 30 μg of CpG oligonucleotide (Invitrogen) as an adjuvant in a volume of 50 μl. Control groups received only CpG or PBS. Each mouse received three injections administered at 4 weeks interval. At 30 days after the last injection, mice were challenged in the right footpad with 2 × 106 metacyclic promastigotes in 50 μl of PBS. After challenge, the lesion development was monitored at weekly intervals by measuring footpad swelling with a metric caliper, and the lesion size was calculated by subtracting the size of the contralateral uninfected footpad.
DTH reaction to Leishmania antigens.
The delayed-type hypersensitivity (DTH) reaction was monitored by injecting leishmanial total antigens (LTA; the equivalent of 2 × 106 promastigotes) in a final volume of 50 μl into the contralateral uninfected hind footpad. LTA were prepared as described previously (27). Footpad swelling was measured with a metric caliper at 24, 48, and 72 h postinfection.
Parasite quantification.
Parasite load was quantified by a limiting-dilution technique adapted from the work of Laskay et al. (18). Briefly, the excised footpad or popliteal draining lymph nodes were homogenized, and serial 10-fold dilutions were plated in triplicate in 96-well flat-bottom microtiter plates (Nunc, Roskilde, Denmark) containing Schneider's Drosophila medium supplemented with Grace's insect tissue culture medium (both from Gibco-BRL, Paisley, Scotland) supplemented with 100 U of penicillin/ml, 100 μg of streptomycin/ml, 2 mM l-glutamine, and 10% heat-inactivated fetal calf serum. The number of viable parasites was determined microscopically after 10 days at 26°C from the reciprocal of the highest dilution at which promastigotes could be detected.
Antibody measurement.
Serum samples collected from immunized mice were analyzed for the presence of specific anti-rLACK, and rLmPDI antibodies by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well high-binding Costar plates (Nunc) were coated with 10 μg of rLACK/ml and 5 μg of rLmPDI/ml (in 100 μl of 0.1 M carbonate-bicarbonate buffer [pH 9.5]) overnight at 4°C. The plates were washed thrice with 1× PBS-T, blocked with 0.5% gelatin PBS-T at 37°C for 1 h, and then washed three times with PBS-T. Sera were added at twofold serial dilutions starting at 1:100 and incubated for 2 h at 37°C. The plates were washed six times, and horseradish peroxidase-conjugated goat anti-mouse total immunoglobulin G (IgG) (Sigma) or IgG1 or IgG2a (Becton Dickinson) was added for 1 h at 37°C at dilutions of 1:2,000 for total IgG and 1:1,000 for IgG1 or IgG2a. The color was developed with o-phenylenediamine-H2O2 and read on an ELISA plate reader at 492 nm with correction at 620 nm.
Statistical analysis.
To determine the statistical significance of differences between groups, analysis of variance, followed by post hoc tests (Dunnett T3) was performed by using SPSS software (version 10.0). A P value of <0.05 was considered statistically significant.
RESULTS
Production and purification of recombinant proteins.
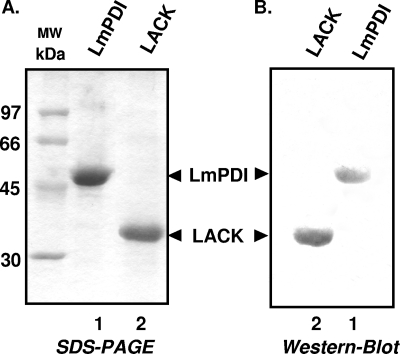
Recombinant proteins LACK and LmPDI were produced in E. coli BL21 cells by using the pET prokaryotic expression system. Proteins were then purified by affinity chromatography over Ni-NTA resin, and purity was assessed by SDS-polyacrylamide gel electrophoresis. Coomassie blue-stained bands of 36 and 50 kDa corresponding, respectively, to purified recombinant LmPDI and LACK proteins are shown in Fig. 1A (lanes 1 and 2, respectively). Western blot analysis showed that the monoclonal anti-histidine antibody strongly reacts with the recombinant LmPDI and LACK proteins (Fig. 1B, lanes 1 and 2, respectively).
FIG. 1.
Expression of recombinant proteins in E. coli. (A) Recombinant LmPDI and LACK proteins (lanes 1 and 2, respectively) were synthesized in BL21 cells, purified by affinity chromatography over Ni-NTA resin, and analyzed by SDS-polyacrylamide gel electrophoresis, followed by Coomassie blue staining. (B) Reactivity of the anti-His against the purified recombinant LmPDI and LACK (lanes 1 and 2, respectively). MW, molecular mass markers.
LmPDI and LACK induce antibody responses in the four immunized strains.
To assess whether the purified recombinant Leishmania antigens were immunogenic in the four mouse strains, sera collected at the end of the immunization were evaluated by ELISA for specific antibodies against the corresponding antigen. Table 1 shows the specific antibody levels of total IgG, IgG1, and IgG2a specific to LmPDI and LACK, measured 2 weeks after the third injection and tested at dilutions of 1:100 and 1:800 (Table 1).
TABLE 1.
Antibody levels of total IgG, IgG1, and IgG2a specific to LmPDI and LACK
| Mouse strain | Mean OD ± SDa |
|||||
|---|---|---|---|---|---|---|
| IgG |
IgG1 |
IgG2a |
||||
| rLmPDI | rLACK | rLmPDI | rLACK | rLmPDI | rLACK | |
| PWK | 0.92 ± 0.28 | 1.02 ± 0.44 | 0.73 ± 0.78 | 2.04 ± 0.9 | 2.14 ± 0.49 | 0.79 ± 0.4 |
| BALB/c | 1.52 ± 0.47 | 1.42 ± 0.54 | 2.44 ± 0.38 | 1.97 ± 1.10 | 2.16 ± 0.98 | 1.17 ± 0.48 |
| C57BL/6 | 1.06 ± 0.13 | 0.67 ± 0.19 | 1.27 ± 0.32 | 0.13 ± 0.16 | 0.47 ± 0.38 | 0.10 ± 0.06 |
| MAI | 0.38 ± 0.48 | 0.27 ± 0.07 | 0.31 ± 0.37 | 0.18 ± 0.08 | 0.81 ± 1.22 | 0.35 ± 0.21 |
The levels of anti-LmPDI and anti-LACK specific antibodies (IgG1, IgG2a, and total IgG) in the sera of BALB/c, C57BL/6, PWK, and MAI mice are indicated. Mice were immunized three times at 4-week intervals and bled 15 days after the last boost. An ELISA was performed at a 1:800 (rLmPDI) or a 1:100 (rLACK) dilution for each serum sample. The optical density (OD) values obtained with nonvaccinated controls mice were <0.1. The results were obtained from one representative experiment of two performed in different PWK, BALB/c, and C57BL/6 mouse groups.
In the four mouse strains, the immunization protocol was able to induce a positive antibody response against LmPDI and LACK, detected by ELISA at 1:800 and 1:100 serum dilutions, respectively. This result concerned total IgG, as well as the IgG1 and IgG2a, subclasses in BALB/c, C57BL/6, PWK, and MAI immunized mice, although to a lesser extent in the latter strain.
Effect of LACK and LmPDI preimmunization on experimental leishmaniasis in mouse inbred strains.
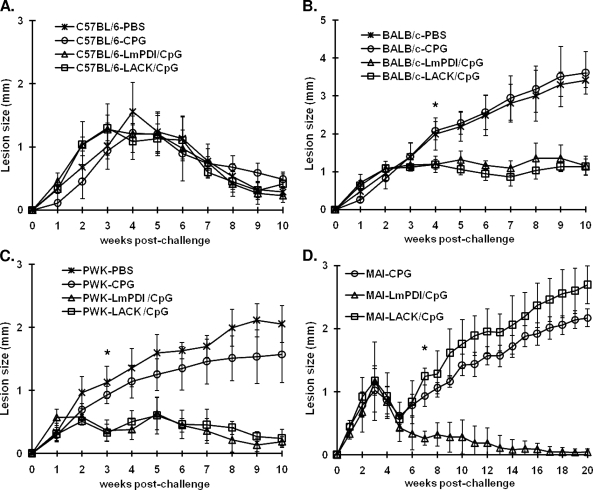
In order to evaluate the impact of the genetic background of inbred strains mice on the potential of vaccine candidates to protect against murine experimental leishmaniasis due to L. major, we compared the disease course in mice from the PWK, MAI, BALB/c, and C57BL/6 strains preimmunized with either of the two Leishmania proteins, namely, LACK and LmPDI. Mice received three injections of either of the purified recombinant proteins mixed with CpG. At 4 weeks after the last boost, mice were challenged with 2 × 106 metacyclic L. major parasites. Lesion development was monitored for up to 10 weeks for BALB/c, C57BL/6, and PWK mice and for up to 20 weeks for MAI mice. For the latter strain, the peculiar aspect of lesion size curve in the immunized groups at week 10 postinfection led us to further extend the monitoring 10 additional weeks. Control groups of mice belonging to each strain received either CpG (three injections) or PBS only, before parasite challenge. Due to the difficulty to get enough MAI mice, the experimental protocol did not include the PBS control group in this strain.
Lesion progression after challenge with L. major.
In C57BL/6 mice, we observed no difference in the disease course between control nonimmunized groups and mice that received either of the two vaccine preparations: LACK+CpG or LmPDI+CpG (Fig. 2A). In BALB/c mice, LmPDI+CpG or LACK+CpG immunization induced a partial effect: there was a lesion size stabilization that was apparent mostly at week 4 postchallenge onward (P < 0.02, Fig. 2B). However, there was no cure or significant lesion size reduction recorded at the end of the observation period. In PWK mice, LmPDI or LACK proteins combined with CpG, induced a significant reduction of lesion size, beginning at week 3, compared to control groups that received only CpG or PBS (P < 0.04 and P < 0.01 for LACK and LmPDI, respectively, Fig. 2C). In addition, PWK immunized mice showed an accelerated cure (10 weeks) compared to nonimmunized control mice in which an apparent cure occurs only after 30 weeks (1). Finally, preimmunization of MAI mice with LmPDI+CpG induced a significant reduction of lesion size (P < 10−3) at week 7 postchallenge onward, compared to CpG control groups. Interestingly, these mice showed complete healing of their lesion at week 20 postinfection. MAI mice injected with LACK+CpG did not show any change in the disease profile compared to control mice (Fig. 2D). In BALB/c, C57BL/6, and PWK mouse strains, CpG only did not significantly alter disease evolution. Mice receiving CpG only show footpad swelling that was comparable to the one developed by PBS only mice. The PBS control group of MAI mice were not included in the present study due to their unavailability.
FIG. 2.
Footpad swelling in mice immunized with LmPDI+CpG or LACK+CpG antigens. Mice (five animals per group) were vaccinated according to indicated vaccine schedule by the subcutaneous route in the right footpad, three times at 4-week intervals. One month after the last immunization, the animals were challenged in the left footpad by subcutaneous injection with 2 × 106 metacyclic L. major parasites, and footpad lesions were measured weekly thereafter in C57BL/6 (A), BALB/c (B), PWK (C), and MAI (D) mice. Control mice were vaccinated with CpG or PBS alone. The results shown here were from one representative experiment, out of two performed in different PWK, BALB/c, and C57BL/6 mouse groups. An asterisk indicates the starting point within the postchallenge time course at which the swelling was significantly different between the LACK+CpG or LmPDI+CpG groups and the CpG or PBS groups (in BALB/c and PWK mice, panels B and C, respectively) and between the LmPDI+CpG and CpG groups (in MAI mice, panel D).
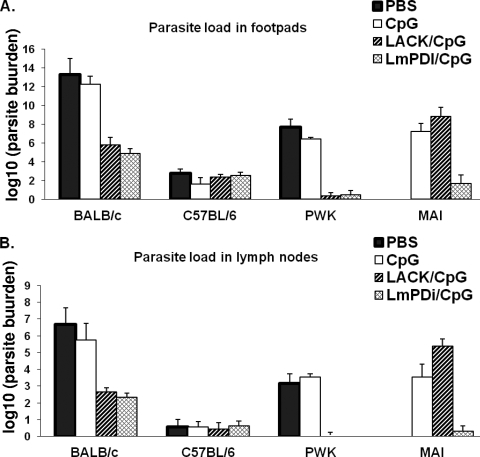
Parasite load quantification.
Parasites infiltrating footpads at the inoculation sites and in the draining lymph nodes were enumerated by limiting dilution at the end of the observation period (week 10 postinfection in PWK, C57BL/6 and BALB/c mice, and at week 20 postinfection in MAI mice) (Fig. 3). Globally, the decrease in parasite load in the footpad and in the draining lymph nodes in vaccinated and protected mice paralleled the reduction in the lesion size induced by vaccine preparation in some mouse strains. Thus, LmPDI+CpG induced a significant decrease in parasite loads in BALB/c mice (seven- and twofold log reductions compared to CpG control mice at the inoculation site and in draining lymph nodes, respectively). In PWK mice, LmPDI+CpG induced ∼6-fold log reduction of parasites in infected footpads of vaccinated mice compared to controls. In addition, no parasite could be detected in the draining lymph nodes of vaccinated animals. In MAI mice there were approximately five- and threefold log reductions compared to controls in infected footpads and in draining lymph nodes, respectively.
FIG. 3.
Parasite burden in the infected footpad and draining lymph node of the four mice strains vaccinated with LACK+CpG and LmPDI+CpG antigens and then challenged with L. major parasites. The parasite burden was determined by limiting-dilution assay at week 10 (PWK, C57BL/6, and BALB/c mice) or week 20 (MAI mice) postchallenge in vaccinated (with LACK+CpG [▨] or LmPDI+CpG [▩]) and control groups (PBS only [▪] or CpG only [□]). The results are expressed as the means ± the standard deviations of the log10 dilutions of infected footpads (A) or draining popliteal lymph nodes (B) positive for L. major promastigotes. The results represent triplicates from five individuals in each group. The results correspond to those obtained from one representative experiment out of two performed in different PWK, BALB/c, and C57BL/6 mouse groups.
With regard to mice immunized with LACK+CpG, there were approximately six- and threefold log reductions in the parasite load in BALB/c mice compared to controls for infected footpads and draining lymph nodes, respectively. In PWK mice there was an ∼6-fold log reduction compared to controls in footpads; no parasite could be recovered in the draining lymph nodes. Interestingly, and in accordance with the clinical observations, the parasite load in MAI mice immunized with LACK+CpG did not significantly decrease compared to controls. Finally, parasite loads were similar in all groups of C57BL/6 mice whatever the antigen used before challenge.
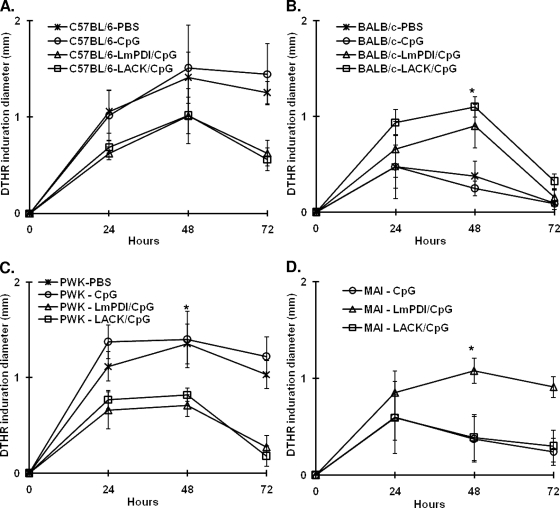
Monitoring the DTH reaction to Leishmania antigens.
The specific DTH reaction to LTA was measured as an indicator of a Th1 response to parasite antigens in L. major infected mice. The dermal infiltration induced by injection of parasite extracts in the contralateral uninfected footpad was evaluated at week 10 after parasite challenge in BALB/c, C57BL/6, and PWK mice and at week 20 after challenge in MAI mice. The footpad swelling was measured at 24, 48, and 72 h after LTA injection. At 48 h, the DTH reaction to LTA was significantly stronger (i.e., larger dermal induration) in BALB/c mice vaccinated with either LmPDI+CpG (P < 0.008) or LACK+CpG (P < 10−3) (Fig. 4B) and in MAI mice vaccinated with LmPDI+CpG (P < 0.003) compared to CpG-only control mice (Fig. 4D). Unexpectedly, the DTH reaction was almost negative in C57BL/6 and PWK mice vaccinated with LmPDI+CpG or LACK+CpG, whereas, as expected for these resistant strains, it was positive in the PBS-only and/or the CpG-only control groups (Fig. 4A and C, respectively). This reduction in DTH induced by the preimmunization protocol was statistically significant only in LmPDI-immunized PWK mice (P < 0.03).
FIG. 4.
Leishmania specific DTH reaction. Groups of C57BL/6 (A), BALB/c (B), PWK (C), and MAI (D) mice immunized with PBS, CpG, LACK+CpG, or LmPDI+CpG antigens and challenged with L. major parasites were injected in the uninfected contralateral hind footpad with LTA. Footpad swelling indicating a specific DTH reaction was measured (in millimeters) with a metric caliper at 24, 48, and 72 h. The results correspond to those obtained from one representative experiment out of two performed in different PWK, BALB/c, and C57BL/6 mouse groups and are represented as means ± the standard deviations. An asterisk indicates that the DTH reaction was significantly different between the LACK+CpG or LmPDI+CpG groups and the CpG or PBS groups (in BALB/c and PWK mice, panels B and C, respectively) and between LmPDI+CpG and CpG groups (in MAI mice, panel D).
Finally, in BALB/c, C57BL/6, and PWK mice the DTH reactions in CpG-only control mice were comparable to those observed in their respective PBS-only control groups.
In conclusion, the four mouse strains showed contrasted responses to the vaccinating antigens (summarized in Table 2), thus confirming the important role played by the genetic background in the immune responses to selected antigens and hence on the potential of the latter to serve as candidate vaccines.
TABLE 2.
Summary of data obtained with different vaccine preparations in four mouse strains
| Vaccine prepn | Findinga in: |
|||
|---|---|---|---|---|
| C57BL/6 mice | BALB/c mice | PWK mice | MAI mice | |
| LmPDI+CpG | No effect | Partial effect* | Protection† | Protection‡ |
| LACK+CpG | No effect | Partial effect* | Protection† | No effect |
| CpG alone | No effect | No effect | No effect | No effect |
*, Smaller lesions with no cure; †, smaller lesions with accelerated cure; ‡, smaller lesions with cure.
DISCUSSION
Cutaneous leishmaniasis due to L. major is an autoresolutive disease in humans and leads to a solid and life-long immunity that protects against reinfection (22). However, and despite enormous efforts, no vaccine is yet available, and this failure is likely due to the fact that immunity induced by the disease is primarily cell mediated (antibodies are not protective) and that correlates of protection are still poorly understood.
Hence, the success of a vaccine development strategy against L. major depends on the identification of the proper antigen(s) to use and the design of an optimal immunization protocol. An accurate preclinical model would allow a reasonably safe extrapolation to humans of the positive results generated in experimental animals and justify the implementation of clinical trials in humans. Although nonhuman primates would offer an advantage for candidate vaccine selection, the use of monkeys is restricted by ethical and technical considerations, and only a few studies were carried out with this model (7). Thus, small animal models are still needed to evaluate Leishmania vaccines and to identify correlates of protection that could predict vaccine efficacy in higher animals and humans. BALB/c mice, which are exquisitely sensitive to infection by L. major, were the most studied model to evaluate different vaccine candidates. The results were variable and sometimes contradictory, depending on the assay design. Actually, considering the very particular mechanism of BALB/c mouse susceptibility to L. major infection, conclusions drawn from disease-modulating experiments with this strain can hardly be extrapolated to human disease. In fact, the T-helper cell polarization is less sharply defined in humans than in mice. Hence, the immune response to the parasite in patients with cutaneous leishmaniasis is rather characterized by the production of a mixture of Th1 and Th2 cytokines (35).
The C57BL/6 mouse model would appear more appropriate since it takes into account two main features of the natural infection: low dose parasite challenge (100 to 1,000 metacyclic promastigotes) and inoculation into a dermal site, along with sand fly saliva components (3). The healing lesions that develop in these mice are more relevant to the lesions that develop in the natural rodent reservoirs of L. major and in humans. Inoculation of L. major parasites to C57BL/6 mice induces a Th1 immune profile resulting in gamma interferon production, macrophage activation, parasite killing, and resolution of the experimental lesion, as well as resistance to a subsequent infection (3, 32). Vaccine evaluation studies were less frequently conducted in C57BL/6 mice than in BALB/c mice. In fact, since C57BL/6 mice develop small and rapidly healing lesions, this strain is still inappropriate for a good estimation of the protective power of a vaccine candidate.
To our knowledge, only a few studies have conducted comparative evaluation of a candidate vaccine tested in parallel on the BALB/c and C57BL/6. Some of these studies have led to discordant results. Thus, vaccination with an avirulent L. major clone was protective against a virulent challenge in C57BL/6 but only partially protective in BALB/c and BALB/c H-2b and H-2k mice (26). Membrane antigens were also shown to be protective against a virulent challenge with L. mexicana in C57BL/6 and CBA but not in NZB and C57BL/10 mice (21). Vaccination with the L. infantum acidic ribosomal P0 protein (LiP0)+CpG induced protection against cutaneous leishmaniasis in C57BL/6 mice but did not prevent progressive disease in BALB/c mice, although LiP0 protein vaccination induced a Th1 immune response in both strains (14). In this last study, it is important that the vaccination protocols used in the two strains were different.
These results stress the important role played by the host genetics not only in determining susceptibility to infection but also in shaping an immune protective response after vaccination. Thus, identifying additional models of experimental leishmaniasis is urgently needed. Our group has characterized two mouse strains, named PWK and MAI, derived from founders of feral origin that are susceptible to L. major infection (1). In the present study, the two strains were used to evaluate two vaccine candidates (i.e., LACK and LmPDI) comparatively with the two classic strains BALB/c and C57BL/6.
The LACK antigen, one of the most extensively studied antigens in term of vaccine potential, is a conserved 36-kDa member of the tryptophan-aspartic acid repeat family of proteins, expressed in both leishmanial life cycle stages (28), which is involved in many regulatory functions, such as binding to multiprotein complexes involved in DNA replication and RNA synthesis (11). This antigen has the ability to induce Th2 type immune responses favored by the expansion of IL-4-secreting T cells (15, 19). The immunization of mice with a truncated recombinant LACK protein with IL-12 as an adjuvant conferred protection against infection (28) but with a short duration (12).
The LmPDI antigen (50 kDa), identified in our laboratory as a virulence factor of L. major parasites (5), is encoded by a single-copy gene and constitutes a member of the thioredoxin superfamily, which is composed of several redox proteins playing a key role in disulfide bond formation, isomerization, reduction within the endoplasmic reticulum, and displaying a chaperone activity (9). These molecules are essential for assisting unfolded or incorrectly folded proteins to attain their native state.
Our results show that the two vaccine preparations were immunogenic in the four strains as they induce specific antibody responses, although weak responses were detected in MAI mice.
We show that LmPDI+CpG or LACK+CpG were partially protective in BALB/c mice since they only stabilize the progression of the lesions. The protection induced by LACK is in accordance with previously described results showing that a partial protection could be induced by LACK in combination with IL-12 (13, 28). In PWK mice, which are characterized by the development of a large chronic nodule that ultimately regresses only at week 30 postinoculation, the two vaccine preparations were able to reduce lesions progression and accelerate cure that occurred within only 10 weeks postchallenge.
For MAI mice, LACK+CpG had no effect, whereas LmPDI+CpG vaccination was significantly protective since lesions completely disappeared at the end of the vaccination protocol. It is known that nonimmunized MAI control mice infected with L. major are not able to heal spontaneously (1). The transient lesion size reduction observed in vaccinated and unvaccinated MAI mice during the third week postinfection might be an effect of CpG. Disease progression reappeared in the fifth week postinfection in mice vaccinated with CpG only or with LACK+CpG, whereas reduction in lesion size continued until cure in mice receiving LmPDI+CpG. It is interesting that neither of the two vaccine preparations could modulate disease evolution or protect mice against L. major infection in mice from the resistant C57BL/6 strain, stressing the large variability in antigen potency in different mice genetic backgrounds.
Since control mice that received only CpG or PBS and were then challenged with L. major did not show any significant alteration in disease evolution (i.e., lesion size reduction), one could assume that the protective effect induced by some preimmunization protocols in BALB/c, PWK, and MAI mouse groups were not due to the CpG immunostimulant but rather reflect the effect of the Leishmania protein vaccine candidate. CpG is an adjuvant that is recognized by cells of the innate immune system through Toll-like receptor 9, and it has the capacity to stimulate Th1-type responses. In the murine model, addition of CpG to live vaccine improves the immune responses and limits lesion development (25). Moreover, some studies reported that the use of L. major with CpG was a safe practice in a nonhuman primate model of cutaneous leishmaniasis (10, 38), suggesting that this approach might be effective in humans.
Previous studies in the experimental mouse model have shown that the protection induced by immune manipulation or vaccine candidates, featured by lesion size reduction, is paralleled by a significant reduction in the parasite load in the lesion and in the lymph node draining the inoculation site. Our results show a similar strong correlation between the lesion size reduction and the decrease of the parasite load. Indeed, vaccination with LACK+CpG and LmPDI+CpG reduced drastically this load in BALB/c and PWK mice, whereas it did not have any effect in C57BL/6 mice. This strong correlation is also true in vaccinated MAI mice receiving LmPDI+CpG. In contrast, mice receiving LACK+CpG did not show any reduction in the lesion size or in the parasite load. Measurement of the parasite load, in addition to the reduction in lesion size, is important to ascertain any antiparasitic effect of an immunomodulation. Actually, lesion size reduction may merely reflect a reduction in the inflammatory cell infiltration irrespective of the parasite load. Thus, in deficient mice such as SCID or IL-12−/− mice, high dermal parasite loads were associated with little or no pathology (4). In addition, the initial phase of infection (5 weeks) in experimental murine leishmaniasis is clinically silent due to absence of inflammation despite active parasite multiplication within macrophages (4).
With regard to the DTH reaction considered as an in vivo measure of the cellular response to Leishmania antigens of the Th1 type, it was positive in BALB/c and MAI mice in which vaccine preparations exerted a partially protective effect compared to control groups (PBS or CpG only) in which the DTH was negative. Interestingly, and quite unexpectedly, preimmunized groups in PWK and C57BL/6 mice did not develop positive DTH reaction to LTA despite cure in contrast to nonimmunized control groups. Discordance between cutaneous DTH (called the LST leishmanin skin test in humans) and disease or vice versa, is a well-known situation in natural (6, 29, 34) or experimental (8, 20, 30) disease.
The unexpected result observed in PWK and C57BL/6 suggests that preimmunization of the two mouse strains inhibits the development of a parasite-specific DTH reaction despite promoting accelerated cure. Several hypotheses could account for this unexpected feature. (i) On the one hand, it could indicate that LTA-specific Th1 cells in these two resistant strains were sequestered at the parasite inoculation site, the draining lymph nodes, and/or other secondary lymphoid organs of these mice. These cells would be thus not available for homing in the LTA inoculation site in the contralateral footpad. Actually, this hypothesis is unlikely since this feature was not observed in PBS only or CpG only control PWK or C57BL/6 mice. (ii) Alternatively, it may indicate that the mechanisms underlying the development of a Leishmania-specific DTH reaction and those responsible for a cure are different and might be activated (or inactivated) separately (30). (iii) On the other hand, one may consider the possibility that the negativated cutaneous DTH reaction in preimmunized PWK and C57BL/6 mice is due to some functional inhibition of Th1 cells at the site of LTA inoculation. Several reports have previously ascribed such functional inhibition to Foxp3+ CD4+ CD25+ Treg cells. These cells home on the dermal site of infection in experimental leishmaniasis in C57BL/6 mice (2). They are thought to play an important role in preventing sterile cure and in maintaining residual parasites at the inoculation site even after cure. Whether the preimmunization protocols with LACK+GpG or LmPDI+CpG that we used have a stimulatory effect on Leishmania specific Treg cells in resistant inbred strains is only speculative and merits further investigation. However, one should mention that one study has reported that live parasites with CpG protect against experimental leishmaniasis in C57BL/6 and reduce the accumulation Foxp3+ CD4+ CD25+ Treg cells at the lesion site (40).
In conclusion, using four inbred mouse strains and two Leishmania antigen preparations mixed with CpG immunostimulant, we showed that the genetic background of the experimental animals has a major impact on the ability of a given antigen to protect against a parasite challenge. It is likely that the set of proteins that could potentially protect against experimental leishmaniasis or modulate favorably disease evolution differs from one strain to another. These results may have important implications for the preclinical evaluation of candidate vaccines in mice prior to clinical trials in humans: thus, rather than using a single mouse strain, candidate vaccines should be comparatively evaluated in a range of various inbred strains differing in their genetic background. It is tempting to assume that a vaccine candidate that confers protection to the larger set of murine inbred strains is more likely to be protective in the outbred human population.
Acknowledgments
This study was supported by the Ministry for Higher Education, Research, and Technology in Tunisia and by a UBS-Optimus grant. F.B. received a fellowship from Agence Universitaire de la Francophonie and was partially supported by UBS-Optimus.
We thank Aurelie Millet-Slaoui and Ines Lakhal-Naouar for their valuable help and Nissaf Ben-Alaya for statistical analysis advice. We are also grateful to Z. Benlasfar and his collaborators (Animal Facilities) for their help in animal handling and housing.
Footnotes
Published ahead of print on 2 September 2009.
REFERENCES
- 1.Babay, B. E., H. Louzir, C. Kebaier, S. Boubaker, K. Dellagi, and P. A. Cazenave. 2004. Inbred strains derived from feral mice reveal new pathogenic mechanisms of experimental leishmaniasis due to Leishmania major. Infect. Immun. 72:4603-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belkaid, Y. 2003. The role of CD4+ CD25+ regulatory T cells in Leishmania infection. Expert Opin. Biol. Ther. 3:875-885. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid, Y., S. Kamhawi, G. Modi, J. Valenzuela, N. Noben-Trauth, E. Rowton, J. Ribeiro, and D. L. Sacks. 1998. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 188:1941-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belkaid, Y., S. Mendez, R. Lira, N. Kadambi, G. Milon, and D. Sacks. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969-977. [DOI] [PubMed] [Google Scholar]
- 5.Ben Achour, Y., M. Chenik, H. Louzir, and K. Dellagi. 2002. Identification of a disulfide isomerase protein of Leishmania major as a putative virulence factor. Infect. Immun. 70:3576-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben Salah, A., H. Louzir, S. Chlif, M. Mokni, A. Zaatour, M. Raouene, R. B. Ismail, and K. Dellagi. 2005. The predictive validity of naturally acquired delayed-type hypersensitivity to leishmanin in resistance to Leishmania major-associated cutaneous leishmaniasis. J. Infect. Dis. 192:1981-1987. [DOI] [PubMed] [Google Scholar]
- 7.Campos-Neto, A., R. Porrozzi, K. Greeson, R. N. Coler, J. R. Webb, Y. A. Seiky, S. G. Reed, and G. Grimaldi, Jr. 2001. Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and nonhuman primate models of the human disease. Infect. Immun. 69:4103-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhaliwal, J. S., F. Y. Liew, and F. E. Cox. 1985. Specific suppressor T cells for delayed-type hypersensitivity in susceptible mice immunized against cutaneous leishmaniasis. Infect. Immun. 49:417-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrari, D. M., and H. D. Soling. 1999. The protein disulphide-isomerase family: unravelling a string of folds. Biochem. J. 339(Pt. 1):1-10. [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn, B., V. Wang, D. L. Sacks, R. A. Seder, and D. Verthelyi. 2005. Prevention and treatment of cutaneous leishmaniasis in primates by using synthetic type D/A oligodeoxynucleotides expressing CpG motifs. Infect. Immun. 73:4948-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez-Aseguinolaza, G., S. Taladriz, A. Marquet, and V. Larraga. 1999. Molecular cloning, cell localization and binding affinity to DNA replication proteins of the p36/LACK protective antigen from Leishmania infantum. Eur. J. Biochem. 259:909-916. [DOI] [PubMed] [Google Scholar]
- 12.Gurunathan, S., C. Prussin, D. L. Sacks, and R. A. Seder. 1998. Vaccine requirements for sustained cellular immunity to an intracellular parasitic infection. Nat. Med. 4:1409-1415. [DOI] [PubMed] [Google Scholar]
- 13.Gurunathan, S., D. L. Sacks, D. R. Brown, S. L. Reiner, H. Charest, N. Glaichenhaus, and R. A. Seder. 1997. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J. Exp. Med. 186:1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iborra, S., J. Carrion, C. Anderson, C. Alonso, D. Sacks, and M. Soto. 2005. Vaccination with the Leishmania infantum acidic ribosomal P0 protein plus CpG oligodeoxynucleotides induces protection against cutaneous leishmaniasis in C57BL/6 mice but does not prevent progressive disease in BALB/c mice. Infect. Immun. 73:5842-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julia, V., and N. Glaichenhaus. 1999. CD4+ T cells which react to the Leishmania major LACK antigen rapidly secrete interleukin-4 and are detrimental to the host in resistant B10.D2 mice. Infect. Immun. 67:3641-3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kebaier, C., H. Louzir, M. Chenik, A. Ben Salah, and K. Dellagi. 2001. Heterogeneity of wild Leishmania major isolates in experimental murine pathogenicity and specific immune response. Infect. Immun. 69:4906-4915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kedzierski, L., Y. Zhu, and E. Handman. 2006. Leishmania vaccines: progress and problems. Parasitology 133(Suppl.):S87-S112. [DOI] [PubMed] [Google Scholar]
- 18.Laskay, T., A. Diefenbach, M. Rollinghoff, and W. Solbach. 1995. Early parasite containment is decisive for resistance to Leishmania major infection. Eur. J. Immunol. 25:2220-2227. [DOI] [PubMed] [Google Scholar]
- 19.Launois, P., K. G. Swihart, G. Milon, and J. A. Louis. 1997. Early production of IL-4 in susceptible mice infected with Leishmania major rapidly induces IL-12 unresponsiveness. J. Immunol. 158:3317-3324. [PubMed] [Google Scholar]
- 20.Lechner, A., U. Ritter, R. Varona, G. Marquez, C. Bogdan, and H. Korner. 2007. Protective immunity and delayed type hypersensitivity reaction are uncoupled in experimental Leishmania major infection of CCR6-negative mice. Microbes Infect. 9:291-299. [DOI] [PubMed] [Google Scholar]
- 21.Lezama-Davila, C. M. 1997. Vaccination of different strains of mice against cutaneous leishmaniasis: usefulness of membrane antigens encapsulated into liposomes by intraperitoneal and subcutaneous administration. Arch. Med. Res. 28:47-53. [PubMed] [Google Scholar]
- 22.Liew, F. Y., and C. A. O'Donnell. 1993. Immunology of leishmaniasis. Adv. Parasitol. 32:161-259. [DOI] [PubMed] [Google Scholar]
- 23.Maalej, I. A., M. Chenik, H. Louzir, A. Ben Salah, C. Bahloul, F. Amri, and K. Dellagi. 2003. Comparative evaluation of ELISAs based on ten recombinant or purified Leishmania antigens for the serodiagnosis of Mediterranean visceral leishmaniasis. Am. J. Trop. Med. Hyg. 68:312-320. [PubMed] [Google Scholar]
- 24.Maillard, I., P. Launois, H. Himmelrich, H. Acha-Orbea, H. Diggelmann, R. M. Locksley, and J. A. Louis. 2001. Functional plasticity of the LACK-reactive Vβ4-Vα8 CD4+ T cells normally producing the early IL-4 instructing Th2 cell development and susceptibility to Leishmania major in BALB/c mice. Eur. J. Immunol. 31:1288-1296. [PubMed] [Google Scholar]
- 25.Mendez, S., K. Tabbara, Y. Belkaid, S. Bertholet, D. Verthelyi, D. Klinman, R. A. Seder, and D. L. Sacks. 2003. Coinjection with CpG-containing immunostimulatory oligodeoxynucleotides reduces the pathogenicity of a live vaccine against cutaneous leishmaniasis but maintains its potency and durability. Infect. Immun. 71:5121-5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell, G. F., E. Handman, and T. W. Spithill. 1984. Vaccination against cutaneous leishmaniasis in mice using nonpathogenic cloned promastigotes of Leishmania major and importance of route of injection. Aust. J. Exp. Biol. Med. Sci. 62(Pt. 2):145-153. [DOI] [PubMed] [Google Scholar]
- 27.Mohrs, M., B. Ledermann, G. Kohler, A. Dorfmuller, A. Gessner, and F. Brombacher. 1999. Differences between IL-4- and IL-4 receptor alpha-deficient mice in chronic leishmaniasis reveal a protective role for IL-13 receptor signaling. J. Immunol. 162:7302-7308. [PubMed] [Google Scholar]
- 28.Mougneau, E., F. Altare, A. E. Wakil, S. Zheng, T. Coppola, Z. E. Wang, R. Waldmann, R. M. Locksley, and N. Glaichenhaus. 1995. Expression cloning of a protective Leishmania antigen. Science 268:563-566. [DOI] [PubMed] [Google Scholar]
- 29.Nylen, S., A. Khamesipour, A. Mohammadi, R. Jafari-Shakib, L. Eidsmo, S. Noazin, F. Modabber, and H. Akuffo. 2006. Surrogate markers of immunity to Leishmania major in leishmanin skin test negative individuals from an endemic area re-visited. Vaccine 24:6944-6954. [DOI] [PubMed] [Google Scholar]
- 30.Okwor, I., D. Liu, S. M. Beverley, and J. E. Uzonna. 2009. Inoculation of killed Leishmania major into immune mice rapidly disrupts immunity to a secondary challenge via IL-10-mediated process. Proc. Natl. Acad. Sci. USA. [DOI] [PMC free article] [PubMed]
- 31.Palatnik-de-Sousa, C. B. 2008. Vaccines for leishmaniasis in the fore coming 25 years. Vaccine 26:1709-1724. [DOI] [PubMed] [Google Scholar]
- 32.Reiner, S. L., and R. M. Locksley. 1995. The regulation of immunity to Leishmania major. Annu. Rev. Immunol. 13:151-177. [DOI] [PubMed] [Google Scholar]
- 33.Sacks, D., and N. Noben-Trauth. 2002. The immunology of susceptibility and resistance to Leishmania major in mice. Nat. Rev. Immunol. 2:845-858. [DOI] [PubMed] [Google Scholar]
- 34.Sassi, A., H. Louzir, A. Ben Salah, M. Mokni, A. Ben Osman, and K. Dellagi. 1999. Leishmanin skin test lymphoproliferative responses and cytokine production after symptomatic or asymptomatic Leishmania major infection in Tunisia. Clin. Exp. Immunol. 116:127-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott, P. 1998. Differentiation, regulation, and death of T helper cell subsets during infection with Leishmania major. Immunol. Res. 17:229-238. [DOI] [PubMed] [Google Scholar]
- 36.Spath, G. F., and S. M. Beverley. 2001. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp. Parasitol. 99:97-103. [DOI] [PubMed] [Google Scholar]
- 37.Sypek, J. P., C. L. Chung, S. E. Mayor, J. M. Subramanyam, S. J. Goldman, D. S. Sieburth, S. F. Wolf, and R. G. Schaub. 1993. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J. Exp. Med. 177:1797-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verthelyi, D., R. T. Kenney, R. A. Seder, A. A. Gam, B. Friedag, and D. M. Klinman. 2002. CpG oligodeoxynucleotides as vaccine adjuvants in primates. J. Immunol. 168:1659-1663. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. 2004. The world health report 2004: changing history. World Health Organization, Geneva, Switzerland. http://www.who.int/whr/2004/en/index.html.
- 40.Wu, W., L. Weigand, Y. Belkaid, and S. Mendez. 2006. Immunomodulatory effects associated with a live vaccine against Leishmania major containing CpG oligodeoxynucleotides. Eur. J. Immunol. 36:3238-3247. [DOI] [PubMed] [Google Scholar]